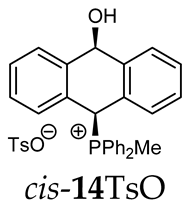
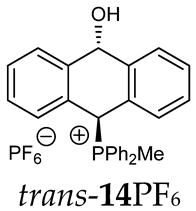
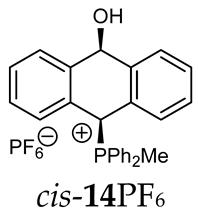
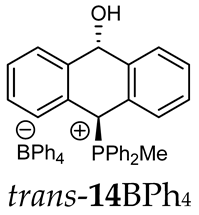
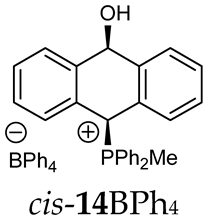
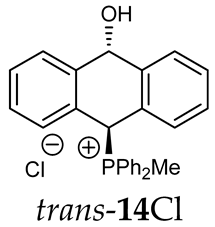
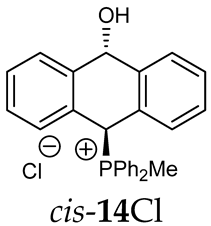
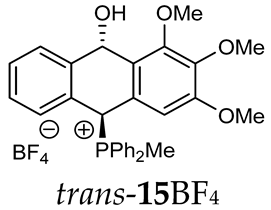
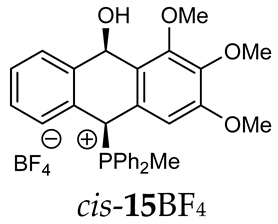
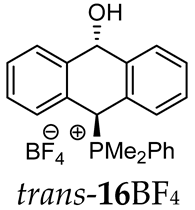
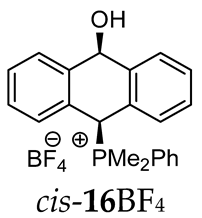
Abstract
The reaction of (ortho-acetalaryl)arylmethanols with various phosphines PR1R2R3 (R1 = R2 = R3 = Ph; R1 = R2 = Ph, R3 = Me and R1 = R2 = Me, R3 = Ph) under acidic conditions (e.g., HCl, HBF4, TsOH) unexpectedly led to the formation of (10-hydroxy-9,10-dihydroanthr-9-yl)phosphonium salts instead of the corresponding anthryl phosphonium salts. The cyclization occurred according to the Friedel–Crafts mechanism but without the usually observed Bradsher dehydration, giving cyclic products in the form of cis/trans isomers and their conformers. In case of electron-rich and less-hindered dimethylphenylphosphine, all four stereoisomers were recorded in 31P{1H} NMR spectra, while for the other phosphines, only the two most stable cis/trans stereoisomers were detected. This study was supported by DFT and NCI calculations in combination with FT-IR analysis.
1. Introduction
Organic electronics and optoelectronics are relatively new fields of basic knowledge and technology that have become a subject of interest to chemists, physicists and process engineers [1,2,3,4]. Therefore, the search for organic fluorescent and semiconducting materials for the construction of new-generation electronic devices, such as organic light-emitting diodes (OLEDs) [5], organic field-effect transistors (OFETs) [6], organic solar cells (OPVs) [7], organic solar concentrators (OSCs) [8], organic lasers [9], etc., has drawn the attention of numerous multidisciplinary joint laboratories. Among the aromatic hydrocarbons, linearly fused acenes are being considered as key organic compounds for achieving these goals [10]. Anthracene and its derivatives are particularly attractive due to their high thermal stability, relatively good solubility, low price and blue photoluminescent and electroluminescent properties [11,12]. Many blue-light-emitting materials with an anthracene core structure have been developed; however, deep blue is still in demand due to the lack of electrically and photochemically stable light-emitting materials [13].
Earlier, we elaborated a new approach to mono-hetero (Z = OR, SR)-substituted acenes IV via the hetero-Friedel–Crafts–Bradsher (F-C-B) cyclization of ortho-O,O-acetals, S,S-dithioacetals and O,S-thioacetals II obtained from diarylmethanols I [14,15,16,17]. In this reaction, a new benzene ring, fused to two other (hetero)aromatic moieties, ArI and ArII, is formed to give acenes IV (Scheme 1, path a). A major advantage of this approach over others known in the literature is the possibility of introducing a large number of substituents of a different electron character, which rapidly change the photophysical properties of the obtained acenes.
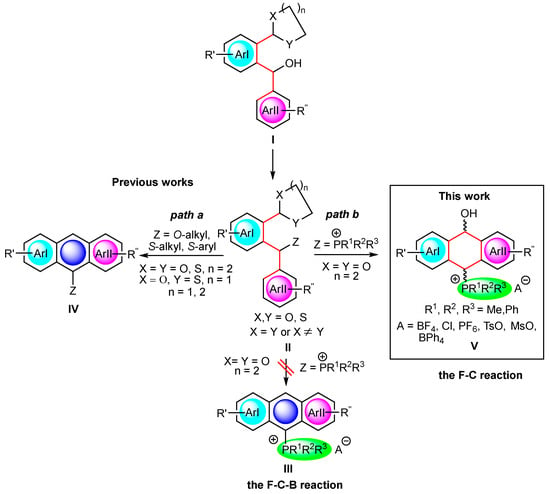
Scheme 1.
A diagram showing the potential for the synthesis of multiply substituted Z-acenes IV (Z = OR, SR; previous works) and dihydroanthrols V (Z = P+R1R2R3; this work) from diarylmethanols I.
In addition to the unique multiple substitution, in this study, we planned to definitely increase the electron donor–acceptor properties of such acenes by introducing strongly electron-withdrawing phosphonium groups (P+R1R2R3) in order to obtain favorable absorption and fluorescence properties [18,19,20]. Thus, following the hetero-F-C-B strategy, we planned to synthesize anthryl phosphonium salts III via intermediate (ortho-acetalaryl)arylmethylphosphonium salts II (Z = P+R1R2R3). However, the change in the nature of the non-ionic Z substituents (Z = OR, SR) to strongly electron-withdrawing and bulky, ionic ones (Z = P+R1R2R3) proved to be a challenge and resulted in the unexpected formation of non-aromatic products V, which are hitherto unknown derivatives of 9,10-dihydro-9-anthrols (Scheme 1, path b). The reaction proceeded via the Friedel–Crafts mechanism without the subsequent and usually observed Bradsher dehydration leading to aromatic species. Advantageously, the synthesis of the latter was a one-pot reaction bypassing the isolation of intermediate phosphonium salts II occurring through the direct treatment of diarylmethanols I with various acids (HBF4, MeSO3H, HCl, TsOH) in the presence of triphenyl-, diphenylmethyl- or dimethylphenylphosphine (Scheme 1).
2. Results and Discussion
Synthesis: Of the many routes described for the synthesis of phosphonium salts, few relate to those with a specific diarylmethyl substituent [21,22,23]. In this study, we focused on the synthesis of phosphonium salts using diarylmethanols I and phosphines as the starting materials under acidic conditions [22]. This approach involving the formal OH/P+R1R2R3 conversion seemed to be the most promising, since in the literature, unsubstituted benzyl and dibenzyl alcohols were converted in this way. However, unsubstituted and multiply substituted dibenzyl alcohols and the corresponding phosphonium salts, containing an acid labile ortho-acetal function, which enabled a subsequent Friedel–Crafts–Bradsher reaction, had not been investigated so far and required a modified synthesis protocol (Scheme 2).
Scheme 2.
Synthesis of phosphonium salts 11A–16A with different anions.
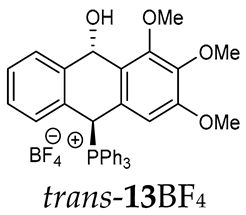
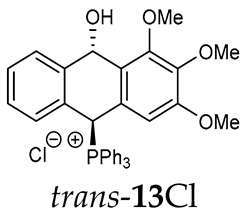
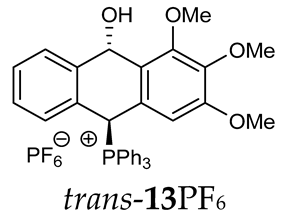
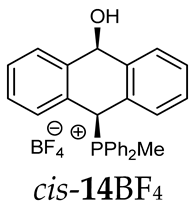
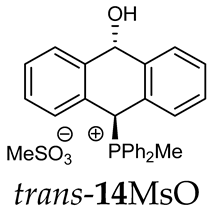
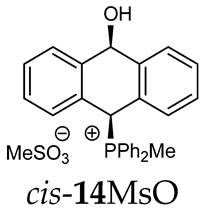
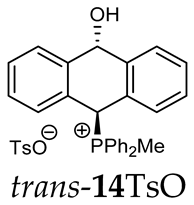
Thus, diarylmethanols 2–4 were first obtained by treating the protected bromobenzaldehyde 1 with n-butyllithium followed by other multiply substituted benzaldehydes. The 1H-NMR spectra of these diarylmethanols showed characteristic signals confirming their structure. For instance, in the spectrum of diarylmethanol 2, broad singlets at 3.82 ppm from the OH group and at 5.69 ppm from the 1,3-dioxane CH proton and a characteristic doublet at 6.41 ppm (J = 3.3 Hz) due to the CHOH proton were observed. Then, the obtained alcohols 2–4 were added dropwise at room temperature to the mixture of the acid HA and phosphine solution and stirred for about 1 h. This strategy gave better results and differed from that described in the literature for the synthesis of phosphonium salts [22]. The reverse addition of acid to the diarylmethanol/phosphine solution resulted in the formation of an impure product. The mixture of alcohol and phosphine in the presence of acid HA spontaneously cyclized to give products 11A–16A (A = anion) due to the presence of the ortho-acetal function, which, under acidic conditions, enabled the Friedel–Crafts (F-C) cyclization. The products were not the expected anthracenes III, as was the case during the previously described intramolecular hetero-Friedel–Crafts–Bradsher reactions [15,16,17], but 9,10-dihydroanthrols with a non-aromatic middle ring containing both 9-hydroxyl and 10-phosphonium groups. The subsequent Bradsher dehydration is usually the next step after the F-C reaction leading to the aromatic system, but not in this case [24]. The structures of the unexpected compounds were confirmed by 1D and 2D NMR, HRMS, IR and UV spectra. They were formed as mixtures of stereoisomers resonating in the 31P{1H} NMR spectrum in the 17–23 ppm range. Interestingly, the trans/cis isomeric ratio varied depending on the phosphine used. In case of bulky triphenylphosphine, this ratio was even 12.3:1 (trans-13Cl:cis-13Cl), while in case of less-hindered diphenylmethylphosphine and dimethylphenylphosphine, it was almost 1.2:1 (trans-14MsO:cis-14MsO and trans-16BF4:cis-16BF4). The intermediates of this reaction were most probably acyclic phosphonium salts 5A–10A, as in other hetero-F-C-B reactions [14,17], but they were not detected by the 31P{1H} NMR spectroscopy and were not isolated due to their rapid cyclization under an acidic conditions. The structure of trans-13Cl was further confirmed by a chloride anion exchange reaction with KPF6, leading to trans-13PF6 (Scheme 2). The high reactivity of phosphonium salts 5A–10A under acidic conditions enabled the use of a one-pot procedure to synthetize salts 11A–16A from diarylmethanols 2–4.
Structure determination: The 1H NMR spectra of 11A–16A showed characteristic signals in the range of 6.39–8.41 ppm due to the PCH group and coupling constants of 2JPH from 7.5 to 11.0 Hz, and they showed signals in the range of 5.03–6.46 ppm and the corresponding coupling constants of 5JPH from 6.7 to 11.3 Hz due to the CHOH proton (Table 1). Relatively large long-range coupling constants 5JPH are characteristic of cyclic bisallyl systems [25,26]. In the 13C{1H} NMR spectra, typical signals from the benzyl group in the range of 78.7–81.9 ppm and 1JCP coupling constants from 55.7 to 65.0 Hz were observed. The JPH and JPC coupling constants were identified by 1H{31P} NMR and 13C{1H,31P} NMR measurements. In some cases, a problem with the identification of PCH protons resulted from their overlapping with aromatic protons; therefore, their position in the spectrum could be indirectly determined by recording the 1H-13C HSQC spectra or by changing the solvent, as was conducted in case of trans-13PF6 (DCM/MeOD).

