Abstract
Ketone bodies (KBs), such as acetoacetate and β-hydroxybutyrate, serve as crucial alternative energy sources during glucose deficiency. KBs, generated through ketogenesis in the liver, are metabolized into acetyl-CoA in extrahepatic tissues, entering the tricarboxylic acid cycle and electron transport chain for ATP production. Reduced glucose metabolism and mitochondrial dysfunction correlate with increased neuronal death and brain damage during cerebral ischemia and neurodegeneration. Both KBs and the ketogenic diet (KD) demonstrate neuroprotective effects by orchestrating various cellular processes through metabolic and signaling functions. They enhance mitochondrial function, mitigate oxidative stress and apoptosis, and regulate epigenetic and post-translational modifications of histones and non-histone proteins. Additionally, KBs and KD contribute to reducing neuroinflammation and modulating autophagy, neurotransmission systems, and gut microbiome. This review aims to explore the current understanding of the molecular mechanisms underpinning the neuroprotective effects of KBs and KD against brain damage in cerebral ischemia and neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease.
1. Introduction
Ketone bodies (KBs) comprise acetone, acetoacetate (AcAc), and β-hydroxybutyrate (BHB). AcAc and BHB serve as crucial alternative energy sources for extrahepatic tissues under various physiological conditions, such as glucose deprivation, fasting, strenuous exercise, the neonatal period, and a low-carbohydrate high-fat ketogenic diet (KD) [1,2]. There are many different types of KDs with varying proportions of macronutrients, which have been well described in recent reviews [3,4]. One representative type of KD is high in fat (70–90% of total calories), moderate protein (7–25%), and low in carbohydrates (3–20%) [3,5]. This high-fat KD (HFKD) is commonly referred to as KD. The very low-calorie KD (VLCKD) is characterized by significant carbohydrate restriction (<50 g/day), adequate protein, and high fat intake, with an average energy intake of 800 kcal/day [4]. Under KD conditions, without or with restriction of calorie, nutritional ketosis is achieved.
In adults, plasma KB concentrations typically range from 0.05–0.1 mM under normal conditions, rising to 1–2 mM after 2 days of fasting and reaching 5–8 mM during prolonged fasting and starvation [1,6,7].
KBs are primarily synthesized in the liver through ketogenesis from acetyl-CoA, produced via fatty acid β-oxidation. The liver can produce up to 300 g of KBs daily, contributing approximately 20% of total energy expenditure during fasting and starvation [2,8,9]. Detailed pathways and regulatory mechanisms of ketogenesis in the liver and ketolysis in extrahepatic tissues have recently been reviewed [2,10].
The human brain, comprising approximately 2% of total body weight, consumes approximately 20% of the total energy expenditure of the body [11]. While glucose is the primary energy source under normal conditions, the brain adapts to KBs during starvation and glucose deprivation. KBs contribute 60–70% of the energy requirements during prolonged fasting [1]. Suckling animal brains exhibit higher circulating KB levels than mature brains, indicating a period of dependence on both KBs and glucose during suckling [12]. Cerebral KB metabolism may play a role in brain development, as the developing brain seems to prioritize KBs for certain metabolic pathways [13]. Brain glial cells, including astrocytes, have been found to produce KBs from fatty acids and ketogenic amino acids via ketogenesis [14,15]. Ketogenic and ketolytic pathways in the brain are briefly described in Section 2.
Although diabetic ketoacidosis is a pathological condition, mild ketonemia resulting from caloric restriction (CR), fasting, vigorous exercise, and KD, has proven beneficial and extends a healthy lifespan [16,17,18,19,20]. Moreover, accumulating evidence has demonstrated that KBs and KD have the potential to treat various diseases such as metabolic diseases including obesity, type II diabetes, and non-alcoholic fatty liver disease, cancer, cardiovascular diseases, and neurological diseases [2,3,10,21,22,23,24]. As the beneficial effects of dietary intervention with KD are mediated through KB production, interest in KBs and KD has been increasing. KBs and KD have been used in the treatment of various neurological diseases, including epilepsy, cerebral ischemia, Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), Huntington’s disease (HD), depression, mood and anxiety disorders, and autism spectrum disorder (ASD) [11,25,26,27,28,29,30,31,32,33,34]. Section 3 describes the latest findings from recent preclinical and clinical studies on the therapeutic potential of KBs and KD in cerebral ischemia and the two most common neurodegenerative diseases, AD and PD.
KBs and KD have been shown to have a variety of beneficial effects on the brain through many different mechanisms [11,35,36]. They exhibit neuroprotective effects by improving mitochondrial function and alleviating oxidative stress. KBs and KD also induce post-translational modifications of epigenetic proteins, reduce neuroinflammation, and affect neurotransmission systems and the gut microbiome [2,9,20,37]. The molecular mechanisms by which KBs exert beneficial effects on health and disease have previously been reviewed with a focus on cancer treatment [10]. In Section 4 of this review, the current knowledge of the molecular mechanisms underlying the neuroprotective effects of KBs and KD is discussed.
2. Ketone Body Metabolism in the Brain
2.1. Brain KBs Supplied by Ketogenesis in Liver and Astrocytes
The brain comprises two primary cell types: neurons and glial cells. While neurons are central to neurotransmission, glial cells, including astrocytes, oligodendrocytes, and microglia, provide essential physical and chemical support, maintaining an optimal environment for robust neuronal function.
Recent emphasis on the role of KBs alongside glucose as energy sources for brain cells has emerged [38]. Although the liver is the primary supplier of KBs to the brain, astrocytes also contribute to KB synthesis [14,39,40]. In instances such as cerebral ischemia or hypoxia, both hepatic and astrocytic ketogenesis increase, offering neuroprotective effects by enhancing brain energy metabolism and synaptic functions [14,15,41,42]. AMP-activated protein kinase (AMPK), a master energy sensor, plays a crucial role in regulating astrocytic ketogenesis [43], influencing processes like lipid mobilization and KB export.
The ketogenic pathways in the liver and astrocytes exhibit similarities, as illustrated in Figure 1, which provides detailed insights into hepatic ketogenesis. Acetyl-CoA, generated through fatty acid β-oxidation during low-glucose conditions, initiates ketogenesis in mitochondria [2,10,44]. Carnitine palmitoyl transferase 1/2 (CPT1/2) facilitates the transport of fatty acyl-CoA to the mitochondria. Additionally, ketogenic amino acids, particularly leucine, contribute to acetyl-CoA production for ketogenesis in liver hepatocytes and brain astrocytes [2,35].
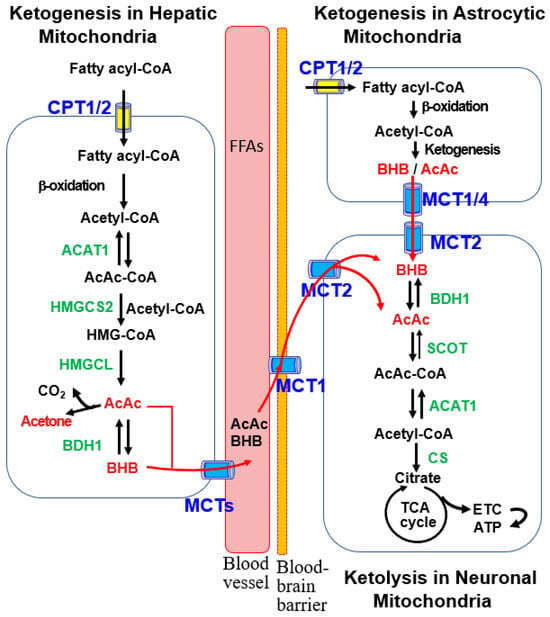
Figure 1.
Ketogenic and ketolytic pathways in the liver and brain cells. Ketogenesis primarily occurs in the mitochondria of hepatocytes with acetyl-CoA produced by β-oxidation of fatty acyl-CoA. This process proceeds through stepwise reactions catalyzed by acetoacetyl-CoA thiolase 1 (ACAT1), 3-hydroxy-methylglutaryl-CoA synthase 2 (HMGCS2), 3-hydroxy-methylglutaryl-CoA lyase (HMGCL), and β-hydroxybutyrate dehydrogenase 1 (BDH1). The resulting ketone bodies (KBs), acetoacetate (AcAc) and β-hydroxybutyrate (BHB), are released into circulating blood via monocarboxylic acid transporters (MCTs). After uptake into the brain via MCT1, ketolysis occurs in the mitochondria of brain neurons, where BHB oxidation to acetyl-CoA is catalyzed by BDH1, succinyl-CoA:3-oxoacid-CoA transferase (SCOT), and ACAT1. After conversion of acetyl-CoA to citrate by citrate synthase (CS), ATP is generated through the tricarboxylic acid (TCA) cycle and the electron transport chain (ETC). KBs are also produced in the mitochondria of brain astrocytes, as described in the hepatic ketogenic pathway, and are provided to neurons as an energy source.
The hepatic ketogenesis diagram (Figure 1) illustrates the condensation of two acetyl-CoA molecules, forming acetoacetyl-CoA (AcAc-CoA) catalyzed by acetyl-CoA acetyltransferase 1 (ACAT1) [44]. Subsequently, 3-hydroxy-3-methylglutaryl (HMG)-CoA synthase 2 (HMGCS2) catalyzes the formation of HMG-CoA, the rate-limiting step in ketogenesis. HMG-CoA is further metabolized to AcAc and BHB by HMG-CoA lyase (HMGCL) and BHB dehydrogenase 1 (BDH1) [44]. AcAc and BHB are then transported to the brain through monocarboxylate transporters (MCTs) for ketolysis and ATP production [7,11,35]. As BHB constitutes approximately 70% of the plasma KB pool [7], the terms “KBs” and “BHB” are used interchangeably.
2.2. Ketolysis in the Brain
Liver-derived blood KBs, including BHB and AcAc, traverse the blood-brain barrier (BBB) to reach the brain through facilitated diffusion mediated by MCTs [7,11,35]. MCT1 is located at the BBB on microvascular endothelial cells and astrocytes. Astrocytes also express MCT4, while MCT2 is mainly localized in neurons [11,35]. Neuronal BHB transport is also facilitated by sodium-dependent MCT1 (SMCT1).
Within brain neuron mitochondria, BHB, supplied by the liver and astrocytes, undergoes oxidation to AcAc via brain mitochondrial BDH1. Subsequently, AcAc is converted to AcAc-CoA by succinyl-CoA:3-oxoacid-CoA transferase (SCOT), a product of the Oxct1 gene [2,10]. ACAT1 catalyzes AcAc-CoA to generate two acetyl-CoA molecules, entering the tricarboxylic acid (TCA) cycle to produce ATP through the electron transport chain (ETC) (Figure 1).
The extent of KB uptake and ketolysis in the brain is largely influenced by blood KB concentrations [11,45]. MCT1/2 expression regulates the brain’s uptake and utilization of KBs. Previous studies demonstrated increased MCT1/2 expression during ketolysis and after brain injury. Additionally, a KD was found to elevate MCT1 expression and subsequent BHB uptake in the brain [46]. After traumatic brain injury, MCT2 expression is induced in cerebral vasculature, correlating with increased cerebral KB uptake [47]. Studies in mice suggest that KD consumption differentially impacts neuronal and astrocytic gene transcription, influencing mitochondrial function and inflammation, potentially associating with neurodegenerative diseases [48].
Consistent with glucose hypometabolism in the AD brain, glycolytic gene expression is diminished in neurons and oligodendrocytes of patients with AD. Conversely, ketolytic gene expression remains unchanged in neurons, astrocytes, or microglia but shows a significant decrease in oligodendrocytes [49]. Hypometabolism in oligodendrocytes, resulting from impaired glycolytic and ketolytic pathways, is linked to dysmyelination, suggesting potential involvement in AD pathology.
3. Therapeutic Potential of KBs and KD in Cerebral Ischemia and Neurodegenerative Diseases
Ketosis induced by KBs and KD intake, ketone ester supplementation, and the administration of medium-chain triglycerides (MCTG) have been utilized in both animal models and humans for treating various neurological diseases, including neurodegenerative diseases. This section outlines recent preclinical and clinical studies on the application of ketogenic therapy for treating cerebral ischemia and two most common neurodegenerative diseases, AD and PD. An overview of clinical studies on the beneficial effects of KD and KBs on neurodegenerative diseases, including AD, PD, MS, and HD is presented in Table 1.

Table 1.
Overview of clinical studies examining the beneficial effects of the KDs on neurodegenerative diseases.
3.1. Cerebral Ischemia
Cerebral ischemia, also known as brain ischemia or ischemic stroke, is a detrimental condition where blood flow to the brain is impaired, leading to reduced glucose and oxygen supply and damage or death of brain cells. If left untreated, cerebral ischemia can result in cerebral infarction, hypoxic-ischemic encephalopathy, permanent disability, or death [29,45]. Acute brain injury caused by cerebral ischemia may also induce protein aggregates, increasing the risk of neurodegenerative diseases [65,66].
Several studies have reported the neuroprotective effects of KBs and KD against neuronal damage in various cerebral ischemia animal models [29,45]. BHB pretreatment through infusion or KD intake reduces infarct volume and improves neurological function after focal ischemia in rats and mice [67,68]. Immediate administration of BHB after reperfusion significantly improves ischemia-related neurological scores in mice [69]. Intravenous BHB administration after middle cerebral artery occlusion (MCAO) significantly reduces the infarct area and neurological deficits [42]. Consumption of a KD 3 days prior to stroke induction improves mobility in endothelin-1 model rats, indicating the benefit of KD preconditioning [70]. Similarly, a recent study reported that pre-treatment with KD or post-treatment with BHB improved motor function and reduced infarct volume in a mouse model of cerebral stroke [71]. Moreover, pre-injury KD consumption prevented neurodegeneration due to cardiac arrest-induced cerebral ischemia [72].
The transport and metabolism of KBs are upregulated in both fetuses and neonates [12,45]. It is suggested that KBs and KD may play a role in protecting the brain from damage caused by neonatal hypoxic-ischemic encephalopathy. Overall, these results suggest that KBs and KD may have neuroprotective effects against brain injury caused by cerebral ischemia.
3.2. Alzheimer’s Disease
AD, the most common neurodegenerative disease, is characterized by amyloid plaque formation due to amyloid-beta (Aβ) deposition, neurofibrillary tangle formation due to hyperphosphorylation of tau protein, and progressive brain shrinkage due to neuronal loss [3,25]. Although the causes of AD are not yet fully understood, it is proposed that a combination of harmful factors such as age-related changes in the brain, oxidative stress, inflammation, blood vessel damage, and lack of energy supply to the brain are involved. Depending on the stage of the disease, AD patients exhibit a variety of symptoms, including memory loss, decreased learning ability, decreased motor skills, cognitive impairment, and lack of physical control.
A previous study showed that ketogenesis induced by 2-deoxy-D-glucose reduced Aβ deposition and oxidative stress in the 3xTgAD mouse model [73]. Recently, the diverse effects of a KD and KBs have also been reported in different animal models of AD. Diets supplemented with BHB or ketone ester were shown to improve energy utilization and reduce oxidized proteins and lipids, normalizing abnormal behavior in a 3xTgAD mouse model [74,75]. Although KD consumption for 1 month did not affect Aβ levels in a young amyloid precursor protein (APP)/presenilin-1 (PS-1) knock-in AD mouse model, this diet improved motor performance in AD mice [76]. KD consumption for 3 months led to improvements in motor function but not in learning and amyloid or tau deposits in APP/PS-1 and Tg4510 AD mouse models [77]. Another study found that KB administration for 2 months improved learning and memory and lowered oxidative stress in an APP mouse model [78]. Similarly, KD or ketone esters significantly reduced Aβ deposition and phosphorylated tau accumulation, improving Aβ- or tau-dependent pathology and exhibiting anxiolytic and cognition-sparing properties in AD mouse models [79,80].
