Effect of Caffeine on the Inflammatory-Dependent Changes in the GnRH/LH Secretion in a Female Sheep Model
Abstract
1. Introduction
2. Results
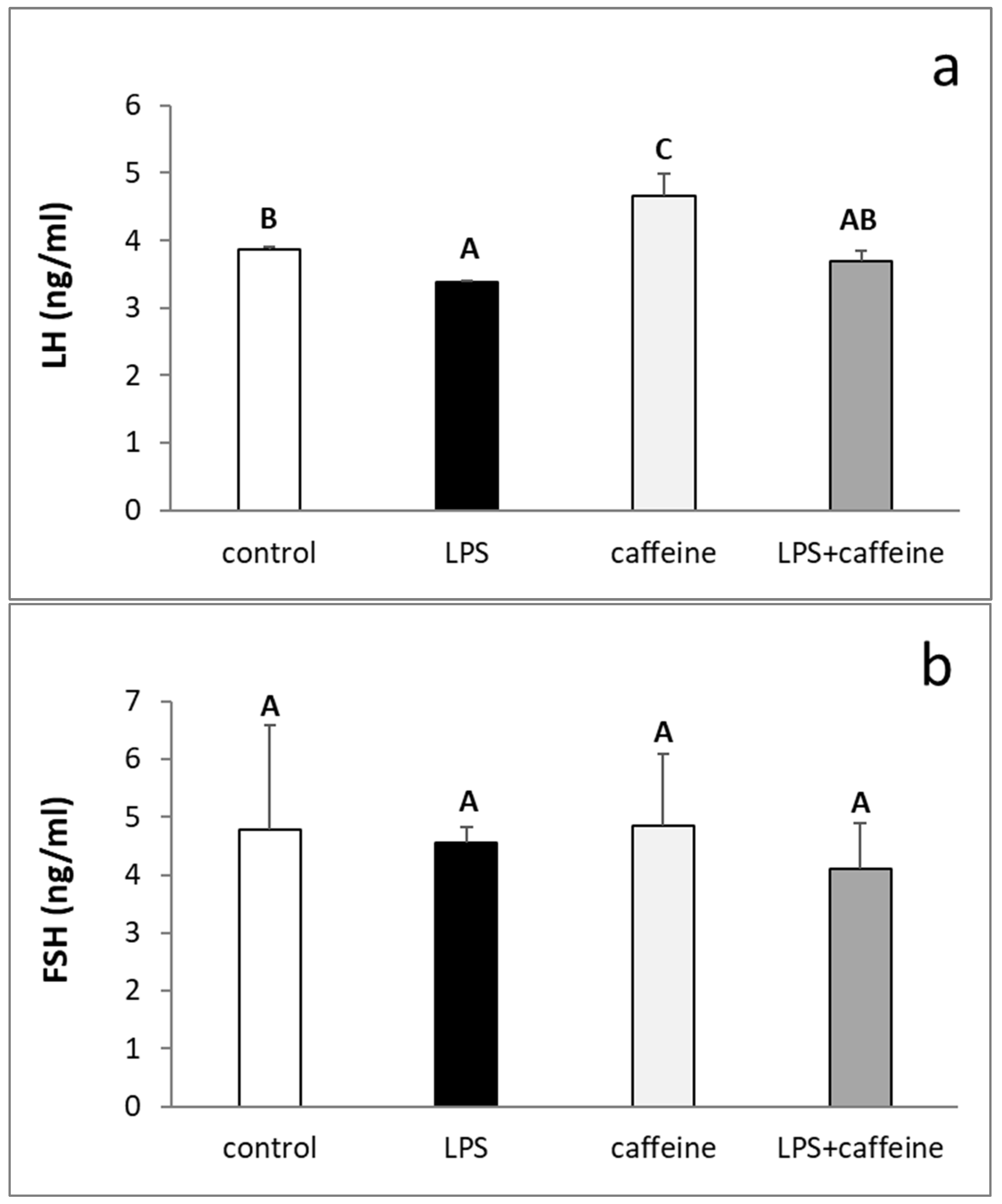
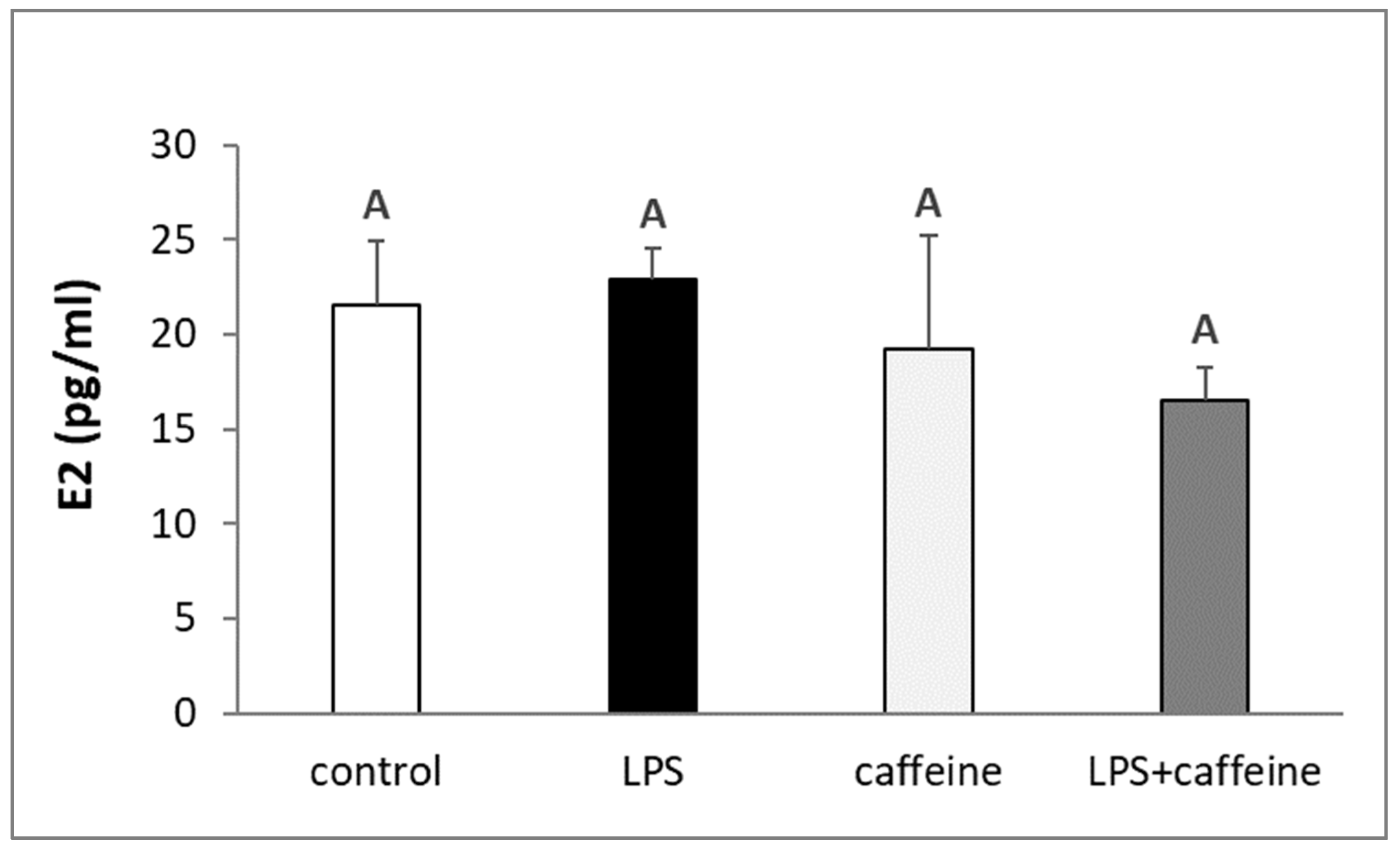
2.1. Effect of Caffeine and LPS Administration on the Circulating Concentration of LH, FSH, Estradiol, and Cortisol
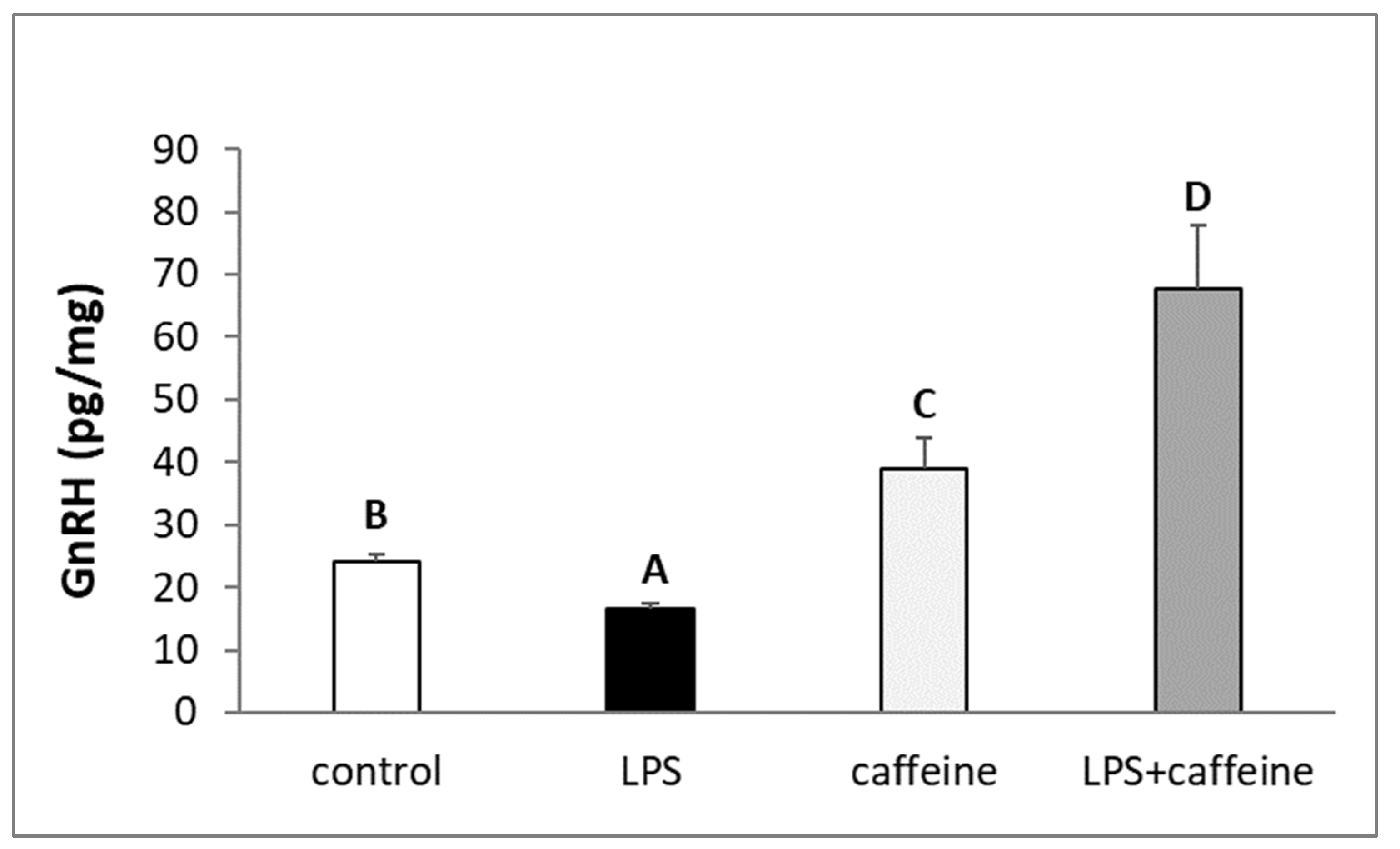
2.2. Effect of Caffeine and LPS Administration on GnRH Content in the Preoptic Area of the Hypothalamus
2.3. Effect of Caffeine and LPS Administration on the Concentration of Neurotransmitters Regulating the Release of GnRH and Their Metabolites in the Median Eminence
2.4. Effect of Caffeine and LPS Administration on the Gene Expression in the Hypothalamus and Anterior Pituitary
3. Discussion
3.1. Effect of Caffeine on the GnRH/LH Secretion in Non-LPS Treated Ewes
3.2. Influence of Caffeine on the Synthesis of Neurotransmitters Involved in the Regulation of GnRH Secretion in the Hypothalamus
3.3. Possible Involvement of Cortisol in Caffeine-Mediated Effect on GnRH/LH Secretion
3.4. The Effect of Inflammation on the Responsiveness of the Hypothalamic–Pituitary Unit to Caffeine Action
3.5. Pharmacokinetics of Caffeine
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Assays
4.2.1. Radioimmunoassay of Hormones
4.2.2. ELISA Assay for the GnRH and Estradiol
4.2.3. HPLC Assays for Neurotransmitters and Their Metabolites
4.2.4. Relative Gene Expression Assay
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uccella, S.; Dottermusch, M.; Erickson, L.; Warmbier, J.; Montone, K.; Saeger, W. Inflammatory and infectious disorders in endocrine pathology. Endocr. Pathol. 2023, 34, 406–436. [Google Scholar] [CrossRef] [PubMed]
- Druvefors, E.; Landerholm, K.; Hammar, U.; Myrelid, P.; Andersson, R.E. Impaired fertility in women with inflammatory bowel disease: A national cohort study from Sweden. J. Crohns. Colitis. 2021, 15, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Goldsmith, L.T.; Taylor, R.N.; Bellet, D.; Taylor, H.S. Inflammation in reproductive disorders. Reprod. Sci. 2009, 16, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, M.; Cronin, J.G.; Healey, G.D.; Gabler, C.; Heuwieser, W.; Streyl, D.; Bromfield, J.J.; Miyamoto, A.; Fergani, C.; Dobson, H. Innate immunity and inflammation of the bovine female reproductive tract in health and disease. Reproduction 2014, 148, R41–R51. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.P.; Krawczyńska, A.; Bochenek, J.; Haziak, K.; Romanowicz, K.; Misztal, T.; Antushevich, H.; Herman, A.; Tomaszewska-Zaremba, D. The effect of rivastigmine on the LPS-induced suppression of GnRH/LH secretion during the follicular phase of the estrous cycle in ewes. Anim. Reprod. Sci. 2013, 138, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Haziak, K.; Herman, A.P.; Wojtulewicz, K.; Pawlina, B.; Paczesna, K.; Bochenek, J.; Tomaszewska-Zaremba, D. Effect of CD14/TLR4 antagonist on GnRH/LH secretion in ewe during central inflammation induced by intracerebroventricular administration of LPS. J. Anim. Sci. Biotechnol. 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.P.; Misztal, T.; Romanowicz, K.; Tomaszewska-Zaremba, D. Central injection of exogenous IL-1β in the control activities of hypothalamic-pituitary-gonadal axis in anestrous ewes. Reprod. Domest. Anim. 2012, 47, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.P.; Skipor, J.; Krawczyńska, A.; Bochenek, J.; Wojtulewicz, K.; Antushevich, H.; Herman, A.; Paczesna, K.; Romanowicz, K.; Tomaszewska-Zaremba, D. Peripheral inhibitor of AChE, neostigmine, prevents the inflammatory dependent suppression of GnRH/LH secretion during the follicular phase of the estrous cycle. BioMed Res. Int. 2017, 2017, 6823209. [Google Scholar] [CrossRef]
- Tomaszewska-Zaremba, D.; Herman, A. The role of immunological system in the regulation of gonadoliberin and gonadotropin secretion. Reprod. Biol. 2009, 9, 11–23. [Google Scholar] [CrossRef]
- Fürst, R.; Zündorf, I. Plant-derived anti-inflammatory compounds: Hopes and disappointments regarding the translation of preclinical knowledge into clinical progress. Mediat. Inflamm. 2014, 2014, 146832. [Google Scholar] [CrossRef]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The safety of ingested caffeine: A comprehensive review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef]
- Saraiva, S.M.; Jacinto, T.A.; Gonçalves, A.C.; Gaspar, D.; Silva, L.R. Overview of caffeine effects on human health and emerging delivery strategies. Pharmaceuticals 2023, 16, 1067. [Google Scholar] [CrossRef]
- Markova, E.V.; Knyazheva, M.A.; Tikhonova, M.A.; Amstislavskaya, T.G. Structural and functional characteristics of the hippocampus in depressive-like recipients after transplantation of in vitro caffeine-modulated immune cells. Neurosci. Lett. 2022, 786, 136790. [Google Scholar] [CrossRef]
- Szczepkowska, A.; Bochenek, J.; Wójcik, M.; Tomaszewska-Zaremba, D.; Antushevich, H.; Tomczyk, M.; Skipor, J.; Herman, A.P. Effect of caffeine on adenosine and ryanodine receptor gene expression in the hypothalamus, pituitary, and choroid plexus in ewes under basal and LPS challenge conditions. J. Anim. Feed. Sci. 2023, 32, 17–23. [Google Scholar] [CrossRef]
- Caldani, M.; Batailler, M.; Thiéry, J.C.; Dubois, M.P. LHRH immunoreactive structures in the sheep brain. Histochemistry 1988, 89, 129–139. [Google Scholar] [CrossRef]
