Evaluation of Osteogenic Potential for Rat Adipose-Derived Stem Cells under Xeno-Free Environment
Abstract
:1. Introduction
2. Results
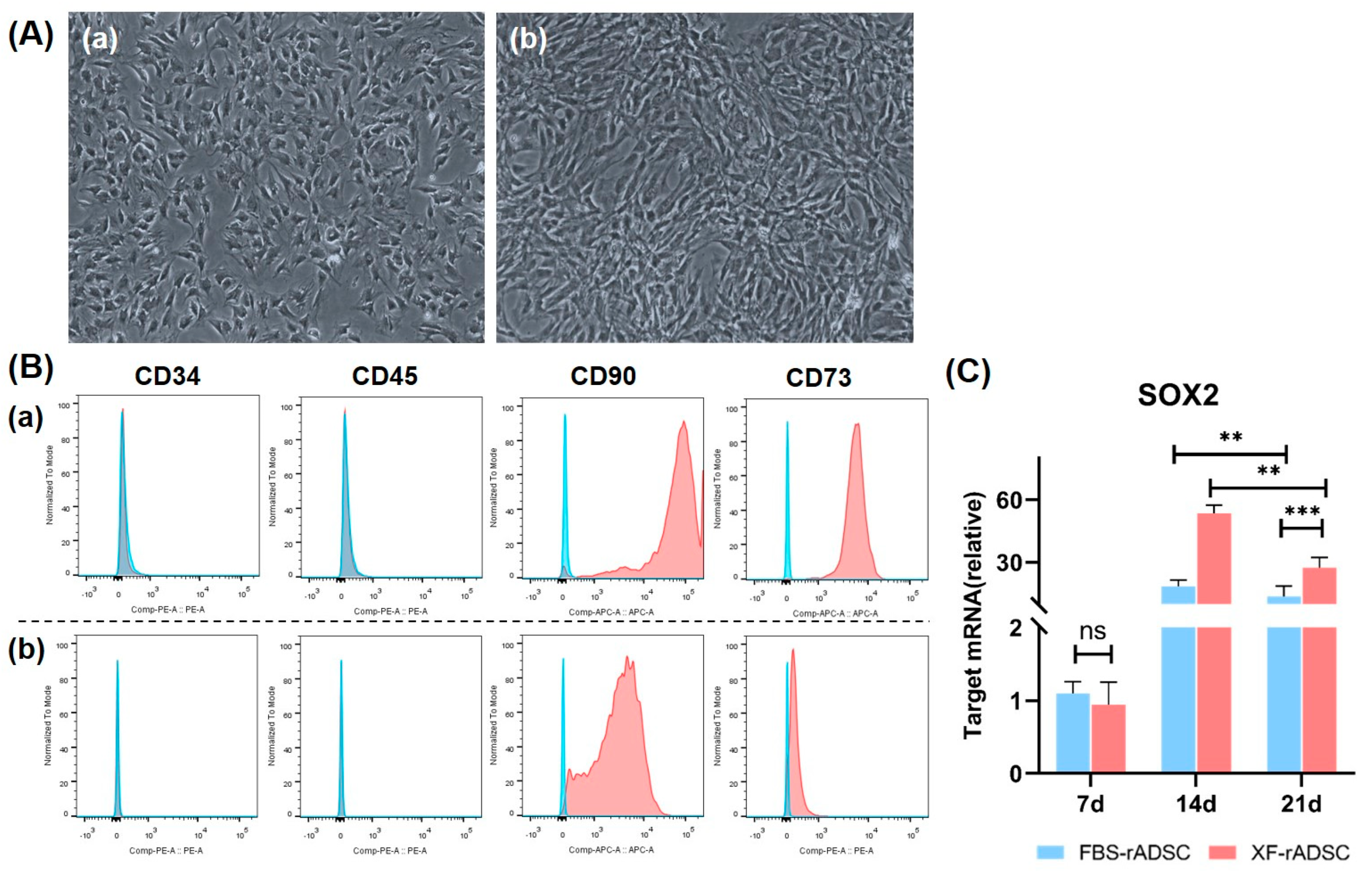
2.1. Characteristics of rADSC under 2-Dimensional Culture by Medium Supplemented with or without Xeno Components
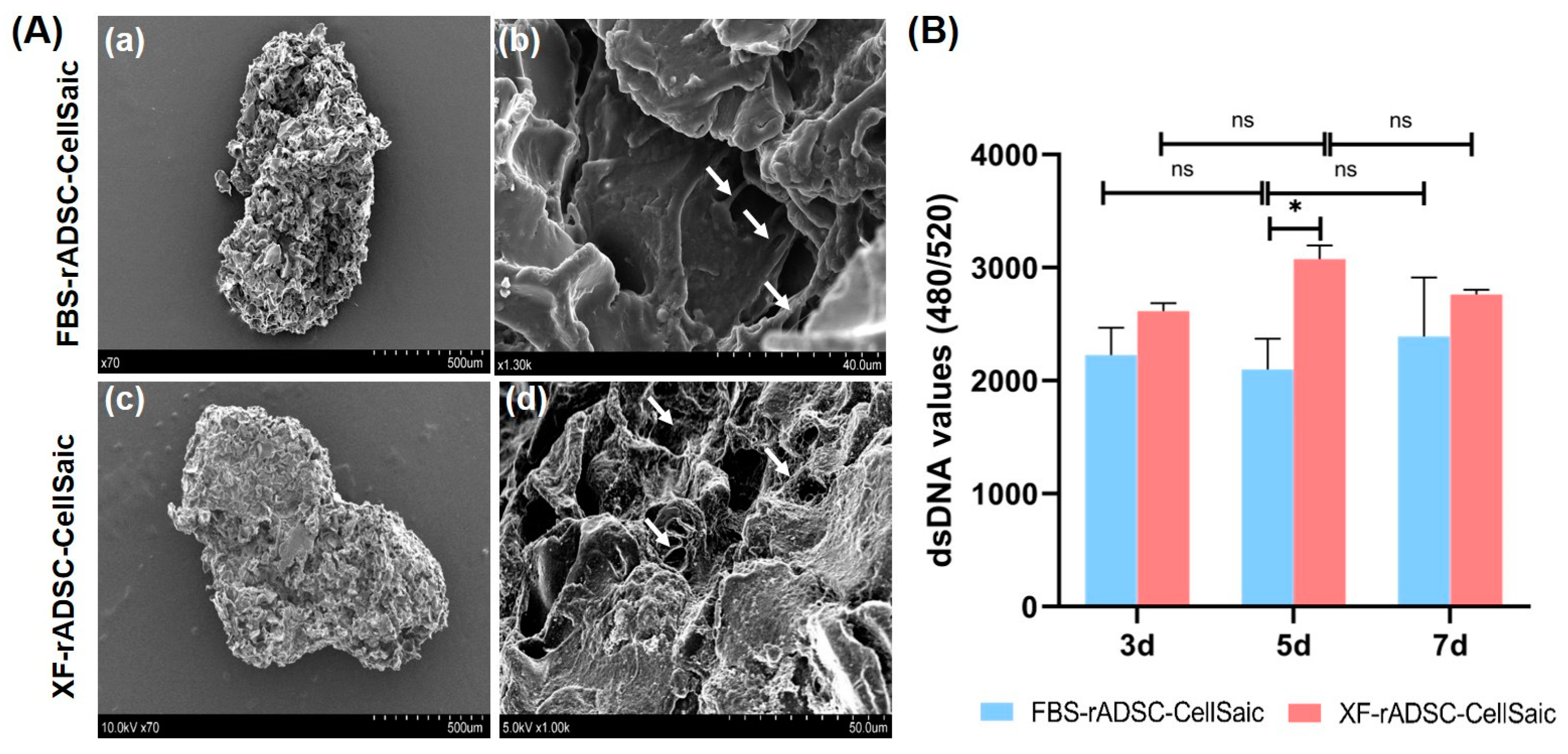
2.2. Formation and Evaluation of 3-Dimensional Aggregates of rADSC and Cellnest (rADSC-CellSaic)
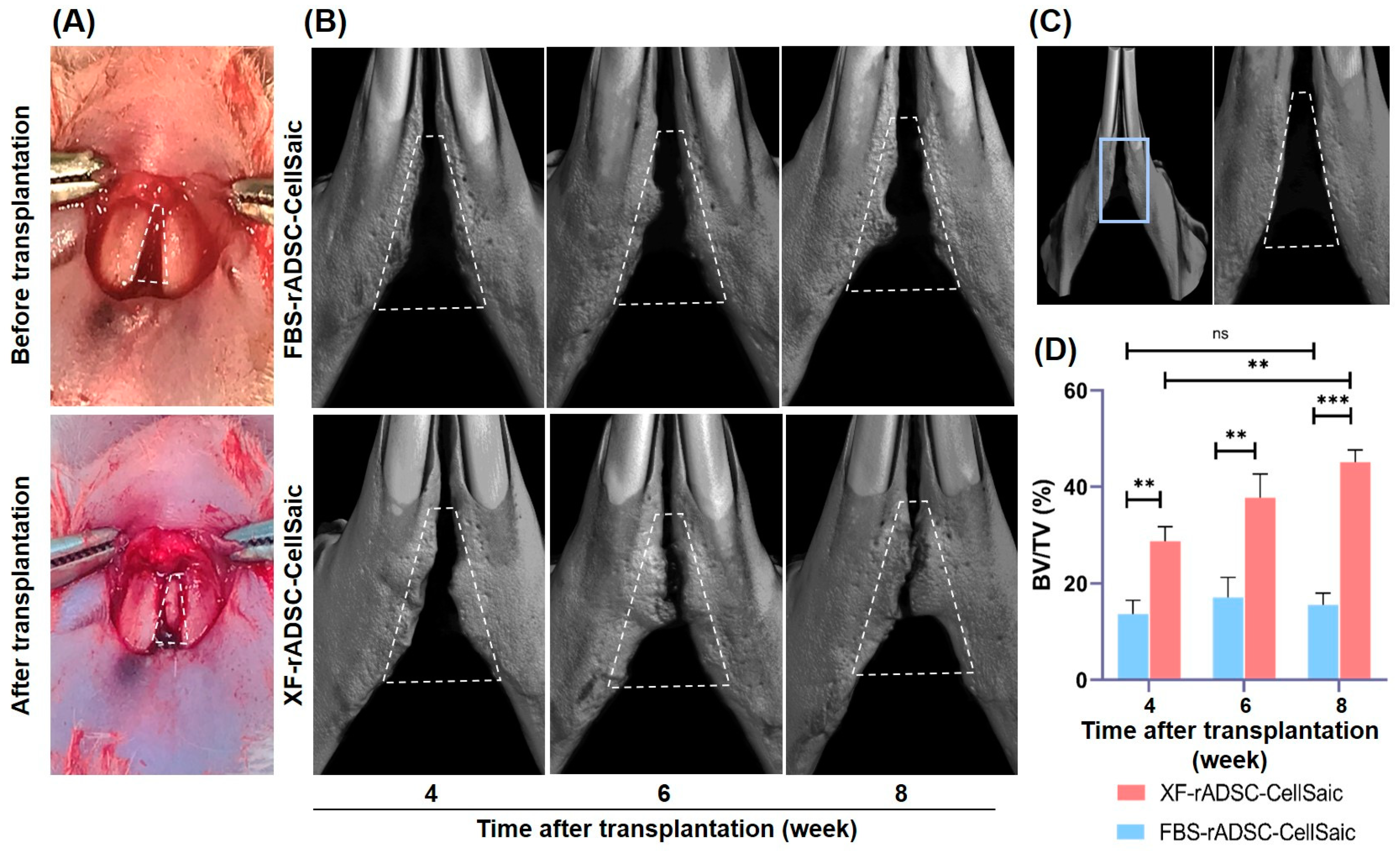
2.3. Bone Formation in Rat Mandibular Congenital Bone Defects by the Transplantation of rADSC-CellSaic after Osteogenic Differentiation
3. Discussion
4. Materials and Methods
4.1. Isolation of rADSC
4.2. Two-Dimensional Culture of rADSC by the Medium with or without Xenogeneic Components
4.3. Preparation and Evaluation of rADSC-CellSaic In Vitro
4.4. Transplantation of rADSC-CellSaic after Osteogenic Differentiation into Rat Mandibular Congenital Bone Defect
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamada, Y.; Nakamura, S.; Ito, K.; Umemura, E.; Hara, K.; Nagasaka, T.; Abe, A.; Baba, S.; Furuichi, Y.; Izumi, Y. Injectable bone tissue engineering using expanded mesenchymal stem cells. Stem Cells 2013, 31, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhang, T.; Lin, Y.; Cai, X. Vascularization in craniofacial bone tissue engineering. J. Dent. Res. 2018, 97, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.; Helder, M.N.; Bravenboer, N.; Ten Bruggenkate, C.M.; Jin, J.; Klein-Nulend, J.; Schulten, E.A. Bone tissue regeneration in the oral and maxillofacial region: A review on the application of stem cells and new strategies to improve vascularization. Stem Cells Int. 2019, 2019, 6279721. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Shang, F.; Yu, Y.; Liu, S.; Ming, L.; Zhang, Y.; Zhou, Z.; Zhao, J.; Jin, Y. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact. Mater. 2021, 6, 666–683. [Google Scholar] [CrossRef]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef]