Clinical studies have shown improvements in verbal memory and cognitive scores in small groups of mild cognitive impairment (MCI) patients who received KD for 6 weeks and in a single-arm study of AD patients with KD administration for 12 weeks [50,57,81]. In a recent randomized crossover trial, 12 weeks of a modified KD significantly improved daily function and quality, but memory improvements were modest [60]. In a pilot clinical study, the modified KD was shown to improve metabolic biomarkers and cerebrospinal fluid Aβ42 [52]. Clinical trials comprising patients with MCI or mild to moderate AD have shown that KD supplementation with ketone ester or MCTG improves short-term cognitive abilities, including memory, language, and attention [51,53,54,56,58,59]. Additionally, an MCTG-based KD improved memory scores and overall cognitive function in patients with mild and moderate AD without significant adverse effects on metabolic parameters [54,55,57,58,82].
Interestingly, an MCTG-based KD reversed cognitive impairment and improved cognitive scores in ApoE4-negative AD patients, but not in ApoE4-positive patients [54,55,59]. These observations suggest that the ApoE4 genotype may play a role in the outcome of KD consumption. Therefore, disease severity, genotype, degree, and duration of KBs and KD may influence treatment outcomes in patients with AD.
3.3. Parkinson’s Disease
PD is a neurological disorder characterized by motor deficits resulting from unintentional or uncontrollable movements, including tremors, balance problems, and coordination difficulties [3,25,26]. PD progresses over time, causing significant difficulties in walking and speaking. PD is associated with the loss of dopaminergic neurons in the brain. Although the causes of PD are not yet fully understood, genetic factors, environmental toxins, oxidants, and aging have been suggested as potential causes of dopaminergic neuronal loss in PD.
In a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD animal model, BHB infusion significantly reduced the loss of dopaminergic neurons and motor deficits [83]. The administration of BHB or KD before MPTP injury improved motor function and reduced dopaminergic neuron loss [84,85]. BHB treatment significantly improved motor dysfunction and dopaminergic neurons in lipopolysaccharide (LPS)-induced PD model rats by inhibiting microglial activation [86]. KD also protected dopaminergic neurons by increasing glutathione levels in PD model rats injected with 6-hydroxydopamine (6-OHDA) [87].
In a pilot clinical study, 8 weeks of KD consumption improved cognitive function but not motor function compared to consumption of a low-fat diet [63]. In a randomized controlled trial, PD patients who consumed KD also showed significant improvement in non-motor symptoms compared to those that consumed a low-fat diet [62]. Additionally, one study found that KD for 28 days improved both motor and non-motor symptoms [61]. These preclinical and clinical observations suggest that KBs and KD may have therapeutic potential in the treatment of PD.
4. Molecular Mechanisms of the Neuroprotective Effects of KBs and KD
4.1. KBs as an Energy Source for the Brain
Glucose is recognized as the primary energy source for the brain. Consequently, impaired glucose metabolism has been proposed as a contributor to aging and several neurological diseases, including AD and PD [88,89,90]. Blood glucose crosses the BBB through glucose transporters (GLUTs). Impaired glucose transport and metabolism have been observed in some patients with MCI and AD long before symptoms manifest [39,88,89,90]. Deficiencies in GLUT1 and GLUT3 may account for lower glucose uptake in AD brains compared to normal brains.
In contrast to glucose, the utilization of KBs remains unaffected in the brains of elderly individuals and patients with AD. As illustrated in Figure 1, the ketolysis of brain KBs and mitochondrial ATP production through the ETC can supply energy for neurotransmission [17,44]. Therefore, KBs may compensate for the deficiency in brain glucose in MCI and AD, thereby delaying cognitive decline [90,91]. Moreover, in situations where both glucose and KBs are available, certain brain regions preferentially take up KBs, indicating that KBs are the preferred energy source for the brain in certain circumstances [92].
A correlation has been shown between increased blood KB concentration and enhanced memory. Consequently, KB supplementation facilitates the preservation or improvement of brain function and neuronal survival, offering therapeutic potential for the treatment of neurodegenerative diseases [39,82,89,90]. BHB serves as a more efficient energy source than glucose during nutritional deficiencies. BHB reduces the NAD+/NADH ratio, oxidizes the coenzyme Q/QH2 couples, and increases the ∆G’ of ATP hydrolysis, thereby promoting ATP production [26,93] (Figure 2A). During aging and neurodegenerative diseases, BHB can emerge as a crucial energy source for the brain, mitigating the energy crisis [11,88,94]. Additionally, KB utilization through fasting, ketone esters, and a KD has been shown to enhance brain network stability in young adults, suggesting the potential of KBs in protecting the brain during aging [95]. In summary, the therapeutic applications of KBs and KD are primarily related to their role in energy metabolism.
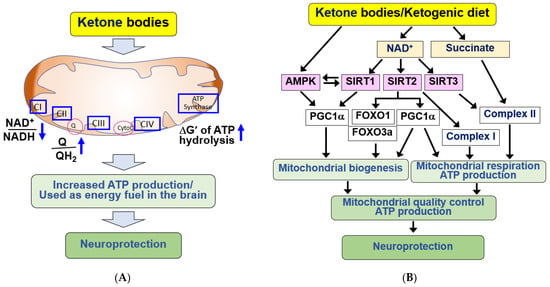
Figure 2.
Neuroprotective effects of KBs and KD by providing energy source and improving mitochondrial dysfunction in the brain. (A) KBs serve as energy fuel for the brain. KBs promote ATP production by decreasing the NAD+/NADH ratio and increasing the coenzyme Q/QH2 ratio and ΔG’ of ATP hydrolysis. KBs exert neuroprotective effects through its use as an energy fuel. (B) KBs exert beneficial effects by improving mitochondrial function. KBs and KD improve mitochondrial dysfunction by increasing mitochondrial biogenesis through SIRT1/2/3, AMPK, PGC1α, and FOXO1/3a pathways after increasing NAD+ production. KBs and KD also increase mitochondrial respiration through PGC1α, complex I/II. The signaling pathways mediated by KBs and KD improve mitochondrial quality control and increase ATP production, resulting in neuroprotection. All arrows indicate activation.
4.2. Beneficial Effects of KBs and KD on Mitochondrial Function
Substantial evidence supports the notion that mitochondrial dysfunctions, including impaired ETC and ATP production, oxidative stress from excessive reactive oxygen species (ROS), mitochondrial DNA damage due to mutations, and defective mitochondrial dynamics and biogenesis, are primary contributors to the pathogenesis of neurodegenerative diseases [39,82,96]. In AD brains, complex IV of the ETC is impaired, whereas in PD brains, complex I activity is compromised. This impaired mitochondrial oxidative phosphorylation reduces cerebral glucose metabolism, leading to neurodegenerative diseases [26,97].
KBs and KD have demonstrated the ability to enhance mitochondrial function in cerebral ischemia and neurodegenerative diseases [26,69,98,99,100,101]. The molecular mechanism underlying the beneficial effects of KBs and KD involves increased NADH oxidation via KB catabolism [39,82]. Elevated NAD+ levels play crucial roles, such as stimulating mitochondrial biogenesis and respiration, by serving as a cofactor for NAD+-dependent enzymes in cellular signaling processes [102] (Figure 2B).
Sirtuins (SIRTs, class III histone deacetylases, HDACs) and the DNA damage repair enzyme, poly-ADP-ribose polymerase-1 (PARP-1), are representative NAD+-dependent enzymes activated by KBs, playing pivotal roles in mitochondrial function [102,103,104]. Brain metabolism by KBs upregulates cytosolic SIRT1/2 by increasing NAD+, and SIRT1/2 stimulates mitochondrial biogenesis through peroxisome proliferator activated receptor γ coactivator 1α (PGC1α) [82,105,106,107]. SIRT1 plays a key role in mediating diverse protective effects of KBs in the brain [108]. SIRT1 activates AMPK through deacetylation and activation of the AMPK kinase, liver kinase B1 (LKB1). AMPK also regulates mitochondrial respiration and activates SIRT1 [109]. Therefore, AMPK and SIRT1 activate each other to form a positive feedback loop.
BHB activates AMPK and regulates mitochondrial biogenesis [107]. AMPK and SIRT1 can directly affect PGC1α activity through phosphorylation and deacetylation, respectively [105]. Activation of PGC1α positively regulates mitochondrial dynamics, biogenesis, and oxidative phosphorylation. SIRT1/2 also positively affect mitochondrial ATP production [105,107]. In cortical neurons, D-BHB improves mitochondrial function and stimulates mitochondrial biogenesis, autophagy, and mitophagy by upregulating FOXO1, FOXO3a, and PGC1α, in a SIRT2-dependent manner [107].
BHB-induced NAD+ production in brain mitochondria enhances complex I integrity and activity [110,111,112,113]. Under conditions of complex I deficiency caused by mitochondrial (mt) DNA mutations, KB exposure significantly restores the stability and activity of complex I, increasing ATP synthesis. This suggests that KBs promote the repair of complex I damage [111,114]. Mitochondrial SIRT3 can bind to complexes I and II, increasing their activity [103], indicating that SIRT3 activation mediates the effects of BHB on the ETC and ATP production. BHB catabolism generates succinate, the oxidative fuel for succinate dehydrogenase, supplying electrons directly to complex II, bypassing complex I abnormalities, stabilizing the complex, and reducing ROS production [26,67,83,115,116]. Therefore, the neuroprotective effect of BHB is linked to the improvement of mitochondrial complexes I and II and the resulting enhancement of ATP production [69]. Similar to KBs, KD-induced nutritional ketosis increases both mitochondrial respiration and homeostasis, suggesting its therapeutic potential for the treatment of chronic and degenerative diseases with mitochondrial dysfunction [99]. Similar to KBs, KD-induced nutritional ketosis increases both mitochondrial respiration and homeostasis, suggesting its therapeutic potential for the treatment of chronic and degenerative diseases with mitochondrial dysfunction [99].
4.3. Anti-Apoptotic and Antioxidant Effects of KBs and KD in the Brain
Apoptotic cell death in brain tissue is a key feature of neurodegeneration, and blocking apoptosis increases neuronal survival, slowing the neurodegenerative process [114]. Oxidative stress is also associated with neurodegenerative progression and neuronal loss through apoptosis. Mitochondria are a major source of ROS generation and play a crucial role in regulating apoptotic cell death [93,97]. The induction of apoptotic cell death is often preceded by the opening of the mitochondrial permeability transition pore (mPTP). This event causes mitochondrial dysfunction, releasing cytochrome C into the cytoplasm, activating caspases and apoptosis [114].
KBs and KD have been reported to alleviate oxidative stress and apoptosis in various cell systems and animal models of neurodegenerative diseases. The anti-apoptotic and antioxidant effects of KBs have undergone extensive study in the brain in vitro and in vivo [83,110,117,118,119]. The signaling pathways responsible for these effects are summarized in Figure 3A. Firstly, treatment with KBs affects mitochondrial function by preventing prolonged mPTP opening. Additionally, BHB catabolism increases the coenzyme Q/QH2 ratio, reducing mitochondrial ROS generation [93,97]. KBs attenuate apoptotic cell death by reducing mitochondrial ROS production, mPTP opening, and cytochrome C release in neurons isolated from rat brains [114,118]. KBs and KD may protect neurons from brain damage by improving mitochondrial function during acute cerebral ischemia and chronic neurological disorders, including AD and PD [45,83,120].
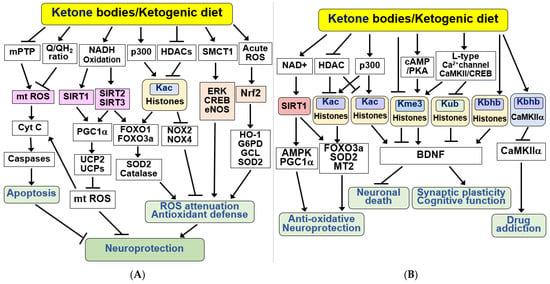
Figure 3.
Signaling functions of KBs and KD for anti-apoptosis, antioxidant defense, and epigenetic and post-translational modifications. (A) KBs exert beneficial effects by inducing anti-apoptotic and antioxidant defenses. KBs and KD protect neurons from apoptosis by inhibiting the mitochondrial permeability transition pore (mPTP), mt ROS production, and caspase activation. KBs and KD induce antioxidant defenses through the SIRT1/2/3, PGC1α, FOXO1/3a, and ERK/CREB/eNOS pathways and acetylation of histones, resulting in stress resistance and neuroprotection. The antioxidant Nrf2 signaling pathway is activated via acute ROS. (B) KBs and KD exhibit their beneficial effects through epigenetic and post-translational modifications of histones and non-histone proteins. KBs exert their neuroprotective effects through the SIRT1/AMPK/PGC1α pathway in a NAD+-dependent manner. Additionally, KBs regulate HDAC and p300 to promote acetylation at lysine 27 of histone H3 (H3K27Ac, Kac). KBs increase brain-derived neurotrophic factor (BDNF) expression by inducing acetylation (Kac), increasing trimethylation at lysine 4 (H3K4me3, Kme3), decreasing trimethylation of H3K27 (H3K27me3, Kme3), reducing ubiquitination at lysine 119 of histone H2A (H2AK119ub, Kub), and increasing β-hydroxybutyrylation on lysine 9 of histone H3 (H3K9bhb, Kbhb). KBs also increase the Kbhb of CaMKIIα, thereby reducing CaMKIIα activity and drug addiction. All arrows indicate activation and truncated cut lines indicate inhibition.
KBs have been reported to reduce glutamate-induced cell death and free radical formation by increasing NADH oxidation (resulting in elevated NAD+ levels) and enhancing mitochondrial respiration in neocortical neurons [110]. BHB and KD were found to activate SIRT1 and rescue premature aging in a mouse model of Cockayne syndrome [121]. SIRT1 activation following status epilepticus in rats has been shown to enhance PGC1α, superoxide dismutase 2 (SOD2), and uncoupling protein 2 (UCP2) [106]. Increased expression and activity of UCPs (UCP2, 4, and 5) were found in the brains of mice fed a KD [104,115]. Increased UCPs can promote mitochondrial respiration and reduce ROS production by uncoupling oxidative phosphorylation and ATP production [115,122].
SIRT3 activation plays an important role in regulating mitochondrial redox homeostasis by increasing NAD+ levels and subsequently deacetylating FOXO3a and SOD2 [82,98,119,123,124,125,126]. PGC1α activation also mediates the antioxidant effects of SIRT3 in the brain, preventing dopaminergic neuron death [126]. KBs produced locally by astrocytes can attenuate oxidative stress in spinal cord injury by inhibiting class I HDACs, which regulate the expression of antioxidant genes, including FOXO1/3a and its targets, such as SOD2 and catalase [119]. BHB and KD inhibition of class I HDACs show neuroprotection against oxidative stress by downregulating NADPH oxidase (NOX) 2 and 4 [119]. BHB can also induce antioxidant defense by reducing the cytoplasmic NADP+/NADPH ratio [20,26,127]. NADPH is used for the biosynthesis of antioxidants, such as reduced glutathione. A KD improves antioxidant capacity by reducing mitochondrial production of ROS and increasing mitochondrial glutathione synthesis in the hippocampus by upregulating glutamate-cysteine ligase (GCL) [128].
The protective effect of BHB is mediated via the upregulation of the KB transporter, SMCT1, and activation of the extracellular signal-regulated kinase (ERK)/cAMP response element binding protein (CREB)/endothelial nitric oxide synthase (eNOS) pathway [129]. The antioxidant nuclear factor erythroid 2-related factor (Nrf2) signaling pathway is activated by acute ROS. BHB also protects the brain from oxidative stress by promoting Nrf2 transcriptional activity and expression of its target antioxidant genes [130]. Nrf2 activation can reduce several pathogenic processes associated with neurodegenerative diseases by upregulating antioxidant defenses, improving mitochondrial function, and suppressing inflammation [131]. Fasting-induced BHB or BHB supplementation prevents ischemic retinal degeneration by upregulating Nrf2 and its target genes, including glucose-6-phosphate dehydrogenase (G6PD), GCL, and SOD2 [132]. Similarly, KD reduced Aβ deposition and neuronal ferroptosis and improved cognitive function in APP/PS-1 mice through Nrf2 activation [133]. Recently, BHB was reported to alleviate oxidative stress and ferroptosis in dopaminergic neurons in a PD model induced by MPTP [134]. This process likely involves the regulation of the zinc finger protein 36 (ZFP36)/acyl-CoA synthetase long-chain family member 4 (ACSL4) axis.
