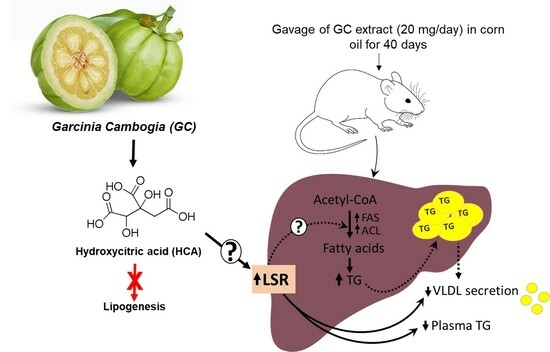
Garcinia cambogia Extract Increased Hepatic Levels of Lipolysis-Stimulated Lipoprotein Receptor and Lipids in Mice on Normal Diet
Abstract
1. Introduction
2. Results
2.1. In Vivo Study of GCE Treatment in Mice
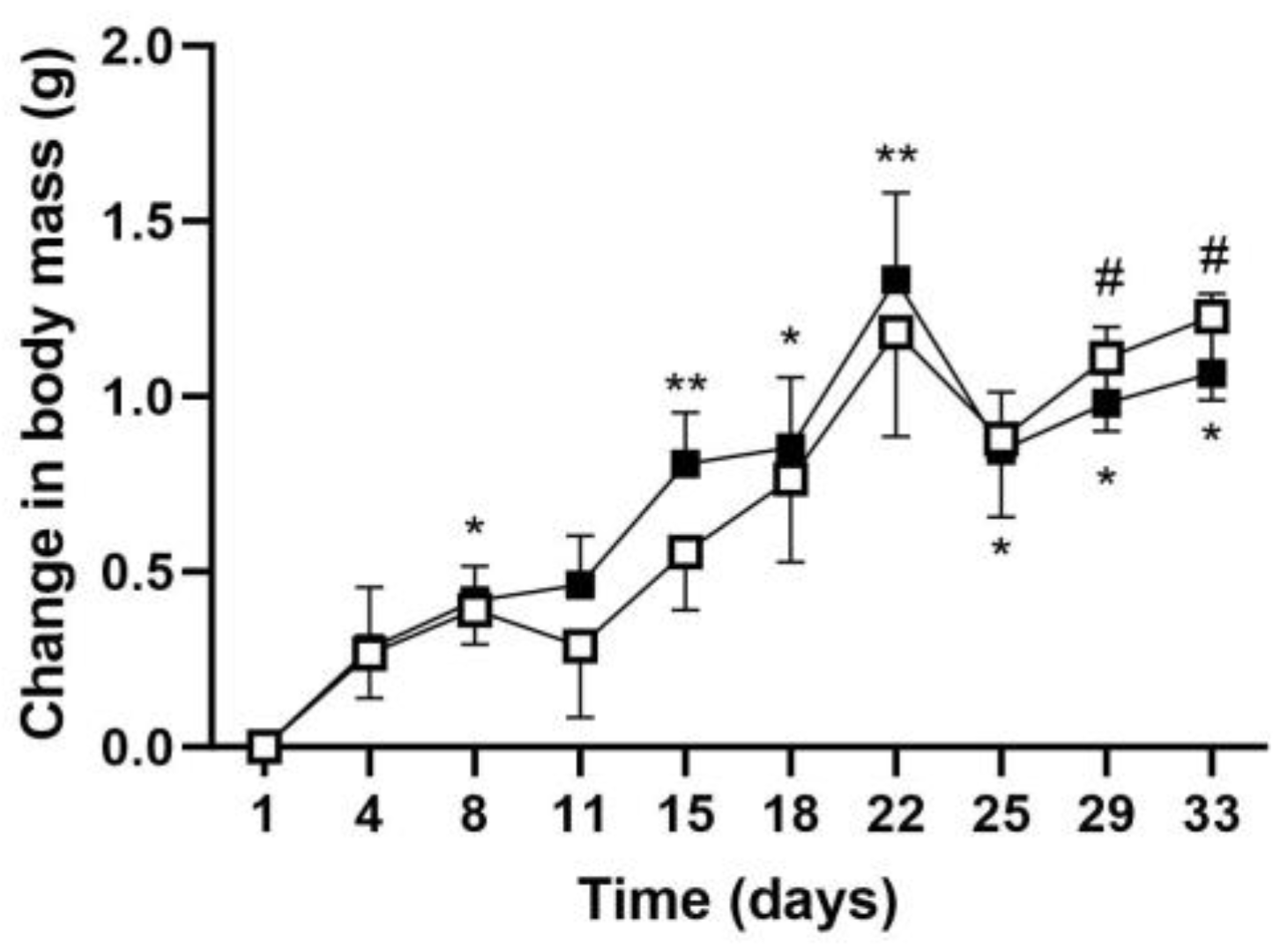
2.1.1. Body Mass Gain Measurement
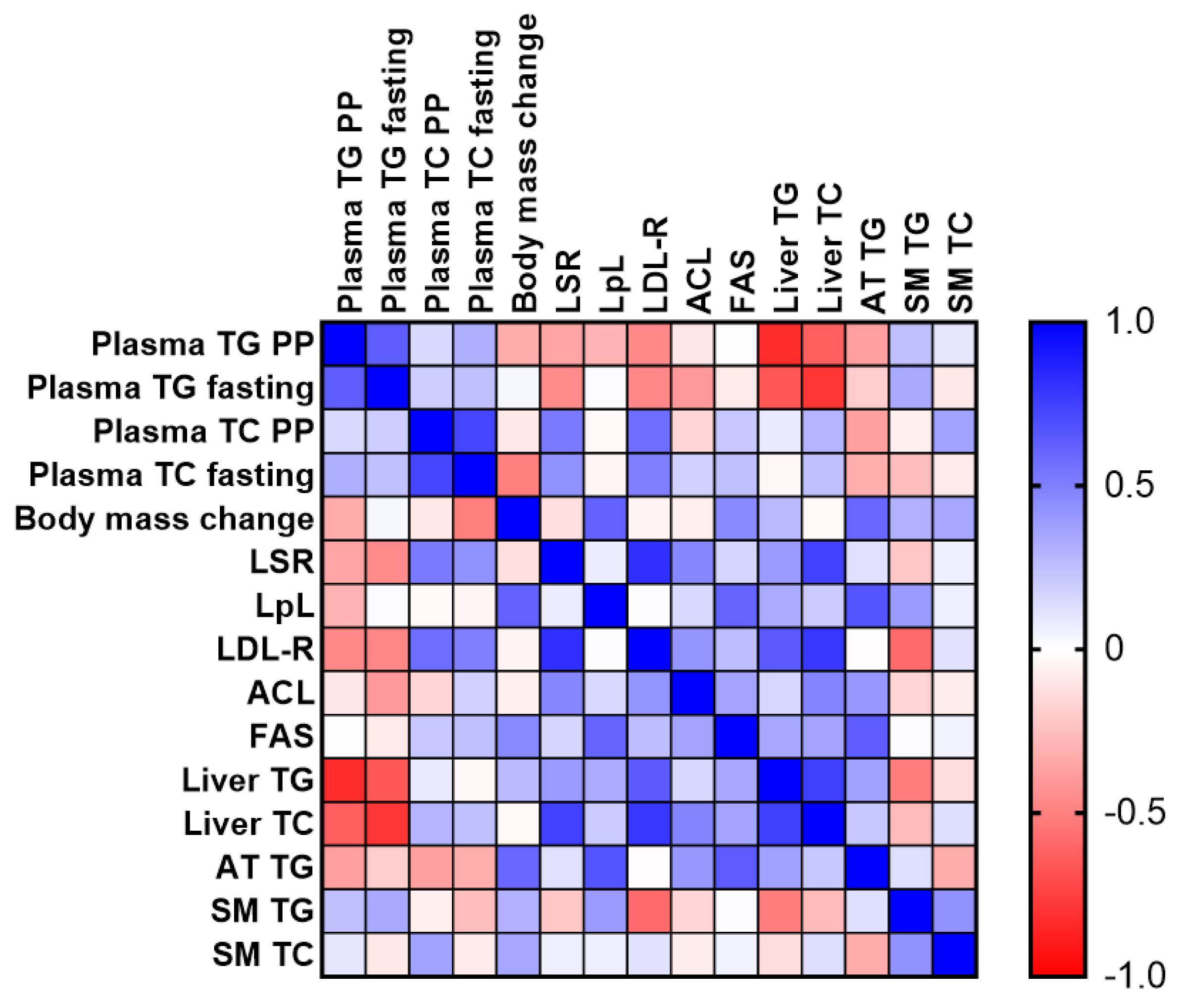
2.1.2. Plasma Lipid, Lipoprotein, and Glucose Measurements
2.1.3. Analysis of Tissue Lipid Content
2.1.4. Changes in Hepatic Protein Levels in GCE-Treated Mice
2.2. Treatment of Hepa1-6 Cells with HCA
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture Studies
4.3. Animals and Diets
4.4. GCE Treatment
4.5. Lipid and Glucose Analyses of Plasma and Tissues
4.6. Lipoprotein Profiles
4.7. Immunoblotting Studies
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pittler, M.H.; Schmidt, K.; Ernst, E. Adverse events of herbal food supplements for body weight reduction: Systematic review. Obes. Rev. 2005, 6, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Hoyo, A.; Gutiérrez-Salmeán, G. New Dietary Supplements for Obesity: What We Currently Know. Curr. Obes. Rep. 2016, 5, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Lewis, Y.S.; Neelakantan, S. (−)-Hydroxycitric acid—The principal acid in the fruits of Garcinia cambogia desr. Phytochemistry 1965, 4, 619–625. [Google Scholar] [CrossRef]
- Golzarand, M.; Omidian, M.; Toolabi, K. Effect of Garcinia cambogia supplement on obesity indices: A systematic review and dose-response meta-analysis. Complement. Ther. Med. 2020, 52, 102451. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.; Hung, S.K.; Perry, R.; Wider, B.; Ernst, E. The use of Garcinia extract (hydroxycitric acid) as a weight loss supplement: A systematic review and meta-analysis of randomised clinical trials. J. Obes. 2011, 2011, 509038. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.B.; Semwal, D.K.; Vermaak, I.; Viljoen, A. A comprehensive scientific overview of Garcinia cambogia. Fitoterapia 2015, 102, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Cheema-Dhadli, S.; Halperin, M.L.; Leznoff, C.C. Inhibition of enzymes which interact with citrate by (−)hydroxycitrate and 1,2,3,-tricarboxybenzene. Eur. J. Biochem. 1973, 38, 98–102. [Google Scholar] [CrossRef]