- Klonoff-Cohen, H.; Bleha, J.; Lam-Kruglick, P. A prospective study of the effects of female and male caffeine consumption on the reproductive endpoints of IVF and gamete intra-Fallopian transfer. Hum. Reprod. 2002, 17, 1746–1754. [Google Scholar] [CrossRef]
- Wilcox, A.; Weinberg, C.; Baird, D. Caffeinated beverages and decreased fertility. Lancet 1988, 2, 1453–1456. [Google Scholar] [CrossRef]
- Ezzat, A.R.; el-Gohary, Z.M. Hormonal and histological effects of chronic caffeine administration on the pituitary-gonadal and pituitary-adrenocortical axes in male rabbits. Funct. Dev. Morphol. 1994, 4, 45–50. [Google Scholar]
- Oluwole, O.F.; Salami, S.A.; Ogunwole, E.; Raji, Y. Implication of caffeine consumption and recovery on the reproductive functions of adult male Wistar rats. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 483–491. [Google Scholar] [CrossRef]
- Dorostghoal, M.; Majd, N.E.; Nooraei, P. Maternal caffeine consumption has irreversible effects on reproductive parameters and fertility in male offspring rats. Clin. Exp. Reprod. Med. 2012, 39, 144–152. [Google Scholar] [CrossRef]
- Bu, F.L.; Feng, X.; Yang, X.Y.; Ren, J.; Cao, H.J. Relationship between caffeine intake and infertility: A systematic review of controlled clinical studies. BMC Womens Health. 2020, 20, 125. [Google Scholar] [CrossRef]
- Scaramuzzi, R.J.; Downing, J.A.; Williamson, S.; Pollard, I. The circulating concentrations of FSH, LH and prolactin in the oestradiol-implanted ovariectomized ewe treated with caffeine. Anim. Reprod. Sci. 1997, 45, 273–282. [Google Scholar] [CrossRef]
- Rawlings, N.C.; Jeffcoate, I.A.; Currie, W.D.; Cook, S.J. Control of the surge release of LH and FSH in estradiol- and progesterone-treated ovariectomized ewes. Can. J. Anim. Sci. 1988, 68, 1089–1096. [Google Scholar] [CrossRef]
- Pollock, B.G.; Wylie, M.; Stack, J.A.; Sorisio, D.A.; Thompson, D.S.; Kirshner, M.A.; Folan, M.M.; Condifer, K.A. Inhibition of caffeine metabolism by estrogen replacement therapy in postmenopausal women. J. Clin. Pharmacol. 1999, 39, 936–940. [Google Scholar] [CrossRef]
- Xu, K.; Xu, Y.; Brown-Jermyn, D.; Chen, J.F.; Ascherio, A.; Dluzen, D.E.; Schwarzschild, M.A. Estrogen prevents neuroprotection by caffeine in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J. Neurosci. 2006, 26, 535–541. [Google Scholar] [CrossRef]
- Lucero, J.; Harlow, B.L.; Barbieri, R.L.; Sluss, P.; Cramer, D.W. Early follicular phase hormone levels in relation to patterns of alcohol, tobacco, and coffee use. Fertil. Steril. 2001, 76, 723–729. [Google Scholar] [CrossRef]