- Naderi, N.; Combellack, E.J.; Griffin, M.; Sedaghati, T.; Javed, M.; Findlay, M.W.; Wallace, C.G.; Mosahebi, A.; Butler, P.E.; Seifalian, A.M.; et al. The regenerative role of adipose-derived stem cells (ADSC) in plastic and reconstructive surgery. Int. Wound J. 2017, 14, 112–124. [Google Scholar] [CrossRef]
- Yoshida, Y.; Matsubara, H.; Fang, X.; Hayashi, K.; Nomura, I.; Ugaji, S.; Hamada, T.; Tsuchiya, H. Adipose-derived stem cell sheets accelerate bone healing in rat femoral defects. PLoS ONE 2019, 14, e0214488. [Google Scholar] [CrossRef]
- Kinzebach, S.; Bieback, K. Expansion of mesenchymal stem/stromal cells under xenogenic-free culture conditions. In Mesenchymal Stem Cells-Basics and Clinical Application I; Springer: Berlin/Heidelberg, Germany, 2013; pp. 33–57. [Google Scholar]
- Dessels, C.; Potgieter, M.; Pepper, M.S. Making the switch: Alternatives to fetal bovine serum for adipose-derived stromal cell expansion. Front. Cell Dev. Biol. 2016, 4, 115. [Google Scholar] [CrossRef] [PubMed]
- Mackensen, A.; Dräger, R.; Schlesier, M.; Mertelsmann, R.; Lindemann, A. Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol. Immunother. 2000, 49, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Tekkatte, C.; Gunasingh, G.P.; Cherian, K.; Sankaranarayanan, K. “Humanized” stem cell culture techniques: The animal serum controversy. Stem Cells Int. 2011, 2011, 504723. [Google Scholar] [CrossRef] [PubMed]
- Phetfong, J.; Tawonsawatruk, T.; Seenprachawong, K.; Srisarin, A.; Isarankura-Na-Ayudhya, C.; Supokawej, A. Re-using blood products as an alternative supplement in the optimisation of clinical-grade adipose-derived mesenchymal stem cell culture. Bone Jt. Res. 2017, 6, 414–422. [Google Scholar] [CrossRef]
- Schallmoser, K.; Strunk, D. Preparation of pooled human platelet lysate (pHPL) as an efficient supplement for animal serum-free human stem cell cultures. JoVE 2009, 32, e1523. [Google Scholar]
- Azouna, N.B.; Jenhani, F.; Regaya, Z.; Berraeis, L.; Othman, T.B.; Ducrocq, E.; Domenech, J. Phenotypical and functional characteristics of mesenchymal stem cells from bone marrow: Comparison of culture using different media supplemented with human platelet lysate or fetal bovine serum. Stem Cell Res. Ther. 2012, 3, 1–14. [Google Scholar] [CrossRef]
