Cannabidiol at Nanomolar Concentrations Negatively Affects Signaling through the Adenosine A2A Receptor
Abstract
:1. Introduction
2. Results
2.1. Homogenous Binding Assays
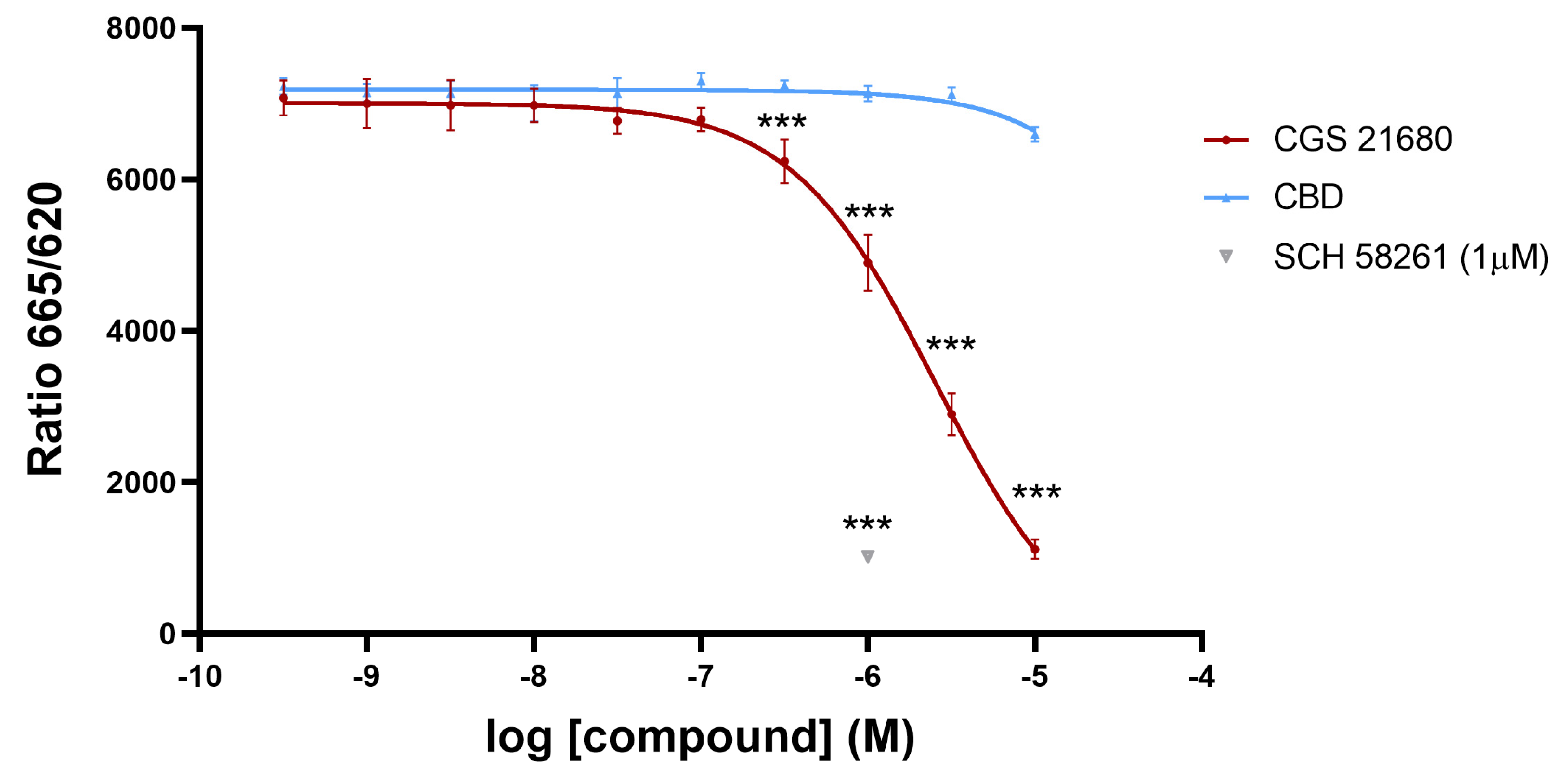
2.2. Signaling Assays
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Transient Transfection
4.3. Homogeneous Time-Resolved Fluorescence (HTRF) Binding Assay
4.4. Non-Radioactive Homogeneous Time-Resolved FRET-Based Binding Assays
4.5. Signal Detection and Data Analysis
4.6. cAMP Determination
4.7. ERK1/2 Phosphorylation Assays
4.8. Data Handling and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, R.; Wolff, H. Structure of Cannabidiol. IV. The Position of the Linkage between the Two Rings. J. Am. Chem. Soc. 1940, 62, 1770–1775. [Google Scholar] [CrossRef]
- Adams, R.; Hunt, M. Structure of Cannabidiol, a Product Isolated from the Marihuana Extract of Minnesota Wild Hemp. I. J. Am. Chem. Soc. 1940, 62, 196–200. [Google Scholar] [CrossRef]
- Mechoulam, R.; Shvo, Y. Hashish. I. The structure of cannabidiol. Tetrahedron 1963, 19, 2073–2078. [Google Scholar] [CrossRef]
- Monti, J.M. Hypnoticlike effects of cannabidiol in the rat. Psychopharmacology 1977, 55, 263–265. [Google Scholar] [CrossRef]
- Warner, W.; Harris, L.S.; Carchman, R.A. Inhibition of cortiocosteroidogenesis by delta-9-tetrahydrocannabinol. Endocrinology 1977, 101, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, H.; Harclerode, J.; Nyquist, S.E. Effects of chronic administration of delta-9-tetrahydrocannabinol and cannabidiol on rat testicular esterase isozymes. Life Sci. 1977, 20, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.R.; O’Sullivan, S.E.; England, T.J. The effects of acute and sustained cannabidiol dosing for seven days on the haemodynamics in healthy men: A randomised controlled trial. Br. J. Clin. Pharmacol. 2020, 86, 1125–1138. [Google Scholar] [CrossRef] [PubMed]
- Dragun, T.; Brown, C.V.; Tulppo, M.P.; Obad, A.; Dujić, Ž. The Influence of Oral Cannabidiol on 24-h Ambulatory Blood Pressure and Arterial Stiffness in Untreated Hypertension: A Double-Blind, Placebo-Controlled, Cross-Over Pilot Study. Adv. Ther. 2023, 40, 3495–3511. [Google Scholar] [CrossRef] [PubMed]
- Resstel, L.B.M.; Tavares, R.F.; Lisboa, S.F.S.; Joca, S.R.L.; Corrêa, F.M.A.; Guimarães, F.S. 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 2009, 156, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.R.; Cinquina, V.; Gómez, A.; Layunta, R.; Santos, M.; Fernández-Ruiz, J.; Martínez-Orgado, J. Cannabidiol administration after hypoxia–ischemia to newborn rats reduces long-term brain injury and restores neurobehavioral function. Neuropharmacology 2012, 63, 776–783. [Google Scholar] [CrossRef]
- Lafuente, H.; Pazos, M.R.; Alvarez, A.; Mohammed, N.; Santos, M.; Arizti, M.; Alvarez, F.J.; Martinez-Orgado, J.A. Effects of cannabidiol and hypothermia on short-term brain damage in new-born piglets after acute hypoxia-ischemia. Front. Neurosci. 2016, 10, 323. [Google Scholar] [CrossRef]
