PKC Inhibition Improves Human Penile Vascular Function and the NO/cGMP Pathway in Diabetic Erectile Dysfunction: The Role of NADPH Oxidase
Abstract
1. Introduction
2. Results
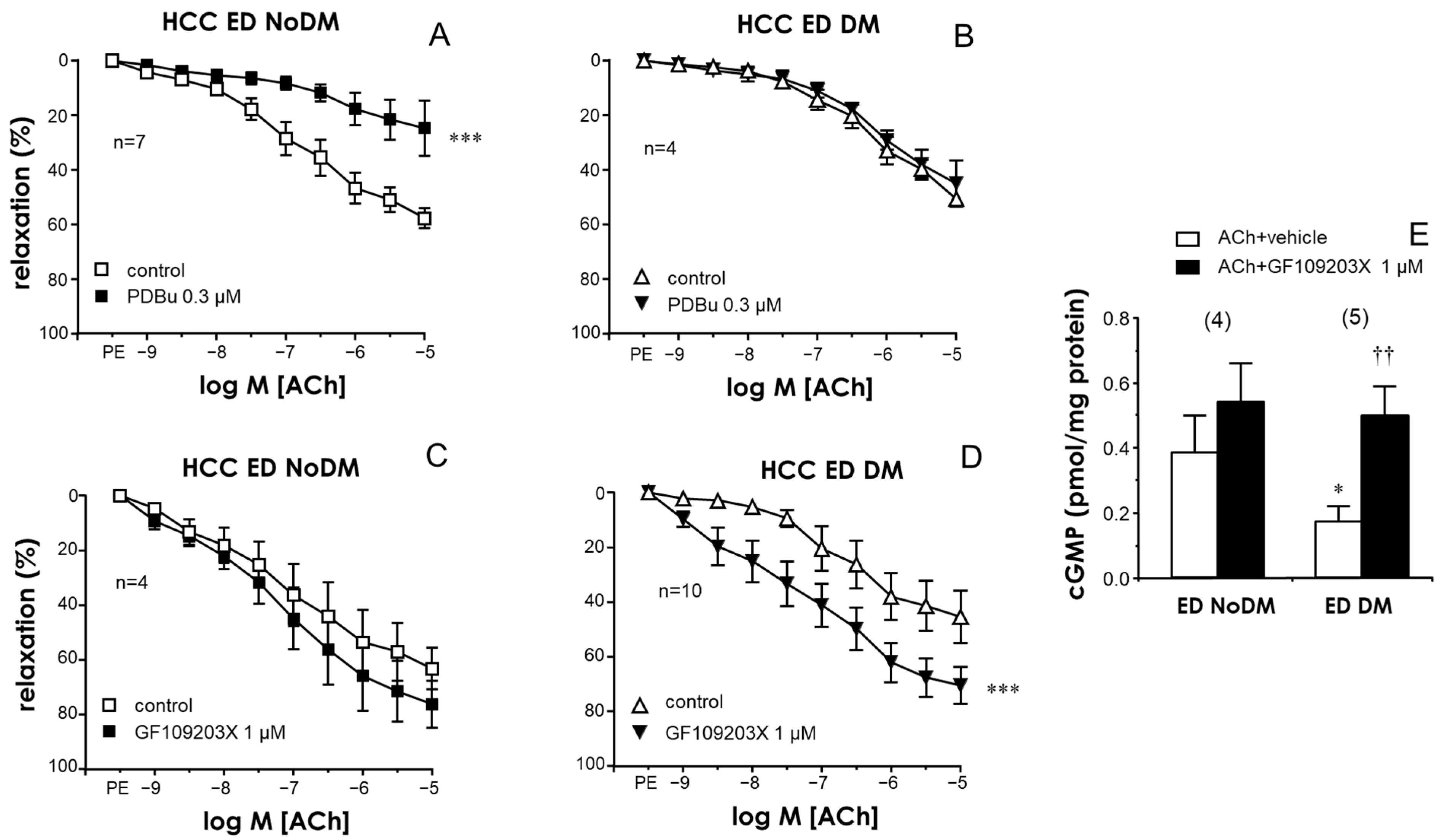
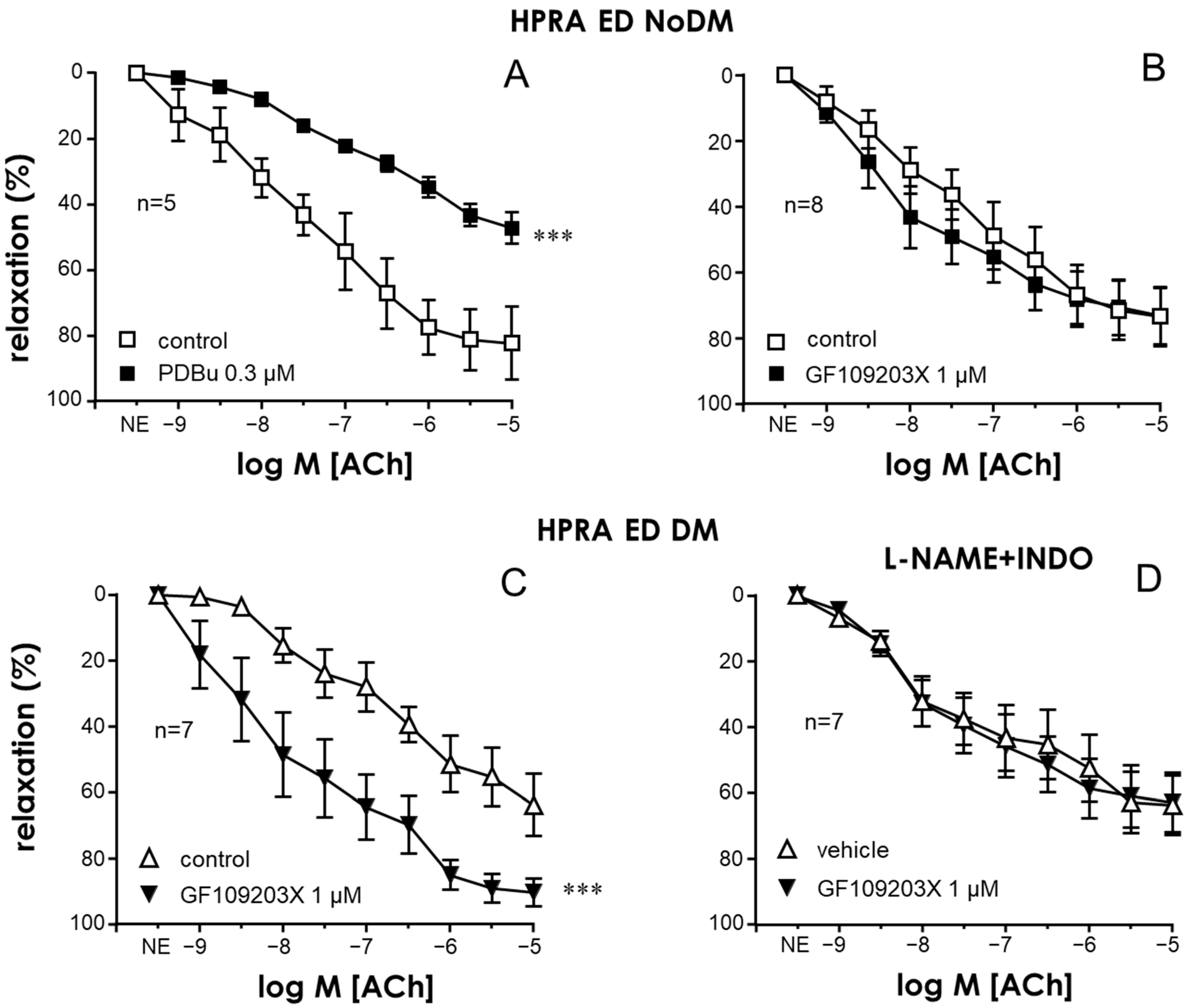
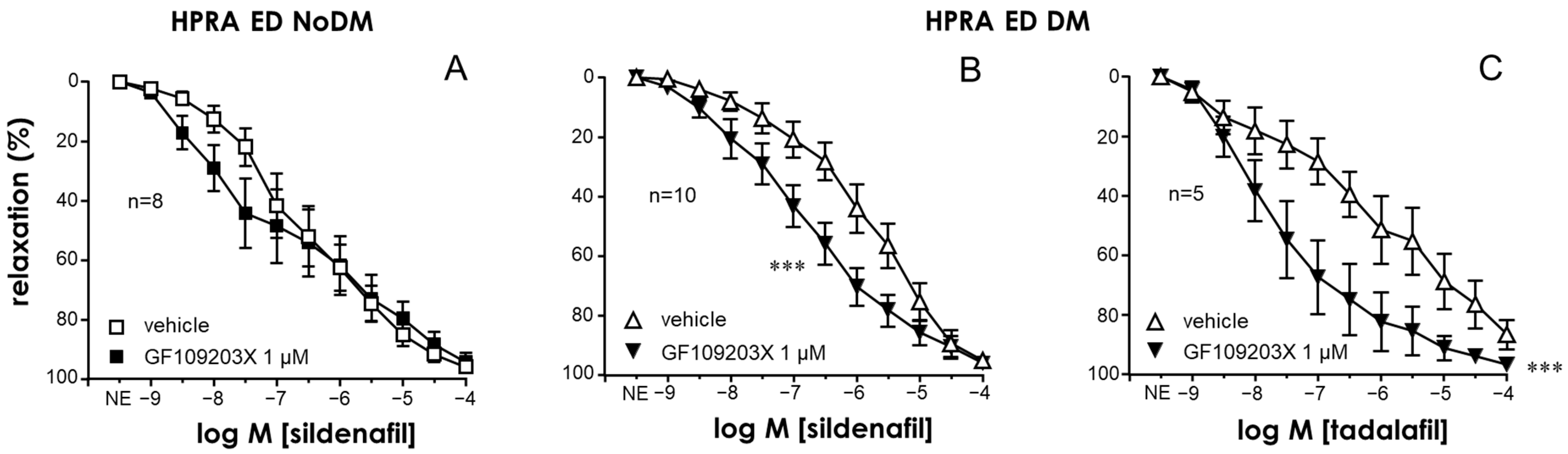
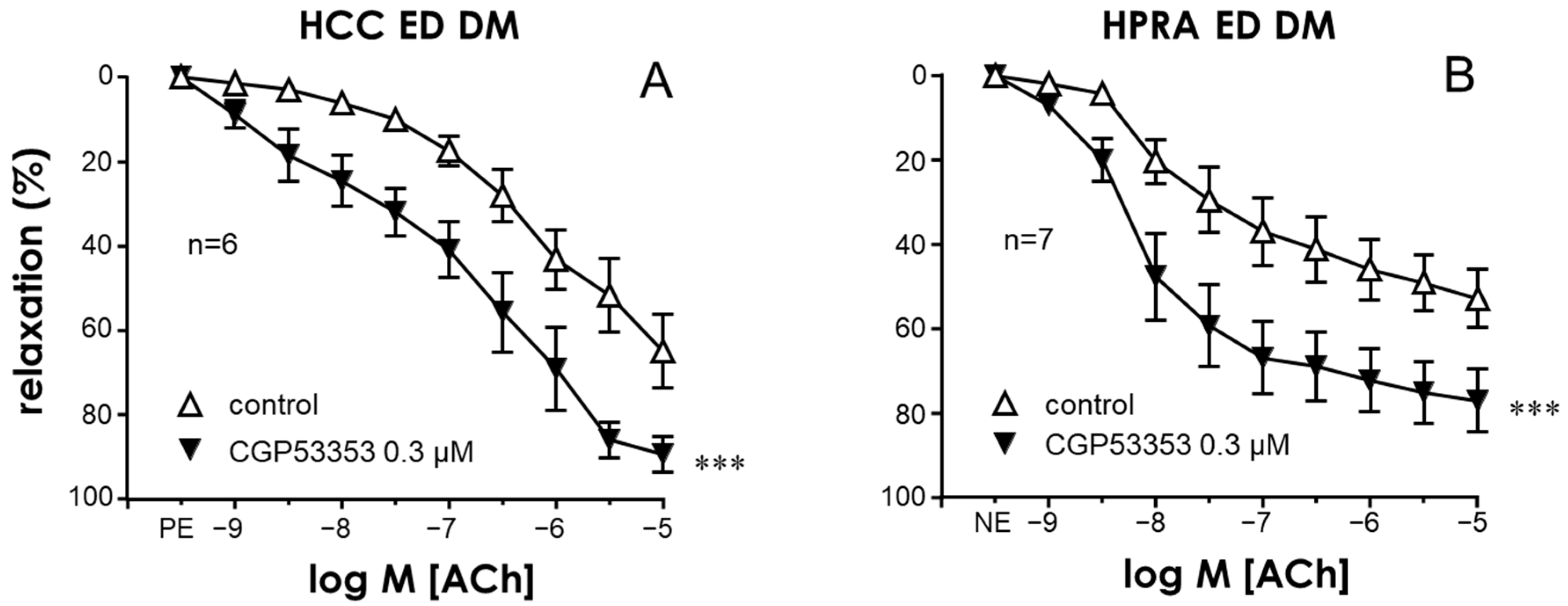
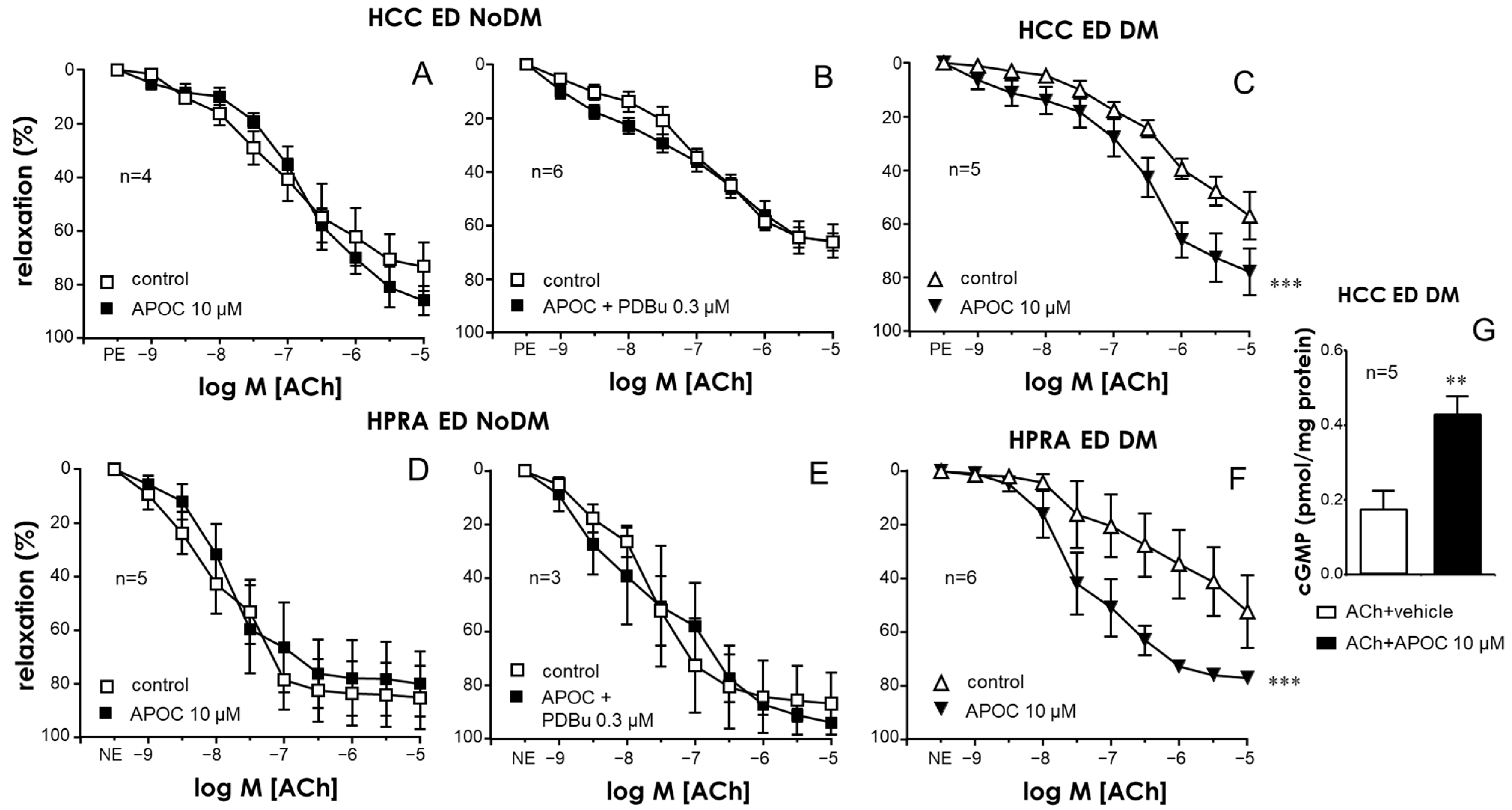
2.1. Activation of PKC Impaired Endothelial Relaxations in the CC and PRAs in the Absence of Diabetes While PKC Inhibition Improved These Responses Only in Tissues from Diabetic Patients
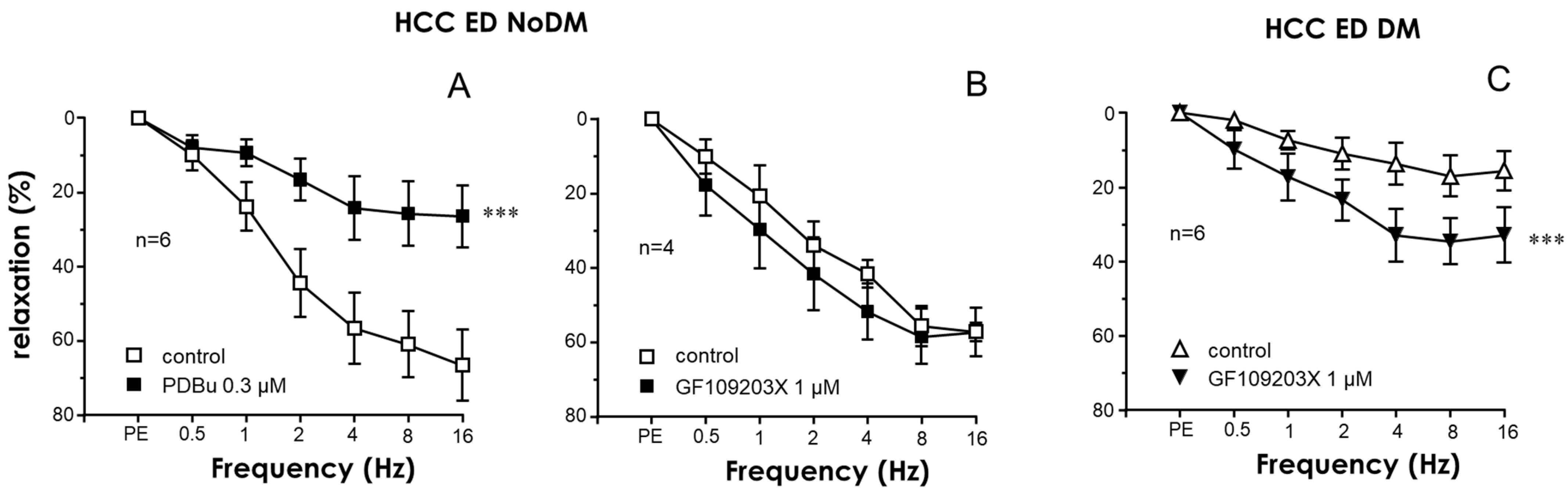
2.2. Nitrergic Neurogenic Relaxation Was Enhanced by PKC Inhibition in the CC of Diabetic ED Patients
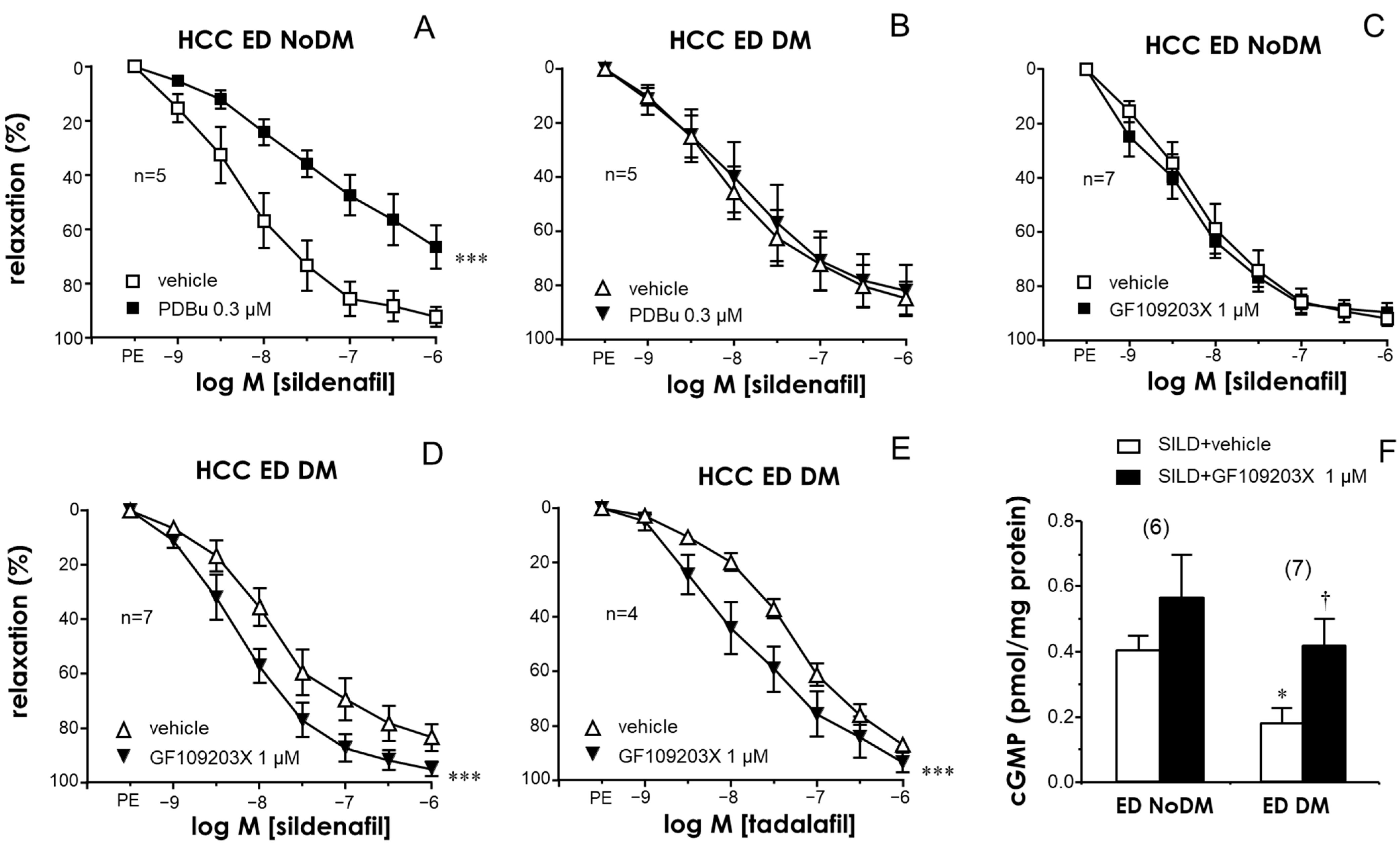
2.3. PKC Inhibition Enhanced the Relaxant Efficacy of PDE5 Inhibitors in the CC and PRAs of ED Patients with Diabetes
2.4. Specific Inhibition of the PKCβ2 Isoform Improved Endothelial Relaxation in the CC and PRAs of Diabetic ED Patients
2.5. NADPH-Oxidase Was Involved in the Endothelial Impairment Caused by PKC in Penile Vascular Tissues
3. Discussion
4. Materials and Methods
4.1. Human Tissues
4.1.1. Functional Evaluation of CC
4.1.2. Functional Evaluation of Human Penile Resistance Arteries (PRAs)
4.1.3. Cyclic GMP Determinations
4.2. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global Estimates for the Prevalence of Diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Defeudis, G.; Mazzilli, R.; Tenuta, M.; Rossini, G.; Zamponi, V.; Olana, S.; Faggiano, A.; Pozzilli, P.; Isidori, A.M.; Gianfrilli, D. Erectile Dysfunction and Diabetes: A Melting Pot of Circumstances and Treatments. Diabetes Metab. Res. Rev. 2022, 38, e3494. [Google Scholar] [CrossRef]
- Cayetano-Alcaraz, A.A.; Tharakan, T.; Chen, R.; Sofikitis, N.; Minhas, S. The Management of Erectile Dysfunction in Men with Diabetes Mellitus Unresponsive to Phosphodiesterase Type 5 Inhibitors. Andrology 2023, 11, 257–269. [Google Scholar] [CrossRef]
