Enhancement of the Rates for Insertion of Zinc(II) Ions into a Cationic Porphyrin Catalyzed by Poly(glutamate)
Abstract
:1. Introduction
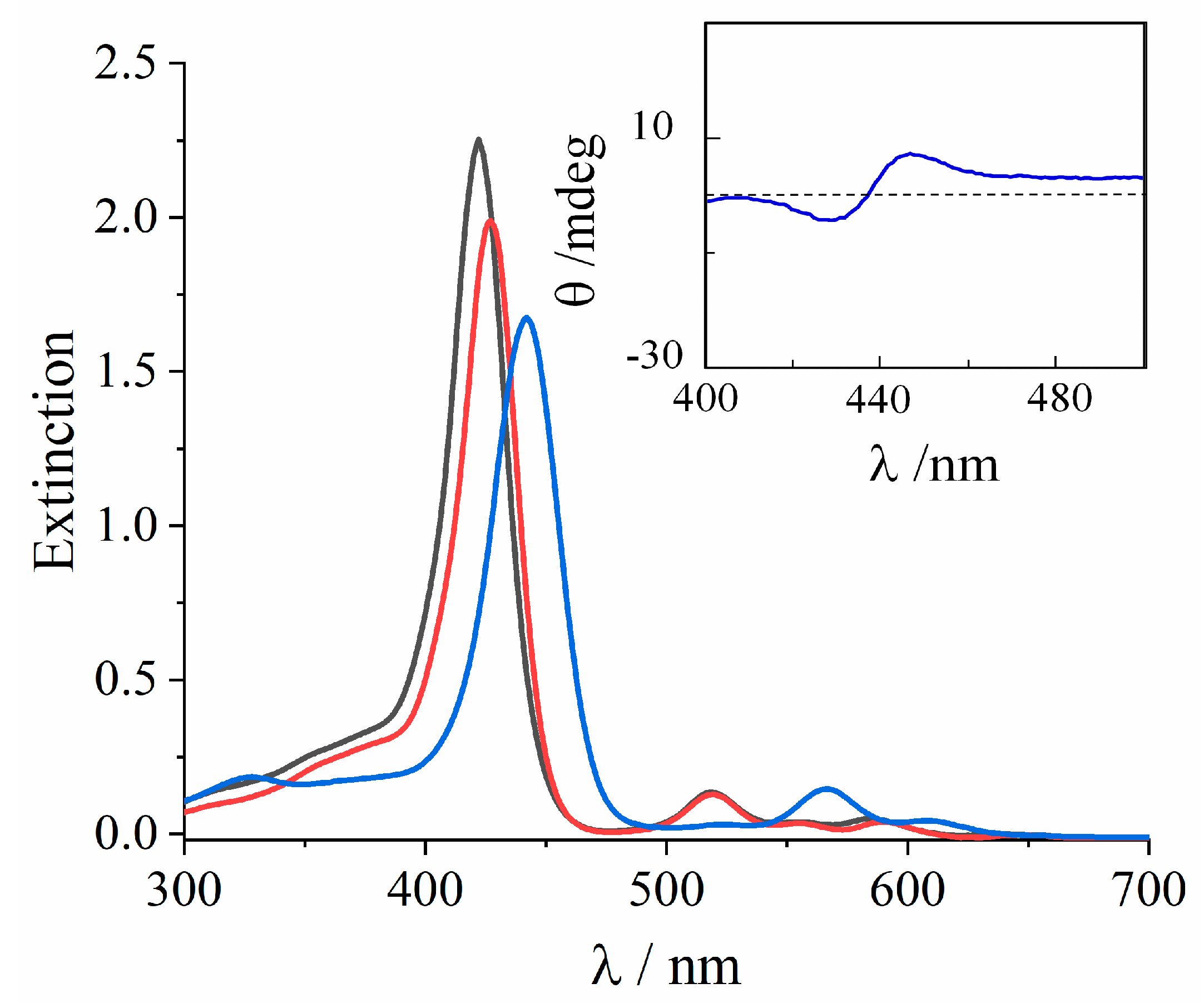
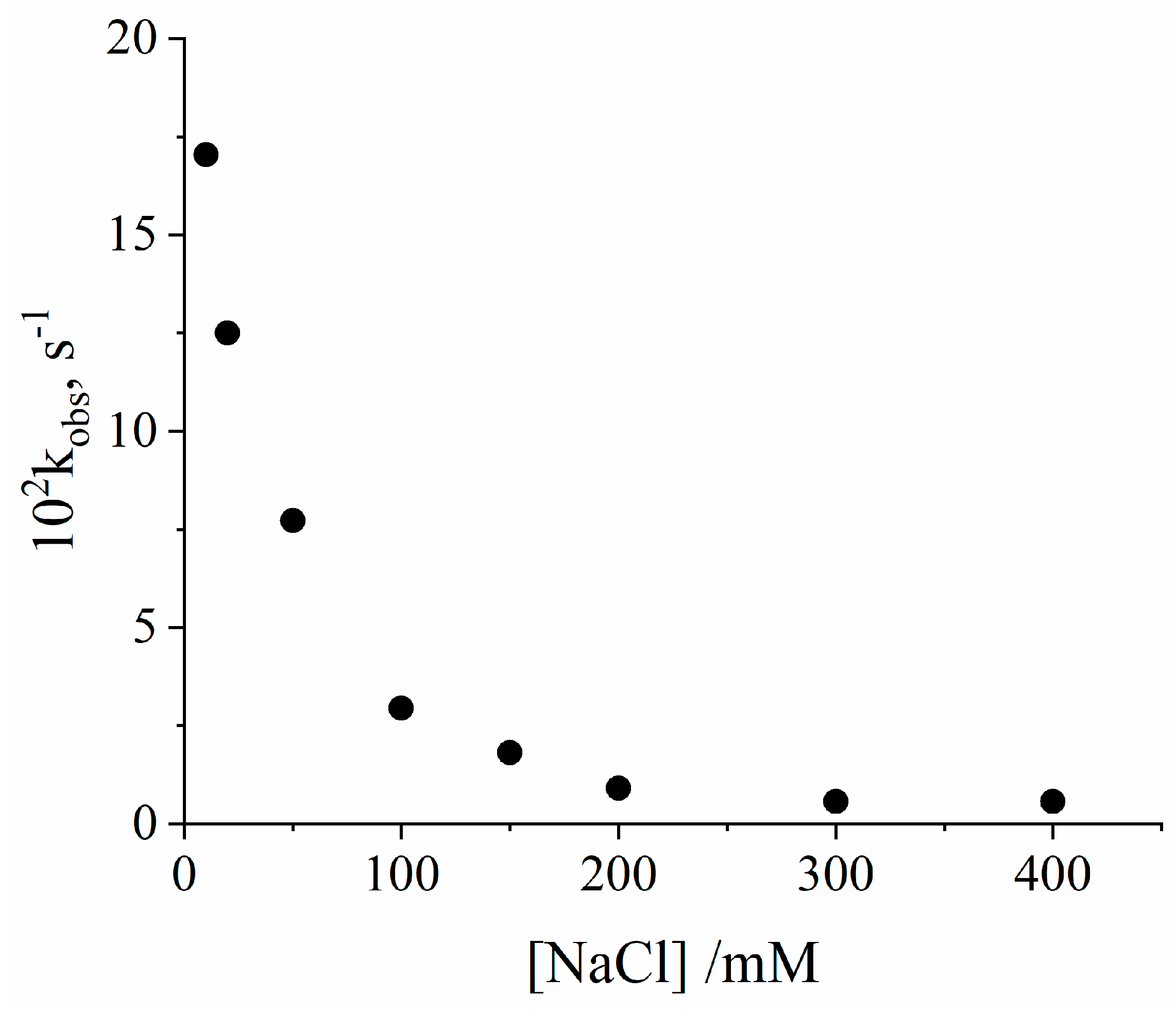
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hambright, P. Chemistry of Water-Soluble Porphyrins. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: New York, NY, USA, 2000; Volume 3, pp. 129–210. [Google Scholar]
- Schneider, W. Kinetics and mechanism of metalloporphyrin formation. In Proceedings of the Biochemistry; Springer: Berlin/Heidelberg, Germany, 1975; pp. 123–166. [Google Scholar]
- Funahashi, S.; Inada, Y.; Inamo, M. Dynamic study of metal-ion incorporation into porphyrins based on the dynamic characterization of metal ions and on sitting-atop complex formation. Anal. Sci. 2001, 17, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Pasternack, R.F.; Vogel, G.C.; Skowronek, C.A.; Harris, R.K.; Miller, J.G. Copper(II) incorporation into tetraphenylporphine in dimethylsulfoxide. Inorg. Chem. 1981, 20, 3763–3765. [Google Scholar] [CrossRef]
- Baker, H.; Hambrigh, P.; Wagner, L.; Ross, L. Metal-ion interactions with porphyrins exchange and substitution-reactions. Inorg. Chem. 1973, 12, 2200–2202. [Google Scholar] [CrossRef]
- Hambright, P. Coordination chemistry of metalloporphyrins. Coord. Chem. Rev. 1971, 6, 247–268. [Google Scholar] [CrossRef]
- Kassner, R.J.; Wang, J.H. Kinetic Studies on the Incorporation of Iron(II) into Porphyrins. J. Am. Chem. Soc. 1966, 88, 5170–5173. [Google Scholar] [CrossRef]
- Stein, T.P.; Plane, R.A. Incorporation of zinc ion into a synthetic water-soluble porphyrin. J. Am. Chem. Soc. 1969, 91, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Occhiuto, I.; De Luca, G.; Trapani, M.; Scolaro, L.M.; Pasternack, R.F. Peripheral Stepwise Degradation of a Porphyrin J-Aggregate. Inorg. Chem. 2012, 51, 10074–10076. [Google Scholar] [CrossRef]
