Akt Is Controlled by Bag5 through a Monoubiquitination to Polyubiquitination Switch
Abstract
:1. Introduction
2. Results
2.1. Identification of BAG5 as an Akt-Interacting Protein
2.2. BAG5 Switches Akt Monoubiquitination to Polyubiquitination and Promotes degradation
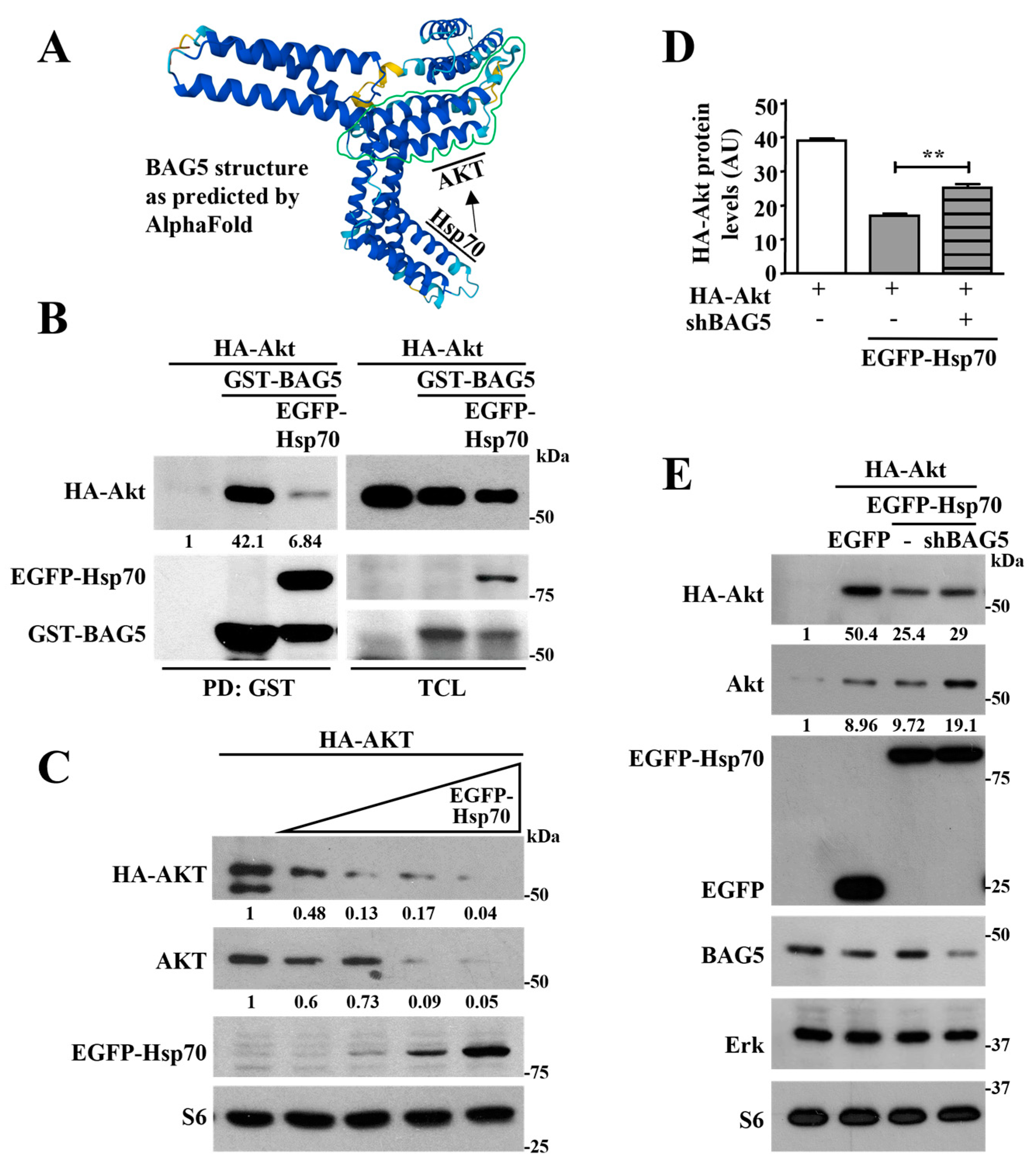
2.3. Hsp70 Mediates Akt Degradation Promoted by BAG5
2.4. Profile of Deubiquitinases and E3 Ligases Linked to BAG5 Expression in Breast, Lung, Uterine, and Ovarian Cancer Patients
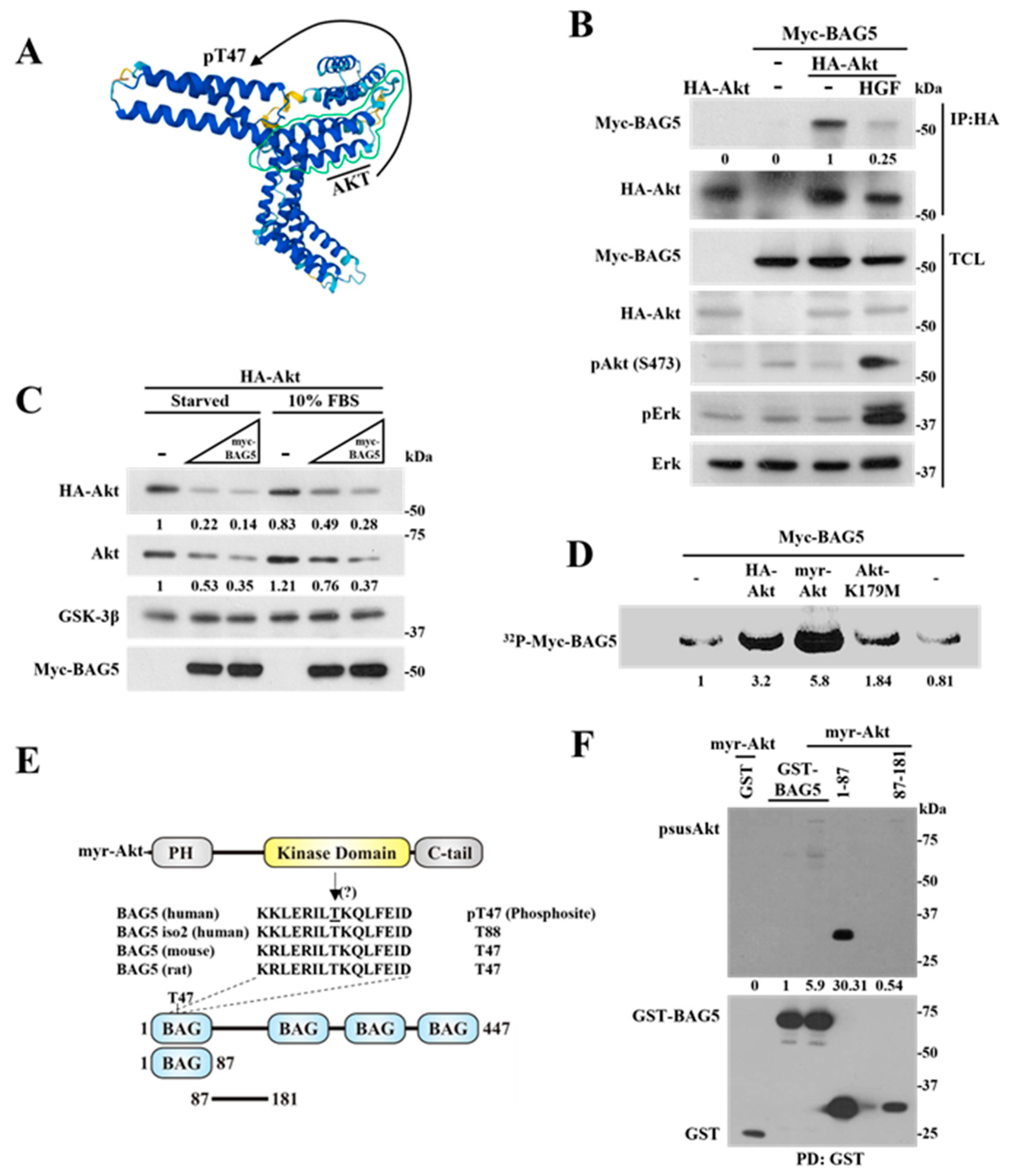
2.5. Phosphorylation of BAG5 by Akt Correlates with a Reduction in the Interaction between Them
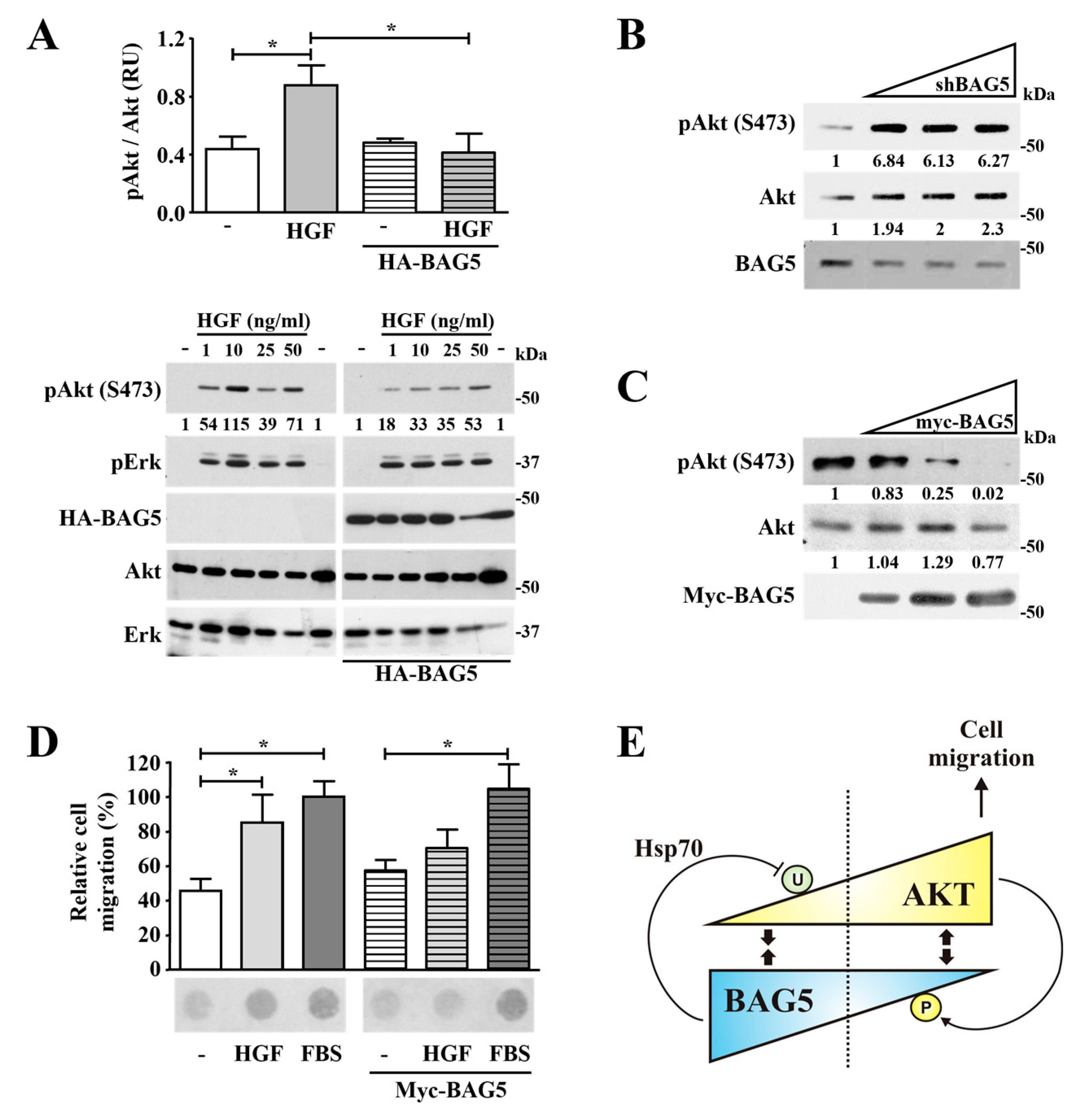
2.6. BAG5 Modulates Akt Phosphorylation and HGF-Dependent Cell Migration
3. Discussion
4. Materials and Methods
4.1. Yeast Two-Hybrid Screening
4.2. Plasmids and DNA Constructs
4.3. Cell Culture and Transfection
4.4. Immunoprecipitation, Pull-Down, and Western Blot Assays
4.5. Akt Stability and In Vivo Ubiquitination Assay
4.6. TCGA Data Mining Guided by BAG5
4.7. BAG5 Phosphorylation Assays
4.8. Akt Phosphorylation
4.9. Chemotaxis Assays
4.10. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Hemmings, B.A. PKB/Akt-dependent regulation of cell motility. J. Natl. Cancer Inst. 2013, 105, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Bracho-Valdes, I.; Moreno-Alvarez, P.; Valencia-Martinez, I.; Robles-Molina, E.; Chavez-Vargas, L.; Vazquez-Prado, J. mTORC1- and mTORC2-interacting proteins keep their multifunctional partners focused. IUBMB Life 2011, 63, 896–914. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, P.A.; di Blasio, L.; Primo, L. PDK1: A signaling hub for cell migration and tumor invasion. Biochim. Biophys. Acta 2015, 1856, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Gan, W.; Chin, Y.R.; Ogura, K.; Guo, J.; Zhang, J.; Wang, B.; Blenis, J.; Cantley, L.C.; Toker, A.; et al. PtdIns(3,4,5)P3-Dependent Activation of the mTORC2 Kinase Complex. Cancer Discov. 2015, 5, 1194–1209. [Google Scholar] [CrossRef]
