Changes in Diversity and Composition of Rhizosphere Bacterial and Fungal Community between Resistant and Susceptible Pakchoi under Plasmodiophora brassicae
Abstract
:1. Introduction
2. Results
2.1. Phenotypes of Pakchoi Infected with P. brassicae
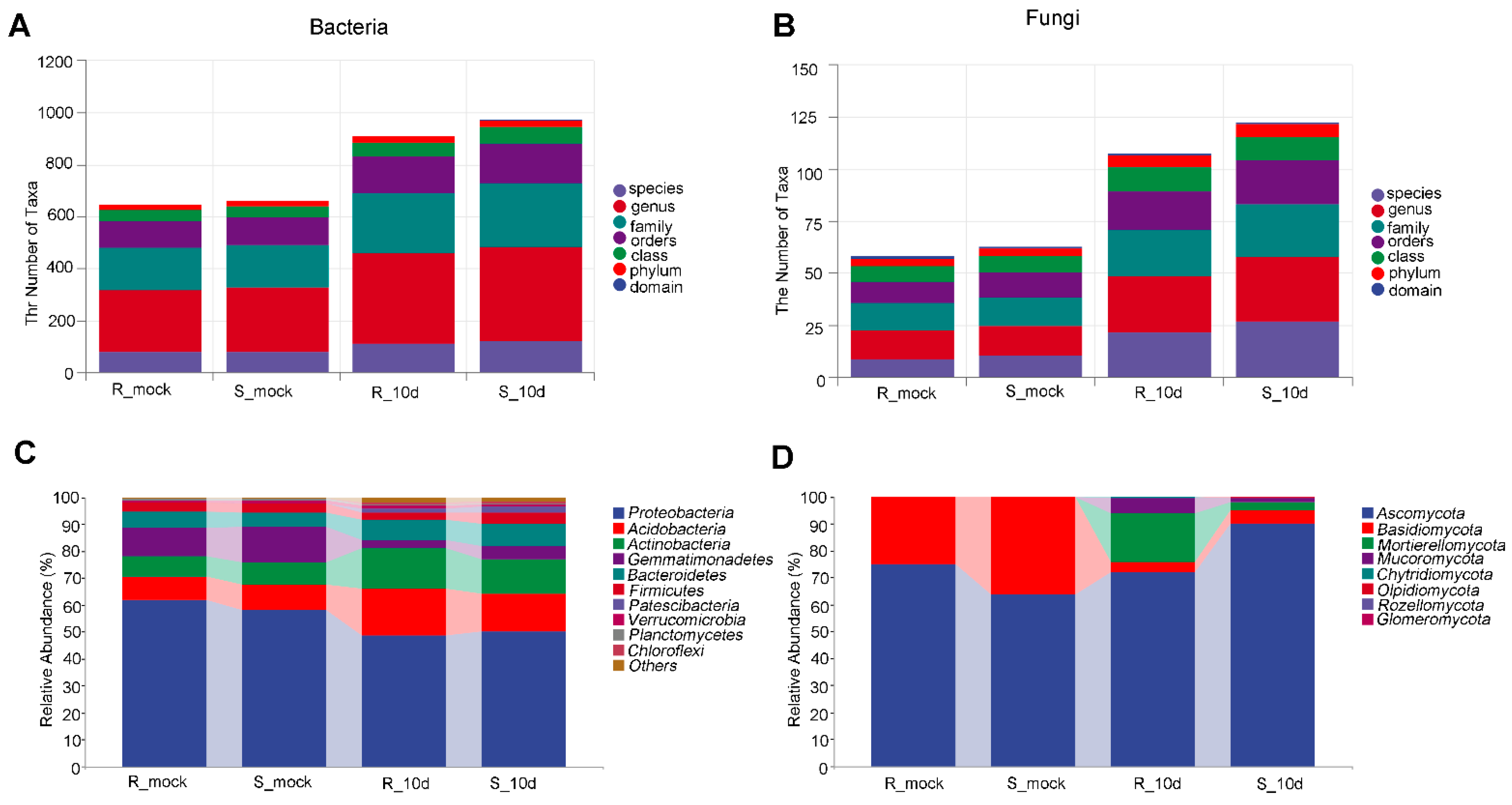
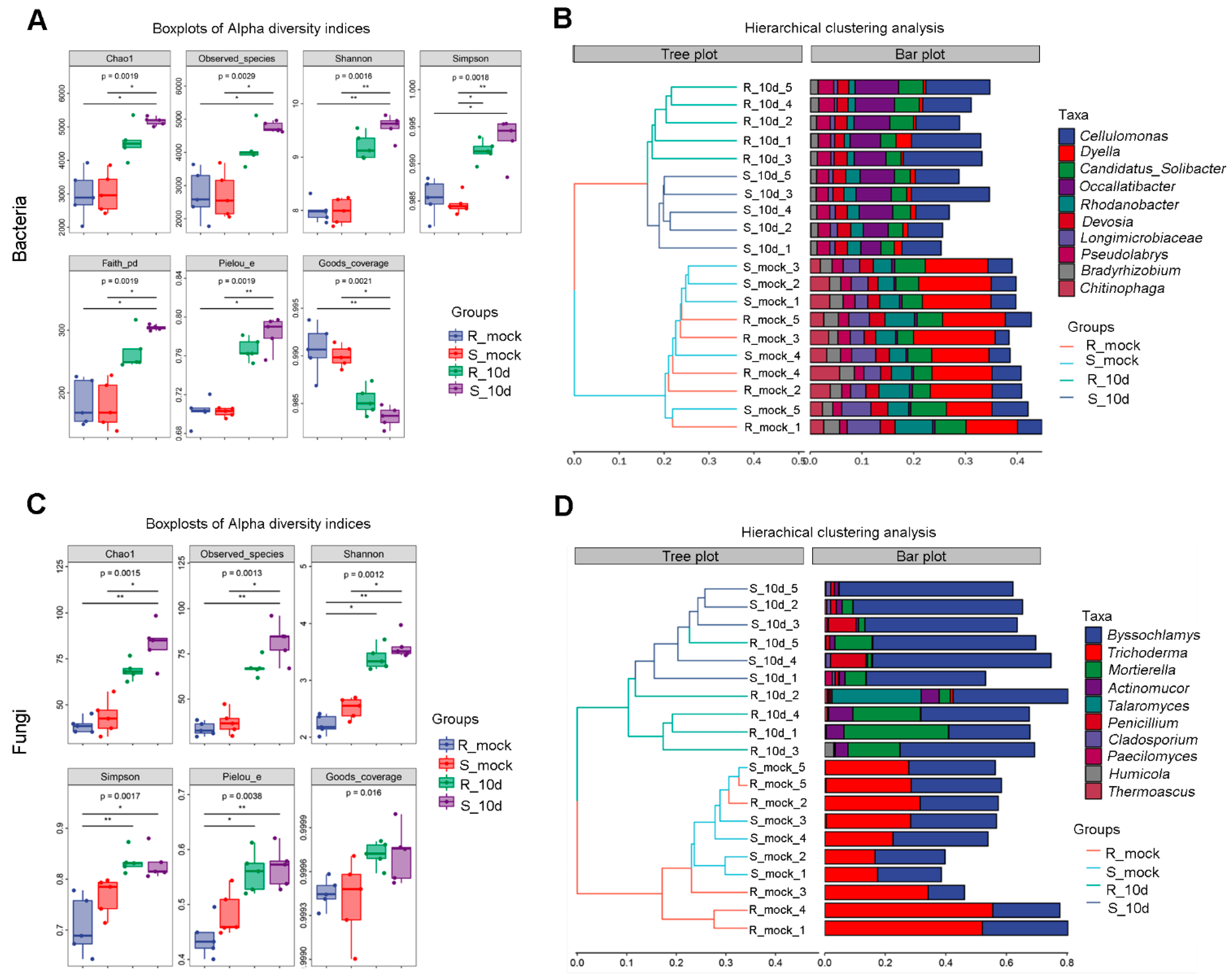
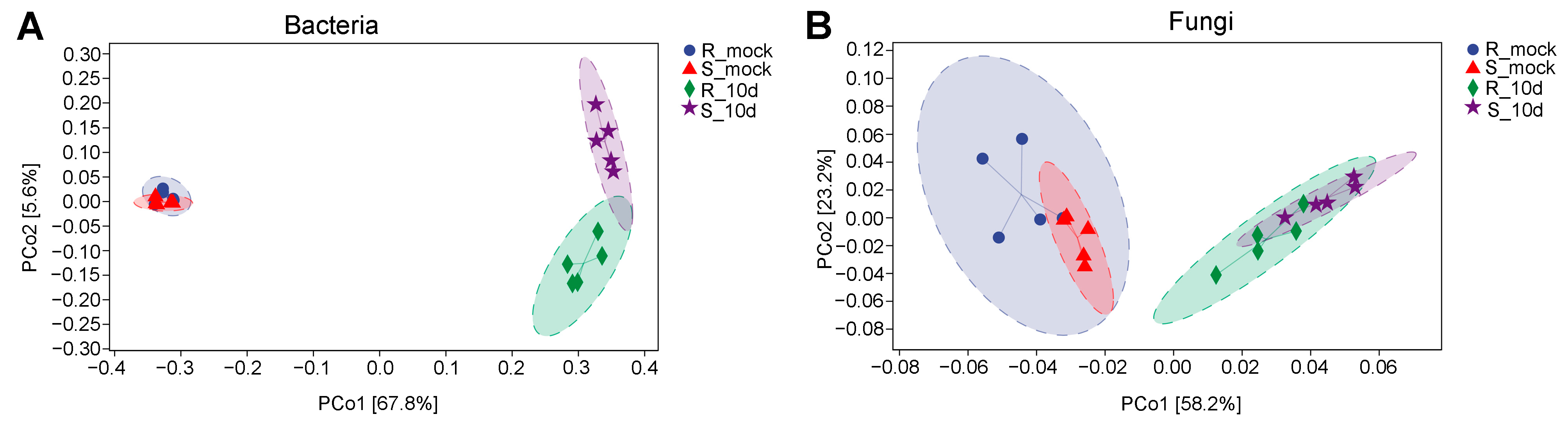
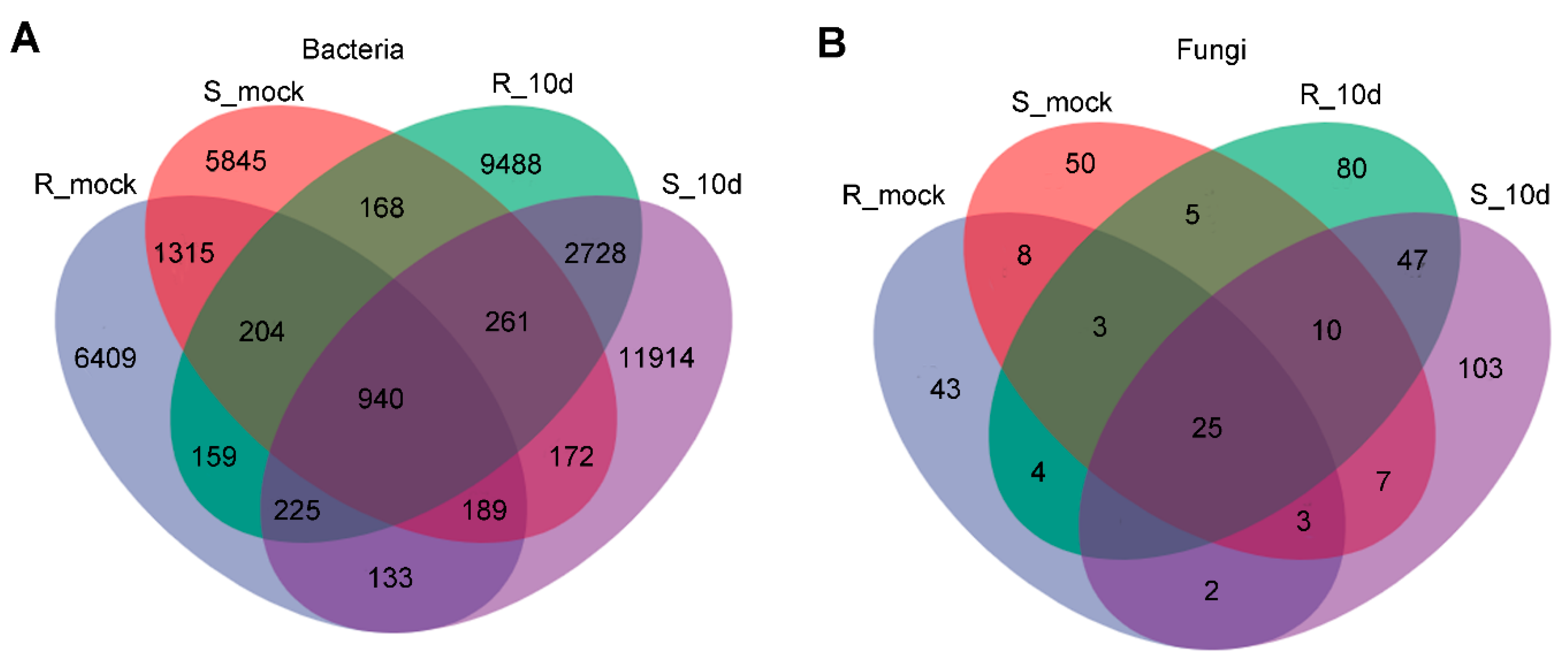
2.2. Microbial Diversity Analysis of Rhizosphere Soil
2.3. Microbial Diversity Analysis of Rhizosphere Soil
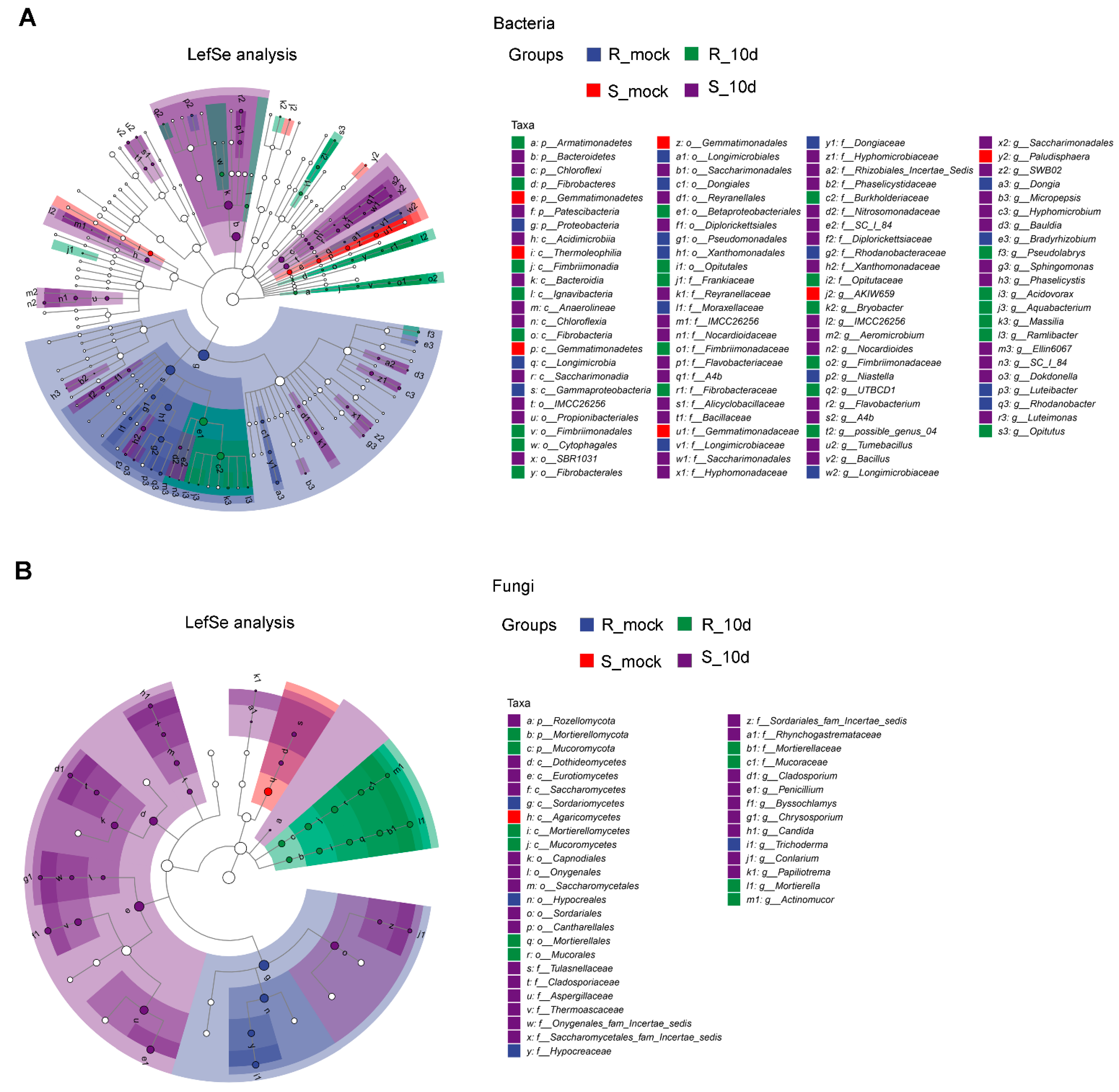
2.4. Species Differences Analysis
3. Discussion
4. Materials and Methods
4.1. Pakchoi Materials
4.2. P. brassicae Inoculation
4.3. Microbial Diversity Sequencing
4.4. Biodiversity Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavalier-Smith, T.; Chao, E.E.Y. Phylogeny and classification of Phylum Cercozoa (Protozoa). Protist 2003, 154, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, K.; Asano, T. Life cycle of Plasmodiophora brassicae. J. Plant Growth Regul. 2009, 28, 203–211. [Google Scholar] [CrossRef]