- Sullivan, A.C.; Hamilton, J.G.; Miller, O.N.; Wheatley, V.R. Inhibition of lipogenesis in rat liver by (−)-hydroxycitrate. Arch. Biochem. Biophys. 1972, 150, 183–190. [Google Scholar] [CrossRef]
- Triscari, J.; Sullivan, A.C. Comparative effects of (−)-hydroxycitrate and (+)-allo-hydroxycitrate on acetyl CoA carboxylase and fatty acid and cholesterol synthesis in vivo. Lipids 1977, 12, 357–363. [Google Scholar] [CrossRef]
- Watson, J.A.; Fang, M.; Lowenstein, J.M. Tricarballylate and hydroxycitrate: Substrate and inhibitor of ATP: Citrate oxaloacetate lyase. Arch. Biochem. Biophys. 1969, 35, 209–217. [Google Scholar] [CrossRef]
- Watson, J.A.; Lowenstein, J.M. Citrate and conversion of carbohydrate into fat. J. Biol. Chem. 1970, 22, 5993–6002. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Jiang, L.; Zhou, Y.; Li, Z.; Shao, M.; Li, W.; Liu, Y. Deficiency in hepatic ATP-citrate lyase affects VLDL-triglyceride mobilization and liver fatty acid composition in mice. J. Lipid Res. 2010, 51, 2516–2526. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. The SREBP pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 1997, 89, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. A century of cholesterol and coronaries: From plaques to genes to statins. Cell 2015, 161, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. A receptor-mediated pathway for cholesterol homeostasis. Science 1986, 232, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.W.; Rall, S.C.J. Type III hyperlipoproteinemia (dysbetalipoproteinemia): The role of apolipoprotein E in normal and abnormal lipoprotein metabolism. In The Metabolic and Molecular BasIs of Inherited Disease, 7th ed.; Scriver, C.R., Beaudet, A.L., Sly, W.S., Valle, D., Eds.; McGraw-Hill: New York, NY, USA, 1995; pp. 1953–1980. [Google Scholar]