Table 1.
1H and 13C{1H} NMR data for compounds 11A–16A 1.
The position of the PCH protons in the 1H NMR spectra depended mostly on the type of anion and phosphine used. According to the studies of non-cyclic benzyl and benzhydryl triphenylphosphonium salts in solution, by Ammer et al., the strong deshielding effect for PCH protons was due to the high acidity of the C–H bond. The effect was influenced by both the substituents in the aromatic rings and the type of X− anion that formed PC-H---X− hydrogen bonds. The strongest interactions, according to the literature observations, which were carried out for benzhydryl triphenylphosphonium salt, took place for X = Cl (δPCH = 8.25 ppm) in contrast to X = BF4 (δPCH = 6.23 ppm in CD2Cl2) [22]. In this case, the chloride anion, being the smallest one, interacted more strongly with the PCH hydrogen atom. In the rings substituted with methoxy groups of trans-13Cl and trans-13BF4, the PCH protons resonated at 8.41 ppm and 8.15 ppm (in CD2Cl2), respectively. A full overview of the shielding/deshielding effects for 11A–16A is presented in Table 1. The highest differences in the PCH chemical shifts were observed for the group of cyclic diphenylmethylphosphonium salts 14, between the weakly complexing and large BPh4− anions in trans-14BPh4/cis-14BPh4 (6.59/6.39 ppm) and the small Cl− anions in trans-14Cl/cis-14Cl (8.32/8.09 ppm).
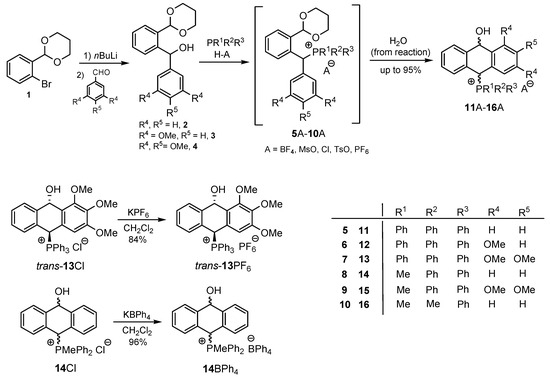
The 31P NMR spectra of 11A–16A were within the range of 17–23 ppm. To provide further confirmation of the non-aromatic character of the middle ring in the products 11A–16A, the fully aromatic phosphonium salt 18I was prepared by reacting anthryldiphenylphosphine 17 with methyl iodide in DMF and was characterized by 1H, 31P and 13C NMR, UV and HRMS spectra. It is currently the second known isolated phosphonium salt with an anthryl substituent at the phosphorus atom [27]. A comparison of the spectroscopic data for 18I and 11A–16A showed a lack of characteristic signals due to CHOH benzyl protons in the range of 5.09–6.46 ppm in the 1H NMR spectrum and a lack of the signal from the CHOH carbon atom in the 13C{1H} NMR spectrum of 18I. It is noteworthy that in the 1H NMR spectrum, a strongly deshielded signal at 9.11 ppm from the C(9)H carbon atom was recorded [28], which was not observed in the spectra of salts 11A–16A. The 31P{1H} NMR spectrum of 18I was characterized by a singlet at 17.6 ppm, and the mass spectrum fully corresponded to the anthracene structure (Scheme 3).
Scheme 3.
Synthesis of the phosphonium salt 18I.
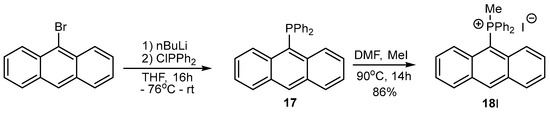
Finally, the UV absorption spectra of 14TsO and 18I showed different structures of both compounds. The spectrum of 18I with an anthryl group showed absorption bands at 265, 351, 370, 390, 406 and 432 nm, which were absent in the absorption spectrum of 14TsO with a 9,10-dihydroanthryl group, where only strong absorption bands at 260, 268 and 275 nm were observed (Figure 1).
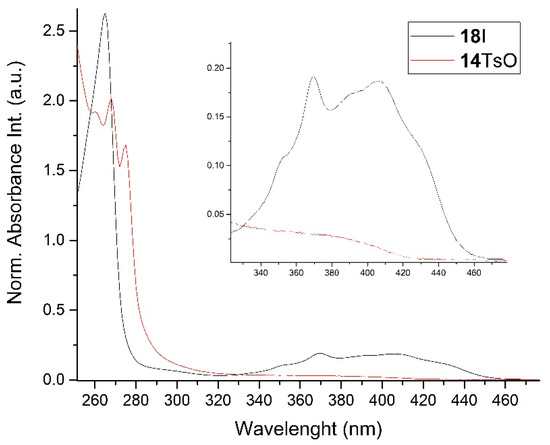
Figure 1.
The absorption spectra of 9,10-dihydroanthracene 14TsO (red line) and anthracene 18I (black line) in dichloromethane (c = 10−5 mol/dm3).
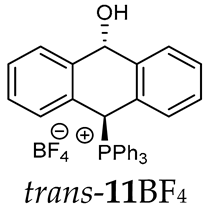
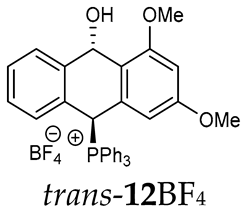
The isomeric compounds 11A–16A were spectroscopically characterized as a mixture. Attempts to separate them failed due to their identical Rf values. Moreover, both in the crude reaction mixture and during the purification of triphenylphosphonium salts 12A and 13A with a silica gel column, a partial decomposition occurred due to cleavage of the P-C bond and the formation of triphenylphosphine, which was then oxidized to phosphine oxide with air oxygen. Decomposition of the products was partially avoided by using neutral alumina instead of silica gel, which enriched the mixture in one more stable isomer (e.g., trans-13BF4) The introduction of three methoxy substituents onto the side benzene ring in the triphenylphosphonium series increased a steric hindrance and, consequently, significantly prevented the formation of the minor isomer (trans-11BF4 vs trans-13BF4).
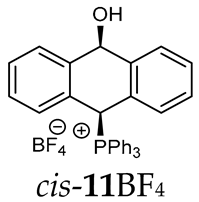
1H-1H NOESY spectra allowed for the determination of the cis or trans configuration of the major and minor isomer (Figure 2). This experiment, carried out for the salt 14BF4, revealed the proximity of the PCH3 and the CHOH protons, which indicated that the major isomer with characteristic CHOH hydrogen at 5.03–6.46 ppm in the 1H NMR spectra was the trans-A isomer, while the minor isomer with CHOH protons at 6.27–6.46 ppm was the cis isomer (Figure 3). This proximity was confirmed by DFT calculation, carried out for the trans-A isomer of 14Cl.
Figure 2.
1H-1H NOESY spectra of the cis(minor)/trans(major) mixture of the phosphonium salt 14BF4.

Figure 3.
The reaction of phosphines with diarylmethanols under acidic conditions (HA), leading to four stereomeric phosphonium salts A–D. All four stereoisomers were observed for R1 = R2 = Me, R3 = Ph, while A and C, as most stable, were only detected for R1 = Me, R2 = R3 = Ph and for R1 = R2 = R3 = Ph.
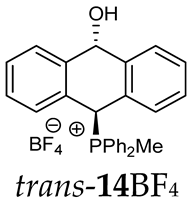
As we have seen, a larger substituent, such as the triphenylphosphonium group, forced the formation of a trans-A isomer with a preferred pseudo-axial phosphonium group and a pseudo-equatorial hydroxyl group in ratios of 12.3–2.3:1 for 11A–13A. Replacing the phenyl group with a methyl group in the triphenylphosphonium group reduced this hindrance, and the 1.2–1.4:1 ratio of the two isomers equalized, as observed for compounds 14A, 15A and 16A (Table 1).
The 31P{1H} NMR temperature experiments (from 25 to 130 °C in DMSO) carried out for the salt 14BF4 revealed two signals due to cis/trans isomers and no coalescence phenomenon at the tested temperature, which indicated the presence of two configurational isomers, such as A or B and C or D.
Since the 31P{1H} NMR spectra of the salts with triphenyl- and diphenylmethylphosphine groups did not allow for the observation of all four A, B, C and D isomeric forms of the corresponding salts, we increased the electron density on the phosphorus atom, reduced the steric hindrance at this atom by choosing the less-bulky dimethylphenylphosphine and lowered the reaction temperature. Thus, we started our measurements at −15 °C by treating the mixture of dimethylphenylphosphine and HBF4 with the alcohol 7. After increasing the temperature to room temperature, the reaction progress was monitored by 31P{1H} NMR (Figure 4). After one hour, two strong singlets were observed, which were due the substrates, i.e., dimethylphenylphosphine at −46.2 ppm and protonated phosphine at −0.2 ppm, as well as four singlets, which were due to the four A–D isomers of the salt 16BF4. The singlets at 23.4 ppm (c) and 22.45 ppm (d) corresponded to the trans-A and cis-C isomers, respectively, while the singlets at 26.0 ppm (a) and 25.1 ppm (b) were due to their conformers (B and D). As the reaction progressed, we observed the disappearance of singlets from the substrates and singlets a and b from the kinetic products. After 6 days, the spectrum showed trace a and b singlets and dominant c and d singlets originating from thermodynamically more stable trans and cis conformational isomers A and C (see calculations). For a comparison, in the case of the triphenylphosphonium salt 11BF4 and diphenylmethylphosphonium salt 14BF4, no signals from the corresponding starting phosphines and/or their protonated forms were obtained in the 31P{1H} NMR spectra. Instead, only two singlets in each case, due to the formation of the thermodynamic products in ratios of 3.6:1 and 1.4:1, respectively, were observed after 1 h at room temperature. The electron density on the phosphorus atom was lower in the case of the latter two phosphines than in the case of dimethylphenylphosphine, in which the positive induction effect of the two methyl groups predominated; therefore, protonation of the phosphorus atom by HBF4 did not occur in a visible way for these two phosphines. Finally, it can be concluded that there was no equilibration between the thermodynamic stereoisomers A and C via benzyl carboacation due to the lack of a change in the ratio of c and d signals during the equilibration process (Figure 3, Figure 4 and Figure 5).

Figure 4.
Reaction progress of the alcohol 4 with dimethylphenylphosphine, diphenylmethylphosphine and triphenylphosphine with HBF4 in CH2Cl2 at room temperature monitored with 31P{1H} NMR. The singlets a–d represent the corresponding products and intermediates A–D.
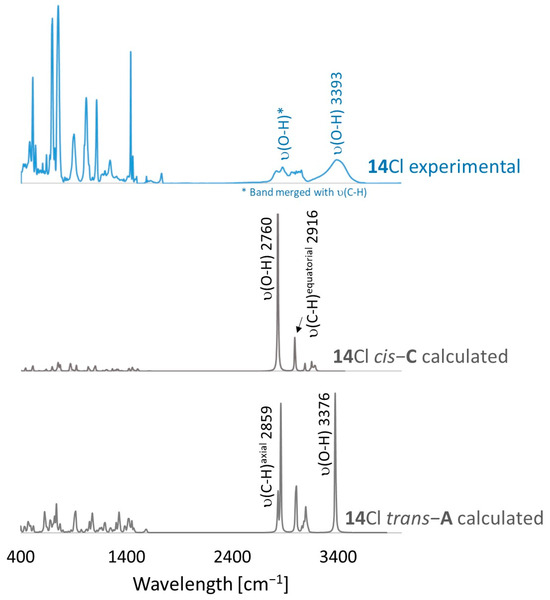
Figure 5.
A comparison of experimental (blue line) and theoretical (grey lines) FT-IR spectra for 14Cl.