- Hwang, S.; Strelkov, S.E.; Feng, J.; Gossen, B.D.; Howard, R.J. Plasmodiophora brassicae: A review of an emerging pathogen of the Canadian canola (Brassica napus) crop. Mol. Plant Pathol. 2012, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jo, E.J.; Choi, Y.H.; Jang, K.S.; Choi, G.J. Pathotype Classification of Plasmodiophora brassicae Isolates Using Clubroot-Resistant Cultivars of Chinese Cabbage. Plant Pathol. J. 2016, 32, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zuzak, K.; Harding, M.; Strelkov, S.; Hwang, S.; Feindel, D.; Feng, J. DNA sequence dimorphisms in populations of the clubroot pathogen Plasmodiophora brassicae. Plant Dis. 2018, 102, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wang, X.; Xiao, Y.; Wei, S.; Wang, D.; Huang, Y.; Wang, W.; Yang, H. Specific genes identified in pathotype 4 of the clubroot pathogen plasmodiophora brassicae. Plant Dis. 2019, 103, 495–503. [Google Scholar] [CrossRef]
- Zamani-Noor, N.; Wallenhammar, A.; Kaczmarek, J.; Patar, U.R.; Zouhar, M.; Manasova, M.; Jędryczka, M. Pathotype characterization of Plasmodiophora brassicae, thecCause of clubroot in central Europe and Sweden (2016–2020). Pathogens 2022, 11, 1440. [Google Scholar] [CrossRef]
