Effects of Different Guests on Pyrolysis Mechanism of α-CL−20/Guest at High Temperatures by Reactive Molecular Dynamics Simulations at High Temperatures
Abstract
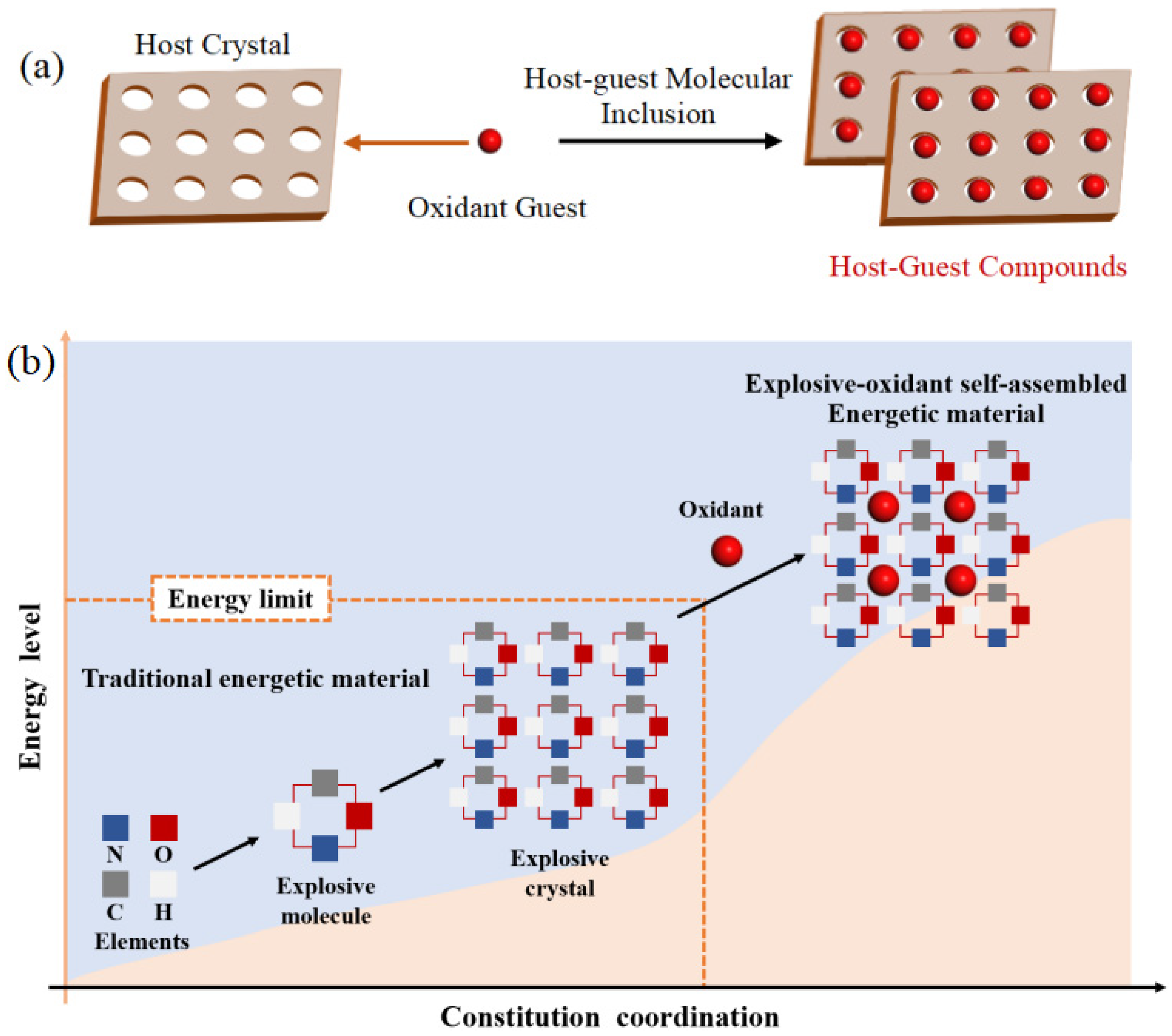
1. Introduction
2. Results and Discussion
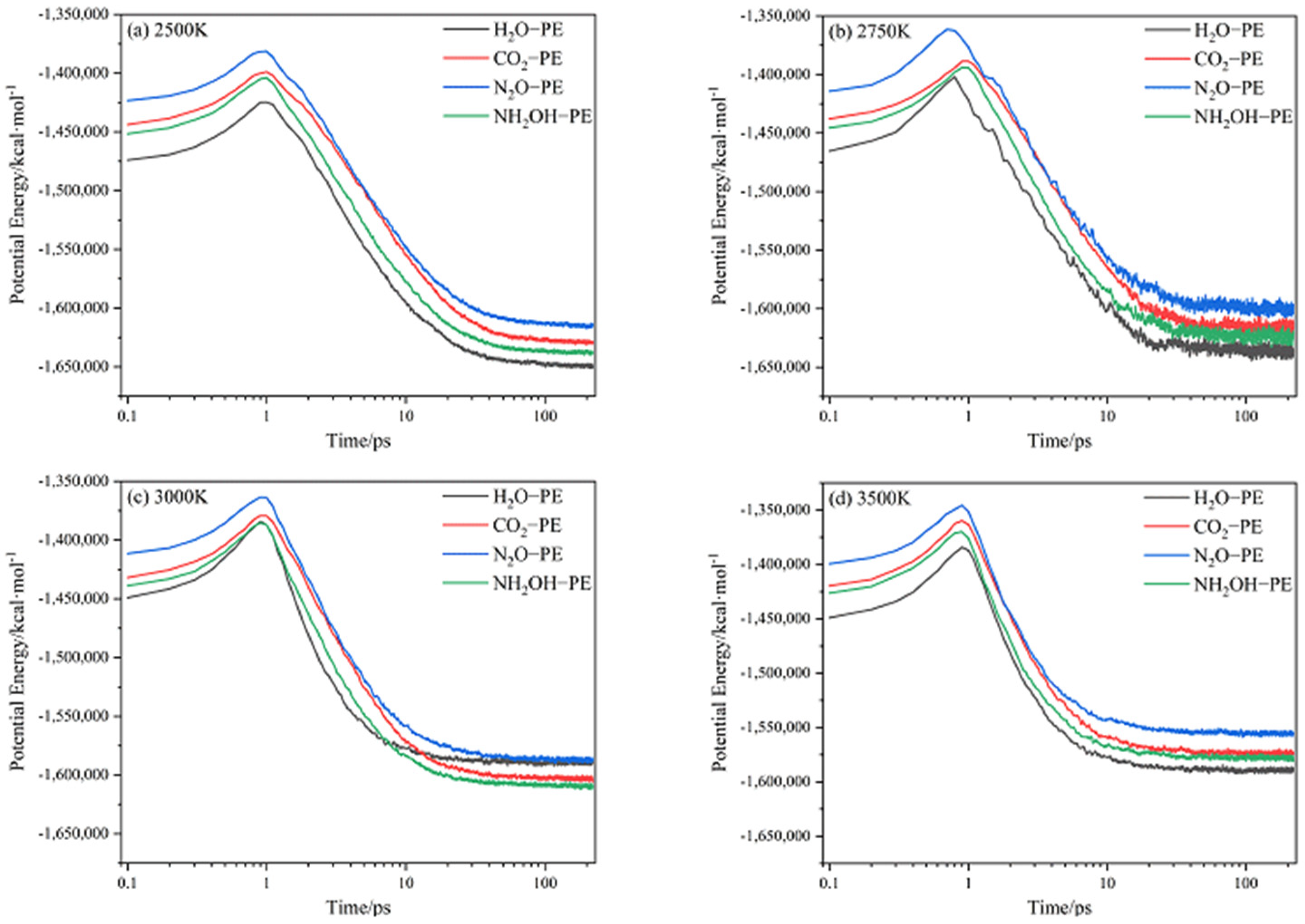
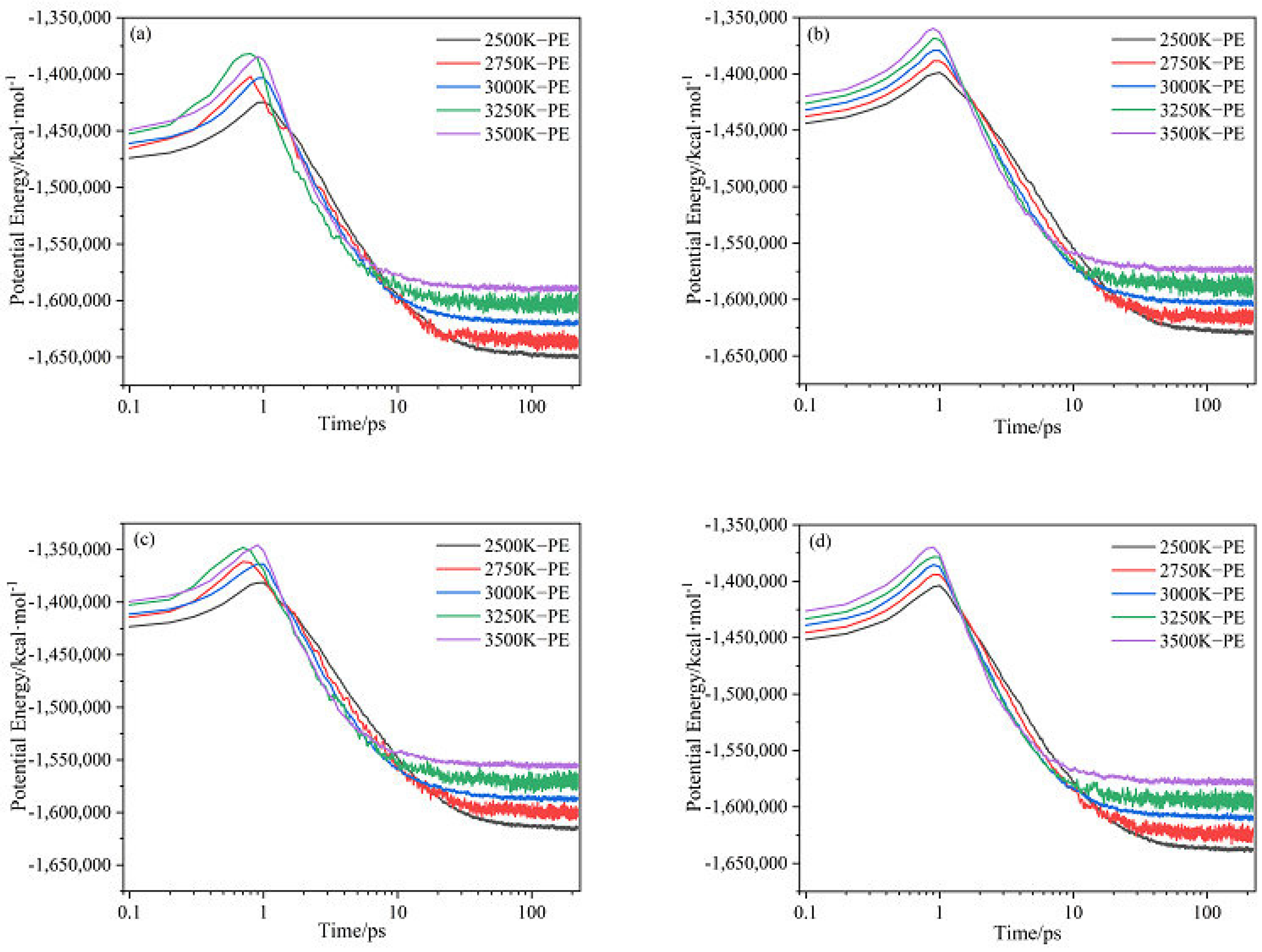
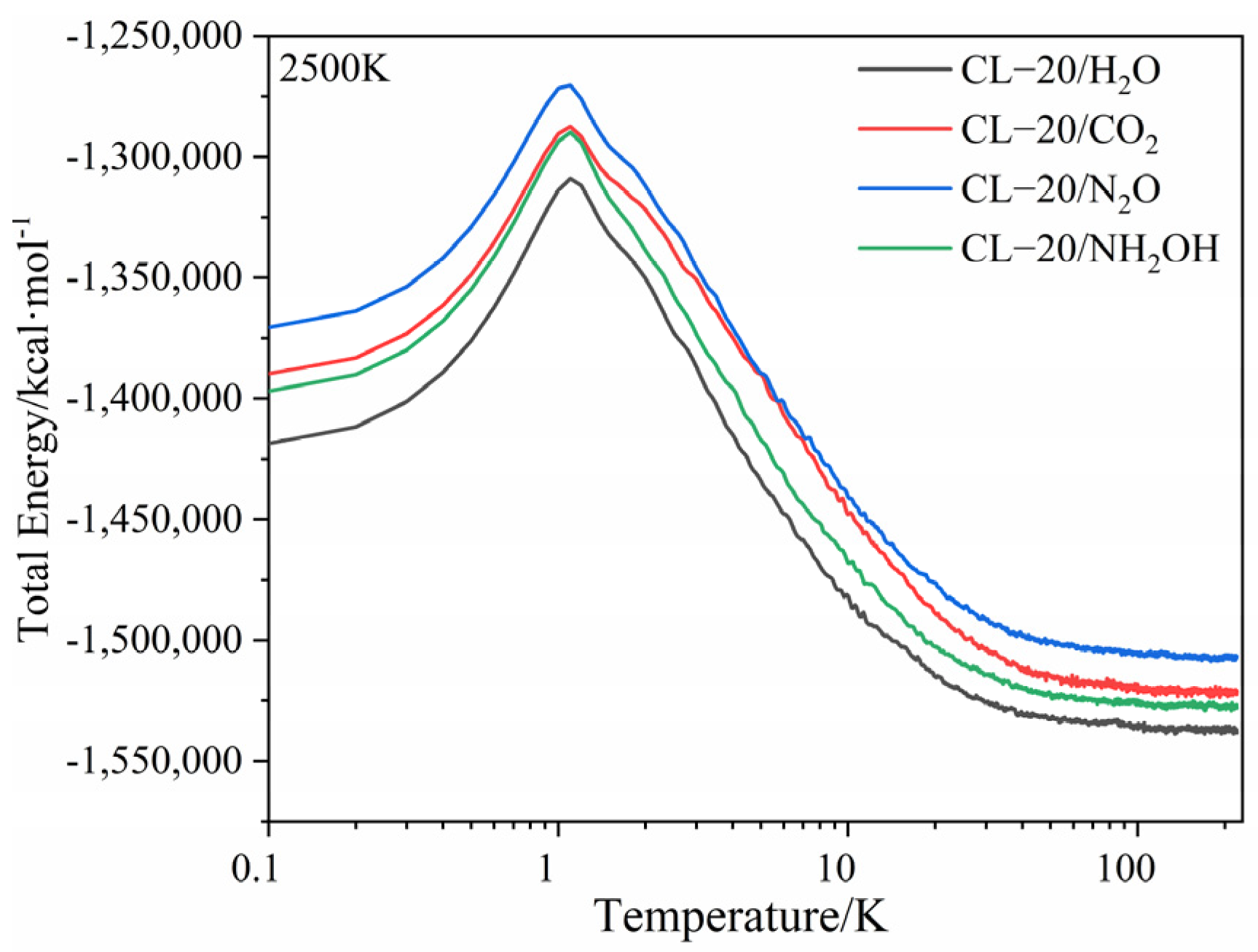
2.1. Potential Energy (PE) and Total Energy for CL-20/H2O, CL-20/CO2, CL-20/N2O, CL-20/NH2OH Systems
2.2. Initial Decomposition Stage
2.2.1. Initial Reaction Path of CL-20/Nitrogen-Guest
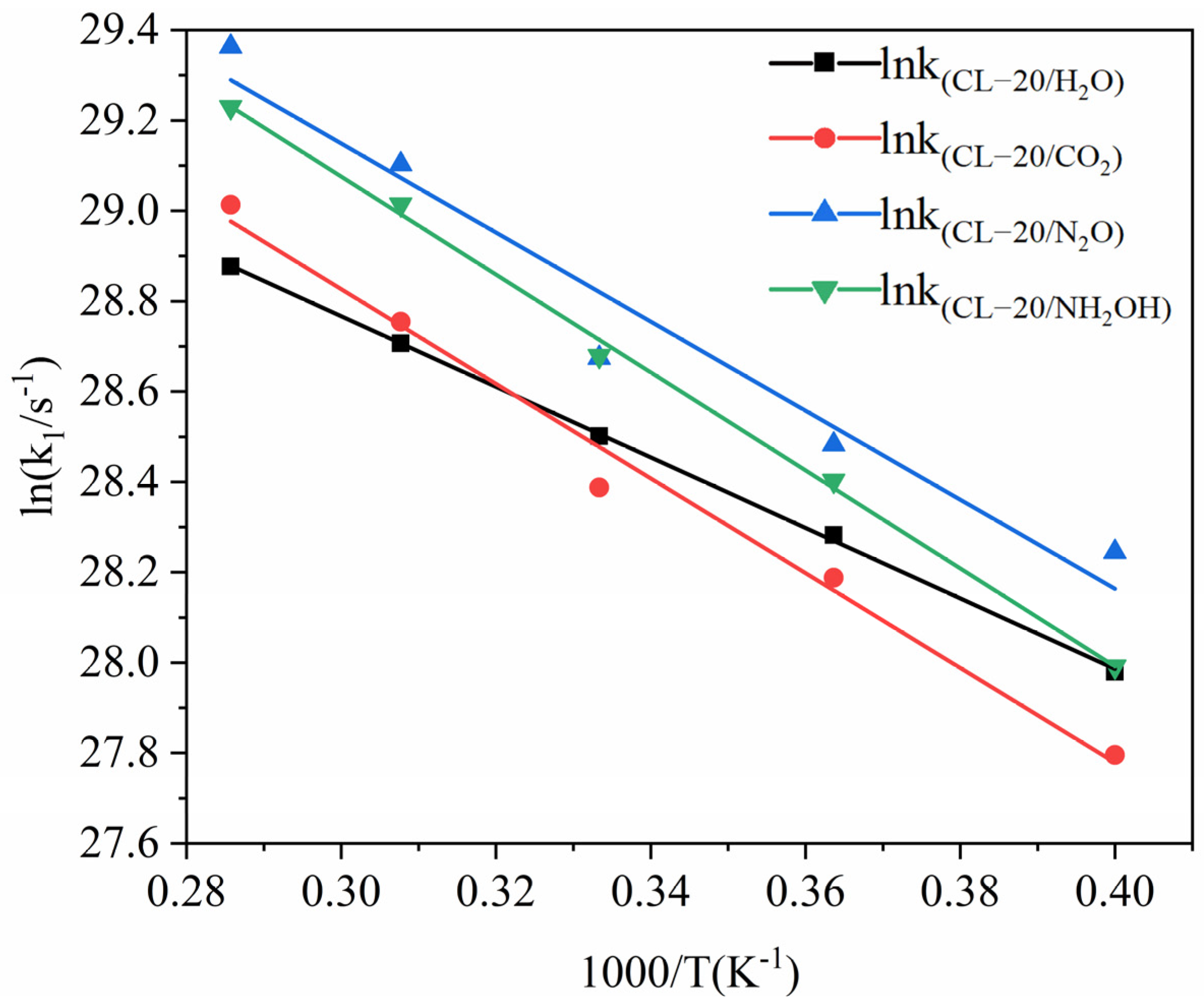
2.2.2. Effect of Nitrogen-Guest on the k1
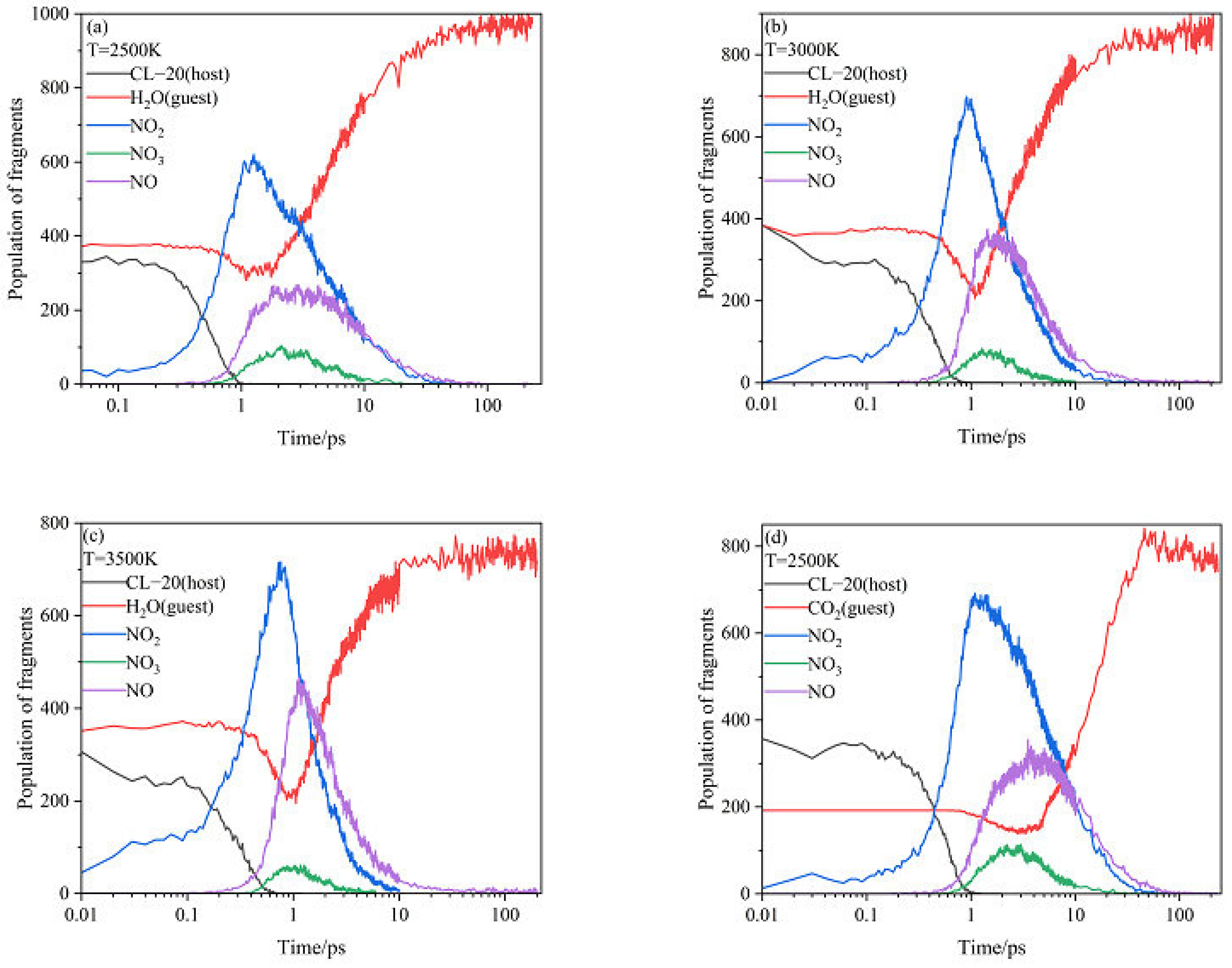
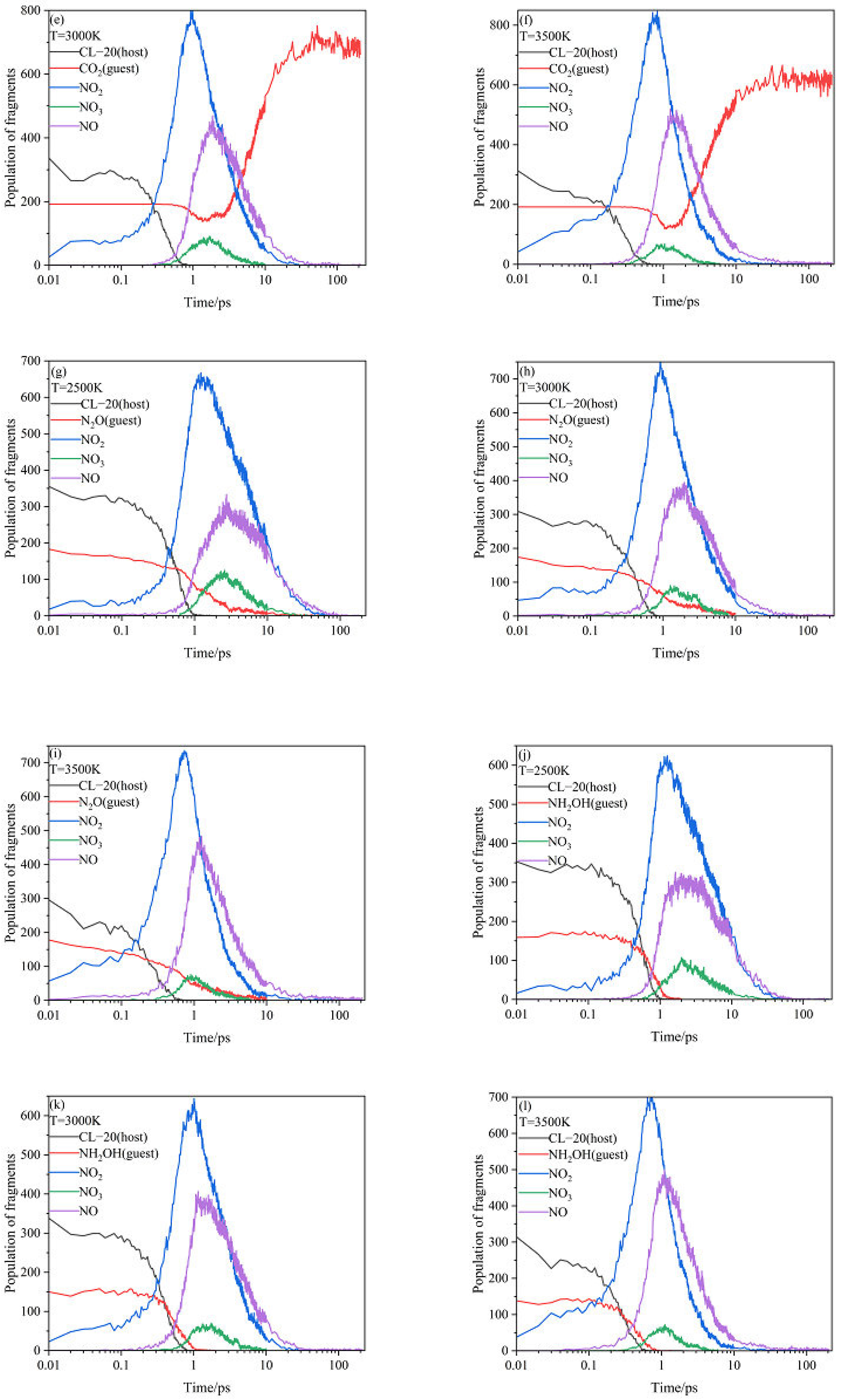
2.3. Intermediate Decomposition Stage
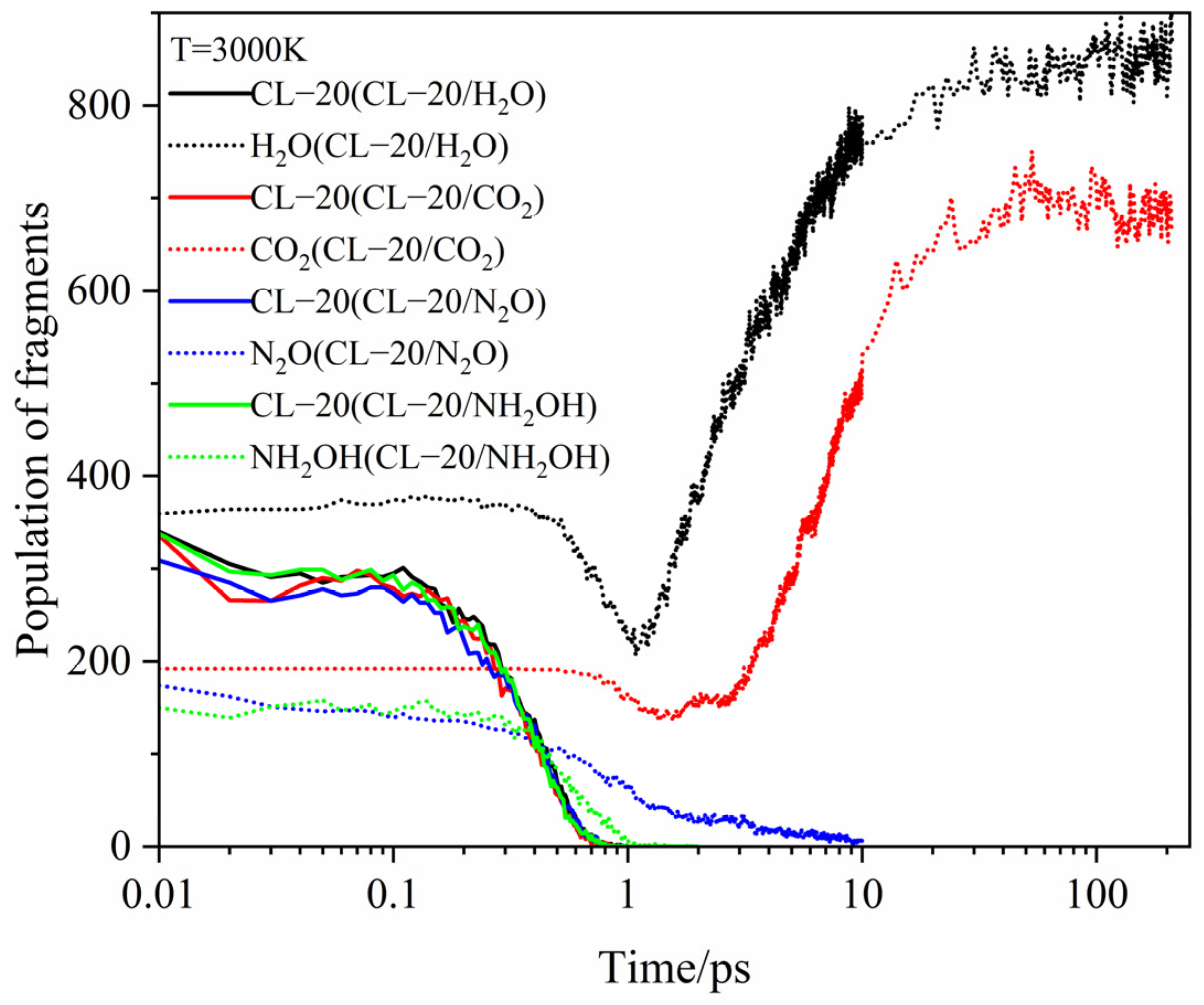
2.3.1. Effect of Nitrogen-Guest on the Main Intermediate Products
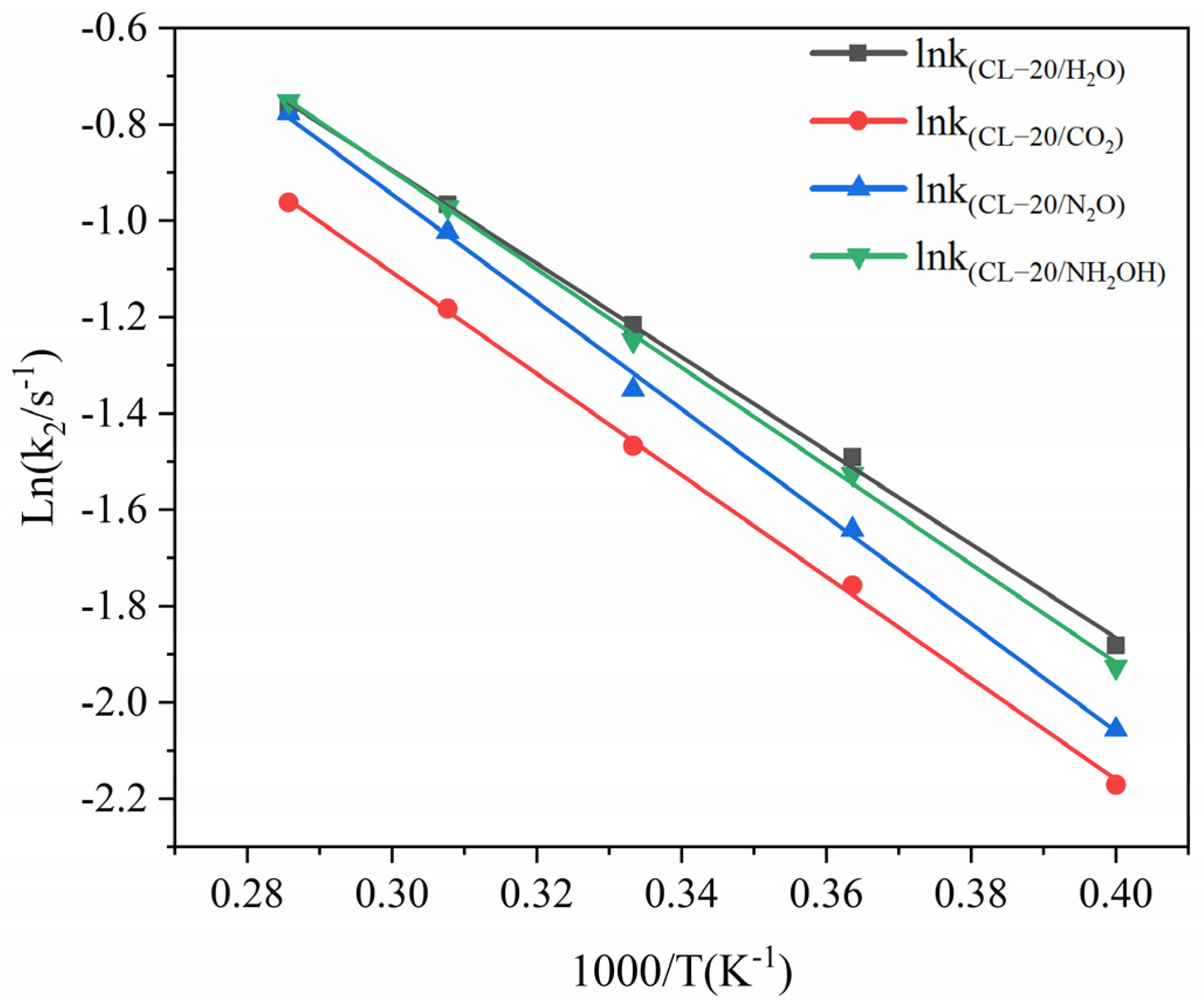
2.3.2. Effect of Nitrogen-Guest on the k2
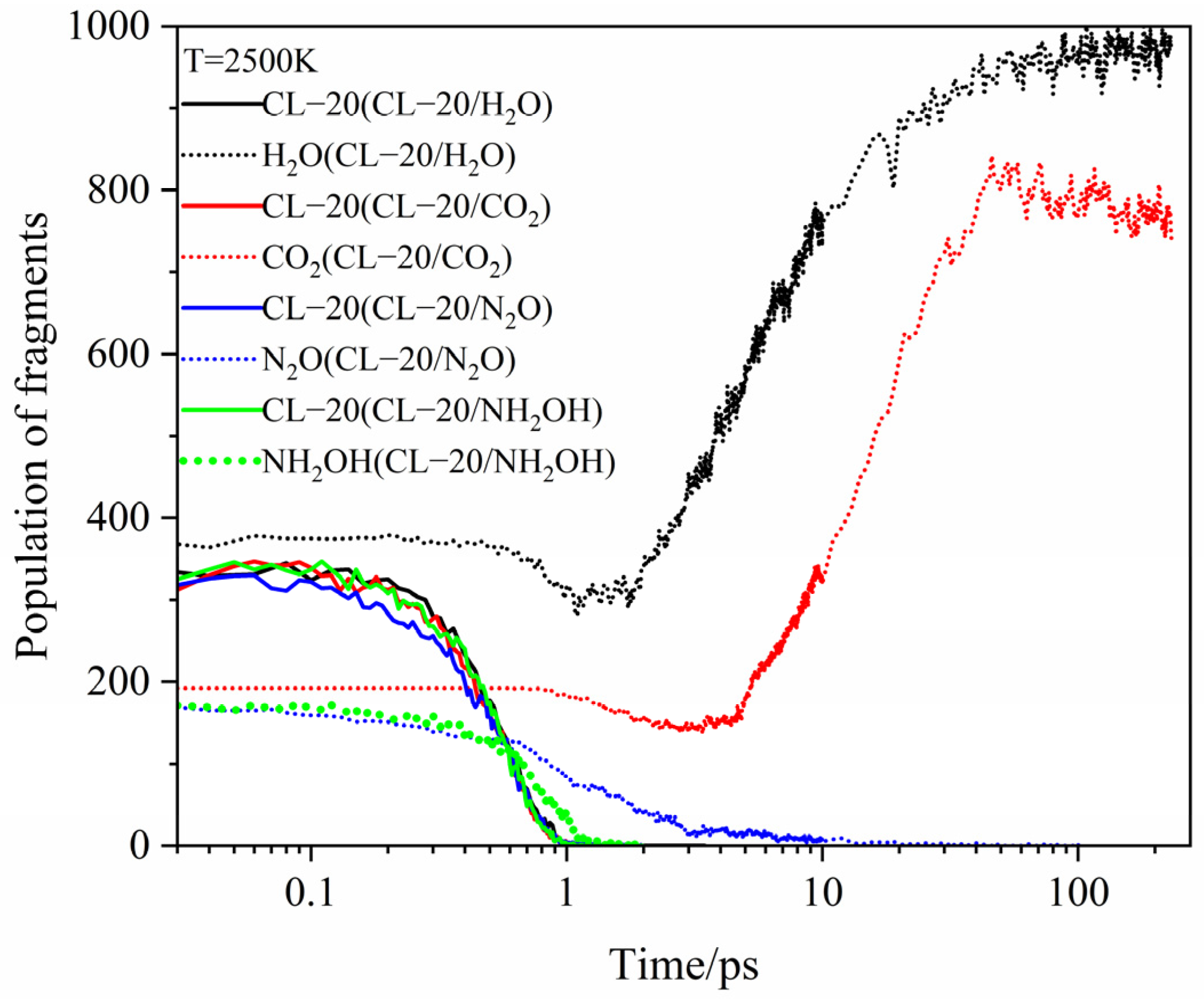
2.4. Final Product Evolution Stage
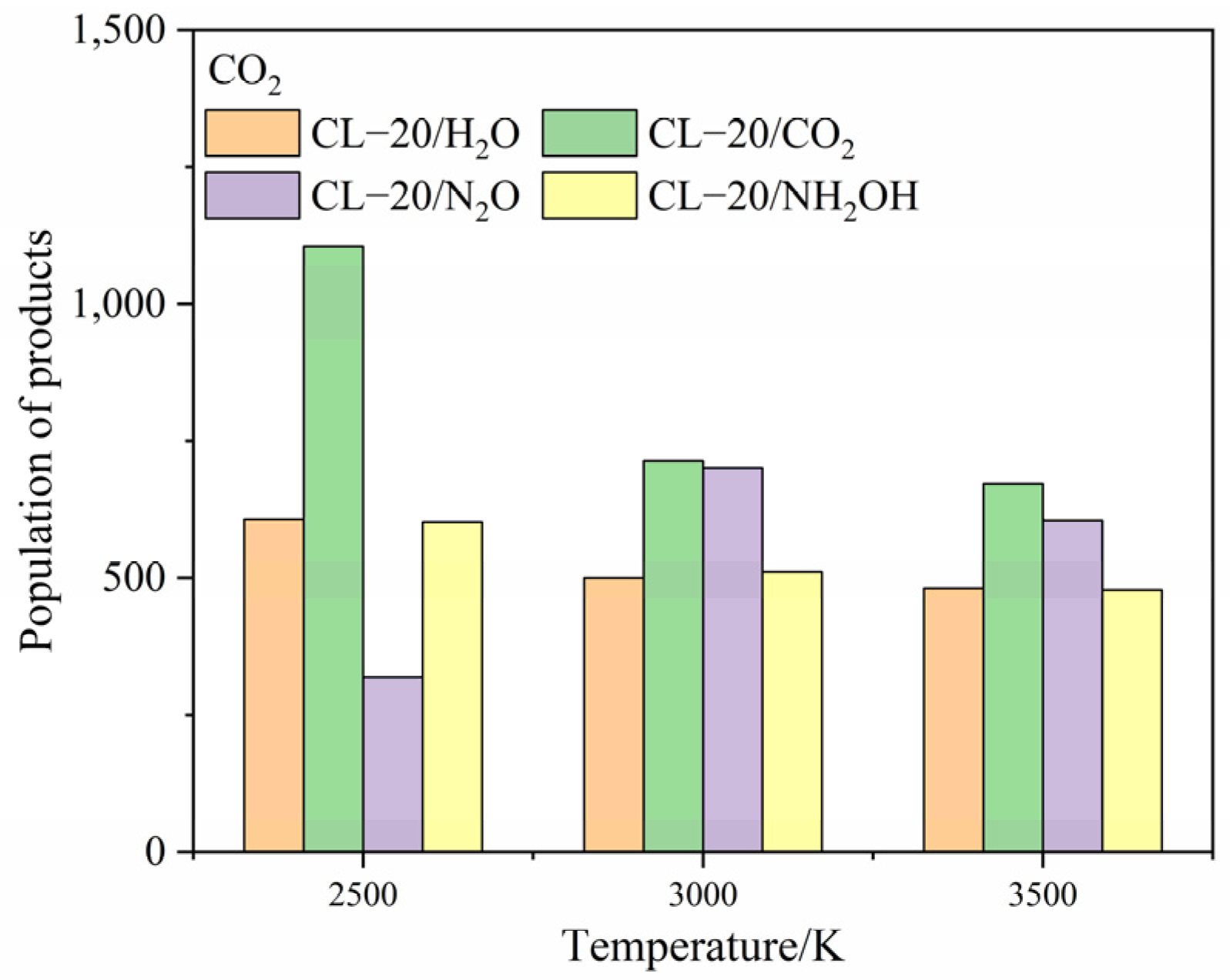
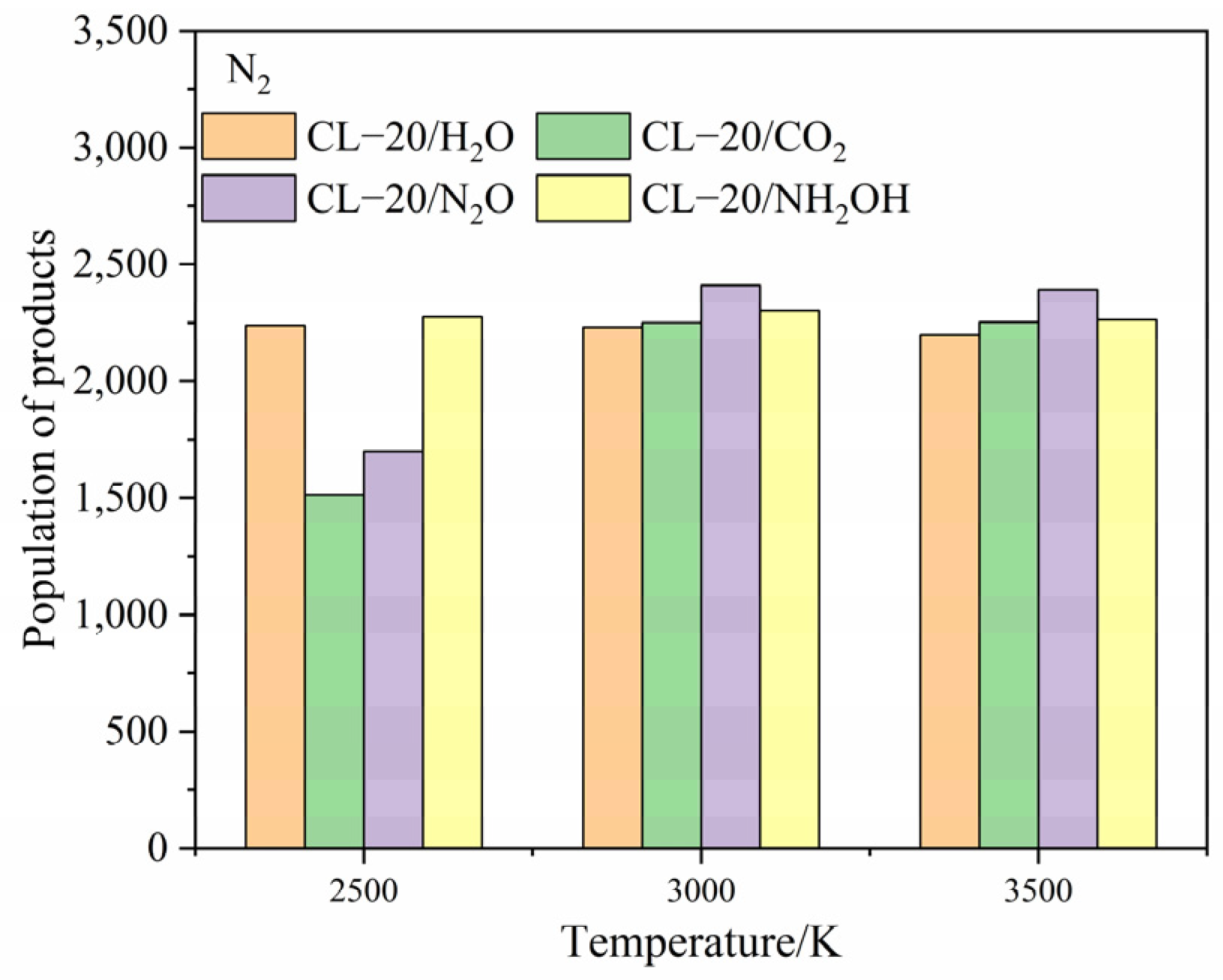
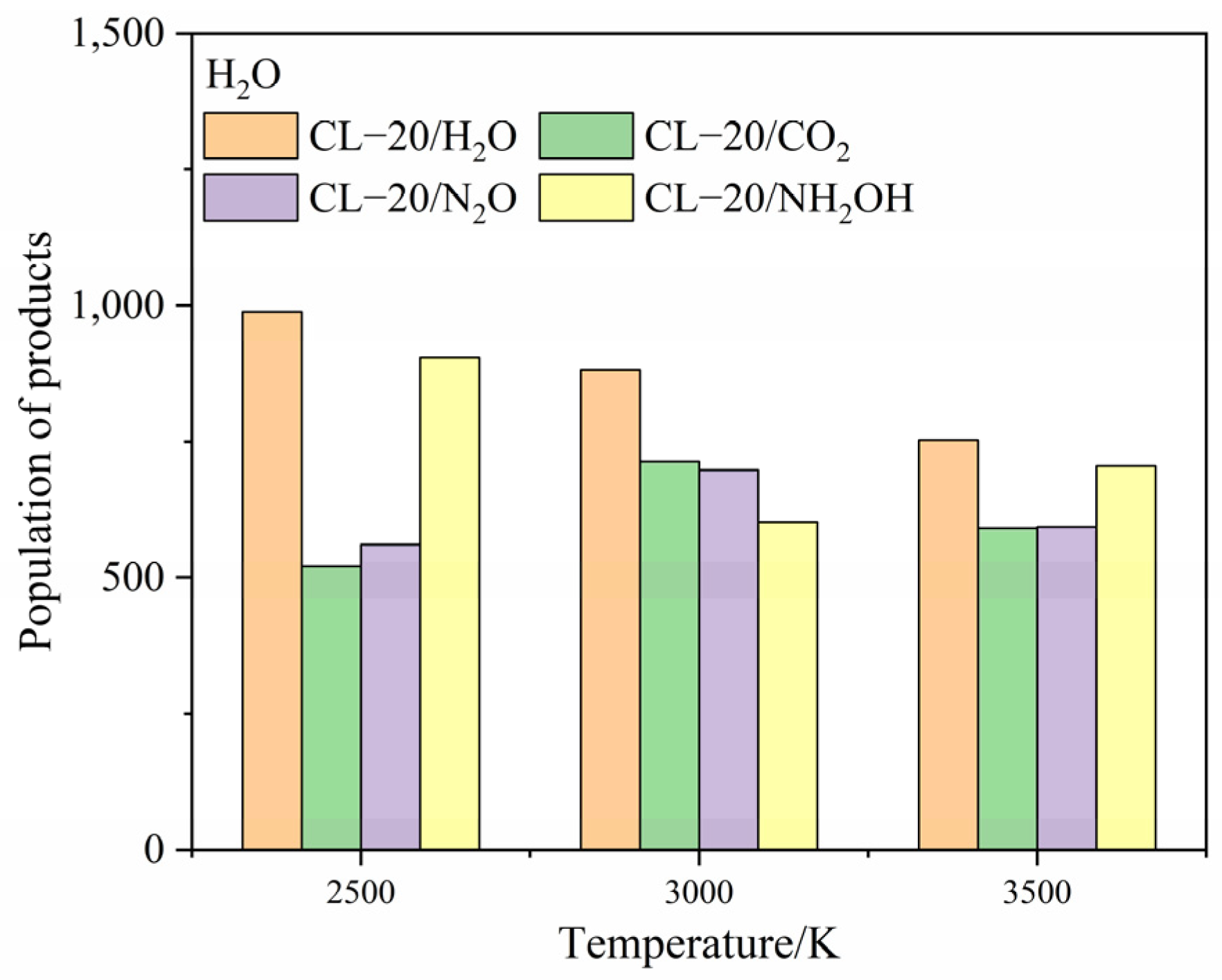
2.4.1. Effect of Nitrogen-Guest on the Final Products
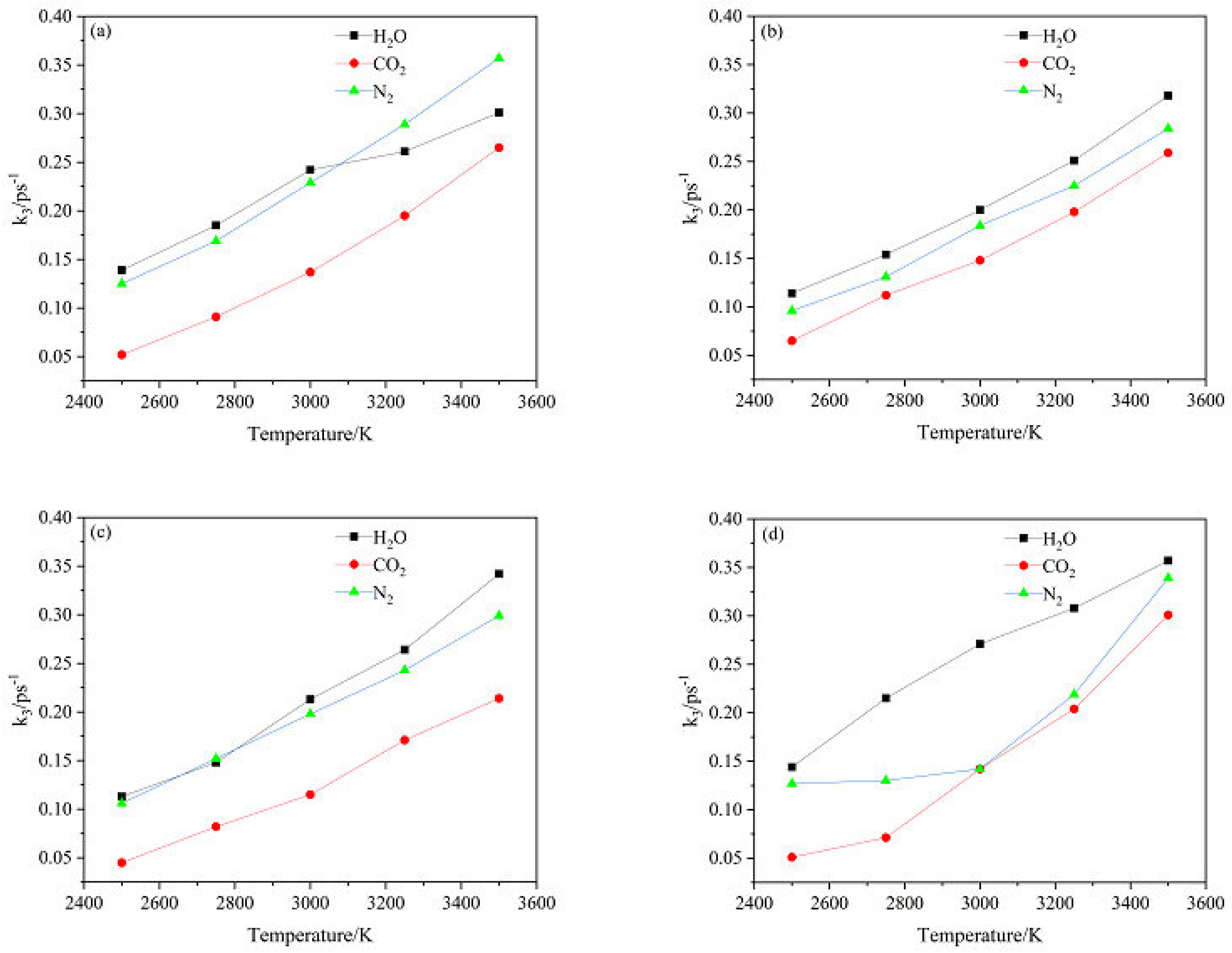
2.4.2. Effect of Nitrogen-Guest on the k3
3. Discussion
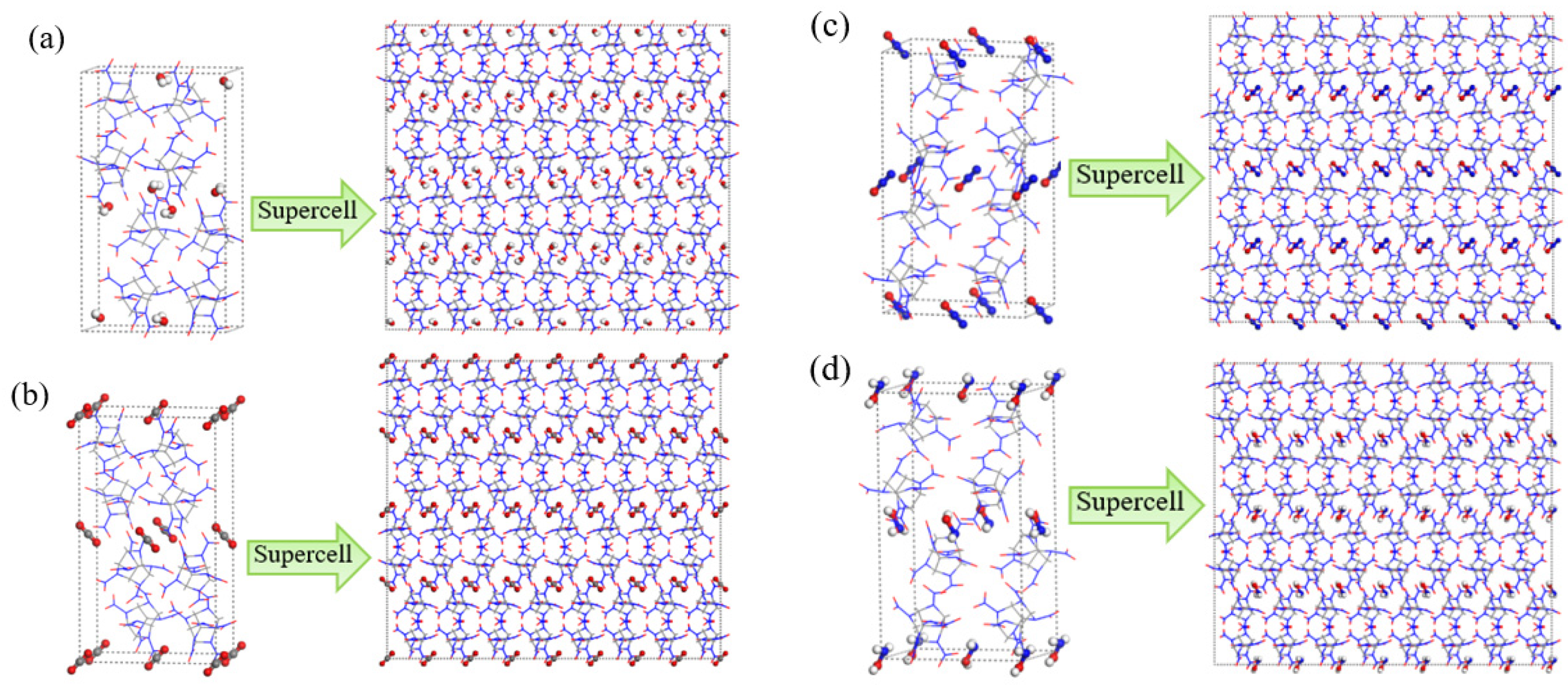
4. Computational Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Song, S.; Huang, C.; Qi, X.; Wang, K.; Liu, Y.; Zhang, Q. Hunting for advanced high-energy-density materials with well-balanced energy and safety through an energetic host-guest inclusion strategy. J. Mater. 2019, 7, 19248–19257. [Google Scholar] [CrossRef]
- Song, S.; Wang, Y.; He, W.; Wang, K.; Yan, M.; Yan, Q.; Zhang, Q. Melamine n-oxide based self-assembled energetic materials with balanced energy & sensitivity and enhanced combustion behavior. Chem. Eng. J. 2020, 395, 125114. [Google Scholar] [CrossRef]
