Early Castration in Horses Does Not Impact Osteoarticular Metabolism
Abstract
1. Introduction
2. Results
2.1. Investigation of ESR1 and AR Detection in Horse Bone and Cartilage Tissues
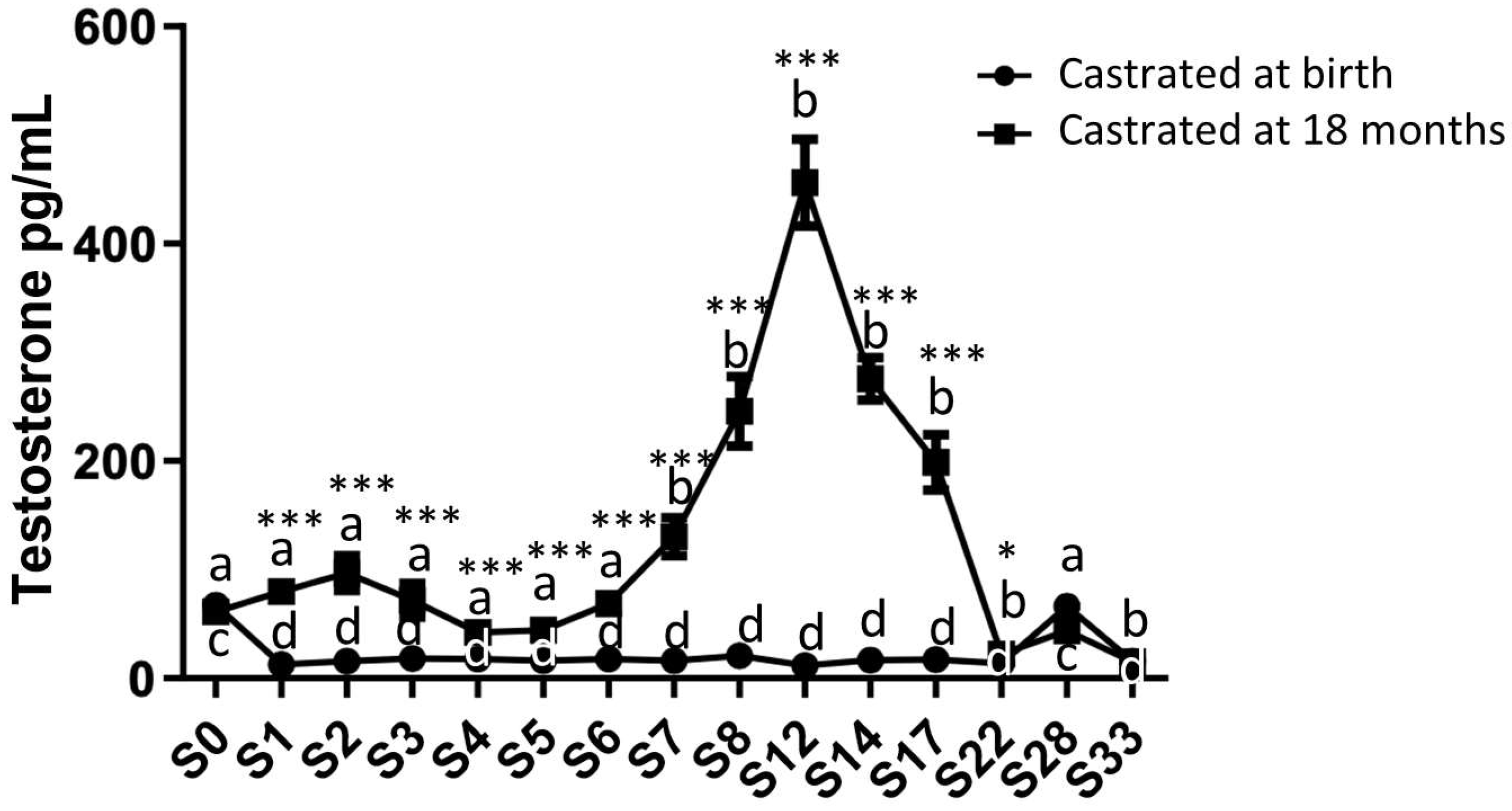
2.2. Analysis of Circulating Levels and Testicular Synthesis of Testosterone in Both Study Groups
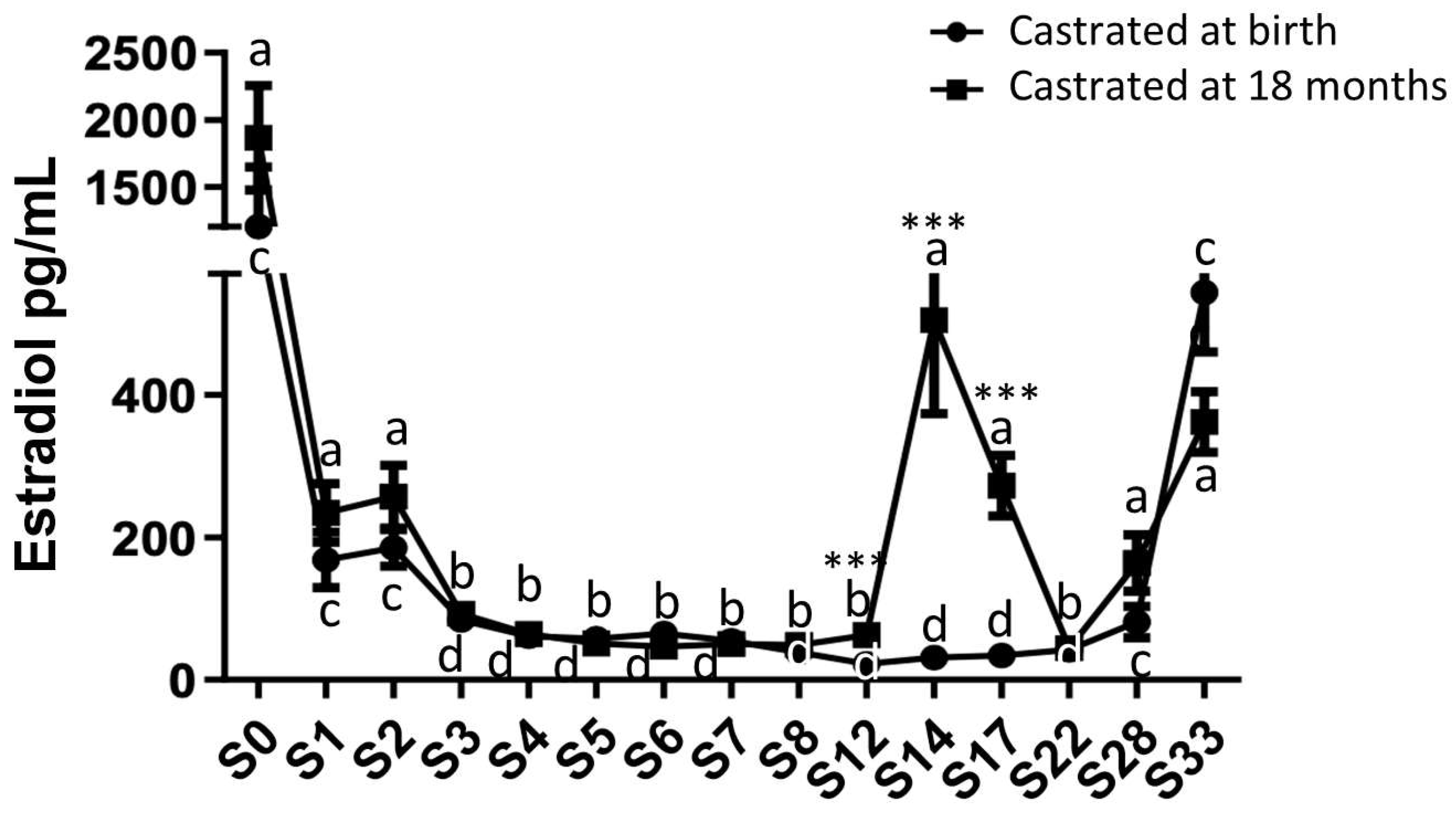