FT-IR for 14Cl in combination with DFT and NCI calculation: The analysis of the FT-IR spectrum measured for 14Cl (Table 2, Figure 5 and see Supplementary Materials) proved that the hydroxyl group was covalently bound to the 9-carbon atom of the non-aromatic ring, as evidenced by the presence of υ(C-O) vibrations, both equatorial and axial. By comparing the experimental FT-IR spectrum with the frequencies calculated (DFT-B3LYP 6-31++G(d,p)) for the most stable A and C isomers of 14Cl (Figure 3) in the ground state, in the gas phase, it can be concluded that the trans stereoisomer A predominated in the obtained sample, as evidenced by the position of the vibration of the hydroxyl group (υ(O-H) 3393 cm−1), which corresponded to the position of the vibration calculated for the trans-A isomer (3376 cm−1). The analysis of the calculated frequencies indicated that the υ(O-H) vibration in the cis-C isomer was shifted towards lower wavelengths (calculated value 2760 cm−1), and in the trans-A isomer, it was observed at a higher wavelength (3376 cm−1). The coexistence of the cis form (in addition to the trans form) can be proven by broadening and strengthening the entire υ(C-H) vibration band (2700–2900 cm−1), in which the υ(O-H)cis vibration may be hidden. Importantly, the presence of two bands for both the υ(C-H) and υ(C-O) vibrations proves the co-occurrence of the axial and equatorial forms. In order to explain (1) why the Bradsher dehydration leading to the aromatic system did not occur as the next step after the F-C reaction and (2) thermodynamic stability of the two stereoisomers A and C present in the product of this reaction, DFT calculations for the four possible A–D stereoisomers of 14Cl (Figure 3) in the ground state, in the gas phase, were made. The optimized geometries of the trans-A, trans-B, cis-C and cis-D isomers of 14Cl are presented in Figure 6. According to the DFT calculations, the trans-A isomer is more thermodynamically stable than its conformer B and cis-D isomer by 4.64 and 26.61 kcal/mol, respectively, and only more stable by 1.12 kcal/mol than the cis-C isomer (Figure 7). Thus, 14Cl exists predominantly in two forms as trans-A and cis-C isomers. As illustrated in Figure 6, in the case of each stereoisomer, the Cl− anion is the acceptor in a strong hydrogen bond, with the hydroxyl group acting as the donor. The distance between H and Cl− is shorter than the sum of the van der Waals radii of these atoms by ca. as much as 0.7 Å and 1.0 Å in trans and cis isomers, respectively. In addition, for the two conformers, A, B and the isomer C, the anion acts as an acceptor for two unconventional but very short C-H∙∙∙Cl− hydrogen bonds (dH∙∙∙Cl < the sum of the van der Waals radii of the H and Cl atoms by ca. 0.5 Å in the case of the trans-A, 0.8 Å and 0.5 Å in the case of the trans-B and 0.9 Å and 0.2 Å in the case of the cis-C isomers). In the trans-A and B isomers, the mutual combination of the hydrogen bond of the type O-H∙∙∙Cl− with P-C-H∙∙∙Cl− (in B) or O-C-H∙∙∙Cl− (in A) hydrogen bonds leads to the formation of rings with descriptors in the form of S21(5) or S21(5), respectively. In the cis-C isomer, a combination of three hydrogen bonds, i.e., one O-H∙∙∙Cl− and two C-H∙∙∙Cl−, leads to the formation of a S32(9) ring. This type of intramolecular ring does not occur only in the highest-energy cis-D isomer, which results from the lack of hydrogen bonds other than O-H∙∙∙Cl−. Practically all of the above hydrogen bonds, and especially O-H∙∙∙Cl−, are visible on three-dimensional NCI diagrams (Figure 6) as dark or light-blue, disk-shaped isosurfaces, indicating strong attractive interactions. The above analysis of the strongest non-covalent interactions occurring in the 14Cl isomers allowed us to conclude that the Bradsher dehydration associated with the elimination of the hydroxyl group could not occur due to (1) its participation in strong hydrogen bonds and the motifs formed by them (Figure 6) and, especially, (2) combinations of the many weak, non-covalent van der Waals interactions visible in the two isomers trans-A and cis-C as greenish isosurfaces, which prevent the middle ring from flattening to form an aromatic system (Figure 7). In the minor trans-B and cis-D isomers, the number of these interactions is much smaller (Figure 7), which causes their interconversion to the more stable isomers trans-A and cis-C.

Table 2.
FT-IR characteristic experimental and theoretical vibrations for the compounds 14Cl.
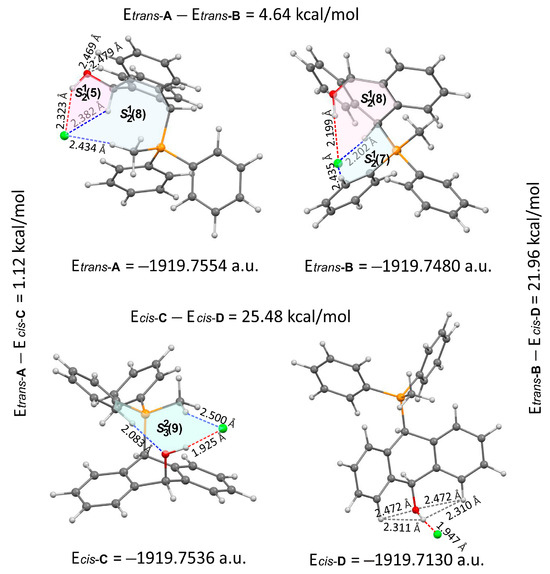
Figure 6.
Molecular structures of four stereoisomers A–D of 14Cl. The dashed red and blue lines denote O-H∙∙∙Cl− and C-H∙∙∙Cl− hydrogen bonds, and the grey dashed lines denote short non-covalent interactions between atoms of hydroxyl groups and aromatic H atoms.
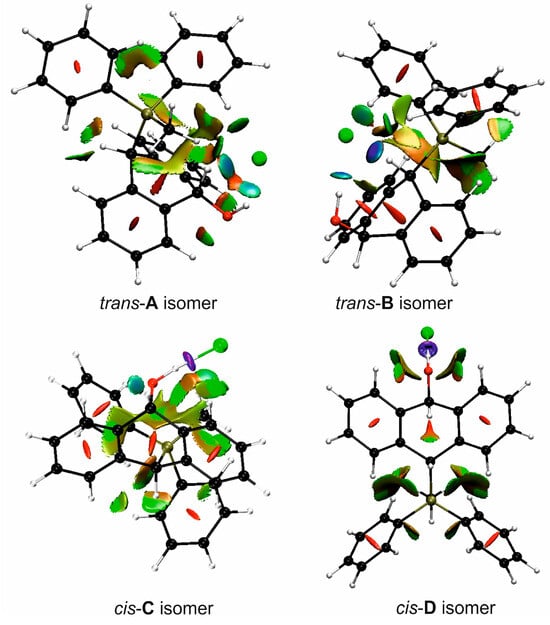
Figure 7.
Color-filled reduced density gradient (RDG) isosurfaces depicting non-covalent interactions in four stereoisomers A–D of 14Cl.
Reaction mechanism: The reaction between diarylmethanol 19 and phosphine PR1R2R3 under acidic conditions in the first step led to protonation of the OH group to give 20 followed by the dehydration and formation of the carbocation 21, which reacted with the phosphine to give the phosphonium salt 22. However, in the case of electron-rich phosphine PMe2Ph, unlike PMe2Ph and PPh3, the phosphorus atom was also protonated. In this case, signals from both the phosphine and its protonated form were observed in the 31P{1H} NMR (Figure 4). In contrast, the phosphonium salt 22 was never detected because it underwent a rapid protonation to give 23 followed by opening the acetal ring and the formation of the carbocation 24. Then, the ring closed according to the Friedel–Crafts reaction to give 25 and aromatization of the benzene ring brought 26. Next, protonation of the propandiol moiety gave 27, which easily lost the diol to obtain the carbocation 28. The 1H NMR spectrum of the crude mixture showed signals from free propanediol and no signal from the aldehyde group, indicating the removal of the protecting group. Interestingly, the carbocation 28 did not undergo further aromatization of the middle ring, typically via the Bradsher dehydration, to give the anthracene 30 via the dicationic species 29, but it unexpectedly reacted with water originating from the dehydration of 20 (in the case of anhydrous acids, e.g., HCl in Et2O, HBF4.Et2O) and/or from the aqueous acid solutions (e.g., HPF6(aq), HClaq, p-TosH.H2O) to give (10-hydroxy-9,10-dihydroanthr-9-yl)phosphonium salts 31 (Scheme 4).
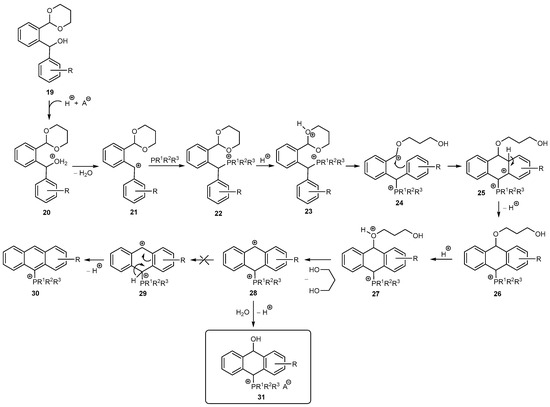
Scheme 4.
Plausible mechanism for the synthesis of phosphonium salts 31.
Attempts to dehydrate the isolated anthrols to anthracenes under strongly acidic conditions failed. For instance, 14BF4 did not aromatize with various acids (HCl, H2SO4) to obtain 18BF4 at room temperature and even at higher temperatures. The reverse reaction, i.e., the addition of water to the activated by a phosphonium group anthracene 18I to form 14I, even at higher temperatures, also did not occur (Scheme 5).
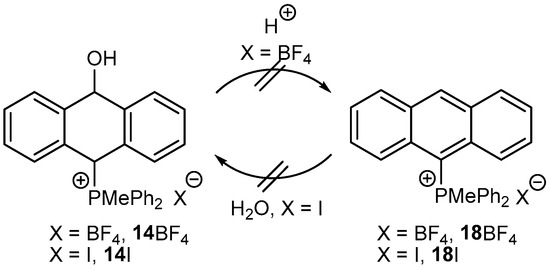
Scheme 5.
The reactivity study of the anthrol 14BF4 to acid and the anthracene 18I to water.
3. Materials and Methods
Organic solvents were purchased from commercial sources (ChemPur, Piekary Śląskie, Poland; POCh, Gliwice, Poland) and used as-received or dried using standard procedures. Tetrahydrofuran (THF) was purchased from J.T. Baker and purified using a Solvent Purification System (MBraun SPS-800). All reagents were from obtained commercial suppliers (Sigma-Aldrich, Merck–USA, Beijing, China) and used without further purification. An Avance NEO 400 NMR spectrometer, operating at 400.15 MHz for 1H, 100.63 MHz for 13C, 128.40 MHz for 31P, 376.55 MHz for 19F and 192.64 MHz for 11B, was used, which consisted of a narrow-bore 9.4 Tesla Ascend superconducting magnet and an Avance NEO console from Bruker Corporation (Karlsruhe, Germany) operating with a SampleCase Plus autosampler. The spectrometer was equipped with 5 mm high-resolution dual-channel 2H/1H(19F)/BB i-Probe (Bruker) with a z-gradient coil capable of tuning to nuclei 19F/31P-199Hg and 17O-109Ag on the BB channel with an automatic tuning and matching system (ATMA). The spectrometer used a BCU-I, controlled by a Bruker Smart Variable Temperature (BSVT) system for the temperature regulation and stabilization. The spectrometer was operated using the TopSpin 4.1 program. High-resolution mass spectrometry (HRMS) measurements were performed using an SQ Detector 2 mass spectrometer (Waters, Milford, MA, USA). Melting points were measured using a Boetius apparatus. Thin-layer chromatography (TLC) was performed on precoated Merck 60 (F254 60, Darmstad, Germany) silica gel plates with a fluorescent indicator with detection by means of UV light at 254 and 360 nm or on aluminum cards (Fluka Chemie AG, Buchs, Switzerland) with a fluorescent indicator with detection by means of UV light at 254 nm. Column chromatography was performed on Merck silica gel (Kieselgel 60, Darmstad, Germany, 230–400 mesh) or using a Pure FlashPrep 850 Chromatography System (Büchi, Flawil, Switzerland). The UV-Vis absorption spectra were recorded in 1 cm cuvettes on a Shimadzu UV-2700 spectrophotometer (Kioto, Japan) using two types of light source: a D2 64604 deuterium lamp and a Wl L6380 halogen lamp (Kioto, Japan, 220–600 nm).
