Tissue Engineering for Gastrointestinal and Genitourinary Tracts
Abstract
1. Introduction
2. Tissue Engineering
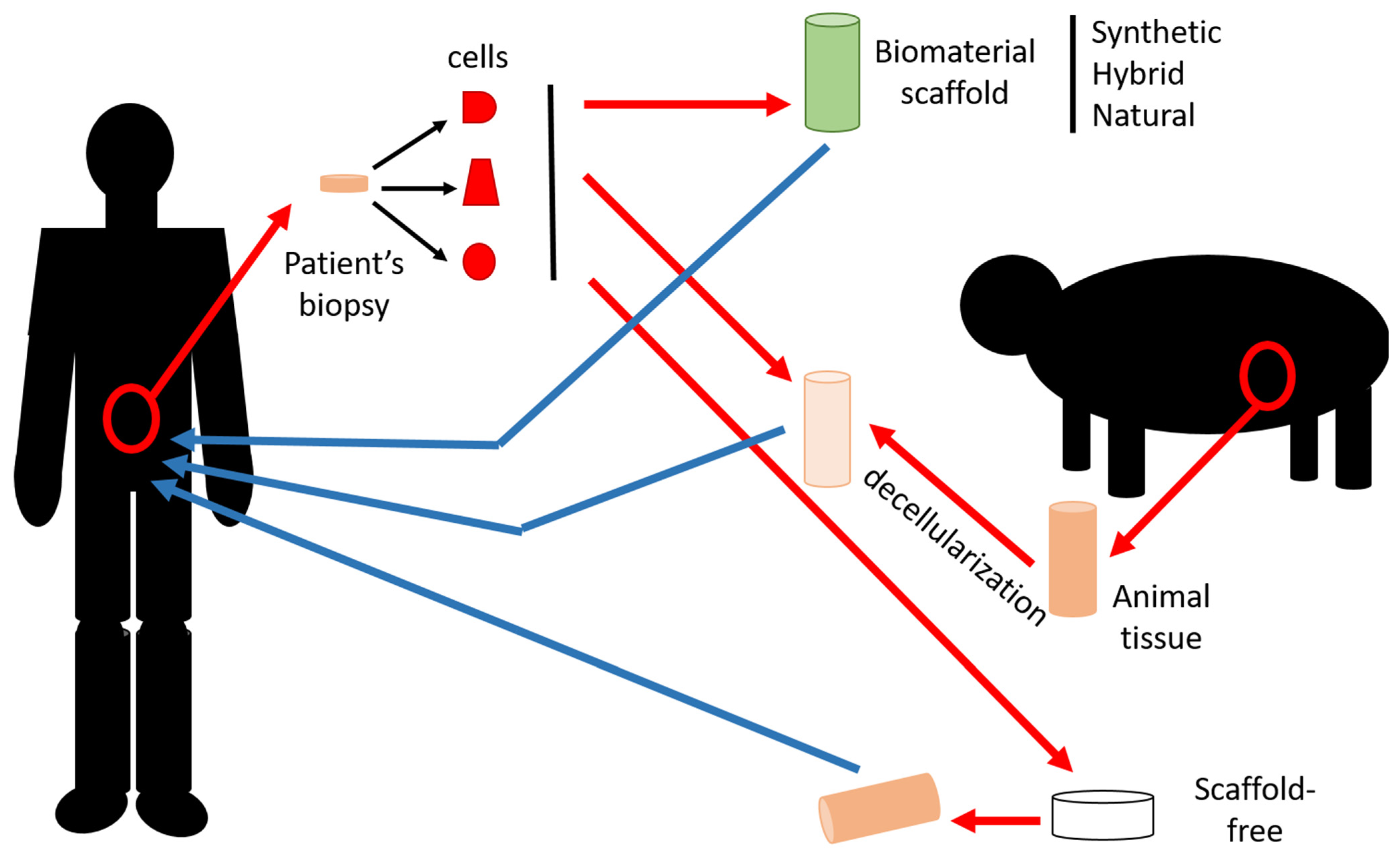
2.1. Materials
2.1.1. Synthetic Materials
2.1.2. Natural Materials
2.1.3. Hybrid Materials
2.1.4. Acellular Matrix
2.2. Cells
2.2.1. Embryonic Stem Cells
2.2.2. Neonatal Stem Cells
2.2.3. Adult Stem Cells
2.2.4. Adult Somatic Cells
2.2.5. Induced Pluripotent Stem Cells
2.2.6. Cell Culture Condition Improvements
2.3. Techniques
2.4. The Self-Assembly Technique
3. Digestive Tract
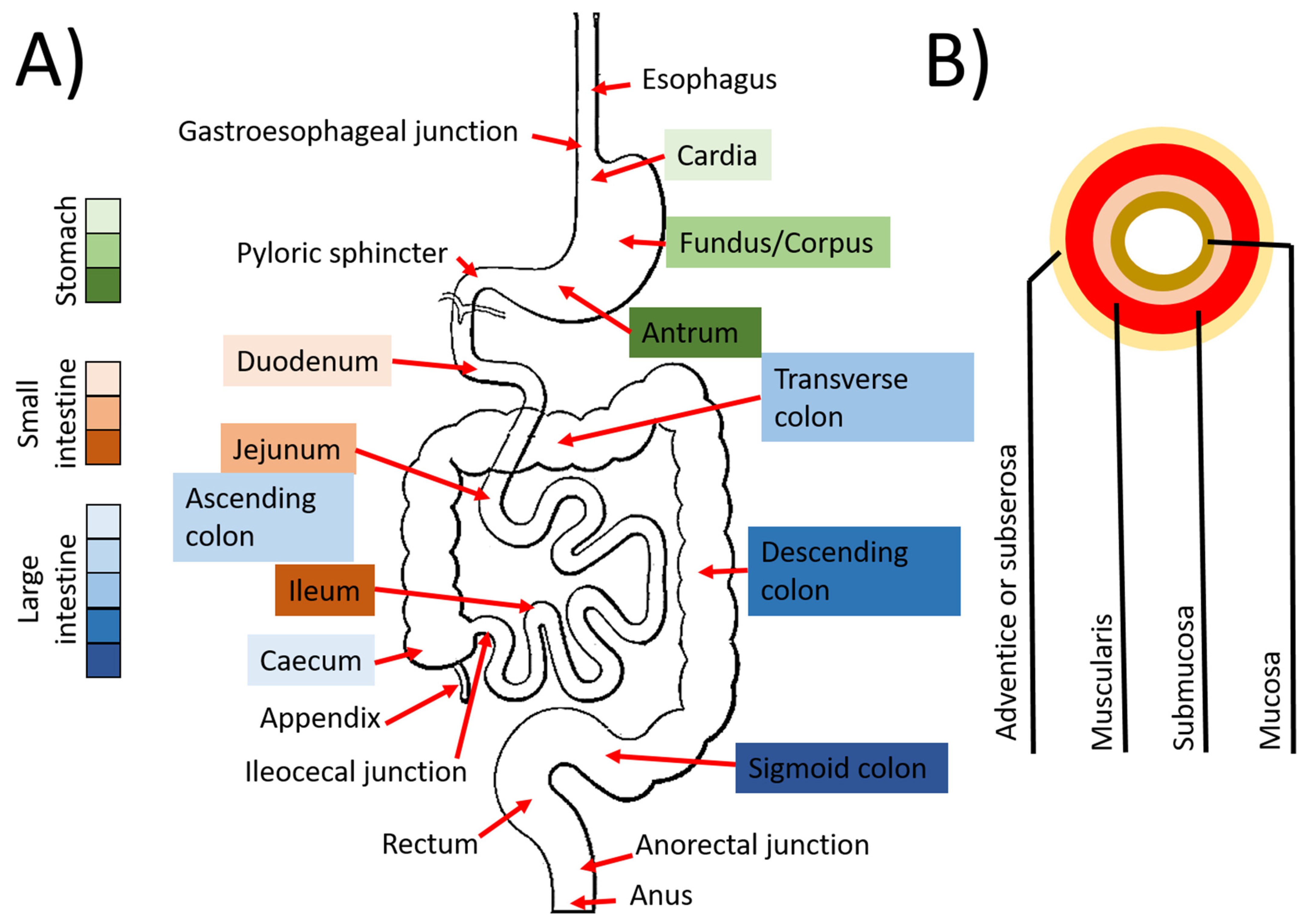
3.1. Digestive Tract
3.1.1. Esophagus
3.1.2. Stomach
3.1.3. The Intestines
3.1.4. Tissue Engineering
4. Urinary Tract
4.1. Ureters
4.2. Bladder
4.3. Urethra
4.4. Reconstruction of the Bladder, Ureters and Urethra
4.4.1. Biomaterials
4.4.2. Cells Used for Urologic Tissue Engineering
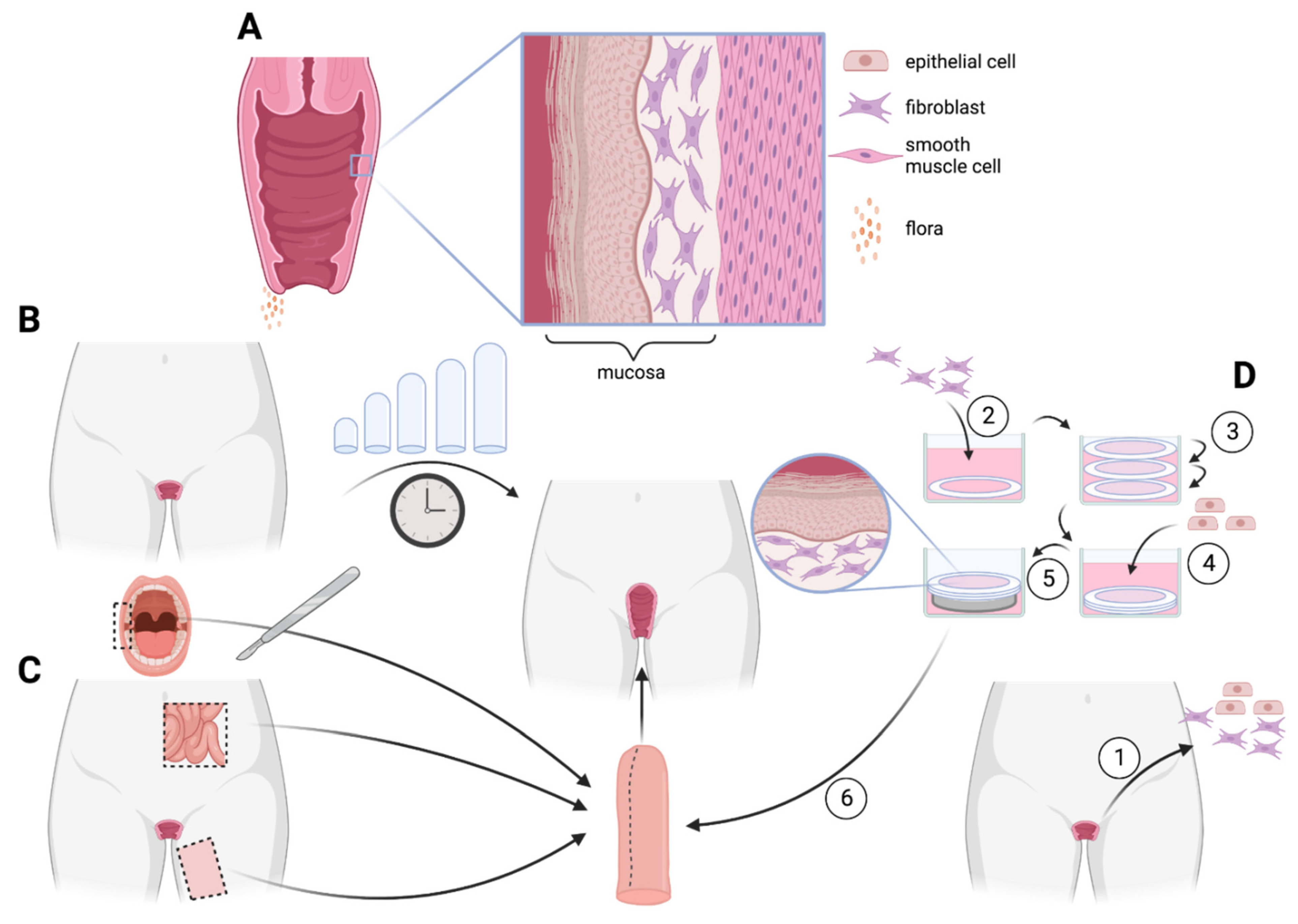
5. Vagina
5.1. Anatomy
5.2. Pathologies
5.3. Current Surgical Treatment
5.4. Tissue Engineering
5.4.1. Decellularized Tissues
5.4.2. Synthetic Biomaterials
5.4.3. Self-Assembly
5.4.4. Use of Stents
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Boys, A.J.; Barron, S.L.; Tilev, D.; Owens, R.M. Building Scaffolds for Tubular Tissue Engineering. Front. Bioeng. Biotechnol. 2020, 8, 589960. [Google Scholar] [CrossRef] [PubMed]
- Farzamfar, S.; Elia, E.; Chabaud, S.; Naji, M.; Bolduc, S. Prospects and Challenges of Electrospun Cell and Drug Delivery Vehicles to Correct Urethral Stricture. Int. J. Mol. Sci. 2022, 23, 519. [Google Scholar] [CrossRef] [PubMed]
- Panja, N.; Maji, S.; Choudhuri, S.; Ali, K.A.; Hossain, C.M. 3D Bioprinting of Human Hollow Organs. AAPS PharmSciTech 2022, 23, 139. [Google Scholar] [CrossRef] [PubMed]
- Saba, I.; Jakubowska, W.; Bolduc, S.; Chabaud, S. Engineering Tissues without the Use of a Synthetic Scaffold: A Twenty-Year History of the Self-Assembly Method. BioMed Res. Int. 2018, 2018, 5684679. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Zarrintaj, P.; Oftadeh, M.O.; Keramati, F.; Fouladiha, H.; Sohrabi-Jahromi, S.; Ziraksaz, Z. Tissue engineering; strategies, tissues, and biomaterials. Biotechnol. Genet. Eng. Rev. 2017, 33, 144–172. [Google Scholar] [CrossRef]
- Alberti, C. From the intestinal neobladder to the bioartificial bladder: Remarks on some biological implications. Minerva Urol. Nefrol. 2000, 52, 219–222. [Google Scholar]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.; Dolivo, D.M.; Yang, T.; Dominko, T.; et al. Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef]
- Yao, S.; Shang, Y.; Ren, B.; Deng, S.; Wang, Z.; Peng, Y.; Huang, Z.; Ma, S.; Peng, C.; Hou, S. A novel natural-derived tilapia skin collagen mineralized with hydroxyapatite as a potential bone-grafting scaffold. J. Biomater. Appl. 2022, 37, 219–237. [Google Scholar] [CrossRef]
- Dearth, C.L.; Keane, T.J.; Carruthers, C.A.; Reing, J.E.; Huleihel, L.; Ranallo, C.A.; Kollar, E.W.; Badylak, S.F. The effect of terminal sterilization on the material properties and in vivo remodeling of a porcine dermal biologic scaffold. Acta Biomater. 2016, 33, 78–87. [Google Scholar] [CrossRef]
- Perez Davila, S.; Gonzalez Rodriguez, L.; Chiussi, S.; Serra, J.; Gonzalez, P. How to Sterilize Polylactic Acid Based Medical Devices? Polymers 2021, 13, 2115. [Google Scholar] [CrossRef] [PubMed]
- Zamuner, A.; Cavo, M.; Scaglione, S.; Messina, G.M.L.; Russo, T.; Gloria, A.; Marletta, G.; Dettin, M. Design of Decorated Self-Assembling Peptide Hydrogels as Architecture for Mesenchymal Stem Cells. Materials 2016, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gareta, E.; Abduldaiem, Y.; Sawadkar, P.; Kyriakidis, C.; Lali, F.; Greco, K.V. Decellularised scaffolds: Just a framework? Current knowledge and future directions. J. Tissue Eng. 2020, 11, 2041731420942903. [Google Scholar] [CrossRef]
- White, L.J.; Taylor, A.J.; Faulk, D.M.; Keane, T.J.; Saldin, L.T.; Reing, J.E.; Swinehart, I.T.; Turner, N.J.; Ratner, B.D.; Badylak, S.F. The impact of detergents on the tissue decellularization process: A ToF-SIMS study. Acta Biomater. 2017, 50, 207–219. [Google Scholar] [CrossRef]
- Ceonzo, K.; Gaynor, A.; Shaffer, L.; Kojima, K.; Vacanti, C.A.; Stahl, G.L. Polyglycolic acid-induced inflammation: Role of hydrolysis and resulting complement activation. Tissue Eng. 2006, 12, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, L.; Morandi, A.; Giannoni, E.; Chiarugi, P. Lactate: A Metabolic Driver in the Tumour Landscape. Trends Biochem. Sci. 2019, 44, 153–166. [Google Scholar] [CrossRef]
- Keane, T.J.; Badylak, S.F. Biomaterials for tissue engineering applications. Semin. Pediatr. Surg. 2014, 23, 112–118. [Google Scholar] [CrossRef]
- Lee, E.J.; Kasper, F.K.; Mikos, A.G. Biomaterials for tissue engineering. Ann. Biomed. Eng. 2014, 42, 323–337. [Google Scholar] [CrossRef]
- Jin, M.; Shi, J.; Zhu, W.; Yao, H.; Wang, D.A. Polysaccharide-Based Biomaterials in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2021, 27, 604–626. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Elkasabgy, N.A. Biomaterials for Tissue Engineering Applications and Current Updates in the Field: A Comprehensive Review. AAPS PharmSciTech 2022, 23, 267. [Google Scholar] [CrossRef]
- Khan, H.M.; Liao, X.; Sheikh, B.A.; Wang, Y.; Su, Z.; Guo, C.; Li, Z.; Zhou, C.; Cen, Y.; Kong, Q. Smart biomaterials and their potential applications in tissue engineering. J. Mater. Chem. B 2022, 10, 6859–6895. [Google Scholar] [CrossRef] [PubMed]
- Asri, N.A.N.; Mahat, M.M.; Zakaria, A.; Safian, M.F.; Abd Hamid, U.M. Fabrication Methods of Electroactive Scaffold-Based Conducting Polymers for Tissue Engineering Application: A Review. Front. Bioeng. Biotechnol. 2022, 10, 876696. [Google Scholar] [CrossRef] [PubMed]
- Caneparo, C.; Sorroza-Martinez, L.; Chabaud, S.; Fradette, J.; Bolduc, S. Considerations for the clinical use of stem cells in genitourinary regenerative medicine. World J. Stem Cells 2021, 13, 1480–1512. [Google Scholar] [CrossRef] [PubMed]
- Suh, H. Tissue restoration, tissue engineering and regenerative medicine. Yonsei Med. J. 2000, 41, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Denker, H.W. Embryonic stem cells: An exciting field for basic research and tissue engineering, but also an ethical dilemma? Cells Tissues Organs 1999, 165, 246–249. [Google Scholar] [CrossRef]
- de Vries, R.B.; Oerlemans, A.; Trommelmans, L.; Dierickx, K.; Gordijn, B. Ethical aspects of tissue engineering: A review. Tissue Eng. Part B Rev. 2008, 14, 367–375. [Google Scholar] [CrossRef]
- Bishop, A.E.; Buttery, L.D.; Polak, J.M. Embryonic stem cells. J. Pathol. 2002, 197, 424–429. [Google Scholar] [CrossRef]
- Hayward, C.J.; Fradette, J.; Galbraith, T.; Remy, M.; Guignard, R.; Gauvin, R.; Germain, L.; Auger, F.A. Harvesting the potential of the human umbilical cord: Isolation and characterisation of four cell types for tissue engineering applications. Cells Tissues Organs 2013, 197, 37–54. [Google Scholar] [CrossRef]
- Hayward, C.J.; Fradette, J.; Morissette Martin, P.; Guignard, R.; Germain, L.; Auger, F.A. Using human umbilical cord cells for tissue engineering: A comparison with skin cells. Differentiation 2014, 87, 172–181. [Google Scholar] [CrossRef]
- Liau, L.L.; Ruszymah, B.H.I.; Ng, M.H.; Law, J.X. Characteristics and clinical applications of Wharton’s jelly-derived mesenchymal stromal cells. Curr. Res. Transl. Med. 2020, 68, 5–16. [Google Scholar] [CrossRef]
- de Souza Dobuchak, D.; Stricker, P.E.F.; de Oliveira, N.B.; Mogharbel, B.F.; da Rosa, N.N.; Dziedzic, D.S.M.; Irioda, A.C.; Athayde Teixeira de Carvalho, K. The Neural Multilineage Differentiation Capacity of Human Neural Precursors from the Umbilical Cord-Ready to Bench for Clinical Trials. Membranes 2022, 12, 873. [Google Scholar] [CrossRef] [PubMed]
- Gerdfaramarzi, M.S.; Bazmi, S.; Kiani, M.; Afshar, L.; Fadavi, M.; Enjoo, S.A. Ethical challenges of cord blood banks: A scoping review. J. Med. Life 2022, 15, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Kalaszczynska, I.; Ferdyn, K. Wharton’s jelly derived mesenchymal stem cells: Future of regenerative medicine? Recent findings and clinical significance. BioMed. Res. Int. 2015, 2015, 430847. [Google Scholar] [CrossRef] [PubMed]
- Ranjbaran, H.; Abediankenari, S.; Mohammadi, M.; Jafari, N.; Khalilian, A.; Rahmani, Z.; Momeninezhad Amiri, M.; Ebrahimi, P. Wharton’s Jelly Derived-Mesenchymal Stem Cells: Isolation and Characterization. Acta Med. Iran. 2018, 56, 28–33. [Google Scholar]
- Grompe, M. Adult versus embryonic stem cells: It’s still a tie. Mol. Ther. 2002, 6, 303–305. [Google Scholar] [CrossRef]
- Wagers, A.J.; Weissman, I.L. Plasticity of adult stem cells. Cell 2004, 116, 639–648. [Google Scholar] [CrossRef]
- Vasanthan, J.; Gurusamy, N.; Rajasingh, S.; Sigamani, V.; Kirankumar, S.; Thomas, E.L.; Rajasingh, J. Role of Human Mesenchymal Stem Cells in Regenerative Therapy. Cells 2020, 10, 54. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Artiles, M.; Bunnell, B.A. Adipose Stem Cells in Regenerative Medicine: Looking Forward. Front. Bioeng. Biotechnol. 2021, 9, 837464. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, J.; Huang, Y.; Wu, C.; Xie, H. Urine-derived stem cells: Applications in skin, bone and articular cartilage repair. Burn. Trauma 2021, 9, tkab039. [Google Scholar] [CrossRef]