2.3. Analysis of Circulating Levels and Testicular Synthesis of Estradiol in Both Study Groups
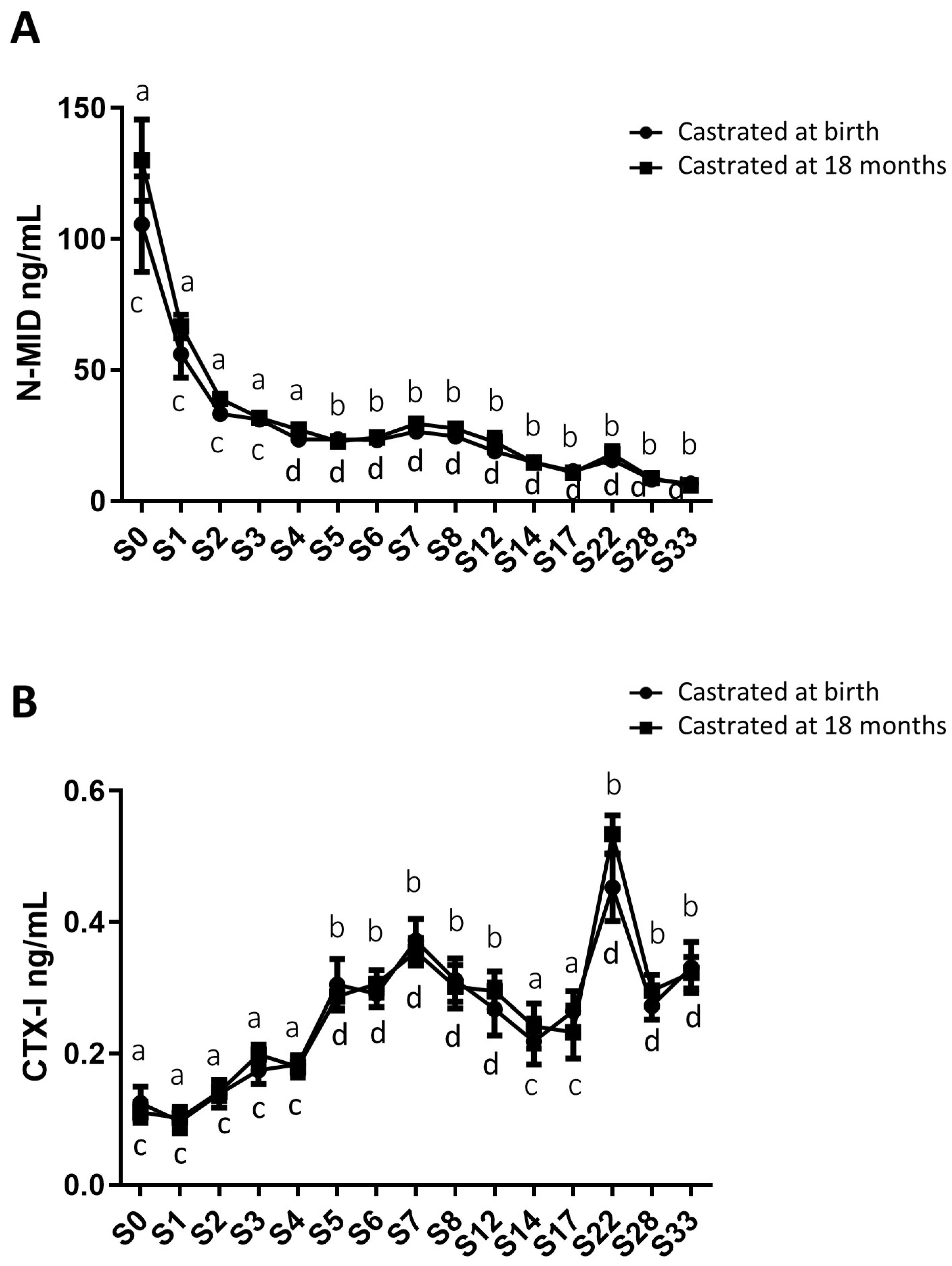
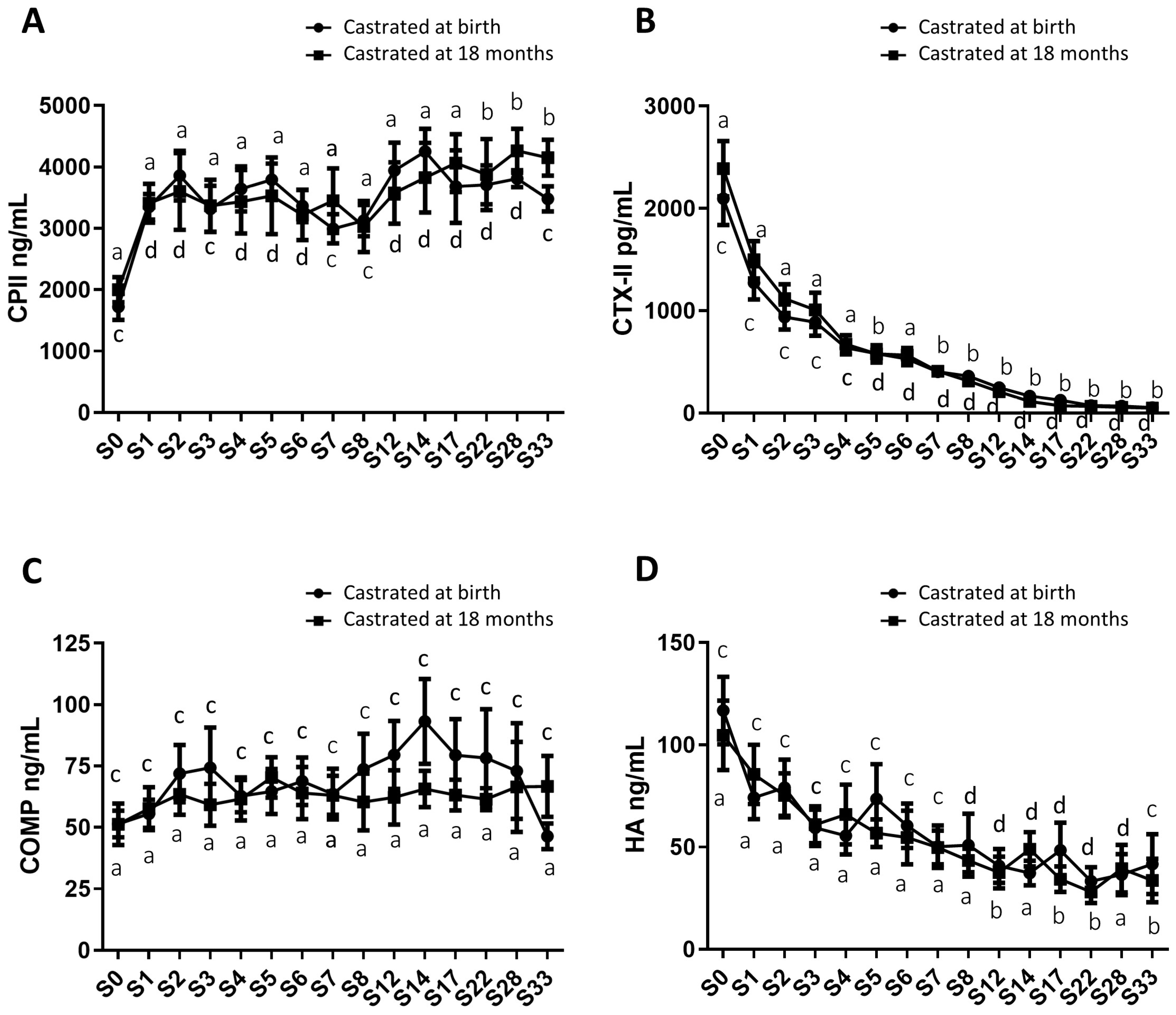
2.4. Analysis of Circulating Levels of Osteoarticular Metabolism Biomarkers in Both Study Groups
3. Discussion
4. Materials and Methods
4.1. Animals and Sample Collection
- GROUP 1: Traditional castration: 11 foals were castrated at 18 months (5 from cohort 1, 3 from cohort 2, and 3 from cohort 3, respectively). Horses in this group were castrated at 18 months and served as the control group for the other experimental group.
- GROUP 2: Early castration: 11 neonates were castrated at birth (5 from cohort 1, 3 from cohort 2, and 3 from cohort 3, respectively). Horses underwent early castration 3 days after birth.