Fourier Transform Infrared Spectroscopy: The Fourier transform mid-infrared spectra of the compounds 14Cl were measured at a 2 cm−1 resolution and by 32 scans on a Nicolet-Nexus spectrometer in the region of 4000–400 cm−1 using the KBr pellet technique. The data were processed using spectrum software OMNIC 8.2.0.387 (Thermo Fisher Scientific Inc., Waltham, MA, USA). IR frequencies predicted for the 14Cl trans-A and cis-C isomers were compared with experimental frequencies measured for the synthesized 14Cl compounds. A precomputed vibrational scaling factor of 0.966 was used.
DFT and NCI calculations. The molecular structures of the four stereoisomers A–D of 14Cl− were calculated by the DFT method using a gradient-corrected three-parameter hybrid functional (B3LYP) with the 6-31++G(d,p) basis set. Full-geometry optimizations of the compounds in the gas phase were performed using their starting geometries prepared manually. The above computations were performed using the GAUSSIAN09 quantum chemistry package. In order to check the structural optimizations, the calculated vibrational frequencies of the compounds were used (no imaginary frequencies). The IR frequencies predicted for the 4Cl trans-A and cis-C isomers were compared with the experimental frequencies measured for the synthesized 14Cl compound. A precomputed vibrational scaling factor of 0.966 was used. The calculations in the framework of the noncovalent interactions (NCI) method were performed with the Multiwfn 3.8 version (A Multifunctional Wavefunction Analyzer) software. This method is based on the relationship between the electron density (ρ) and the reduced density gradient (RDG) and is a very useful tool for the analysis and visualization of different types of non-covalent interactions. The Mercury program was employed to visualize the chemical structures of the investigated isomers, and the VMD (Visual Molecular Dynamics)) 1.9.3. software and Multiwfn 3.8 version software were used for the visualization of their RDG isosurfaces.
3.1. General Procedure for Synthesis of o-Acetalaryl(aryl)methanols
2-(2-Bromophenyl)-1,3-dioxane 1 (5 mmol, 1.21 g) was placed in a round-bottom flask and dissolved in dry THF (50 mL) at −78 °C under an argon atmosphere. Next, 2.2 mL of n-BuLi (5.5 mmol, 2.5 M in hexane) was added. The resulting mixture was stirred for 15 min under argon, and then aromatic aldehyde (5.5 mmol) was added in dry THF. Stirring was continued for 2 h at −78 °C, and the reaction mixture was warmed to room temperature. Saturated aqueous NH4Cl solution was added, and the solvent was evaporated. The residue was diluted with ethyl acetate (3 × 30 mL), washed with water (40 mL) and dried over anhydrous MgSO4. After filtration, ethyl acetate was removed in a vacuum, and the crude product 2, 3 or 4 was purified by column chromatography over silica gel with a mixture of hexane/ethyl acetate (4:1 v/v) as an eluent.
(2-(1,3-Dioxan-2-yl)phenyl)phenylmethanol (2). White solid, m.p: 84–85 °C, yield: 85%. 1H NMR (CD2Cl2) δ 7.71–7.64 (m, 1H, Ar), 7.54–7.33 (m, 7H, Ar), 7.33–7.25 (m, 1H, Ar), 6.41 (d, J = 3.3 Hz, 1H, CHOH), 5.69 (s, 1H, CHO), 4.29 (ddd, J = 11.5, 4.4, 2.9 Hz, 2H, OCH2), 3.98 (ddd, J = 12.1, 6.1, 2.6 Hz, 2H, OCH2), 3.83 (s, 1H, OH), 2.34–2.20 (m, 1H, CH2), 1.48 (m, 1H, CH2) ppm. 13C{1H} NMR (CD2Cl2) δ 143.4, 142.6, 136.2, 129.2, 128.8, 128.2, 127.5, 127.0, 126.6, 101.1, 71.7, 67.6, 67.5, 25.7 ppm. HRMS (EI, 70 eV) m/z calc. for [M]+ C17H18O3 270.1256, found 270.1257.
(2-(1,3-Dioxan-2-yl)phenyl)(3,5-dimethoxyphenyl)methanol (3) [17]. Colorless oil, yield: 85%. 1H NMR (CD2Cl2) δ 7.62 (dd, J = 5.6, 3.5 Hz, 1H), 7.35 (dd, J = 5.8, 3.4 Hz, 2H), 7.26 (dd, J = 5.7, 3.4 Hz, 1H), 6.65 (dd, J = 2.4, 0.8 Hz, 2H), 6.44 (dd, J = 2.4, 2.4 Hz, 1H), 6.30 (d, J = 3.0 Hz, 1H), 5.70 (s, 1H), 4.29 (ddd, J = 9.5, 7.4, 3.6 Hz, 2H), 4.05–3.94 (m, 2H), 3.81 (s, 6H), 2.34–2.19 (m, 1H), 1.54–1.44 (m, 1H) ppm. 13C{1H} NMR (CD2Cl2) δ 160.8 (C), 145.8 (C), 142.4 (C), 136.1 (C), 129.2 (CH), 128.7 (CH), 127.5 (CH), 126.9 (CH), 104.5 (CH), 101.1 (CH), 98.9 (CH), 71.6 (CH), 67.6 (CH2), 67.5 (CH2), 55.3 (CH3), 25.7 (CH2) ppm. HRMS (EI, 70 eV) m/z calc. for [M]+ C19H22O5 330.1467, found 330.1470.
(2-(1,3-Dioxan-2-yl)phenyl)(3,4,5-trimethoxyphenyl)methanol (4) [17]. Colorless oil, yield: 88%. 1H NMR (C6D6) δ 7.85–7.75 (m, 1H), 7.48–7.36 (m, 1H), 7.20–7.11 (m, 1H), 7.04 (s, 2H), 6.63 (d, J = 3.4 Hz, 1H), 5.63 (s, 1H), 4.02–3.74 (m, 2H), 3.95 (s, 3H), 3.54–3.27 (m, 2H), 3.46 (s, 6H), 2.00–1.74 (m, 1H), 0.72–0.59 (m, 1H) ppm. 13C{1H} NMR (C6D6) δ 154.8, 144.3, 139.8, 138.9, 137.5, 130.2, 130.3, 128.5, 128.3, 32.4, 105.2, 102.6, 72.8, 68.1, 68.0, 61.2, 56.4, 26.3 ppm. HRMS (EI, 70 eV) m/z calc. for [M]+ C20H24O6 360.1573, found 360.1571.
3.2. General Procedures for Synthesis of (10-hydroxy-9,10-dihydroanthr-9-yl)phosphonium Salts
3.2.1. Synthesis of Phosphonium Salts 12A and 13A
To a solution of triphenyl phosphine (1 mmol) and dry acid (HBF4·Et2O/MeSO3H/HCl in Et2O) (1 mmol) in DCM (3 mL), a solution of o-acetalaryl(aryl)methanol 3 or 4 (1 mmol) in 3 mL of DCM was added at room temperature. The mixture was stirred under an argon atmosphere for 1 h, and then the solvent was removed with a vacuum pump. The crude products 12 and 13 were purified by flash chromatography over neutral Al2O3 with a mixture of ethyl acetate/MeOH (1:5 v/v).
Trans-(10-hydroxy-2,4-dimethoxy-9,10-dihydroanthr-9-yl)triphenylphosphonium tetrafluoroborate (trans-12BF4). Yellowish amorphous solid, yield: 54%. 1H NMR (200 MHz, CDCl3) δ 7.90–7.71 (m, 4H), 7.70–7.53 (m, 11H), 7.47 (dd, J = 11.0, 3.6 Hz, 1H, CHP), 7.36–7.16 (m, 2H), 6.87 (d, J = 7.5 Hz, 1H), 6.73 (d, J = 7.8 Hz, 1H), 6.35 (d, J = 2.3 Hz, 1H), 6.29 (d, J = 2.3 Hz, 2H), 5.03 (d, J = 7.4 Hz, 1H, CHOH), 3.73 (s, 6H) ppm. 13C{1H} NMR (CD2Cl2) δ 161.2 (C), 143.4 (d, J = 4.9 Hz, C), 141.9 (d, J = 6.2 Hz, C), 135.9 (d, J = 3.1 Hz, CH), 134.5 (d, J = 9.3 Hz, CH), 133.8 (d, J = 16.9 Hz, CH), 130.5 (d, J = 12.2 Hz, CH), 129.2 (d, J = 8.7 Hz, CH), 123.1 (d, J = 3.1 Hz, CH), 122.6 (d, J = 3.1 Hz, CH), 115.7 (d, J = 81.3 Hz, C), 104.9 (CH), 88.6 (CHOH), 81.9 (d, J = 58.2 Hz, PCH), 55.4 (OCH3) ppm. 31P{1H} NMR (81 MHz, CDCl3) δ 18.5 ppm. HRMS (ESI+) m/z calc. for [M]+ C34H30O3P+ 517.1927, found 517.1925.
Trans-(10-hydroxy-2,3,4-trimethoxy-9,10-dihydroanthr-9-yl)triphenylphosphonium tetrafluoroborate (trans-13BF4). Yellowish amorphous solid, yield: 57%. 1H NMR (500 MHz, Acetone-d6) δ 8.15 (dd, J = 10.1, 2.3 Hz, 1H, CHP), 7.97–7.88 (m, 3H), 7.86-7.61 (m, 13H), 7.40 (dd, J = 7.7, 7.7 Hz, 1H), 7.26 (dd, J = 7.7, 7.7 Hz, 1H), 7.08 (d, J = 7.7 Hz, 1H), 6.79 (d, J = 7.7 Hz, 1H), 6.66 (s, 2H), 5.49 (dd, J = 6.7, 2.3 Hz, 1H, CHOH), 3.72 (s, 6H, OMe), 3.66 (s, 3H, OMe) ppm. 13C{1H} NMR (50 MHz, CDCl3) δ 152.2 (C), 142.1 (d, J = 4.9 Hz, C), 134.4 (d, J = 3.0 Hz), 134.2 (d, J = 6.5 Hz), 133.1 (d, J = 9.2 Hz, CH), 129.2 (d, J = 12.3 Hz, CH), 129.8 (CH), 127.7 (CH), 121.6, 114.7 (d, J = 81.1 Hz, C), 102.6 (CH), 87.6 (CH), 80.1 (d, J = 57.1 Hz, CHP), 59.4 (OCH3), 55.0 (OCH3) ppm. 31P{1H} NMR (Acetone-d6) δ 18.6 ppm. 19F{1H} NMR (Acetone-d6) δ −150.70 ppm. HRMS (ESI+) m/z calc. for [M]+ C35H30O3P 529.1933, found 529.1927.
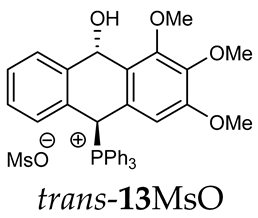
Trans-(10-hydroxy-2,3,4-trimethoxy-9,10-dihydroanthr-9-yl)triphenylphosphonium methanesulfonate (trans-13MsO). Yellow amorphous solid, yield: 38%. 1H NMR (CDCl3) δ 8.20 (d, J = 10.2 Hz, 1H, PCH), 7.87–7.82 (m, 2H), 7.76–7.59 (m, 10H), 7.55 (dd, J = 7.5, 7.5 Hz, 1H), 7.46 (dd, J = 6.5, 6.5 Hz, 1H), 7.39–7.26 (m, 3H), 7.23 (dd, J = 7.5, 7.5 Hz, 1H), 6.89 (d, J = 7.7 Hz, 1H), 6.81 (d, J = 7.7 Hz, 1H), 5.09 (d, J = 7.3 Hz, 1H, CHOH), 3.80 (s, 6H, OMe), 3.79 (s, 3H, OMe), 2.71 (s, 3H, SMe) ppm. 13C{1H} NMR (CDCl3) δ 153.5, 143.4 (d, J = 5.1 Hz), 138.3, 135.6 (d, J = 3.0 Hz), 135.3 (d, J = 6.5 Hz), 134.6 (d, J = 9.3 Hz), 133.7 (d, J = 19.5 Hz), 132,1 (J = 10 Hz), 132.1 (d, J = 2.8 Hz), 131.4 (d, J = 2.0 Hz), 130.5 (d, J = 12.2 Hz), 128.6 (s, J = 12.2 Hz), 128.5 (d, J = 7.1 Hz), 123.3, 122.8, 116.3 (d, J = 81.0 Hz), 104.2, 88.8 (CHOH), 81.6 (d, J = 55.8 Hz, PCH), 39.3 ppm. 31P{1H} NMR (CDCl3) δ 17.1 ppm. HRMS (ESI+) m/z calc. for [M]+ C35H30O3P+ 529.1933, found 529.1920.