Taken together, these observations suggest that KBs function as stress response molecules, mediating the beneficial effects of a KD through anti-apoptotic and antioxidant defenses, and enhancing stress resistance and neuroprotection.
4.4. KBs and KD as Epigenetic and Post-Translational Modifiers of Histones and Non-Histone Proteins
SIRT1 is associated with the health and longevity effects of CR and mediates the neurological benefits of a KD [26]. SIRT1 induces the FOXO3a-dependent antioxidant genes catalase and SOD2 [108,124] (Figure 3B). Additionally, KD has been shown to induce SIRT1-dependent deacetylation of p53, thereby inhibiting proapoptotic p53 function and protecting neurons from apoptosis [121]. SIRT3 regulates cellular metabolism during fasting, CR, and exercise. Upregulated SIRT3 may mediate the KB-induced improvement in learning and memory in ApoE4 mice by increasing neuronal energy metabolism [135]. Intermittent fasting-induced ketosis increases SIRT3 activity and prevents hyperexcitability and hippocampal synapse dysfunction in APP mutant mice [136]. Consistently, dietary KB supplementation was found to induce SIRT3 expression and prevent seizures and early death in a SIRT3 haploidic AD mouse model [137]. BHB and KD protect against GABAergic (producing γ-aminobutyric acid, GABA) neuronal degeneration and excitotoxicity through SIRT3-mediated mechanisms [97].
Aging is a major risk factor for neurodegenerative diseases, and the epigenetic changes associated with aging can cause neurodegeneration [138]. HDAC inhibitors have shown potential in the prevention and control of cognitive dysfunction, memory deficits, and neuroprotection in AD and PD animal models [139,140]. Epigenetic regulation by KD may extend lifespan and protect against age-related diseases [141,142]. BHB exerts its effects by regulating gene expression and enzyme activity through epigenetic and post-translational modifications, including acetylation, beta-hydroxybutyrylation, and methylation [10,143]. BHB induces histone acetylation through the inhibition of HDACs 1, 3, and 4 (classes I and IIa) and increases the activation of transcriptional target genes, including FOXO3a, SOD2, and metallothionein 2 (MT2), in response to ketogenic states [144]. BHB has been shown to improve Aβ-induced cell viability reduction and downregulation of tyrosine kinase receptor A by inhibiting HDAC1/3 in SH-SY5Y cells [145]. Inhibition of class I and II HDACs by BHB in mice contributes to the life-extending properties of CR [20]. Similarly, administration of BHB to C. elegans extends their lifespan, delays Aβ toxicity in AD, and reduces α-synuclein aggregation in PD [16]. This finding indicates that BHB may extend lifespan and prevent age-related neurodegeneration through HDAC inhibition [16,20].
BHB and KD improved hyperbaric oxygen-induced spatial memory impairment through increased histone acetylation [146]. KBs promote hyperacetylation of histones and non-histone proteins by increasing acetyl-CoA, a substrate for p300 histone acetyltransferase (HAT) [10,147]. Recent studies have shown that BHB promotes stroke recovery in rodents by reversing stroke-induced GABA transporter-1 (GAT-1) downregulation and dysfunction through HDAC2/3 inhibition, enhancing neural circuit excitability and functional plasticity [148]. Consistently, BHB has been shown to mediate exercise-stimulated expression of brain-derived neurotrophic factor (BDNF) through HDAC2/3 inhibition and H3 acetylation in the hippocampus [149]. BDNF is a neurotrophic factor that promotes neuronal differentiation and survival, synaptic plasticity, and cognitive function [114].
BHB and KD improve hyperbaric oxygen-induced spatial memory impairment through increased histone acetylation [146]. KBs promote hyperacetylation of histones and non-histone proteins by increasing acetyl-CoA, a substrate for p300 histone acetyltransferase (HAT) [10,147]. Recent studies have shown that BHB promotes stroke recovery in rodents by reversing stroke-induced GABA transporter-1 (GAT-1) downregulation and dysfunction through HDAC2/3 inhibition, enhancing neural circuit excitability and functional plasticity [148]. Consistently, BHB has been shown to mediate exercise-stimulated expression of brain-derived neurotrophic factor (BDNF) through HDAC2/3 inhibition and H3 acetylation in the hippocampus [149]. BDNF is a neurotrophic factor that promotes neuronal differentiation and survival, synaptic plasticity, and cognitive function [114].
In addition to histone acetylation, KD and KBs affect histone methylation as well as DNA methylation [143,150]. The anti-seizure effect of KD is associated with inhibition of DNA methyltransferase (DNMT) through increased adenosine, which causes global DNA hypomethylation [142,150,151]. BHB has been shown to enhance BDNF expression by increasing histone H3 lysine 4 trimethylation (H3K4me3) and decreasing histone H2A lysine 119 ubiquitination (H2AK119ub) at the BDNF promoter in hippocampal neurons [152]. The BHB-induced decrease in H2AK119ub levels depends on L-type Ca2+ channels and Ca2+-calmodulin-dependent protein kinase II (CaMKII)/CREB signaling, whereas an increase in H3K4me3 levels depends on the activation of cAMP/protein kinase A (PKA) signaling. In addition, BHB promotes BDNF expression through increased acetylation of H3 lysine 27 (H3K27Ac), which is mediated by cAMP/PKA/CREB signaling and decreased trimethylation of H3K27 (H3K27me3) by H3K27me3-specific demethylase [153].
Protein beta-hydroxybutyrylation plays important roles in various cellular processes [154,155]. Xie et al. showed that BHB induces lysine beta-hydroxybutyrylation (Kbhb) on histones and upregulates genes involved in starvation response metabolic pathways [156]. In mouse models of depression, H3K9bhb is reduced, and KD or BHB administration exerts antidepressant effects by increasing H3K9bhb levels and BDNF expression, linking BHB and BDNF via H3K9bhb [157]. A recent study revealed that KD consumption and BHB treatment in rats reduced cocaine-induced reinstatement through beta-hydroxybutyrylation of CaMKIIα in the hippocampus [158]. Beta-hydroxybutyrylation of CaMKIIα inhibited phosphorylation and activity of CaMKIIα. Taken together, these results suggest that BHB and KD protect the brain from neurodegeneration through epigenetic and post-translational modifications of histones and non-histone proteins.
4.5. KBs and KD as Anti-Neuroinflammatory Signaling Modulators in the Brain
Neuroinflammation constitutes a pivotal pathological feature in ischemic stroke and neurodegenerative diseases. The management of neuroinflammation emerges as a crucial strategy to prevent and treat nerve damage in neurological diseases [86,159,160,161]. KBs function as signaling molecules, orchestrating various cellular responses, encompassing inflammatory and immune functions [2,10]. BHB and KD alleviate motor dysfunction and dopaminergic neuron loss in diverse AD and PD animal models by inhibiting microglial activation and the expression of pro-inflammatory cytokines [85,86,162,163]. Similarly, KD pre-treatment or BHB post-treatment enhances motor function and mitigates brain damage by suppressing microglia-mediated proinflammatory responses in a cerebral stroke mouse model [71]. The signaling pathways underlying KBs/KD-induced anti-neuroinflammatory neuroprotection are summarized in Figure 4A.
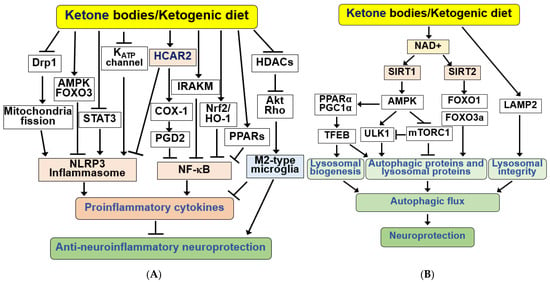
Figure 4.
Signaling functions of KBs and KD for anti-neuroinflammation and autophagic flux. (A) KBs exert anti-inflammatory responses by activating hydroxycarboxylic acid receptor 2 (HCAR2), which inhibits nuclear factor kappa B (NF-κB) and NOD-like receptor protein 3 (NLRP3) inflammasomes. KBs and KD inhibit the NLRP3 inflammasome through KATP channels, Drp1, AMPK/FOXO3, and STAT3 pathways, and NF-κB through COX-1/PGD2, IRAKM, PPARs, and Nrf2/HO-1 pathways. KBs and KD also suppress inflammation through Akt/RhoGTPase-mediated ramification via HDAC inhibition and M2 microglial polarization. (B) KBs and KD induce autophagy and lysosomal proteins through SIRT1/2, AMPK, ULK1, mTORC1, and FOXO1/3a pathways and maintain lysosomal integrity through LAMP2. These changes increase autophagic flux, resulting in increased neuroprotection. All arrows indicate activation and truncated cut lines indicate inhibition.
BHB functions by binding to and activating the hydroxycarboxylic acid receptor 2 (HCAR2), also known as GPR109A, a Gi/o-type G protein-coupled receptor (GPCR) [164]. HCAR2 is required for the neuroprotective effects of BHB and KD in AD, PD, and ischemic stroke models. HCAR2 levels increase in microglia and the substantia nigra under neuroinflammatory conditions in patients with PD [86,104,165]. The anti-inflammatory effects of HCAR2 ligands, such as BHB, nicotinic acid (niacin), and butyrate, have been identified in neurodegenerative diseases [2,161,164,166].
Upon transportation to the brain, BHB binds to HCAR2 on microglial, neutrophil, and monocyte-derived cells, consequently reducing neuroinflammation [161,164]. HCAR2 activation induces a neuroprotective phenotype in macrophages dependent on prostaglandin D2 (PGD2) production by cyclooxygenase 1 (COX1) [160]. The BHB/HCAR2 signaling pathway inhibits inflammatory responses by suppressing nuclear factor-kappa B (NF-κB) activation [86,160,167]. Knockdown of HCAR2 or treatment with pertussis toxin (PTX), an inhibitor of Gi proteins, in microglia abolishes the effects of BHB on NF-κB activation and proinflammatory cytokine release [167]. In the brain, the anti-inflammatory effects of BHB through HCAR2 and NF-κB prove neuroprotective.
In a mouse model of transient MCAO, KD pre-treatment or BHB post-treatment mitigated ischemic brain damage, reduced infarct volume, and suppressed hyperactivation of microglia by inhibiting NF-κB activation through the interleukin-1 (IL-1) receptor-associated kinase M (IRAKM)-dependent pathway [71]. Similarly, recent research showed that KD consumption enhanced cognitive function, reduced amyloid plaques, and lowered proinflammatory cytokine levels in APP/PS-1 mice by activating Nrf2/HO-1 and inhibiting the NF-κB signaling pathway [168]. Additionally, KD activated peroxisome proliferator-activated receptors (PPARs), inhibiting proinflammatory NF-κB activation and COX2 expression in the mouse hippocampus [169,170].
BHB injection inhibits microglial activation, reduces amyloid plaques, and enhances cognitive function in a 5xFAD mouse model [171]. HCAR2 expression was higher in the brains of 5xFAD mice than in wild-type mice. BHB inhibited the expression of pro-inflammatory cytokines, suppressed APP expression, and increased the levels of the Aβ-degrading enzyme, neprilysin, in an HCAR2-dependent manner [171]. Moreover, KD consumption for 4 months in 5xFAD mice improved cognitive function, prevented synaptic and neuronal loss, reduced Aβ deposition, and diminished microgliosis and neuroinflammation [172].
The activation of NOD-like receptor family pyrin domain-containing protein 3 (NLRP3) inflammasome is associated with inflammatory responses. Previously, BHB was demonstrated to inhibit the NLRP3 inflammasome in macrophages and in vivo inflammatory models [173,174]. Consistently, BHB administration reduced amyloid plaques and microgliosis, inhibited NLRP3 inflammasome activation, and curtailed proinflammatory IL-1β release in 5xFAD mice [175]. BHB inhibited endoplasmic reticulum (ER) stress-induced inflammasome formation through AMPK activation and reduced ROS levels through the AMPK-FOXO3 antioxidant signaling pathway [176]. Systemic BHB administration reduces diabetic retinal damage by inhibiting NLRP3 inflammasome and ER stress through retinal HCAR2 activation [173].
KD improves brain ischemia tolerance and inhibits NLRP3 inflammasome activation by preventing dynamin-related protein 1 (Drp1)-mediated mitochondrial fission [177]. BHB administration has also been demonstrated to exert an anxiolytic effect by suppressing NLRP3-induced neuroinflammation in rodent models of post-traumatic stress disorders [178]. Similarly, BHB has been shown to reverse the loss of dopaminergic neurons and glial activation and inhibit microglial pyroptosis by negatively regulating STAT3-mediated NLRP3, resulting in the downregulation of pro-inflammatory cytokines [85].
BHB alleviates the neurodegenerative processes by regulating activated microglia and astrocytes [159,161,179,180]. BHB exhibits anti-inflammatory neuroprotection by inducing anti-inflammatory M2-type macrophage polarization through HDAC inhibition-triggered Akt/RhoGTPase axis [181].
4.6. KBs and KD as Regulators of Autophagy
Autophagy, a lysosome-dependent degradation process, is a key mechanism in maintaining cellular homeostasis and survival. Impaired autophagy in the brain results in the accumulation of abnormal protein aggregates and neuronal loss, culminating in neurodegenerative disorders [182]. Therefore, maintaining proper autophagy is essential to prevent accelerated neurodegeneration [108,182].
BHB and KD have been shown to stimulate autophagic flux in glucose-starved cultured neurons and hypoglycemic rat brains [183]. BHB and KD protect against neuronal damage under hypoglycemic and ischemic stroke conditions by improving impaired autophagic flux and reversing reduced monomeric Aβ levels [184,185,186]. The hippocampus and prefrontal cortex respond differentially to changes in autophagy markers and levels of KB utilization upon KD administration, suggesting a potential interconnection between KB metabolism and autophagy [187]. Moreover, the ketogenic enzyme, HMGCS2, promotes the autophagic clearance of APP and tau/phosphorylated tau, mimicked by KB consumption during AD pathophysiology [188,189]. These observations indicate that HMGCS2-induced KB production plays a crucial role in autophagy regulation. Consistently, KD increased the expression of autophagy-related proteins and ameliorated neuronal death in an epilepsy rat model [190]. Collectively, the neuroprotective effects of KBs and KD appear to be linked to their ability to stimulate autophagic flux and correct autophagy defects.
The signaling pathways for neuroprotection by KBs and KD through autophagy regulation are summarized in Figure 4B. BHB has been shown to increase NAD+ levels, stimulating lysosomal autophagy by activating SIRT1/2. The SIRT1–AMPK pathway can stimulate neuronal autophagy by regulating several autophagy proteins, including unc-51-like autophagy-activating kinase (ULK1) and mechanistic target of rapamycin (mTORC1). AMPK activates ULK1 by inducing phosphorylation but inhibits mTORC1 [191]. ULK1 increases autophagy by initiating autophagosome formation; however, the mTORC1 signaling pathway inhibits autophagy by inhibiting ULK1 and other autophagic proteins. NAD+-dependent SIRT2 activation also increases FOXO1/3a activity, influencing autophagy. The activation and expression of autophagy proteins play a role in preventing neurodegenerative disorders by increasing autophagic flux [108].