- Bennion, J.C.; Chowdhury, N.; Kampf, J.W.; Matzger, A.J. Hydrogen peroxide solvates of 2, 4, 6, 8, 10, 12-hexanitro-2, 4, 6, 8, 10, 12-hexaazaisowurtzitane. Angew. Chem. 2016, 128, 13312–13315. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, S.; Huang, S.; Tian, Y.; Liu, Y.; Zhang, H.; Sun, J. Host–guest energetic materials constructed by incorporating oxidizing gas molecules into an organic lattice cavity toward achieving highly-energetic and low-sensitivity performance. Chem. Commun. 2019, 55, 909–912. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, H.; Wang, Z.; Xu, J.; Huang, S.; Tian, Y.; Sun, J. Smart host–guest energetic material constructed by stabilizing energetic fuel hydroxylamine in lattice cavity of 2,4,6,8,10,12-hexanitrohexaazaisowurtzitane significantly enhanced the detonation, safety, propulsion, and combustion performances. ACS Appl. Mater. Interfaces. 2021, 13, 61324–61333. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Y.; Zhang, Y.; Ma, H. Energetic host-guest inclusion compounds: An effective design paradigm for high-energy materials. CrystEngComm 2022, 24, 3667–3674. [Google Scholar] [CrossRef]
- Muravyev, N.V.; Wozniak, D.R.; Piercey, D.G. Progress and performance of energetic materials: Open dataset, tool, and implications for synthesis. J. Mater. Chem. A 2022, 10, 11054–11073. [Google Scholar] [CrossRef]
- Nielsen, A.T.; Chafin, A.P.; Christian, S.L.; Moore, D.W.; Nadler, M.P.; Nissan, R.A.; Vanderah, D.J.; Gilardi, R.D.; George, C.F.; Flippen-Anderson, J.L. Synthesis of polyazapolycyclic caged polynitramines. Tetrahedron 1998, 54, 11793–11812. [Google Scholar] [CrossRef]
- Liu, G.; Li, H.; Gou, R.J.; Zhang, C. Packing structures of CL-20-based cocrystals. Cryst. Growth Des. 2018, 18, 7065–7078. [Google Scholar] [CrossRef]