- Schliep, K.C.; Schisterman, E.F.; Mumford, S.L.; Pollack, A.Z.; Zhang, C.; Ye, A.; Stanford, J.B.; Hammoud, A.O.; Porucznik, C.A.; Wactawski-Wende, J. Caffeinated beverage intake and reproductive hormones among premenopausal women in the BioCycle Study. Am. J. Clin. Nutr. 2012, 95, 488–497. [Google Scholar] [CrossRef]
- Herman, A.P.; Herman, A.; Skipor, J.; Krawczyńska, A.; Bochenek, J.; Tomaszewska-Zaremba, D. Caffeine stimulates in vitro pituitary LH secretion in lipopolysaccharide-treated ewes. Reprod. Biol. 2015, 15, 20–26. [Google Scholar] [CrossRef]
- McCall, A.L.; Millington, W.R.; Wurtman, R.J. Blood-brain barrier transport of caffeine: Dose-related restriction of adenine transport. Life Sci. 1982, 31, 2709–2715. [Google Scholar] [CrossRef]
- Lin, Z.; Jiang, D.; Liu, P.; Ge, Y.; Moghekar, A.; Lu, H. Blood–brain barrier permeability in response to caffeine challenge. Magn. Reson. Med. 2022, 88, 2259–2266. [Google Scholar] [CrossRef]
- Cao, Y.J.; Peng, Y.Y. Caffeine and carbonyl cyanide m-chlorophenylhydrazone increased evoked and spontaneous release of luteinizing hormone-releasing hormone from intact presynaptic terminals. Neuroscience 1999, 92, 1511–1521. [Google Scholar] [CrossRef]
- Uemura, T.; Nishimura, J.; Yamaguchi, H.; Hiruma, H.; Kimura, F.; Minaguchi, H. Effects of noradrenaline on GnRH-secreting immortalized hypothalamic (GT1-7) neurons. Endocr. J. 1997, 44, 73–78. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Herbison, A.E. Dopamine regulation of gonadotropin-releasing hormone neuron excitability in male and female mice. Endocrinology 2013, 154, 340–350. [Google Scholar] [CrossRef]
- Ciechanowska, M.; Łapot, M.; Paruszewska, E.; Radawiec, W.; Przekop, F. The influence of dopaminergic system inhibition on biosynthesis of gonadotrophin-releasing hormone (GnRH) and GnRH receptor in anoestrous sheep; hierarchical role of kisspeptin and RFamide-related peptide-3 (RFRP-3). Reprod. Fertil. Dev. 2018, 30, 672–680. [Google Scholar] [CrossRef]
- Kim, H.S.; Yumkham, S.; Choi, J.H.; Son, G.H.; Kim, K.; Ryu, S.H.; Suh, P. Serotonin stimulates GnRH secretion through the c-Src-PLC γ1 pathway in GT1–7 hypothalamic cells. J. Endocrinol. 2006, 190, 581–591. [Google Scholar] [CrossRef]
- Bhattarai, J.P.; Roa, J.; Herbison, A.E.; Han, S.K. Serotonin acts through 5-HT1 and 5-HT2 receptors to exert biphasic actions on GnRH neuron excitability in the mouse. Endocrinology 2014, 155, 513–524. [Google Scholar] [CrossRef]
- Lovallo, W.R.; Farag, N.H.; Vincent, A.S.; Thomas, T.L.; Wilson, M.F. Cortisol responses to mental stress, exercise, and meals following caffeine intake in men and women. Pharmacol. Biochem. Behav. 2006, 83, 441–447. [Google Scholar] [CrossRef]