Autophagy is enhanced by KBs and KD through the transcription factor EB (TFEB), a key regulator of lysosomal biogenesis [107]. Although the mechanisms by which TFEB is regulated are poorly understood, the recruitment of retinoid X receptor α, PPARα, and PGC1α to the PPAR binding site of the Tfeb gene promoter has been proposed to upregulate TFEB in brain cells [192]. KBs/KD-mediated activation of PPARα and PGC1α and subsequent TFEB induction seem to increase lysosomal proteins and lysosomal biogenesis. D-BHB administration has also been shown to reduce autophagic protein content and cleavage of the lysosome-associated membrane protein 2 (LAMP2) in rat brains injected with N-methyl-D-aspartate (NMDA), thereby increasing lysosomal autophagic degradation and preventing NMDA-induced brain damage [193]. Similarly, D-BHB treatment after MCAO significantly reduced brain damage by preventing LAMP2 cleavage and stimulating autophagic flux in the ischemic core and penumbra [186]. These results suggest that the neuroprotection induced by D-BHB prevents lysosomal rupture, enabling functional autophagy.
4.7. KBs and KD as Modulators of Neurotransmission Systems
KBs influence various brain functions and neuronal excitability by regulating neurotransmitter systems [28,194]. The therapeutic impact of KBs on refractory epilepsy may stem from the modulation of neuronal excitability and a decrease in neuronal firing rates [194]. Mitochondrial ketolysis reduces the release of excitatory glutamate, lowering extracellular glutamate levels and elevates inhibitory GABA levels [28]. In cultured neurons, the replacement of glucose with BHB leads to changes in metabolic pathways, enhancing the TCA cycle flux, producing more α-ketoglutarate, and ultimately converting to GABA in GABAergic neurons [38,195] (Figure 5A).
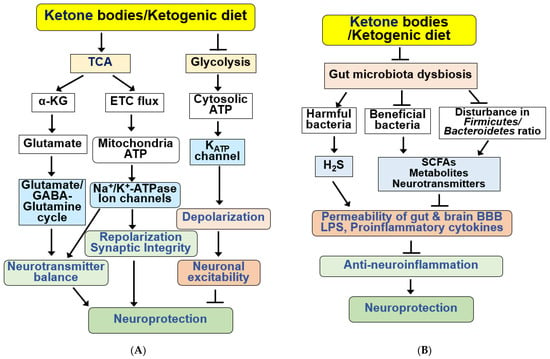
Figure 5.
Functions of KBs and KD for the regulation of neurotransmission systems and gut microbiota. (A) KBs and KD exert various beneficial effects on the regulation of neurotransmission systems. KBs/KD produce α-ketoglutarate (α-KG) through the TCA cycle and regulate neurotransmitter balance by regulating the glutamate–GABA/glutamate–glutamine cycle. KBs and KD also produce mitochondrial ATP and regulate synaptic integrity through ion channels and Na+/K+-ATPase. KBs and KD inhibit neuronal excitability through hyperpolarization by inhibiting glycolysis, reducing cytoplasmic ATP levels, and opening KATP channels. KBs and KD regulate sympathetic function through FFAR3 and N-type Ca2+ channels and norepinephrine release. (B) KBs and KD improve gut microbiota dysbiosis, increasing beneficial bacteria and reducing harmful proinflammatory bacteria. KB and KD also reverse disturbance in the ratio of Firmicutes to Bacteroidetes. Bacteria-derived SCFAs, metabolites, neurotransmitters, H2S, and LPS regulate the permeability of the gut-brain BBB and the production of proinflammatory cytokines. These alterations by KBs and KD result in anti-neuroinflammatory neuroprotection. All arrows indicate activation and truncated cut lines indicate inhibition.
BHB stimulates neurotransmitter recycling in the glutamate–glutamine cycle between neurons and astrocytes [38]. BHB prevents impaired synaptic function, long-term potentiation, and GABAergic activity in hippocampal slices exposed to Aβ and reduces neuronal hyperexcitability in a transgenic APP/PS-1 mouse model [196]. KB-induced mitochondrial ATP production through the TCA cycle and ETC flux can stimulate Na+/K+-ATPase and play a role in repolarization of the neuronal membrane, thereby regulating neurotransmitter levels [28].
When blood glucose levels are high, active glycolysis increases the cytosolic ATP levels and closes ATP-sensitive K+ (KATP) channels in the plasma membrane, depolarizing the membrane. In response to changes in membrane potential, voltage-gated Ca2+ channels open, and Ca2+ flows into the cell. Ca2+ is also released from the ER in response to an initial increase in Ca2+ concentration in the cytoplasm. Cytosolic Ca2+ concentration is sufficiently high to trigger neurotransmitter release via exocytosis, thereby increasing neuronal excitability. BHB treatment decreases neuronal excitability by inhibiting glycolysis, reducing cytosolic ATP levels, opening KATP channels, causing neuronal membrane hyperpolarization, and decreasing neuronal activity [28,35,197,198]. However, KBs and KD can increase ATP production, and KBs may regulate the opening of KATP channels through various other mechanisms [17,194,197].
BHB treatment reduces NMDA-induced intracellular Ca2+ increase in glutamatergic neurons [198]. Although BHB had no direct effect on NMDA-evoked neurotransmitters, BHB was found to increase neurotransmitter release upon stimulation with the KATP channel blocker, glibenclamide, indicating the involvement of KATP channels in the effects exhibited by BHB [198]. Additionally, KBs have been shown to inhibit glutamate transport by binding to Cl− ions and acting as allosteric modulators of vesicular glutamate transporters [28,194,199].
Interestingly, KD consumption has been shown to improve emotional symptoms, such as anxiety and depression, in patients with AD by modulating the glutamatergic neurotransmission system [32]. Similarly, a KD improves cognitive function in patients with AD and mood disorders in 3xTgAD mice [58,74,79]. Overstimulation of NMDA receptors by glutamate results in neuronal cell death owing to increased Ca2+ concentrations and ROS production [32]. The BHB produced by KD ingestion has been shown to reduce the toxicity of amyloid plaques by acting as a glutamate inhibitor at extrasynaptic NMDA receptors. BHB can reduce anxiety and depression in AD pathology by improving the activity of glutamate at the synaptic level with better ATP efficiency and reducing oxidative stress and inflammation [32,195,200].
BHB has been found to bind to free fatty acid receptor 3 (FFAR3, GPR41), a type of GPCR for short-chain fatty acids (SCFAs) that regulates sympathetic nerve activity [201,202]. BHB inhibits voltage-dependent N-type Ca2+ channels, and reduces norepinephrine release through FFAR3 activation in rat sympathetic neurons [28,202]. Recently, SCFAs and FFAR3 were reported to enhance cognition and alleviate disease in the 5xFAD mouse model of AD, suggesting that metabolite-sensing FFAR3 may play a protective role against AD [203]. Overall, KBs and KD may enhance neural activity and brain function by regulating neurotransmission.
4.8. KBs and KD as Regulators of the Gut Microbiota
The gut microbiome is associated with neurodegenerative diseases by affecting neuroinflammation and metabolic homeostasis [37,204]. Previously, patients with MCI were demonstrated to have increased colonization with pathogenic Proteobacteria and Firmicutes phyla and a lower abundance of Bacteroidetes than cognitively normal subjects [37,204,205]. High levels of harmful bacteria are positively associated with AD biomarkers [205]. Additionally, Gram-negative bacteria containing highly inflammatory LPS are abundant in patients with MCI and AD [205,206]. Similar to AD, disturbances in the gut microbiome (dysbiosis) have been associated with many other neurological disorders, such as PD, epilepsy, ASD, MS, and ischemic stroke [81,204,207,208,209,210].
Gut microbiota have been shown to play an important role in brain function through the gut–brain axis [211,212,213]. The gut microbiota can directly or indirectly influence host metabolism, cognition, and neuroinflammation by producing microbe-derived metabolites, such as SCFAs, lactate, and neurotransmitters, including GABA, dopamine, and serotonin [204,214,215,216].
KD exerts beneficial effects in various neurodegenerative diseases by affecting the diversity and abundance of the gut microbiota and attenuating pro-inflammatory responses [114,204,212,215,217]. A KD alters gut microbiota composition in several neurological diseases [37,204,205,218]. A KD also increases the relative abundance of potentially beneficial gut microorganisms, such as A. muciniphila and Lactobacillus, and reduces the abundance of harmful proinflammatory bacteria, such as Desulfovibrio, Turicibacter, and the Lachnobacterium genus of the Firmicutes phylum [37,205,215,219] (Figure 5B). Desulfovibrio produces hydrogen sulfide (H2S), which destroys the intestinal mucosal barrier; therefore, reduction in this microorganism by KD may play a role in its beneficial effects [215].
Modified mediterranean KD (MMKD) was shown to improve AD biomarkers, including Aβ and tau protein, in the cerebrospinal fluid of patients with MCI, which correlated with changes in gut microbiome and SCFAs [205]. The abundance of A. muciniphila and butyrate significantly increases after MMKD consumption in patients with MCI [205]. Therefore, the effect of KD on the brain is likely mediated by the SCFAs produced by A. muciniphila and Lactobacillus [219,220]. Additionally, the phylum Actinobacteria, family Bifidobacteriaceae, and genus Bifidobacterium were found to be decreased in the MCI group after MMKD compared to their cognitively normal counterparts, which was consistent with the decrease in lactate and acetate levels in the MCI-MMKD group [205]. Similarly, BHB produced by KD and ketone ester supplementation reduces Bifidobacteria and pro-inflammatory Th17 cells in the intestine [221].
KD consumption has been shown to reverse the common autism phenotype of a low proportion of Firmicutes to Bacteroides species and improve behavioral symptoms in a mouse model of ASD [215,222]. Disturbances in the ratio of Firmicutes to Bacteroidetes may be associated with gut dysbiosis and negative health outcomes [205]. The ameliorating effect of a KD on ASD-like behaviors is related to reduced levels of pro-inflammatory cytokines and microbiota remodeling [223]. A KD was found to increase the putatively beneficial microbiota, A. muciniphila and Blautia, and reverse the increase in Lactobacillus in the feces of the ASD mouse model. Gut microbial dysbiosis and changes in microbial metabolites have been observed in patients with PD [224]. In patients with PD, non-motor symptoms and gastrointestinal dysfunction often precede motor symptoms [225]. Recent studies revealed that KD and MCTG attenuated impaired motor function, loss of dopaminergic neurons, and inflammation by altering gut microbiota and metabolites in an MPTP-induced mouse model of PD [226,227]. Compared with MPTP-control diet groups, the relative abundance of SCFA-producing bacteria, such as Blautia and Romboutsia, increased in the KD group with MPTP-MCTG, while the relative abundance of Desulfomicrobium decreased significantly [227].
Some studies have reported that KD has negative effects on the gut microbiota [37]. KD has been shown to reduce the overall diversity of the gut microbiome due to its low carbohydrate content [228]. As microorganisms utilize fiber as their primary energy source, some portions of the microbial community cannot survive in KDs with low carbohydrate content. Signs of intestinal dysbiosis were observed in a group of AD rats and patients with MCI after KD consumption [37]. Intermittent fasting reduced hippocampal Aβ deposition, whereas KD worsened gut dysbiosis by increasing Proteobacteria and failed to improve memory function in an AD model of rats receiving Aβ(25–35) infusion [229]. A KD also resulted in a further increase in Enterobacteriaceae among patients with MCI [205]. Plasma KB levels increase, while plasma and cecal SCFA concentrations decrease in KD [230]. As the neuroprotective effect of a KD exhibited through the intestinal microbial community is influenced by individual factors, such as sex, age, and race, additional studies are needed to identify the underlying mechanism [114].
4.9. Adverse Effects of the KD
Although ketosis induced by KBs and KD can provide beneficial effects on various diseases, some adverse effects of KD have been reported. A KD causes a variety of gastrointestinal discomforts, including nausea, vomiting, constipation, diarrhea, low appetite, weight loss, headache, hyperuricemia, and development of dyslipidemia [5,82]. Therefore, more studies, especially long-term studies, are needed to investigate the positive effects, efficacy, and safety regarding the therapeutic application of KBs and KD for neurodegenerative diseases.
5. Conclusions
Recently, researchers and clinicians have paid considerable attention to KBs and KD due to their therapeutic potential across various diseases, including metabolic disorders, cancer, cardiovascular diseases, and neurological disorders. Notably, data from both preclinical and clinical trials substantiate the efficacy of KBs and KD in the prevention and treatment of neurodegenerative diseases, such as AD and PD.
KBs and KD confer neuroprotective effects by supplying alternative energy substrates to neurons and enhancing mitochondrial structure and function (Figure 6). Additionally, KBs and KD exhibit positive impacts by reducing oxidative stress and apoptosis, promoting autophagic flux, and regulating gene expression and other cellular functions through epigenetic and post-translational modifications of histones and non-histone proteins. KBs and KD also suppress neuroinflammation and modulate neurotransmitter systems and the gut microbiota. Through these diverse effects, KBs and KD demonstrate significant therapeutic potential for cerebral ischemia and various neurodegenerative diseases.
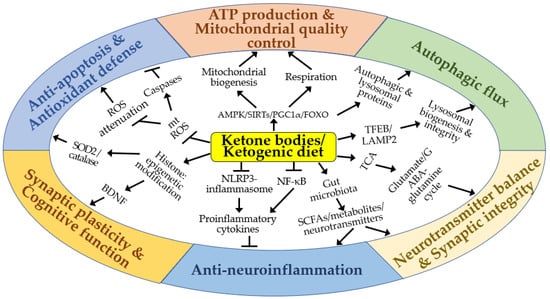
Figure 6.
A schematic diagram of the molecular mechanisms by which ketone bodies and ketogenic diet may exert neuroprotective effects in vitro and in vivo. Arrows and truncated lines indicate activation and inhibition, respectively.
The molecular mechanisms governing KB levels and the functions of KBs and KD in the brain remain incompletely understood in the context of cerebral ischemia and other neurodegenerative diseases. Consequently, further studies are imperative to elucidate the roles and mechanisms of action of KBs and KD in treating cerebral ischemia and diverse forms of neurodegenerative diseases.