4.2. Biomarker Assays
4.3. Immunohistochemistry
4.3.1. Testicular Tissue
4.3.2. Osteochondral Tissue
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carreau, S.; Genissel, C.; Bilinska, B.; Levallet, J. Sources of Oestrogen in the Testis and Reproductive Tract of the Male. Int. J. Androl. 1999, 22, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.H.; Hales, D.B. Overview of Steroidogenic Enzymes in the Pathway from Cholesterol to Active Steroid Hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef]
- Andersson, A.-M.; Toppari, J.; Haavisto, A.-M.; Petersen, J.H.; Simell, T.; Simell, O.; Skakkeb, N.E. Longitudinal Reproductive Hormone Profiles in Infants: Peak of Inhibin B Levels in Infant Boys Exceeds Levels in Adult Men. J. Clin. Endocrinol. Metab. 1998, 83, 675–681. [Google Scholar] [CrossRef]
- Zirkin, B.R.; Papadopoulos, V. Leydig Cells: Formation, Function, and Regulation. Biol. Reprod. 2018, 99, 101–111. [Google Scholar] [CrossRef]
- Vermeulen, A.; Kaufman, J.M.; Goemaere, S.; Van Pottelberg, I. Estradiol in Elderly Men. Aging Male 2002, 5, 98–102. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the Human Tissue-Specific Expression by Genome-Wide Integration of Transcriptomics and Antibody-Based Proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Cauley, J.A. Estrogen and Bone Health in Men and Women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef]
- Gokhale, J.A.; Frenkel, S.R.; Dicesare, P.E. Estrogen and Osteoarthritis. Am. J. Orthop. 2004, 33, 71–80. [Google Scholar]
- Lorentzon, M.; Swanson, C.; Andersson, N.; Mellström, D.; Ohlsson, C. Free Testosterone Is a Positive, Whereas Free Estradiol Is a Negative, Predictor of Cortical Bone Size in Young Swedish Men: The GOOD Study: Estradiol predicts cortical bone size in men. J. Bone Miner. Res. 2005, 20, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Roman-Blas, J.A.; Castañeda, S.; Largo, R.; Herrero-Beaumont, G. Osteoarthritis Associated with Estrogen Deficiency. Arthritis Res. Ther. 2009, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Bord, S. Estrogen Receptors and Are Differentially Expressed in Developing Human Bone. J. Clin. Endocrinol. Metab. 2001, 86, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.B.; Krum, S.A. Estrogen Receptors Alpha and Beta in Bone. Bone 2016, 87, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Nasatzky, E.; Schwartz, Z.; Soskolne, W.A.; Brooks, B.P.; Dean, D.D.; Boyan, B.D.; Ornoy, A. Evidence for Receptors Specific for 17 Beta-Estradiol and Testosterone in Chondrocyte Cultures. Connect. Tissue Res. 1994, 30, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, O.; Chrysis, D.; Pajulo, O.; Boman, A.; Holst, M.; Rubinstein, J.; Martin Ritzén, E.; Sävendahl, L. Localization of Estrogen Receptors-Alpha and -Beta and Androgen Receptor in the Human Growth Plate at Different Pubertal Stages. J. Endocrinol. 2003, 177, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Vanderschueren, D.; Vandenput, L.; Boonen, S.; Lindberg, M.K.; Bouillon, R.; Ohlsson, C. Androgens and Bone. Endocr. Rev. 2004, 25, 389–425. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Monroe, D.G. Regulation of Bone Metabolism by Sex Steroids. Cold Spring Harb. Perspect. Med. 2018, 8, a031211. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Imai, Y.; Matsumoto, T.; Sato, S.; Takeuchi, K.; Igarashi, K.; Harada, Y.; Azuma, Y.; Krust, A.; Yamamoto, Y.; et al. Estrogen Prevents Bone Loss via Estrogen Receptor α and Induction of Fas Ligand in Osteoclasts. Cell 2007, 130, 811–823. [Google Scholar] [CrossRef]
- Juul, A. The Effects of Oestrogens on Linear Bone Growth. Hum. Reprod. Update 2001, 7, 303–313. [Google Scholar] [CrossRef]
- Tanner, J.M.; Davies, P.S.W. Clinical Longitudinal Standards for Height and Height Velocity for North American Children. J. Pediatr. 1985, 107, 317–329. [Google Scholar] [CrossRef]
- Cadogan, J.; Blumsohn, A.; Barker, M.E.; Eastell, R. A Longitudinal Study of Bone Gain in Pubertal Girls: Anthropometric and Biochemical Correlates. J. Bone Miner. Res. 1998, 13, 1602–1612. [Google Scholar] [CrossRef]
- Lu, W.P.; Cowell, T.; Lloyd-Jones, A.; Briody, N.; Howman-Giles, R. Volumetric Bone Mineral Density in Normal Subjects, Aged 5–27 Years. J. Clin. Endocrinol. Metab. 1996, 81, 1586–1590. [Google Scholar] [PubMed]