- Rubinsztein, D.C.; Cohen, J.C.; Berger, G.M.; van der Westhuyzen, D.R.; Coetzee, G.A.; Gevers, W. Chylomicron remnant clearance from the plasma is normal in familial hypercholesterolemic homozygotes with defined receptor defects. J. Clin. Investig. 1990, 86, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Kita, T.; Goldstein, J.L.; Brown, M.S.; Watanabe, Y.; Hornick, C.A.; Havel, R.J. Hepatic uptake of chylomicron remnants in WHHL rabbits: A mechanism genetically distinct from the low density lipoprotein receptor. Proc. Natl. Acad. Sci. USA 1982, 79, 3623–3627. [Google Scholar] [CrossRef] [PubMed]
- Rohlmann, A.; Gotthardt, M.; Hammer, R.E.; Herz, J. Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J. Clin. Investig. 1998, 101, 689–695. [Google Scholar] [CrossRef]
- Yen, F.T.; Roitel, O.; Bonnard, L.; Notet, V.; Pratte, D.; Stenger, C.; Magueur, E.; Bihain, B.E. Lipolysis stimulated lipoprotein receptor: A novel molecular link between hyperlipidemia, weight gain, and atherosclerosis in mice. J. Biol. Chem. 2008, 283, 25650–25659. [Google Scholar] [CrossRef]
- Stenger, C.; Hanse, M.; Pratte, D.; Mbala, M.L.; Akbar, S.; Koziel, V.; Escanyé, M.-C.; Kriem, B.; Malaplate-Armand, C.; Olivier, J.-L.; et al. Up-regulation of hepatic lipolysis stimulated lipoprotein receptor by leptin: A potential lever for controlling lipid clearance during the postprandial phase. FASEB J. 2010, 24, 4218–4228. [Google Scholar] [CrossRef]
- Akbar, S.; Pincon, A.; Lanhers, M.C.; Claudepierre, T.; Corbier, C.; Gregory-Pauron, L.; Malaplate-Armand, C.; Visvikis, A.; Oster, T.; Yen, F.T. Expression profile of hepatic genes related to lipid homeostasis in LSR heterozygous mice contribute to their increased response to high-fat diet. Physiol. Genom. 2016, 48, 928–935. [Google Scholar] [CrossRef][Green Version]
- Xie, T.; Stathopoulou, M.G.; Akbar, S.; Oster, T.; Siest, G.; Yen, F.T.; Visvikis-Siest, S. Effect of LSR polymorphism on blood lipid levels and age-specific epistatic interaction with the APOE common polymorphism. Clin. Genet. 2018, 93, 846–852. [Google Scholar] [CrossRef]