- Lovallo, W.R.; Whitsett, T.L.; Al’absi, M.; Sung, B.H.; Vincent, A.S.; Wilson, M.F. Caffeine stimulation of cortisol secretion across the waking hours in relation to caffeine intake levels. Psychosom. Med. 2005, 67, 734–739. [Google Scholar] [CrossRef]
- Oakley, A.E.; Breen, K.M.; Clarke, I.J.; Karsch, F.J.; Wagenmaker, E.R.; Tilbrook, A.J. Cortisol reduces gonadotropin-releasing hormone pulse frequency in follicular phase ewes: Influence of ovarian steroids. Endocrinology 2009, 150, 341–349. [Google Scholar] [CrossRef]
- Phumsatitpong, C.; Wagenmaker, E.R.; Moenter, S.M. Neuroendocrine interactions of the stress and reproductive axes. Front. Neuroendocrinol. 2021, 63, 100928. [Google Scholar] [CrossRef]
- Rodovalho-Callegari, F.V.; Rodrigues-Santos, I.; Lucion, A.B.; Rodovalho, G.V.; Leite, C.M.; De Paula, B.B.; Pestana-Oliveira, N.; Anselmo-Franci, J.A. Acute stress anticipates and amplifies the luteinizing hormone pre-ovulatory surge in rats: Role of noradrenergic neurons. Brain Res. 2022, 1781, 147805. [Google Scholar] [CrossRef]
- Szczepkowska, A.; Wójcik, M.; Tomaszewska-Zaremba, D.; Antushevich, H.; Krawczyńska, A.; Wiechetek, W.; Skipor, J.; Herman, A.P. Acute Effect of caffeine on the synthesis of pro-inflammatory cytokines in the hypothalamus and choroid plexus during endotoxin-induced inflammation in a female sheep model. Int. J. Mol. Sci. 2021, 22, 13237. [Google Scholar] [CrossRef] [PubMed]
- Horrigan, L.A.; Kelly, J.P.; Connor, T.J. Caffeine suppresses TNF-alpha production via activation of the cyclic AMP/protein kinase A pathway. Int. Immunopharmacol. 2004, 4, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Lazzara, F.; Romano, G.L.; Platania, C.B.M.; Drago, F.; Bucolo, C. Caffeine protects against retinal inflammation. Front. Pharmacol. 2022, 12, 824885. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Shi, R.; Guo, H. Tumor necrosis factor α reduces gonadotropin-releasing hormone release through increase of forkhead box protein O1 activity. Neuroreport 2020, 31, 473–477. [Google Scholar] [CrossRef]
- Barabás, K.; Szabó-Meleg, E.; Ábrahám, I.M. Effect of inflammation on female gonadotropin-releasing hormone (GnRH) neurons: Mechanisms and consequences. Int. J. Mol. Sci. 2020, 21, 529. [Google Scholar] [CrossRef]
- Finlay, J.M.; Zigmond, M.J. The effects of stress on central dopaminergic neurons: Possible clinical implications. Neurochem. Res. 1997, 22, 1387–1394. [Google Scholar] [CrossRef]
- Buhr, T.J.; Reed, C.H.; Wee, O.M.; Lee, J.H.; Yuan, L.L.; Fleshner, M.; Valentine, R.J.; Clark, P.J. The persistence of stress-induced physical inactivity in rats: An investigation of central monoamine neurotransmitters and skeletal muscle oxidative stress. Front. Behav. Neurosci. 2023, 17, 1169151. [Google Scholar] [CrossRef]