- Saint Martin, S.; Marre, S.; Guionneau, P.; Cansell, F.; Renouard, J.; Marchetto, V.; Aymonier, C. Host-guest inclusion compound from nitramine crystals exposed to condensed carbon dioxide. Chem.-Eur. J. 2010, 16, 13473–13478. [Google Scholar] [CrossRef]
- Millar, D.I.; Maynard-Casely, H.E.; Allan, D.R.; Cumming, A.S.; Lennie, A.R.; Mackay, A.J.; Oswald, I.D.H.; Tang, C.C.; Pulham, C.R. Crystal engineering of energetic materials: Co-crystals of CL-20. CrystEngComm 2012, 14, 3742–3749. [Google Scholar] [CrossRef]
- Bennion, J.C.; Siddiqi, Z.R.; Matzger, A.J. A melt castable energetic cocrystal. Chem. Commun. 2017, 53, 6065–6068. [Google Scholar] [CrossRef] [PubMed]
- Bolton, O.; Simke, L.R.; Pagoria, P.F.; Matzger, A.J. High power explosive with good sensitivity: A 2: 1 cocrystal of CL-20: HMX. Cryst. Growth Des. 2012, 12, 4311–4314. [Google Scholar] [CrossRef]
- Han, Q.; Zhu, W. Effect of particle size on the thermal decomposition of nano ε-CL-20 by ReaxFF-lg molecular dynamics simulations. Chem. Phys. Lett. 2020, 761, 138067. [Google Scholar] [CrossRef]
- Spitzer, D.; Risse, B.; Schnell, F.; Pichot, V.; Klaumünzer, M.; Schaefer, M.R. Continuous engineering of nano-cocrystals for medical and energetic applications. Sci. Rep. 2014, 4, 6575. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.L.; Urtiew, P.A.; Ornellas, D.L.; Moody, G.L.; Scribner, K.J.; Hoffman, D.M. CL-20 Performance Exceeds that of HMX and its Sensitivity is Moderate. Propell. Explos. Pyrot. 1997, 22, 249–255. [Google Scholar] [CrossRef]
- van der Heijden, A.E.; Bouma, R.H. Crystallization and Characterization of RDX, HMX, and CL-20. Cryst. Growth Des. 2004, 4, 999–1007. [Google Scholar] [CrossRef]
- Pu, L.; Xu, J.; Song, G.; Tian, Y.; Zhang, H.; Liu, X.; Sun, J. Investigation on the thermal expansion of α-CL-20 with different water contents. J. Therm. Anal. Calorim. 2015, 122, 1355–1364. [Google Scholar] [CrossRef]