Trans-(10-hydroxy-2,3,4-trimethoxy-9,10-dihydroanthr-9-yl)triphenylphosphonium chloride (trans-13Cl). Orange amorphous solid, yield: 18%. 1H NMR (CD2Cl2) δ 8.41 (dd, J = 10.2, 2.6 Hz, 1H, PCH), 7.97–7.88 (m, 3H), 7.80–7.65 (m, 9H), 7.40–7.27 (m, 7H), 6.99 (d, J = 7.7 Hz, 1H), 6.77 (dd, J = 7.7, 1.7 Hz, 1H), 5.25 (dd, J = 7.1, 2.4 Hz, 1H, CHOH), 3.80 (s, 6H), 3.76 (s, 3H) ppm. 13C NMR (CD2Cl2) δ 153.6, 143.4 (d, J = 5.1 Hz), 138.4, 137.3 (d, J = 11.0 Hz), 135.7 (d, J = 3.2 Hz), 135.3 (d, J = 6.5 Hz), 134.6 (d, J = 9.5 Hz), 133.8, 133.6, 131.4 (d, J = 2.0 Hz), 130.7 (d, J = 3.6 Hz), 130.4 (d, J = 12.2 Hz), 129.1 (d, J = 3.2 Hz), 128.8, 128.5 (d, J = 6.8 Hz), 128.3, 123.0 (d, J = 3.0 Hz), 122.9 (d, J = 3.4 Hz), 116.2 (d, J = 81.2 Hz), 104.2, 88.8, 81.8 (d, J = 55.9 Hz), 60.4, 56.3 ppm. 31P{1H} NMR (CD2Cl2) δ 18.3 ppm. HRMS (ESI+) m/z calc. for [M]+ C35H30O3P+ 529.1933, found 529.1920.
3.2.2. Synthesis of Phosphonium Salts 11A and 14A–16A
To a solution of phosphine (1 mmol) and acid (HBF4·Et2O/MeSO3H/HCl(aq)/pTsOH·H2O/HPF6(aq)) (1 mmol) in DCM (5 mL), a solution of o-acetalaryl(aryl)methanol 2, 3 or 4 (1 mmol) in 5 mL of DCM was added at room temperature. The mixture was stirred under an argon atmosphere for 1 h, and then water (5 mL) was added, extracted with DCM (10 mL) and dried over anhydrous MgSO4. After separating the drying agent, the resulting solution was concentrated using a vacuum pump. The residue was washed with ethyl acetate twice, and the residual solvent was removed by a vacuum pump.
(10-Hydroxy-9,10-dihydroanthr-9-yl)triphenylphosphonium tetrafluoroborate (11BF4). Yellowish amorphous solid, yield: 79%. Isomer ratio trans/cis: 3.6:1. 1H NMR (CDCl3) δ 7.90–7.80 (m, 6H, major + minor) 7.72–7.57 (m, 23H, major + minor, CHP major), 7.40 (d, 2JPH = 8.0 Hz, 1H, CHP, minor), 7.37–7.24 (m, 6H, major + minor), 7.21 (dd, J = 7.5, 7.5 Hz, 2H, major), 7.18–7.11 (m, 2H, major), 7.06 (dd, J = 7.7, 7.7 Hz, 2H, minor), 7.00 (d, J = 7.6 Hz, 1H, minor), 6.87 (d, 3JHH = 7.6 Hz, 1H, major), 6.75 (d, 3JHH = 7.7 Hz, 1H, major), 6.55 (d, JHH = 7.8 Hz, 1H, minor), 6.46 (d, JHH = 7.5 Hz, 2H, minor), 6.36 (dd, 5JPH = 11.3, 5JHH = 2.3 Hz, 1H, CHOH, minor), 5.15 (dd, 5JPH = 7.5, 5JHH = 2.3 Hz, 1H, CHOH, major) ppm. 13C{1H} NMR (CDCl3) δ 143.5 (d, J = 5.0 Hz, C), 141.6 (d, J = 5.0 Hz, C), 139.4 (d, J = 6.1 Hz, C), 135.8 (d, J = 3.2 Hz), 134.7 (d, J = 9.4 Hz), 134.5 (d, J = 9.5 Hz), 131.2 (C), 130.8, 130.6 (d, J = 8.7 Hz), 130.5 (d, J = 12.1 Hz), 129.2 (d, J = 3.1 Hz), 129.0, 128.8, 128.6, 127.5, 127.3, 123.7, 123.0, 122.9 (d, J = 3.0 Hz) 122.3, 115.9 (d, J = 81.1 Hz, C), 115.8 (d, J = 83.0 Hz, C), 88.7 (CHOH, major), 88.4 (CHOH, minor), 81.6 (d, 1JCP = 57.0 Hz, CHP, major), 79.9 (d, 1JCP = 56.0 Hz, CHP, minor) ppm. 31P{1H} NMR (CDCl3) δ 18.5 (minor), 18.2 (major) ppm. 19F{1H} NMR (CDCl3) δ −152.42 (minor), −152.47 (major) ppm. 11B{1H} NMR (CD2Cl2) δ −0.98 ppm. HRMS (ESI+) m/z calc. for [M]+ C32H26OP+ 457.1716, found 457.1714.
(10-Hydroxy-9,10-dihydroanthr-9-yl)methyldiphenylphosphonium tetrafluoroborate (14BF4). Yellow amorphous solid, yield: 95%. Isomer ratio trans/cis: 1.4:1. 1H NMR (CD2Cl2) δ 7.98–7.86 (m, 2H, major), 7.86–7.60 (m, 18H, major + minor), 7.50 (dd, J = 7.6, 7.6 Hz, 1H, minor), 7.47–7.33 (m, 7H, major + minor), 7.28 (dd, J = 7.6, 7.6 Hz, 2H, minor), 7.24–7.18 (m, 2H, major), 7.15 (dd, 2JPH = 9.8, 5JHH = 2.7 Hz, 1H, CHP, major), 7.05 (d, JHH = 7.1 Hz, 1H, major), 6.99 (d, JHH = 7.4 Hz, 1H, major), 6.96–6.86 (m, 2H, major + minor), 6.80 (d, J = 7.8 Hz, 1H, minor), 6.46 (d, 5JPH = 11.0 Hz, 1H, CHOH, minor), 5.71 (dd, 5JPH = 7.1, 5JHH = 2.7 Hz, 1H, CHOH, major), 3.82 (s, 1H, OH, major), 2.55 (d, J = 13.3 Hz, 3H, PMe, major), 2.41 (d, J = 13.4 Hz, 3H, PMe, minor), 1.78 (s, 1H, OH, minor). 13C{1H} NMR (CD2Cl2) δ 142.8 (d, J = 4.9 Hz, C), 140.9 (d, J = 6.8 Hz, C), 139.8 (d, J = 6.2 Hz, C), 138.0 (C), 135.8 (d, J = 3.2 Hz, CH), 135.7 (d, J = 3.1 Hz, CH), 135.4 (d, J = 3.5 Hz, CH), 133.6 (d, J = 9.0 Hz, CH), 133.4 (d, J = 9.0 Hz, CH), 133.3 (d, J = 9.6 Hz, CH), 133.2 (d, J = 9.6 Hz, CH), 132.5 (C), 131.0 (C), 130.7 (d, J = 3.4 Hz, CH), 130.5 (CH), 130.4 (d, J = 2.9 Hz, CH), 130.3 (CH), 130.3 (CH), 130.2 (CH), 129.2 (d, J = 4.1 Hz, CH), 129.1 (CH), 128.8 (CH), 128.1 (CH), 127.2 (CH), 124.0 (C), 123.3 (d, J = 2.9 Hz, C), 122.5 (d, J = 3.3 Hz, C), 122.0 (C), 117.4 (d, J = 81.6 Hz C), 117.3 (d, J = 80.6 Hz, C), 115.7 (d, J = 61.1 Hz, C), 114.9 (d, J = 83.0 Hz, C), 88.8 (CHOH, major), 88.3 (d, J = 2.7 Hz CHOH, minor), 80.3 (d, J = 59.5 Hz, CHP, major), 78.9 (d, J = 65.0 Hz, CHP, minor), 4.9 (d, J = 55.0 Hz, PCH3, minor), 4.6 (d, J = 52.9 Hz, PCH3, major) ppm. 31P{1H} NMR (162 MHz, CD2Cl2) δ 20.3 (major), 19.9 (minor) ppm. 19F{1H} NMR (376 MHz, CD2Cl2) δ −150.69, −150.74 ppm. HRMS (ESI+) m/z calc. for [M]+ C27H24OP 395.1565, found 395.1580.
(10-hydroxy-9,10-dihydroanthr-9-yl)methyldiphenylphosphonium methanesulfonate (14MsO). Yellow amorphous solid, yield: 83%. Isomer ratio trans/cis: 1.2:1. 1H NMR (CD2Cl2) δ 7.94–7.71 (m, 7H, major + minor), 7.71–7.58 (m, 4H, major + minor), 7.54 (dd, J = 8.8, 2.7 Hz, 1H, major, PCH), 7.47 (dd, J = 7.6 Hz, 1H, major), 7.41–7.31 (m, 11H), 7.28 (dd, J = 8.3, 2.5 Hz, 1H, minor, PCH), 7.26–7.20 (m, 9H, major + minor), 7.17 (dd, J = 6.6, 2.0 Hz, 1H, major), 7.13 (d, J = 7.6 Hz, 1H, minor), 7.01–6.93 (d, J = 13.6 Hz, 1H, major), 6.88 (d, J = 7.8 Hz, 1H, minor), 6.85–6.79 (m, 2H, major + minor), 6.42 (dd, J = 11.0, 2.5 Hz, CHOH, 1H, minor), 5.64 (dd, J = 7.2, 2.7 Hz, 1H, CHOH, major), 2.69 (d, J = 13.6 Hz, 3H, PMe, major), 2.69 (s, 6H, SMe, major + minor)), 2.57 (d, J = 13.6 Hz, 3H, minor) ppm. 13C NMR (101 MHz, CD2Cl2) δ 142.9 (d, J = 4.8 Hz), 141.0 (d, J = 6.8 Hz), 140.1 (d, J = 6.2 Hz), 138.2, 135.5 (d, J = 3.2 Hz), 135.4 (d, J = 3.1 Hz), 135.2 (dd, J = 2.8 Hz), 133.8 (d, J = 9.0 Hz), 133.6 (d, J = 9.0 Hz), 133.5, 133.4 (d, J = 9.4 Hz), 133.0 (d, J = 2.4 Hz), 131.5, 130.5 (d, J = 3.7 Hz), 130.4, 130.3 (d, J = 2.6 Hz), 130.2 (d, J = 3.0 Hz), 130.0, 129.1, 129.1 (d, J = 2.8 Hz), 128.9, 128.8, 128.7, 128.0, 127.2, 123.9 (d, J = 2.6 Hz), 123.1 (d, J = 3.0 Hz), 122.7 (d, J = 3.2 Hz), 122.1 (d, J = 2.5 Hz), 117.7 (d, J = 80.7 Hz), 116.2 (d, J = 66.1 Hz), 115.4 (d, J = 67.6 Hz), 88.5 (CHOH, major), 88.1 (d, J = 2.8 Hz, CHOH, minor), 80.4 (d, J = 58.4 Hz, PCH, major), 78.9 (d, J = 64.1 Hz, PCH, minor), 39.4 (s, SMe), 5.1 (d, J = 54.2 Hz), 4.9 (d, J = 52.1 Hz). 31P{1H} NMR (162 MHz, CD2Cl2) δ 20.7 (s, major), 20.3 (s, minor) ppm. HRMS (ESI+) m/z calc. for [M]+ C27H24OP+ 395.1559, found 395.1562.
