A Newly Developed Chemically Defined Serum-Free Medium Suitable for Human Primary Keratinocyte Culture and Tissue Engineering Applications
Abstract
1. Introduction
2. Results and Discussion
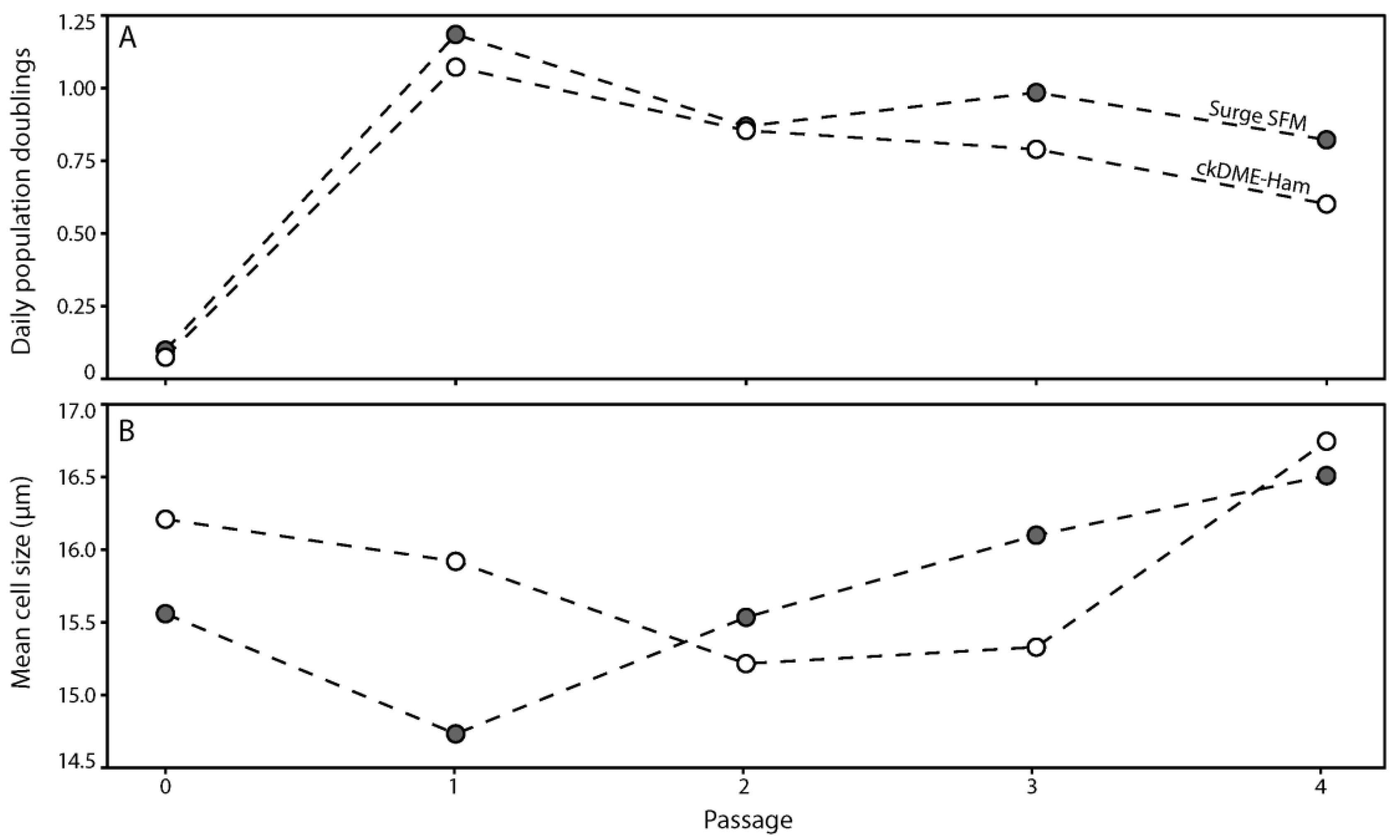
2.1. Surge SFM Supports the Growth of Freshly Isolated Human Skin Keratinocytes in Culture
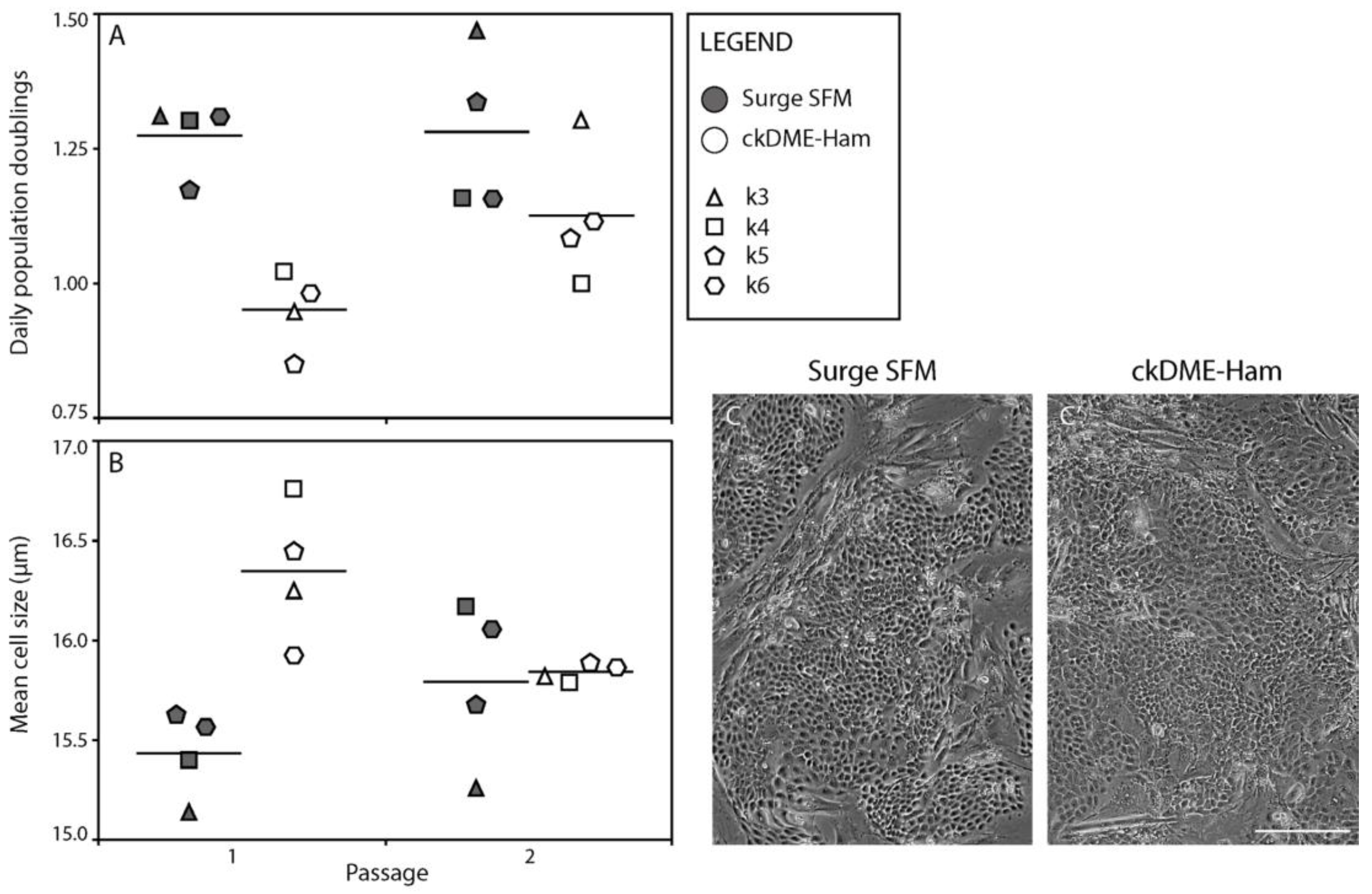
2.2. Surge SFM Supports the Growth of Cryopreserved Primary Human Keratinocytes in Culture
2.3. Surge SFM Supports the Retention of Epithelial Stem Cells in Culture
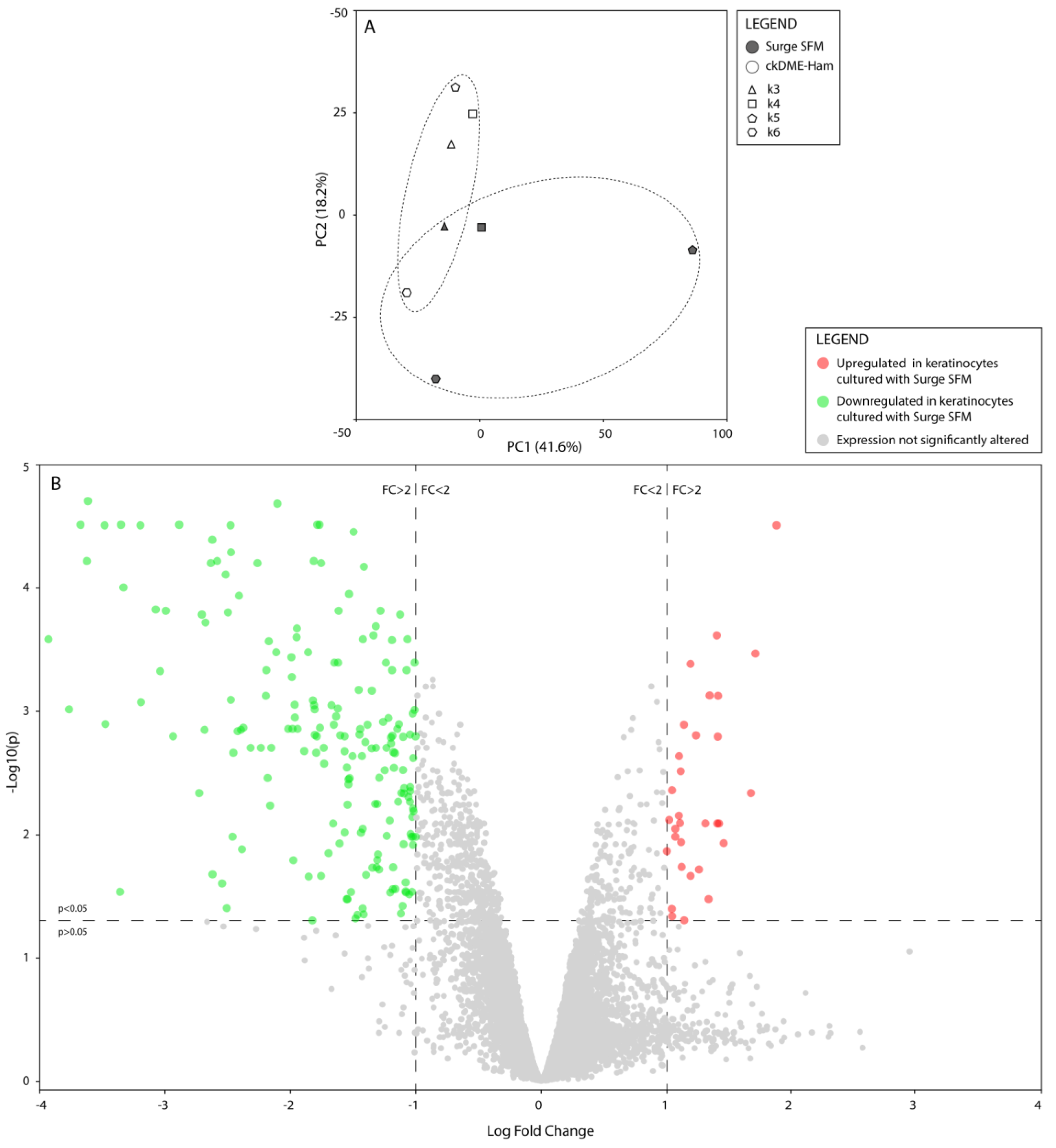
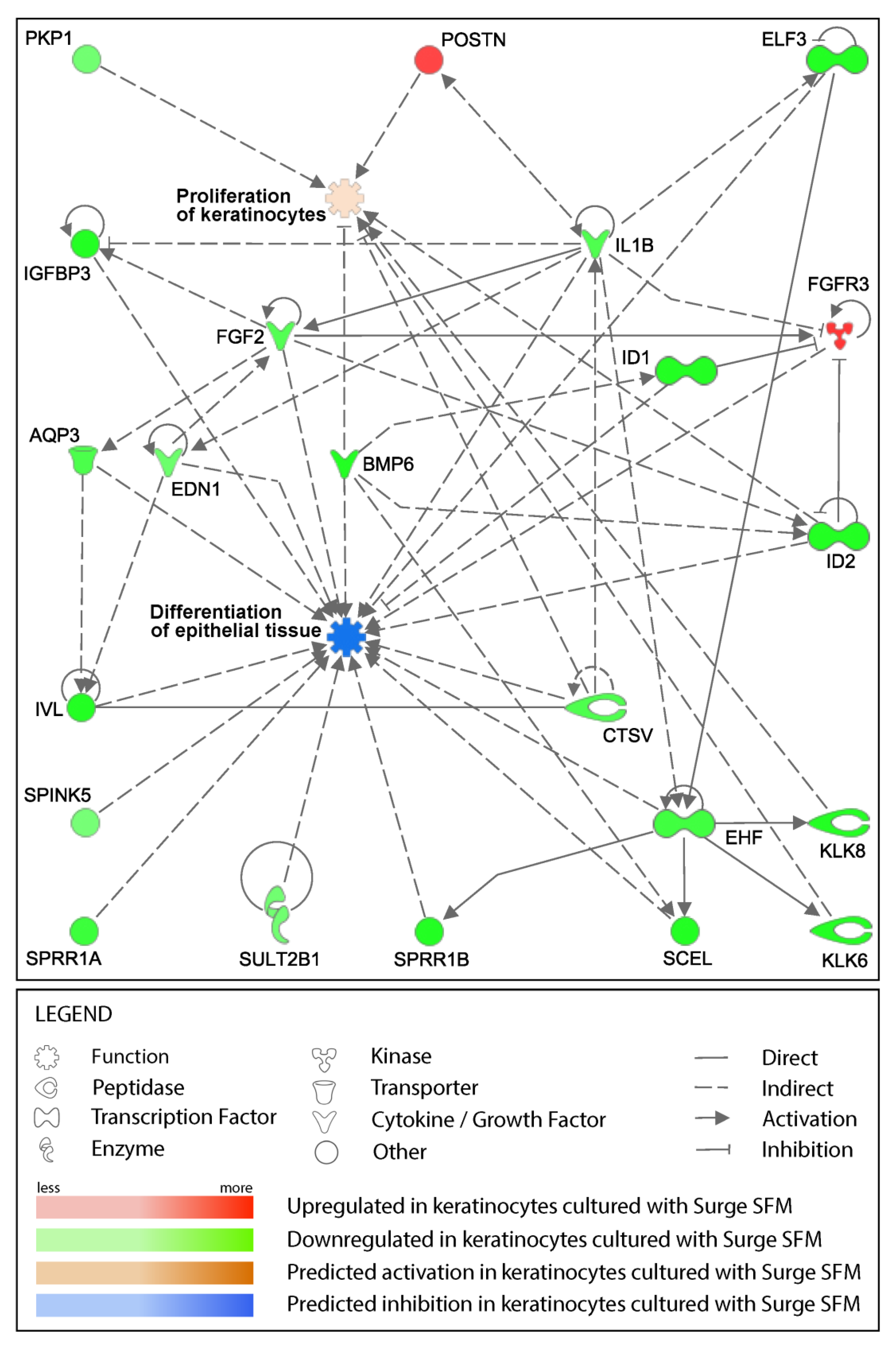
2.4. Surge SFM’s Effect on Keratinocytes’ Transcriptome Is Minute but Could Promote Growth and Stifle Differentiation through EHF Regulation
2.5. Surge SFM Supports the Formation of a Properly Stratified Neo-Epithelium on Tissue-Engineered Skin Substitutes
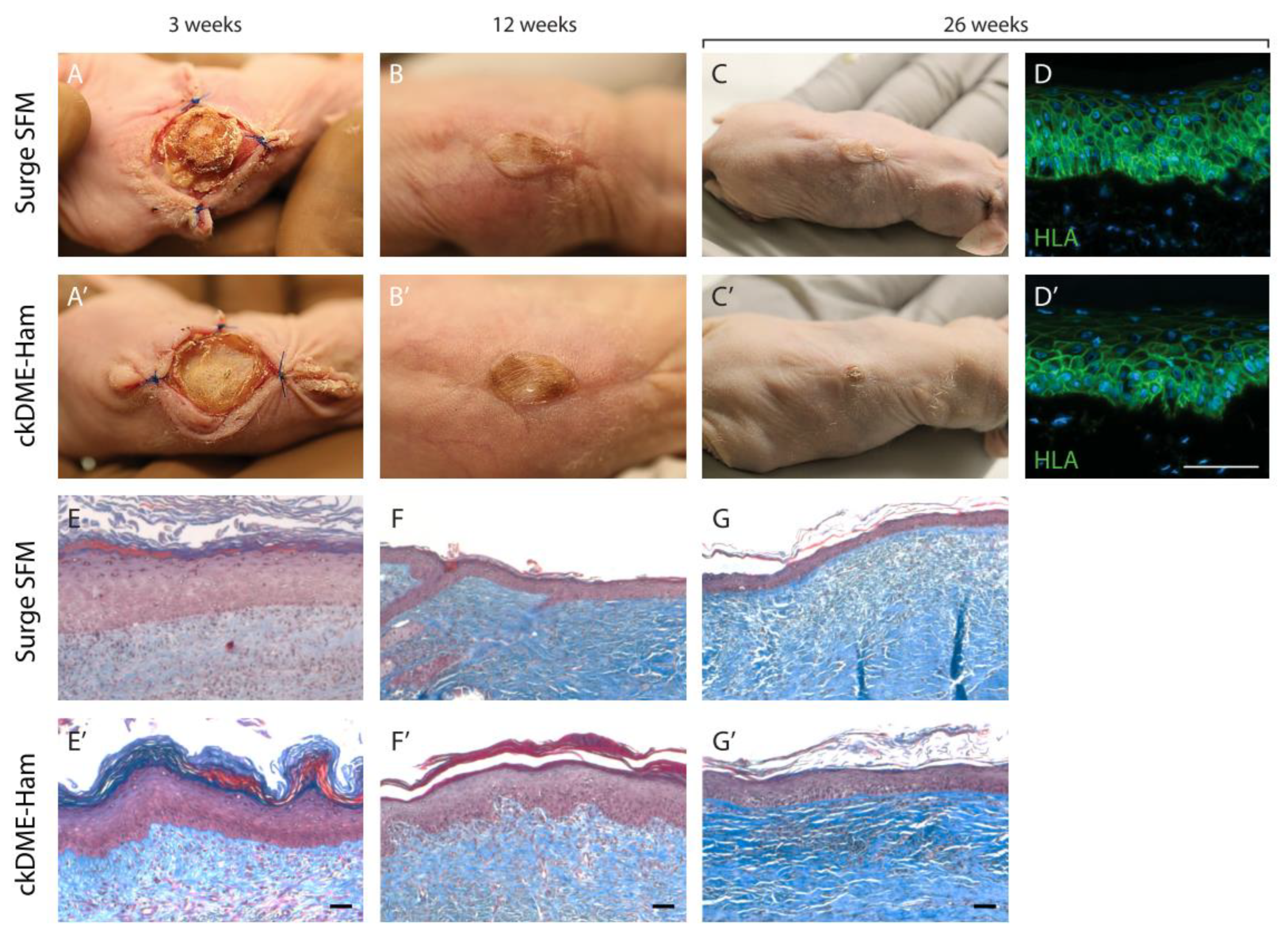
2.6. Self-Assembled Skin Substitutes Produced with Surge SFM Display Long-Term Graft Persistence on Athymic Mice
3. Materials and Methods
3.1. Cell Populations
3.2. Media
3.3. Keratinocyte Culture
3.4. Cryopreserved Cells
3.5. Serum-Free Medium Development
3.6. Population Doublings
3.7. Colony-Forming Efficiency
3.8. Flow Cytometry Analysis
3.9. Microarray Assay and Gene Expression Analyses
3.10. Skin Substitute Production
3.11. Skin Substitute Grafting on Nude Mice
3.12. Histological and Immunofluorescence Analyses
3.13. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, H. Cyclic AMP in relation to proliferation of the epidermal cell: A new view. Cell 1978, 15, 801–811. [Google Scholar] [CrossRef]
- Green, H.; Rheinwald, J.; Sun, T. Properties of an epithelial cell type in culture: The epidermal keratinocyte and its dependence on products of the fibroblast. Prog. Clin. Biol. Res. 1977, 17, 493–500. [Google Scholar]
- Rheinwald, J.; Green, H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature 1977, 265, 421–424. [Google Scholar] [CrossRef]