- Khazaei, S.; Keshavarz, G.; Bozorgi, A.; Nazari, H.; Khazaei, M. Adipose tissue-derived stem cells: A comparative review on isolation, culture, and differentiation methods. Cell Tissue Bank. 2022, 23, 1–16. [Google Scholar] [CrossRef]
- Dromard, C.; Bourin, P.; Andre, M.; De Barros, S.; Casteilla, L.; Planat-Benard, V. Human adipose derived stroma/stem cells grow in serum-free medium as floating spheres. Exp. Cell Res. 2011, 317, 770–780. [Google Scholar] [CrossRef]
- Moutos, F.T.; Estes, B.T.; Guilak, F. Multifunctional hybrid three-dimensionally woven scaffolds for cartilage tissue engineering. Macromol. Biosci. 2010, 10, 1355–1364. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, S.; Mei, D.; Li, J.; Zhang, J.; Yang, S.; Guan, S. Application of 3D printing technology in bone tissue engineering: A review. Curr. Drug Deliv. 2021, 18, 847–861. [Google Scholar] [CrossRef]
- Gelmi, A.; Schutt, C.E. Stimuli-Responsive Biomaterials: Scaffolds for Stem Cell Control. Adv. Healthc. Mater. 2021, 10, e2001125. [Google Scholar] [CrossRef] [PubMed]
- Hippler, M.; Lemma, E.D.; Bertels, S.; Blasco, E.; Barner-Kowollik, C.; Wegener, M.; Bastmeyer, M. 3D Scaffolds to Study Basic Cell Biology. Adv. Mater. 2019, 31, e1808110. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Kim, H.D.; Amirthalingam, S.; Kim, S.L.; Lee, S.S.; Rangasamy, J.; Hwang, N.S. Biomimetic Materials and Fabrication Approaches for Bone Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700612. [Google Scholar] [CrossRef]
- Kim, J.J.; Hou, L.; Huang, N.F. Vascularization of three-dimensional engineered tissues for regenerative medicine applications. Acta Biomater. 2016, 41, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Tabata, Y. Preparation of stem cell aggregates with gelatin microspheres to enhance biological functions. Acta Biomater. 2011, 7, 2797–2803. [Google Scholar] [CrossRef]
- Tajima, S.; Tabata, Y. Preparation and functional evaluation of cell aggregates incorporating gelatin microspheres with different degradabilities. J. Tissue Eng. Regen. Med. 2013, 7, 801–811. [Google Scholar] [CrossRef]
