Omega-3 Recovers Cannabinoid 1 Receptor Expression in the Adult Mouse Brain after Adolescent Binge Drinking
Abstract
1. Introduction
2. Results
N-3 and Brain CB1 Receptor Expression
3. Discussion
3.1. Long-Lasting Effect of Adolescent Binge Drinking on CB1 Receptor Expression
3.2. N-3 Recovers CB1 Receptor Expression in the Brain
4. Material & Methods
4.1. Generation of CB1-KO
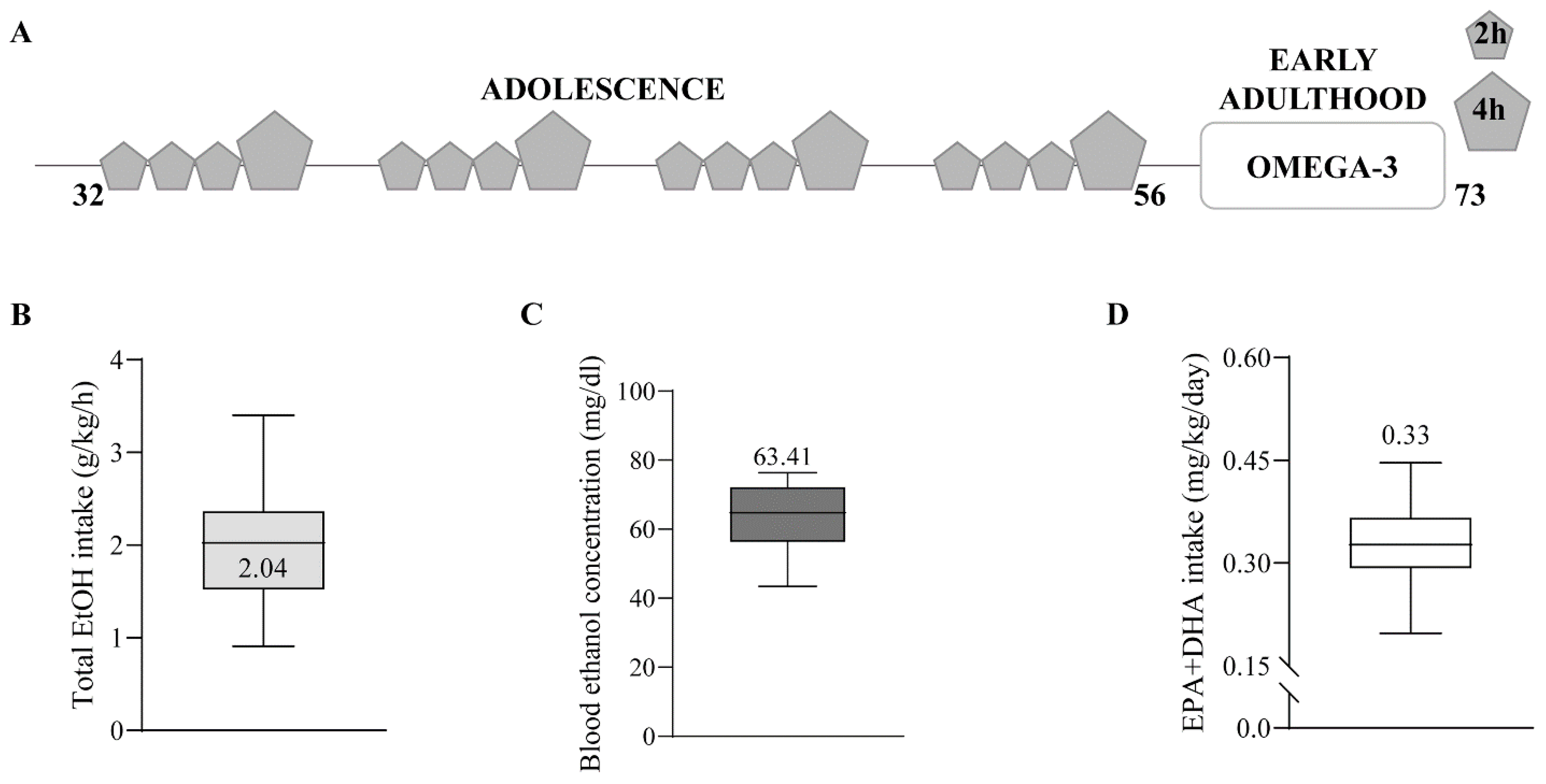
4.2. Animal Treatment
4.3. Antibody Characterization
4.4. Immunohistochemistry for Light Microscopy
4.5. Semiquantitative Analysis of CB1 Receptor Optical Density
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, H.-C.; Mackie, K. An introduction to the endogenous cannabinoid system. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rodríguez, A.; Bonilla-Del Río, I.; Puente, N.; Gómez-Urquijo, S.M.; Fontaine, C.J.; Egaña-Huguet, J.; Elezgarai, I.; Ruehle, S.; Lutz, B.; Robin, L.M.; et al. Localization of the cannabinoid type-1 receptor in subcellular astrocyte compartments of mutant mouse hippocampus. Glia 2018, 66, 1417–1431. [Google Scholar] [CrossRef] [PubMed]
- Hebert-Chatelain, E.; Desprez, T.; Serrat, R.; Bellocchio, L.; Soria-Gomez, E.; Busquets-Garcia, A.; Pagano Zottola, A.C.; Delamarre, A.; Cannich, A.; Vincent, P.; et al. A cannabinoid link between mitochondria and memory. Nature 2016, 539, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Katona, I.; Freund, T.F. Multiple functions of endocannabinoid signaling in the brain. Annu. Rev. Neurosci. 2012, 35, 529–558. [Google Scholar] [CrossRef]
- Carbia, C.; Cadaveira, F.; Caamaño-Isorna, F.; Rodríguez-Holguín, S.; Corral, M. Binge drinking during adolescence and young adulthood is associated with deficits in verbal episodic memory. PLoS ONE 2017, 12, e0171393. [Google Scholar] [CrossRef] [PubMed]
- Lutz, B.; Marsicano, G.; Maldonado, R.; Hillard, C.J. The endocannabinoid system in guarding against fear, anxiety and stress. Nat. Rev. Neurosci. 2015, 16, 705–718. [Google Scholar] [CrossRef]
- Pava, M.; Woodward, J. A review of the interactions between alcohol and the endocannabinoid system: Implications for alcohol dependence and future directions for research. Alcohol 2012, 46, 185–204. [Google Scholar] [CrossRef]
- Rico-Barrio, I.; Peñasco, S.; Lekunberri, L.; Serrano, M.; Egaña-Huguet, J.; Mimenza, A.; Soria-Gomez, E.; Ramos, A.; Buceta, I.; Gerrikagoitia, I.; et al. Environmental enrichment rescues endocannabinoid-dependent synaptic plasticity lost in young adult male mice after ethanol exposure during adolescence. Biomedicines 2021, 9, 825. [Google Scholar] [CrossRef]