- Trapani, M.; Occhiuto, I.G.; Zagami, R.; De Luca, G.; Castriciano, M.A.; Romeo, A.; Scolaro, L.M.; Pasternack, R.F. Mechanism for Copper(II)-Mediated Disaggregation of a Porphyrin J-Aggregate. Acs Omega 2018, 3, 18843–18848. [Google Scholar] [CrossRef]
- Pasternack, R.F.; Gibbs, E.J.; Santucci, R.; Schaertel, S.; Ellinas, P.; Mah, S.C. Influence of dna on the rate of porphyrin metalation. J. Am. Chem. Soc.—Chem. Commun. 1987, 23, 1771–1774. [Google Scholar] [CrossRef]
- Morawetz, H. Chemical reaction rates reflecting physical properties of polymer solutions. Acc. Chem. Res. 1970, 3, 354. [Google Scholar] [CrossRef]
- Manning, G.S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q. Rev. Biophys. 1978, 11, 179–246. [Google Scholar] [CrossRef] [PubMed]
- Morawetz, H.; Shafer, J.A. Characterization of counterion distribution in polyelectrolyte solutions. ii. the effect of the distribution of electrostatic potential on the solvolysis of cationic esters in polymeric acid solution1,2. J. Phys. Chem. 1963, 67, 1293–1297. [Google Scholar] [CrossRef]
- Bellacchio, E.; Gurrieri, S.; Lauceri, R.; Magrì, A.; Scolaro, L.M.; Purrello, R.; Romeo, A. Nanomolar determination of copper(II) and zinc(II) using supramolecular complexes of meso-tetrakis(4-N-methylpyridyl)porphin on polyglutamate. Chem. Commun. 1998, 13, 1333–1334. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, D.; Liang, R.P.; Qiu, J.D. Nitrogen-Doped Graphene Quantum Dots as a New Catalyst Accelerating the Coordination Reaction between Cadmium(II) and 5,10,15,20-Tetrakis(1-methyl-4-pyridinio)porphyrin for Cadmium(II) Sensing. Anal. Chem. 2015, 87, 10894–10901. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Li, Y.Q.; Huang, Z.C.; Liang, R.P.; Qiu, J.D.; Liu, J.W. Efficient DNA-Catalyzed Porphyrin Metalation for Fluorescent Ratiometric Pb2+Detection. Anal. Chem. 2019, 91, 11403–11408. [Google Scholar] [CrossRef] [PubMed]