- Lowenstein, J.M. Effect of (−)-hydroxycitrate on fatty acid synthesis by rat liver in vivo. J. Biol. Chem. 1971, 246, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, M.; Hrupka, B.; Langhans, W. Effect of hydroxycitrate on food intake and body weight regain after a period of restrictive feeding in male rats. Physiol. Behav. 2001, 74, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.D.; Huypens, P.; Stewart, L.K.; Gettys, T.W. Implications of crosstalk between leptin and insulin signaling during the development of diet-induced obesity. Biochim. Biophys. Acta 2009, 1792, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Bihain, B.E.; Yen, F.T. Free fatty acids activate a high-affinity saturable pathway for degradation of low-density lipoproteins in fibroblasts from a subject homozygous for familial hypercholesterolemia. Biochemistry 1992, 31, 4628–4636. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.T.; Mann, C.J.; Guermani, L.M.; Hannouche, N.F.; Hubert, N.; Hornick, C.A.; Bordeau, V.N.; Agnani, G.; Bihain, B.E. Identification of a lipolysis-stimulated receptor that is distinct from the LDL receptor and the LDL receptor-related protein. Biochemistry 1994, 33, 1172–1180. [Google Scholar] [CrossRef]
- Mahapatra, S.; Mallik, S.B.; Rao, G.V.; Reddy, G.C.; Row, T.N.G. Garcinia lactone. Acta Crystallogr. 2007, E63, o3869. [Google Scholar] [CrossRef]
- Narvekar, P.; Berriel Diaz, M.; Krones-Herzig, A.; Hardeland, U.; Strzoda, D.; Stohr, S.; Frohme, M.; Herzig, S. Liver-specific loss of lipolysis-stimulated lipoprotein receptor triggers systemic hyperlipidemia in mice. Diabetes 2009, 58, 1040–1049. [Google Scholar] [CrossRef]
- de Faria, E.; Fong, L.G.; Komaromy, M.; Cooper, A.D. Relative roles of the LDL receptor, the LDL receptor-like protein, and hepatic lipase in chylomicron remnant removal by the liver. J. Lipid Res. 1996, 37, 197–209. [Google Scholar] [CrossRef]
- Berkhout, T.A.; Havekes, L.M.; Pearce, N.J.; Groot, P.H. The effect of (−)-hydroxycitrate on the activity of the low-density-lipoprotein receptor and 3-hydroxy-3-methylglutaryl-CoA reductase levels in the human hepatoma cell line Hep G2. Biochem. J. 1990, 272, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.C.; Triscari, J.; Hamilton, J.G.; Miller, O.N.; Wheatley, V.R. Effect of (−)-hydroxycitrate upon the accumulation of lipid in the rat: I. Lipogenesis. Lipids 1974, 9, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Lee, H.N.; Kim, Y.J.; Park, T. Garcinia cambogia extract ameliorates visceral adiposity in C57BL/6J mice fed on a high-fat diet. Biosci. Biotechnol. Biochem. 2008, 72, 1772–1780. [Google Scholar] [CrossRef]