- Ma, J.X.; Wang, B.; Li, H.S.; Yu, J.; Hu, H.M.; Ding, C.F.; Chen, W.Q. Uncovering the Mechanisms of Leech and Centipede Granules in the Treatment of Diabetes Mellitus-Induced Erectile Dysfunction Utilising Network Pharmacology. J. Ethnopharmacol. 2021, 265, 113358. [Google Scholar] [CrossRef]
- Angulo, J.; González-Corrochano, R.; Cuevas, P.; Fernández, A.; La Fuente, J.M.; Rolo, F.; Allona, A.; Sáenz de Tejada, I. Diabetes Exacerbates the Functional Deficiency of NO/CGMP Pathway Associated with Erectile Dysfunction in Human Corpus Cavernosum and Penile Arteries. J. Sex. Med. 2010, 7, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Sevilleja-Ortiz, A.; El Assar, M.; García-Gómez, B.; La Fuente, J.M.; Alonso-Isa, M.; Romero-Otero, J.; Martínez-Salamanca, J.I.; Fernández, A.; Rodríguez-Mañas, L.; Angulo, J. STIM/Orai Inhibition as a Strategy for Alleviating Diabetic Erectile Dysfunction through Modulation of Rat and Human Penile Tissue Contractility and In Vivo Potentiation of Erectile Responses. J. Sex. Med. 2022, 19, 1733–1749. [Google Scholar] [CrossRef] [PubMed]
- Schjørring, O.; Kun, A.; Flyvbjerg, A.; Kirkeby, H.J.; Jensen, J.B.; Simonsen, U. Flow-Evoked Vasodilation Is Blunted in Penile Arteries from Zucker Diabetic Fatty Rats. J. Sex. Med. 2012, 9, 1789–1800. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.; Cuevas, P.; Fernández, A.; Gabancho, S.; Allona, A.; Martín-Morales, A.; Moncada, I.; Videla, S.; de Tejada, I.S. Diabetes Impairs Endothelium-Dependent Relaxation of Human Penile Vascular Tissues Mediated by NO and EDHF. Biochem. Biophys. Res. Commun. 2003, 312, 1202–1208. [Google Scholar] [CrossRef]
- Bivalacqua, T.J.; Hellstrom, W.J.G.; Kadowitz, P.J.; Champion, H.C. Increased Expression of Arginase II in Human Diabetic Corpus Cavernosum: In Diabetic-Associated Erectile Dysfunction. Biochem. Biophys. Res. Commun. 2001, 283, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.-F.; Chen, S.-J.; Tsai, M.-C.; Lin, C.-S. Potential Role of Protein Kinase C in the Pathophysiology of Diabetes-Associated Atherosclerosis. Front. Pharmacol. 2021, 12, 716332. [Google Scholar] [CrossRef] [PubMed]