- Mitrirattanakul, S.; López-Valdés, H.E.; Liang, J.; Matsuka, Y.; Mackie, K.; Faull, K.F.; Spigelman, I. Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcohol. Clin. Exp. Res. 2007, 31, 855–867. [Google Scholar] [CrossRef]
- Vinod, K.Y.; Kassir, S.A.; Hungund, B.L.; Cooper, T.B.; Mann, J.J.; Arango, V. Selective alterations of the CB1 receptors and the fatty acid amide hydrolase in the ventral striatum of alcoholics and suicides. J. Psychiatr. Res. 2010, 44, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Ceccarini, J.; Hompes, T.; Verhaeghen, A.; Casteels, C.; Peuskens, H.; Bormans, G.; Claes, S.; Van Laere, K. Changes in cerebral CB1 receptor availability after acute and chronic alcohol abuse and monitored abstinence. J. Neurosci. 2014, 34, 2822–2831. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, J.; Zanotti-Fregonara, P.; Umhau, J.C.; George, D.T.; Rallis-Frutos, D.; Lyoo, C.H.; Li, C.T.; Hines, C.S.; Sun, H.; Terry, G.E.; et al. Reduced cannabinoid CB 1 receptor binding in alcohol dependence measured with positron emission tomography. Mol. Psychiatry 2013, 18, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, P.; Colombo, G.; Carai, M. Blockade of the cannabinoid cb1 receptor and alcohol dependence: Preclinical evidence and preliminary clinical data. CNS Neurol. Disord. Drug Targets 2010, 9, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Peñasco, S.; Rico-Barrio, I.; Puente, N.; Fontaine, C.J.; Ramos, A.; Reguero, L.; Gerrikagoitia, I.; Rodríguez de Fonseca, F.; Barrondo, S.; Aretxabala, X.; et al. Intermittent ethanol exposure during adolescence impairs cannabinoid type 1 receptor- dependent long-term depression and recognition memory in adult mice. Neuropsychopharmacology 2020, 45, 309–318. [Google Scholar] [CrossRef]
- Joffre, C.; Rey, C.; Layé, S. N-3 polyunsaturated fatty acids and the resolution of neuroinflammation. Front. Pharmacol. 2019, 10, 1022. [Google Scholar] [CrossRef]
- Patten, A.R.; Brocardo, P.S.; Christie, B.R. Omega-3 supplementation can restore glutathione levels and prevent oxidative damage caused by prenatal ethanol exposure. J. Nutr. Biochem. 2013, 24, 760–769. [Google Scholar] [CrossRef]
- Patten, A.R.; Sickmann, H.M.; Dyer, R.A.; Innis, S.M.; Christie, B.R. Omega-3 fatty acids can reverse the long-term deficits in hippocampal synaptic plasticity caused by prenatal ethanol exposure. Neurosci. Lett. 2013, 551, 7–11. [Google Scholar] [CrossRef]
- Serrano, M.; Rico-Barrio, I.; Grandes, P. The effect of omega-3 fatty acids on alcohol-induced damage. Front. Nutr. 2023, 10, 1068343. [Google Scholar] [CrossRef]