(10-hydroxy-9,10-dihydroanthr-9-yl)methyldiphenylphosphonium para-toluenesulfonate (14TsO). Yellow amorphous solid, yield: 85%. Isomer ratio trans/cis: 1.3:1. 1H NMR (CD2Cl2) δ 8.09–7.49 (m, 23H, major + minor), 7.48–7.17 (m, 13H, major + minor) 7.14 (d, J = 7.7 Hz, 1H, major), 7.10–7.03 (m, 6H, major + minor), 6.92 (d, J = 6.4 Hz, 1H, major), 6.83–6.75 (m, 4H, major + minor), 6.27 (dd, 5JPH = 10.7; 5JHH =2.9 Hz, 1H, CHOH, minor), 5.59 (dd, 5JPH = 7.2; 5JHH =2.7 Hz, 1H, CHOH, major), 2.69 (d, 2JPH = 13.6 Hz, 3H, major), 2.57 (d, 2JPH = 13.6 Hz, 3H, minor), 2.32 (s, 6H, major + minor) ppm. 13C{1H} NMR (CD2Cl2) δ 145.1, 142.8 (d, J = 4.9 Hz), 141.1 (d, J = 7.0 Hz), 140.3 (d, J = 6.2 Hz), 138.7, 138.4, 135.2 (d, J = 3.0 Hz), 135.1 (d, J = 3.1 Hz), 135.0 (d, J = 3.2 Hz), 134.9 (d, J = 3.2 Hz), 133.9 (d, J = 9.0 Hz), 133.7 (d, J = 4.3 Hz), 133.6 (d, J = 4.8 Hz), 133.5 (d, J = 9.6 Hz), 133.3 (d, J = 2.3 Hz), 131.7, 130.2 (d, J = 11.0 Hz), 130.0 (d, J = 13.2 Hz), 129.0, 128.89, 128.8, 128.7 (d, J = 2.0 Hz), 128.4, 128.0, 127.2, 125.9, 123.6 (d, J = 2.4 Hz), 122.9 (d, J = 3.2 Hz), 122.1 (d, J = 2.7 Hz), 117.8 (d, J = 80.6 Hz), 116.5 (d, J = 60.3 Hz), 115.7 (d, J = 61.7 Hz), 88.3 (CHOH, major), 87.8 (d, 4JCP = 3.1 Hz, CHOH, minor), 80.4 (d, J = 57.9 Hz, CHP, major), 78.7 (d, 1JCP = 64.0 Hz, CHP, minor), 21.1, 4.8 (d, 1JCP = 51.7 Hz), 4.8 (d, J = 51.9 Hz) ppm. 31P{1H} NMR (162 MHz, CD2Cl2) δ 21.0 (major), 20.5 (minor) ppm. HRMS (ESI+) m/z calc. for [M]+ C27H24OP+ 395.1559, found 395.1565.
(10-Hydroxy-9,10-dihydroanthr-9-yl)methyldiphenylphosphonium chloride (14Cl). White solid, mp: 78–80 °C, yield: 82%. Isomer ratio trans/cis: 1.4:1. 1H NMR (CD2Cl2) δ 8.32 (d, J = 7.8 Hz, 1H, major), 8.12 (d, J = 8.1 Hz, 2H, minor), 8.09 (d, J = 8.0 Hz, 1H, minor), 7.97–7.64 (m, 11H, major, minor), 7.64–7.54 (m, 5H, major, minor), 7.43 (dd, J = 7.4, 7.4 Hz, 2H, minor), 7.39–7.24 (m, 9H, major, minor), 7.23–7.11 (m, 4H, major, minor), 7.07 (d, J = 7.6 Hz, 1H, minor), 6.94–6.86 (m, 2H, major, minor), 6.71 (d, J = 7.6 Hz, 2H, minor), 6.34 (d, J = 11.2, 1H, minor), 5.46 (d, J = 7.6 Hz, 1H, major), 2.98 (d, J = 13.8 Hz, 3H, major), 2.87 (d, J = 13.9 Hz, 3H, minor) ppm. 13C{1H} NMR (101 MHz, CD2Cl2) δ 142.8 (d, J = 4.9 Hz, C), 141.0 (d, J = 7.0 Hz, C), 140.1 (d, J = 6.2 Hz, C), 138.4 (C), 135.2 (d, J = 3.1 Hz, CH), 135.1 (d, J = 2.9 Hz, CH), 134.9 (d, J = 3.5 Hz, CH), 134.9 (d, J = 4.1 Hz, CH), 134.0 (d, J = 7.5 Hz, CH), 133.4 (d, J = 6.3 Hz, CH), 133.8 (d, J = 2.9 Hz, CH), 133.7 (d, J = 3.3 Hz, CH), 132.0 (d, J = 12.6 Hz, CH), 131.9 (d, 5.6 Hz, CH), 130.2 (d, J = 3.5 Hz, CH), 130.1 (d, J = 12.3 Hz, CH), 130.0 (d, J = 12.3 Hz, CH), 129.9 (CH), 129.8 (d, J = 2.8 Hz, CH), 128.9 (d, J = 2.9 Hz, CH), 128.8 (d, J = 2.7 Hz, CH), 128.6 (CH), 128.6 (CH), 128.4 (d, J = 4.1 Hz, CH), 128.3 (CH), 127.9 (CH), 127.2 (CH), 123.7 (d, J = 2.2 Hz, CH), 123.2 (d, J = 3.5 Hz, CH), 122.8 (d, J = 3.2 Hz, CH), 122.2 (d, J = 2.7 Hz, CH), 117.8 (d, J = 79.5 Hz, PC), 117.2 (d, J = 53.8 Hz, PC), 115.7 (d, J = 81.9 Hz, PC), 88.3 (CHOH), 87.9 (d, J = 2.8 Hz, CHOH), 80.9 (d, J = 57.1 Hz, PCH), 79.4 (d, J = 62.6 Hz, PCH), 6.2 (d, J = 50.6 Hz, PCH3), 6.1 (d, J = 53.0 Hz, PCH3) ppm. 31P{1H} NMR (162 MHz, CD2Cl2) δ 20.7 (s, major), 20.5 (s, minor) ppm. IR (cm−1): 3393, 2876, 1457, 1438, 1114, 1016, 900, 750. HRMS (ESI+) m/z calc. for [M]+ C27H24OP+ 395.1559, found 395.1561.
(10-Hydroxy-9,10-dihydroanthr-9-yl)methyldiphenylphosphonium hexafluorophosphate (14PF6). White solid, mp: 102–104 °C, yield: 83%. Isomer ratio trans/cis: 1.4:1. 1H NMR (CD2Cl2) δ 7.97–7.88 (m, 2H, major, minor), 7.88–7.60 (m, 18H, major, minor), 7.51 (dd, J = 7.1, 7.1, 1H, major), 7.44 (dd, J = 7.6, 7.6 Hz, 1H, major), 7.41–7.33 (m, 6H, major, minor), 7.28 (dd, J = 8.0, 8.0 Hz, 2H, major, minor), 7.26 (d, J = 11.1 Hz, 1H, PCH, major), 7.24–7.16 (m, 3H, major, minor), 7.04 (dd, J = 9.1, 2.8 Hz, 1H, PCH minor), 7.01 (d, J = 8.6 Hz, 2H, major), 6.89 (d, J = 7.0 Hz, 2H, minor), 6.76 (d, J = 7.9 Hz, 1H, minor), 6.46 (dd, J = 11.1, 2.5 Hz, 1H. CH(OH), minor), 5.74 (dd, J = 7.1, 2.7 Hz, 1H, CH(OH), major), 2.57 (d, J = 13.3 Hz, 3H, PCH3, major), 2.42 (d, J = 13.4 Hz, 3H, PCH3, minor) ppm. 13C{1H} NMR (CD2Cl2) δ 142.7 (d, J = 4.7 Hz), 140.8 (d, J = 6.7 Hz), 139.7 (d, J = 6.3 Hz), 137.9, 135.8 (d, J = 3.2 Hz), 135.9 (d, J = 3.0 Hz), 135.5 (d, J = 3.6 Hz), 135.4 (d, J = 3.5 Hz), 133.6 (d, J = 8.7 Hz), 133.4 (d, J = 9.1 Hz), 133.3 (d, J = 9.9 Hz), 133.2 (d, J = 9.5 Hz), 132. 4 (d, J = 2.6 Hz), 132.0 (d, J = 2.8 Hz), 130.8, 130.69 (d, J = 19.8 Hz), 130.4 (d, J = 2.8 Hz), 130.4 (d, J = 16.6 Hz), 130.3 (d, J = 17.0 Hz), 129.2 (d, J = 13.4 Hz), 129.2 (d, J = 3.2 Hz), 128.8, 128.0, 127.2, 124.1 (d, J = 2.4 Hz), 123.4 (d, J = 3.2 Hz), 122.4 (d, J = 3.3 Hz), 122.0, 117.2 (d, J = 80.2 Hz, PC), 117.3 (d, J = 81.4 Hz, PC), 114.7 (d, J = 83.0 Hz, PC), 115.3 (d, J = 81.8 Hz, PC), 88.8 (CHOH, major), 88.4 (d, J = 2.7 Hz, CHOH, minor), 80.4 (d, J = 59.7 Hz, PCH, major), 79.0 (d, J = 64.8 Hz, PCH, minor), 5.01 (d, J = 55.3 Hz, PCH3, minor), 4.8 (d, J = 53.0 Hz, PCH3, major) ppm. 31P{1H} NMR (CD2Cl2) δ 20.2 (s, major), 19.7 (s, minor), −144.4 (hept, J = 711.2 Hz) ppm. 19F{1H} NMR (376 MHz, CD2Cl2) δ −72.59 (d, J = 711.2 Hz) ppm. IR (cm−1): 3424, 2924, 1439, 1118, 1023, 896, 839, 746, 689, 557. HRMS (ESI+) m/z calc. for [M]+ C27H24OP+ 395.1559, found 395.1559.