- Ceprián, M.; Vargas, C.; García-Toscano, L.; Penna, F.; Jiménez-Sánchez, L.; Achicallende, S.; Elezgarai, I.; Grandes, P.; Hind, W.; Ruth Pazos, M.; et al. Cannabidiol Administration Prevents Hypoxia-Ischemia-Induced Hypomyelination in Newborn Rats. Front. Pharmacol. 2019, 10, 1131. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Orgado, J.; Villa, M.; del Pozo, A. Cannabidiol for the Treatment of Neonatal Hypoxic-Ischemic Brain Injury. Front. Pharmacol. 2021, 11, 584533. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Bosquez-Berger, T.; Gudorf, J.A.; Kuntz, C.P.; Desmond, J.A.; Schlebach, J.P.; VanNieuwenhze, M.S.; Straiker, A. Structure-Activity Relationship Study of Cannabidiol-Based Analogs as Negative Allosteric Modulators of the μ-Opioid Receptor. J. Med. Chem. 2023, 66, 9466–9494. [Google Scholar] [CrossRef] [PubMed]
- Ibeas Bih, C.; Chen, T.; Nunn, A.V.W.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef] [PubMed]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef]
- Zaccara, G.; Schmidt, D. Antiepileptic Drugs in Clinical Development: Differentiate or Die? Curr. Pharm. Des. 2018, 23, 5593–5605. [Google Scholar] [CrossRef]
- Sultan, S.R.; Millar, S.A.; England, T.J.; O’Sullivan, S.E. A systematic review and meta-analysis of the haemodynamic effects of cannabidiol. Front. Pharmacol. 2017, 8, 245415. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Sagredo, O.; Pazos, M.R.; García, C.; Pertwee, R.; Mechoulam, R.; Martínez-Orgado, J. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013, 75, 323–333. [Google Scholar] [CrossRef]
- Espejo-Porras, F.; Fernández-Ruiz, J.; Pertwee, R.G.; Mechoulam, R.; García, C. Motor effects of the non-psychotropic phytocannabinoid cannabidiol that are mediated by 5-HT1A receptors. Neuropharmacology 2013, 75, 155–163. [Google Scholar] [CrossRef]
- Ružić Zečević, D.; Folić, M.; Tantoush, Z.; Radovanović, M.; Babić, G.; Janković, S.M. Investigational cannabinoids in seizure disorders, what have we learned thus far? Expert Opin. Investig. Drugs 2018, 27, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Marsh, E.; Friedman, D.; Thiele, E.; Laux, L.; Sullivan, J.; Miller, I.; Flamini, R.; Wilfong, A.; Filloux, F.; et al. Cannabidiol in patients with treatment-resistant epilepsy: An open-label interventional trial. Lancet Neurol. 2016, 15, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Wise, J. FDA approves its first cannabis based medicine. BMJ 2018, 361, k2827. [Google Scholar] [CrossRef] [PubMed]
- McGuire, P.; Robson, P.; Cubala, W.J.; Vasile, D.; Morrison, P.D.; Barron, R.; Taylor, A.; Wright, S. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: A multicenter randomized controlled trial. Am. J. Psychiatry 2018, 175, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Massi, P.; Solinas, M.; Cinquina, V.; Parolaro, D. Cannabidiol as potential anticancer drug. Br. J. Clin. Pharmacol. 2013, 75, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, J.; Julian, M.; Carrascosa, A. Role of the Cannabinoid System in Pain Control and Therapeutic Implications for the Management of Acute and Chronic Pain Episodes. Curr. Neuropharmacol. 2006, 4, 239–257. [Google Scholar] [CrossRef]