- Milne, G.L.; Morrow, J.D.; Picklo, M.J. Elevated oxidation of docosahexaenoic acid, 22:6 (n-3), in brain regions of rats undergoing ethanol withdrawal. Neurosci. Lett. 2006, 405, 172–174. [Google Scholar] [CrossRef]
- Tajuddin, N.; Moon, K.H.; Marshall, S.A.; Nixon, K.; Neafsey, E.J.; Kim, H.Y.; Collins, M.A. Neuroinflammation and neurodegeneration in adult rat brain from binge ethanol exposure: Abrogation by docosahexaenoic acid. PLoS ONE 2014, 9, e101223. [Google Scholar] [CrossRef] [PubMed]
- Lafourcade, M.; Larrieu, T.; Mato, S.; Duffaud, A.; Sepers, M.; Matias, I.; De Smedt-Peyrusse, V.; Labrousse, V.F.; Bretillon, L.; Matute, C.; et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat. Neurosci. 2011, 14, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Manduca, A.; Bara, A.; Larrieu, T.; Lassalle, O.; Joffre, C.; Layé, S.; Manzoni, O.J. Amplification of mGlu5-endocannabinoid signaling rescues behavioral and synaptic deficits in a mouse model of adolescent and adult dietary polyunsaturated fatty acid imbalance. J. Neurosci. 2017, 37, 6851–6868. [Google Scholar] [CrossRef]
- Thomazeau, A.; Bosch-Bouju, C.; Manzoni, O.; Layé, S. Nutritional n-3 PUFA deficiency abolishes endocannabinoid gating of hippocampal long-term potentiation. Cereb. Cortex 2017, 27, 2571–2579. [Google Scholar] [CrossRef]
- Pan, J.P.; Zhang, H.Q.; Wang, W.; Guo, Y.F.; Xiao, N.; Cao, X.H.; Liu, L.J. Some subtypes of endocannabinoid/endovanilloid receptors mediate docosahexaenoic acid-induced enhanced spatial memory in rats. Brain Res. 2011, 1412, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Artmann, A.; Petersen, G.; Hellgren, L.I.; Boberg, J.; Skonberg, C.; Nellemann, C.; Hansen, S.H.; Hansen, H.S. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2008, 1781, 200–212. [Google Scholar] [CrossRef]
- Wood, J.A.T.; Williams, J.S.; Pandarinathan, L.; Janero, D.R.; Lammi-Keefe, C.J.; Makriyannis, A. Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid-system metabolites in brain and plasma. J. Lipid Res. 2010, 51, 1416–1423. [Google Scholar] [CrossRef]
- Watson, J.E.; Kim, J.S.; Das, A. Emerging class of omega-3 fatty acid endocannabinoids & their derivatives. Prostaglandins Other Lipid Mediat. 2019, 143, 106337. [Google Scholar] [CrossRef]
- Soria-Gómez, E.; Bellocchio, L.; Reguero, L.; Lepousez, G.; Martin, C.; Bendahmane, M.; Ruehle, S.; Remmers, F.; Desprez, T.; Matias, I.; et al. The endocannabinoid system controls food intake via olfactory processes. Nat. Neurosci. 2014, 17, 407–415. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, A.; Puente, N.; Elezgarai, I.; Ruehle, S.; Lutz, B.; Reguero, L.; Gerrikagoitia, I.; Marsicano, G.; Grandes, P. Anatomical characterization of the cannabinoid CB1 receptor in cell-type–specific mutant mouse rescue models. J. Comp. Neurol. 2017, 525, 302–318. [Google Scholar] [CrossRef]