- Jubaidi, F.F.; Zainalabidin, S.; Taib, I.S.; Abdul Hamid, Z.; Mohamad Anuar, N.N.; Jalil, J.; Mohd Nor, N.A.; Budin, S.B. The Role of PKC-MAPK Signalling Pathways in the Development of Hyperglycemia-Induced Cardiovascular Complications. Int. J. Mol. Sci. 2022, 23, 8582. [Google Scholar] [CrossRef] [PubMed]
- Nangle, M.R.; Cotter, M.A.; Cameron, N.E. Protein Kinase Cβ Inhibition and Aorta and Corpus Cavernosum Function in Streptozotocin-Diabetic Mice. Eur. J. Pharmacol. 2003, 475, 99–106. [Google Scholar] [CrossRef]
- Angulo, J.; Cuevas, P.; Fernández, A.; Allona, A.; Moncada, I.; Martín-Morales, A.; La Fuente, J.M.; Sáenz De Tejada, I. Enhanced Thromboxane Receptor-Mediated Responses and Impaired Endothelium-Dependent Relaxation in Human Corpus Cavernosum from Diabetic Impotent Men: Role of Protein Kinase C Activity. J. Pharmacol. Exp. Ther. 2006, 319, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Wang, D.; Li, D.; Huang, J.; Ma, F.; Zhang, H.; Sheng, Y.; Zhang, C.; Ha, X. Protein Kinase C: A Potential Therapeutic Target for Endothelial Dysfunction in Diabetes. J. Diabetes Complicat. 2023, 37, 108565. [Google Scholar] [CrossRef]
- Castela, Â.; Costa, C. Molecular Mechanisms Associated with Diabetic Endothelial-Erectile Dysfunction. Nat. Rev. Urol. 2016, 13, 266–274. [Google Scholar] [CrossRef]
- Kizub, I.V.; Klymenko, K.I.; Soloviev, A.I. Protein Kinase C in Enhanced Vascular Tone in Diabetes Mellitus. Int. J. Cardiol. 2014, 174, 230–242. [Google Scholar] [CrossRef]
- Inoguchi, T.; Sonta, T.; Tsubouchi, H.; Etoh, T.; Kakimoto, M.; Sonoda, N.; Sato, N.; Sekiguchi, N.; Kobayashi, K.; Sumimoto, H.; et al. Protein Kinase C-Dependent Increase in Reactive Oxygen Species (ROS) Production in Vascular Tissues of Diabetes: Role of Vascular NAD(P)H Oxidase. J. Am. Soc. Nephrol. 2003, 14, S227–S232. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Xie, S.; Wang, J.; Xia, Z.; Nie, R. Inhibition of Protein Kinase C β2 Prevents Tumor Necrosis Factor-α-Induced Apoptosis and Oxidative Stress in Endothelial Cells: The Role of NADPH Oxidase Subunits. J. Vasc. Res. 2012, 49, 144–159. [Google Scholar] [CrossRef]