- Madsen, R.R.; Toker, A. PI3K signaling through a biochemical systems lens. J. Biol. Chem. 2023, 299, 105224. [Google Scholar] [CrossRef]
- Liao, Y.; Wei, Y.; Zhou, X.; Yang, J.Y.; Dai, C.; Chen, Y.J.; Agarwal, N.K.; Sarbassov, D.; Shi, D.; Yu, D.; et al. Peptidyl-prolyl cis/trans isomerase Pin1 is critical for the regulation of PKB/Akt stability and activation phosphorylation. Oncogene 2009, 28, 2436–2445. [Google Scholar] [CrossRef]
- Wang, G.; Long, J.; Gao, Y.; Zhang, W.; Han, F.; Xu, C.; Sun, L.; Yang, S.C.; Lan, J.; Hou, Z.; et al. SETDB1-mediated methylation of Akt promotes its K63-linked ubiquitination and activation leading to tumorigenesis. Nat. Cell Biol. 2019, 21, 214–225. [Google Scholar] [CrossRef]
- Noguchi, M.; Hirata, N.; Suizu, F. The links between AKT and two intracellular proteolytic cascades: Ubiquitination and autophagy. Biochim. Biophys. Acta 2014, 1846, 342–352. [Google Scholar] [CrossRef]
- Yang, W.L.; Jin, G.; Li, C.F.; Jeong, Y.S.; Moten, A.; Xu, D.; Feng, Z.; Chen, W.; Cai, Z.; Darnay, B.; et al. Cycles of ubiquitination and deubiquitination critically regulate growth factor-mediated activation of Akt signaling. Sci. Signal 2013, 6, ra3. [Google Scholar] [CrossRef]
- Chan, C.H.; Li, C.F.; Yang, W.L.; Gao, Y.; Lee, S.W.; Feng, Z.; Huang, H.Y.; Tsai, K.K.; Flores, L.G.; Shao, Y.; et al. The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis. Cell 2012, 149, 1098–1111. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.L.; Wang, J.; Chan, C.H.; Lee, S.W.; Campos, A.D.; Lamothe, B.; Hur, L.; Grabiner, B.C.; Lin, X.; Darnay, B.G.; et al. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science 2009, 325, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Clement, E.; Inuzuka, H.; Nihira, N.T.; Wei, W.; Toker, A. Skp2-dependent reactivation of AKT drives resistance to PI3K inhibitors. Sci. Signal 2018, 11, eaao3810. [Google Scholar] [CrossRef] [PubMed]