- Winters, B.D.; Krüger, J.M.; Huang, X.; Gallaher, Z.R.; Ishikawa, M.; Czaja, K.; Krueger, J.M.; Huang, Y.H.; Schlüter, O.M.; Dong, Y. Cannabinoid receptor 1-expressing neurons in the nucleus accumbens. Proc. Natl. Acad. Sci. USA 2012, 109, 2717–2725. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.L.; Medina, K.L.; Padula, C.B.; Tapert, S.F.; Brown, S. Impact of adolescent alcohol and drug use on neuropsychological functioning in young adulthood: 10-year outcomes. J. Child Adolesc. Subst. Abus. 2011, 20, 135–154. [Google Scholar] [CrossRef]
- Lees, B.; Debenham, J.; Squeglia, L.M. Alcohol and cannabis use and the developing brain. Alcohol Res. Curr. Rev. 2021, 41, 11. [Google Scholar] [CrossRef]
- Coleman, L.G.; He, J.; Lee, J.; Styner, M.; Crews, F. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol. Clin. Exp. Res. 2011, 35, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Guerri, C.; Pascual, M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol 2010, 44, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, J.; Cabañero, D.; Puente, N.; García-Gutiérrez, M.S.; Grandes, P.; Maldonado, R. Role of the endocannabinoid system in drug addiction. Biochem. Pharmacol. 2018, 157, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Flores-Bonilla, A.; Richardson, H. Sex differences in the neurobiology of alcohol use disorder. Alcohol Res. 2020, 40, 04. [Google Scholar] [CrossRef]
- Alfonso-Loeches, S.; Pascual, M.; Guerri, C. Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology 2013, 311, 27–34. [Google Scholar] [CrossRef]
- Maynard, M.E.; Barton, E.A.; Robinson, C.R.; Wooden, J.I.; Leasure, J.L. Sex differences in hippocampal damage, cognitive impairment, and trophic factor expression in an animal model of an alcohol use disorder. Brain Struct. Funct. 2018, 223, 195–210. [Google Scholar] [CrossRef]
- Moore, C.F.; Lynch, W.J. Alcohol preferring (P) rats as a model for examining sex differences in alcohol use disorder and its treatment. Pharmacol. Biochem. Behav. 2015, 132, 1–9. [Google Scholar] [CrossRef]
- Egertova, M.; Giang, D.K.; Cravatt, B.F.; Elphick, M.R. A new perspective on cannabinoid signalling: Complimentary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proc. R. Soc. London Ser. B Biol. Sci. 1998, 265, 2081–2085. [Google Scholar] [CrossRef]