- MacDonald, S.M.; Burnett, A.L. Physiology of Erection and Pathophysiology of Erectile Dysfunction. Urol. Clin. N. Am. 2021, 48, 513–525. [Google Scholar] [CrossRef]
- Meir, J.; Huang, L.; Mahmood, S.; Whiteson, H.; Cohen, S.; Aronow, W.S. The Vascular Complications of Diabetes: A Review of Their Management, Pathogenesis, and Prevention. Expert Rev. Endocrinol. Metab. 2024, 19, 11–20. [Google Scholar] [CrossRef]
- La Sala, L.; Prattichizzo, F.; Ceriello, A. The Link between Diabetes and Atherosclerosis. Eur. J. Prev. Cardiol. 2019, 26, 15–24. [Google Scholar] [CrossRef]
- Malavige, L.S.; Levy, J.C. Erectile Dysfunction in Diabetes Mellitus. J. Sex. Med. 2009, 6, 1232–1247. [Google Scholar] [CrossRef]
- Maalmi, H.; Herder, C.; Bönhof, G.J.; Strassburger, K.; Zaharia, O.-P.; Rathmann, W.; Burkart, V.; Szendroedi, J.; Roden, M.; Ziegler, D. Differences in the Prevalence of Erectile Dysfunction between Novel Subgroups of Recent-Onset Diabetes. Diabetologia 2022, 65, 552–562. [Google Scholar] [CrossRef]
- Feldman, H.A.; Goldstein, I.; Hatzichristou, D.G.; Krane, R.J.; McKinlay, J.B. Impotence and Its Medical and Psychosocial Correlates: Results of the Massachusetts Male Aging Study. J. Urol. 1994, 151, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Jian, Z.; Gao, X.; Jin, X.; Wang, M.; Xiang, L.; Li, H.; Wang, K. Type 2 Diabetes Mellitus Increases Risk of Erectile Dysfunction Independent of Obesity and Dyslipidemia: A Mendelian Randomization Study. Andrology 2022, 10, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-B.; Niu, Z.-H.; Fan, W.-M.; Sheng, C.-S.; Chen, Q. Type 2 Diabetes Mellitus and the Risk of Male Infertility: A Mendelian Randomization Study. Front. Endocrinol. 2023, 14, 1279058. [Google Scholar] [CrossRef]
- Fonseca, V.; Seftel, A.; Denne, J.; Fredlund, P. Impact of Diabetes Mellitus on the Severity of Erectile Dysfunction and Response to Treatment: Analysis of Data from Tadalafil Clinical Trials. Diabetologia 2004, 47, 1914–1923. [Google Scholar] [CrossRef]
- Goldstein, I.; Stecher, V.; Carlsson, M. Treatment Response to Sildenafil in Men with Erectile Dysfunction Relative to Concomitant Comorbidities and Age. Int. J. Clin. Pract. 2017, 71, e12939. [Google Scholar] [CrossRef]