(10-Hydroxy-2,3,4-trimethoxy-9,10-dihydroanthr-9-yl)methyldiphenylphosphonium tetrafluoroborate (15BF4). Yellowish amorphous solid, yield: 73%. Isomer ratio trans/cis: 3.1:1. 1H NMR (500 MHz, CD2Cl2) δ 7.91–7.83 (m, 2H, CHPh, major + minor), 7.83–7.78 (m, 2H, CHPh, major + minor), 7.78–7.61 (m, 14H CHPh, 2H CHAn, major + minor), 7.58 (ddd, JHH = 7.9, 7.9 Hz, JPH = 3.6 Hz 2H, CHPh, minor), 7.50 (dd, JHH = 7.5, 7.5 Hz, 1H, CHAn, minor), 7.40 (dd, JHH = 7.5, 7.5 Hz, 1H, CHAn, major), 7.35 (dd, JHH = 7.6, 7.6 Hz, 1H, CHAn, minor), 7.33 (dd, JHH = 8.1, 8.1 Hz, 1H, CHAn, major), 7.27 (d, J = 7.7 Hz, 1H, CHAn, minor), 7.19 (dd, 2JPH = 9.8, JHH = 2.8 Hz, 1H, CHP, major), 7.01 (d, 3JHH = 7.6 Hz, 1H, CHAn, major), 6.93 (dd, 3JHH = 7.7, 4JPH = 1.2 Hz, 1H, CHAn, major), 6.88 (dd, 2JPH = 8.7, JHH = 2.8 Hz, 1H, CHP, minor), 6.70 (d, 3JHH = 7.8 Hz, 1H, CHAn, minor), 6.42 (s, 2H, major), 6.41 (dd, 5JPH = 7.1, JHH = 2.8 Hz, 1H, CHOH, minor), 6.29 (s, 2H, minor), 5.67 (dd, 5JPH = 7.0, 3JHH = 2.8 Hz, 1H, CHOH, major), 3.77 (s, 3H, minor), 3.75 (s, 6H, major), 3.71 (s, 3H, major), 3.68 (s, 6H, minor), 2.50 (d, J = 13.3 Hz, 3H, major), 2.34 (d, J = 13.4 Hz, 3H, minor), 1.87 (br s, 1H, OH) ppm. 13C{1H} NMR (101 MHz, CD2Cl2) δ 153.6 (C), 153.5 (C), 142.6 (d, J = 4.9 Hz, C), 140.3 (d, J = 6.7 Hz, C), 138.7 (C), 138.4 (C), 135.7 (d, J = 3.1 Hz, C), 135.6 (d, J = 3.1 Hz, CH), 135.5 (d, J = 6.4 Hz, CH), 135.4 (d, J = 3.1 Hz, CH), 135.3 (d, J = 3.1 Hz, CH), 133.5 (d, J = 9.1 Hz, CH), 133.4 (d, J = 9.0 Hz, CH), 133.2 (d, J = 9.6 Hz, CH), 133.2 (J = 9.5 Hz, CH), 132.7 (C), 131.0 (C), 130.6 (d, J = 3.5 Hz, CH), 130.4 (d, J = 11.9 Hz, CH), 130.3 (d, J = 10.2 Hz, CH), 130.2 (d, J = 10.2 Hz, CH), 130.2 (d, J = 12.4 Hz, CH), 129.4 (d, J = 3.0 Hz, CH), 129.2 (d, J = 3.1 Hz, CH), 124.1 (d, J = 2.4 Hz, CH), 123.2 (d, J = 3.0 Hz, CH), 122.5 (d, J = 3.1 Hz, CH), 122.2 (d, J = 2.3 Hz, CH), 117.5 (d, J = 81.9 Hz, PCPh, minor), 117.4 (d, J = 80.3 Hz, PCPh, major), 115.5 (d, J = 81.6 Hz, PCPh, major), 115.4 (d, J = 82.3 Hz, PCPh, minor), 105.3 (CH), 104.1 (CH), 88.9 (CHOH, major), 88.2 (d, J = 2.4 Hz, CHOH, minor), 80.2 (d, J = 59.4 Hz, PCH, major), 78.7 (d, J = 64.2 Hz, PCH, minor), 60.4 (OMe), 60.3 (OMe), 56.1 (OMe), 56.1 (OMe), 4.6 (d, J = 53.2 Hz, PMe), 4.4 (d, J = 55.2 Hz, PMe) ppm. 31P{1H} NMR (162 MHz, CD2Cl2) δ 20.3 (major), 19.6 (minor) ppm. 19F{1H} NMR (CD2Cl2) δ −151.38, −151.43 ppm. HRMS (ESI+) m/z calc. for [M+] C30H30O4P+ 485.1882, found 485.1900.
(10-Hydroxy-9,10-dihydroanthr-9-yl)dimethylphenylphosphonium tetrafluoroborate (16BF4). White amorphous solid, yield: 75%. Isomer ratio trans/cis: 1.2:1. 1H NMR (CD2Cl2) δ 7.89–7.76 (m, 2H), 7.69–7.55 (m, 7H), 7.53–7.29 (m, 11H), 7.24–7.14 (m, 4H), 7.03–6.95 (m, 3H), 6.66 (dd, J = 9.6, 2.8 Hz, 1H, major), 6.50–6.38 (m, 2H), 5.74 (dd, J = 7.0, 2.8 Hz, 1H, minor), 2.36 (d, J = 12.8 Hz, 3H, major), 2.35 (d, J = 13.9 Hz, 3H, major), 2.28 (d, J = 13.8 Hz, 3H, minor), 2.16 (d, J = 13.8 Hz, 3H, minor) ppm. 13C{1H} NMR (101 MHz, CD2Cl2) δ 139.9, 138.2, 135.2 (d, J = 9.6 Hz), 132.8, 132.2 (d, J = 9.0 Hz), 132.0 (d, J = 9.0 Hz), 131.2, 130.5, 130.1, 130.0, 130.0, 129.9, 129.23, 129.0, 128.8, 128.7, 128.1, 127.1, 123.6 (d, J = 82.4 Hz), 122.2, 121.8, 115.9, 88.5, 88.0, 80.3 (d, J = 60.1 Hz), 79.1 (d, J = 64.4 Hz), 5.5 (d, J = 52.3 Hz), 4.9 (d, J = 50.3 Hz), 3.6 (d, J = 53.8 Hz), 3.4 (d, 54.9 Hz) ppm. 31P{1H} NMR (CD2Cl2) δ 23.9 (major), 23.1 (minor) ppm. 11B{1H} NMR (CD2Cl2) δ −1.02 ppm. HRMS (ESI+) m/z calc. for [M]+ C22H22OP+ 333.1403, found 333.1405.
3.2.3. General Procedure for Replacing Chloride Anion with PF6− or BPh4−: Synthesis of Phosphonium Salts trans-13PF6 and 14BPh4
To a solution of the corresponding chloride trans-13Cl or 14Cl (1 mmol) in DCM (3 mL), 1 equiv KPF6 or KBPh4 was added and stirred for 3 h at room temperature. The resulting KCl was separated, and the filtrate was concentrated to a white precipitate that was dried using a vacuum pump.
Trans-(10-hydroxy-2,3,4-trimethoxy-9,10-dihydroanthr-9-yl)triphenylphosphonium hexafluorophosphate (trans-13PF6). White amorphous solid, yield: 83%. 1H NMR (CDCl3) δ 7.89–7.80 (m, 3H), 7.70–7.52 (m, 12H, Ar, PCH), 7.40–7.27 (m, 3H), 7.20 (dd, J = 7.4 Hz, 1H), 6.93 (d, J = 7.7 Hz, 1H), 6.64 (dd, J = 7.8, 2.0 Hz, 1H), 5.18 (dd, 5JPH = 7.3, 5JHH = 2.7 Hz, 1H, CHOH), 3.78 (s, 9H, OMe) ppm. 13C{1H} NMR (methanol-d4) δ 153.4, 143.6 (d, J = 5.0 Hz), 137.9, 137.1 (d, J = 10.8 Hz), 136.2 (d, J = 6.9 Hz), 135.5 (d, J = 3.3 Hz), 134.6, 134.4 (d, J = 9.2 Hz), 133.3 (d, J = 19.7 Hz), 131.2, 130.6, 130.2 (d, J = 12.1 Hz), 128.7, 128.6, 128.3 (d, J = 6.9 Hz), 127.9, 123.0 (d, J = 4.0 Hz), 122.7 (d, J = 9.0 Hz), 122.3 (d, J = 3.0 Hz), 121.8 (d, J = 4.0 Hz) 116.2 (d, J = 81.6 Hz), 103.9, 103.7, 88.7 (CHOH), 81.2 (d, J = 57.7 Hz. CHP), 59.7, 55.3 ppm. 31P{1H} NMR (methanol-d4) δ 18.03, −144.29 (hept, J = 713.0 Hz) ppm. 19F{1H} NMR (methanol-d4) δ −74.00 (d, J = 707.9 Hz) ppm. HRMS (ESI+) m/z calc. for [M]+ C35H30O3P 529.1933, found 529.1925.
(10-Hydroxy-9,10-dihydroanthr-9-yl)methyldiphenylphosphonium tetraphenylborate (14BPh4). White solid, mp: 74–76 °C, yield: 88%. Isomer ratio trans/cis: 1.5:1. 1H NMR (CD2Cl2) δ 7.90 (dd, J = 8.3, 8.3 Hz, 2H, major, minor), 7.83 (dd, J = 8.3, 8.3 Hz, 2H, major, minor), 7.73–7.65 (m, 4H, major, minor), 7.63–7.37 (m, 32H, major, minor), 7.33 (dd, J = 8.2, 8.2 Hz, 1H, major), 7.26–7.15 (m, 1H major, 2H minor), 7.06 (d, J = 7.7 Hz, 1H, major), 7.00 (t, J = 7.3 Hz, 22H, major, minor), 6.95 (d, J = 7.8 Hz, 1H, major), 6.89–6.81 (m, 8H, major, minor), 6.59 (d, J = 7.4 Hz, 1H major PCH, 1H minor), 6.49 (d, J = 7.9 Hz, 1H, minor), 6.43 (d, J = 11.0 Hz, 1H, minor, CHOH), 6.38 (d, J = 9.7 Hz, 1H, minor, PCH), 5.78 (dd, J = 7.4, 3.1 Hz, 1H, major, CHOH), 1.63 (d, J = 12.9 Hz, 3H, major), 1.61 (d, J = 13.0 Hz, 3H, minor) ppm. 13C{1H} NMR (CD2Cl2) δ 164.8, 164.3, 163.8, 163.3, 142.6 (d, J = 6.0 Hz), 140.8 (d, J = 6.0 Hz), 139.4 (d, J = 6.0 Hz), 137.7, 136.0, 135.6 (d, J = 3.2 Hz), 135.5 (d, J = 3.2 Hz), 133.5 (d, J = 8.9 Hz), 133.3 (d, J = 9.1 Hz), 132.0 (d, J = 9.5 Hz), 133.0 (d, J = 9.5 Hz), 131.1 (d, J = 3.2 Hz), 130.6 (d, J = 5.1 Hz), 130.5 (d, J = 4.7 Hz), 130.4 (d, J = 4.7 Hz), 130.3 (d, J = 4.7 Hz), 129.4 (d, J = 9.1 Hz), 129.3 (d, J = 3.2 Hz), 128.9, 128.4 (d, J = 6.6 Hz), 128.1, 127.2, 125.8 (d, J = 2.8 Hz), 125.7 (d, J = 2.8 Hz), 124.2, 123.6 (d, J = 2.8 Hz), 122.1 (d, J = 3.3 Hz), 121.8, 117.5 (d, J = 24.6 Hz), 116.6 (d, J = 23.7 Hz), 114.7 (d, J = 81.0 Hz), 114.2 (d, J = 81.0 Hz), 89.0, 88.5, 80.3 (d, J = 60.6 Hz), 79.0 (d, J = 65.2 Hz), 4.1 (d, J = 55.0 Hz), 3.5 (d, J = 53.0 Hz) ppm. 31P{1H} NMR (CD2Cl2) δ 19.7 (s, major), 19.1 (s, minor) ppm. 11B{1H} NMR (CD2Cl2) δ −6.54 ppm. IR (cm−1): 3446, 3054, 1579, 1437, 1117, 1030, 1018, 893, 734, 705, 611. HRMS (ESI+) m/z calc. for [M]+ C27H24OP+ 395.1559, found 395.1560.
3.3. Synthesis of Phosphonium Salt 18I
To a solution of 9-bromoanthracene (0.62 g, 2.4 mmol) in dry THF (10 mL), n-butyllithium (0.9 mL, 2.7 M solution in hexane) was added dropwise at −78 °C and stirred for 30 min under argon. Then, a solution of chlorodiphenylphosphine (0.532 g, 2.4 mmol) in dry THF (7 mL) was added. The mixture was left at room temperature overnight, and then the solvent was evaporated and DMF (15 mL) and methyl iodide (2.2 mL, 3.6 mmol) were added. The homogeneous solution was heated under argon overnight at 90 °C. The solvent and excess methyl iodide were evaporated using a vacuum line. The obtained yellow precipitate was purified using a column filled with neutral aluminum oxide, eluting with a mixture of ethyl acetate/methanol (1:1 v/v).
Anthr-9-yl-methyldiphenylphosphonium iodide (18I). Yellow solid, mp: 138–140 °C, yield: 74%. 1H NMR (CD2Cl2) δ 9.11 (s, 1H), 8.34–8.26 (m, 2H), 7.95–7.86 (m, 2H), 7.85–7.71 (m, 8H), 7.67–7.59 (m, 4H), 7.52–7.44 (m, 2H), 3.17 (d, J = 12.4 Hz, 3H) ppm. 13C{1H} NMR (CD2Cl2) δ 140.2, 139.0, 135.5, 135.12, 132.6 (d, J = 10.9 Hz), 131.6, 130.9 (d, J = 13.0 Hz), 130.8, 129.5, 126.1, 124.6 (d, J = 9.1 Hz), 122.3 (d, J = 85.8 Hz), 16.8 (d, J = 59.7 Hz) ppm. 31P{1H} NMR (CD2Cl2) δ 17.4 ppm. HRMS (ESI+) m/z calc. for [M]+ C27H22P+ 377.1459, found 377.1462.