- Khalsa, J.H.; Bunt, G.; Blum, K.; Maggirwar, S.B.; Galanter, M.; Potenza, M.N. Review: Cannabinoids as Medicinals. Curr. Addict. Rep. 2022, 9, 630–646. [Google Scholar] [CrossRef]
- Rosenberg, E.C.; Louik, J.; Conway, E.; Devinsky, O.; Friedman, D. Quality of Life in Childhood Epilepsy in pediatric patients enrolled in a prospective, open-label clinical study with cannabidiol. Epilepsia 2017, 58, e96–e100. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Felder, C.C.; Glass, M. Cannabinoid receptors and their endogenous agonists. Annu. Rev. Pharmacol. Toxicol. 2003, 38, 179–200. [Google Scholar] [CrossRef]
- McPartland, J.M.; Glass, M.; Pertwee, R.G. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: Interspecies differences. Br. J. Pharmacol. 2007, 152, 583–593. [Google Scholar] [CrossRef]
- Thomas, B.F.; Gilliam, A.F.; Burch, D.F.; Roche, M.J.; Seltzman, H.H. Comparative Receptor Binding Analyses of Cannabinoid Agonists and Antagonists. J. Pharmacol. Exp. Ther. 1998, 285, 285–292. [Google Scholar] [PubMed]
- Navarro, G.; Varani, K.; Lillo, A.; Vincenzi, F.; Rivas-Santisteban, R.; Raïch, I.; Reyes-Resina, I.; Ferreiro-Vera, C.; Borea, P.A.; Sánchez de Medina, V.; et al. Pharmacological data of cannabidiol- and cannabigerol-type phytocannabinoids acting on cannabinoid CB1, CB2 and CB1/CB2 heteromer receptors. Pharmacol. Res. 2020, 159, 104940. [Google Scholar] [CrossRef]
- Martínez-Pinilla, E.; Varani, K.; Reyes-Resina, I.; Angelats, E.; Vincenzi, F.; Ferreiro-Vera, C.; Oyarzabal, J.; Canela, E.I.; Lanciego, J.L.; Nadal, X.; et al. Binding and signaling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2 receptors. Front. Pharmacol. 2017, 8, 744. [Google Scholar] [CrossRef] [PubMed]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Gonzalez, A.; Sánchez-Morales, A.; Casajuana-Martin, N.; Gómez-Ventura, M.; Cordomí, A.; Busqué, F.; Alibés, R.; Pardo, L.; Franco, R. Design of Negative and Positive Allosteric Modulators of the Cannabinoid CB2 Receptor Derived from the Natural Product Cannabidiol. J. Med. Chem. 2021, 64, 9354–9364. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Ferraz-De-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Mariano-Souza, D.P.; Quinteiro-Filho, W.M.; Akamine, A.T.; Almeida, V.I.; Quevedo, J.; Dal-Pizzol, F.; et al. Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: Role for the adenosine A(2A) receptor. Eur. J. Pharmacol. 2012, 678, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Carrier, E.J.; Auchampach, J.A.; Hillard, C.J. Inhibition of an equilibrative nucleoside transporter by cannabidiol: A mechanism of cannabinoid immunosuppression. Proc. Natl. Acad. Sci. USA 2006, 103, 7895–7900. [Google Scholar] [CrossRef]