- Kim, N.; Azadzoi, K.M.; Goldstein, I.; Saenz de Tejada, I. A Nitric Oxide-like Factor Mediates Nonadrenergic-Noncholinergic Neurogenic Relaxation of Penile Corpus Cavernosum Smooth Muscle. J. Clin. Investig. 1991, 88, 112–118. [Google Scholar] [CrossRef]
- Rajfer, J.; Aronson, W.J.; Bush, P.A.; Dorey, F.J.; Ignarro, L.J. Nitric Oxide as a Mediator of Relaxation of the Corpus Cavernosum in Response to Nonadrenergic, Noncholinergic Neurotransmission. N. Engl. J. Med. 1992, 326, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Ballard, S.A.; Gingell, C.J.; Tang, K.; Turner, L.A.; Price, M.E.; Naylor, A.M. Effects of Sildenafil on the Relaxation of Human Corpus Cavernosum Tissue In Vitro and on the Activities of Cyclic Nucleotide Phosphodiesterase Isozymes. J. Urol. 1998, 159, 2164–2171. [Google Scholar] [CrossRef] [PubMed]
- Saenz de Tejada, I.; Angulo, J.; Cuevas, P.; Fernández, A.; Moncada, I.; Allona, A.; Lledó, E.; Körschen, H.; Niewöhner, U.; Haning, H.; et al. The Phosphodiesterase Inhibitory Selectivity and the In Vitro and In Vivo Potency of the New PDE5 Inhibitor Vardenafil. Int. J. Impot. Res. 2001, 13, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Hatzimouratidis, K.; Salonia, A.; Adaikan, G.; Buvat, J.; Carrier, S.; El-Meliegy, A.; McCullough, A.; Torres, L.O.; Khera, M. Pharmacotherapy for Erectile Dysfunction: Recommendations from the Fourth International Consultation for Sexual Medicine (ICSM 2015). J. Sex. Med. 2016, 13, 465–488. [Google Scholar] [CrossRef] [PubMed]
- Karakus, S.; Burnett, A.L. The Medical and Surgical Treatment of Erectile Dysfunction: A Review and Update. Can. J. Urol. 2020, 27, 28–35. [Google Scholar] [PubMed]
- Martínez-Salamanca, J.I.; La Fuente, J.M.; Cardoso, J.; Fernández, A.; Cuevas, P.; Wright, H.M.; Angulo, J. Nebivolol Potentiates the Efficacy of PDE5 Inhibitors to Relax Corpus Cavernosum and Penile Arteries from Diabetic Patients by Enhancing the NO/CGMP Pathway. J. Sex. Med. 2014, 11, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, P.; King, G.L. Activation of Protein Kinase C Isoforms and Its Impact on Diabetic Complications. Circ. Res. 2010, 106, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Idris, I.; Donnelly, R. Protein Kinase C β Inhibition: A Novel Therapeutic Strategy for Diabetic Microangiopathy. Diabetes Vasc. Dis. Res. 2006, 3, 172–178. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Bakris, G.L.; Toto, R.D.; McGill, J.B.; Hu, K.; Anderson, P.W. The Effect of Ruboxistaurin on Nephropathy in Type 2 Diabetes. Diabetes Care 2005, 28, 2686–2690. [Google Scholar] [CrossRef]
- Aiello, L.P. Inhibition of PKC by Oral Administration of Ruboxistaurin Is Well Tolerated and Ameliorates Diabetes-Induced Retinal Hemodynamic Abnormalities in Patients. Investig. Ophthalmol. Vis. Sci. 2006, 47, 86–92. [Google Scholar] [CrossRef]
- Casellini, C.M.; Barlow, P.M.; Rice, A.L.; Casey, M.; Simmons, K.; Pittenger, G.; Bastyr, E.J.; Wolka, A.M.; Vinik, A.I. A 6-Month, Randomized, Double-Masked, Placebo-Controlled Study Evaluating the Effects of the Protein Kinase C-β Inhibitor Ruboxistaurin on Skin Microvascular Blood Flow and Other Measures of Diabetic Peripheral Neuropathy. Diabetes Care 2007, 30, 896–902. [Google Scholar] [CrossRef]
- Mehta, N.N.; Sheetz, M.; Price, K.; Comiskey, L.; Amrutia, S.; Iqbal, N.; Mohler, E.R.; Reilly, M.P. Selective PKC Beta Inhibition with Ruboxistaurin and Endothelial Function in Type-2 Diabetes Mellitus. Cardiovasc. Drugs Ther. 2009, 23, 17–24. [Google Scholar] [CrossRef]
- Toullec, D.; Pianetti, P.; Coste, H.; Bellevergue, P.; Grand-Perret, T.; Ajakane, M.; Baudet, V.; Boissin, P.; Boursier, E.; Loriolle, F. The Bisindolylmaleimide GF 109203X Is a Potent and Selective Inhibitor of Protein Kinase C. J. Biol. Chem. 1991, 266, 15771–15781. [Google Scholar] [CrossRef] [PubMed]
- Martiny-Baron, G.; Kazanietz, M.G.; Mischak, H.; Blumberg, P.M.; Kochs, G.; Hug, H.; Marmé, D.; Schächtele, C. Selective Inhibition of Protein Kinase C Isozymes by the Indolocarbazole Gö 6976. J. Biol. Chem. 1993, 268, 9194–9197. [Google Scholar] [CrossRef] [PubMed]