- Rheinwald, J.; Green, H. Serial cultivation of strains of human epidemal keratinocytes: The formation keratinizing colonies from single cells. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Cortez Ghio, S.; Cantin-Warren, L.; Guignard, R.; Larouche, D.; Germain, L. Are the Effects of the Cholera Toxin and Isoproterenol on Human Keratinocytes’ Proliferative Potential Dependent on Whether They Are Co-Cultured with Human or Murine Fibroblast Feeder Layers? Int. J. Mol. Sci. 2018, 19, 2174. [Google Scholar] [CrossRef] [PubMed]
- Germain, L.; Larouche, D.; Nedelec, B.; Perreault, I.; Duranceau, L.; Bortoluzzi, P.; Beaudoin Cloutier, C.; Genest, H.; Caouette-Laberge, L.; Dumas, A. Autologous bilayered self-assembled skin substitutes (SASSs) as permanent grafts: A case series of 14 severely burned patients indicating clinical effectiveness. Eur. Cells Mater. 2018, 36, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Llames, S.; García, E.; García, V.; del Río, M.; Larcher, F.; Jorcano, J.; López, E.; Holguín, P.; Miralles, F.; Otero, J. Clinical results of an autologous engineered skin. Cell Tissue Bank. 2006, 7, 47–53. [Google Scholar] [CrossRef]
- Kljenak, A.; Tominac Trcin, M.; Bujić, M.; Dolenec, T.; Jevak, M.; Mršić, G.; Zmiš, G.; Barčot, Z.; Muljačić, A.; Popović, M. Fibrin gel as a scaffold for skin substitute–production and clinical experience. Acta Clin. Croat. 2016, 55, 279–288. [Google Scholar] [CrossRef]
- Cortez Ghio, S.; Larouche, D.; Doucet, E.; Germain, L. The role of cultured autologous bilayered skin substitutes as epithelial stem cell niches after grafting: A systematic review of clinical studies. Burns Open 2021, 5, 56–66. [Google Scholar] [CrossRef]
- Hawkes, P. Fetal bovine serum: Geographic origin and regulatory relevance of viral contamination. Bioresour. Bioprocess. 2015, 2, 34. [Google Scholar] [CrossRef]
- Baker, M. Reproducibility: Respect your cells! Nature 2016, 537, 433–435. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, J.; Mellor, D.; Brands, R.; Fischer, R.; Gruber, F.; Gstraunthaler, G.; Hellebrekers, L.; Hyllner, J.; Jonker, F.; Prieto, P. The humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxicol. Vitr. 2004, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khodabukus, A.; Baar, K. The effect of serum origin on tissue engineered skeletal muscle function. J. Cell. Biochem. 2014, 115, 2198–2207. [Google Scholar] [CrossRef] [PubMed]