- Jeong, S.I.; Ko, E.K.; Yum, J.; Jung, C.H.; Lee, Y.M.; Shin, H. Nanofibrous poly(lactic acid)/hydroxyapatite composite scaffolds for guided tissue regeneration. Macromol. Biosci. 2008, 8, 328–338. [Google Scholar] [CrossRef]
- Abbass, M.M.; El-Rashidy, A.A.; Sadek, K.M.; Moshy, S.E.; Radwan, I.A.; Rady, D.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Hydrogels and dentin–pulp complex regeneration: From the benchtop to clinical translation. Polymers 2020, 12, 2935. [Google Scholar] [CrossRef]
- Lerman, M.J.; Lembong, J.; Muramoto, S.; Gillen, G.; Fisher, J.P. The evolution of polystyrene as a cell culture material. Tissue Eng. Part B Rev. 2018, 24, 359–372. [Google Scholar] [CrossRef]
- Serafin, A.; Culebras, M.; Collins, M.N. Synthesis and evaluation of alginate, gelatin, and hyaluronic acid hybrid hydrogels for tissue engineering applications. Int. J. Biol. Macromol. 2023, 233, 123438. [Google Scholar] [CrossRef]
- Nakamura, K. CellSaic, a cell aggregate-like technology using recombinant peptide pieces for MSC transplantation. Curr. Stem Cell Res. Ther. 2019, 14, 52–56. [Google Scholar] [CrossRef]
- Kogawa, R.; Nakamura, K.; Mochizuki, Y. A new islet transplantation method combining mesenchymal stem cells with recombinant peptide pieces, microencapsulated islets, and mesh bags. Biomedicines 2020, 8, 299. [Google Scholar] [CrossRef]
- Lyu, J.; Hashimoto, Y.; Honda, Y.; Matsumoto, N. Comparison of osteogenic potentials of dental pulp and bone marrow mesenchymal stem cells using the new cell transplantation platform, cellsaic, in a rat congenital cleft-jaw model. Int. J. Mol. Sci. 2021, 22, 9478. [Google Scholar] [CrossRef]
- Sasayama, S.; Hara, T.; Tanaka, T.; Honda, Y.; Baba, S. Osteogenesis of multipotent progenitor cells using the epigallocatechin gallate-modified gelatin sponge scaffold in the rat congenital cleft-jaw model. Int. J. Mol. Sci. 2018, 19, 3803. [Google Scholar] [CrossRef] [PubMed]