- Tsou, K.; Brown, S.; Sañudo-Peña, M.C.; Mackie, K.; Walker, J.M. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 1998, 83, 393–411. [Google Scholar] [CrossRef]
- Lees, B.; Meredith, L.R.; Kirkland, A.E.; Bryant, B.E.; Squeglia, L.M. Effect of alcohol use on the adolescent brain and behavior. Pharmacol. Biochem. Behav. 2020, 192, 172906. [Google Scholar] [CrossRef]
- Risher, M.L.; Fleming, R.L.; Boutros, N.; Semenova, S.; Wilson, W.A.; Levin, E.D.; Markou, A.; Swartzwelder, H.S.; Acheson, S.K. Long-term effects of chronic intermittent ethanol exposure in adolescent and adult rats: Radial-arm maze performance and operant food reinforced responding. PLoS ONE 2013, 8, e62940. [Google Scholar] [CrossRef] [PubMed]
- Rico-Barrio, I.; Peñasco, S.; Puente, N.; Ramos, A.; Fontaine, C.J.; Reguero, L.; Giordano, M.E.; Buceta, I.; Terradillos, I.; Lekunberri, L.; et al. Cognitive and neurobehavioral benefits of an enriched environment on young adult mice after chronic ethanol consumption during adolescence. Addict. Biol. 2019, 24, 969–980. [Google Scholar] [CrossRef]
- Li, N.; Chen, T.W.; Guo, Z.V.; Gerfen, C.R.; Svoboda, K. A motor cortex circuit for motor planning and movement. Nature 2015, 519, 51–56. [Google Scholar] [CrossRef]
- Schmahmann, J.D. The cerebellum and cognition. Neurosci. Lett. 2019, 688, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Buceta, I.; Elezgarai, I.; Rico-Barrio, I.; Gerrikagoitia, I.; Puente, N.; Grandes, P. Deletion of the cannabinoid CB1 receptor impacts on the ultrastructure of the cerebellar parallel fiber-Purkinje cell synapses. J. Comp. Neurol. 2019, 528, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Albergaria, C.; Silva, N.T.; Darmohray, D.; Carey, M.R. Cannabinoids modulate associative cerebellar learning via alterations in behavioral state. eLife 2020, 9, e61821. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Kano, M. Endogenous cannabinoid signaling through the CB1 receptor is essential for cerebellum-dependent discrete motor learning. J. Neurosci. 2006, 26, 8829–8837. [Google Scholar] [CrossRef]
- El Manira, A.; Kyriakatos, A. The role of endocannabinoid signaling in motor control. Physiology 2010, 25, 230–238. [Google Scholar] [CrossRef]
- Reddy, V.D.; Padmavathi, P.; Bulle, S.; Hebbani, A.V.; Marthadu, S.B.; Venugopalacharyulu, N.C.; Maturu, P.; Varadacharyulu, N.C. Association between alcohol-induced oxidative stress and membrane properties in synaptosomes: A protective role of vitamin E. Neurotoxicology Teratol. 2017, 63, 60–65. [Google Scholar] [CrossRef]