4. Conclusions
In summary, we performed a study in which we attempted to obtain anthryl phosphonium salts that, due to the presence of a positively charged phosphonium group, would exhibit a strong donor–acceptor effect. However, the Friedel–Crafts–Bradsher reaction usually used for this purpose, after the cyclization step, stopped at the stage of the intermediate anthrol, which did not undergo the further the expected Bradsher dehydration reaction, leading to a fully aromatic system, which is the first example of such behavior of diarylmethyl derivatives in the literature. Quaternary phosphonium salts are extensively used in organic synthesis as Lewis acid organocatalysts [21,29,30], reagents [23,31] and ionic liquids [32], and they also demonstrate a strong antimicrobial activity [33,34]. Therefore, the results obtained provide stimulus for further research into this phenomenon, especially in terms of studying the intramolecular interactions involving various anions and other 1,2-disubstituted anthrols.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25031741/s1.
Author Contributions
Conceptualization, K.O., E.R.-S. and P.B.; synthesis, K.O.; methodology, K.O., E.R.-S. and P.B.; software E.R.-S., M.T. and K.O.; DFT calculations, E.R.-S. and M.T.; validation, K.O., E.R.-S. and P.B.; formal analysis, K.O., E.R.-S. and P.B.; investigations, K.O. and E.R.-S.; resources K.O., E.R.-S., V.V., B.D., Ł.K. and M.K.; data curation, K.O., E.R.-S. and M.T.; writing—original draft preparation, K.O., E.R.-S., P.B. and M.T.; writing—review and editing, K.O., E.R.-S. and P.B.; visualization, K.O., E.R.-S. and M.T.; supervision, K.O., E.R.-S. and P.B.; project administration, P.B.; funding acquisition, P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out within the research project (2019-2025) No. 2019/33/B/ST4/02843, financed by the National Science Centre (Poland). Purchase of the Avance 400 Neo NMR and Avance III 500 NMR spectrometer used to obtain the results included in this publication was supported by funds from the EU Regional Operational Program of the Lodz Region, RPLD.01.01.00-10-0008/18.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and supplementary material.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kousseff, C.J.; Halaksa, R.; Parr, Z.S.; Nielsen, C.B. Mixed Ionic and Electronic Conduction in Small-Molecule Semiconductors. Chem. Rev. 2022, 122, 4397–4419. [Google Scholar] [CrossRef]
- Peng, Z.; Stingelin, N.; Ade, H.; Michels, J.J. A materials physics perspective on structure–processing–function relations in blends of organic semiconductors. Nat. Rev. Mater. 2023, 8, 439–455. [Google Scholar] [CrossRef]
- Zhu, J.; Wen, W.; Tian, Z.; Zhang, X.; Wang, S. Covalent organic framework: A state-of-the-art review of electrochemical sensing applications. Talanta 2023, 260, 124613. [Google Scholar] [CrossRef] [PubMed]
- Khasbaatar, A.; Xu, Z.; Lee, J.-H.; Campillo-Alvarado, G.; Hwang, C.; Onusaitis, B.N.; Diao, Y. From Solution to Thin Film: Molecular Assembly of π-Conjugated Systems and Impact on (Opto)electronic Properties. Chem. Rev. 2023, 123, 8395–8487. [Google Scholar] [CrossRef]
- Kumar, K. Charge transporting and thermally activated delayed fluorescence materials for OLED applications. Phys. Chem. Chem. Phys. 2024. [Google Scholar] [CrossRef]
- Nawaz, A.; Merces, L.; Ferro, L.M.M.; Sonar, P.; Bufon, C.C.B. Impact of Planar and Vertical Organic Field-Effect Transistors on Flexible Electronics. Adv. Mater. 2023, 35, 2204804. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Y.; Meng, L.; Han, C.; Wan, X.; Chen, Y. Recent Progress in All-Small-Molecule Organic Solar Cells. Small 2023, 19, 2205594. [Google Scholar] [CrossRef]
- Essahili, O.; Ouafi, M.; Moudam, O. Recent progress in organic luminescent solar concentrators for agrivoltaics: Opportunities for rare-earth complexes. Sol. Energy 2022, 245, 58–66. [Google Scholar] [CrossRef]
- Gunnarsson, W.B.; Roh, K.; Zhao, L.; Murphy, J.P.; Grede, A.J.; Giebink, N.C.; Rand, B.P. Toward Nonepitaxial Laser Diodes. Chem. Rev. 2023, 123, 7548–7584. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.E. Functionalized Acenes and Heteroacenes for Organic Electronics. Chem. Rev. 2006, 106, 5028–5048. [Google Scholar] [CrossRef]
- Deori, U.; Ravindran, E.; Nanda, G.P.; Ramanath, S.G.; Acharya, U.; Gandeepan, P.; Rajamalli, P. Design Strategy for Enabling Multifunctional Properties of Anthracene-based Emitters. Chem. Asian J. 2023, 18, e202300352. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Cao, W.; Zhang, M.; Li, F. Efficient blue fluorescent OLEDs based on a D–π–A emitter with the hybridized localized and charge-transfer state. J. Mater. Chem. C 2023, 11, 6695–6701. [Google Scholar] [CrossRef]
- Siddiqui, I.; Kumar, S.; Tsai, Y.-F.; Gautam, P.; Shahnawaz; Kesavan, K.; Lin, J.-T.; Khai, L.; Chou, K.-H.; Choudhury, A.; et al. Status and Challenges of Blue OLEDs: A Review. Nanomaterials 2023, 13, 2521. [Google Scholar] [CrossRef] [PubMed]
- Owsianik, K.; Różycka-Sokołowska, E.; Bałczewski, P. O,S-Acetals in a New Modification of oxo-Friedel–Crafts–Bradsher Cyclization—Synthesis of Fluorescent (Hetero)acenes and Mechanistic Considerations. Molecules 2023, 28, 2474. [Google Scholar] [PubMed]
- Bałczewski, P.; Kowalska, E.; Różycka-Sokołowska, E.; Skalik, J.; Owsianik, K.; Koprowski, M.; Marciniak, B.; Guziejewski, D.; Ciesielski, W. Mono-Aryl/Alkylthio-Substituted (Hetero)acenes of Exceptional Thermal and Photochemical Stability by the Thio-Friedel–Crafts/Bradsher Cyclization Reaction. Chem. Eur. J. 2019, 25, 14148–14161. [Google Scholar] [CrossRef] [PubMed]
- Bałczewski, P.; Kowalska, E.; Różycka-Sokołowska, E.; Uznański, P.; Wilk, J.; Koprowski, M.; Owsianik, K.; Marciniak, B. Organosulfur Materials with High Photo- and Photo-Oxidation Stability: 10-Anthryl Sulfoxides and Sulfones and Their Photophysical Properties Dependent on the Sulfur Oxidation State. Materials 2021, 14, 3506. [Google Scholar] [CrossRef] [PubMed]
- Bałczewski, P.; Kowalska, E.; Skalik, J.; Koprowski, M.; Owsianik, K.; Różycka-Sokołowska, E. Ultrasound-assisted synthesis of RO- and RS-substituted (hetero)acenes via oxo- and thio-Friedel-Crafts/Bradsher reactions. Ultrason. Sonochem. 2019, 58, 104640. [Google Scholar] [CrossRef]
- Koprowski, M.; Owsianik, K.; Knopik, Ł.; Vivek, V.; Romaniuk, A.; Różycka-Sokołowska, E.; Bałczewski, P. Comprehensive Review on Synthesis, Properties, and Applications of Phosphorus (PIII, PIV, PV) Substituted Acenes with More Than Two Fused Benzene Rings. Molecules 2022, 27, 6611. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, A.; Chou, P.-T.; Koshevoy, I.O. Cationic Organophosphorus Chromophores: A Diamond in the Rough among Ionic Dyes. Chem. Eur. J. 2021, 27, 537–552. [Google Scholar] [CrossRef]
- Belyaev, A.; Cheng, Y.-H.; Liu, Z.-Y.; Karttunen, A.J.; Chou, P.-T.; Koshevoy, I.O. A Facile Molecular Machine: Optically Triggered Counterion Migration by Charge Transfer of Linear Donor-π-Acceptor Phosphonium Fluorophores. Angew. Chem. Int. Ed. 2019, 58, 13456–13465. [Google Scholar] [CrossRef]
- Noroozi-Shad, N.; Gholizadeh, M.; Sabet-Sarvestani, H. Quaternary phosphonium salts in the synthetic chemistry: Recent progress, development, and future perspectives. J. Mol. Struct. 2022, 1257, 132628. [Google Scholar] [CrossRef]
- Ammer, J.; Nolte, C.; Karaghiosoff, K.; Thallmair, S.; Mayer, P.; de Vivie-Riedle, R.; Mayr, H. Ion-Pairing of Phosphonium Salts in Solution: C–H⋅⋅⋅Halogen and C–H⋅⋅⋅π Hydrogen Bonds. Chem. Eur. J. 2013, 19, 14612–14630. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.N.; Massarwe, F.; Shioukhi, I.; Masarwa, A. Sequential Selective C–H and C(sp3)–+P Bond Functionalizations: An Entry to Bioactive Arylated Scaffolds. Angew. Chem. Int. Ed. 2021, 60, 26199–26209. [Google Scholar] [CrossRef] [PubMed]
- Bradsher, C.K. 9-Methyl- and 10-Methyl-1,2-benzanthracene. J. Am. Chem. Soc. 1940, 62, 1077–1078. [Google Scholar] [CrossRef]
- Villemin, E.; Marchand-Brynaert, J. Cycloaddition/Aromatization Sequence for the Synthesis of 2,3-Disubstituted Benzenephosphonates. Synthesis 2012, 44, 1923–1927. [Google Scholar] [CrossRef][Green Version]
- Ohmori, H.; Nakai, S.; Masui, M. Anodic Phosphonylation of Anthracene. Chem. Pharm. Bull. 1979, 27, 1271–1273. [Google Scholar] [CrossRef][Green Version]
- Yamaguchi, S.; Akiyama, S.; Tamao, K. The coordination number–photophysical properties relationship of trianthrylphosphorus compounds: Doubly locked fluorescence of anthryl groups. J. Organomet. Chem. 2002, 646, 277–281. [Google Scholar] [CrossRef]
- Nikitin, K.; Jennings, E.V.; Al Sulaimi, S.; Ortin, Y.; Gilheany, D.G. Dynamic Cross-Exchange in Halophosphonium Species: Direct Observation of Stereochemical Inversion in the Course of an SN2 Process. Angew. Chem. Int. Ed. 2018, 57, 1480–1484. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, H.; Guo, H. Recent Advances in Phosphonium Salt Catalysis. Adv. Synth. Catal. 2021, 363, 2023–2036. [Google Scholar] [CrossRef]
- Werner, T. Phosphonium Salt Organocatalysis. Adv. Synth. Catal. 2009, 351, 1469–1481. [Google Scholar] [CrossRef]
- Maddigan-Wyatt, J.; Hooper, J.F. Phosphorus Compounds as Precursors and Catalysts for Radical C–C Bond-Forming Reactions. Adv. Synth. Catal. 2021, 363, 924–936. [Google Scholar] [CrossRef]
- Khazalpour, S.; Yarie, M.; Kianpour, E.; Amani, A.; Asadabadi, S.; Seyf, J.Y.; Rezaeivala, M.; Azizian, S.; Zolfigol, M.A. Applications of phosphonium-based ionic liquids in chemical processes. J. Iran. Chem. Soci. 2020, 17, 1775–1917. [Google Scholar] [CrossRef]
- Ibrahim, M.K.; Haria, A.; Mehta, N.V.; Degani, M.S. Antimicrobial potential of quaternary phosphonium salt compounds: A review. Future Med. Chem. 2023, 15, 2113–2141. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).