- Dong, J.; Li, W.; Du, X.; He, X.; Deng, B.; Zheng, H.; Tian, Y.; Sheng, J.; Fang, C. Garcinia cambogia water extract alleviates insulin resistance and hepatic lipid accumulation in mice fed a high-fat diet. Food Nutr. Res. 2023, 67, 8977. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, M.S.; Park, Y.B.; Kim, S.R.; Lee, M.K.; Jung, U.J. Garcinia cambogia attenuates diet-induced adiposity but exacerbates hepatic collagen accumulation and inflammation. World J. Gastroenterol. 2013, 19, 4689–4701. [Google Scholar] [CrossRef]
- Sripradha, R.; Sridhar, M.G.; Maithilikarpagaselvi, N. Antihyperlipidemic and antioxidant activities of the ethanolic extract of Garcinia cambogia on high fat diet-fed rats. J. Complement. Integr. Med. 2016, 13, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, M.R.; Cleary, M.P.; Gruen, R.; Blase, D.; Stern, J.S.; Triscari, J.; Sullivan, A.C. Effect of (−)-hydroxycitrate on development of obesity in the Zucker obese rat. Am. J. Physiol. 1981, 240, E72–E78. [Google Scholar] [CrossRef]
- Chuah, L.O.; Ho, W.Y.; Beh, B.K.; Yeap, S.K. Updates on Antiobesity Effect of Garcinia Origin (−)-HCA. Evid.-Based Complement. Altern. Med. 2013, 2013, 751658. [Google Scholar] [CrossRef] [PubMed]
- Licholai, J.A.; Nguyen, K.P.; Fobbs, W.C.; Schuster, C.J.; Ali, M.A.; Kravitz, A.V. Why do mice overeat high-fat diets? How high-fat diet alters the regulation of daily caloric intake in mice. Obesity 2018, 26, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, M.; Münch, S.; Westerterp-Plantenga, M.; Langhans, W. Effects of hydroxycitrate, conjugated linoleic acid, and guar gum on food intake, body weight regain, and metabolism after body weight loss in male rats. Nutr. Res. 2004, 24, 659–669. [Google Scholar] [CrossRef]
- Bilal, T.; Gursel, F.E.; Ateş, A.; Altiner, A. Effect of Garcinia cambogia extract on body weight gain, feed intake and feed conversion ratio, and serum non-esterified fatty acids and C-reactive protein levels in rats fed with atherogenic diet. Iran. J. Vet. Res. 2012, 13, 330–333. [Google Scholar] [CrossRef]