- Moon, K.H.; Tajuddin, N.; Brown, J.; Neafsey, E.J.; Kim, H.Y.; Collins, M.A. Phospholipase A2, Oxidative Stress, and Neurodegeneration in Binge Ethanol-Treated Organotypic Slice Cultures of Developing Rat Brain. Alcohol. Clin. Exp. Res. 2014, 38, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, B.S.; Nagre, N.; Xie, S.; Subbanna, S. Elevation of endogenous anandamide impairs ltp, learning and memory through cb1 receptor signaling in mice. Hippocampus 2014, 24, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, S.; Oliva, J.M.; Pérez-Rial, S.; Palomo, T.; Manzanares, J. Chronic ethanol consumption regulates cannabinoid CB1 receptor gene expression in selected regions of rat brain. Alcohol Alcohol. 2004, 39, 88–92. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Basavarajappa, B.S.; Saito, M.; Cooper, T.B.; Hungund, B.L. Chronic ethanol inhibits the anandamide transport and increases extracellular anandamide levels in cerebellar granule neurons. Eur. J. Pharmacol. 2003, 466, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, B.; Bermúdez-Silva, F.J.; Bilbao, A.; Alvarez-Jaimes, L.; Sanchez-Vera, I.; Giuffrida, A.; Serrano, A.; Baixeras, E.; Khaturia, S.; Navarro, M.; et al. Regulation of brain anandamide by acute administration of ethanol. Biochem. J. 2007, 404, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Mikasova, L.; Groc, L.; Choquet, D.; Manzoni, O.J. Altered surface trafficking of presynaptic cannabinoid type 1 receptor in and out synaptic terminals parallesls receptor desensitization.pdf. Proc. Natl. Acad. Sci. USA 2008, 105, 18596–18601. [Google Scholar] [CrossRef]
- Bonilla-Del Río, I.; Puente, N.; Mimenza, A.; Ramos, A.; Serrano, M.; Lekunberri, L.; Gerrikagoitia, I.; Christie, B.R.; Nahirney, P.C.; Grandes, P. Acute Δ9-tetrahydrocannabinol prompts rapid changes in cannabinoid CB1 receptor immunolabeling and subcellular structure in CA1 hippocampus of young adult male mice. J. Comp. Neurol. 2020, 529, 2332–2346. [Google Scholar] [CrossRef]
- Leishman, E.; Manchanda, M.; Thelen, R.; Miller, S.; Mackie, K.; Bradshaw, H.B. Cannabidiol’s Upregulation of N-acyl Ethanolamines in the Central Nervous System Requires N-acyl Phosphatidyl Ethanolamine-Specific Phospholipase D. Cannabis Cannabinoid Res. 2018, 3, 228–241. [Google Scholar] [CrossRef]
- Waddell, J.; McKenna, M.C.; Kristian, T. Brain ethanol metabolism and mitochondria. Curr. Top. Biochem. Res. 2022, 23, 1–13. [Google Scholar]
- Isaac, A.R.; de Velasco, P.C.; Fraga, K.Y.D.; Tavares-do-Carmo, M.d.G.; Campos, R.M.P.; Iannotti, F.A.; Verde, R.; Martins, D.B.G.; Santos, T.A.; Ferreira, B.K.; et al. Maternal omega-3 intake differentially affects the endocannabinoid system in the progeny`s neocortex and hippocampus: Impact on synaptic markers. J. Nutr. Biochem. 2021, 96, 108782. [Google Scholar] [CrossRef] [PubMed]