Author Contributions
Conceptualization, J.J., S.R.K., J.E.L., E.-J.Y. and I.K.; writing—original draft preparation, J.J., S.R.K., J.E.L., E.-J.Y. and I.K.; writing—review and editing, J.J., S.R.K., J.E.L., S.L., H.J.S., W.C., K.-S.Y., S.S.K., E.-J.Y. and I.K.; supervision, E.-J.Y. and I.K.; project administration, I.K.; funding acquisition, I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basic Science Research Program through the National Research Foundation of Korea (NRF) grants funded by the Korean government (NRF-2021R1F1A1049054 and NRF-2018R1A6A1A03025124).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Aβ, amyloid-beta; AcAc, acetoacetate; ACAT1, acetyl-CoA acetyltransferase 1; ACSL4, acyl-CoA synthetase long-chain family member 4; AD, Alzheimer’s disease; AMPK, AMP-activated protein kinase; APP, amyloid precursor protein; ASD, autism spectrum disorder; BBB, blood-brain barrier; BDH1, β-hydroxybutyrate dehydrogenase 1; BDNF, BHB, β-hydroxy butyrate; CaMKIIα, Ca2+-calmodulin-dependent protein kinase IIα; COX-1, cyclooxygenase 1; COX-2, cyclooxygenase 2; CPT1, carnitine palmitoyl transferase 1; CR, calorie restriction; CREB, cAMP response element binding protein; CS, citrate synthase; D-BHB, D-beta-hydroxybutyrate; DNMT1, DNA methyltransferase 1; Drp1, dynamin-related protein 1; eNOS; endothelial nitric oxide synthase; ER, endoplasmic reticulum; ERK1/2, extracellular signal-regulated kinase 1/2; ETC, electron transport chain; FFAR3, free fatty acid receptor 3; FOXO3, forkhead box O3; G6PD, glucose-6-phosphate dehydrogenase; GABA, gamma-aminobutyric acid; GAT-1, GABA transporter-1; GCL, glutamate-cysteine ligase; GLUT1, glucose transporter 1; GPCR, G-protein coupled receptor; HD, Huntington’s disease; H2S, hydrogen sulfide; HCAR2, hydroxycarboxylic acid receptor 2; HDAC, histone deacetylase; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; HMGCL, 3-hydroxy-3-methylglutaryl-CoA lyase; HMGCS2, 3-hydroxy-3-methylglutaryl-CoA synthase 2; HO-1, heme oxygenase-1; IRAKM, interleukin-1 (IL-1) receptor-associated kinase M; KATP, ATP-sensitive K+; KB, ketone body; Kbhb, lysine beta-hydroxybutyrylation; KD, ketogenic diet; LAMP2, lysosome-associated membrane protein 2; LKB1, liver kinase B1; LPS, lipopolysaccharide; MC, multiple sclerosis; MCAO, middle cerebral artery occlusion; MCI, mild cognitive impairment; MCT, monocarboxylate transporter; MCTG, medium-chain triglyceride; MMKD, modified mediterranean-ketogenic diet; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; mPTP, mitochondrial permeability transition pore; MS, multiple sclerosis; mt, mitochondrial; MT2, metallothionein 2; mTOR, mechanistic target of rapamycin; mTORC1, mTOR complex 1; NF-κB, nuclear factor kappa B; NLRP3, NOD-like receptor pyrin domain-containing protein 3; NMDA, N-methyl-D-aspartate; NOX2, NADPH oxidase 2; Nrf2, nuclear factor erythroid 2-related factor 2; 6-OHDA, 6-hydroxydopamine; Oxct1, 3-oxoacid-CoA transferase 1; PARP-1, poly-ADP-ribose polymerase-1; PD, Parkinson’s disease; PGC-1α, peroxisome proliferator activated receptor γ coactivator 1α; PGD2, prostaglandin D2; PKA, protein kinase A; PPARs, peroxisome proliferator activated receptors; PS-1, presenilin-1; ROS reactive oxygen species; SCFA, short-chain fatty acid; SCOT, succinyl-CoA:3-oxoacid-CoA transferase; SIRT, sirtuin; SMCT1, sodium-dependent MCT1; SOD2, superoxide dismutase 2; STAT3, signal transducer and activator of transcription 3; TCA, tricarboxylic acid; TFEB, transcription factor EB; UCP2, uncoupling protein 2; ULK1, unc-51 like kinase 1; ZEP36, zinc finger protein 36.
References
- Cahill, G.F., Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Puchalska, P.; Crawford, P.A. Metabolic and Signaling Roles of Ketone Bodies in Health and Disease. Annu. Rev. Nutr. 2021, 41, 49–77. [Google Scholar] [CrossRef]
- Pietrzak, D.; Kasperek, K.; Rekawek, P.; Piatkowska-Chmiel, I. The Therapeutic Role of Ketogenic Diet in Neurological Disorders. Nutrients 2022, 14, 1952. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Tozzi, R.; Risi, R.; Tuccinardi, D.; Mariani, S.; Basciani, S.; Spera, G.; Lubrano, C.; Gnessi, L. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes. Rev. 2020, 21, e13024. [Google Scholar] [CrossRef] [PubMed]
- Wlodarek, D. Role of Ketogenic Diets in Neurodegenerative Diseases (Alzheimer’s Disease and Parkinson’s Disease). Nutrients 2019, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Abdul Kadir, A.; Clarke, K.; Evans, R.D. Cardiac ketone body metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165739. [Google Scholar] [CrossRef]
- Dabek, A.; Wojtala, M.; Pirola, L.; Balcerczyk, A. Modulation of Cellular Biochemistry, Epigenetics and Metabolomics by Ketone Bodies. Implications of the Ketogenic Diet in the Physiology of the Organism and Pathological States. Nutrients 2020, 12, 788. [Google Scholar] [CrossRef]
- Tozzi, R.; Cipriani, F.; Masi, D.; Basciani, S.; Watanabe, M.; Lubrano, C.; Gnessi, L.; Mariani, S. Ketone Bodies and SIRT1, Synergic Epigenetic Regulators for Metabolic Health: A Narrative Review. Nutrients 2022, 14, 3145. [Google Scholar] [CrossRef]
- Wang, L.; Chen, P.; Xiao, W. beta-hydroxybutyrate as an Anti-Aging Metabolite. Nutrients 2021, 13, 3420. [Google Scholar] [CrossRef]
- Hwang, C.Y.; Choe, W.; Yoon, K.S.; Ha, J.; Kim, S.S.; Yeo, E.J.; Kang, I. Molecular Mechanisms for Ketone Body Metabolism, Signaling Functions, and Therapeutic Potential in Cancer. Nutrients 2022, 14, 4932. [Google Scholar] [CrossRef]
- Jensen, N.J.; Wodschow, H.Z.; Nilsson, M.; Rungby, J. Effects of Ketone Bodies on Brain Metabolism and Function in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8767. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.L. Cerebral ketone metabolism during development and injury. Epilepsy Res. 2012, 100, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Nehlig, A. Brain uptake and metabolism of ketone bodies in animal models. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Blazquez, C. Ketone body synthesis in the brain: Possible neuroprotective effects. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Iizumi, T.; Mashima, K.; Abe, T.; Suzuki, N. Roles and regulation of ketogenesis in cultured astroglia and neurons under hypoxia and hypoglycemia. ASN Neuro 2014, 6, 1759091414550997. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.; Canfield, J.; Copes, N.; Rehan, M.; Lipps, D.; Bradshaw, P.C. D-beta-hydroxybutyrate extends lifespan in C. elegans. Aging 2014, 6, 621–644. [Google Scholar] [CrossRef] [PubMed]
- Gano, L.B.; Patel, M.; Rho, J.M. Ketogenic diets, mitochondria, and neurological diseases. J. Lipid Res. 2014, 55, 2211–2228. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef]
- Newman, J.C.; Covarrubias, A.J.; Zhao, M.; Yu, X.; Gut, P.; Ng, C.P.; Huang, Y.; Haldar, S.; Verdin, E. Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab. 2017, 26, 547–557.e8. [Google Scholar] [CrossRef]
- Veech, R.L.; Bradshaw, P.C.; Clarke, K.; Curtis, W.; Pawlosky, R.; King, M.T. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life 2017, 69, 305–314. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, J.; Xu, D.; Zhou, Y.; Qu, Z.; Yang, Q.; Lv, Q. Low carbohydrate ketogenic diets reduce cardiovascular risk factor levels in obese or overweight patients with T2DM: A meta-analysis of randomized controlled trials. Front. Nutr. 2022, 9, 1092031. [Google Scholar] [CrossRef] [PubMed]
- Risi, R.; Tozzi, R.; Watanabe, M. Beyond weight loss in nonalcoholic fatty liver disease: The role of carbohydrate restriction. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Yurista, S.R.; Chong, C.R.; Badimon, J.J.; Kelly, D.P.; de Boer, R.A.; Westenbrink, B.D. Therapeutic Potential of Ketone Bodies for Patients With Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Bi, D.; Zhang, Y.; Kong, C.; Du, J.; Wu, X.; Wei, Q.; Qin, H. Ketogenic diet for human diseases: The underlying mechanisms and potential for clinical implementations. Signal Transduct. Target. Ther. 2022, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Dynka, D.; Kowalcze, K.; Paziewska, A. The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients 2022, 14, 5003. [Google Scholar] [CrossRef] [PubMed]
- Norwitz, N.G.; Hu, M.T.; Clarke, K. The Mechanisms by Which the Ketone Body D-beta-Hydroxybutyrate May Improve the Multiple Cellular Pathologies of Parkinson’s Disease. Front. Nutr. 2019, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.S.; Lin, S.J.; Liao, P.Y.; Suresh, D.; Hsu, T.R.; Wang, P.Y. Role of Ketogenic Diets in Multiple Sclerosis and Related Animal Models: An Updated Review. Adv. Nutr. 2022, 13, 2002–2014. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, Z.; D’Agostino, D.P.; Diamond, D.; Kindy, M.S.; Rogers, C.; Ari, C. Therapeutic Potential of Exogenous Ketone Supplement Induced Ketosis in the Treatment of Psychiatric Disorders: Review of Current Literature. Front. Psychiatry 2019, 10, 363. [Google Scholar] [CrossRef]
- Makievskaya, C.I.; Popkov, V.A.; Andrianova, N.V.; Liao, X.; Zorov, D.B.; Plotnikov, E.Y. Ketogenic Diet and Ketone Bodies against Ischemic Injury: Targets, Mechanisms, and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 2576. [Google Scholar] [CrossRef]
- Brenton, J.N.; Banwell, B.; Bergqvist, A.G.C.; Lehner-Gulotta, D.; Gampper, L.; Leytham, E.; Coleman, R.; Goldman, M.D. Pilot study of a ketogenic diet in relapsing-remitting MS. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e565. [Google Scholar] [CrossRef]
- Phillips, M.C.L.; McManus, E.J.; Brinkhuis, M.; Romero-Ferrando, B. Time-Restricted Ketogenic Diet in Huntington’s Disease: A Case Study. Front. Behav. Neurosci. 2022, 16, 931636. [Google Scholar] [CrossRef] [PubMed]
- de la Rubia Orti, J.E.; Fernandez, D.; Platero, F.; Garcia-Pardo, M.P. Can Ketogenic Diet Improve Alzheimer’s Disease? Association with Anxiety, Depression, and Glutamate System. Front. Nutr. 2021, 8, 744398. [Google Scholar] [CrossRef] [PubMed]
- Dietch, D.M.; Kerr-Gaffney, J.; Hockey, M.; Marx, W.; Ruusunen, A.; Young, A.H.; Berk, M.; Mondelli, V. Efficacy of low carbohydrate and ketogenic diets in treating mood and anxiety disorders: Systematic review and implications for clinical practice. BJPsych Open 2023, 9, e70. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.W.Y.; Corley, M.J.; Pang, A.; Arakaki, G.; Abbott, L.; Nishimoto, M.; Miyamoto, R.; Lee, E.; Yamamoto, S.; Maunakea, A.K.; et al. A modified ketogenic gluten-free diet with MCT improves behavior in children with autism spectrum disorder. Physiol. Behav. 2018, 188, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Achanta, L.B.; Rae, C.D. beta-Hydroxybutyrate in the Brain: One Molecule, Multiple Mechanisms. Neurochem. Res. 2017, 42, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Moller, N. Ketone Body, 3-Hydroxybutyrate: Minor Metabolite—Major Medical Manifestations. J. Clin. Endocrinol. Metab. 2020, 105, 2884–2892. [Google Scholar] [CrossRef] [PubMed]
- Kaviyarasan, S.; Chung Sia, E.L.; Retinasamy, T.; Arulsamy, A.; Shaikh, M.F. Regulation of gut microbiome by ketogenic diet in neurodegenerative diseases: A molecular crosstalk. Front. Aging Neurosci. 2022, 14, 1015837. [Google Scholar] [CrossRef]
- Andersen, J.V.; Schousboe, A. Milestone Review: Metabolic dynamics of glutamate and GABA mediated neurotransmission—The essential roles of astrocytes. J. Neurochem. 2023, 166, 109–137. [Google Scholar] [CrossRef]
- Morris, G.; Maes, M.; Berk, M.; Carvalho, A.F.; Puri, B.K. Nutritional ketosis as an intervention to relieve astrogliosis: Possible therapeutic applications in the treatment of neurodegenerative and neuroprogressive disorders. Eur. Psychiatry 2020, 63, e8. [Google Scholar] [CrossRef]
- Silva, B.; Mantha, O.L.; Schor, J.; Pascual, A.; Placais, P.Y.; Pavlowsky, A.; Preat, T. Glia fuel neurons with locally synthesized ketone bodies to sustain memory under starvation. Nat. Metab. 2022, 4, 213–224. [Google Scholar] [CrossRef]
- Koch, K.; Berressem, D.; Konietzka, J.; Thinnes, A.; Eckert, G.P.; Klein, J. Hepatic Ketogenesis Induced by Middle Cerebral Artery Occlusion in Mice. J. Am. Heart Assoc. 2017, 6, e005556. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Suzuki, M.; Kitamura, Y.; Mori, S.; Sato, K.; Dohi, S.; Sato, T.; Matsuura, A.; Hiraide, A. Beta-hydroxybutyrate, a cerebral function improving agent, protects rat brain against ischemic damage caused by permanent and transient focal cerebral ischemia. Jpn. J. Pharmacol. 2002, 89, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, C.; Woods, A.; de Ceballos, M.L.; Carling, D.; Guzman, M. The AMP-activated protein kinase is involved in the regulation of ketone body production by astrocytes. J. Neurochem. 1999, 73, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Longo, R.; Peri, C.; Cricri, D.; Coppi, L.; Caruso, D.; Mitro, N.; De Fabiani, E.; Crestani, M. Ketogenic Diet: A New Light Shining on Old but Gold Biochemistry. Nutrients 2019, 11, 2497. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Sun, L.; Wang, H. Ketogenic Diet for Neonatal Hypoxic-Ischemic Encephalopathy. ACS Chem. Neurosci. 2023, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Leino, R.L.; Gerhart, D.Z.; Duelli, R.; Enerson, B.E.; Drewes, L.R. Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem. Int. 2001, 38, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.L.; Giza, C.C. Induction of monocarboxylate transporter 2 expression and ketone transport following traumatic brain injury in juvenile and adult rats. Dev. Neurosci. 2006, 28, 447–456. [Google Scholar] [CrossRef]
- Koppel, S.J.; Pei, D.; Wilkins, H.M.; Weidling, I.W.; Wang, X.; Menta, B.W.; Perez-Ortiz, J.; Kalani, A.; Manley, S.; Novikova, L.; et al. A ketogenic diet differentially affects neuron and astrocyte transcription. J. Neurochem. 2021, 157, 1930–1945. [Google Scholar] [CrossRef]