- Pacheco, R.; Martinez-Navio, J.M.M.; Lejeune, M.; Climent, N.; Oliva, H.; Gatell, J.M.M.; Gallart, T.; Mallol, J.; Lluis, C.; Franco, R. CD26, adenosine deaminase, and adenosine receptors mediate costimulatory signals in the immunological synapse. Proc. Natl. Acad. Sci. USA 2005, 102, 9583–9588. [Google Scholar] [CrossRef]
- Beamer, E.; Gölöncsér, F.; Horváth, G.; Bekő, K.; Otrokocsi, L.; Koványi, B.; Sperlágh, B. Purinergic mechanisms in neuroinflammation: An update from molecules to behavior. Neuropharmacology 2016, 104, 94–104. [Google Scholar] [CrossRef]
- Haskó, G.; Pacher, P.; Vizi, E.S.; Illes, P. Adenosine receptor signaling in the brain immune system. Trends Pharmacol. Sci. 2005, 26, 511–516. [Google Scholar] [CrossRef]
- Ohta, A.; Sitkovsky, M. Methylxanthines, inflammation, and cancer: Fundamental mechanisms. Handb. Exp. Pharmacol. 2011, 200, 469–481. [Google Scholar] [CrossRef]
- Vincenzi, F.; Corciulo, C.; Targa, M.; Casetta, I.; Gentile, M.; Granieri, E.; Borea, P.A.; Popoli, P.; Varani, K. A2A adenosine receptors are up-regulated in lymphocytes from amyotrophic lateral sclerosis patients. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, F.; Corciulo, C.; Targa, M.; Merighi, S.; Gessi, S.; Casetta, I.; Gentile, M.; Granieri, E.; Borea, P.A.; Varani, K. Multiple sclerosis lymphocytes upregulate A2A adenosine receptors that are antiinflammatory when stimulated. Eur. J. Immunol. 2013, 43, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Miyamoto, M.; Miyamoto, T.; Uchiyama, T.; Watanabe, Y.; Suzuki, S.; Kadowaki, T.; Fujita, H.; Matsubara, T.; Sakuramoto, H.; et al. Istradefylline improves daytime sleepiness in patients with Parkinson’s disease: An open-label, 3-month study. J. Neurol. Sci. 2017, 380, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.A.; Winnick, A.; Welschmeyer, A.; Kaneb, A.; Berardino, K.; Cornett, E.M.; Kaye, A.D.; Viswanath, O.; Urits, I. Istradefylline to treat patients with parkinson’s disease experiencing “off” episodes: A comprehensive review. Neurol. Int. 2020, 12, 109–129. [Google Scholar] [CrossRef]
- Mizuno, Y.; Kondo, T. Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson’s disease. Mov. Disord. 2013, 28, 1138–1141. [Google Scholar] [CrossRef]
- Cunha, R.A. How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem. 2016, 139, 1019–1055. [Google Scholar] [CrossRef]
- Gui, L.; Duan, W.; Tian, H.; Li, C.; Zhu, J.; Chen, J.F.; Zheng, J. Adenosine A2Areceptor deficiency reduces striatal glutamate outflow and attenuates brain injury induced by transient focal cerebral ischemia in mice. Brain Res. 2009, 1297, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W. Purines and neuroprotection. Adv. Exp. Med. Biol. 2002, 513, 249–280. [Google Scholar] [CrossRef] [PubMed]
- Nobre, H.V., Jr.; de Andrade Cunha, G.M.; de Vasconcelos, L.M.; Magalhães, H.I.F.; Neto, R.N.O.; Maia, F.D.; de Moraes, M.O.; Leal, L.K.A.M.; de Barros Viana, G.S. Caffeine and CSC, adenosine A2A antagonists, offer neuroprotection against 6-OHDA-induced neurotoxicity in rat mesencephalic cells. Neurochem. Int. 2010, 56, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-F.; Sonsalla, P.K.; Pedata, F.; Melani, A.; Domenici, M.R.; Popoli, P.; Geiger, J.; Lopes, L.V.; de Mendonça, A. Adenosine A2A receptors and brain injury: Broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Prog. Neurobiol. 2007, 83, 310–331. [Google Scholar] [CrossRef] [PubMed]