- Chu, M.; Song, T.; Falk, K.C.; Zhang, X.; Liu, X.; Chang, A.; Lahlali, R.; McGregor, L.; Gossen, B.D.; Peng, G.; et al. Fine mapping of Rcr1 and analyses of its effect on transcriptome patterns during infection by Plasmodiophora brassicae. BMC Genom. 2014, 15, 1166. [Google Scholar] [CrossRef]
- Chang, A.; Lamara, M.; Wei, Y.; Hu, H.; Parkin, I.A.P.; Gossen, B.D.; Peng, G.; Yu, F. Clubroot resistance gene Rcr6 in Brassica nigra resides in a genomic region homologous to chromosome A08 in B. rapa. BMC Plant Biol. 2019, 19, 224. [Google Scholar] [CrossRef]
- Zhu, H.; Zhai, W.; Li, X.; Zhu, Y. Two QTLs controlling Clubroot resistance identified from Bulked Segregant Sequencing in Pakchoi (Brassica campestris ssp. chinensis Makino). Sci. Rep. 2019, 9, 9228. [Google Scholar] [CrossRef]
- Ce, F.; Mei, J.; He, H.; Zhao, Y.; Hu, W.; Yu, F.; Li, Q.; Ren, X.; Si, J.; Song, H.; et al. Identification of candidate genes for clubroot-resistance in Brassica oleracea using 1uantitative trait loci-sequencing. Front. Plant Sci. 2021, 12, 703520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiang, X.; Huang, F.; Yu, W.; Zhou, X.; Li, B.; Zhang, Y.; Chen, P.; Zhang, C. Fine mapping of clubroot resistance loci CRA8.1 and candidate gene analysis in Chinese cabbage (Brassica rapa L.). Front. Plant Sci. 2022, 13, 898108. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Philp, J.; Li, J.; Wei, Y.; Li, H.; Yang, K.; Ryder, M.; Toh, R.; Zhou, Y.; Denton, M.D.; et al. Trichoderma harzianum inoculation reduces the incidence of clubroot disease in Chinese Cabbage by regulating the rhizosphere microbial Community. Microorganisms 2020, 8, 1325. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; He, Y.; Li, Y.; Ren, T.; Liu, H.; Huang, J.; Jiang, D.; Hsiang, T.; Zheng, L. Two new biocontrol agents against clubroot caused by Plasmodiophora brassicae. Front. Microbiol. 2020, 10, 3099. [Google Scholar] [CrossRef] [PubMed]
- Arif, S.; Liaquat, F.; Yang, S.; Shah, I.H.; Zhao, L.; Xiong, X.; Garcia, D.; Zhang, Y. Exogenous inoculation of endophytic bacterium Bacillus cereus suppresses clubroot (Plasmodiophora brassicae) occurrence in pak choi (Brassica campestris sp. chinensis L.). Planta 2021, 253, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, X.; Cheng, J.; Xie, J.; Lin, Y.; Jiang, D.; Fu, Y.; Chen, T. Application of Trichoderma Hz36 and Hk37 as biocontrol agents against clubroot caused by Plasmodiophora brassicae. J. Fungi 2022, 8, 777. [Google Scholar] [CrossRef]