- Fedyanin, I.V.; Lyssenko, K.A.; Fershtat, L.L.; Muravyev, N.V.; Makhova, N.N. Crystal Solvates of Energetic 2, 4, 6, 8, 10, 12-Hexanitro-2, 4, 6, 8, 10, 12-hexaazaisowurtzitane Molecule with [bmim]-Based Ionic Liquids. Cryst. Growth Des. 2019, 19, 3660–3669. [Google Scholar] [CrossRef]
- Zharkov, M.N.; Kuchurov, I.V.; Zlotin, S.G. Micronization of CL-20 using supercritical and liquefied gases. CrystEngComm 2020, 22, 7549–7555. [Google Scholar] [CrossRef]
- Yudin, N.V.; Sinditskii, V.P.; Filatov, S.A.; Serushkin, V.V.; Kostin, N.A.; Ivanyan, M.V.; Zhang, J.G. Solvate of 2, 4, 6, 8, 10, 12-Hexanitro-2, 4, 6, 8, 10, 12-Hexaazaisowurtzitane (CL-20) with both N2O4 and Stable NO2 Free Radical. ChemPlusChem 2020, 85, 1994–2000. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wei, S.H.; Zhang, C. Review of the intermolecular interactions in energetic molecular cocrystals. Cryst. Growth Des. 2020, 20, 7065–7079. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, S.; Liu, Y.; Li, J.; Zhu, W. Effects of noncovalent interactions on the impact sensitivity of hns-based cocrystals: A DFT study. Cryst. Growth Des. 2018, 19, 756–767. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, W. Insight into the roles of small molecules in CL-20 based host-guest crystals: A comparative DFT-D study. CrystEngComm. 2020, 22, 6228–6238. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, L.; Yang, K.; Geng, D.; Lu, J.; Wu, J. Mechanism of the improvement of the energy of host-guest explosives by incorporation of small guest molecules: HNO3 and H2O2 promoted C–N bond cleavage of the ring of ICM-102. Sci. Rep. 2021, 11, 10559. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, L.; Geng, D.; Yang, K.; Lu, J.; Wu, J. A quantum-based molecular dynamics study of the ICM-102/HNO3 host-guest reaction at high temperatures. Phys. Chem. Chem. Phys. 2020, 22, 27002–27012. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, L.; Geng, D.; Yang, K.; Lu, J.; Wu, J.; Yu, B. Reaction mechanism of embedding oxidizing small molecules in energetic materials to improve the energy by reactive molecular dynamics simulations. J. Phys. Chem. C 2019, 123, 29144–29154. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, S.L.; Yu, Y.; Chen, J. Revealing solid properties of high-energy-density molecular cocrystals from the cooperation of hydrogen bonding and molecular polarizability. Sci. Rep. 2019, 9, 1257. [Google Scholar] [CrossRef]
- Rom, N.; Zybin, S.V.; Van Duin, A.C.; Goddard, W.A., III; Zeiri, Y.; Katz, G.; Kosloff, R. Density-dependent liquid nitromethane decomposition: Molecular dynamics simulations based on ReaxFF. J. Phys. Chem. A 2011, 115, 10181–10202. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Geng, D.; Wu, J.; Lu, J.; Wang, C. Thermal decomposition mechanism of CL-20 at different temperatures by ReaxFF reactive molecular dynamics simulations. J. Phys. Chem. A 2018, 122, 3971–3979. [Google Scholar] [CrossRef]
- Zhang, L.; Zybin, S.V.; Van Duin, A.C.; Dasgupta, S.; Goddard, W.A., III; Kober, E.M. Carbon cluster formation during thermal decomposition of octahydro-1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetrazocine and 1, 3, 5-triamino-2, 4, 6-trinitrobenzene high explosives from ReaxFF reactive molecular dynamics simulations. J. Phys. Chem. A 2009, 113, 10619–10640. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bai, C.; Sun, H.; Goddard, W.A., III. Mechanism and kinetics for the initial steps of pyrolysis and combustion of 1, 6-dicyclopropane-2, 4-hexyne from ReaxFF reactive dynamics. J. Phys. Chem. A 2021, 115, 4941–4950. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ye, C.; Xiang, D. Theoretical studies on the role of guest in α-CL-20/guest crystals. Molecules 2022, 27, 3266. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Gou, R.; Chen, M.; Zhang, S.; Chen, Y.; Hu, W. ReaxFF/lg molecular dynamics study on thermal decomposition mechanism of 1-methyl-2, 4, 5-trinitroimidazole. Comput. Theor. Chem. 2022, 1209, 113594. [Google Scholar] [CrossRef]