- De Miguel, M.P.; Fuentes-Julian, S.; Blazquez-Martinez, A.; Pascual, C.Y.; Aller, M.A.; Arias, J.; Arnalich-Montiel, F. Immunosuppressive properties of mesenchymal stem cells: Advances and applications. Curr. Mol. Med. 2012, 12, 574–591. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, A.; Garcia-Sanchez, D.; Dotta, M.; Rodriguez-Rey, J.C.; Perez-Campo, F.M. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine. World J. Stem Cells 2020, 12, 1529–1552. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, R.; Hinley, J.; Stahlschmidt, J.; Southgate, J. Tissue engineering potential of urothelial cells from diseased bladders. J. Urol. 2011, 186, 2014–2020. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Zhao, R.; Doi, A.; Ng, K.; Unternaehrer, J.; Cahan, P.; Huo, H.; Loh, Y.H.; Aryee, M.J.; Lensch, M.W.; et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat. Biotechnol. 2011, 29, 1117–1119. [Google Scholar] [CrossRef]
- Tapia, N.; Scholer, H.R. Molecular Obstacles to Clinical Translation of iPSCs. Cell Stem Cell 2016, 19, 298–309. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Brunet, A. Aging and reprogramming: A two-way street. Curr. Opin. Cell Biol. 2012, 24, 744–756. [Google Scholar] [CrossRef]
- Kang, E.; Wang, X.; Tippner-Hedges, R.; Ma, H.; Folmes, C.D.; Gutierrez, N.M.; Lee, Y.; Van Dyken, C.; Ahmed, R.; Li, Y.; et al. Age-Related Accumulation of Somatic Mitochondrial DNA Mutations in Adult-Derived Human iPSCs. Cell Stem Cell 2016, 18, 625–636. [Google Scholar] [CrossRef]
- Timpano, S.; Guild, B.D.; Specker, E.J.; Melanson, G.; Medeiros, P.J.; Sproul, S.L.J.; Uniacke, J. Physioxic human cell culture improves viability, metabolism, and mitochondrial morphology while reducing DNA damage. FASEB J. 2019, 33, 5716–5728. [Google Scholar] [CrossRef]
- Chabaud, S.; Saba, I.; Baratange, C.; Boiroux, B.; Leclerc, M.; Rousseau, A.; Bouhout, S.; Bolduc, S. Urothelial cell expansion and differentiation are improved by exposure to hypoxia. J. Tissue Eng. Regen. Med. 2017, 11, 3090–3099. [Google Scholar] [CrossRef]
- Caneparo, C.; Baratange, C.; Chabaud, S.; Bolduc, S. Conditioned medium produced by fibroblasts cultured in low oxygen pressure allows the formation of highly structured capillary-like networks in fibrin gels. Sci. Rep. 2020, 10, 9291. [Google Scholar] [CrossRef]
- Bhat, S.; Viswanathan, P.; Chandanala, S.; Prasanna, S.J.; Seetharam, R.N. Expansion and characterization of bone marrow derived human mesenchymal stromal cells in serum-free conditions. Sci. Rep. 2021, 11, 3403. [Google Scholar] [CrossRef] [PubMed]
- Caneparo, C.; Chabaud, S.; Fradette, J.; Bolduc, S. Evaluation of a Serum-Free Medium for Human Epithelial and Stromal Cell Culture. Int. J. Mol. Sci. 2022, 23, 35. [Google Scholar] [CrossRef] [PubMed]
- Caneparo, C.; Chabaud, S.; Bolduc, S. Reconstruction of Vascular and Urologic Tubular Grafts by Tissue Engineering. Processes 2021, 9, 513. [Google Scholar] [CrossRef]
- Gould, B.S. Collagen Formation and Fibrogenesis with Special Reference to the Role of Ascorbic Acid. Int. Rev. Cytol. 1963, 15, 301–361. [Google Scholar] [CrossRef] [PubMed]
- Switzer, B.R.; Summer, G.K. Collagen synthesis in human skin fibroblasts: Effects of ascorbate, -ketoglutarate and ferrous ion on proline hydroxylation. J. Nutr. 1972, 102, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Hata, R.; Senoo, H. L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fibroblasts. J. Cell Physiol. 1989, 138, 8–16. [Google Scholar] [CrossRef]
- L’Heureux, N.; Germain, L.; Labbe, R.; Auger, F.A. In vitro construction of a human blood vessel from cultured vascular cells: A morphologic study. J. Vasc. Surg. 1993, 17, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Bouhout, S.; Perron, E.; Gauvin, R.; Bernard, G.; Ouellet, G.; Cattan, V.; Bolduc, S. In vitro reconstruction of an autologous, watertight, and resistant vesical equivalent. Tissue Eng. Part A 2010, 16, 1539–1548. [Google Scholar] [CrossRef]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar]
- Bedard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, E.; Chabaud, S.; Bolduc, S. Innovative Human Three-Dimensional Tissue-Engineered Models as an Alternative to Animal Testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef]
- Simon, F.; Bergeron, D.; Larochelle, S.; Lopez-Valle, C.A.; Genest, H.; Armour, A.; Moulin, V.J. Enhanced secretion of TIMP-1 by human hypertrophic scar keratinocytes could contribute to fibrosis. Burns 2012, 38, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Corriveau, M.P.; Boufaied, I.; Lessard, J.; Chabaud, S.; Senecal, J.L.; Grodzicky, T.; Chartier, S.; Raymond, Y.; Moulin, V.J. The fibrotic phenotype of systemic sclerosis fibroblasts varies with disease duration and severity of skin involvement: Reconstitution of skin fibrosis development using a tissue engineering approach. J. Pathol. 2009, 217, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Bourland, J.; Fradette, J.; Auger, F.A. Tissue-engineered 3D melanoma model with blood and lymphatic capillaries for drug development. Sci. Rep. 2018, 8, 13191. [Google Scholar] [CrossRef] [PubMed]
- Gibot, L.; Galbraith, T.; Huot, J.; Auger, F.A. Development of a tridimensional microvascularized human skin substitute to study melanoma biology. Clin. Exp. Metastasis 2013, 30, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Jean, J.; Lapointe, M.; Soucy, J.; Pouliot, R. Development of an in vitro psoriatic skin model by tissue engineering. J. Derm. Sci 2009, 53, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Dakiw Piaceski, A.; Larouche, D.; Ghani, K.; Bisson, F.; Cortez Ghio, S.; Larochelle, S.; Moulin, V.J.; Caruso, M.; Germain, L. Translating the combination of gene therapy and tissue engineering for treating recessive dystrophic epidermolysis bullosa. Eur. Cell Mater. 2018, 35, 73–86. [Google Scholar] [CrossRef]
- Roy, V.; Lamontagne, R.; Talagas, M.; Touzel-Deschenes, L.; Khuong, H.T.; Saikali, S.; Dupre, N.; Gros-Louis, F. Biofabrication of a three dimensional human-based personalized neurofibroma model. Biotechnol. J. 2021, 16, e2000250. [Google Scholar] [CrossRef]
- Pare, B.; Touzel-Deschenes, L.; Lamontagne, R.; Lamarre, M.S.; Scott, F.D.; Khuong, H.T.; Dion, P.A.; Bouchard, J.P.; Gould, P.; Rouleau, G.A.; et al. Early detection of structural abnormalities and cytoplasmic accumulation of TDP-43 in tissue-engineered skins derived from ALS patients. Acta Neuropathol. Commun. 2015, 3, 5. [Google Scholar] [CrossRef]
- Saba, I.; Barat, C.; Chabaud, S.; Reyjon, N.; Leclerc, M.; Jakubowska, W.; Orabi, H.; Lachhab, A.; Pelletier, M.; Tremblay, M.J.; et al. Immunocompetent Human 3D Organ-Specific Hormone-Responding Vaginal Mucosa Model of HIV-1 Infection. Tissue Eng. Part C Methods 2021, 27, 152–166. [Google Scholar] [CrossRef]
- Bureau, M.; Pelletier, J.; Rousseau, A.; Bernard, G.; Chabaud, S.; Bolduc, S. Demonstration of the direct impact of ketamine on urothelium using a tissue engineered bladder model. Can. Urol. Assoc. J. 2015, 9, E613–E617. [Google Scholar] [CrossRef]
- Ringuette Goulet, C.; Bernard, G.; Chabaud, S.; Couture, A.; Langlois, A.; Neveu, B.; Pouliot, F.; Bolduc, S. Tissue-engineered human 3D model of bladder cancer for invasion study and drug discovery. Biomaterials 2017, 145, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Roussel, C.; Chabaud, S.; Lessard-Lord, J.; Cattero, V.; Pellerin, F.A.; Feutry, P.; Bochard, V.; Bolduc, S.; Desjardins, Y. UPEC Colonic-Virulence and Urovirulence Are Blunted by Proanthocyanidins-Rich Cranberry Extract Microbial Metabolites in a Gut Model and a 3D Tissue-Engineered Urothelium. Microbiol. Spectr. 2022, 10, e0243221. [Google Scholar] [CrossRef] [PubMed]
- Caneparo, C.; Brownell, D.; Chabaud, S.; Bolduc, S. Genitourinary Tissue Engineering: Reconstruction and Research Models. Bioengineering 2021, 8, 99. [Google Scholar] [CrossRef]
- Messier, B.; Leblond, C.P. Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. Am. J. Anat. 1960, 106, 247–285. [Google Scholar] [CrossRef]
- Seery, J.P. Stem cells of the oesophageal epithelium. J. Cell Sci. 2002, 115, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, P.F.; Simoes e Silva, A.C.; Pereira, R.M. Current knowledge on esophageal atresia. World J. Gastroenterol. 2012, 18, 3662–3672. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Huang, F.L.; Yu, S.J. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J. Surg. 2018, 41, 210–215. [Google Scholar] [CrossRef]
- Watanabe, M.; Otake, R.; Kozuki, R.; Toihata, T.; Takahashi, K.; Okamura, A.; Imamura, Y. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg. Today 2020, 50, 12–20. [Google Scholar] [CrossRef]
- Hamza, A.F. Colonic replacement in cases of esophageal atresia. Semin. Pediatr. Surg. 2009, 18, 40–43. [Google Scholar] [CrossRef]
- Burrington, J.D.; Stephens, C.A. Esophageal replacement with a gastric tube in infants and children. J. Pediatr. Surg. 1968, 3, 24–52. [Google Scholar] [CrossRef] [PubMed]
- Scharli, A.F. Esophageal reconstruction by elongation of the lesser gastric curvature. Pediatr. Surg. Int. 1996, 11, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Londono, R.; Badylak, S.F. Regenerative Medicine Strategies for Esophageal Repair. Tissue Eng. Part B Rev. 2015, 21, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.A. Normal histology of the stomach. Am. J. Surg. Pathol. 1986, 10, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.A.; DeLaForest, A.; Battle, M.A. Patterning the gastrointestinal epithelium to confer regional-specific functions. Dev. Biol. 2018, 435, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Norwood, D.A.; Montalvan, E.E.; Dominguez, R.L.; Morgan, D.R. Gastric Cancer: Emerging Trends in Prevention, Diagnosis, and Treatment. Gastroenterol. Clin. N. Am. 2022, 51, 501–518. [Google Scholar] [CrossRef]