- Dufour, M.; Dormond-Meuwly, A.; Pythoud, C.; Demartines, N.; Dormond, O. Reactivation of AKT signaling following treatment of cancer cells with PI3K inhibitors attenuates their antitumor effects. Biochem. Biophys. Res. Commun. 2013, 438, 32–37. [Google Scholar] [CrossRef]
- Facchinetti, V.; Ouyang, W.; Wei, H.; Soto, N.; Lazorchak, A.; Gould, C.; Lowry, C.; Newton, A.C.; Mao, Y.; Miao, R.Q.; et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. Embo J. 2008, 27, 1932–1943. [Google Scholar] [CrossRef]
- Oh, W.J.; Wu, C.C.; Kim, S.J.; Facchinetti, V.; Julien, L.A.; Finlan, M.; Roux, P.P.; Su, B.; Jacinto, E. mTORC2 can associate with ribosomes to promote cotranslational phosphorylation and stability of nascent Akt polypeptide. Embo J. 2010, 29, 3939–3951. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef]
- Wu, Y.T.; Ouyang, W.; Lazorchak, A.S.; Liu, D.; Shen, H.M.; Su, B. mTOR complex 2 targets Akt for proteasomal degradation via phosphorylation at the hydrophobic motif. J. Biol. Chem. 2011, 286, 14190–14198. [Google Scholar] [CrossRef]
- Martin, D.; Salinas, M.; Fujita, N.; Tsuruo, T.; Cuadrado, A. Ceramide and reactive oxygen species generated by H2O2 induce caspase-3-independent degradation of Akt/protein kinase B. J. Biol. Chem. 2002, 277, 42943–42952. [Google Scholar] [CrossRef]
- Riesterer, O.; Zingg, D.; Hummerjohann, J.; Bodis, S.; Pruschy, M. Degradation of PKB/Akt protein by inhibition of the VEGF receptor/mTOR pathway in endothelial cells. Oncogene 2004, 23, 4624–4635. [Google Scholar] [CrossRef]
- Suizu, F.; Hiramuki, Y.; Okumura, F.; Matsuda, M.; Okumura, A.J.; Hirata, N.; Narita, M.; Kohno, T.; Yokota, J.; Bohgaki, M.; et al. The E3 ligase TTC3 facilitates ubiquitination and degradation of phosphorylated Akt. Dev. Cell 2009, 17, 800–810. [Google Scholar] [CrossRef]
- Dwane, L.; Gallagher, W.M.; Ni Chonghaile, T.; O’Connor, D.P. The Emerging Role of Non-traditional Ubiquitination in Oncogenic Pathways. J. Biol. Chem. 2017, 292, 3543–3551. [Google Scholar] [CrossRef] [PubMed]
- Solit, D.B.; Basso, A.D.; Olshen, A.B.; Scher, H.I.; Rosen, N. Inhibition of heat shock protein 90 function down-regulates Akt kinase and sensitizes tumors to Taxol. Cancer Res. 2003, 63, 2139–2144. [Google Scholar] [PubMed]