- Strachan, A.; van Duin, A.C.; Chakraborty, D.; Dasgupta, S.; Goddard, W.A., III. Shock waves in high-energy materials: The initial chemical events in nitramine RDX. Phys. Rev. Lett. 2003, 91, 098301. [Google Scholar] [CrossRef]
- Strachan, A.; Kober, E.M.; Van Duin, A.C.; Oxgaard, J.; Goddard, W.A., III. Thermal decomposition of RDX from reactive molecular dynamics. J. Chem. Phys. 2005, 122, 054502. [Google Scholar] [CrossRef]


| Host–Guest Crystal | Temperatures | Initial Reaction Paths | Frequency |
|---|---|---|---|
| CL-20/H2O | 2500 | C6H6O12N12 → C6H6O10N11 + NO2 | 29 |
| C6H6O12N12 → C6H5O12N12 + H | 21 | ||
| H2O → H + OH | 20 | ||
| 3000 | C6H6O12N12 → C6H6O10N11 + NO2 | 51 | |
| C6H6O12N12 → C6H5O12N12 + H | 31 | ||
| H2O → H + OH | 20 | ||
| 3500 | C6H6O12N12 → C6H6O10N11 + NO2 | 69 | |
| C6H6O12N12 → C6H5O12N12 + H | 41 | ||
| H2O → H + OH | 27 | ||
| CL-20/CO2 | 2500 | C6H6O12N12 → C6H6O10N11 + NO2 | 41 |
| C6H6O12N12 → C6H5O12N12 + H | 25 | ||
| 3000 | C6H6O12N12 → C6H6O10N11 + NO2 | 60 | |
| C6H6O12N12 → C6H5O12N12 + H | 38 | ||
| 3500 | C6H6O12N12 → C6H6O10N11 + NO2 | 65 | |
| C6H6O12N12 → C6H5O12N12 + H | 34 | ||
| CL-20/N2O | 2500 | C6H6O12N12 → C6H6O10N11 + NO2 | 30 |
| C6H6O12N12 → C6H5O12N12 + H | 25 | ||
| N2O → N + NO | 5 | ||
| N2O → N2 + O | 18 | ||
| 3000 | C6H6O12N12 → C6H6O10N11 + NO2 | 63 | |
| C6H6O12N12 → C6H5O12N12 + H | 31 | ||
| N2O → N + NO | 5 | ||
| N2O → N2 + O | 34 | ||
| 3500 | C6H6O12N12 → C6H6O10N11 + NO2 | 77 | |
| C6H6O12N12 → C6H5O12N12 + H | 49 | ||
| N2O → N + NO | 11 | ||
| N2O → N2 + O | 24 | ||
| CL-20/NH2OH | 2500 | C6H6O12N12 → C6H6O10N11 + NO2 | 31 |
| C6H6O12N12 → C6H5O12N12 + H | 22 | ||
| NH2OH → NH2 + OH | 8 | ||
| NH2OH → NH2O + H | 11 | ||
| 3000 | C6H6O12N12 → C6H6O10N11 + NO2 | 41 | |