- Chu, W.-L.; Lin, W.-C.; Yang, L.-C. Effects of Garcinia cambogia compounded supplements on the formation of body fat induced by a high energy diet in obese rats. Obes. Res. Open J. 2021, 8, 18–25. [Google Scholar] [CrossRef]
- Han, J.H.; Jang, K.W.; Myung, C.S. Garcinia cambogia attenuates adipogenesis by affecting CEBPB and SQSTM1/p62-mediated selective autophagic degradation of KLF3 through RPS6KA1 and STAT3 suppression. Autophagy 2022, 18, 518–539. [Google Scholar] [CrossRef] [PubMed]
- Aas, V.; Kase, E.T.; Solberg, R.; Jensen, J.; Rustan, A.C. Chronic hyperglycaemia promotes lipogenesis and triacylglycerol accumulation in human skeletal muscle cells. Diabetologia 2004, 47, 1452–1461. [Google Scholar] [CrossRef]
- Erikci Ertunc, M.; Hotamisligil, G.S. Lipid signaling and lipotoxicity in metabolic inflammation: Indications for metabolic disease pathogenesis and treatment. J. Lipid Res. 2016, 57, 2099–2114. [Google Scholar] [CrossRef]
- Ishihara, K.; Oyaizu, S.; Onuki, K.; Lim, K.; Fushiki, T. Chronic (−)-hydroxycitrate administration spares carbohydrate utilization and promotes lipid oxidation during exercise in mice. J. Nutr. 2000, 130, 2990–2995. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; He, J.; Watkins, S.; Kelley, D.E. Skeletal muscle lipid content and insulin resistance: Evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab. 2001, 86, 5755–5761. [Google Scholar] [CrossRef]
- Lobb, A. Hepatoxicity associated with weight-loss supplements: A case for better post-marketing surveillance. World J. Gastroenterol. 2009, 15, 1786–1787. [Google Scholar] [CrossRef]
- Vitalone, A.; Menniti-Ippolito, F.; Moro, P.A.; Firenzuoli, F.; Raschetti, R.; Mazzanti, G. Suspected adverse reactions associated with herbal products used for weight loss: A case series reported to the Italian National Institute of Health. Eur. J. Clin. Pharmacol. 2011, 67, 215–224. [Google Scholar] [CrossRef]
- Sharma, A.; Akagi, E.; Njie, A.; Goyal, S.; Arsene, C.; Krishnamoorthy, G.; Ehrinpreis, M. Acute Hepatitis due to Garcinia cambogia Extract, an Herbal Weight Loss Supplement. Case Rep. Gastrointest. Med. 2018, 2018, 9606171. [Google Scholar] [CrossRef]