- Koren, J., 3rd; Jinwal, U.K.; Jin, Y.; O’Leary, J.; Jones, J.R.; Johnson, A.G.; Blair, L.J.; Abisambra, J.F.; Chang, L.; Miyata, Y.; et al. Facilitating Akt clearance via manipulation of Hsp70 activity and levels. J. Biol. Chem. 2010, 285, 2498–2505. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, A.; Handa, N.; Ohsawa, N.; Shida, M.; Kigawa, T.; Hayashi, F.; Shirouzu, M.; Yokoyama, S. The C-terminal BAG domain of BAG5 induces conformational changes of the Hsp70 nucleotide-binding domain for ADP-ATP exchange. Structure 2010, 18, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Kalia, S.K.; Lee, S.; Smith, P.D.; Liu, L.; Crocker, S.J.; Thorarinsdottir, T.E.; Glover, J.R.; Fon, E.A.; Park, D.S.; Lozano, A.M. BAG5 inhibits parkin and enhances dopaminergic neuron degeneration. Neuron 2004, 44, 931–945. [Google Scholar] [CrossRef]
- Kalia, L.V.; Kalia, S.K.; Chau, H.; Lozano, A.M.; Hyman, B.T.; McLean, P.J. Ubiquitinylation of alpha-synuclein by carboxyl terminus Hsp70-interacting protein (CHIP) is regulated by Bcl-2-associated athanogene 5 (BAG5). PLoS ONE 2011, 6, e14695. [Google Scholar] [CrossRef]
- Friesen, E.L.; Zhang, Y.T.; Earnshaw, R.; De Snoo, M.L.; O’Hara, D.M.; Agapova, V.; Chau, H.; Ngana, S.; Chen, K.S.; Kalia, L.V.; et al. BAG5 Promotes Alpha-Synuclein Oligomer Formation and Functionally Interacts With the Autophagy Adaptor Protein p62. Front. Cell Dev. Biol. 2020, 8, 716. [Google Scholar] [CrossRef]
- Bellacosa, A.; Chan, T.O.; Ahmed, N.N.; Datta, K.; Malstrom, S.; Stokoe, D.; McCormick, F.; Feng, J.; Tsichlis, P. Akt activation by growth factors is a multiple-step process: The role of the PH domain. Oncogene 1998, 17, 313–325. [Google Scholar] [CrossRef]
- Kabbage, M.; Dickman, M.B. The BAG proteins: A ubiquitous family of chaperone regulators. Cell Mol. Life Sci. 2008, 65, 1390–1402. [Google Scholar] [CrossRef]