| C6H6O12N12 → C6H5O12N12 + H | 31 | ||
| NH2OH → NH2 + OH | 27 | ||
| NH2OH → NH2O + H | 15 | ||
| 3500 | C6H6O12N12 → C6H6O10N11 + NO2 | 69 | |
| C6H6O12N12 C6H5O12N12 + H | 48 | ||
| C6H6O10N11 → C6H6O8N10 + NO2 | 6 | ||
| C6H5O12N12 → C6H5O10N11 + NO2 | 7 | ||
| C6H6O12N12 → C6H4O12N12 + 2H | 5 | ||
| NH2OH → NH2 + OH | 35 | ||
| NH2OH → NH2O + H | 20 |
| Host–Guest Crystal | T/K | k1/ps−1 |
|---|---|---|
| CL-20/H2O | 2500 | 1.417 |
| 2750 | 1.918 | |
| 3000 | 2.388 | |
| 3250 | 2.932 | |
| 3500 | 3.476 | |
| CL-20/CO2 | 2500 | 1.179 |
| 2750 | 1.745 | |
| 3000 | 2.131 | |
| 3250 | 3.075 | |
| 3500 | 3.984 | |
| CL-20/N2O | 2500 | 1.848 |
| 2750 | 2.344 | |
| 3000 | 2.839 | |
| 3250 | 4.357 | |
| 3500 | 5.653 | |
| CL-20/NH2OH | 2500 | 1.434 |
| 2750 | 2.163 | |
| 3000 | 2.851 | |
| 3250 | 3.985 | |
| 3500 | 4.944 |
| Host–Guest Crystal | T/K | Umax | U∞ | ΔUexo | k2/ps−1 |
|---|---|---|---|---|---|
| CL-20/H2O | 2500 | −1,424,794 | −1,650,929 | 226,135 | 0.1523 |
| 2750 | −1,413,953 | −1,636,452 | 222,499 | 0.2251 | |
| 3000 | −1,403,193 | −1,621,948 | 218,755 | 0.2963 | |
| 3250 | −1,397,567 | −1,606,748 | 209,181 | 0.38057 | |
| 3500 | −1,384,266 | −1,591,748 | 207,482 | 0.46484 | |
| CL-20/CO2 | 2500 | −1,398,845 | −1,630,254 | 231,409 | 0.11404 |
| 2750 | −1,389,461 | −1,617,052 | 227,591 | 0.17252 | |
| 3000 | −1,379,278 | −1,603,764 | 224,486 | 0.23059 | |
| 3250 | −1,3694,15 | −1,589,817 | 220,402 | 0.30645 | |
| 3500 | −1,359,754 | −1,575,870 | 216,116 | 0.38217 | |
| CL-20/N2O | 2500 | −1,381,272 | −1,616,532 | 235,260 | 0.12789 |
| 2750 | −1,372,536 | −1,602,557 | 230,021 | 0.19377 | |
| 3000 | −1,363,624 | −1,588,473 | 224,849 | 0.25909 | |
| 3250 | −1,354,660 | −1,572,699 | 218,039 | 0.35959 | |
| 3500 | −1,345,497 | −1,556,825 | 211,328 | 0.46010 | |
| CL-20/NH2OH | 2500 | −1,403,762 | −1,639,940 | 236,178 | 0.14567 |
| 2750 | −1,395,173 | −1,625,769 | 230,596 | 0.21743 | |