- Wessman, S.; Levings, R. Benefits and risks due to animal serum used in cell culture production. Dev. Biol. Stand. 1999, 99, 3. [Google Scholar]
- Asher, D. Bovine sera used in the manufacture of biologicals: Current concerns and policies of the US Food and Drug Administration regarding the transmissible spongiform encephalopathies. Dev. Biol. Stand. 1999, 99, 41. [Google Scholar]
- Shailer, C.; Corrin, K. Serum supply: Policies and controls operating in New Zealand. Dev. Biol. Stand. 1999, 99, 71–77. [Google Scholar]
- Gstraunthaler, G.; Lindl, T.; van der Valk, J. A severe case of fraudulent blending of fetal bovine serum strengthens the case for serum-free cell and tissue culture applications. Altern. Lab. Anim. 2014, 42, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Gstraunthaler, G.; Lindl, T.; van der Valk, J. A plea to reduce or replace fetal bovine serum in cell culture media. Cytotechnology 2013, 65, 791–793. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, J.; Brunner, D.; De Smet, K.; Svenningsen, Å.; Honegger, P.; Knudsen, L.; Lindl, T.; Noraberg, J.; Price, A.; Scarino, M. Optimization of chemically defined cell culture media–replacing fetal bovine serum in mammalian in vitro methods. Toxicol. Vitr. 2010, 24, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Mak, V.; Cumpstone, M.; Kennedy, A.; Harmon, C.; Guy, R.; Potts, R. Barrier function of human keratinocyte cultures grown at the air-liquid interface. J. Investig. Dermatol. 1991, 96, 323–327. [Google Scholar] [CrossRef]
- Shepherd, B.; Enis, D.; Wang, F.; Suarez, Y.; Pober, J.; Schechner, J.; Shepherd, B.; Enis, D.; Wang, F.; Suarez, Y. Vascularization and engraftment of a human skin substitute using circulating progenitor cell-derived endothelial cells. FASEB J. 2006, 20, 1739–1741. [Google Scholar] [CrossRef] [PubMed]