- Pedata, F.; Pugliese, A.M.; Melani, A.; Gianfriddo, M. A2A receptors in neuroprotection of dopaminergic neurons. Neurology 2003, 61, S49–S50. [Google Scholar] [CrossRef]
- Xu, K.; Di Luca, D.G.; Orrú, M.; Xu, Y.; Chen, J.-F.; Schwarzschild, M.A. Neuroprotection by caffeine in the MPTP model of parkinson’s disease and its dependence on adenosine A 2A receptors. Neuroscience 2016, 322, 129–137. [Google Scholar] [CrossRef]
- Saura, J.; Angulo, E.; Ejarque, A.; Casado, V.; Tusell, J.M.; Moratalla, R.; Chen, J.-F.F.; Schwarzschild, M.A.; Lluis, C.; Franco, R.; et al. Adenosine A2A receptor stimulation potentiates nitric oxide release by activated microglia. J. Neurochem. 2005, 95, 919–929. [Google Scholar] [CrossRef]
- Abbracchio, M.P.; Cattabeni, F. Brain adenosine receptors as targets for therapeutic intervention in neurodegenerative diseases. Ann. N. Y. Acad. Sci. 1999, 890, 79–92. [Google Scholar] [CrossRef]
- Oñatibia-Astibia, A.; Franco, R.; Martínez-Pinilla, E. Health benefits of methylxanthines in neurodegenerative diseases. Mol. Nutr. Food Res. 2017, 61, 1600670. [Google Scholar] [CrossRef]
- Schwarzschild, M.A.; Chen, J.-F.; Ascherio, A. Caffeinated clues and the promise of adenosine A(2A) antagonists in PD. Neurology 2002, 58, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Lunet, N.; Santos, C.; Santos, J.; Vaz-Carneiro, A. Caffeine exposure and the risk of Parkinson’s disease: A systematic review and meta-analysis of observational studies. J. Alzheimer’s Dis. 2010, 20 (Suppl. S1), S221–S238. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Guo, X.; Park, Y.; Huang, X.; Sinha, R.; Freedman, N.D.; Hollenbeck, A.R.; Blair, A.; Chen, H. Caffeine intake, smoking, and risk of parkinson disease in men and women. Am. J. Epidemiol. 2012, 175, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; O’Reilly, É.J.; Hughes, K.C.; Gao, X.; Schwarzschild, M.A.; Ascherio, A. Differences in Parkinson’s Disease Risk with Caffeine Intake and Postmenopausal Hormone Use. J. Park. Dis. 2017, 7, 677–684. [Google Scholar] [CrossRef]
- Eskelinen, M.H.; Kivipelto, M. Caffeine as a protective factor in dementia and Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 20, S167–S174. [Google Scholar] [CrossRef] [PubMed]
- Sindi, S.; Kåreholt, I.; Eskelinen, M.; Hooshmand, B.; Lehtisalo, J.; Soininen, H.; Ngandu, T.; Kivipelto, M. Healthy dietary changes in midlife are associated with reduced dementia risk later in life. Nutrients 2018, 10, 1649. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, M.H.; Ngandu, T.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Midlife coffee and tea drinking and the risk of late-life dementia: A population-based CAIDE study. J. Alzheimer’s Dis. 2009, 16, 85–91. [Google Scholar] [CrossRef]