- Kothadia, J.P.; Kaminski, M.; Samant, H.; Olivera-Martinez, M. Hepatotoxicity Associated with Use of the Weight Loss Supplement Garcinia cambogia: A Case Report and Review of the Literature. Case Rep. Hepatol. 2018, 2018, 6483605. [Google Scholar] [CrossRef]
- Haber, S.L.; Awwad, O.; Phillips, A.; Park, A.E.; Pham, T.M. Garcinia cambogia for weight loss. Am. J. Health Syst. Pharm. 2018, 75, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, G.; Lombardi, N.; Bettiol, A.; Marconi, E.; Risaliti, F.; Bertoni, M.; Ippolito, F.M.; Maggini, V.; Gallo, E.; Firenzuoli, F.; et al. Acute liver injury following Garcinia cambogia weight-loss supplementation: Case series and literature review. Intern. Emerg. Med. 2018, 13, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Layeghkhavidaki, H.; Lanhers, M.C.; Akbar, S.; Gregory-Pauron, L.; Oster, T.; Grova, N.; Appenzeller, B.; Jasniewski, J.; Feidt, C.; Corbier, C.; et al. Inhibitory action of benzo[a]pyrene on hepatic lipoprotein receptors in vitro and on liver lipid homeostasis in mice. PLoS ONE 2014, 9, e102991. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, P.; Shyamala Devi, C.S. The modulating effect of Garcinia cambogia extract on ethanol-induced peroxidative damage in rats. Indian J. Pharmacol. 2001, 33, 87–91. [Google Scholar]
- Mahmoud, G.S.; Abd El-Aziz, E.A.; Muhammed, A.A. The protective effect of garcinia on cardiovascular system of propylthiouracil induced hypothyroid rat model. IOSR-JDMS 2016, 15, 103–112. [Google Scholar] [CrossRef]
- Markwell, M.A.; Haas, S.M.; Tolbert, N.E.; Bieber, L.L. Protein determination in membrane and lipoprotein samples: Manual and automated procedures. Methods Enzymol. 1981, 72, 296–303. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]
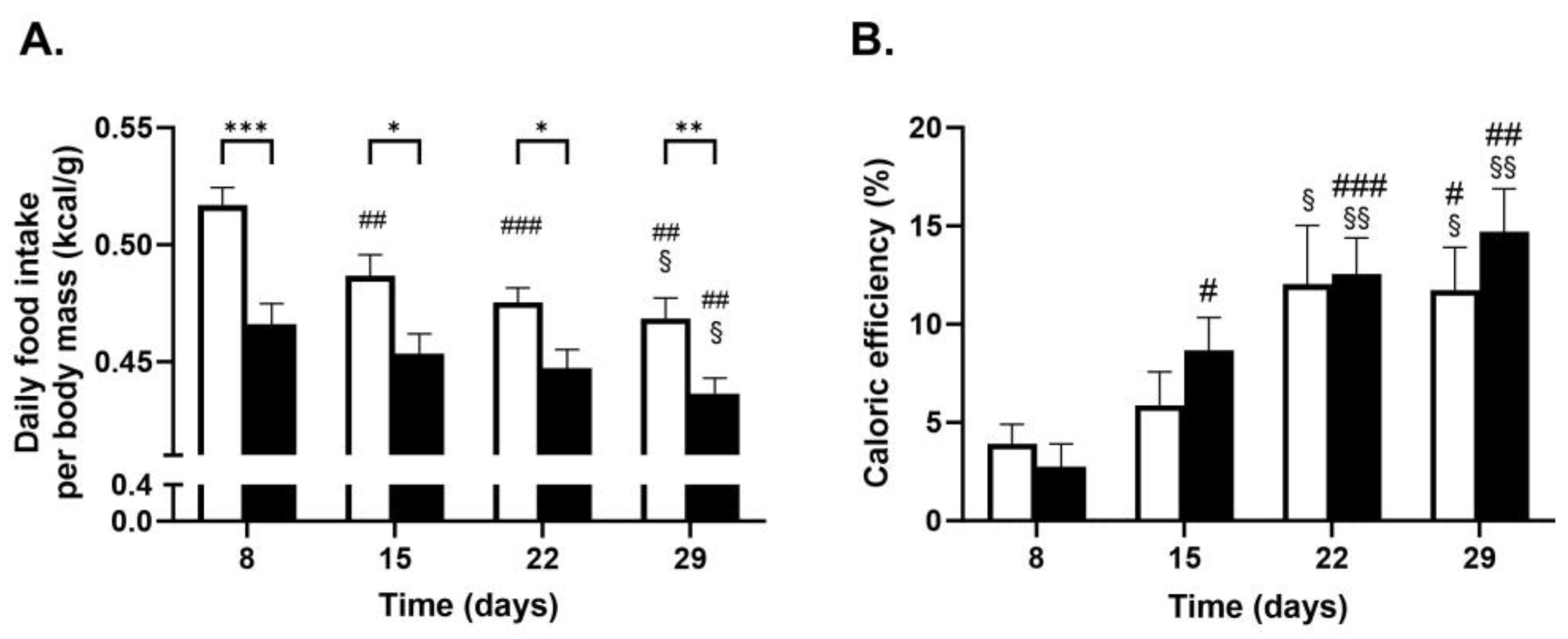



| Food Intake (kcal/Day) | Fat Intake (% kcal/Day) | |||
|---|---|---|---|---|
| Day | CTRL | GCE | CTRL | GCE |
| 8 | 12.8 ± 0.14 | 11.9 ± 0.22 ** | 23.1 ± 0.09 | 23.7 ± 0.16 ** |
| 15 | 12.3 ± 0.15 | 11.9 ± 0.22 | 23.4 ± 0.10 | 23.8 ± 0.16 |