- Krasna, M.; Planinsek, F.; Knezevic, M.; Arnez, Z.; Jeras, M. Evaluation of a fibrin-based skin substitute prepared in a defined keratinocyte medium. Int. J. Pharm. 2005, 291, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.; Kagan, R.; Greenhalgh, D.; Warner, P.; Yakuboff, K.; Palmieri, T.; Warden, G. Cultured skin substitutes reduce requirements for harvesting of skin autograft for closure of excised, full-thickness burns. J. Trauma Acute Care Surg. 2006, 60, 821–829. [Google Scholar]
- Bisson, F.; Rochefort, É.; Lavoie, A.; Larouche, D.; Zaniolo, K.; Simard-Bisson, C.; Damour, O.; Auger, F.; Guérin, S.; Germain, L. Irradiated human dermal fibroblasts are as efficient as mouse fibroblasts as a feeder layer to improve human epidermal cell culture lifespan. Int. J. Mol. Sci. 2013, 14, 4684–4704. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, D.; Kwon, S.; Park, E.; Huh, C.; Youn, S.; Kim, S.; Park, K. Protective effects of EGCG on UVB-induced damage in living skin equivalents. Arch. Pharmacol. Res. 2005, 28, 784. [Google Scholar] [CrossRef] [PubMed]
- Cortez Ghio, S.; Le-Bel, G.; Lavoie, A.; Larouche, D.; Germain, L. Isolation and culture of human keratinocytes. In Skin Tissue Engineering; Springer: Berlin/Heidelberg, Germany, 2019; pp. 3–13. [Google Scholar]
- Lamb, R.; Ambler, C. Keratinocytes propagated in serum-free, feeder-free culture conditions fail to form stratified epidermis in a reconstituted skin model. PLoS ONE 2013, 8, e52494. [Google Scholar] [CrossRef]
- Zöller, N.; Valesky, E.; Butting, M.; Hofmann, M.; Kippenberger, S.; Bereiter-Hahn, J.; Bernd, A.; Kaufmann, R. Clinical application of a tissue-cultured skin autograft: An alternative for the treatment of non-healing or slowly healing wounds? Dermatology 2014, 229, 190–198. [Google Scholar] [CrossRef]
- Boyce, S.; Ham, R. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J. Investig. Dermatol. 1983, 81, S33–S40. [Google Scholar] [CrossRef]