- Doong, H.; Vrailas, A.; Kohn, E.C. What’s in the ‘BAG’?--A functional domain analysis of the BAG-family proteins. Cancer Lett. 2002, 188, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Takayama, S.; Reed, J.C. Molecular chaperone targeting and regulation by BAG family proteins. Nat. Cell Biol. 2001, 3, E237–E241. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Randhawa, P.K.; Masternak, M.M. Role of BAG5 in Protein Quality Control: Double-Edged Sword? Front. Aging 2022, 3, 844168. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Haiyan, G.; Haidong, G. BAG5 regulates PTEN stability in MCF-7 cell line. BMB Rep. 2013, 46, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Tahrir, F.G.; Knezevic, T.; White, M.K.; Gordon, J.; Cheung, J.Y.; Khalili, K.; Feldman, A.M. GRP78 Interacting Partner Bag5 Responds to ER Stress and Protects Cardiomyocytes From ER Stress-Induced Apoptosis. J. Cell Biochem. 2016, 117, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.D.; Solit, D.B.; Chiosis, G.; Giri, B.; Tsichlis, P.; Rosen, N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J. Biol. Chem. 2002, 277, 39858–39866. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Matsushima-Nishiwaki, R.; Yamada, N.; Migita, S.; Hioki, T.; Mizutani, D.; Kozawa, O. Heat shock protein 70 positively regulates transforming growth factor-alpha-induced hepatocellular carcinoma cell migration via the AKT signaling pathway. Heliyon 2020, 6, e05002. [Google Scholar] [CrossRef]
- Paul, I.; Ahmed, S.F.; Bhowmik, A.; Deb, S.; Ghosh, M.K. The ubiquitin ligase CHIP regulates c-Myc stability and transcriptional activity. Oncogene 2013, 32, 1284–1295. [Google Scholar] [CrossRef]
- Shang, Y.; Zhao, X.; Xu, X.; Xin, H.; Li, X.; Zhai, Y.; He, D.; Jia, B.; Chen, W.; Chang, Z. CHIP functions an E3 ubiquitin ligase of Runx1. Biochem. Biophys. Res. Commun. 2009, 386, 242–246. [Google Scholar] [CrossRef]
- Qian, S.B.; McDonough, H.; Boellmann, F.; Cyr, D.M.; Patterson, C. CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature 2006, 440, 551–555. [Google Scholar] [CrossRef]