- Volk, N.; Lacy, B. Anatomy and Physiology of the Small Bowel. Gastrointest. Endosc. Clin. N. Am. 2017, 27, 1–13. [Google Scholar] [CrossRef]
- Hounnou, G.; Destrieux, C.; Desme, J.; Bertrand, P.; Velut, S. Anatomical study of the length of the human intestine. Surg. Radiol. Anat. 2002, 24, 290–294. [Google Scholar] [CrossRef]
- Krause, W.J. Brunner’s glands: A structural, histochemical and pathological profile. Prog. Histochem. Cytochem. 2000, 35, 259–367. [Google Scholar] [CrossRef]
- Azzouz, L.L.; Sharma, S. Physiology, Large Intestine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Irving, M.H.; Catchpole, B. ABC of colorectal diseases. Anatomy and physiology of the colon, rectum, and anus. BMJ 1992, 304, 1106–1108. [Google Scholar] [CrossRef]
- Wang, Y.H.W.; Wiseman, J. Anatomy, Abdomen and Pelvis, Rectum. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- van der Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.F. Extracellular matrix components and integrins in relationship to human intestinal epithelial cell differentiation. Prog. Histochem. Cytochem. 1997, 31, 76. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Cavalcoli, F.; Rausa, E.; Invernizzi, P.; Braga, M.; Vecchi, M. Understanding short bowel syndrome: Current status and future perspectives. Dig. Liver Dis. 2020, 52, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Che, Z.; Ye, Z.; Zhang, X.; Lin, B.; Yang, W.; Liang, Y.; Zeng, J. Mesenchymal stem/stromal cells in the pathogenesis and regenerative therapy of inflammatory bowel diseases. Front. Immunol. 2022, 13, 952071. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.U.; Neaga, A.; West, B.; Safran, J.; Brown, P.; Btaiche, I.; Kuzma-O’Reilly, B.; Teitelbaum, D.H. Pediatric short bowel syndrome: Redefining predictors of success. Ann. Surg. 2005, 242, 403–409; discussion 409–412. [Google Scholar] [CrossRef]
- Grant, D.; Abu-Elmagd, K.; Mazariegos, G.; Vianna, R.; Langnas, A.; Mangus, R.; Farmer, D.G.; Lacaille, F.; Iyer, K.; Fishbein, T.; et al. Intestinal transplant registry report: Global activity and trends. Am. J. Transpl. 2015, 15, 210–219. [Google Scholar] [CrossRef]
- Arakelian, L.; Kanai, N.; Dua, K.; Durand, M.; Cattan, P.; Ohki, T. Esophageal tissue engineering: From bench to bedside. Ann. N. Y. Acad. Sci. 2018, 1434, 156–163. [Google Scholar] [CrossRef]
- Vogt, C.D.; Panoskaltsis-Mortari, A. Tissue engineering of the gastroesophageal junction. J. Tissue Eng. Regen. Med. 2020, 14, 855–868. [Google Scholar] [CrossRef]
- San Roman, A.K.; Shivdasani, R.A. Boundaries, junctions and transitions in the gastrointestinal tract. Exp. Cell Res. 2011, 317, 2711–2718. [Google Scholar] [CrossRef]
- Badylak, S.F.; Vorp, D.A.; Spievack, A.R.; Simmons-Byrd, A.; Hanke, J.; Freytes, D.O.; Thapa, A.; Gilbert, T.W.; Nieponice, A. Esophageal reconstruction with ECM and muscle tissue in a dog model. J. Surg. Res. 2005, 128, 87–97. [Google Scholar] [CrossRef]
- Ohki, T.; Yamato, M.; Murakami, D.; Takagi, R.; Yang, J.; Namiki, H.; Okano, T.; Takasaki, K. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut 2006, 55, 1704–1710. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Miyazaki, S.; Miyata, G.; Satomi, S.; Hori, Y. Autologous buccal keratinocyte implantation for the prevention of stenosis after EMR of the esophagus. Gastrointest. Endosc. 2007, 66, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Hori, Y.; Nakada, A.; Uji, M.; Nishizawa, Y.; Yamamoto, K.; Kobayashi, T.; Shimada, H.; Kida, N.; Sato, T.; et al. Use of adipose tissue-derived stromal cells for prevention of esophageal stricture after circumferential EMR in a canine model. Gastrointest. Endosc. 2011, 73, 777–784. [Google Scholar] [CrossRef]
- Ohki, T.; Yamato, M.; Ota, M.; Takagi, R.; Murakami, D.; Kondo, M.; Sasaki, R.; Namiki, H.; Okano, T.; Yamamoto, M. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology 2012, 143, 582–588.e582. [Google Scholar] [CrossRef] [PubMed]
- Perrod, G.; Rahmi, G.; Pidial, L.; Camilleri, S.; Bellucci, A.; Casanova, A.; Viel, T.; Tavitian, B.; Cellier, C.; Clement, O. Cell Sheet Transplantation for Esophageal Stricture Prevention after Endoscopic Submucosal Dissection in a Porcine Model. PLoS ONE 2016, 11, e0148249. [Google Scholar] [CrossRef]
- Takeoka, Y.; Matsumoto, K.; Taniguchi, D.; Tsuchiya, T.; Machino, R.; Moriyama, M.; Oyama, S.; Tetsuo, T.; Taura, Y.; Takagi, K.; et al. Regeneration of esophagus using a scaffold-free biomimetic structure created with bio-three-dimensional printing. PLoS ONE 2019, 14, e0211339. [Google Scholar] [CrossRef]
- Mark, J.B.; Briggs, H.C. Segmental Replacement of the Thoracic Esophagus with Woven Teflon. J. Surg. Res. 1964, 4, 400–402. [Google Scholar] [CrossRef]
- Lynen Jansen, P.; Klinge, U.; Anurov, M.; Titkova, S.; Mertens, P.R.; Jansen, M. Surgical mesh as a scaffold for tissue regeneration in the esophagus. Eur. Surg. Res. 2004, 36, 104–111. [Google Scholar] [CrossRef]
- Beckstead, B.L.; Pan, S.; Bhrany, A.D.; Bratt-Leal, A.M.; Ratner, B.D.; Giachelli, C.M. Esophageal epithelial cell interaction with synthetic and natural scaffolds for tissue engineering. Biomaterials 2005, 26, 6217–6228. [Google Scholar] [CrossRef]
- Liang, J.H.; Zhou, X.; Zheng, Z.B.; Liang, X.L. Long-term form and function of neoesophagus after experimental replacement of thoracic esophagus with nitinol composite artificial esophagus. ASAIO J. 2010, 56, 232–234. [Google Scholar] [CrossRef]
- Gong, C.; Hou, L.; Zhu, Y.; Lv, J.; Liu, Y.; Luo, L. In vitro constitution of esophageal muscle tissue with endocyclic and exolongitudinal patterns. ACS Appl. Mater. Interfaces 2013, 5, 6549–6555. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, M.; Miyazawa, M.; Okamoto, K.; Okada, K.; Akimoto, N.; Sato, H.; Koyama, I.; Yamaguchi, S.; Ikada, Y. A bioabsorbable polymer patch for the treatment of esophageal defect in a porcine model. J. Gastroenterol. 2013, 48, 822–829. [Google Scholar] [CrossRef]
- Diemer, P.; Markoew, S.; Le, D.Q.; Qvist, N. Poly-epsilon-caprolactone mesh as a scaffold for in vivo tissue engineering in rabbit esophagus. Dis. Esophagus 2015, 28, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.J.; Yeong, W.Y.; Tan, X.; An, J.; Chian, K.S.; Leong, K.F. Characterization, mechanical behavior and in vitro evaluation of a melt-drawn scaffold for esophageal tissue engineering. J. Mech. Behav. Biomed. Mater. 2016, 57, 246–259. [Google Scholar] [CrossRef]
- Hou, L.; Gong, C.; Zhu, Y. In vitro construction and in vivo regeneration of esophageal bilamellar muscle tissue. J. Biomater. Appl. 2016, 30, 1373–1384. [Google Scholar] [CrossRef]
- Park, S.Y.; Choi, J.W.; Park, J.K.; Song, E.H.; Park, S.A.; Kim, Y.S.; Shin, Y.S.; Kim, C.H. Tissue-engineered artificial oesophagus patch using three-dimensionally printed polycaprolactone with mesenchymal stem cells: A preliminary report. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 712–717. [Google Scholar] [CrossRef]
- Kuppan, P.; Sethuraman, S.; Krishnan, U.M. In vitro co-culture of epithelial cells and smooth muscle cells on aligned nanofibrous scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 191–205. [Google Scholar] [CrossRef]
- Dorati, R.; De Trizio, A.; Marconi, S.; Ferrara, A.; Auricchio, F.; Genta, I.; Modena, T.; Benazzo, M.; Benazzo, A.; Volpato, G.; et al. Design of a Bioabsorbable Multilayered Patch for Esophagus Tissue Engineering. Macromol. Biosci. 2017, 17, 1600426. [Google Scholar] [CrossRef]
- Tan, Y.J.; Tan, X.; Yeong, W.Y.; Tor, S.B. Hybrid microscaffold-based 3D bioprinting of multi-cellular constructs with high compressive strength: A new biofabrication strategy. Sci. Rep. 2016, 6, 39140. [Google Scholar] [CrossRef]
- La Francesca, S.; Aho, J.M.; Barron, M.R.; Blanco, E.W.; Soliman, S.; Kalenjian, L.; Hanson, A.D.; Todorova, E.; Marsh, M.; Burnette, K.; et al. Long-term regeneration and remodeling of the pig esophagus after circumferential resection using a retrievable synthetic scaffold carrying autologous cells. Sci. Rep. 2018, 8, 4123. [Google Scholar] [CrossRef]
- Wei, Q.; Jin, J.; Wang, X.; Shen, Q.; Zhou, M.; Bu, S.; Zhu, Y. The growth and pluripotency of mesenchymal stem cell on the biodegradable polyurethane synthesized with ferric catalyst. J. Biomater. Sci. Polym. Ed. 2018, 29, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.J.; Ju, H.W.; Yeon, Y.K.; Lee, J.S.; Lee, Y.J.; Seo, Y.B.; Chan Hum, P. Development of an omentum-cultured oesophageal scaffold reinforced by a 3D-printed ring: Feasibility of an in vivo bioreactor. Artif. Cells Nanomed. Biotechnol. 2018, 46, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Wanczyk, H.; Sharma, I.; Mitchell, A.; Sayej, W.N.; Finck, C. Polyurethane scaffolds seeded with autologous cells can regenerate long esophageal gaps: An esophageal atresia treatment model. J. Pediatr. Surg. 2019, 54, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.G.; Wu, Y.; Park, S.A.; Cho, H.; Choi, J.J.; Kwon, S.K.; Shin, J.W.; Chung, E.J. Tissue-Engineered Esophagus via Bioreactor Cultivation for Circumferential Esophageal Reconstruction. Tissue Eng. Part A 2019, 25, 1478–1492. [Google Scholar] [CrossRef]
- Zhuravleva, M.; Gilazieva, Z.; Grigoriev, T.E.; Shepelev, A.D.; Kh Tenchurin, T.; Kamyshinsky, R.; Krasheninnikov, S.V.; Orlov, S.; Caralogli, G.; Archipova, S.; et al. In vitro assessment of electrospun polyamide-6 scaffolds for esophageal tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 253–268. [Google Scholar] [CrossRef]