- Takami, Y.; Yamaguchi, R.; Ono, S.; Hyakusoku, H. Clinical application and histological properties of autologous tissue-engineered skin equivalents using an acellular dermal matrix. J. Nippon. Med. Sch. 2014, 81, 356–363. [Google Scholar] [CrossRef]
- Barrandon, Y.; Green, H. Cell size as a determinant of the clone-forming ability of human keratinocytes. Proc. Natl. Acad. Sci. USA 1985, 82, 5390–5394. [Google Scholar] [CrossRef]
- Belleudi, F.; Nanni, M.; Raffa, S.; Torrisi, M. HPV16 E5 deregulates the autophagic process in human keratinocytes. Oncotarget 2015, 6, 9370. [Google Scholar] [CrossRef]
- Funayama, E.; Chodon, T.; Oyama, A.; Sugihara, T. Keratinocytes promote proliferation and inhibit apoptosis of the underlying fibroblasts: An important role in the pathogenesis of keloid. J. Investig. Dermatol. 2003, 121, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, D.; Kim, J.; Choi, S.; Park, W.; Ha, K.; Kim, T.; Choe, J.; Won, M.; Kwon, Y.; et al. A miRNA-101-3p/Bim axis as a determinant of serum deprivation-induced endothelial cell apoptosis. Cell Death Dis. 2017, 8, e2808. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, A.; Fugère, C.; Beauparlant, A.; Goyer, B.; Larouche, D.; Paquet, C.; Desgagné, M.; Sauvé, S.; Robitaille, H.; Dunnwald, M.; et al. Human epithelial stem cells persist witihin tissue-engineered skin produced by the self-assembly approach. Tissue Eng. Part A 2013, 19, 1023–1038. [Google Scholar] [CrossRef]
- Paquet, C.; Larouche, D.; Bisson, F.; Proulx, S.; Simard-Bisson, C.; Gaudreault, M.; Robitaille, H.; Carrier, P.; Martel, I.; Duranceau, L.; et al. Tissue engineering of skin and cornea: Development of new models for in vitro studies. Ann. N. Y. Acad. Sci. 2010, 1197, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Larouche, D.; Lavoie, A.; Paquet, C.; Simard-Bisson, C.; Germain, L. Identification of epithelial stem cells in vivo and in vitro using keratin 19 and BrdU. In Epidermal Cells; Springer: Berlin/Heidelberg, Germany, 2010; pp. 383–400. [Google Scholar]