- Ding, L.; Zhou, H.; Liang, H.; Tan, L.; Zhao, H.; Chen, X.; Ren, Z. Efficacy of Streptomyces melanosporofaciens strain X216 at controlling clubroot disease on oilseed rape. Front. Microbiol. 2023, 14, 1249813. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; Da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Shenton, M.; Iwamoto, C.; Kurata, N.; Ikeo, K. Effect of wild and cultivated rice genotypes on rhizosphere bacterial community composition. Rice 2016, 9, 42. [Google Scholar] [CrossRef]
- Wei, F.; Zhao, L.; Xu, X.; Feng, H.; Shi, Y.; Deakin, G.; Feng, Z.; Zhu, H. Cultivar-dependent variation of the cotton rhizosphere and endosphere microbiome under field conditions. Front. Plant Sci. 2019, 10, 1659. [Google Scholar] [CrossRef]
- Tie, Z.; Wang, P.; Chen, W.; Tang, B.; Yu, Y.; Liu, Z.; Zhao, S.; Khan, F.H.; Zhang, X.; Xi, H. Different responses of the rhizosphere microbiome to Verticillium dahliae infection in two cotton cultivars. Front. Microbiol. 2023, 14, 1229454. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, H.; Zhao, L.; Wang, M.; Sun, M. Diversity and structure of the rhizosphere microbial communities of wild and cultivated ginseng. BMC Microbiol. 