- Sharma, H.S. Blood–brain and spinal cord barriers in stress. In Blood-Spinal Cord and Brain Barriers in Health and Disease; Sharma, H.S., Westman, J., Eds.; Academic Press: Cambridge, MA, USA, 2004; pp. 231–298. [Google Scholar]
- Counis, R.; Garrel, G.; Laverriere, J.N.; Simon, V.; Bleux, C.; Magre, S.; Cohen-Tannoudji, J. The GnRH receptor and the response of gonadotrope cells to GnRH pulse frequency code. A story of an atypical adaptation of cell function relying on a lack of receptor homologous desensitization. Folia Histochem. Cytobiol. 2009, 47, S81–S87. [Google Scholar] [CrossRef]
- Grzegorzewski, J.; Bartsch, F.; Köller, A.; König, M. Pharmacokinetics of caffeine: A systematic analysis of reported data for application in metabolic phenotyping and liver function testing. Front. Pharmacol. 2022, 12, 752826. [Google Scholar] [CrossRef]
- Szlapinski, S.K.; Charrette, A.; Guthrie, N.; Hilmas, C.J. Paraxanthine safety and comparison to caffeine. Front. Toxicol. 2023, 5, 1117729. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Purpura, M.; Wells, S.D.; Liao, K.; Godavarthi, A. Paraxanthine supplementation increases muscle mass, strength, and endurance in mice. Nutrients 2022, 14, 893. [Google Scholar] [CrossRef] [PubMed]
- Horrigan, L.A.; Kelly, J.P.; Connor, T.J. Immunomodulatory effects of caffeine: Friend or foe? Pharmacol. Ther. 2006, 111, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Sugimoto, N.; Katakura, M.; Matsuzaki, K.; Tanigami, H.; Yachie, A.; Ohno-Shosaku, T.; Shido, O. Theobromine up-regulates cerebral brain-derived neurotrophic factor and facilitates motor learning in mice. J. Nutr. Biochem. 2017, 39, 110–116. [Google Scholar] [CrossRef]
- Martínez-Pinilla, E.; Oñatibia-Astibia, A.; Franco, R. The relevance of theobromine for the beneficial effects of cocoa consumption. Front. Pharmacol. 2015, 6, 30. [Google Scholar] [CrossRef]
- Jilani, T.N.; Preuss, C.V.; Sharma, S. Theophylline. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Müller, C.E.; Jacobson, K.A. Xanthines as adenosine receptor antagonists. Handb. Exp. Pharmacol. 2011, 200, 151–199. [Google Scholar]
- Mascali, J.J.; Cvietusa, P.; Negri, J.; Borish, L. Anti-inflammatory effects of theophylline: Modulation of cytokine production. Ann. Allergy Asthma Immunol. 1996, 77, 34–38. [Google Scholar] [PubMed]
- Pollard, I.; Williamson, S.; Downing, J.; Scaramuzzi, R. Pharmacokinetics of caffeine in the oestrogen-implanted ovariectomized ewe. J. Vet. Pharmacol. Ther. 1996, 19, 113–117. [Google Scholar] [CrossRef]
- Wójcik, M.; Zięba, D.A.; Tomczyk, M.; Bochenek, J.; Antushevich, H.; Krawczyńska, A.; Herman, A.P. Time-dependent effect of inflammation on the gene expression of pro-inflammatory cytokines and their receptors at the different levels of the somatotropic axis in ewe. J. Anim. Feed. Sci. 2023, 32, 400–412. [Google Scholar] [CrossRef]