- Lindsay, J.; Laurin, D.; Verreault, R.; Hébert, R.; Helliwell, B.; Hill, G.B.; McDowell, I. Risk factors for Alzheimer’s disease: A prospective analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002, 156, 445–453. [Google Scholar] [CrossRef]
- Maia, L.; De Mendonça, A. Does caffeine intake protect from Alzheimer’s disease? Eur. J. Neurol. 2002, 9, 377–382. [Google Scholar] [CrossRef]
- Santos, C.; Costa, J.; Santos, J.; Vaz-Carneiro, A.; Lunet, N. Caffeine intake and dementia: Systematic review and meta-analysis. J. Alzheimer’s Dis. 2010, 20 (Suppl. S1), S187–S204. [Google Scholar] [CrossRef]
- Merighi, S.; Travagli, A.; Nigro, M.; Pasquini, S.; Cappello, M.; Contri, C.; Varani, K.; Vincenzi, F.; Borea, P.A.; Gessi, S. Caffeine for Prevention of Alzheimer’s Disease: Is the A2A Adenosine Receptor Its Target? Biomolecules 2023, 13, 967. [Google Scholar] [CrossRef]
- Willingham, S.B.; Hotson, A.N.; Miller, R.A. Targeting the A2AR in cancer; early lessons from the clinic. Curr. Opin. Pharmacol. 2020, 53, 126–133. [Google Scholar] [CrossRef]
- Sitkovsky, M.V.; Hatfield, S.; Abbott, R.; Belikoff, B.; Lukashev, D.; Ohta, A. Hostile, Hypoxia-A2-Adenosinergic Tumor Biology as the Next Barrier to Overcome for Tumor Immunologists. Cancer Immunol. Res. 2014, 2, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.; Kini, R.; Ohta, A.; Subramanian, M.; Madasu, M.; Sitkovsky, M. The development and immunosuppressive functions of CD4+ CD25+ FoxP3+ regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front. Immunol. 2012, 3, 190. [Google Scholar] [CrossRef]
- Franco, R.; Rivas-Santisteban, R.; Navarro, G.; Reyes-Resina, I. Adenosine Receptor Antagonists to Combat Cancer and to Boost Anti-Cancer Chemotherapy and Immunotherapy. Cells 2021, 10, 2831. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, S.M.; Sitkovsky, M. A2A adenosine receptor antagonists to weaken the hypoxia-HIF-1α driven immunosuppression and improve immunotherapies of cancer. Curr. Opin. Pharmacol. 2016, 29, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T. Protean Agonists. Ann. N. Y. Acad. Sci. 1997, 812, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Seidel, M.G.; Klinger, M.; Freissmuth, M.; Höller, C. Activation of mitogen-activated protein kinase by the A(2A)-adenosine receptor via a rap1-dependent and via a p21(ras)-dependent pathway. J. Biol. Chem. 1999, 274, 25833–25841. [Google Scholar] [CrossRef]
- Dickenson, J.M.; Blank, J.L.; Hill, S.J. Human adenosine A1 receptor and P2Y2-purinoceptor-mediated activation of the mitogen-activated protein kinase cascade in transfected CHO cells. Br. J. Pharmacol. 1998, 124, 1491–1499. [Google Scholar] [CrossRef]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef]
- Fronik, P.; Gaiser, B.I.; Sejer Pedersen, D. Bitopic Ligands and Metastable Binding Sites: Opportunities for G Protein-Coupled Receptor (GPCR) Medicinal Chemistry. J. Med. Chem. 2017, 60, 4126–4134. [Google Scholar] [CrossRef]