- Soliman, S.; Laurent, J.; Kalenjian, L.; Burnette, K.; Hedberg, B.; La Francesca, S. A multilayer scaffold design with spatial arrangement of cells to modulate esophageal tissue growth. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 324–331. [Google Scholar] [CrossRef]
- Pisani, S.; Croce, S.; Chiesa, E.; Dorati, R.; Lenta, E.; Genta, I.; Bruni, G.; Mauramati, S.; Benazzo, A.; Cobianchi, L.; et al. Tissue Engineered Esophageal Patch by Mesenchymal Stromal Cells: Optimization of Electrospun Patch Engineering. Int. J. Mol. Sci. 2020, 21, 1764. [Google Scholar] [CrossRef]
- Purushotham, A.D.; Carachi, R.; Gorham, S.D.; French, D.A.; Shivas, A.A. Use of a collagen coated vicryl tube in reconstruction of the porcine esophagus. Eur. J. Pediatr. Surg. 1991, 1, 80–84. [Google Scholar] [CrossRef]
- Natsume, T.; Ike, O.; Okada, T.; Takimoto, N.; Shimizu, Y.; Ikada, Y. Porous collagen sponge for esophageal replacement. J. Biomed. Mater. Res. 1993, 27, 867–875. [Google Scholar] [CrossRef]
- Takimoto, Y.; Nakamura, T.; Teramachi, M.; Kiyotani, T.; Shimizu, Y. Replacement of long segments of the esophagus with a collagen-silicone composite tube. ASAIO J. 1995, 41, M605–M608. [Google Scholar] [CrossRef]
- Grikscheit, T.; Ochoa, E.R.; Srinivasan, A.; Gaissert, H.; Vacanti, J.P. Tissue-engineered esophagus: Experimental substitution by onlay patch or interposition. J. Thorac. Cardiovasc. Surg. 2003, 126, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chan-Park, M.B.; Sin Chian, K. The growth improvement of porcine esophageal smooth muscle cells on collagen-grafted poly(DL-lactide-co-glycolide) membrane. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 75, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chian, K.S.; Chan-Park, M.B.; Mhaisalkar, P.S.; Ratner, B.D. Protein bonding on biodegradable poly(L-lactide-co-caprolactone) membrane for esophageal tissue engineering. Biomaterials 2006, 27, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Leong, M.F.; Ong, W.F.; Chan-Park, M.B.; Chian, K.S. Esophageal epithelium regeneration on fibronectin grafted poly(L-lactide-co-caprolactone) (PLLC) nanofiber scaffold. Biomaterials 2007, 28, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Nakase, Y.; Nakamura, T.; Kin, S.; Nakashima, S.; Yoshikawa, T.; Kuriu, Y.; Sakakura, C.; Yamagishi, H.; Hamuro, J.; Ikada, Y.; et al. Intrathoracic esophageal replacement by in situ tissue-engineered esophagus. J. Thorac. Cardiovasc. Surg. 2008, 136, 850–859. [Google Scholar] [CrossRef]
- Lv, J.; Chen, L.; Zhu, Y.; Hou, L.; Liu, Y. Promoting epithelium regeneration for esophageal tissue engineering through basement membrane reconstitution. ACS Appl. Mater. Interfaces 2014, 6, 4954–4964. [Google Scholar] [CrossRef]
- Nam, H.; Jeong, H.J.; Jo, Y.; Lee, J.Y.; Ha, D.H.; Kim, J.H.; Chung, J.H.; Cho, Y.S.; Cho, D.W.; Lee, S.J.; et al. Multi-layered Free-form 3D Cell-printed Tubular Construct with Decellularized Inner and Outer Esophageal Tissue-derived Bioinks. Sci. Rep. 2020, 10, 7255. [Google Scholar] [CrossRef]
- Takimoto, Y.; Nakamura, T.; Yamamoto, Y.; Kiyotani, T.; Teramachi, M.; Shimizu, Y. The experimental replacement of a cervical esophageal segment with an artificial prosthesis with the use of collagen matrix and a silicone stent. J. Thorac. Cardiovasc. Surg. 1998, 116, 98–106. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nakamura, T.; Shimizu, Y.; Matsumoto, K.; Takimoto, Y.; Kiyotani, T.; Sekine, T.; Ueda, H.; Liu, Y.; Tamura, N. Intrathoracic esophageal replacement in the dog with the use of an artificial esophagus composed of a collagen sponge with a double-layered silicone tube. J. Thorac. Cardiovasc. Surg. 1999, 118, 276–286. [Google Scholar] [CrossRef]
- Natsume, T.; Ike, O.; Okada, T.; Shimizu, Y.; Ikada, Y.; Tamura, K. Experimental studies of a hybrid artificial esophagus combined with autologous mucosal cells. ASAIO Trans. 1990, 36, M435–M437. [Google Scholar]
- Kajitani, M.; Wadia, Y.; Hinds, M.T.; Teach, J.; Swartz, K.R.; Gregory, K.W. Successful repair of esophageal injury using an elastin based biomaterial patch. ASAIO J. 2001, 47, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Komuro, H.; Nakamura, T.; Kaneko, M.; Nakanishi, Y.; Shimizu, Y. Application of collagen sponge scaffold to muscular defects of the esophagus: An experimental study in piglets. J. Pediatr. Surg. 2002, 37, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, G.; Morhardt, D.; Cristofaro, V.; Algarrahi, K.; Yang, X.; Costa, K.; Alegria, C.G.; Sullivan, M.P.; Mauney, J.R. Evaluation of Bilayer Silk Fibroin Grafts for Tubular Esophagoplasty in a Porcine Defect Model. Tissue Eng. Part A 2021, 27, 103–116. [Google Scholar] [CrossRef]
- Badylak, S.; Meurling, S.; Chen, M.; Spievack, A.; Simmons-Byrd, A. Resorbable bioscaffold for esophageal repair in a dog model. J. Pediatr. Surg. 2000, 35, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Isch, J.A.; Engum, S.A.; Ruble, C.A.; Davis, M.M.; Grosfeld, J.L. Patch esophagoplasty using AlloDerm as a tissue scaffold. J. Pediatr. Surg. 2001, 36, 266–268. [Google Scholar] [CrossRef]
- Marzaro, M.; Vigolo, S.; Oselladore, B.; Conconi, M.T.; Ribatti, D.; Giuliani, S.; Nico, B.; Perrino, G.; Nussdorfer, G.G.; Parnigotto, P.P. In vitro and in vivo proposal of an artificial esophagus. J. Biomed. Mater. Res. A 2006, 77, 795–801. [Google Scholar] [CrossRef]
- Lopes, M.F.; Cabrita, A.; Ilharco, J.; Pessa, P.; Paiva-Carvalho, J.; Pires, A.; Patricio, J. Esophageal replacement in rat using porcine intestinal submucosa as a patch or a tube-shaped graft. Dis. Esophagus 2006, 19, 254–259. [Google Scholar] [CrossRef]
- Bhrany, A.D.; Beckstead, B.L.; Lang, T.C.; Farwell, D.G.; Giachelli, C.M.; Ratner, B.D. Development of an esophagus acellular matrix tissue scaffold. Tissue Eng. 2006, 12, 319–330. [Google Scholar] [CrossRef]
- Urita, Y.; Komuro, H.; Chen, G.; Shinya, M.; Kaneko, S.; Kaneko, M.; Ushida, T. Regeneration of the esophagus using gastric acellular matrix: An experimental study in a rat model. Pediatr. Surg. Int. 2007, 23, 21–26. [Google Scholar] [CrossRef]
- Bhrany, A.D.; Lien, C.J.; Beckstead, B.L.; Futran, N.D.; Muni, N.H.; Giachelli, C.M.; Ratner, B.D. Crosslinking of an oesophagus acellular matrix tissue scaffold. J. Tissue Eng. Regen. Med. 2008, 2, 365–372. [Google Scholar] [CrossRef]
- Nieponice, A.; McGrath, K.; Qureshi, I.; Beckman, E.J.; Luketich, J.D.; Gilbert, T.W.; Badylak, S.F. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest. Endosc. 2009, 69, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.Q.; Tan, B.; Tan, M.Y.; Luo, J.C.; Deng, L.; Chen, X.H.; Li, X.Q.; Zuo, X.; Zhi, W.; Yang, P.; et al. Grafts of porcine small intestinal submucosa with cultured autologous oral mucosal epithelial cells for esophageal repair in a canine model. Exp. Biol. Med. (Maywood) 2009, 234, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Doede, T.; Bondartschuk, M.; Joerck, C.; Schulze, E.; Goernig, M. Unsuccessful alloplastic esophageal replacement with porcine small intestinal submucosa. Artif. Organs 2009, 33, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Gaujoux, S.; Le Balleur, Y.; Bruneval, P.; Larghero, J.; Lecourt, S.; Domet, T.; Lambert, B.; Zohar, S.; Prat, F.; Cattan, P. Esophageal replacement by allogenic aorta in a porcine model. Surgery 2010, 148, 39–47. [Google Scholar] [CrossRef]
- Badylak, S.F.; Hoppo, T.; Nieponice, A.; Gilbert, T.W.; Davison, J.M.; Jobe, B.A. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: A regenerative medicine approach with a biologic scaffold. Tissue Eng. Part A 2011, 17, 1643–1650. [Google Scholar] [CrossRef]
- Clough, A.; Ball, J.; Smith, G.S.; Leibman, S. Porcine small intestine submucosa matrix (Surgisis) for esophageal perforation. Ann. Thorac. Surg. 2011, 91, e15–e16. [Google Scholar] [CrossRef]
- Hoppo, T.; Badylak, S.F.; Jobe, B.A. A novel esophageal-preserving approach to treat high-grade dysplasia and superficial adenocarcinoma in the presence of chronic gastroesophageal reflux disease. World J. Surg. 2012, 36, 2390–2393. [Google Scholar] [CrossRef]
- Tan, B.; Wei, R.Q.; Tan, M.Y.; Luo, J.C.; Deng, L.; Chen, X.H.; Hou, J.L.; Li, X.Q.; Yang, Z.M.; Xie, H.Q. Tissue engineered esophagus by mesenchymal stem cell seeding for esophageal repair in a canine model. J. Surg. Res. 2013, 182, 40–48. [Google Scholar] [CrossRef]
- Keane, T.J.; Londono, R.; Carey, R.M.; Carruthers, C.A.; Reing, J.E.; Dearth, C.L.; D’Amore, A.; Medberry, C.J.; Badylak, S.F. Preparation and characterization of a biologic scaffold from esophageal mucosa. Biomaterials 2013, 34, 6729–6737. [Google Scholar] [CrossRef]
- Totonelli, G.; Maghsoudlou, P.; Georgiades, F.; Garriboli, M.; Koshy, K.; Turmaine, M.; Ashworth, M.; Sebire, N.J.; Pierro, A.; Eaton, S.; et al. Detergent enzymatic treatment for the development of a natural acellular matrix for oesophageal regeneration. Pediatr. Surg. Int. 2013, 29, 87–95. [Google Scholar] [CrossRef]
- Nieponice, A.; Ciotola, F.F.; Nachman, F.; Jobe, B.A.; Hoppo, T.; Londono, R.; Badylak, S.; Badaloni, A.E. Patch esophagoplasty: Esophageal reconstruction using biologic scaffolds. Ann. Thorac. Surg. 2014, 97, 283–288. [Google Scholar] [CrossRef]