- Jiang, J.; Ballinger, C.A.; Wu, Y.; Dai, Q.; Cyr, D.M.; Hohfeld, J.; Patterson, C. CHIP is a U-box-dependent E3 ubiquitin ligase: Identification of Hsc70 as a target for ubiquitylation. J. Biol. Chem. 2001, 276, 42938–42944. [Google Scholar] [CrossRef] [PubMed]
- Kohn, A.D.; Takeuchi, F.; Roth, R.A. Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J. Biol. Chem. 1996, 271, 21920–21926. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Murakami, H.; Asai, N.; Morone, N.; Watanabe, T.; Kawai, K.; Murakumo, Y.; Usukura, J.; Kaibuchi, K.; Takahashi, M. Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev. Cell 2005, 9, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Gandino, L.; Longati, P.; Medico, E.; Prat, M.; Comoglio, P.M. Phosphorylation of serine 985 negatively regulates the hepatocyte growth factor receptor kinase. J. Biol. Chem. 1994, 269, 1815–1820. [Google Scholar] [CrossRef]
- Holmes, D. PI3K pathway inhibitors approach junction. Nat. Rev. Drug Discov. 2011, 10, 563–564. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Soda, M.; Hatakeyama, S.; Akagi, T.; Hashikawa, T.; Nakayama, K.I.; Takahashi, R. CHIP is associated with Parkin, a gene responsible for familial Parkinson’s disease, and enhances its ubiquitin ligase activity. Mol. Cell 2002, 10, 55–67. [Google Scholar] [CrossRef]
- Bruchmann, A.; Roller, C.; Walther, T.V.; Schäfer, G.; Lehmusvaara, S.; Visakorpi, T.; Klocker, H.; Cato, A.C.B.; Maddalo, D. Bcl-2 associated athanogene 5 (Bag5) is overexpressed in prostate cancer and inhibits ER-stress induced apoptosis. BMC Cancer 2013, 13, 96. [Google Scholar] [CrossRef]
- Bae, S.; Kim, S.Y.; Jung, J.H.; Yoon, Y.; Cha, H.J.; Lee, H.; Kim, K.; Kim, J.; An, I.S.; Um, H.D.; et al. Akt is negatively regulated by the MULAN E3 ligase. Cell Res. 2012, 22, 873–885. [Google Scholar] [CrossRef]
- Li, W.; Bengtson, M.H.; Ulbrich, A.; Matsuda, A.; Reddy, V.A.; Orth, A.; Chanda, S.K.; Batalov, S.; Joazeiro, C.A. Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle’s dynamics and signaling. PLoS ONE 2008, 3, e1487. [Google Scholar] [CrossRef]