- Stupnicki, R.; Madej, A. Radioimmunoassay of LH in blood plasma of farm animals. Endokrynologie 1976, 68, 6–13. [Google Scholar]
- L’Hermite, M.; Niswender, G.D.; Reichert, L.E.; Midgley, A.R. Serum follicle stimulating hormone in sheep as measured by radioimmunoassay. Biol. Reprod. 1972, 6, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Kokot, F.; Stupnicki, R. Metody Radioimmunologiczne i Radiokompetyncyjne Stosowane w Klinice, 2nd ed.; PZWL: Warsaw, Poland, 1985. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Bruce, B. Comprehensive Neurochemical Profiling of Brain Tissue Samples; Thermo Sciebtific Application Note 1060; Bruce Bailey Thermo Fisher Scientific: Chelmsford, MA, USA, 2016. [Google Scholar]
- Wojtulewicz, K.; Tomczyk, M.; Wójcik, M.; Bochenek, J.; Antushevich, H.; Krawczyńska, A.; Herman, A.P. Circadian and seasonal changes in the expression of clock genes in the ovine pars tuberalis. J. Anim. Feed. Sci. 2023, 32, 363–371. [Google Scholar] [CrossRef]

| Control | LPS | Caffeine | LPS + Caffeine | |
|---|---|---|---|---|
| Norepinephrine (NE) | 6.24 ± 0.93 A | 8.85 ± 1.52 A | 18.30 ± 4.87 B | 14.03 ± 3.10 AB |
| Dopamine (DA) | 13.60 ± 1.65 A | 22.17 ± 2.74 B | 23.00 ± 3.00 B | 35.54 ± 5.08 C |
| 5-Hydroxyindoleacetic Acid (5-HIAA) | 0.66 ± 0.13 A | 1.09 ± 0.11 A | 1.11 ± 0.29 A | 1.12 ± 0.19 A |
| Homovanillic acid (HVA) | 1.63 ± 0.14 A | 3.05 ± 0.26 B | 1.75 ± 0.25 A | 2.67 ± 0.49 B |
| Serotonin (5-HT) | 0.35 ± 0.06 A | 0.37 ± 0.03 A | 0.72 ± 0.09 B | 0.43 ± 0.03 A |
| Tryptophan (TRP) | 33.45 ± 2.45 A | 64.64 ± 2.63 B | 30.44 ± 5.80 A | 69.88 ± 8.40 B |
| Anterior Pituitary | ||||
|---|---|---|---|---|
| Gene | Control | LPS | Caffeine | LPS + Caffeine |
| GnRHR | 1.00 ± 0.2 B | 0.44 ± 0.1 A | 0.74 ± 0.08 B | 0.49 ± 0.09 A |
| LHβ | 1.00 ± 0.09 B | 0.64 ± 0.04 A | 1.38 ± 0.11 C | 0.89 ± 0.07 AB |
| FSHβ | 1.00 ± 0.27 A | 0.91 ± 0.18 A | 1.18 ± 0.46 A | 1.13 ± 0.45 A |
| GnRH Relative Gene Expression | ||||
|---|---|---|---|---|
| Hypothalamic Structure | Control | LPS | Caffeine | LPS + Caffeine |
| POA | 1.00 ± 0.05 B | 0.76 ± 0.03 A | 1.11 ± 0.02 C | 1.30 ± 0.13 BC |
| AHA | 1.00 ± 0.35 A | 1.00 ± 0.31 A | 0.85 ± 0.20 A | 0.82 ± 0.28 A |
| MBH | 1.00 ± 0.28 A | 1.12 ± 0.41 A | 0.81 ± 0.25 A | 0.55 ± 0.15 A |
| ME | 1.00 ± 0.28 B | 0.30 ± 0.05 A | 1.98 ± 0.16 C | 1.48 ± 0.12 BC |
| Group | No. of Animals | Experimental Treatment I | Dose [ng/kg] | Experimental Treatment II | Dose [mg/kg] |
|---|---|---|---|---|---|
| control | 6 | NaCl | 0 | NaCl | 0 |
| LPS | 6 | LPS | 400 | NaCl | 0 |