- Larouche, D.; Hayward, C.; Cuffley, K.; Germain, L. Keratin 19 as a stem cell marker in vivo and in vitro. In Epidermal Cells; Springer: Berlin/Heidelberg, Germany, 2005; pp. 103–110. [Google Scholar]
- Michel, M.; Torok, N.; Godbout, M.; Lussier, M.; Gaudreau, P.; Royal, A.; Germain, L. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: Keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J. Cell Sci. 1996, 109, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Rubin, A.; Barajas, B.; Furlan-Magaril, M.; Lopez-Pajares, V.; Mumbach, M.; Howard, I.; Kim, D.; Boxer, L.; Cairns, J.; Spivakov, M.; et al. Lineage-specific dynamic and pre-established enhancer–promoter contacts cooperate in terminal differentiation. Nat. Genet. 2017, 49, 1522–1528. [Google Scholar] [CrossRef]
- Zomer, H.; Trentin, A. Skin wound healing in humans and mice: Challenges in translational research. J. Dermatol. Sci. 2018, 90, 3–12. [Google Scholar] [CrossRef]
- Larouche, D.; Cantin-Warren, L.; Desgagné, M.; Guignard, R.; Martel, I.; Ayoub, A.; Lavoie, A.; Gauvin, R.; Auger, F.; Moulin, V. Improved methods to produce tissue-engineered skin substitutes suitable for the permanent closure of full-thickness skin injuries. BioResearch Open Access 2016, 5, 320–329. [Google Scholar] [CrossRef]
- Germain, L.; Rouabhia, M.; Guignard, R.; Carrier, L.; Bouvard, V.; Auger, F. Improvement of human keratinocyte isolation and culture using thermolysin. Burns 1993, 19, 99–104. [Google Scholar] [CrossRef]
- Hayman, E.; Ruoslahti, E. Distribution of fetal bovine serum fibronectin and endogenous rat cell fibronectin in extracellular matrix. J. Cell Biol. 1979, 83, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Larouche, D.; Paquet, C.; Fradette, J.; Carrier, P.; Auger, F.; Germain, L. Regeneration of skin and cornea by tissue engineering. In Stem Cells in Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2009; pp. 233–256. [Google Scholar]
- Box, G.; Hunter, J.; Hunter, W. Statistics for Experimenters: Design, Innovation, and Discovery; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Barrandon, Y.; Green, H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl. Acad. Sci. USA 1987, 84, 2302–2306. [Google Scholar] [CrossRef] [PubMed]