- Boot, A.M.; de Ridder, M.A.J.; van der Sluis, I.M.; van Slobbe, I.; Krenning, E.P.; de Muinck Keizer-Schrama, S.M.P.F. Peak Bone Mineral Density, Lean Body Mass and Fractures. Bone 2010, 46, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Behre, H.M.; Kliesch, S.; Leifke, E.; Link, T.M.; Nieschlag, E. Long-Term Effect of Testosterone Therapy on Bone Mineral Density in Hypogonadal Men. J. Clin. Endocrinol. Metab. 1997, 82, 2386–2390. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Cunningham, G.R.; Hayes, F.J.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Montori, V.M. Testosterone Therapy in Men with Androgen Deficiency Syndromes: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2010, 95, 2536–2559. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Korach, K. Oestrogen Receptor Deficiency: Consequences for Growth. Acta Paediatr. 1996, 85, 39–43. [Google Scholar] [CrossRef]
- Smith, E.P.; Boyd, J.; Frank, G.R.; Takahashi, H.; Cohen, R.M.; Specker, B.; Williams, T.C.; Lubahn, D.B.; Korach, K.S. Estrogen Resistance Caused by a Mutation in the Estrogen-Receptor Gene in a Man. N. Engl. J. Med. 1994, 331, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Carani, C.; Qin, K.; Simoni, M.; Faustini-Fustini, M.; Serpente, S.; Boyd, J.; Korach, K.S.; Simpson, E.R. Effect of Testosterone and Estradiol in a Man with Aromatase Deficiency. N. Engl. J. Med. 1997, 337, 91–95. [Google Scholar] [CrossRef]
- Moverare, S.; Venken, K.; Eriksson, A.-L.; Andersson, N.; Skrtic, S.; Wergedal, J.; Mohan, S.; Salmon, P.; Bouillon, R.; Gustafsson, J.-A.; et al. Differential Effects on Bone of Estrogen Receptor and Androgen Receptor Activation in Orchidectomized Adult Male Mice. Proc. Natl. Acad. Sci. USA 2003, 100, 13573–13578. [Google Scholar] [CrossRef]
- Notini, A.J.; McManus, J.F.; Moore, A.; Bouxsein, M.; Jimenez, M.; Chiu, W.M.; Glatt, V.; Kream, B.E.; Handelsman, D.J.; Morris, H.A.; et al. Osteoblast Deletion of Exon 3 of the Androgen Receptor Gene Results in Trabecular Bone Loss in Adult Male Mice. J. Bone Miner. Res. 2007, 22, 347–356. [Google Scholar] [CrossRef]
- Peng, Z.; Li, X.; Mäkelä, S.; Väänänen, H.K.; Poutanen, M. Skeletal Changes in Transgenic Male Mice Expressing Human Cytochrome P450 Aromatase. J. Bone Miner. Res.. 2004, 19, 1320–1328. [Google Scholar] [CrossRef]
- Vico, L.; Vanacker, J.-M. Sex Hormones and Their Receptors in Bone Homeostasis: Insights from Genetically Modified Mouse Models. Osteoporos. Int. 2010, 21, 365–372. [Google Scholar] [CrossRef]
- Khosla, S.; Amin, S.; Orwoll, E. Osteoporosis in Men. Endocr. Rev. 2008, 29, 441–464. [Google Scholar] [CrossRef] [PubMed]
- Nevitt, M.C.; Cummings, S.R.; Lane, N.E.; Hochberg, M.C.; Scott, J.C.; Pressman, A.R.; Genant, H.K.; Cauley, J.A. Association of Estrogen Replacement Therapy with the Risk of Osteoarthritis of the Hip in Elderly White Women. Study of Osteoporotic Fractures Research Group. Arch. Intern. Med. 1996, 156, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Mhaouty-Kodja, S.; Naulé, L.; Capela, D. Sexual Behavior: From Hormonal Regulation to Endocrine Disruption. Neuroendocrinology 2018, 107, 400–416. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, K.M.; McCue, P.M.; Nayden, D.K.; Osawa, Y.; Roser, J.F. Localization of Aromatase in Equine Leydig Cells. Domest. Anim. Endocrinol. 1994, 11, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Raeside, J.I. The Isolation of Estrone Sulfate and Estradiol-17 Beta Sulfate from Stallion Testes. Can. J. Biochem. 1969, 47, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Line, S.W.; Hart, B.L.; Sanders, L. Effect of Prepubertal versus Postpubertal Castration on Sexual and Aggressive Behavior in Male Horses. J. Am. Vet. Med. Assoc. 1985, 186, 249–251. [Google Scholar]
- Moll, H.D.; Pelzer, K.D.; Pleasant, R.S.; Modransky, P.D.; May, K.A. A Survey of Equine Castration Complications. J. Equine Vet. Sci. 1995, 15, 522–526. [Google Scholar] [CrossRef]
- Cognie, J.; Freret, S.; Lansade, L.; Parias, C.; Barriere, P.; Gesbert, A.; Reigner, F.; Deleuze, S. Early Castration in Foals: Consequences on Physical and Behavioural Development. Equine Vet. J. 2023, 55, 214–221. [Google Scholar] [CrossRef]
- Sims, N.A.; Clément-Lacroix, P.; Minet, D.; Fraslon-Vanhulle, C.; Gaillard-Kelly, M.; Resche-Rigon, M.; Baron, R. A Functional Androgen Receptor Is Not Sufficient to Allow Estradiol to Protect Bone after Gonadectomy in Estradiol Receptor–Deficient Mice. J. Clin. Investig. 2003, 111, 1319–1327. [Google Scholar] [CrossRef]