- Iwazawa, R.; Kozakai, S.; Kitahashi, T.; Nakamura, K.; Hata, K.-I. The therapeutic effects of adipose-derived stem cells and recombinant peptide pieces on mouse model of DSS colitis. Cell Transplant. 2018, 27, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Shahabipour, F.; Banach, M.; Johnston, T.P.; Pirro, M.; Sahebkar, A. Novel approaches toward the generation of bioscaffolds as a potential therapy in cardiovascular tissue engineering. Int. J. Cardiol. 2017, 228, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Yagyuu, T.; Kirita, T.; Hattori, K.; Tadokoro, M.; Ohgushi, H. Unique and reliable rat model for the assessment of cell therapy: Bone union in the rat mandibular symphysis using bone marrow stromal cells. J. Tissue Eng. Regen. Med. 2015, 9, 276–285. [Google Scholar] [CrossRef]
- Bui, H.T.H.; Nguyen, L.T.; Than, U.T.T. Influences of xeno-free media on mesenchymal stem cell expansion for clinical application. Tissue Eng. Regen. Med. 2021, 18, 15–23. [Google Scholar] [CrossRef]
- Lindroos, B.; Boucher, S.; Chase, L.; Kuokkanen, H.; Huhtala, H.; Haataja, R.; Vemuri, M.; Suuronen, R.; Miettinen, S. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy 2009, 11, 958–972. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Shang, H.; Khurgel, M.; Katz, A. Low serum and serum-free culture of multipotential human adipose stem cells. Cytotherapy 2007, 9, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, X.; Song, W.; Pan, T.; Wang, H.; Ning, T.; Wei, Q.; Xu, H.H.; Wu, B.; Ma, D. Effects of 3-dimensional bioprinting alginate/gelatin hydrogel scaffold extract on proliferation and differentiation of human dental pulp stem cells. J. Endod. 2019, 45, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N.; Wilde, C.; Haque, T.; Francis, W.; Seifalian, A.M.; Thornton, C.A.; Xia, Z.; Whitaker, I.S. Adipogenic differentiation of adipose-derived stem cells in 3-dimensional spheroid cultures (microtissue): Implications for the reconstructive surgeon. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Patrikoski, M.; Juntunen, M.; Boucher, S.; Campbell, A.; Vemuri, M.C.; Mannerström, B.; Miettinen, S. Development of fully defined xeno-free culture system for the preparation and propagation of cell therapy-compliant human adipose stem cells. Stem Cell Res. Ther. 2013, 4, 1–15. [Google Scholar] [CrossRef]