- Poghosyan, T.; Sfeir, R.; Michaud, L.; Bruneval, P.; Domet, T.; Vanneaux, V.; Luong-Nguyen, M.; Gaujoux, S.; Gottrand, F.; Larghero, J.; et al. Circumferential esophageal replacement using a tube-shaped tissue-engineered substitute: An experimental study in minipigs. Surgery 2015, 158, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Urbani, L.; Maghsoudlou, P.; Milan, A.; Menikou, M.; Hagen, C.K.; Totonelli, G.; Camilli, C.; Eaton, S.; Burns, A.; Olivo, A.; et al. Long-term cryopreservation of decellularised oesophagi for tissue engineering clinical application. PLoS ONE 2017, 12, e0179341. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, H.; Umeda, S.; Takama, Y.; Terasawa, T.; Nakayama, Y. Patch esophagoplasty using an in-body-tissue-engineered collagenous connective tissue membrane. J. Pediatr. Surg. 2018, 53, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Catry, J.; Luong-Nguyen, M.; Arakelian, L.; Poghosyan, T.; Bruneval, P.; Domet, T.; Michaud, L.; Sfeir, R.; Gottrand, F.; Larghero, J.; et al. Circumferential Esophageal Replacement by a Tissue-engineered Substitute Using Mesenchymal Stem Cells: An Experimental Study in Mini Pigs. Cell Transpl. 2017, 26, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Luc, G.; Charles, G.; Gronnier, C.; Cabau, M.; Kalisky, C.; Meulle, M.; Bareille, R.; Roques, S.; Couraud, L.; Rannou, J.; et al. Decellularized and matured esophageal scaffold for circumferential esophagus replacement: Proof of concept in a pig model. Biomaterials 2018, 175, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Urbani, L.; Camilli, C.; Phylactopoulos, D.E.; Crowley, C.; Natarajan, D.; Scottoni, F.; Maghsoudlou, P.; McCann, C.J.; Pellegata, A.F.; Urciuolo, A.; et al. Multi-stage bioengineering of a layered oesophagus with in vitro expanded muscle and epithelial adult progenitors. Nat. Commun. 2018, 9, 4286. [Google Scholar] [CrossRef]
- Marzaro, M.; Algeri, M.; Tomao, L.; Tedesco, S.; Caldaro, T.; Balassone, V.; Contini, A.C.; Guerra, L.; Federici D’Abriola, G.; Francalanci, P.; et al. Successful muscle regeneration by a homologous microperforated scaffold seeded with autologous mesenchymal stromal cells in a porcine esophageal substitution model. Ther. Adv. Gastroenterol. 2020, 13, 1756284820923220. [Google Scholar] [CrossRef]
- Chaitin, H.; Lu, M.L.; Wallace, M.B.; Kang, Y. Development of a Decellularized Porcine Esophageal Matrix for Potential Applications in Cancer Modeling. Cells 2021, 10, 1055. [Google Scholar] [CrossRef]
- Xu, R.; Fang, X.; Wu, S.; Wang, Y.; Zhong, Y.; Hou, R.; Zhang, L.; Shao, L.; Pang, Q.; Zhang, J.; et al. Development and Prospect of Esophageal Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 853193. [Google Scholar] [CrossRef]
- Model, L.; Wiesel, O. A narrative review of esophageal tissue engineering and replacement: Where are we? Ann. Transl. Med. 2021, 9, 910. [Google Scholar] [CrossRef]
- Chian, K.S.; Leong, M.F.; Kono, K. Regenerative medicine for oesophageal reconstruction after cancer treatment. Lancet Oncol. 2015, 16, e84–e92. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudlou, P.; Eaton, S.; De Coppi, P. Tissue engineering of the esophagus. Semin. Pediatr. Surg. 2014, 23, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Levin, D. Bench to Bedside: Approaches for Engineered Intestine, Esophagus, and Colon. Gastroenterol. Clin. N. Am. 2019, 48, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Kanetaka, K.; Eguchi, S. Regenerative medicine for the upper gastrointestinal tract. Regen. Ther. 2020, 15, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.C.; Shibuya, S.; Durkin, N.; De Coppi, P. Regenerative medicine for childhood gastrointestinal diseases. Best Pract. Res. Clin. Gastroenterol. 2022, 56-57, 101769. [Google Scholar] [CrossRef] [PubMed]
- Stange, D.E.; Koo, B.K.; Huch, M.; Sibbel, G.; Basak, O.; Lyubimova, A.; Kujala, P.; Bartfeld, S.; Koster, J.; Geahlen, J.H.; et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 2013, 155, 357–368. [Google Scholar] [CrossRef]
- Tanaka, S.; Kanetaka, K.; Fujii, M.; Ito, S.; Sakai, Y.; Kobayashi, S.; Yamanouchi, K.; Fujita, F.; Kuroki, T.; Eguchi, S. Cell sheet technology for the regeneration of gastrointestinal tissue using a novel gastric perforation rat model. Surg. Today 2017, 47, 114–121. [Google Scholar] [CrossRef]
- Sala, F.G.; Kunisaki, S.M.; Ochoa, E.R.; Vacanti, J.; Grikscheit, T.C. Tissue-engineered small intestine and stomach form from autologous tissue in a preclinical large animal model. J. Surg. Res. 2009, 156, 205–212. [Google Scholar] [CrossRef]
- Maemura, T.; Kinoshita, M.; Shin, M.; Miyazaki, H.; Tsujimoto, H.; Ono, S.; Hase, K.; Saitoh, D. Assessment of a tissue-engineered gastric wall patch in a rat model. Artif. Organs 2012, 36, 409–417. [Google Scholar] [CrossRef]
- Hori, Y.; Nakamura, T.; Matsumoto, K.; Kurokawa, Y.; Satomi, S.; Shimizu, Y. Experimental study on in situ tissue engineering of the stomach by an acellular collagen sponge scaffold graft. ASAIO J. 2001, 47, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Nakamura, T.; Kimura, D.; Kaino, K.; Kurokawa, Y.; Satomi, S.; Shimizu, Y. Functional analysis of the tissue-engineered stomach wall. Artif. Organs 2002, 26, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Grikscheit, T.; Srinivasan, A.; Vacanti, J.P. Tissue-engineered stomach: A preliminary report of a versatile in vivo model with therapeutic potential. J. Pediatr. Surg. 2003, 38, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Maemura, T.; Ogawa, K.; Shin, M.; Mochizuki, H.; Vacanti, J.P. Assessment of tissue-engineered stomach derived from isolated epithelium organoid units. Transpl. Proc. 2004, 36, 1595–1599. [Google Scholar] [CrossRef]
- Maemura, T.; Shin, M.; Kinoshita, M.; Majima, T.; Ishihara, M.; Saitoh, D.; Ichikura, T. A tissue-engineered stomach shows presence of proton pump and G-cells in a rat model, resulting in improved anemia following total gastrectomy. Artif. Organs 2008, 32, 234–239. [Google Scholar] [CrossRef]
- Araki, M.; Tao, H.; Sato, T.; Nakajima, N.; Hyon, S.H.; Nagayasu, T.; Nakamura, T. Development of a new tissue-engineered sheet for reconstruction of the stomach. Artif. Organs 2009, 33, 818–826. [Google Scholar] [CrossRef]
- Speer, A.L.; Sala, F.G.; Matthews, J.A.; Grikscheit, T.C. Murine tissue-engineered stomach demonstrates epithelial differentiation. J. Surg. Res. 2011, 171, 6–14. [Google Scholar] [CrossRef]
- Barker, N.; Huch, M.; Kujala, P.; van de Wetering, M.; Snippert, H.J.; van Es, J.H.; Sato, T.; Stange, D.E.; Begthel, H.; van den Born, M.; et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010, 6, 25–36. [Google Scholar] [CrossRef]
- Katano, T.; Ootani, A.; Mizoshita, T.; Tanida, S.; Tsukamoto, H.; Ozeki, K.; Ebi, M.; Mori, Y.; Kataoka, H.; Kamiya, T.; et al. Establishment of a long-term three-dimensional primary culture of mouse glandular stomach epithelial cells within the stem cell niche. Biochem. Biophys. Res. Commun. 2013, 432, 558–563. [Google Scholar] [CrossRef]
- McCracken, K.W.; Cata, E.M.; Crawford, C.M.; Sinagoga, K.L.; Schumacher, M.; Rockich, B.E.; Tsai, Y.H.; Mayhew, C.N.; Spence, J.R.; Zavros, Y.; et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014, 516, 400–404. [Google Scholar] [CrossRef]
- Bartfeld, S.; Bayram, T.; van de Wetering, M.; Huch, M.; Begthel, H.; Kujala, P.; Vries, R.; Peters, P.J.; Clevers, H. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 2015, 148, 126–136.e126. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Aihara, E.; Feng, R.; Engevik, A.; Shroyer, N.F.; Ottemann, K.M.; Worrell, R.T.; Montrose, M.H.; Shivdasani, R.A.; Zavros, Y. The use of murine-derived fundic organoids in studies of gastric physiology. J. Physiol. 2015, 593, 1809–1827. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.K.; Ninomiya, N.; Sekine, M.; Komazaki, S.; Wang, P.C.; Asashima, M.; Kurisaki, A. Generation of stomach tissue from mouse embryonic stem cells. Nat. Cell Biol. 2015, 17, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Schlaermann, P.; Toelle, B.; Berger, H.; Schmidt, S.C.; Glanemann, M.; Ordemann, J.; Bartfeld, S.; Mollenkopf, H.J.; Meyer, T.F. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut 2016, 65, 202–213. [Google Scholar] [CrossRef]
- McCracken, K.W.; Aihara, E.; Martin, B.; Crawford, C.M.; Broda, T.; Treguier, J.; Zhang, X.; Shannon, J.M.; Montrose, M.H.; Wells, J.M. Wnt/beta-catenin promotes gastric fundus specification in mice and humans. Nature 2017, 541, 182–187. [Google Scholar] [CrossRef]
- Ueno, T.; de la Fuente, S.G.; Abdel-Wahab, O.I.; Takahashi, T.; Gottfried, M.; Harris, M.B.; Tatewaki, M.; Uemura, K.; Lawson, D.C.; Mantyh, C.R.; et al. Functional evaluation of the grafted wall with porcine-derived small intestinal submucosa (SIS) to a stomach defect in rats. Surgery 2007, 142, 376–383. [Google Scholar] [CrossRef]
- Nakatsu, H.; Ueno, T.; Oga, A.; Nakao, M.; Nishimura, T.; Kobayashi, S.; Oka, M. Influence of mesenchymal stem cells on stomach tissue engineering using small intestinal submucosa. J. Tissue Eng. Regen. Med. 2015, 9, 296–304. [Google Scholar] [CrossRef]
- Maemura, T.; Shin, M.; Kinoshita, M. Tissue engineering of the stomach. J. Surg. Res. 2013, 183, 285–295. [Google Scholar] [CrossRef]