- Rouge, M.; Elkhatib, R.; Delalande, C.; Cognié, J.; Reigner, F.; Barriere, P.; Deleuze, S.; Cousty, M.; Legendre, F.; Galera, P.; et al. Investigation of Equine Testis Contribution to Vitamin D Bioactivation. Domest. Anim. Endocrinol. 2022, 79, 106691. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, R.; Wang, W.; Lu, W.; Xiao, Y.; Wang, D.; Dong, Z. Hormone Inhibition during Mini-Puberty and Testicular Function in Male Rats. Int. J. Endocrinol. Metab. 2015, 13, e25465. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.S.; Hughes, I.A.; Reyes, F.I.; Faiman, C. Pituitary-Gonadal Relations in Infancy: 2. Patterns of Serum Gonadal Steroid Concentrations in Man from Birth to Two Years of Age. J. Clin. Endocrinol. Metab. 1976, 42, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Jost, A.; Vigier, B.; Prépin, J.; Perchellet, J.P. Studies on Sex Differentiation in Mammals. In Recent Progress in Hormone Research, Proceedings of the 1972 Laurentian Hormone Conference; Elsevier: Amsterdam, The Netherlands, 1973; pp. 1–41. ISBN 978-0-12-571129-6. [Google Scholar]
- Wilson, C.A.; Davies, D.C. The Control of Sexual Differentiation of the Reproductive System and Brain. Reproduction 2007, 133, 331–359. [Google Scholar] [CrossRef]
- Gao, W.; Alcauter, S.; Elton, A.; Hernandez-Castillo, C.R.; Smith, J.K.; Ramirez, J.; Lin, W. Functional Network Development During the First Year: Relative Sequence and Socioeconomic Correlations. Cereb. Cortex 2015, 25, 2919–2928. [Google Scholar] [CrossRef] [PubMed]
- Knickmeyer, R.C.; Gouttard, S.; Kang, C.; Evans, D.; Wilber, K.; Smith, J.K.; Hamer, R.M.; Lin, W.; Gerig, G.; Gilmore, J.H. A Structural MRI Study of Human Brain Development from Birth to 2 Years. J. Neurosci. 2008, 28, 12176–12182. [Google Scholar] [CrossRef]
- Lyall, A.E.; Shi, F.; Geng, X.; Woolson, S.; Li, G.; Wang, L.; Hamer, R.M.; Shen, D.; Gilmore, J.H. Dynamic Development of Regional Cortical Thickness and Surface Area in Early Childhood. Cereb. Cortex 2015, 25, 2204–2212. [Google Scholar] [CrossRef]
- Barnes, R.J.; Nathanielsz, P.W.; Rossdale, P.D.; Comline, R.S.; Silver, M. Plasma Progestagens and Oestrogens in Fetus and Mother in Late Pregnancy. J. Reprod. Fertil. Suppl. 1975, 617–623. [Google Scholar]
- Legacki, E.L.; Ball, B.A.; Corbin, C.J.; Loux, S.C.; Scoggin, K.E.; Stanley, S.D.; Conley, A.J. Equine Fetal Adrenal, Gonadal and Placental Steroidogenesis. Reproduction 2017, 154, 445–454. [Google Scholar] [CrossRef]
- Schlinger, B.A.; Lane, N.I.; Grisham, W.; Thompson, L. Androgen Synthesis in a Songbird: A Study of Cyp17 (17alpha-Hydroxylase/C17,20-Lyase) Activity in the Zebra Finch. Gen. Comp. Endocrinol. 1999, 113, 46–58. [Google Scholar] [CrossRef]
- Witchel, S.F.; Pinto, B.; Burghard, A.C.; Oberfield, S.E. Update on Adrenarche. Curr. Opin. Pediatr. 2020, 32, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Turcu, A.F.; Auchus, R.J. Adrenal Steroidogenesis and Congenital Adrenal Hyperplasia. Endocrinol. Metab. Clin. N. Am. 2015, 44, 275–296. [Google Scholar] [CrossRef]
- Labrie, F.; Bélanger, A.; Cusan, L.; Gomez, J.L.; Candas, B. Marked Decline in Serum Concentrations of Adrenal C19 Sex Steroid Precursors and Conjugated Androgen Metabolites during Aging. J. Clin. Endocrinol. Metab. 1997, 82, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Migeon, C.J.; Keller, A.R.; Lawrence, B.; Shepard, T.H. Dehydroepiandrosterone and Androsterone Levels in Human Plasma: Effect of Age and Sex; Day-to-Day and Diurnal Variations. J. Clin. Endocrinol. Metab. 1957, 17, 1051–1062. [Google Scholar] [CrossRef]
- Remer, T.; Manz, F.; Hartmann, M.F.; Schoenau, E.; Wudy, S.A. Prepubertal Healthy Children’s Urinary Androstenediol Predicts Diaphyseal Bone Strength in Late Puberty. J. Clin. Endocrinol. Metab. 2009, 94, 575–578. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holló, I.; Fehér, T.; Szücs, J. Serum Dehydroepiandrosterone, Androsterone and Cortisol Level in Primary Postmenopausal and Other Type Osteoporosis. Acta Medica Acad. Sci. Hung. 1970, 27, 155–160. [Google Scholar]
- Zhao, H.; Tian, Z.; Hao, J.; Chen, B. Extragonadal Aromatization Increases with Time after Ovariectomy in Rats. Reprod. Biol. Endocrinol. 2005, 3, 6. [Google Scholar] [CrossRef]