- Kasper, M.; Stosiek, P.; Typlt, H.; Karsten, U. Histological evaluation of three new monoclonal anti-cytokeratin antibodies. 1. Normal tissues. Eur. J. Cancer Clin. Oncol. 1987, 23, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Becton, Dickinson and Company. FlowJoTM Software for Windows v.10.0.7; Becton, Dickinson and Company: Ashland, OR, USA, 2019. [Google Scholar]
- Xia, J.; Gill, E.; Hancock, R. NetworkAnalyst for statistical, visual and network-based meta-analysis of gene expression data. Nat. Protoc. 2015, 10, 823–844. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Goyer, B.; Larouche, D.; Kim, D.; Veillette, N.; Pruneau, V.; Bernier, V.; Auger, F.; Germain, L. Immune tolerance of tissue-engineered skin produced with allogeneic or xenogeneic fibroblasts and syngeneic keratinocytes grafted on mice. Acta Biomater. 2019, 90, 192–204. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Heisterkamp, S.; Van Willigen, B.; Maintainer, R. Package ‘nlme’. Linear and Nonlinear Mixed Effects Models; R Core Team: Vienna, Austria, 2017; Version 3. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P.; Heiberger, R.; Schuetzenmeister, A.; Scheibe, S.; Hothorn, M. Package ‘multcomp’: Simultaneous inference in general parametric models. In Project for Statistical Computing; R Core Team: Vienna, Austria, 2016. [Google Scholar]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated marginal means, aka least-squares means. R Package 2018, 1, 3. [Google Scholar]
- Auxenfans, C.; Thépot, A.; Justin, V.; Hautefeuille, A.; Shahabeddin, L.; Damour, O.; Hainaut, P. Characterisation of human fibroblasts as keratinocyte feeder layer using p63 isoforms status. Bio-Med. Mater. Eng. 2009, 19, 365–372. [Google Scholar] [CrossRef]
- Watt, F.; Mattey, D.; Garrod, D. Calcium-induced reorganization of desmosomal components in cultured human keratinocytes. J. Cell Biol. 1984, 99, 2211–2215. [Google Scholar] [CrossRef]