- Dror, R.O.; Pan, A.C.; Arlow, D.H.; Borhani, D.W.; Maragakis, P.; Shan, Y.; Xu, H.; Shaw, D.E. Pathway and mechanism of drug binding to G-protein-coupled receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 13118–13123. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, A.; Blackmer, T. G-protein-coupled receptor pharmacology: Examining the edges between theory and proof. Curr. Opin. Drug Discov. Dev. 2007, 10, 446–451. [Google Scholar]
- Serrano-Marín, J.; Reyes-Resina, I.; Martínez-Pinilla, E.; Navarro, G.; Franco, R. Natural Compounds as Guides for the Discovery of Drugs Targeting G-Protein-Coupled Receptors. Molecules 2020, 25, 5060. [Google Scholar] [CrossRef] [PubMed]
- Shonberg, J.; Kling, R.C.; Gmeiner, P.; Löber, S. GPCR crystal structures: Medicinal chemistry in the pocket. Bioorg. Med. Chem. 2015, 23, 3880–3906. [Google Scholar] [CrossRef]
- Goupil, E.; Laporte, S.A.; Hébert, T.E. A Simple Method to Detect Allostery in GPCR Dimers. Methods Cell Biol. 2013, 117, 165–179. [Google Scholar] [CrossRef]
- Gomes, I.; IJzerman, A.P.; Ye, K.; Maillet, E.L.; Devi, L.A. G Protein-Coupled Receptor Heteromerization: A Role in Allosteric Modulation of Ligand Binding. Mol. Pharmacol. 2011, 79, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fernández, N.; Gómez-Acero, L.; Sarasola, L.I.; Argerich, J.; Chevigné, A.; Jacobson, K.A.; Ciruela, F.; Fernández-Dueñas, V.; Aso, E. Cannabidiol negatively modulates adenosine A2A receptor functioning in living cells. Acta Neuropsychiatr. 2023, 1–5. [Google Scholar] [CrossRef]
- Gao, Z.G.; Toti, K.S.; Campbell, R.; Suresh, R.R.; Yang, H.; Jacobson, K.A. Allosteric Antagonism of the A2A Adenosine Receptor by a Series of Bitopic Ligands. Cells 2020, 9, 1200. [Google Scholar] [CrossRef]
- Hua, T.; Li, X.; Wu, L.; Iliopoulos-Tsoutsouvas, C.; Wang, Y.; Wu, M.; Shen, L.; Johnston, C.A.; Nikas, S.P.; Song, F.; et al. Activation and Signaling Mechanism Revealed by Cannabinoid Receptor-Gi Complex Structures. Cell 2020, 180, 655–665.e18. [Google Scholar] [CrossRef]
- Xing, C.; Zhuang, Y.; Xu, T.H.; Feng, Z.; Zhou, X.E.; Chen, M.; Wang, L.; Meng, X.; Xue, Y.; Wang, J.; et al. Cryo-EM Structure of the Human Cannabinoid Receptor CB2-Gi Signaling Complex. Cell 2020, 180, 645–654.e13. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hua, T.; Vemuri, K.; Ho, J.H.; Wu, Y.; Wu, L.; Popov, P.; Benchama, O.; Zvonok, N.; Locke, K.; et al. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell 2019, 176, 459–467.e13. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Navarro, G.; Gómez-Autet, M.; Redondo, L.; Fernández-Ruiz, J.; Pérez-Benito, L.; Cordomí, A.; Pardo, L.; Franco, R.; Jagerovic, N. Discovery of Homobivalent Bitopic Ligands of the Cannabinoid CB2 Receptor. Chem. Eur. J. 2020, 26, 15839–15842. [Google Scholar] [CrossRef]
- Stanley, N.; Pardo, L.; Fabritiis, G. De The pathway of ligand entry from the membrane bilayer to a lipid G protein-coupled receptor. Sci. Rep. 2016, 6, 22639. [Google Scholar] [CrossRef]