- Muroga, Y.; Nakaya, A.; Inoue, A.; Itoh, D.; Abiru, M.; Wada, K.; Takada, M.; Ikake, H.; Shimizu, S. Conformation of Poly(γ-Glutamic Acid) in Aqueous Solution. Biopolymers. 2016, 105, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, M.; Holtzer, A. The Helix-Coil Transition in Solutions of Polyglutamic Acid. J. Am. Chem. Soc. 1964, 86, 538–543. [Google Scholar] [CrossRef]
- Thorpe, S.L.; Snyder, G.N.; Mammana, A. Spectroscopic study of porphyrin self-assembly: Role of pH, time, and chiral template. Chirality 2020, 32, 5–16. [Google Scholar] [CrossRef]
- Pasternack, R.F.; Collings, P.J. Resonance light-scattering—A new technique for studying chromophore aggregation. Science 1995, 269, 935–939. [Google Scholar] [CrossRef]
- Parkash, J.; Robblee, J.H.; Agnew, J.; Gibbs, E.; Collings, P.; Pasternack, R.F.; de Paula, J.C. Depolarized resonance light scattering by porphyrin and chlorophyll J-aggregates. Biophys. J. 1998, 74, 2089–2099. [Google Scholar] [CrossRef]
- Hambright, P.; Chock, P.B. Metal-porphyrin interactions. III. Dissociative-interchange mechanism for metal ion incorporation into porphyrin molecules. J. Am. Chem. Soc. 1974, 96, 3123–3131. [Google Scholar] [CrossRef] [PubMed]
- Castriciano, M.A.; Samperi, M.; Camiolo, S.; Romeo, A.; Scolaro, L.M. Unusual Stepwise Protonation and J-Aggregation of meso-Tetrakis(N-methylpyridinium-4-yl)porphine on Binding Poly(sodium vinylsulfonate). Chem. Eur. J. 2013, 19, 12161–12168. [Google Scholar] [CrossRef] [PubMed]
- Chapot, D.; Bocquet, L.; Trizac, E. Electrostatic potential around charged finite rodlike macromolecules: Nonlinear Poisson-Boltzmann theory. J. Colloid. Interface Sci. 2005, 285, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Tuinier, R. Approximate solutions to the Poisson-Boltzmann equation in spherical and cylindrical geometry. J. Colloid. Interface Sci. 2003, 258, 45–49. [Google Scholar] [CrossRef]
- Pasternack, R.F.; Huber, P.R.; Boyd, P.; Engasser, G.; Francesconi, L.; Gibbs, E.; Fasella, P.; Cerio Venturo, G.; Hinds, L. Aggregation of meso-substituted water-soluble porphyrins. J. Am. Chem. Soc. 1972, 94, 4511–4517. [Google Scholar] [CrossRef]