- Kouroedov, A.; Eto, M.; Joch, H.; Volpe, M.; Lüscher, T.F.; Cosentino, F. Selective Inhibition of Protein Kinase Cbeta2 Prevents Acute Effects of High Glucose on Vascular Cell Adhesion Molecule-1 Expression in Human Endothelial Cells. Circulation 2004, 110, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.; Peiró, C.; Cuevas, P.; Gabancho, S.; Fernández, A.; González-Corrochano, R.; La Fuente, J.M.; Baron, A.D.; Chen, K.S.; De Tejada, I.S. The Novel Antioxidant, AC3056 (2,6-Di-t-Butyl-4-((Dimethyl-4-Methoxyphenylsilyl)Methyloxy)Phenol), Reverses Erectile Dysfunction in Diabetic Rats and Improves NO-Mediated Responses in Penile Tissue from Diabetic Men. J. Sex. Med. 2009, 6, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New Insights into Oxidative Stress and Inflammation during Diabetes Mellitus-Accelerated Atherosclerosis. Redox Biol. 2019, 20, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Koya, D.; Sugimoto, T.; Isono, M.; Araki, S.I.; Kashiwagi, A.; Haneda, M. Translocation of Glomerular P47phox and P67phox by Protein Kinase C-Beta Activation Is Required for Oxidative Stress in Diabetic Nephropathy. Diabetes 2003, 52, 2603–2614. [Google Scholar] [CrossRef] [PubMed]
- Jeremy, J.Y.; Jones, R.A.; Koupparis, A.J.; Hotston, M.; Persad, R.; Angelini, G.D.; Shukla, N. Reactive Oxygen Species and Erectile Dysfunction: Possible Role of NADPH Oxidase. Int. J. Impot. Res. 2007, 19, 265–280. [Google Scholar] [CrossRef]
- Paterniti, I.; Galuppo, M.; Mazzon, E.; Impellizzeri, D.; Esposito, E.; Bramanti, P.; Cuzzocrea, S. Protective Effects of Apocynin, an Inhibitor of NADPH Oxidase Activity, in Splanchnic Artery Occlusion and Reperfusion. J. Leukoc. Biol. 2010, 88, 993–1003. [Google Scholar] [CrossRef]
- Drummond, G.R.; Sobey, C.G. Endothelial NADPH Oxidases: Which NOX to Target in Vascular Disease? Trends Endocrinol. Metab. 2014, 25, 452–463. [Google Scholar] [CrossRef]
- Kendrick, D.J.; Mishra, R.C.; John, C.M.; Zhu, H.L.; Braun, A.P. Effects of Pharmacological Inhibitors of NADPH Oxidase on Myogenic Contractility and Evoked Vasoactive Responses in Rat Resistance Arteries. Front. Physiol. 2022, 12, 752366. [Google Scholar] [CrossRef] [PubMed]

| Non-Diabetic | Diabetic | p Value | |