- Saito, E.R.; Miller, J.B.; Harari, O.; Cruchaga, C.; Mihindukulasuriya, K.A.; Kauwe, J.S.K.; Bikman, B.T. Alzheimer’s disease alters oligodendrocytic glycolytic and ketolytic gene expression. Alzheimers Dement. 2021, 17, 1474–1486. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Dangelo, K.; Couch, S.C.; Benoit, S.C.; Clegg, D.J. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol. Aging 2012, 33, 425.e19–425.e27. [Google Scholar] [CrossRef]
- Rebello, C.J.; Keller, J.N.; Liu, A.G.; Johnson, W.D.; Greenway, F.L. Pilot feasibility and safety study examining the effect of medium chain triglyceride supplementation in subjects with mild cognitive impairment: A randomized controlled trial. BBA Clin. 2015, 3, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Neth, B.J.; Mintz, A.; Whitlow, C.; Jung, Y.; Solingapuram Sai, K.; Register, T.C.; Kellar, D.; Lockhart, S.N.; Hoscheidt, S.; Maldjian, J.; et al. Modified ketogenic diet is associated with improved cerebrospinal fluid biomarker profile, cerebral perfusion, and cerebral ketone body uptake in older adults at risk for Alzheimer’s disease: A pilot study. Neurobiol. Aging 2020, 86, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Fortier, M.; Castellano, C.A.; St-Pierre, V.; Myette-Cote, E.; Langlois, F.; Roy, M.; Morin, M.C.; Bocti, C.; Fulop, T.; Godin, J.P.; et al. A ketogenic drink improves cognition in mild cognitive impairment: Results of a 6-month RCT. Alzheimers Dement. 2021, 17, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Reger, M.A.; Henderson, S.T.; Hale, C.; Cholerton, B.; Baker, L.D.; Watson, G.S.; Hyde, K.; Chapman, D.; Craft, S. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol. Aging 2004, 25, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.T.; Vogel, J.L.; Barr, L.J.; Garvin, F.; Jones, J.J.; Costantini, L.C. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: A randomized, double-blind, placebo-controlled, multicenter trial. Nutr. Metab. 2009, 6, 31. [Google Scholar] [CrossRef]
- Newport, M.T.; VanItallie, T.B.; Kashiwaya, Y.; King, M.T.; Veech, R.L. A new way to produce hyperketonemia: Use of ketone ester in a case of Alzheimer’s disease. Alzheimers Dement. 2015, 11, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.K.; Sullivan, D.K.; Mahnken, J.D.; Burns, J.M.; Swerdlow, R.H. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimers Dement. 2018, 4, 28–36. [Google Scholar] [CrossRef]
- Ota, M.; Matsuo, J.; Ishida, I.; Takano, H.; Yokoi, Y.; Hori, H.; Yoshida, S.; Ashida, K.; Nakamura, K.; Takahashi, T.; et al. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease. Neurosci. Lett. 2019, 690, 232–236. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Y.; Zhang, X.; Liu, L.; Zhou, B.; Mo, R.; Li, Y.; Li, H.; Li, F.; Tao, Y.; et al. Medium-chain triglycerides improved cognition and lipid metabolomics in mild to moderate Alzheimer’s disease patients with APOE4(-/-): A double-blind, randomized, placebo-controlled crossover trial. Clin. Nutr. 2020, 39, 2092–2105. [Google Scholar] [CrossRef]
- Phillips, M.C.L.; Deprez, L.M.; Mortimer, G.M.N.; Murtagh, D.K.J.; McCoy, S.; Mylchreest, R.; Gilbertson, L.J.; Clark, K.M.; Simpson, P.V.; McManus, E.J.; et al. Randomized crossover trial of a modified ketogenic diet in Alzheimer’s disease. Alzheimers Res. Ther. 2021, 13, 51. [Google Scholar] [CrossRef]
- Vanitallie, T.B.; Nonas, C.; Di Rocco, A.; Boyar, K.; Hyams, K.; Heymsfield, S.B. Treatment of Parkinson disease with diet-induced hyperketonemia: A feasibility study. Neurology 2005, 64, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C.L.; Murtagh, D.K.J.; Gilbertson, L.J.; Asztely, F.J.S.; Lynch, C.D.P. Low-fat versus ketogenic diet in Parkinson’s disease: A pilot randomized controlled trial. Mov. Disord. 2018, 33, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Shidler, M.D.; Summer, S.S.; Sullivan, P.G.; Duker, A.P.; Isaacson, R.S.; Espay, A.J. Nutritional ketosis for mild cognitive impairment in Parkinson’s disease: A controlled pilot trial. Clin. Park. Relat. Disord. 2019, 1, 41–47. [Google Scholar] [CrossRef]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. Brain Ischemia as a Prelude to Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 636653. [Google Scholar] [CrossRef] [PubMed]
- Kahl, A.; Blanco, I.; Jackman, K.; Baskar, J.; Milaganur Mohan, H.; Rodney-Sandy, R.; Zhang, S.; Iadecola, C.; Hochrainer, K. Cerebral ischemia induces the aggregation of proteins linked to neurodegenerative diseases. Sci. Rep. 2018, 8, 2701. [Google Scholar] [CrossRef]
- Puchowicz, M.A.; Zechel, J.L.; Valerio, J.; Emancipator, D.S.; Xu, K.; Pundik, S.; LaManna, J.C.; Lust, W.D. Neuroprotection in diet-induced ketotic rat brain after focal ischemia. J. Cereb. Blood Flow Metab. 2008, 28, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Ye, L.; Sharma, K.; Jin, Y.; Harrison, M.M.; Caldwell, T.; Berthiaume, J.M.; Luo, Y.; LaManna, J.C.; Puchowicz, M.A. Diet-Induced Ketosis Protects Against Focal Cerebral Ischemia in Mouse. Adv. Exp. Med. Biol. 2017, 977, 205–213. [Google Scholar] [CrossRef]
- Lehto, A.; Koch, K.; Barnstorf-Brandes, J.; Viel, C.; Fuchs, M.; Klein, J. ss-Hydroxybutyrate Improves Mitochondrial Function After Transient Ischemia in the Mouse. Neurochem. Res. 2022, 47, 3241–3249. [Google Scholar] [CrossRef]
- Shaafi, S.; Sharifi-Bonab, M.; Ghaemian, N.; Mokhtarkhani, M.; Akbari, H. Early Motor-Behavioral Outcome of Ischemic Stroke with Ketogenic Diet Preconditioning: Interventional Animal Study. J. Stroke Cerebrovasc. Dis. 2019, 28, 1032–1039. [Google Scholar] [CrossRef]
- Lin, C.; Wang, S.; Xie, J.; Zhu, J.; Xu, J.; Liu, K.; Chen, J.; Yu, M.; Zhong, H.; Huang, K.; et al. Ketogenic diet and beta-Hydroxybutyrate alleviate ischemic brain injury in mice via an IRAKM-dependent pathway. Eur. J. Pharmacol. 2023, 955, 175933. [Google Scholar] [CrossRef] [PubMed]
- Tai, K.K.; Nguyen, N.; Pham, L.; Truong, D.D. Ketogenic diet prevents cardiac arrest-induced cerebral ischemic neurodegeneration. J. Neural Transm. 2008, 115, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Chen, S.; Mao, Z.; Cadenas, E.; Brinton, R.D. 2-Deoxy-D-glucose treatment induces ketogenesis, sustains mitochondrial function, and reduces pathology in female mouse model of Alzheimer’s disease. PLoS ONE 2011, 6, e21788. [Google Scholar] [CrossRef] [PubMed]
- Pawlosky, R.J.; Kemper, M.F.; Kashiwaya, Y.; King, M.T.; Mattson, M.P.; Veech, R.L. Effects of a dietary ketone ester on hippocampal glycolytic and tricarboxylic acid cycle intermediates and amino acids in a 3xTgAD mouse model of Alzheimer’s disease. J. Neurochem. 2017, 141, 195–207. [Google Scholar] [CrossRef]
- Pawlosky, R.J.; Kashiwaya, Y.; King, M.T.; Veech, R.L. A Dietary Ketone Ester Normalizes Abnormal Behavior in a Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1044. [Google Scholar] [CrossRef]
- Beckett, T.L.; Studzinski, C.M.; Keller, J.N.; Paul Murphy, M.; Niedowicz, D.M. A ketogenic diet improves motor performance but does not affect beta-amyloid levels in a mouse model of Alzheimer’s disease. Brain Res. 2013, 1505, 61–67. [Google Scholar] [CrossRef]
- Brownlow, M.L.; Benner, L.; D’Agostino, D.; Gordon, M.N.; Morgan, D. Ketogenic diet improves motor performance but not cognition in two mouse models of Alzheimer’s pathology. PLoS ONE 2013, 8, e75713. [Google Scholar] [CrossRef]
- Yin, J.X.; Maalouf, M.; Han, P.; Zhao, M.; Gao, M.; Dharshaun, T.; Ryan, C.; Whitelegge, J.; Wu, J.; Eisenberg, D.; et al. Ketones block amyloid entry and improve cognition in an Alzheimer’s model. Neurobiol. Aging 2016, 39, 25–37. [Google Scholar] [CrossRef]
- Kashiwaya, Y.; Bergman, C.; Lee, J.H.; Wan, R.; King, M.T.; Mughal, M.R.; Okun, E.; Clarke, K.; Mattson, M.P.; Veech, R.L. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1530–1539. [Google Scholar] [CrossRef]
- Van der Auwera, I.; Wera, S.; Van Leuven, F.; Henderson, S.T. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutr. Metab. 2005, 2, 28. [Google Scholar] [CrossRef]
- Gough, S.M.; Casella, A.; Ortega, K.J.; Hackam, A.S. Neuroprotection by the Ketogenic Diet: Evidence and Controversies. Front. Nutr. 2021, 8, 782657. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Puri, B.K.; Carvalho, A.; Maes, M.; Berk, M.; Ruusunen, A.; Olive, L. Induced Ketosis as a Treatment for Neuroprogressive Disorders: Food for Thought? Int. J. Neuropsychopharmacol. 2020, 23, 366–384. [Google Scholar] [CrossRef] [PubMed]
- Tieu, K.; Perier, C.; Caspersen, C.; Teismann, P.; Wu, D.C.; Yan, S.D.; Naini, A.; Vila, M.; Jackson-Lewis, V.; Ramasamy, R.; et al. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Investig. 2003, 112, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, B. Neuroprotective and anti-inflammatory activities of ketogenic diet on MPTP-induced neurotoxicity. J. Mol. Neurosci. 2010, 42, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Yin, X.; Wang, M.; Wang, Y.; Li, F.; Gao, Y.; Han, G.; Gao, Z.; Wang, Z. beta-Hydroxybutyrate alleviates pyroptosis in MPP(+)/MPTP-induced Parkinson’s disease models via inhibiting STAT3/NLRP3/GSDMD pathway. Int. Immunopharmacol. 2022, 113, 109451. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.P.; Wang, J.F.; Xue, W.J.; Liu, H.M.; Liu, B.R.; Zeng, Y.L.; Li, S.N.; Huang, B.X.; Lv, Q.K.; Wang, W.; et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflamm. 2015, 12, 9. [Google Scholar] [CrossRef]
- Cheng, B.; Yang, X.; An, L.; Gao, B.; Liu, X.; Liu, S. Ketogenic diet protects dopaminergic neurons against 6-OHDA neurotoxicity via up-regulating glutathione in a rat model of Parkinson’s disease. Brain Res. 2009, 1286, 25–31. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef]
- Kapogiannis, D.; Avgerinos, K.I. Brain glucose and ketone utilization in brain aging and neurodegenerative diseases. Int. Rev. Neurobiol. 2020, 154, 79–110. [Google Scholar] [CrossRef]
- Myette-Cote, E.; Soto-Mota, A.; Cunnane, S.C. Ketones: Potential to achieve brain energy rescue and sustain cognitive health during ageing. Br. J. Nutr. 2022, 128, 407–423. [Google Scholar] [CrossRef]
- Croteau, E.; Castellano, C.A.; Fortier, M.; Bocti, C.; Fulop, T.; Paquet, N.; Cunnane, S.C. A cross-sectional comparison of brain glucose and ketone metabolism in cognitively healthy older adults, mild cognitive impairment and early Alzheimer’s disease. Exp. Gerontol. 2018, 107, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Svart, M.; Gormsen, L.C.; Hansen, J.; Zeidler, D.; Gejl, M.; Vang, K.; Aanerud, J.; Moeller, N. Regional cerebral effects of ketone body infusion with 3-hydroxybutyrate in humans: Reduced glucose uptake, unchanged oxygen consumption and increased blood flow by positron emission tomography. A randomized, controlled trial. PLoS ONE 2018, 13, e0190556. [Google Scholar] [CrossRef] [PubMed]
- Veech, R.L.; Chance, B.; Kashiwaya, Y.; Lardy, H.A.; Cahill, G.F., Jr. Ketone bodies, potential therapeutic uses. IUBMB Life 2001, 51, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Poff, A.M.; Moss, S.; Soliven, M.; D’Agostino, D.P. Ketone Supplementation: Meeting the Needs of the Brain in an Energy Crisis. Front. Nutr. 2021, 8, 783659. [Google Scholar] [CrossRef] [PubMed]
- Mujica-Parodi, L.R.; Amgalan, A.; Sultan, S.F.; Antal, B.; Sun, X.; Skiena, S.; Lithen, A.; Adra, N.; Ratai, E.M.; Weistuch, C.; et al. Diet modulates brain network stability, a biomarker for brain aging, in young adults. Proc. Natl. Acad. Sci. USA 2020, 117, 6170–6177. [Google Scholar] [CrossRef]
- Sultana, M.A.; Hia, R.A.; Akinsiku, O.; Hegde, V. Peripheral Mitochondrial Dysfunction: A Potential Contributor to the Development of Metabolic Disorders and Alzheimer’s Disease. Biology 2023, 12, 1019. [Google Scholar] [CrossRef]
- Norwitz, N.G.; Jaramillo, J.G.; Clarke, K.; Soto, A. Ketotherapeutics for neurodegenerative diseases. Int. Rev. Neurobiol. 2020, 155, 141–168. [Google Scholar] [CrossRef]
- Yin, J.; Han, P.; Tang, Z.; Liu, Q.; Shi, J. Sirtuin 3 mediates neuroprotection of ketones against ischemic stroke. J. Cereb. Blood Flow Metab. 2015, 35, 1783–1789. [Google Scholar] [CrossRef]
- Miller, V.J.; Villamena, F.A.; Volek, J.S. Nutritional Ketosis and Mitohormesis: Potential Implications for Mitochondrial Function and Human Health. J. Nutr. Metab. 2018, 2018, 5157645. [Google Scholar] [CrossRef]
- Qu, C.; Keijer, J.; Adjobo-Hermans, M.J.W.; van de Wal, M.; Schirris, T.; van Karnebeek, C.; Pan, Y.; Koopman, W.J.H. The ketogenic diet as a therapeutic intervention strategy in mitochondrial disease. Int. J. Biochem. Cell Biol. 2021, 138, 106050. [Google Scholar] [CrossRef]
- Gureev, A.P.; Silachev, D.N.; Sadovnikova, I.S.; Krutskikh, E.P.; Chernyshova, E.V.; Volodina, D.E.; Samoylova, N.A.; Potanina, D.V.; Burakova, I.Y.; Smirnova, Y.D.; et al. The Ketogenic Diet but not Hydroxycitric Acid Keeps Brain Mitochondria Quality Control and mtDNA Integrity Under Focal Stroke. Mol. Neurobiol. 2023, 60, 4288–4303. [Google Scholar] [CrossRef] [PubMed]
- Elamin, M.; Ruskin, D.N.; Sacchetti, P.; Masino, S.A. A unifying mechanism of ketogenic diet action: The multiple roles of nicotinamide adenine dinucleotide. Epilepsy Res. 2020, 167, 106469. [Google Scholar] [CrossRef] [PubMed]
- Lombard, D.B.; Tishkoff, D.X.; Bao, J. Mitochondrial sirtuins in the regulation of mitochondrial activity and metabolic adaptation. Handb. Exp. Pharmacol. 2011, 206, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Hasan-Olive, M.M.; Lauritzen, K.H.; Ali, M.; Rasmussen, L.J.; Storm-Mathisen, J.; Bergersen, L.H. A Ketogenic Diet Improves Mitochondrial Biogenesis and Bioenergetics via the PGC1alpha-SIRT3-UCP2 Axis. Neurochem. Res. 2019, 44, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Canto, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef]