- Hennings, H.; Michael, D.; Cheng, C.; Steinert, P.; Holbrook, K.; Yuspa, S. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell 1980, 19, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Safoine, M.; Côté, A.; Leloup, R.; Hayward, C.J.; Campagna, M.-A.P.; Ruel, J.; Fradette, J. Engineering naturally-derived human connective tissues for clinical applications using a serum-free production system. Biomed. Mater. 2022, 17, 055011. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghio, S.C.; Barbier, M.A.; Doucet, E.J.; Debbah, I.; Safoine, M.; Le-Bel, G.; Cartier, A.; Jolibois, E.; Morissette, A.; Larouche, D.; et al. A Newly Developed Chemically Defined Serum-Free Medium Suitable for Human Primary Keratinocyte Culture and Tissue Engineering Applications. Int. J. Mol. Sci. 2023, 24, 1821. https://doi.org/10.3390/ijms24031821
Ghio SC, Barbier MA, Doucet EJ, Debbah I, Safoine M, Le-Bel G, Cartier A, Jolibois E, Morissette A, Larouche D, et al. A Newly Developed Chemically Defined Serum-Free Medium Suitable for Human Primary Keratinocyte Culture and Tissue Engineering Applications. International Journal of Molecular Sciences. 2023; 24(3):1821. https://doi.org/10.3390/ijms24031821
Chicago/Turabian StyleGhio, Sergio Cortez, Martin A. Barbier, Emilie J. Doucet, Imad Debbah, Meryem Safoine, Gaëtan Le-Bel, Andréanne Cartier, Emilie Jolibois, Amélie Morissette, Danielle Larouche, and et al. 2023. "A Newly Developed Chemically Defined Serum-Free Medium Suitable for Human Primary Keratinocyte Culture and Tissue Engineering Applications" International Journal of Molecular Sciences 24, no. 3: 1821. https://doi.org/10.3390/ijms24031821
APA StyleGhio, S. C., Barbier, M. A., Doucet, E. J., Debbah, I., Safoine, M., Le-Bel, G., Cartier, A., Jolibois, E., Morissette, A., Larouche, D., Fradette, J., Guérin, S. L., Garnier, A., & Germain, L. (2023). A Newly Developed Chemically Defined Serum-Free Medium Suitable for Human Primary Keratinocyte Culture and Tissue Engineering Applications. International Journal of Molecular Sciences, 24(3), 1821. https://doi.org/10.3390/ijms24031821