- Cruciani, S.; Santaniello, S.; Montella, A.; Ventura, C.; Maioli, M. Orchestrating stem cell fate: Novel tools for regenerative medicine. World J. Stem Cells 2019, 11, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Kellner, K.; Liebsch, G.; Klimant, I.; Wolfbeis, O.S.; Blunk, T.; Schulz, M.B.; Göpferich, A. Determination of oxygen gradients in engineered tissue using a fluorescent sensor. Biotechnol. Bioeng. 2002, 80, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Tanioka, H.; Miyagawa, S.; Mori, D.; Watanabe, K.I.; Ueno, T.; Toda, K.; Shibuya, T.; Kuratani, T.; Sawa, Y. New cell delivery system CellSaic with adipose-derived stromal cells promotes functional angiogenesis in critical limb ischemia model mice. J. Artif. Organs. 2021, 24, 343–350. [Google Scholar] [CrossRef]
- Garnero, P. The Role of Collagen Organization on the Properties of Bone. Calcif. Tissue Int. 2015, 97, 229–240. [Google Scholar] [CrossRef]
- Ueyama, Y.; Yagyuu, T.; Maeda, M.; Imada, M.; Akahane, M.; Kawate, K.; Tanaka, Y.; Kirita, T. Maxillofacial bone regeneration with osteogenic matrix cell sheets: An experimental study in rats. Arch. Oral Biol. 2016, 72, 138–145. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Jo, J.-I.; Hashimoto, Y. Evaluation of Osteogenic Potential for Rat Adipose-Derived Stem Cells under Xeno-Free Environment. Int. J. Mol. Sci. 2023, 24, 17532. https://doi.org/10.3390/ijms242417532
Sun Y, Jo J-I, Hashimoto Y. Evaluation of Osteogenic Potential for Rat Adipose-Derived Stem Cells under Xeno-Free Environment. International Journal of Molecular Sciences. 2023; 24(24):17532. https://doi.org/10.3390/ijms242417532
Chicago/Turabian StyleSun, Yuzhu, Jun-Ichiro Jo, and Yoshiya Hashimoto. 2023. "Evaluation of Osteogenic Potential for Rat Adipose-Derived Stem Cells under Xeno-Free Environment" International Journal of Molecular Sciences 24, no. 24: 17532. https://doi.org/10.3390/ijms242417532
APA StyleSun, Y., Jo, J.-I., & Hashimoto, Y. (2023). Evaluation of Osteogenic Potential for Rat Adipose-Derived Stem Cells under Xeno-Free Environment. International Journal of Molecular Sciences, 24(24), 17532. https://doi.org/10.3390/ijms242417532