- Rosenheck, K.; Doty, P. The far ultraviolet absorption spectra of polypeptide and protein solutions and their dependence on conformation. Proc. Natl. Acad. Sci. USA 1961, 47, 1775–1785. [Google Scholar] [CrossRef]




| 105 [H+]/M | 103 kobs/s−1 a |
|---|---|
| 8.91 | 0.16 ± 0.01 |
| 5.24 | 0.28 ± 0.02 |
| 3.34 | 0.55 ± 0.04 |
| 2.63 | 1.24 ± 0.01 |
| 1.86 | 3.12 ± 0.03 |
| 1.66 | 5.10 ± 0.06 |
| 1.39 | 7.48 ± 0.04 |
| 1.20 | 11.90 ± 0.05 |
| 1.00 | 19.60 ± 0.06 |
| 0.79 | 30.00 ± 0.01 |
| 0.56 | 51.50 ± 0.03 |
| 0.35 | 82.40 ± 0.08 |
| 0.27 | 129 ± 0.009 |
| 0.21 | 151 ± 0.004 |
| 0.18 | 161 ± 0.003 |
| 0.11 | 169 ± 0.007 |
| 0.06 | 176 ± 0.008 |
| 0.42 b | 0.098 ± 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagami, R.; Castriciano, M.A.; Romeo, A.; Monsù Scolaro, L. Enhancement of the Rates for Insertion of Zinc(II) Ions into a Cationic Porphyrin Catalyzed by Poly(glutamate). Int. J. Mol. Sci. 2023, 24, 17371. https://doi.org/10.3390/ijms242417371
Zagami R, Castriciano MA, Romeo A, Monsù Scolaro L. Enhancement of the Rates for Insertion of Zinc(II) Ions into a Cationic Porphyrin Catalyzed by Poly(glutamate). International Journal of Molecular Sciences. 2023; 24(24):17371. https://doi.org/10.3390/ijms242417371
Chicago/Turabian StyleZagami, Roberto, Maria Angela Castriciano, Andrea Romeo, and Luigi Monsù Scolaro. 2023. "Enhancement of the Rates for Insertion of Zinc(II) Ions into a Cationic Porphyrin Catalyzed by Poly(glutamate)" International Journal of Molecular Sciences 24, no. 24: 17371. https://doi.org/10.3390/ijms242417371
APA StyleZagami, R., Castriciano, M. A., Romeo, A., & Monsù Scolaro, L. (2023). Enhancement of the Rates for Insertion of Zinc(II) Ions into a Cationic Porphyrin Catalyzed by Poly(glutamate). International Journal of Molecular Sciences, 24(24), 17371. https://doi.org/10.3390/ijms242417371