- Jaakola, V.-P.; Griffith, M.T.; Hanson, M.A.; Cherezov, V.; Chien, E.Y.T.; Lane, J.R.; Ijzerman, A.P.; Stevens, R.C. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 2008, 322, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wu, H.; Katritch, V.; Han, G.W.; Jacobson, K.A.; Gao, Z.-G.; Cherezov, V.; Stevens, R.C. Structure of an agonist-bound human A2A adenosine receptor. Science 2011, 332, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Massink, A.; Gutiérrez-de-Terán, H.; Lenselink, E.B.; Ortiz Zacarías, N.V.; Xia, L.; Heitman, L.H.; Katritch, V.; Stevens, R.C.; IJzerman, A.P. Sodium ion binding pocket mutations and adenosine A2A receptor function. Mol. Pharmacol. 2015, 87, 305–313. [Google Scholar] [CrossRef]
- Nadal, X. Methods of Purifying Cannabinoids, Compositions and Kits Thereof. U.S. Patent 9,765,000, 19 September 2017. [Google Scholar]
- Navarro, G.; Cordomí, A.; Brugarolas, M.; Moreno, E.; Aguinaga, D.; Pérez-Benito, L.; Ferre, S.; Cortés, A.; Casadó, V.; Mallol, J.; et al. Cross-communication between Gi and Gs in a G-protein-coupled receptor heterotetramer guided by a receptor C-terminal domain. BMC Biol. 2018, 16, 1–15. [Google Scholar] [CrossRef]
- Medrano, M.; Aguinaga, D.; Reyes-Resina, I.; Canela, E.I.; Mallol, J.; Navarro, G.; Franco, R. Orexin A/Hypocretin Modulates Leptin Receptor-Mediated Signaling by Allosteric Modulations Mediated by the Ghrelin GHS-R1A Receptor in Hypothalamic Neurons. Mol. Neurobiol. 2018, 55, 4718–4730. [Google Scholar] [CrossRef]
- Martinez-Pinilla, E.; Rabal, O.; Reyes-Resina, I.; Zamarbide, M.; Navarro, G.; Sanchez-Arias, J.A.; de Miguel, I.; Lanciego, J.L.; Oyarzabal, J.; Franco, R. Two Affinity Sites of the Cannabinoid Subtype 2 Receptor Identified by a Novel Homogeneous Binding Assay. J. Pharmacol. Exp. Ther. 2016, 358, 580–587. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raïch, I.; Lillo, J.; Ferreiro-Vera, C.; Sánchez de Medina, V.; Navarro, G.; Franco, R. Cannabidiol at Nanomolar Concentrations Negatively Affects Signaling through the Adenosine A2A Receptor. Int. J. Mol. Sci. 2023, 24, 17500. https://doi.org/10.3390/ijms242417500
Raïch I, Lillo J, Ferreiro-Vera C, Sánchez de Medina V, Navarro G, Franco R. Cannabidiol at Nanomolar Concentrations Negatively Affects Signaling through the Adenosine A2A Receptor. International Journal of Molecular Sciences. 2023; 24(24):17500. https://doi.org/10.3390/ijms242417500
Chicago/Turabian StyleRaïch, Iu, Jaume Lillo, Carlos Ferreiro-Vera, Verónica Sánchez de Medina, Gemma Navarro, and Rafael Franco. 2023. "Cannabidiol at Nanomolar Concentrations Negatively Affects Signaling through the Adenosine A2A Receptor" International Journal of Molecular Sciences 24, no. 24: 17500. https://doi.org/10.3390/ijms242417500
APA StyleRaïch, I., Lillo, J., Ferreiro-Vera, C., Sánchez de Medina, V., Navarro, G., & Franco, R. (2023). Cannabidiol at Nanomolar Concentrations Negatively Affects Signaling through the Adenosine A2A Receptor. International Journal of Molecular Sciences, 24(24), 17500. https://doi.org/10.3390/ijms242417500