|---|---|---|---|
| n | 40 | 36 | |
| Age (years) | 59.1 ± 1.6 | 57.5 ± 1.7 | 0.4666 |
| Hypertension (%) | 18 (45.0) | 17 (47.2) | 1.0000 |
| Dyslipidemia (%) | 7 (17.5) | 10 (27.8) | 0.4090 |
| Obesity (%) | 4 (10.0) | 1 (2.8) | 0.3616 |
| Smoking habit (%) | 14 (35.0) | 6 (16.7) | 0.1164 |
| Cardiovascular disease (%) | 1 (2.5) | 5 (13.9) | 0.0954 |
| Respiratory disease (%) | 2 (5.0) | 2 (5.6) | 1.0000 |
| Pelvic surgery (%) | 11 (27.5) | 7 (19.4) | 0.4338 |
| Peyronie’s disease (%) | 3 (7.5) | 2 (5.6) | 1.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Assar, M.; La Fuente, J.M.; Sosa, P.; Fernández, A.; Pepe-Cardoso, A.J.; Martínez-Salamanca, J.I.; Rodríguez-Mañas, L.; Angulo, J. PKC Inhibition Improves Human Penile Vascular Function and the NO/cGMP Pathway in Diabetic Erectile Dysfunction: The Role of NADPH Oxidase. Int. J. Mol. Sci. 2024, 25, 3111. https://doi.org/10.3390/ijms25063111
El Assar M, La Fuente JM, Sosa P, Fernández A, Pepe-Cardoso AJ, Martínez-Salamanca JI, Rodríguez-Mañas L, Angulo J. PKC Inhibition Improves Human Penile Vascular Function and the NO/cGMP Pathway in Diabetic Erectile Dysfunction: The Role of NADPH Oxidase. International Journal of Molecular Sciences. 2024; 25(6):3111. https://doi.org/10.3390/ijms25063111
Chicago/Turabian StyleEl Assar, Mariam, José M. La Fuente, Patricia Sosa, Argentina Fernández, Augusto J. Pepe-Cardoso, Juan I. Martínez-Salamanca, Leocadio Rodríguez-Mañas, and Javier Angulo. 2024. "PKC Inhibition Improves Human Penile Vascular Function and the NO/cGMP Pathway in Diabetic Erectile Dysfunction: The Role of NADPH Oxidase" International Journal of Molecular Sciences 25, no. 6: 3111. https://doi.org/10.3390/ijms25063111
APA StyleEl Assar, M., La Fuente, J. M., Sosa, P., Fernández, A., Pepe-Cardoso, A. J., Martínez-Salamanca, J. I., Rodríguez-Mañas, L., & Angulo, J. (2024). PKC Inhibition Improves Human Penile Vascular Function and the NO/cGMP Pathway in Diabetic Erectile Dysfunction: The Role of NADPH Oxidase. International Journal of Molecular Sciences, 25(6), 3111. https://doi.org/10.3390/ijms25063111