- Raghavan, S.; Lam, M.T.; Foster, L.L.; Gilmont, R.R.; Somara, S.; Takayama, S.; Bitar, K.N. Bioengineered three-dimensional physiological model of colonic longitudinal smooth muscle in vitro. Tissue Eng. Part C Methods 2010, 16, 999–1009. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Ridaura, V.; Belkaid, Y. Gut microbiota: The link to your second brain. Cell 2015, 161, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Schlieve, C.R.; Fowler, K.L.; Thornton, M.; Huang, S.; Hajjali, I.; Hou, X.; Grubbs, B.; Spence, J.R.; Grikscheit, T.C. Neural Crest Cell Implantation Restores Enteric Nervous System Function and Alters the Gastrointestinal Transcriptome in Human Tissue-Engineered Small Intestine. Stem Cell Rep. 2017, 9, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Workman, M.J.; Mahe, M.M.; Trisno, S.; Poling, H.M.; Watson, C.L.; Sundaram, N.; Chang, C.F.; Schiesser, J.; Aubert, P.; Stanley, E.G.; et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat. Med. 2017, 23, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Somara, S.; Gilmont, R.R.; Dennis, R.G.; Bitar, K.N. Bioengineered internal anal sphincter derived from isolated human internal anal sphincter smooth muscle cells. Gastroenterology 2009, 137, 53–61. [Google Scholar] [CrossRef]
- Yui, S.; Nakamura, T.; Sato, T.; Nemoto, Y.; Mizutani, T.; Zheng, X.; Ichinose, S.; Nagaishi, T.; Okamoto, R.; Tsuchiya, K.; et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat. Med. 2012, 18, 618–623. [Google Scholar] [CrossRef]
- Zani, A.; Cananzi, M.; Fascetti-Leon, F.; Lauriti, G.; Smith, V.V.; Bollini, S.; Ghionzoli, M.; D’Arrigo, A.; Pozzobon, M.; Piccoli, M.; et al. Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX-2 dependent mechanism. Gut 2014, 63, 300–309. [Google Scholar] [CrossRef]
- Sugimoto, S.; Kobayashi, E.; Fujii, M.; Ohta, Y.; Arai, K.; Matano, M.; Ishikawa, K.; Miyamoto, K.; Toshimitsu, K.; Takahashi, S.; et al. An organoid-based organ-repurposing approach to treat short bowel syndrome. Nature 2021, 592, 99–104. [Google Scholar] [CrossRef]
- Finkbeiner, S.R.; Freeman, J.J.; Wieck, M.M.; El-Nachef, W.; Altheim, C.H.; Tsai, Y.H.; Huang, S.; Dyal, R.; White, E.S.; Grikscheit, T.C.; et al. Generation of tissue-engineered small intestine using embryonic stem cell-derived human intestinal organoids. Biol. Open 2015, 4, 1462–1472. [Google Scholar] [CrossRef]
- Costello, C.M.; Hongpeng, J.; Shaffiey, S.; Yu, J.; Jain, N.K.; Hackam, D.; March, J.C. Synthetic small intestinal scaffolds for improved studies of intestinal differentiation. Biotechnol. Bioeng. 2014, 111, 1222–1232. [Google Scholar] [CrossRef]
- Choi, R.S.; Vacanti, J.P. Preliminary studies of tissue-engineered intestine using isolated epithelial organoid units on tubular synthetic biodegradable scaffolds. Transpl. Proc. 1997, 29, 848–851. [Google Scholar] [CrossRef]
- Grikscheit, T.C.; Siddique, A.; Ochoa, E.R.; Srinivasan, A.; Alsberg, E.; Hodin, R.A.; Vacanti, J.P. Tissue-engineered small intestine improves recovery after massive small bowel resection. Ann. Surg. 2004, 240, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rager, T.; Johnson, J.; Enmark, J.; Besner, G.E. Enriched Intestinal Stem Cell Seeding Improves the Architecture of Tissue-Engineered Intestine. Tissue Eng. Part C Methods 2015, 21, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordonez-Moran, P.; Clevers, H.; Lutolf, M.P. Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nelson, T.; Chakroff, J.; Cromeens, B.; Johnson, J.; Lannutti, J.; Besner, G.E. Comparison of polyglycolic acid, polycaprolactone, and collagen as scaffolds for the production of tissue engineered intestine. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 750–760. [Google Scholar] [CrossRef]
- Levin, D.E.; Barthel, E.R.; Speer, A.L.; Sala, F.G.; Hou, X.; Torashima, Y.; Grikscheit, T.C. Human tissue-engineered small intestine forms from postnatal progenitor cells. J. Pediatr. Surg. 2013, 48, 129–137. [Google Scholar] [CrossRef]
- Wieck, M.M.; El-Nachef, W.N.; Hou, X.; Spurrier, R.G.; Holoyda, K.A.; Schall, K.A.; Mojica, S.G.; Collins, M.K.; Trecartin, A.; Cheng, Z.; et al. Human and Murine Tissue-Engineered Colon Exhibit Diverse Neuronal Subtypes and Can Be Populated by Enteric Nervous System Progenitor Cells When Donor Colon Is Aganglionic. Tissue Eng. Part A 2016, 22, 53–64. [Google Scholar] [CrossRef]
- Ladd, M.R.; Costello, C.M.; Gosztyla, C.; Werts, A.D.; Johnson, B.; Fulton, W.B.; Martin, L.Y.; Redfield, E.J.; Crawford, B.; Panaparambil, R.; et al. Development of Intestinal Scaffolds that Mimic Native Mammalian Intestinal Tissue. Tissue Eng. Part A 2019, 25, 1225–1241. [Google Scholar] [CrossRef]
- Watson, C.L.; Mahe, M.M.; Munera, J.; Howell, J.C.; Sundaram, N.; Poling, H.M.; Schweitzer, J.I.; Vallance, J.E.; Mayhew, C.N.; Sun, Y.; et al. An in vivo model of human small intestine using pluripotent stem cells. Nat. Med. 2014, 20, 1310–1314. [Google Scholar] [CrossRef]
- Fukuda, M.; Mizutani, T.; Mochizuki, W.; Matsumoto, T.; Nozaki, K.; Sakamaki, Y.; Ichinose, S.; Okada, Y.; Tanaka, T.; Watanabe, M.; et al. Small intestinal stem cell identity is maintained with functional Paneth cells in heterotopically grafted epithelium onto the colon. Genes Dev. 2014, 28, 1752–1757. [Google Scholar] [CrossRef]
- Cromeens, B.P.; Liu, Y.; Stathopoulos, J.; Wang, Y.; Johnson, J.; Besner, G.E. Production of tissue-engineered intestine from expanded enteroids. J. Surg. Res. 2016, 204, 164–175. [Google Scholar] [CrossRef]
- Zakhem, E.; Tamburrini, R.; Orlando, G.; Koch, K.L.; Bitar, K.N. Transplantation of a Human Tissue-Engineered Bowel in an Athymic Rat Model. Tissue Eng. Part C Methods 2017, 23, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, S.; Ohta, Y.; Fujii, M.; Matano, M.; Shimokawa, M.; Nanki, K.; Date, S.; Nishikori, S.; Nakazato, Y.; Nakamura, T.; et al. Reconstruction of the Human Colon Epithelium In Vivo. Cell Stem Cell 2018, 22, 171–176.e175. [Google Scholar] [CrossRef]
- Capeling, M.M.; Czerwinski, M.; Huang, S.; Tsai, Y.H.; Wu, A.; Nagy, M.S.; Juliar, B.; Sundaram, N.; Song, Y.; Han, W.M.; et al. Nonadhesive Alginate Hydrogels Support Growth of Pluripotent Stem Cell-Derived Intestinal Organoids. Stem Cell Rep. 2019, 12, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Totonelli, G.; Maghsoudlou, P.; Garriboli, M.; Riegler, J.; Orlando, G.; Burns, A.J.; Sebire, N.J.; Smith, V.V.; Fishman, J.M.; Ghionzoli, M.; et al. A rat decellularized small bowel scaffold that preserves villus-crypt architecture for intestinal regeneration. Biomaterials 2012, 33, 3401–3410. [Google Scholar] [CrossRef] [PubMed]
- Kitano, K.; Schwartz, D.M.; Zhou, H.; Gilpin, S.E.; Wojtkiewicz, G.R.; Ren, X.; Sommer, C.A.; Capilla, A.V.; Mathisen, D.J.; Goldstein, A.M.; et al. Bioengineering of functional human induced pluripotent stem cell-derived intestinal grafts. Nat. Commun. 2017, 8, 765. [Google Scholar] [CrossRef]
- Palikuqi, B.; Nguyen, D.T.; Li, G.; Schreiner, R.; Pellegata, A.F.; Liu, Y.; Redmond, D.; Geng, F.; Lin, Y.; Gomez-Salinero, J.M.; et al. Adaptable haemodynamic endothelial cells for organogenesis and tumorigenesis. Nature 2020, 585, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Meran, L.; Massie, I.; Campinoti, S.; Weston, A.E.; Gaifulina, R.; Tullie, L.; Faull, P.; Orford, M.; Kucharska, A.; Baulies, A.; et al. Engineering transplantable jejunal mucosal grafts using patient-derived organoids from children with intestinal failure. Nat. Med. 2020, 26, 1593–1601. [Google Scholar] [CrossRef]
- Tullie, L.; Jones, B.C.; De Coppi, P.; Li, V.S.W. Building gut from scratch-progress and update of intestinal tissue engineering. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 417–431. [Google Scholar] [CrossRef]
- Clevers, H.; Conder, R.K.; Li, V.S.W.; Lutolf, M.P.; Vallier, L.; Chan, S.; Grikscheit, T.C.; Jensen, K.B.; De Coppi, P. Tissue-Engineering the Intestine: The Trials before the Trials. Cell Stem Cell 2019, 24, 855–859. [Google Scholar] [CrossRef]
- Costa, J.; Ahluwalia, A. Advances and Current Challenges in Intestinal in vitro Model Engineering: A Digest. Front. Bioeng. Biotechnol. 2019, 7, 144. [Google Scholar] [CrossRef]
- Bitar, K.N.; Zakhem, E. Bioengineering the gut: Future prospects of regenerative medicine. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Cattan, V.; Bernard, G.; Rousseau, A.; Bouhout, S.; Chabaud, S.; Auger, F.A.; Bolduc, S. Mechanical stimuli-induced urothelial differentiation in a human tissue-engineered tubular genitourinary graft. Eur. Urol. 2011, 60, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, T.; Roy, V.; Bourget, J.M.; Tsutsumi, T.; Picard-Deland, M.; Morin, J.F.; Gauvin, R.; Ismail, A.A.; Auger, F.A.; Gros-Louis, F. Cell Seeding on UV-C-Treated 3D Polymeric Templates Allows for Cost-Effective Production of Small-Caliber Tissue-Engineered Blood Vessels. Biotechnol. J. 2019, 14, e1800306. [Google Scholar] [CrossRef]
- Jakubowska, W.; Chabaud, S.; Saba, I.; Galbraith, T.; Berthod, F.; Bolduc, S. Prevascularized Tissue-Engineered Human Vaginal Mucosa: In Vitro Optimization and In Vivo Validation. Tissue Eng. Part A 2020, 26, 811–822. [Google Scholar] [CrossRef]
- Tremblay, P.L.; Hudon, V.; Berthod, F.; Germain, L.; Auger, F.A. Inosculation of tissue-engineered capillaries with the host’s vasculature in a reconstructed skin transplanted on mice. Am. J. Transpl. 2005, 5, 1002–1010. [Google Scholar] [CrossRef]
- Imbeault, A.; Bernard, G.; Rousseau, A.; Morissette, A.; Chabaud, S.; Bouhout, S.; Bolduc, S. An endothelialized urothelial cell-seeded tubular graft for urethral replacement. Can. Urol. Assoc. J. 2013, 7, E4–E9. [Google Scholar] [CrossRef][Green Version]
- Goyer, B.; Larouche, D.; Kim, D.H.; Veillette, N.; Pruneau, V.; Bernier, V.; Auger, F.A.; Germain, L. Immune tolerance of tissue-engineered skin produced with allogeneic or xenogeneic fibroblasts and syngeneic keratinocytes grafted on mice. Acta Biomater. 2019, 90, 192–204. [Google Scholar] [CrossRef]
- Bohnenpoll, T.; Kispert, A. Ureter growth and differentiation. Semin. Cell Dev. Biol. 2014, 36, 21–30. [Google Scholar] [CrossRef]