- Carrié, I.; Clément, M.; De Javel, D.; Francès, H.; Bourre, J.M. Specific phospholipid fatty acid composition of brain regions in mice: Effects of n-3 polyunsaturated fatty acid deficiency and phospholipid supplementation. J. Lipid Res. 2000, 41, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Brown, J., III; Achille, N.; Neafsey, E.J.; Collins, M.A. Binge ethanol-induced neurodegeneration in rat organotypic brain slice cultures: Effects of PLA2 inhibitor mepacrine and docosahexaenoic acid (DHA). Neurochem. Res. 2009, 34, 260–267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Collins, M.A.; Moon, K.; Tajuddin, N.; Neafsey, E.J.; Kim, Y. Docosahexaenoic acid (DHA) prevents binge ethanol-dependent aquaporin-4 elevations while inhibiting neurodegeneration: Experiments in rat adult-age entorhino-hippocampal slice cultures. Neurotox. Res. 2013, 23, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Marsicano, G.; Wotjak, C.T.; Azad, S.C. The endogenous cannabinoid system controls extinction of aversive memories. Nature 2002, 418, 530–534. [Google Scholar] [CrossRef]

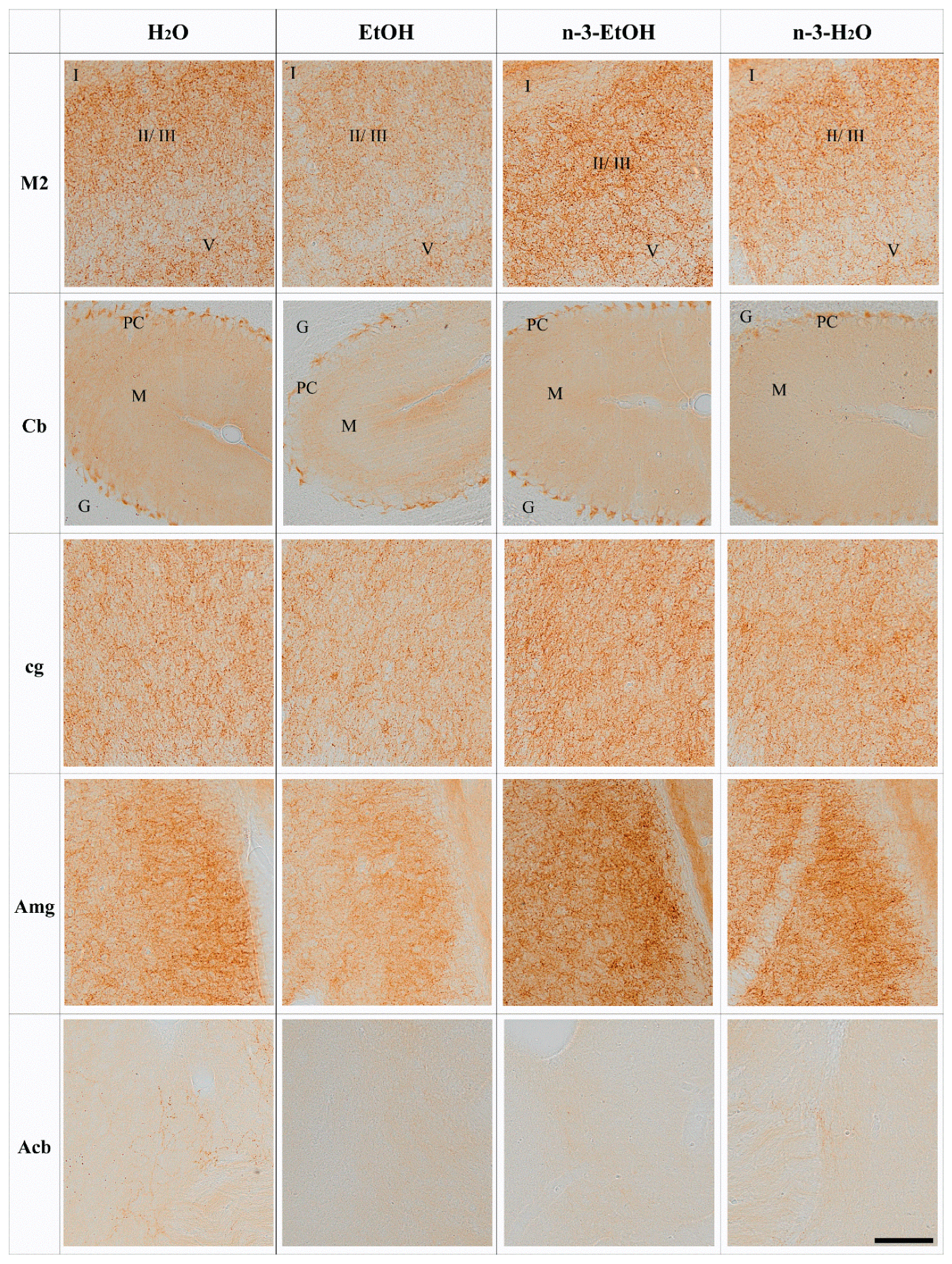

| H2O | EtOH | n-3-EtOH | n-3-H2O | |
|---|---|---|---|---|
| OB | 100.00 ± 3.982 | 102.30 ± 5.493 | 108.50 ± 4.499 | 100.40 ± 6.144 |
| M1 | 100.00 ± 4.074 | 89.57 ± 4.427 | 106.80 ± 6.384 | 92.25 ± 5.785 |
| M2 | 100.00 ± 3.507 | 82.20 ± 3.732 | 106.30 ± 4.604 | 100.50 ± 5.218 |
| Fr3 | 100.00 ± 15.57 | 114.10 ± 14.25 | 130.20 ± 12.08 | 116.60 ± 13.00 |
| Cg1 | 100.00 ± 6.694 | 77.99 ± 9.688 | 77.06 ± 14.08 | 90.67 ± 10.22 |
| cg | 100.00 ± 4.868 | 80.77 ± 4.864 | 97.86 ± 5.274 | 105.20 ± 4.610 |
| CPu | 100.00 ± 11.31 | 79.01 ± 11.61 | 83.25 ± 12.03 | 90.02 ± 15.31 |
| Acb | 100.00 ± 6.028 | 54.18 ± 10.81 | 60.61 ± 8.153 | 66.15 ± 11.04 |
| Amg | 100.00 ± 3.594 | 82.80 ± 3.468 | 102.30 ± 3.977 | 97.69 ± 5.744 |
| DG | 100.00 ± 7.651 | 92.42 ± 4.135 | 96.72 ± 4.383 | 93.04 ± 3.291 |
| CA1 | 100.00 ± 10.62 | 100.80 ± 10.23 | 103.80 ± 5.839 | 98.02 ± 3.491 |
| SN | 100.00 ± 4.788 | 111.20 ± 3.457 | 109.10 ± 3.957 | 99.57 ± 3.918 |
| Ent | 100.00 ± 8.555 | 96.17 ± 4.879 | 104.20 ± 7.367 | 103.70 ± 3.471 |
| Cb | 100.00 ± 5.267 | 76.40 ± 4.445 | 98.43 ± 5.627 | 100.40 ± 4.620 |
| Name | Mouse Line Derived from | Background | Breeding Strategy Used |
|---|---|---|---|