- Wang, S.J.; Zhao, X.H.; Chen, W.; Bo, N.; Wang, X.J.; Chi, Z.F.; Wu, W. Sirtuin 1 activation enhances the PGC-1alpha/mitochondrial antioxidant system pathway in status epilepticus. Mol. Med. Rep. 2015, 11, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Gomora-Garcia, J.C.; Montiel, T.; Huttenrauch, M.; Salcido-Gomez, A.; Garcia-Velazquez, L.; Ramiro-Cortes, Y.; Gomora, J.C.; Castro-Obregon, S.; Massieu, L. Effect of the Ketone Body, D-beta-Hydroxybutyrate, on Sirtuin2-Mediated Regulation of Mitochondrial Quality Control and the Autophagy-Lysosomal Pathway. Cells 2023, 12, 486. [Google Scholar] [CrossRef]
- McCarty, M.F.; DiNicolantonio, J.J.; O’Keefe, J.H. Ketosis may promote brain macroautophagy by activating Sirt1 and hypoxia-inducible factor-1. Med. Hypotheses 2015, 85, 631–639. [Google Scholar] [CrossRef]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Maalouf, M.; Sullivan, P.G.; Davis, L.; Kim, D.Y.; Rho, J.M. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience 2007, 145, 256–264. [Google Scholar] [CrossRef]
- Frey, S.; Geffroy, G.; Desquiret-Dumas, V.; Gueguen, N.; Bris, C.; Belal, S.; Amati-Bonneau, P.; Chevrollier, A.; Barth, M.; Henrion, D.; et al. The addition of ketone bodies alleviates mitochondrial dysfunction by restoring complex I assembly in a MELAS cellular model. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.D.; Kanabus, M.; Anderson, G.; Hargreaves, I.P.; Rutherford, T.; O’Donnell, M.; Cross, J.H.; Rahman, S.; Eaton, S.; Heales, S.J. The ketogenic diet component decanoic acid increases mitochondrial citrate synthase and complex I activity in neuronal cells. J. Neurochem. 2014, 129, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Dabke, P.; Das, A.M. Mechanism of Action of Ketogenic Diet Treatment: Impact of Decanoic Acid and Beta-Hydroxybutyrate on Sirtuins and Energy Metabolism in Hippocampal Murine Neurons. Nutrients 2020, 12, 2379. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Leng, S.X.; Zhang, H. Ketogenic Diet: An Effective Treatment Approach for Neurodegenerative Diseases. Curr. Neuropharmacol. 2022, 20, 2303–2319. [Google Scholar] [CrossRef]
- Sullivan, P.G.; Rippy, N.A.; Dorenbos, K.; Concepcion, R.C.; Agarwal, A.K.; Rho, J.M. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann. Neurol. 2004, 55, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Balietti, M.; Giorgetti, B.; Di Stefano, G.; Casoli, T.; Platano, D.; Solazzi, M.; Bertoni-Freddari, C.; Aicardi, G.; Lattanzio, F.; Fattoretti, P. A ketogenic diet increases succinic dehydrogenase (SDH) activity and recovers age-related decrease in numeric density of SDH-positive mitochondria in cerebellar Purkinje cells of late-adult rats. Micron 2010, 41, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Haces, M.L.; Hernandez-Fonseca, K.; Medina-Campos, O.N.; Montiel, T.; Pedraza-Chaverri, J.; Massieu, L. Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp. Neurol. 2008, 211, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Davis, L.M.; Sullivan, P.G.; Maalouf, M.; Simeone, T.A.; van Brederode, J.; Rho, J.M. Ketone bodies are protective against oxidative stress in neocortical neurons. J. Neurochem. 2007, 101, 1316–1326. [Google Scholar] [CrossRef]
- Kong, G.; Huang, Z.; Ji, W.; Wang, X.; Liu, J.; Wu, X.; Huang, Z.; Li, R.; Zhu, Q. The Ketone Metabolite beta-Hydroxybutyrate Attenuates Oxidative Stress in Spinal Cord Injury by Suppression of Class I Histone Deacetylases. J. Neurotrauma 2017, 34, 2645–2655. [Google Scholar] [CrossRef]
- Kashiwaya, Y.; Takeshima, T.; Mori, N.; Nakashima, K.; Clarke, K.; Veech, R.L. D-beta-hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 5440–5444. [Google Scholar] [CrossRef]
- Scheibye-Knudsen, M.; Mitchell, S.J.; Fang, E.F.; Iyama, T.; Ward, T.; Wang, J.; Dunn, C.A.; Singh, N.; Veith, S.; Hasan-Olive, M.M.; et al. A high-fat diet and NAD(+) activate Sirt1 to rescue premature aging in cockayne syndrome. Cell Metab. 2014, 20, 840–855. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chan, S.L.; de Souza-Pinto, N.C.; Slevin, J.R.; Wersto, R.P.; Zhan, M.; Mustafa, K.; de Cabo, R.; Mattson, M.P. Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromolecular Med. 2006, 8, 389–414. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, P.; Karthikeyan, A.; Lu, J.; Ling, E.A.; Dheen, S.T. Sirtuin 3 regulates Foxo3a-mediated antioxidant pathway in microglia. Neuroscience 2015, 311, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox. Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Tseng, A.H.; Shieh, S.S.; Wang, D.L. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic. Biol. Med. 2013, 63, 222–234. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, X.; Zhang, Q.; Li, Z.; Ma, S.; Bao, J.; Li, Z.; Bai, X.; Zheng, L.; Zhang, Z.; et al. PGC-1alpha/ERRalpha-Sirt3 Pathway Regulates DAergic Neuronal Death by Directly Deacetylating SOD2 and ATP Synthase beta. Antioxid. Redox Signal. 2016, 24, 312–328. [Google Scholar] [CrossRef]
- Veech, R.L.; Todd King, M.; Pawlosky, R.; Kashiwaya, Y.; Bradshaw, P.C.; Curtis, W. The “great” controlling nucleotide coenzymes. IUBMB Life 2019, 71, 565–579. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Milder, J.B.; Liang, L.P.; Patel, M. The ketogenic diet increases mitochondrial glutathione levels. J. Neurochem. 2008, 106, 1044–1051. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Ma, A.; Kang, Y. Rational Application of beta-Hydroxybutyrate Attenuates Ischemic Stroke by Suppressing Oxidative Stress and Mitochondrial-Dependent Apoptosis via Activation of the Erk/CREB/eNOS Pathway. ACS Chem. Neurosci. 2021, 12, 1219–1227. [Google Scholar] [CrossRef]
- Kolb, H.; Kempf, K.; Rohling, M.; Lenzen-Schulte, M.; Schloot, N.C.; Martin, S. Ketone bodies: From enemy to friend and guardian angel. BMC Med. 2021, 19, 313. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Kazantsev, A.G. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018, 285, 3576–3590. [Google Scholar] [CrossRef] [PubMed]
- Izuta, Y.; Imada, T.; Hisamura, R.; Oonishi, E.; Nakamura, S.; Inagaki, E.; Ito, M.; Soga, T.; Tsubota, K. Ketone body 3-hydroxybutyrate mimics calorie restriction via the Nrf2 activator, fumarate, in the retina. Aging Cell 2018, 17, e12699. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Bai, D.; Tang, M.; Zhang, M.; Zhao, L.; Li, J.; Yang, R.; Jiang, G. Ketogenic diet alleviates brain iron deposition and cognitive dysfunction via Nrf2-mediated ferroptosis pathway in APP/PS1 mouse. Brain Res. 2023, 1812, 148404. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yang, Y.; Zhang, B.; Han, G.; Yu, J.; Yu, Q.; Zhang, L. Ketone Body beta-Hydroxybutyric Acid Ameliorates Dopaminergic Neuron Injury Through Modulating Zinc Finger Protein 36/Acyl-CoA Synthetase Long-Chain Family Member Four Signaling Axis-Mediated Ferroptosis. Neuroscience 2023, 509, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Nielsen, M.; Li, S.; Shi, J. Ketones improves Apolipoprotein E4-related memory deficiency via sirtuin 3. Aging 2019, 11, 4579–4586. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, A.; Li, Y.J.; Yang, Y.; Kishimoto, Y.; Zhang, S.; Wang, Y.; Wan, R.; Raefsky, S.M.; Lu, D.; et al. SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat. Commun. 2019, 10, 1886. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Wang, J.; Ghena, N.; Zhao, Q.; Perone, I.; King, T.M.; Veech, R.L.; Gorospe, M.; Wan, R.; Mattson, M.P. SIRT3 Haploinsufficiency Aggravates Loss of GABAergic Interneurons and Neuronal Network Hyperexcitability in an Alzheimer’s Disease Model. J. Neurosci. 2020, 40, 694–709. [Google Scholar] [CrossRef]
- Berson, A.; Nativio, R.; Berger, S.L.; Bonini, N.M. Epigenetic Regulation in Neurodegenerative Diseases. Trends Neurosci. 2018, 41, 587–598. [Google Scholar] [CrossRef]
- Ganai, S.A.; Ramadoss, M.; Mahadevan, V. Histone Deacetylase (HDAC) Inhibitors—Emerging roles in neuronal memory, learning, synaptic plasticity and neural regeneration. Curr. Neuropharmacol. 2016, 14, 55–71. [Google Scholar] [CrossRef]
- Yang, H.; Shan, W.; Zhu, F.; Wu, J.; Wang, Q. Ketone Bodies in Neurological Diseases: Focus on Neuroprotection and Underlying Mechanisms. Front. Neurol. 2019, 10, 585. [Google Scholar] [CrossRef]
- Moreno, C.L.; Mobbs, C.V. Epigenetic mechanisms underlying lifespan and age-related effects of dietary restriction and the ketogenic diet. Mol. Cell. Endocrinol. 2017, 455, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, P.; Nettore, I.C.; Franchini, F.; Palatucci, G.; Muscogiuri, G.; Colao, A.; Macchia, P.E. Epigenome Modulation Induced by Ketogenic Diets. Nutrients 2022, 14, 3245. [Google Scholar] [CrossRef] [PubMed]
- Bandera-Merchan, B.; Boughanem, H.; Crujeiras, A.B.; Macias-Gonzalez, M.; Tinahones, F.J. Ketotherapy as an epigenetic modifier in cancer. Rev. Endocr. Metab. Disord. 2020, 21, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhan, Z.; Zhang, J.; Zhou, F.; An, L. Beta-Hydroxybutyrate Ameliorates Abeta-Induced Downregulation of TrkA Expression by Inhibiting HDAC1/3 in SH-SY5Y Cells. Am. J. Alzheimers Dis. Other Demen. 2020, 35, 1533317519883496. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Huang, X.; Cheng, X.; Lin, X.; Zhao, T.; Wu, L.; Yu, X.; Wu, K.; Fan, M.; Zhu, L. Ketogenic diet improves the spatial memory impairment caused by exposure to hypobaric hypoxia through increased acetylation of histones in rats. PLoS ONE 2017, 12, e0174477. [Google Scholar] [CrossRef] [PubMed]
- Simeone, T.A.; Simeone, K.A.; Stafstrom, C.E.; Rho, J.M. Do ketone bodies mediate the anti-seizure effects of the ketogenic diet? Neuropharmacology 2018, 133, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Yang, D.; Ni, H.Y.; Xu, X.M.; Wu, F.; Lin, L.; Chen, J.; Sun, Y.Y.; Huang, Z.Q.; Li, S.Y.; et al. Ketone bodies promote stroke recovery via GAT-1-dependent cortical network remodeling. Cell Rep. 2023, 42, 112294. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. Elife 2016, 5, e15092. [Google Scholar] [CrossRef]
- Murugan, M.; Boison, D. Ketogenic diet, neuroprotection, and antiepileptogenesis. Epilepsy Res. 2020, 167, 106444. [Google Scholar] [CrossRef]
- Chen, F.; He, X.; Luan, G.; Li, T. Role of DNA Methylation and Adenosine in Ketogenic Diet for Pharmacoresistant Epilepsy: Focus on Epileptogenesis and Associated Comorbidities. Front. Neurol. 2019, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Du, H.; Shang, S.; Zhang, Y.; Lu, X. Beta-Hydroxybutyrate Enhances BDNF Expression by Increasing H3K4me3 and Decreasing H2AK119ub in Hippocampal Neurons. Front. Neurosci. 2020, 14, 591177. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Du, H.; Zhu, X.; Wang, L.; Shang, S.; Wu, X.; Lu, H.; Lu, X. Beta-hydroxybutyrate Promotes the Expression of BDNF in Hippocampal Neurons under Adequate Glucose Supply. Neuroscience 2018, 386, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Cheng, X.; He, Y.; Xie, Y.; Xu, F.; Xu, Y.; Huang, W. Function and mechanism of histone beta-hydroxybutyrylation in health and disease. Front. Immunol. 2022, 13, 981285. [Google Scholar] [CrossRef] [PubMed]
- Cornuti, S.; Chen, S.; Lupori, L.; Finamore, F.; Carli, F.; Samad, M.; Fenizia, S.; Caldarelli, M.; Damiani, F.; Raimondi, F.; et al. Brain histone beta-hydroxybutyrylation couples metabolism with gene expression. Cell Mol. Life Sci. 2023, 80, 28. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhang, D.; Chung, D.; Tang, Z.; Huang, H.; Dai, L.; Qi, S.; Li, J.; Colak, G.; Chen, Y.; et al. Metabolic Regulation of Gene Expression by Histone Lysine beta-Hydroxybutyrylation. Mol. Cell 2016, 62, 194–206. [Google Scholar] [CrossRef]
- Chen, L.; Miao, Z.; Xu, X. beta-hydroxybutyrate alleviates depressive behaviors in mice possibly by increasing the histone3-lysine9-beta-hydroxybutyrylation. Biochem. Biophys. Res. Commun. 2017, 490, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wan, X.; Wu, Z.; Zhou, Y.; Chen, R.; Xu, W.; Zhang, J.; Yang, Z.; Bai, L.; Zhang, J.; et al. beta-hydroxybutyrate reduces reinstatement of cocaine conditioned place preference through hippocampal CaMKII-alpha beta-hydroxybutyrylation. Cell Rep. 2022, 41, 111724. [Google Scholar] [CrossRef]
- Jayashankar, S.S.; Tajul Arifin, K.; Nasaruddin, M.L. beta-Hydroxybutyrate Regulates Activated Microglia to Alleviate Neurodegenerative Processes in Neurological Diseases: A Scoping Review. Nutrients 2023, 15, 524. [Google Scholar] [CrossRef]
- Rahman, M.; Muhammad, S.; Khan, M.A.; Chen, H.; Ridder, D.A.; Muller-Fielitz, H.; Pokorna, B.; Vollbrandt, T.; Stolting, I.; Nadrowitz, R.; et al. The beta-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat. Commun. 2014, 5, 3944. [Google Scholar] [CrossRef]
- Taing, K.; Chen, L.; Weng, H.R. Emerging roles of GPR109A in regulation of neuroinflammation in neurological diseases and pain. Neural Regen. Res. 2023, 18, 763–768. [Google Scholar] [CrossRef]
- Jiang, Z.; Yin, X.; Wang, M.; Chen, T.; Wang, Y.; Gao, Z.; Wang, Z. Effects of Ketogenic Diet on Neuroinflammation in Neurodegenerative Diseases. Aging Dis. 2022, 13, 1146–1165. [Google Scholar] [CrossRef]
- Koh, S.; Dupuis, N.; Auvin, S. Ketogenic diet and Neuroinflammation. Epilepsy Res. 2020, 167, 106454. [Google Scholar] [CrossRef]
- Offermanns, S. Free fatty acid (FFA) and hydroxy carboxylic acid (HCA) receptors. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 407–434. [Google Scholar] [CrossRef]
- Wakade, C.; Chong, R.; Bradley, E.; Thomas, B.; Morgan, J. Upregulation of GPR109A in Parkinson’s disease. PLoS ONE 2014, 9, e109818. [Google Scholar] [CrossRef]
- Lukasova, M.; Malaval, C.; Gille, A.; Kero, J.; Offermanns, S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J. Clin. Investig. 2011, 121, 1163–1173. [Google Scholar] [CrossRef]
- Fu, S.P.; Li, S.N.; Wang, J.F.; Li, Y.; Xie, S.S.; Xue, W.J.; Liu, H.M.; Huang, B.X.; Lv, Q.K.; Lei, L.C.; et al. BHBA suppresses LPS-induced inflammation in BV-2 cells by inhibiting NF-kappaB activation. Mediat. Inflamm. 2014, 2014, 983401. [Google Scholar] [CrossRef]
- Jiang, J.; Pan, H.; Shen, F.; Tan, Y.; Chen, S. Ketogenic diet alleviates cognitive dysfunction and neuroinflammation in APP/PS1 mice via the Nrf2/HO-1 and NF-kappaB signaling pathways. Neural Regen. Res. 2023, 18, 2767–2772. [Google Scholar] [CrossRef]