| 22 | 12.3 ± 0.21 | 11.8 ± 0.21 | 23.4 ± 0.14 | 23.8 ± 0.15 |
| 29 | 12.1 ± 0.23 # | 11.6 ± 0.18 | 23.6 ± 0.16 # | 23.9 ± 0.13 |
| Liver Total Liver Content (mg) | Adipose Tissue µg/mg Dry Weight | Skeletal Muscle µg/mg Dry Weight | Heart µg/mg Dry Weight | |||||
|---|---|---|---|---|---|---|---|---|
| Lipid | CTRL | GCE | CTRL | GCE | CTRL | GCE | CTRL | GCE |
| TC | 1.4 ± 0.1 | 2.0 ± 0.1 ** | nd | nd | 2.5 ± 0.3 | 3.0 ± 0.2 | 5.9 ± 0.5 | 5.8 ± 0.5 |
| TG | 7.8 ± 0.7 | 9.6 ± 0.5 * | 9.6 ± 1.1 | 9.7 ± 0.61 | 11.5 ± 0.7 | 15.0 ± 1.1 * | 19.3 ± 2.5 | 17.9 ± 2.6 |
| PL | 8.5 ± 0.5 | 8.3 ± 0.3 | 3.5 ± 0.4 | 3.1 ± 0.24 | 20.6 ± 2.0 | 24.4 ± 1.6 | 24.2 ± 3.0 | 25.6 ± 3.8 |
| Ratio | ||||||||
| TC/PL | 0.17 ± 0.03 | 0.25 ± 0.02 * | -- | -- | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.25 ± 0.03 | 0.25 ± 0.1 |
| TG/PL | 0.92 ± 0.1 | 1.18 ± 0.1 | 2.77 ± 0.1 | 3.14 ± 0.14 * | 0.53 ± 0.01 | 0.63 ± 0.04 | 0.83 ± 0.2 | 0.63 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanse, M.; Akbar, S.; Layeghkhavidaki, H.; Yen, F.T. Garcinia cambogia Extract Increased Hepatic Levels of Lipolysis-Stimulated Lipoprotein Receptor and Lipids in Mice on Normal Diet. Int. J. Mol. Sci. 2023, 24, 16298. https://doi.org/10.3390/ijms242216298
Hanse M, Akbar S, Layeghkhavidaki H, Yen FT. Garcinia cambogia Extract Increased Hepatic Levels of Lipolysis-Stimulated Lipoprotein Receptor and Lipids in Mice on Normal Diet. International Journal of Molecular Sciences. 2023; 24(22):16298. https://doi.org/10.3390/ijms242216298
Chicago/Turabian StyleHanse, Marine, Samina Akbar, Hamed Layeghkhavidaki, and Frances T. Yen. 2023. "Garcinia cambogia Extract Increased Hepatic Levels of Lipolysis-Stimulated Lipoprotein Receptor and Lipids in Mice on Normal Diet" International Journal of Molecular Sciences 24, no. 22: 16298. https://doi.org/10.3390/ijms242216298
APA StyleHanse, M., Akbar, S., Layeghkhavidaki, H., & Yen, F. T. (2023). Garcinia cambogia Extract Increased Hepatic Levels of Lipolysis-Stimulated Lipoprotein Receptor and Lipids in Mice on Normal Diet. International Journal of Molecular Sciences, 24(22), 16298. https://doi.org/10.3390/ijms242216298