| CB1-KO | CB1-KO (CB1−/−) Originally obtained by crossing CB1f/f mice with CMV-Cre mice (“Cre deleter”) [66] | Predominant C57BL/6-N (9–10 back-crossings) | Female CB1+/− X Male CB1+/− |
| Antibody | Immunogen | Manufacturer, Species, Catalog Number, Rrid | Dilution | Characterization |
|---|---|---|---|---|
| ANTI-CB1 | Recognizes the last 31 amino acids of the C-terminus of the mouse CB1 receptor (NM007726), as provided by the manufacturer: NCBI Reference Sequence: NP_031752.1; 443–473 amino acid residues: MHRAAESCIKSTVKIAKVTMSVSTDTSAEAL | Frontier Institute; Goat polyclonal; #CB1-Go-Af450, RRID: AB_2571592 | 2 µg/mL | On immunoblot, the antibody detects a single protein band at 52 kDa |
| H2O | EtOH | n-3-EtOH | n-3-H2O | |
|---|---|---|---|---|
| OB | 39 | 54 | 45 | 30 |
| M1 | 72f | 69 | 48 | 66 |
| M2 | 72 | 63 | 48 | 66 |
| Fr3 | 18 | 18 | 18 | 18 |
| Cg1 | 18 | 18 | 12 | 18 |
| cg | 102 | 105 | 84 | 70 |
| CPu | 18 | 18 | 15 | 18 |
| Acb | 18 | 18 | 12 | 18 |
| Amg | 90 | 75 | 54 | 60 |
| DG | 51 | 51 | 45 | 36 |
| CA1 | 36 | 36 | 27 | 21 |
| SN | 51 | 51 | 42 | 36 |
| Ent | 36 | 36 | 21 | 24 |
| Cb | 45 | 30 | 24 | 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Llorente, A.; Serrano, M.; Bonilla-Del Río, I.; Lekunberri, L.; Ocerin, G.; Puente, N.; Ramos, A.; Rico-Barrio, I.; Gerrikagoitia, I.; Grandes, P. Omega-3 Recovers Cannabinoid 1 Receptor Expression in the Adult Mouse Brain after Adolescent Binge Drinking. Int. J. Mol. Sci. 2023, 24, 17316. https://doi.org/10.3390/ijms242417316
Martín-Llorente A, Serrano M, Bonilla-Del Río I, Lekunberri L, Ocerin G, Puente N, Ramos A, Rico-Barrio I, Gerrikagoitia I, Grandes P. Omega-3 Recovers Cannabinoid 1 Receptor Expression in the Adult Mouse Brain after Adolescent Binge Drinking. International Journal of Molecular Sciences. 2023; 24(24):17316. https://doi.org/10.3390/ijms242417316
Chicago/Turabian StyleMartín-Llorente, Ane, Maitane Serrano, Itziar Bonilla-Del Río, Leire Lekunberri, Garazi Ocerin, Nagore Puente, Almudena Ramos, Irantzu Rico-Barrio, Inmaculada Gerrikagoitia, and Pedro Grandes. 2023. "Omega-3 Recovers Cannabinoid 1 Receptor Expression in the Adult Mouse Brain after Adolescent Binge Drinking" International Journal of Molecular Sciences 24, no. 24: 17316. https://doi.org/10.3390/ijms242417316
APA StyleMartín-Llorente, A., Serrano, M., Bonilla-Del Río, I., Lekunberri, L., Ocerin, G., Puente, N., Ramos, A., Rico-Barrio, I., Gerrikagoitia, I., & Grandes, P. (2023). Omega-3 Recovers Cannabinoid 1 Receptor Expression in the Adult Mouse Brain after Adolescent Binge Drinking. International Journal of Molecular Sciences, 24(24), 17316. https://doi.org/10.3390/ijms242417316