- Cullingford, T.E. The ketogenic diet; fatty acids, fatty acid-activated receptors and neurological disorders. Prostaglandins Leukot Essent Fat. Acids 2004, 70, 253–264. [Google Scholar] [CrossRef]
- Jeong, E.A.; Jeon, B.T.; Shin, H.J.; Kim, N.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Ketogenic diet-induced peroxisome proliferator-activated receptor-gamma activation decreases neuroinflammation in the mouse hippocampus after kainic acid-induced seizures. Exp. Neurol. 2011, 232, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gong, Y.; Luan, Y.; Li, Y.; Liu, J.; Yue, Z.; Yuan, B.; Sun, J.; Xie, C.; Li, L.; et al. BHBA treatment improves cognitive function by targeting pleiotropic mechanisms in transgenic mouse model of Alzheimer’s disease. FASEB J. 2020, 34, 1412–1429. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, C.; Wu, J.; Liu, P.; Deng, X.; Zhang, Y.; Peng, B.; Zhu, Y. Ketogenic diet ameliorates cognitive impairment and neuroinflammation in a mouse model of Alzheimer’s disease. CNS Neurosci. Ther. 2022, 28, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Trotta, M.C.; Maisto, R.; Guida, F.; Boccella, S.; Luongo, L.; Balta, C.; D’Amico, G.; Herman, H.; Hermenean, A.; Bucolo, C.; et al. The activation of retinal HCA2 receptors by systemic beta-hydroxybutyrate inhibits diabetic retinal damage through reduction of endoplasmic reticulum stress and the NLRP3 inflammasome. PLoS ONE 2019, 14, e0211005. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Shippy, D.C.; Wilhelm, C.; Viharkumar, P.A.; Raife, T.J.; Ulland, T.K. beta-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer’s disease pathology. J. Neuroinflamm. 2020, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.R.; Kim, D.H.; Park, M.H.; Lee, B.; Kim, M.J.; Lee, E.K.; Chung, K.W.; Kim, S.M.; Im, D.S.; Chung, H.Y. beta-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget 2016, 7, 66444–66454. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wang, X.; Zhao, Y.; Yang, Q.; Ding, H.; Dong, Q.; Chen, X.; Cui, M. Ketogenic Diet Improves Brain Ischemic Tolerance and Inhibits NLRP3 Inflammasome Activation by Preventing Drp1-Mediated Mitochondrial Fission and Endoplasmic Reticulum Stress. Front. Mol. Neurosci. 2018, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Yamanashi, T.; Iwata, M.; Shibushita, M.; Tsunetomi, K.; Nagata, M.; Kajitani, N.; Miura, A.; Matsuo, R.; Nishiguchi, T.; Kato, T.A.; et al. Beta-hydroxybutyrate, an endogenous NLRP3 inflammasome inhibitor, attenuates anxiety-related behavior in a rodent post-traumatic stress disorder model. Sci. Rep. 2020, 10, 21629. [Google Scholar] [CrossRef]
- Polito, R.; La Torre, M.E.; Moscatelli, F.; Cibelli, G.; Valenzano, A.; Panaro, M.A.; Monda, M.; Messina, A.; Monda, V.; Pisanelli, D.; et al. The Ketogenic Diet and Neuroinflammation: The Action of Beta-Hydroxybutyrate in a Microglial Cell Line. Int. J. Mol. Sci. 2023, 24, 3102. [Google Scholar] [CrossRef]
- Jin, L.W.; Di Lucente, J.; Ruiz Mendiola, U.; Suthprasertporn, N.; Tomilov, A.; Cortopassi, G.; Kim, K.; Ramsey, J.J.; Maezawa, I. The ketone body beta-hydroxybutyrate shifts microglial metabolism and suppresses amyloid-beta oligomer-induced inflammation in human microglia. FASEB J. 2023, 37, e23261. [Google Scholar] [CrossRef]
- Huang, C.; Wang, P.; Xu, X.; Zhang, Y.; Gong, Y.; Hu, W.; Gao, M.; Wu, Y.; Ling, Y.; Zhao, X.; et al. The ketone body metabolite beta-hydroxybutyrate induces an antidepression-associated ramification of microglia via HDACs inhibition-triggered Akt-small RhoGTPase activation. Glia 2018, 66, 256–278. [Google Scholar] [CrossRef] [PubMed]
- Frake, R.A.; Ricketts, T.; Menzies, F.M.; Rubinsztein, D.C. Autophagy and neurodegeneration. J. Clin. Investig. 2015, 125, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Torres-Esquivel, C.; Montiel, T.; Flores-Mendez, M.; Massieu, L. Effect of beta-Hydroxybutyrate on Autophagy Dynamics During Severe Hypoglycemia and the Hypoglycemic Coma. Front. Cell Neurosci. 2020, 14, 547215. [Google Scholar] [CrossRef] [PubMed]
- Camberos-Luna, L.; Geronimo-Olvera, C.; Montiel, T.; Rincon-Heredia, R.; Massieu, L. The Ketone Body, beta-Hydroxybutyrate Stimulates the Autophagic Flux and Prevents Neuronal Death Induced by Glucose Deprivation in Cortical Cultured Neurons. Neurochem. Res. 2016, 41, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.F.; Nguyen, N.T.K.; Hsia, S.M.; Chen, H.Y.; Lin, S.H.; Lin, C.I. Protective Potential of beta-Hydroxybutyrate against Glucose-Deprivation-Induced Neurotoxicity Involving the Modulation of Autophagic Flux and the Monomeric Abeta Level in Neuro-2a Cells. Biomedicines 2023, 11, 698. [Google Scholar] [CrossRef]
- Montiel, T.; Gomora-Garcia, J.C.; Geronimo-Olvera, C.; Heras-Romero, Y.; Bernal-Vicente, B.N.; Perez-Martinez, X.; Tovar, Y.R.L.B.; Massieu, L. Modulation of the autophagy-lysosomal pathway and endoplasmic reticulum stress by ketone bodies in experimental models of stroke. J. Neurochem. 2023, 166, 87–106. [Google Scholar] [CrossRef]
- Liskiewicz, D.; Liskiewicz, A.; Nowacka-Chmielewska, M.M.; Grabowski, M.; Pondel, N.; Grabowska, K.; Student, S.; Barski, J.J.; Malecki, A. Differential Response of Hippocampal and Cerebrocortical Autophagy and Ketone Body Metabolism to the Ketogenic Diet. Front. Cell Neurosci. 2021, 15, 733607. [Google Scholar] [CrossRef]
- Hu, L.T.; Xie, X.Y.; Zhou, G.F.; Wen, Q.X.; Song, L.; Luo, B.; Deng, X.J.; Pan, Q.L.; Chen, G.J. HMGCS2-Induced Autophagic Degradation of Tau Involves Ketone Body and ANKRD24. J. Alzheimers Dis. 2023, 91, 407–426. [Google Scholar] [CrossRef]
- Hu, L.T.; Zhu, B.L.; Lai, Y.J.; Long, Y.; Zha, J.S.; Hu, X.T.; Zhang, J.H.; Chen, G.J. HMGCS2 promotes autophagic degradation of the amyloid-beta precursor protein through ketone body-mediated mechanisms. Biochem. Biophys. Res. Commun. 2017, 486, 492–498. [Google Scholar] [CrossRef]
- Wang, B.H.; Hou, Q.; Lu, Y.Q.; Jia, M.M.; Qiu, T.; Wang, X.H.; Zhang, Z.X.; Jiang, Y. Ketogenic diet attenuates neuronal injury via autophagy and mitochondrial pathways in pentylenetetrazol-kindled seizures. Brain Res. 2018, 1678, 106–115. [Google Scholar] [CrossRef]
- Egan, D.; Kim, J.; Shaw, R.J.; Guan, K.L. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 2011, 7, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Jana, M.; Modi, K.; Gonzalez, F.J.; Sims, K.B.; Berry-Kravis, E.; Pahan, K. Activation of peroxisome proliferator-activated receptor alpha induces lysosomal biogenesis in brain cells: Implications for lysosomal storage disorders. J. Biol. Chem. 2015, 290, 10309–10324. [Google Scholar] [CrossRef] [PubMed]
- Montiel, T.; Montes-Ortega, L.A.; Flores-Yanez, S.; Massieu, L. Treatment with the Ketone Body D-beta-hydroxybutyrate Attenuates Autophagy Activated by NMDA and Reduces Excitotoxic Neuronal Damage in the Rat Striatum In Vivo. Curr. Pharm. Des. 2020, 26, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodriguez, D.; Gimenez-Cassina, A. Ketone Bodies in the Brain Beyond Fuel Metabolism: From Excitability to Gene Expression and Cell Signaling. Front. Mol. Neurosci. 2021, 14, 732120. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.M.; Risa, O.; Sonnewald, U.; Schousboe, A.; Waagepetersen, H.S. Availability of neurotransmitter glutamate is diminished when beta-hydroxybutyrate replaces glucose in cultured neurons. J. Neurochem. 2009, 110, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Zilberter, M.; Ivanov, A.; Ziyatdinova, S.; Mukhtarov, M.; Malkov, A.; Alpar, A.; Tortoriello, G.; Botting, C.H.; Fulop, L.; Osypov, A.A.; et al. Dietary energy substrates reverse early neuronal hyperactivity in a mouse model of Alzheimer’s disease. J. Neurochem. 2013, 125, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Berg, J.; Yellen, G. Ketogenic diet metabolites reduce firing in central neurons by opening K(ATP) channels. J. Neurosci. 2007, 27, 3618–3625. [Google Scholar] [CrossRef]
- Lund, T.M.; Ploug, K.B.; Iversen, A.; Jensen, A.A.; Jansen-Olesen, I. The metabolic impact of beta-hydroxybutyrate on neurotransmission: Reduced glycolysis mediates changes in calcium responses and KATP channel receptor sensitivity. J. Neurochem. 2015, 132, 520–531. [Google Scholar] [CrossRef]
- Juge, N.; Gray, J.A.; Omote, H.; Miyaji, T.; Inoue, T.; Hara, C.; Uneyama, H.; Edwards, R.H.; Nicoll, R.A.; Moriyama, Y. Metabolic control of vesicular glutamate transport and release. Neuron 2010, 68, 99–112. [Google Scholar] [CrossRef]
- Pflanz, N.C.; Daszkowski, A.W.; James, K.A.; Mihic, S.J. Ketone body modulation of ligand-gated ion channels. Neuropharmacology 2019, 148, 21–30. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef]
- Won, Y.J.; Lu, V.B.; Puhl, H.L., 3rd; Ikeda, S.R. beta-Hydroxybutyrate modulates N-type calcium channels in rat sympathetic neurons by acting as an agonist for the G-protein-coupled receptor FFA3. J. Neurosci. 2013, 33, 19314–19325. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, L.; Schröder, J.; Schuster, I.S.; Nakai, M.; Sun, G.; Sun, Y.B.Y.; Marino, E.; Degli-Esposti, M.A.; Marques, F.Z.; et al. Dietary fiber and microbiota metabolite receptors enhance cognition and alleviate disease in the 5xFAD mouse model of Alzheimer’s disease. J. Neurosci. 2023, 43, 6460–6475. [Google Scholar] [CrossRef]
- Rawat, K.; Singh, N.; Kumari, P.; Saha, L. A review on preventive role of ketogenic diet (KD) in CNS disorders from the gut microbiota perspective. Rev. Neurosci. 2021, 32, 143–157. [Google Scholar] [CrossRef]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. The Role of Gut Microbiota in an Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 915. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Fang, Z.; Chen, M.; Qian, J.; Wang, C.; Zhang, J. The Bridge Between Ischemic Stroke and Gut Microbes: Short-Chain Fatty Acids. Cell Mol. Neurobiol. 2023, 43, 543–559. [Google Scholar] [CrossRef]
- Attaye, I.; van Oppenraaij, S.; Warmbrunn, M.V.; Nieuwdorp, M. The Role of the Gut Microbiota on the Beneficial Effects of Ketogenic Diets. Nutrients 2021, 14, 191. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.t.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Mulero, A.; Tinahones, A.; Bandera, B.; Moreno-Indias, I.; Macias-Gonzalez, M.; Tinahones, F.J. Keto microbiota: A powerful contributor to host disease recovery. Rev. Endocr. Metab. Disord. 2019, 20, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Krakovski, M.A.; Arora, N.; Jain, S.; Glover, J.; Dombrowski, K.; Hernandez, B.; Yadav, H.; Sarma, A.K. Diet-microbiome-gut-brain nexus in acute and chronic brain injury. Front. Neurosci. 2022, 16, 1002266. [Google Scholar] [CrossRef] [PubMed]
- Kincaid, H.J.; Nagpal, R.; Yadav, H. Diet-Microbiota-Brain Axis in Alzheimer’s Disease. Ann. Nutr. Metab. 2021, 77 (Suppl. S2), 21–27. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Mancin, L.; Bianco, A.; Thomas, E.; Mota, J.F.; Piccini, F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes 2019, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.M.; Letchumanan, V.; Tan, L.T.; Hong, K.W.; Wong, S.H.; Ab Mutalib, N.S.; Lee, L.H.; Law, J.W. Ketogenic Diet: A Dietary Intervention via Gut Microbiome Modulation for the Treatment of Neurological and Nutritional Disorders (a Narrative Review). Nutrients 2022, 14, 3566. [Google Scholar] [CrossRef] [PubMed]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef]
- Ma, D.; Wang, A.C.; Parikh, I.; Green, S.J.; Hoffman, J.D.; Chlipala, G.; Murphy, M.P.; Sokola, B.S.; Bauer, B.; Hartz, A.M.S.; et al. Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci. Rep. 2018, 8, 6670. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Q.; Liu, J. Microbiota-gut-brain axis: A novel potential target of ketogenic diet for epilepsy. Curr. Opin. Pharmacol. 2021, 61, 36–41. [Google Scholar] [CrossRef]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef] [PubMed]
- Newell, C.; Bomhof, M.R.; Reimer, R.A.; Hittel, D.S.; Rho, J.M.; Shearer, J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol. Autism. 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Olivito, I.; Avolio, E.; Minervini, D.; Soda, T.; Rocca, C.; Angelone, T.; Iaquinta, F.S.; Bellizzi, D.; De Rango, F.; Bruno, R.; et al. Ketogenic diet ameliorates autism spectrum disorders-like behaviors via reduced inflammatory factors and microbiota remodeling in BTBR T(+) Itpr3(tf)/J mice. Exp. Neurol. 2023, 366, 114432. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, X.; Zhang, H.; Yin, J.; Zhao, P.; Yin, Q.; Wang, Z. Ketogenic diet protects MPTP-induced mouse model of Parkinson’s disease via altering gut microbiota and metabolites. MedComm 2023, 4, e268. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Huang, X.; Tong, H.; Niu, H.; Lu, L. Neuroprotective effect of a medium-chain triglyceride ketogenic diet on MPTP-induced Parkinson’s disease mice: A combination of transcriptomics and metabolomics in the substantia nigra and fecal microbiome. Cell Death Discov. 2023, 9, 251. [Google Scholar] [CrossRef]
- Swidsinski, A.; Dorffel, Y.; Loening-Baucke, V.; Gille, C.; Goktas, O.; Reisshauer, A.; Neuhaus, J.; Weylandt, K.H.; Guschin, A.; Bock, M. Reduced Mass and Diversity of the Colonic Microbiome in Patients with Multiple Sclerosis and Their Improvement with Ketogenic Diet. Front. Microbiol. 2017, 8, 1141. [Google Scholar] [CrossRef]
- Park, S.M.; Zhang, T.; Wu, X.G.; Qiu, J.Y. Ketone production by ketogenic diet and by intermittent fasting has different effects on the gut microbiota and disease progression in an Alzheimer’s disease rat model. J. Clin. Biochem. Nutr. 2020, 67, 188–198. [Google Scholar] [CrossRef]
- Miyamoto, J.; Ohue-Kitano, R.; Mukouyama, H.; Nishida, A.; Watanabe, K.; Igarashi, M.; Irie, J.; Tsujimoto, G.; Satoh-Asahara, N.; Itoh, H.; et al. Ketone body receptor GPR43 regulates lipid metabolism under ketogenic conditions. Proc. Natl. Acad. Sci. USA 2019, 116, 23813–23821. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).