- Juliand, V.; Martin-Rosset, W. (Eds.) The Growing Horse: Nutrition and Prevention of Growth Disorders; EAAP Scientific Series; Wageningen Academic Publishers: Wageningen, The Netherlands, 2005; Volume 114, ISBN 978-90-76998-62-6. [Google Scholar]
- Nelson, L.R.; Bulun, S.E. Estrogen Production and Action. J. Am. Acad. Dermatol. 2001, 45, S116–S124. [Google Scholar] [CrossRef]
- Khosla, S.; Melton, L.J.; Atkinson, E.J.; O’Fallon, W.M.; Klee, G.G.; Riggs, B.L. Relationship of Serum Sex Steroid Levels and Bone Turnover Markers with Bone Mineral Density in Men and Women: A Key Role for Bioavailable Estrogen. J. Clin. Endocrinol. Metab. 1998, 83, 2266–2274. [Google Scholar] [CrossRef][Green Version]
- Falahati-Nini, A.; Riggs, B.L.; Atkinson, E.J.; O’Fallon, W.M.; Eastell, R.; Khosla, S. Relative Contributions of Testosterone and Estrogen in Regulating Bone Resorption and Formation in Normal Elderly Men. J. Clin. Investig. 2000, 106, 1553–1560. [Google Scholar] [CrossRef]
- Verhas, M.; Schoutens, A.; L’hermite-Baleriaux, M.; Dourov, N.; Verschaeren, A.; Mone, M.; Heilporn, A. The Effect of Orchidectomy on Bone Metabolism in Aging Rats. Calcif. Tissue Int. 1986, 39, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Aizawa, T.; Kokubun, S. The Role of Sex Hormones in the Kinetics of Chondrocytes in the Growth Plate: A study in rabbit. J. Bone Jt. Surg. Br. 2005, 87-B, 1278–1284. [Google Scholar] [CrossRef]
- Sniekers, Y.H.; Weinans, H.; Bierma-Zeinstra, S.M.; van Leeuwen, J.P.T.M.; van Osch, G.J.V.M. Animal Models for Osteoarthritis: The Effect of Ovariectomy and Estrogen Treatment—A Systematic Approach. Osteoarthr. Cartil. 2008, 16, 533–541. [Google Scholar] [CrossRef]
- Peshkova, M.; Lychagin, A.; Lipina, M.; Di Matteo, B.; Anzillotti, G.; Ronzoni, F.; Kosheleva, N.; Shpichka, A.; Royuk, V.; Fomin, V.; et al. Gender-Related Aspects in Osteoarthritis Development and Progression: A Review. Int. J. Mol. Sci. 2022, 23, 2767. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Hannon, R.A. Biomarkers of Bone Health and Osteoporosis Risk. Proc. Nutr. Soc. 2008, 67, 157–162. [Google Scholar] [CrossRef]
- Nagasue, K.; Inaba, M.; Okuno, S.; Kitatani, K.; Imanishi, Y.; Ishimura, E.; Miki, T.; Kim, M.; Nishizawa, Y. Serum N-Terminal Midfragment vs. Intact Osteocalcin Immunoradiometric Assay as Markers for Bone Turnover and Bone Loss in Hemodialysis Patients. Biomed. Pharmacother. Biomed. Pharmacother. 2003, 57, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, D.; Intemann, T.; Lauria, F.; Mårild, S.; Molnár, D.; Moreno, L.A.; Sioen, I.; Tornaritis, M.; Veidebaum, T.; Pigeot, I.; et al. Reference Values of Bone Stiffness Index and C-Terminal Telopeptide in Healthy European Children. Int. J. Obes. 2014, 38, S76–S85. [Google Scholar] [CrossRef]
- Rauchenzauner, M.; Schmid, A.; Heinz-Erian, P.; Kapelari, K.; Falkensammer, G.; Griesmacher, A.; Finkenstedt, G.; Högler, W. Sex- and Age-Specific Reference Curves for Serum Markers of Bone Turnover in Healthy Children from 2 Months to 18 Years. J. Clin. Endocrinol. Metab. 2007, 92, 443–449. [Google Scholar] [CrossRef]
- Ambroszkiewicz, J.; Gajewska, J.; Rowicka, G.; Klemarczyk, W.; Chelchowska, M. Assessment of Biochemical Bone Turnover Markers and Bone Mineral Density in Thin and Normal-Weight Children. Cartilage 2018, 9, 255–262. [Google Scholar] [CrossRef]
- Bleasel, J.F.; Poole, A.R.; Heinegård, D.; Saxne, T.; Holderbaum, D.; Ionescu, M.; Jones, P.; Moskowitz, R.W. Changes in Serum Cartilage Marker Levels Indicate Altered Cartilage Metabolism in Families with the Osteoarthritis-Related Type II Collagen Gene COL2A1 Mutation. Arthritis Rheum. 1999, 42, 39–45. [Google Scholar] [CrossRef]
- Mouritzen, U.; Christgau, S.; Lehmann, H.-J.; Tankó, L.B.; Christiansen, C. Cartilage Turnover Assessed with a Newly Developed Assay Measuring Collagen Type II Degradation Products: Influence of Age, Sex, Menopause, Hormone Replacement Therapy, and Body Mass Index. Ann. Rheum. Dis. 2003, 62, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Hoch, J.M.; Mattacola, C.G.; Medina McKeon, J.M.; Howard, J.S.; Lattermann, C. Serum Cartilage Oligomeric Matrix Protein (SCOMP) Is Elevated in Patients with Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Osteoarthr. Cartil. 2011, 19, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Saruga, T.; Sasaki, E.; Inoue, R.; Chiba, D.; Ota, S.; Iwasaki, H.; Uesato, R.; Nakaji, S.; Ishibashi, Y. Usefulness of Serum Hyaluronic Acid Levels as a Predictor of Incidence of Hand Osteoarthritis Analyzed by Longitudinal Analysis from the Iwaki Cohort. Sci. Rep. 2021, 11, 4074. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, E.; Tsuda, E.; Yamamoto, Y.; Maeda, S.; Inoue, R.; Chiba, D.; Fujita, H.; Takahashi, I.; Umeda, T.; Nakaji, S.; et al. Serum Hyaluronic Acid Concentration Predicts the Progression of Joint Space Narrowing in Normal Knees and Established Knee Osteoarthritis—A Five-Year Prospective Cohort Study. Arthritis Res. Ther. 2015, 17, 283. [Google Scholar] [CrossRef]