| caffeine | 6 | NaCl | 0 | caffeine | 30 |
| LPS + caffeine | 6 | LPS | 400 | caffeine | 30 |
| GenBank Acc. No. | Gene | Amplicon Size [bp] | Forward/Reverse | Sequence 5′ → 3′ | Reference |
|---|---|---|---|---|---|
| NM_001034034 | GAPDH glyceraldehyde-3-phosphate dehydrogenase | 134 | forward | AGAAGGCTGGGGCTCACT | [6] |
| reverse | GGCATTGCTGACAATCTTGA | ||||
| U39357 | ACTB actin beta | 168 | forward | CTTCCTTCCTGGGCATGG | [6] |
| reverse | GGGCAGTGATCTCTTTCTGC | ||||
| BC108088.1 | HDAC1 histone deacetylase1 | 115 | forward | CTGGGGACCTACGGGATATT | [8] |
| reverse | GACATGACCGGCTTGAAAAT | ||||
| NM_001009397 | GnRHR gonadotropin-releasing hormone receptor | 150 | forward | TCTTTGCTGGACCACAGTTAT | [6] |
| reverse | GGCAGCTGAAGGTGAAAAAG | ||||
| U02517 | GnRH gonadotropin-releasing hormone | 123 | forward | GCCCTGGAGGAAAGAGAAAT | [6] |
| reverse | GAGGAGAATGGGACTGGTGA | ||||
| X52488 | LHB luteinizing hormone beta-subunit | 184 | forward | AGATGCTCCAGGGACTGCT | [8] |
| reverse | TGCTTCATGCTGAGGCAGTA | ||||
| X15493 | FSHB follicle-stimulating hormone beta-subunit | 131 | forward | TATTGCTACACCCGGGACTT | [8] |
| reverse | TACAGGGAGTCTGCATGGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herman, A.P.; Tomczyk, M.; Wójcik, M.; Bochenek, J.; Antushevich, H.; Herman, A.; Wiechetek, W.; Szczepkowska, A.; Marciniak, E.; Tomaszewska-Zaremba, D. Effect of Caffeine on the Inflammatory-Dependent Changes in the GnRH/LH Secretion in a Female Sheep Model. Int. J. Mol. Sci. 2024, 25, 2663. https://doi.org/10.3390/ijms25052663
Herman AP, Tomczyk M, Wójcik M, Bochenek J, Antushevich H, Herman A, Wiechetek W, Szczepkowska A, Marciniak E, Tomaszewska-Zaremba D. Effect of Caffeine on the Inflammatory-Dependent Changes in the GnRH/LH Secretion in a Female Sheep Model. International Journal of Molecular Sciences. 2024; 25(5):2663. https://doi.org/10.3390/ijms25052663
Chicago/Turabian StyleHerman, Andrzej Przemysław, Monika Tomczyk, Maciej Wójcik, Joanna Bochenek, Hanna Antushevich, Anna Herman, Wiktoria Wiechetek, Aleksandra Szczepkowska, Elżbieta Marciniak, and Dorota Tomaszewska-Zaremba. 2024. "Effect of Caffeine on the Inflammatory-Dependent Changes in the GnRH/LH Secretion in a Female Sheep Model" International Journal of Molecular Sciences 25, no. 5: 2663. https://doi.org/10.3390/ijms25052663
APA StyleHerman, A. P., Tomczyk, M., Wójcik, M., Bochenek, J., Antushevich, H., Herman, A., Wiechetek, W., Szczepkowska, A., Marciniak, E., & Tomaszewska-Zaremba, D. (2024). Effect of Caffeine on the Inflammatory-Dependent Changes in the GnRH/LH Secretion in a Female Sheep Model. International Journal of Molecular Sciences, 25(5), 2663. https://doi.org/10.3390/ijms25052663