- Maccagnano, C.; Pellucchi, F.; Rocchini, L.; Ghezzi, M.; Scattoni, V.; Montorsi, F.; Rigatti, P.; Colombo, R. Ureteral endometriosis: Proposal for a diagnostic and therapeutic algorithm with a review of the literature. Urol. Int. 2013, 91, 1–9. [Google Scholar] [CrossRef]
- Cankurtaran, M.; Oyan, B.; Kilickap, S.; Yavuz, B.B.; Batman, F. Idiopathic fibrosclerosis of bilateral orbits, bilateral ureters, thyroid: A case report and review of the literature. Int. Urol. Nephrol. 2004, 36, 495–498. [Google Scholar] [CrossRef]
- Farci, F.; Manassero, F.; Baldesi, R.; Bartolucci, A.; Boldrini, L.; Selli, C.; Faviana, P. Primary small cell carcinoma of the ureter: Case report and review of the literature. Med. Baltim. 2018, 97, e11113. [Google Scholar] [CrossRef] [PubMed]
- Winalski, C.S.; Lipman, J.C.; Tumeh, S.S. Ureteral neoplasms. Radiographics 1990, 10, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Bertolaccini, L.; Giacomelli, G.; Bozzo, R.E.; Gastaldi, L.; Moroni, M. Inguino-scrotal hernia of a double district ureter: Case report and literature review. Hernia 2005, 9, 291–293. [Google Scholar] [CrossRef]
- Weledji, E.P.; Eyongeta, D.; Ngounou, E. The anatomy of urination: What every physician should know. Clin. Anat. 2019, 32, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.E.; McCloskey, K.D. Lamina propria: The functional center of the bladder? Neurourol. Urodyn. 2014, 33, 9–16. [Google Scholar] [CrossRef]
- Pathak, P.; Ring, J.D.; Delfino, K.R.; Dynda, D.I.; Mathews, R.I. Complete primary repair of bladder exstrophy: A systematic review. J. Pediatr. Urol. 2020, 16, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, D. The epidemiology and pathophysiology of neurogenic bladder. Am. J. Manag. Care 2013, 19, s191–s196. [Google Scholar]
- Daniels, A.M.; Schulte, A.R.; Herndon, C.M. Interstitial Cystitis: An Update on the Disease Process and Treatment. J. Pain Palliat. Care Pharm. 2018, 32, 49–58. [Google Scholar] [CrossRef]
- Li, R.; Leslie, S.W. Cystitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bono, M.J.; Leslie, S.W.; Reygaert, W.C. Urinary Tract Infection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M.D. Bladder Cancer: A Review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Dietrich, B.; Srinivas, S. Urothelial carcinoma: The evolving landscape of immunotherapy for patients with advanced disease. Res. Rep. Urol. 2018, 10, 7–16. [Google Scholar] [CrossRef]
- Stoddard, N.; Leslie, S.W. Histology, Male Urethra. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Pederzoli, F.; Joice, G.; Salonia, A.; Bivalacqua, T.J.; Sopko, N.A. Regenerative and engineered options for urethroplasty. Nat. Rev. Urol. 2019, 16, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Mistry, M.A.; Klarskov, N.; DeLancey, J.O.; Lose, G. A structured review on the female urethral anatomy and innervation with an emphasis on the role of the urethral longitudinal smooth muscle. Int. Urogynecol. J. 2020, 31, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.P.; Mittal, A.G.; Austin, P.F.; Hilliard, M.E. The hypospadias-specific health-related quality of life conceptual framework: A scoping review of the literature. Qual. Life Res. 2021, 30, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Frimberger, D. Diagnosis and management of epispadias. Semin. Pediatr. Surg. 2011, 20, 85–90. [Google Scholar] [CrossRef]
- Mangera, A.; Osman, N.; Chapple, C. Evaluation and management of anterior urethral stricture disease. F1000Research 2016, 5, 153. [Google Scholar] [CrossRef][Green Version]
- Orabi, H.; Bouhout, S.; Morissette, A.; Rousseau, A.; Chabaud, S.; Bolduc, S. Tissue Engineering of Urinary Bladder and Urethra: Advances from Bench to Patients. Sci. World J. 2013, 2013, 154564. [Google Scholar] [CrossRef]
- Malcor, J.D.; Mallein-Gerin, F. Biomaterial functionalization with triple-helical peptides for tissue engineering. Acta Biomater. 2022, 148, 1–21. [Google Scholar] [CrossRef]
- Shaterian, N.; Abdi, F.; Ghavidel, N.; Alidost, F. Role of cesarean section in the development of neonatal gut microbiota: A systematic review. Open Med. Wars 2021, 16, 624–639. [Google Scholar] [CrossRef]
- Ruchelli, E.D.H.; Vagina, D.S. Color Atlas of Fetal and Neonatal Histology; Springer: New York, NY, USA, 2011; pp. 195–198. [Google Scholar] [CrossRef]
- Nilsson, K.; Risberg, B.; Heimer, G. The vaginal epithelium in the postmenopause--cytology, histology and pH as methods of assessment. Maturitas 1995, 21, 51–56. [Google Scholar] [CrossRef]
- Morris, M.; Nicoll, A.; Simms, I.; Wilson, J.; Catchpole, M. Bacterial vaginosis: A public health review. BJOG 2001, 108, 439–450. [Google Scholar] [CrossRef]
- Boskey, E.R.; Telsch, K.M.; Whaley, K.J.; Moench, T.R.; Cone, R.A. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect. Immun. 1999, 67, 5170–5175. [Google Scholar] [CrossRef] [PubMed]
- Vliet, R.; Roelofs, L.A.; Rassouli-Kirchmeier, R.; de Gier, R.P.; Claahsen-van der Grinten, H.L.; Verhaak, C.; Hosman, A.J.; Beerendonk, C.C.; van Lindert, E.J.; Willemsen, M.A.; et al. Clinical outcome of cloacal exstrophy, current status, and a change in surgical management. Eur. J. Pediatr. Surg. 2015, 25, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, D.; Nguyen-Khoa, T.; Gonzalez Briceno, L.; Polak, M. Newborn screening for congenital adrenal hyperplasia in France. Med. Sci. Paris 2021, 37, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Oppelt, P.; Renner, S.P.; Kellermann, A.; Brucker, S.; Hauser, G.A.; Ludwig, K.S.; Strissel, P.L.; Strick, R.; Wallwiener, D.; Beckmann, M.W. Clinical aspects of Mayer-Rokitansky-Kuester-Hauser syndrome: Recommendations for clinical diagnosis and staging. Hum. Reprod. 2006, 21, 792–797. [Google Scholar] [CrossRef]
- Breech, L.L.; Laufer, M.R. Mullerian anomalies. Obs. Gynecol. Clin. N. Am. 2009, 36, 47–68. [Google Scholar] [CrossRef]
- Ketheeswaran, A.; Morrisey, J.; Abbott, J.; Bennett, M.; Dudley, J.; Deans, R. Vaginal Dilation in Mayer-Rokitansky-Kuster-Hauser (MRKH) Syndrome. J. Minim. Invasive Gynecol. 2015, 22, S103–S104. [Google Scholar] [CrossRef]
- Morris, L.; Do, V.; Chard, J.; Brand, A.H. Radiation-induced vaginal stenosis: Current perspectives. Int. J. Womens Health 2017, 9, 273–279. [Google Scholar] [CrossRef]
- Amankwah, Y.A.; Haefner, H.K.; Brincat, C.A. Management of vulvovaginal strictures/shortened vagina. Clin. Obs. Gynecol. 2010, 53, 125–133. [Google Scholar] [CrossRef]
- O’Boyle, A.L.; O’Boyle, J.D.; Calhoun, B.; Davis, G.D. Pelvic organ support in pregnancy and postpartum. Int. Urogynecol. J. Pelvic. Floor Dysfunct. 2005, 16, 69–72; discussion 72. [Google Scholar] [CrossRef]
- Nygaard, I.E.; Heit, M. Stress urinary incontinence. Obs. Gynecol. 2004, 104, 607–620. [Google Scholar] [CrossRef]
- Bean, E.J.; Mazur, T.; Robinson, A.D. Mayer-Rokitansky-Kuster-Hauser syndrome: Sexuality, psychological effects, and quality of life. J. Pediatr. Adolesc. Gynecol. 2009, 22, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Heller-Boersma, J.G.; Schmidt, U.H.; Edmonds, D.K. Psychological distress in women with uterovaginal agenesis (Mayer-Rokitansky-Kuster-Hauser Syndrome, MRKH). Psychosomatics 2009, 50, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Herlin, M.K.; Petersen, M.B.; Brannstrom, M. Mayer-Rokitansky-Kuster-Hauser (MRKH) syndrome: A comprehensive update. Orphanet. J. Rare Dis. 2020, 15, 214. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, D.E.; van der Helm, T.W. The treatment of vaginal atresia. Surg. Gynecol. Obs. 1991, 172, 407–414. [Google Scholar]
- Foster, C.; Daigle, R.; Rowe, C.K. Tissue Engineering Opportunities for Vaginal Replacement in a Pediatric Population. Tissue Eng. Part B Rev. 2022, 28, 476–487. [Google Scholar] [CrossRef]
- Brownell, D.; Chabaud, S.; Bolduc, S. Tissue Engineering Solutions For Vaginal Reconstruction. Tissue Eng. Part A 2022, 28, 48. [Google Scholar]
- Wefer, J.; Sekido, N.; Sievert, K.D.; Schlote, N.; Nunes, L.; Dahiya, R.; Jonas, U.; Tanagho, E.A. Homologous acellular matrix graft for vaginal repair in rats: A pilot study for a new reconstructive approach. World J. Urol. 2002, 20, 260–263. [Google Scholar] [CrossRef]
- Walles, T.; Puschmann, C.; Haverich, A.; Mertsching, H. Acellular scaffold implantation--no alternative to tissue engineering. Int. J. Artif. Organs 2003, 26, 225–234. [Google Scholar] [CrossRef]
- Raya-Rivera, A.M.; Esquiliano, D.; Fierro-Pastrana, R.; Lopez-Bayghen, E.; Valencia, P.; Ordorica-Flores, R.; Soker, S.; Yoo, J.J.; Atala, A. Tissue-engineered autologous vaginal organs in patients: A pilot cohort study. Lancet 2014, 384, 329–336. [Google Scholar] [CrossRef]
- De Filippo, R.E.; Yoo, J.J.; Atala, A. Engineering of vaginal tissue in vivo. Tissue Eng. 2003, 9, 301–306. [Google Scholar] [CrossRef]
- De Filippo, R.E.; Bishop, C.E.; Filho, L.F.; Yoo, J.J.; Atala, A. Tissue engineering a complete vaginal replacement from a small biopsy of autologous tissue. Transplantation 2008, 86, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Orabi, H.; Saba, I.; Rousseau, A.; Bolduc, S. Novel three-dimensional autologous tissue-engineered vaginal tissues using the self-assembly technique. Transl. Res. 2017, 180, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Bastu, E.; Akhan, S.E.; Mutlu, M.F.; Nehir, A.; Yumru, H.; Hocaoglu, E.; Gungor-Ugurlucan, F. Treatment of vaginal agenesis using a modified McIndoe technique: Long-term follow-up of 23 patients and a literature review. Can. J. Plast. Surg. 2012, 20, 241–244. [Google Scholar] [CrossRef]