- Du, K.; Herzig, S.; Kulkarni, R.N.; Montminy, M. TRB3: A tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 2003, 300, 1574–1577. [Google Scholar] [CrossRef]
- Qi, L.; Heredia, J.E.; Altarejos, J.Y.; Screaton, R.; Goebel, N.; Niessen, S.; Macleod, I.X.; Liew, C.W.; Kulkarni, R.N.; Bain, J.; et al. TRB3 links the E3 ubiquitin ligase COP1 to lipid metabolism. Science 2006, 312, 1763–1766. [Google Scholar] [CrossRef]
- Vazquez-Prado, J.; Basile, J.; Gutkind, J.S. Modular architecture and novel protein-protein interactions regulating the RGS-containing Rho guanine nucleotide exchange factors. Methods Enzymol. 2004, 390, 259–285. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.C.; Bhasin, S.; Wu, X.; Lee, J.G.; Maffi, S.; Nichols, C.J.; Lee, K.J.; Taylor, J.P.; Greene, L.E.; Eisenberg, E. Hsp70 dynamics in vivo: Effect of heat shock and protein aggregation. J. Cell Sci. 2004, 117, 4991–5000. [Google Scholar] [CrossRef] [PubMed]
- Chiariello, M.; Marinissen, M.J.; Gutkind, J.S. Multiple mitogen-activated protein kinase signaling pathways connect the cot oncoprotein to the c-jun promoter and to cellular transformation. Mol. Cell Biol. 2000, 20, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Murga, C.; Fukuhara, S.; Gutkind, J.S. A novel role for phosphatidylinositol 3-kinase beta in signaling from G protein-coupled receptors to Akt. J. Biol. Chem. 2000, 275, 12069–12073. [Google Scholar] [CrossRef]
- Guzman-Hernandez, M.L.; Vazquez-Macias, A.; Carretero-Ortega, J.; Hernandez-Garcia, R.; Garcia-Regalado, A.; Hernandez-Negrete, I.; Reyes-Cruz, G.; Gutkind, J.S.; Vazquez-Prado, J. Differential inhibitor of Gbetagamma signaling to AKT and ERK derived from phosducin-like protein: Effect on sphingosine 1-phosphate-induced endothelial cell migration and in vitro angiogenesis. J. Biol. Chem. 2009, 284, 18334–18346. [Google Scholar] [CrossRef]