- Bertoni, L.; Jacquet-Guibon, S.; Branly, T.; Legendre, F.; Desancé, M.; Mespoulhes, C.; Melin, M.; Hartmann, D.-J.; Schmutz, A.; Denoix, J.-M.; et al. An Experimentally Induced Osteoarthritis Model in Horses Performed on Both Metacarpophalangeal and Metatarsophalangeal Joints: Technical, Clinical, Imaging, Biochemical, Macroscopic and Microscopic Characterization. PLoS ONE 2020, 15, e0235251. [Google Scholar] [CrossRef]

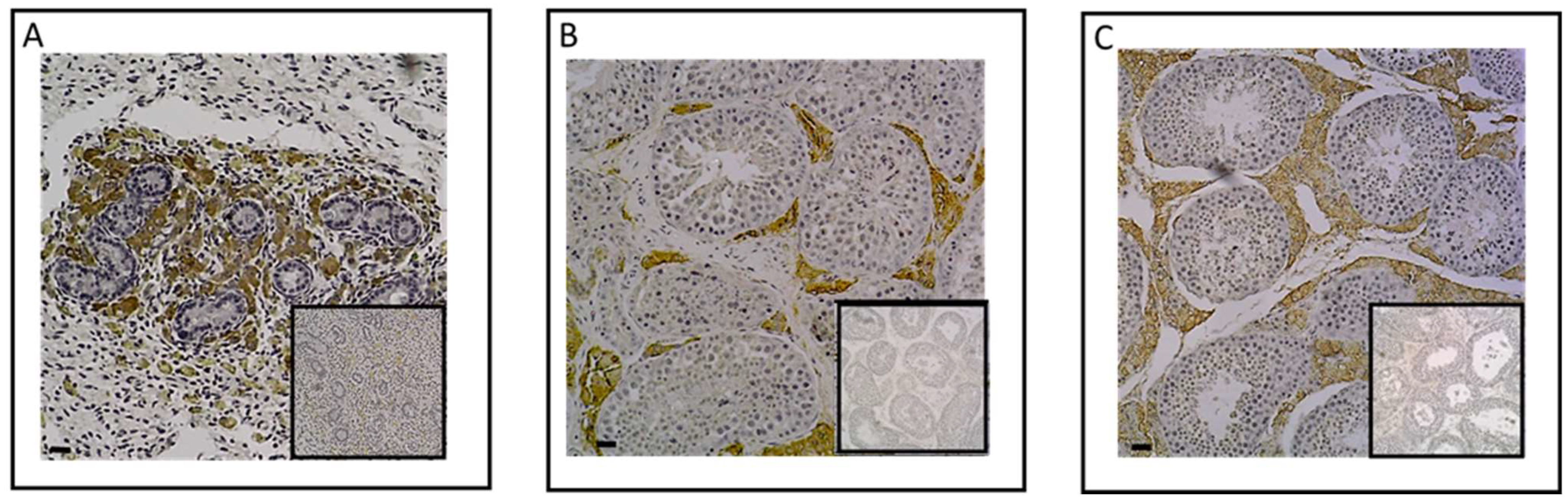
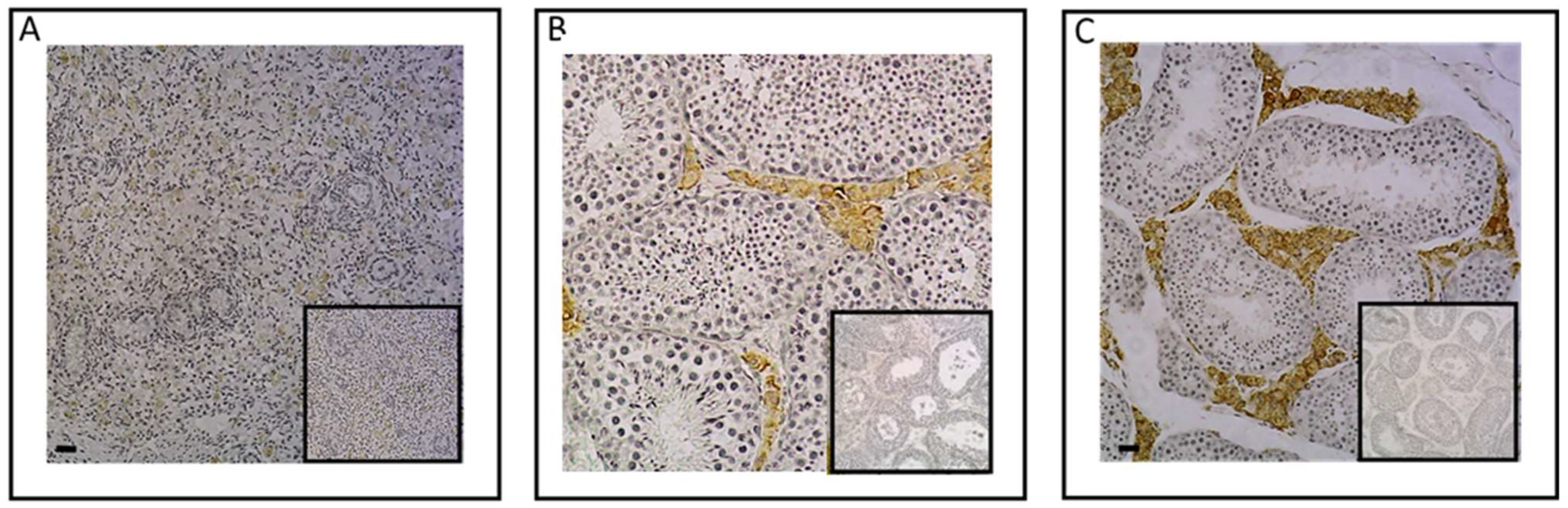

| Biomarker | Reference | Limits of Detection | Type of Sample |
|---|---|---|---|
| Testosterone Sex steroid hormone | ADI-901-176, Enzo Life Sciences (Lyon, France) | 3.9–1000 pg/mL | Plasma |
| 17β-estradiol Sex steroid hormone | 951456, Cayman Chemical, Interchim SA, Montluçon, France | 6.6–4000 pg/mL | Plasma |
| CTX-I Bone catabolism | AC-02F1, Immunodiagnostic Systems, Dijon, France | 0.02 ng/mL | Serum |
| N-MID Osteocalcin Bone anabolism | AC-11F1, Immunodiagnostic Systems, Dijon, France | 0.5 ng/mL | EDTA plasma |
| CPII Cartilage anabolism | 60-1003, IBEX pharmaceuticals Inc, Montréal, Canada | Unspecified | Serum |
| CTX-II Cartilage catabolism | AC-08F1, Immunodiagnostic Systems, Dijon, France | 3.7 pg/mL | Serum |
| COMP Osteoarthritis | MBS006795, MyBioSource, San Diego, USA | 15.6 ng/mL | Serum |
| HA Osteoarthritis | TE1017-2, TECOmedical group, Eurobio Scientific, Les Ulis, France | 2.7 ng/mL | Serum |
| PGE2 Inflammation | KGE004B, R&D, Bio-Techne SAS, Noyal Châtillon sur Seiche, France | 16 pg/mL | Heparin plasma |
| IL-6 Inflammation | EHS0002, FineTest, Clinisciences, Nanterre, France | 3.125 pg/ml | EDTA plasma |
| Primary Antibody | Reference | Dilution | Secondary Antibody Reference | Dilution |
|---|---|---|---|---|
| CYP17A1 | sc-374244 mouse monoclonal IgG, Santa Cruz Biotechnology, Dallas, TX, USA | 1:50 | 7076S, anti-mouse IgG-HRP, Cell Signaling Technology, Leiden, The Netherlands | 1:200 |
| Aromatase | sc-374176 mouse monoclonal IgG, Santa Cruz Biotechnology, Dallas, TX, USA | 1:50 | ||
| ESR1 | GTX22746 mouse monoclonal IgG, Diagomics, Blagnac, France | 1:50 | sc-2005, goat anti-mouse IgG-HRP, Santa Cruz Biotechnology, Dallas, TX, USA | 1:200 |
| AR | sc-815 rabbit polyclonal IgG, Santa Cruz Biotechnology, Dallas, TX, USA | 1:50 | ab 205718, goat pAb anti IgG-HRP, Abcam, Paris, France | 1:200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouge, M.; Legendre, F.; Elkhatib, R.; Delalande, C.; Cognié, J.; Reigner, F.; Barrière, P.; Deleuze, S.; Hanoux, V.; Galéra, P.; et al. Early Castration in Horses Does Not Impact Osteoarticular Metabolism. Int. J. Mol. Sci. 2023, 24, 16778. https://doi.org/10.3390/ijms242316778
Rouge M, Legendre F, Elkhatib R, Delalande C, Cognié J, Reigner F, Barrière P, Deleuze S, Hanoux V, Galéra P, et al. Early Castration in Horses Does Not Impact Osteoarticular Metabolism. International Journal of Molecular Sciences. 2023; 24(23):16778. https://doi.org/10.3390/ijms242316778
Chicago/Turabian StyleRouge, Marion, Florence Legendre, Razan Elkhatib, Christelle Delalande, Juliette Cognié, Fabrice Reigner, Philippe Barrière, Stefan Deleuze, Vincent Hanoux, Philippe Galéra, and et al. 2023. "Early Castration in Horses Does Not Impact Osteoarticular Metabolism" International Journal of Molecular Sciences 24, no. 23: 16778. https://doi.org/10.3390/ijms242316778
APA StyleRouge, M., Legendre, F., Elkhatib, R., Delalande, C., Cognié, J., Reigner, F., Barrière, P., Deleuze, S., Hanoux, V., Galéra, P., & Bouraïma-Lelong, H. (2023). Early Castration in Horses Does Not Impact Osteoarticular Metabolism. International Journal of Molecular Sciences, 24(23), 16778. https://doi.org/10.3390/ijms242316778