- Chabaud, S.; Rousseau, A.; Marcoux, T.L.; Bolduc, S. Inexpensive production of near-native engineered stromas. J. Tissue Eng. Regen. Med. 2017, 11, 1377–1389. [Google Scholar] [CrossRef]

| Date | 1st Author | Biomaterial | Cells | Animal | Ref. |
|---|---|---|---|---|---|
| Scaffold-free | |||||
| 2005 | Badylak | n/a | muscle tissue | dog | [102] |
| 2006 | Ohki | n/a | oral epithelial cells | dog | [103] |
| 2007 | Sakurai | n/a | buccal K | pig | [104] |
| 2011 | Honda | n/a | ASC | dog | [105] |
| 2012 | Ohki | n/a | oral epithelial cells | human | [106] |
| 2016 | Perrod | n/a | ASC cell sheet | pig | [107] |
| 2019 | Takeoka | n/a | organoids | rat | [108] |
| Synthetic biomaterials | |||||
| 1964 | Mark | Teflon | n/a | dog | [109] |
| 2004 | Lynen Jansen | PVDF | n/a | rabbit | [110] |
| 2004 | Lynen Jansen | Vycril | n/a | rabbit | [110] |
| 2005 | Beckstead | PLLA | Ks | in vitro | [111] |
| 2005 | Beckstead | PLGA | Ks | in vitro | [111] |
| 2005 | Beckstead | PCL/PLLA | Ks | in vitro | [111] |
| 2010 | Liang | nitinol | n/a | pig | [112] |
| 2013 | Gong | PU | SMCs | rabbit | [113] |
| 2013 | Aikawa | BAP | n/a | pig | [114] |
| 2015 | Diemer | PCL | SMCs + Epithelial cells | rabbit | [115] |
| 2016 | Tan | PLA/PCL | Fbs | in vitro | [116] |
| 2016 | Hou | PU | n/a | in vitro | [117] |
| 2016 | Park | PCL | MSCs | rabbit | [118] |
| 2016 | Tan | PLA/PCL | various | in vitro | [116] |
| 2017 | Kuppan | PHBV | ECs + MSCs | in vitro | [119] |
| 2017 | Kuppan | PCL | ECs + MSCs | in vitro | [119] |
| 2017 | Dorati | PLA/PCL | Fbs | in vitro | [120] |
| 2017 | Tan | PLGA | various | in vitro | [121] |
| 2018 | La Francesca | PU | ASCs | pig | [122] |
| 2018 | Wei | PU-derived | MSCs | in vitro | [123] |
| 2018 | Chung | PCL | Fbs | rat | [124] |
| 2019 | Jensen | PU | pig Epithelial cells + MSCs | pig | [125] |
| 2019 | Kim | PU/PCL | MSCs | rat | [126] |
| 2019 | Zhuravleva | Polyamide 6 | MSCs, ASCs | in vitro | [127] |
| 2019 | Soliman | PU | MSCs + SMCs | in vitro | [128] |
| 2019 | Kim | PU/PCL | MSCs | rat | [126] |
| 2020 | Pisani | PLA/PCL | MSC | in vitro | [129] |
| Date | 1st Author | Biomaterial | Cells | Animal | Ref. |
|---|---|---|---|---|---|
| Hybrid biomaterials | |||||
| 1991 | Purushotham | Vycril/Coll | n/a | pig | [130] |
| 1993 | Natsume | Silicone/Coll | n/a | dog | [131] |
| 1995 | Takimoto | Silicone/Coll | n/a | dog | [132] |
| 2003 | Grikscheit | PGA/collagen | organoids | rat | [133] |
| 2005 | Zhu | PLGA/Coll | SMCs | in vitro | [134] |
| 2006 | Zhu | PLLC/Coll | various | in vitro | [135] |
| 2007 | Zhu | PLLC/Fibronectin | Epithelial cells | in vitro | [136] |
| 2008 | Nakase | Amniotic + PGA | oralKs + Fbs | dog | [137] |
| 2014 | Lv | PCL/silk | n/a | rabbit | [138] |
| 2017 | Kuppan | PHBV/gelatin | ECs + MSCs | in vitro | [119] |
| 2017 | Kuppan | PCL/gelatin | ECs + MSCs | in vitro | [119] |
| 2017 | Dorati | PLA/PCL + Chitosan | Fbs | in vitro | [120] |
| 2020 | Nam | PCL + ECM bioink | SMCs + Epithelial cells | in vitro | [139] |
| Natural biomaterials | |||||
| 1998 | Takimoto | collagen | n/a | dog | [140] |
| 1999 | Yamamoto | collagen | n/a | dog | [141] |
| 1990 | Natsume | collagen | Mucosal cells | dog | [142] |
| 2001 | Kajitani | Elastin | n/a | pig | [143] |
| 2002 | Komuro | collagen | n/a | pig | [144] |
| 2021 | Gundogdu | silk | n/a | pig | [145] |
| Date | 1st Author | Biomaterial | Cells | Animal | Ref. |
|---|---|---|---|---|---|
| Acellular matrices | |||||
| 2000 | Badylak | SIS | n/a | dog | [146] |
| 2000 | Badylak | Bladder submucosa | n/a | dog | [146] |
| 2001 | Isch | Dermis | n/a | dog | [147] |
| 2005 | Badylak | Pig bladder | n/a | dog | [102] |
| 2005 | Beckstead | Dermis | Ks | in vitro | [111] |
| 2006 | Marzaro | Esophagus | SMCs | pig | [148] |
| 2006 | Lopes | SIS | n/a | rat | [149] |
| 2006 | Bhrany | Esophagus | Epithelial cells | rat | [150] |
| 2007 | Urita | Gastric | n/a | rat | [151] |
| 2008 | Bhrany | Esophagus (crosslink) | Epithelial cells | rat | [152] |
| 2009 | Nieponice | Pig bladder | n/a | dog | [153] |
| 2009 | Wei | SIS | Oral epithelial cells | dog | [154] |
| 2009 | Doede | SIS | n/a | pig | [155] |
| 2010 | Gaujoux | Aorta | n/a | pig | [156] |
| 2011 | Badylak | SIS | n/a | human | [157] |
| 2011 | Clough | SIS | n/a | human | [158] |
| 2012 | Hoppo | SIS | n/a | human | [159] |
| 2013 | Tan | SIS | BMSCs | dog | [160] |
| 2013 | Keane | Esophagus | Perivascular SCs | rat | [161] |
| 2013 | Totonelli | Pig esophagus | n/a | in vitro | [162] |
| 2014 | Nieponice | Bladder submucosa | n/a | human | [163] |
| 2015 | Poghosyan | SIS + HAM | Myoblasts + Epithelial cells | pig | [164] |
| 2017 | Urbani | Rabbit Esophagus | n/a | in vitro | [165] |
| 2017 | Okuyama | Biosheet | n/a | dog | [166] |
| 2017 | Catry | SIS | MSCs | pig | [167] |
| 2018 | Luc | Esophagus | ASCs | pig | [168] |
| 2018 | Urbani | Rat Esophagus | Various | mouse | [169] |
| 2020 | Marzaro | Esophagus | MSCs | pig | [170] |
| 2021 | Chaitin | Esophagus | ESCs + Fbs | in vitro | [171] |
| Date | First Author | Biomaterial | Cells | Animal | Ref. |
|---|---|---|---|---|---|
| Scaffold free | |||||
| 2010 | Stange | n/a | organoids | in vitro | [179] |
| 2017 | Tanaka | n/a | Myoblast sheet | rat | [180] |
| Synthetic biomaterials | |||||
| 2009 | Sala | PGA/PLLA | Stomach cells | pig | [181] |
| 2012 | Maemura | PGA/PLLA | Epi organoids | rat | [182] |
| 2012 | Maemura | PGA | organoids | rat | [182] |
| Hybrid biomaterials | |||||
| 2001 | Hori | PGA/coll | n/a | dog | [183] |
| 2002 | Hori | PGA/coll | n/a | dog | [184] |
| 2003 | Griksheit | PGA/coll | Ep iorganoids | rat | [185] |
| 2004 | Maemura | PGA/PLLA/Coll | Epi organoids | rat | [186] |
| 2008 | Maemura | PGA/PLLA/Coll | Epi organoids | rat | [187] |
| 2009 | Araki | PLC/PGA/Coll | n/a | dog | [188] |
| 2009 | Araki | PDLCL/PGA/Coll | n/a | dog | [188] |
| 2011 | Speer | PGA/PLLA/Coll | organoids | mouse | [189] |
| Natural biomaterials | |||||
| 2010 | Barker | Matrigel | organoids | in vitro | [190] |
| 2013 | Katano | collagen | Epi | in vitro | [191] |
| 2014 | McCracken | Matrigel | Human iPSC | in vitro | [192] |
| 2015 | Bartfeld | Matrigel | organoids | in vitro | [193] |
| 2015 | Schumacher | Matrigel | organoids | in vitro | [194] |
| 2015 | Noguchi | Matrigel | mouse ESC | in vitro | [195] |
| 2016 | Schlaermann | Matrigel | organoids | in vitro | [196] |
| 2017 | McCracken | Matrigel | iPSC | in vitro | [197] |
| Acellular matrices | |||||
| 2007 | Ueno | SIS | n/a | rats | [198] |
| 2015 | Nakatsu | SIS | MSC | rat | [199] |
| Date | 1st Author | Biomaterial | Cells | Animal | Organ | Ref. |
|---|---|---|---|---|---|---|
| Scaffold-free | ||||||
| 2012 | Yui | n/a | organoids | mouse | Int | [207] |
| 2014 | Zani | n/a | AFSC | rat | Int | [208] |
| 2021 | Sugimoto | n/a | organoids | rat | Int | [209] |
| Synthetic biomaterials | ||||||
| 2015 | Finkbeiner | PGA/PLLA | organoids | mouse | Sint | [210] |
| 2017 | Schlieve | PGA | iPSC+organoids+neurons | mouse | Sint | [204] |
| 2014 | Costello | PLGA | Cell lines/organoids | in vitro | SInt | [211] |
| 1997 | Choi | PGA | organoids | mouse | int | [212] |
| 2004 | Grikscheit | PGA | organoids | rat | Int | [213] |
| 2009 | Sala | PGA/PLLA | Int cells | pig | Int | [181] |
| 2015 | Liu | PGA | organoids | mouse | Int | [214] |
| 2016 | Gjorevski | PEG-VS | murine crypt cells | in vitro | Int | [215] |
| 2019 | Liu | PGA | crypt SC | rat | Int | [216] |
| 2019 | Liu | PCL | crypt SC | rat | Int | [216] |
| Hybrid biomaterials | ||||||
| 2013 | Levin | PGA/collagen | organoids | mouse | Sint | [217] |
| 2016 | Wieck | PGA/PLLA/collagen | organoids | mouse | Int | [218] |
| 2019 | Ladd | PGS/collagen | organoids | mouse | Int | [219] |
| Natural biomaterials | ||||||
| 2014 | Watson | collagen | iPSC/ESC organoids | mouse | Sint | [220] |
| 2014 | Fukuda | Matrigel | organoids | mouse | Sint | [221] |
| 2016 | Cromeens | Matrigel | murine crypt cells | in vitro | SInt | [222] |
| 2017 | Workman | collagen | iPSC/ESC organoids | mouse | Sint | [205] |
| 2017 | Zakhem | Chitosan | human cells | rat | Int | [223] |
| 2019 | Liu | Collagen | crypt SC | rat | Int | [216] |
| 2018 | Sugimoto | Matrigel | human organoids | mouse | Int | [224] |
| 2019 | Capeling | Alginate | human HPSC | in vitro | Int | [225] |
| Acellular matrices | ||||||
| 2012 | Totonelli | Rat SInt | AFSC | in vitro | Sint | [226] |
| 2015 | Finkbeiner | Pig Int | organoids | mouse | SInt | [210] |
| 2017 | Kitano | Rat Int | iPSC | rat | Int | [227] |
| 2020 | Palikuqi | Rat Int | various | mouse | Int | [228] |
| 2020 | Meran | Int | Fb + organoids | mouse | Int | [229] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elia, E.; Brownell, D.; Chabaud, S.; Bolduc, S. Tissue Engineering for Gastrointestinal and Genitourinary Tracts. Int. J. Mol. Sci. 2023, 24, 9. https://doi.org/10.3390/ijms24010009
Elia E, Brownell D, Chabaud S, Bolduc S. Tissue Engineering for Gastrointestinal and Genitourinary Tracts. International Journal of Molecular Sciences. 2023; 24(1):9. https://doi.org/10.3390/ijms24010009
Chicago/Turabian StyleElia, Elissa, David Brownell, Stéphane Chabaud, and Stéphane Bolduc. 2023. "Tissue Engineering for Gastrointestinal and Genitourinary Tracts" International Journal of Molecular Sciences 24, no. 1: 9. https://doi.org/10.3390/ijms24010009
APA StyleElia, E., Brownell, D., Chabaud, S., & Bolduc, S. (2023). Tissue Engineering for Gastrointestinal and Genitourinary Tracts. International Journal of Molecular Sciences, 24(1), 9. https://doi.org/10.3390/ijms24010009