- Carretero-Ortega, J.; Walsh, C.T.; Hernandez-Garcia, R.; Reyes-Cruz, G.; Brown, J.H.; Vazquez-Prado, J. Phosphatidylinositol 3,4,5-triphosphate-dependent Rac exchanger 1 (P-Rex-1), a guanine nucleotide exchange factor for Rac, mediates angiogenic responses to stromal cell-derived factor-1/chemokine stromal cell derived factor-1 (SDF-1/CXCL-12) linked to Rac activation, endothelial cell migration, and in vitro angiogenesis. Mol. Pharmacol. 2010, 77, 435–442. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bracho-Valdés, I.; Cervantes-Villagrana, R.D.; Beltrán-Navarro, Y.M.; Olguín-Olguín, A.; Escobar-Islas, E.; Carretero-Ortega, J.; Olivares-Reyes, J.A.; Reyes-Cruz, G.; Gutkind, J.S.; Vázquez-Prado, J. Akt Is Controlled by Bag5 through a Monoubiquitination to Polyubiquitination Switch. Int. J. Mol. Sci. 2023, 24, 17531. https://doi.org/10.3390/ijms242417531
Bracho-Valdés I, Cervantes-Villagrana RD, Beltrán-Navarro YM, Olguín-Olguín A, Escobar-Islas E, Carretero-Ortega J, Olivares-Reyes JA, Reyes-Cruz G, Gutkind JS, Vázquez-Prado J. Akt Is Controlled by Bag5 through a Monoubiquitination to Polyubiquitination Switch. International Journal of Molecular Sciences. 2023; 24(24):17531. https://doi.org/10.3390/ijms242417531
Chicago/Turabian StyleBracho-Valdés, Ismael, Rodolfo Daniel Cervantes-Villagrana, Yarely Mabell Beltrán-Navarro, Adán Olguín-Olguín, Estanislao Escobar-Islas, Jorge Carretero-Ortega, J. Alberto Olivares-Reyes, Guadalupe Reyes-Cruz, J. Silvio Gutkind, and José Vázquez-Prado. 2023. "Akt Is Controlled by Bag5 through a Monoubiquitination to Polyubiquitination Switch" International Journal of Molecular Sciences 24, no. 24: 17531. https://doi.org/10.3390/ijms242417531
APA StyleBracho-Valdés, I., Cervantes-Villagrana, R. D., Beltrán-Navarro, Y. M., Olguín-Olguín, A., Escobar-Islas, E., Carretero-Ortega, J., Olivares-Reyes, J. A., Reyes-Cruz, G., Gutkind, J. S., & Vázquez-Prado, J. (2023). Akt Is Controlled by Bag5 through a Monoubiquitination to Polyubiquitination Switch. International Journal of Molecular Sciences, 24(24), 17531. https://doi.org/10.3390/ijms242417531