| 3000 | −1,385,384 | −1,611,798 | 226,414 | 0.28695 | |
| 3250 | −1,377,669 | −1,595,917 | 218,248 | 0.378106 | |
| 3500 | −1,369,830 | −1,580,037 | 210,207 | 0.47146 |
| Crystal | Method | a/Å | b/Å | c/Å | ρ/g·cm−3 |
|---|---|---|---|---|---|
| CL-20/H2O | from CCDC | 9.477 | 13.139 | 23.380 | 2.081 |
| ReaxFF-lg | 9.370 | 12.993 | 23.119 | 2.153 | |
| CL-20/CO2 | from CCDC | 9.673 | 13.203 | 23.553 | 2.033 |
| ReaxFF-lg | 9.467 | 13.167 | 23.489 | 2.049 | |
| CL-20/N2O | from CCDC | 9.577 | 13.256 | 23.625 | 2.038 |
| ReaxFF-lg | 9.427 | 13.049 | 23.256 | 2.137 | |
| CL-20/NH2OH | from CCDC | 9.789 | 13.123 | 23.509 | 2.000 |
| ReaxFF-lg | 9.602 | 12.873 | 23.059 | 2.119 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Luo, J.; Xiang, D. Effects of Different Guests on Pyrolysis Mechanism of α-CL−20/Guest at High Temperatures by Reactive Molecular Dynamics Simulations at High Temperatures. Int. J. Mol. Sci. 2023, 24, 1840. https://doi.org/10.3390/ijms24031840
Zhou M, Luo J, Xiang D. Effects of Different Guests on Pyrolysis Mechanism of α-CL−20/Guest at High Temperatures by Reactive Molecular Dynamics Simulations at High Temperatures. International Journal of Molecular Sciences. 2023; 24(3):1840. https://doi.org/10.3390/ijms24031840
Chicago/Turabian StyleZhou, Mingming, Jing Luo, and Dong Xiang. 2023. "Effects of Different Guests on Pyrolysis Mechanism of α-CL−20/Guest at High Temperatures by Reactive Molecular Dynamics Simulations at High Temperatures" International Journal of Molecular Sciences 24, no. 3: 1840. https://doi.org/10.3390/ijms24031840
APA StyleZhou, M., Luo, J., & Xiang, D. (2023). Effects of Different Guests on Pyrolysis Mechanism of α-CL−20/Guest at High Temperatures by Reactive Molecular Dynamics Simulations at High Temperatures. International Journal of Molecular Sciences, 24(3), 1840. https://doi.org/10.3390/ijms24031840