2022, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, Z.; Tian, B.; Bi, K.; Chen, T.; Liu, H.; Xie, J.; Cheng, J.; Fu, Y.; Jiang, D. Endosphere microbiome comparison between symptomatic and asymptomatic roots of Brassica napus infected with Plasmodiophora brassicae. PLoS ONE 2017, 12, e185907. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.; Guillerm-Erckelboudt, A.; Gazengel, K.; Linglin, J.; Ourry, M.; Glory, P.; Sarniguet, A.; Daval, S.; Manzanares-Dauleux, M.J.; Mougel, C. Temporal dynamics of bacterial and fungal communities during the infection of Brassica rapa roots by the protist Plasmodiophora brassicae. PLoS ONE 2019, 14, e204195. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, G.; Ma, L.; Liu, T.; Zhang, C.; Xiao, D.; Zheng, H.; Chen, F.; Hou, X. A chromosome-level reference genome of non-heading Chinese cabbage [Brassica campestris (syn. Brassica rapa) ssp. chinensis]. Hortic. Res. 2020, 7. [Google Scholar] [CrossRef]
- Dixon, G.R. The Occurrence and economic Impact of Plasmodiophora brassicae and clubroot disease. J. Plant Growth Regul. 2009, 28, 194–202. [Google Scholar] [CrossRef]
- Kwak, M.; Kong, H.G.; Choi, K.; Kwon, S.; Song, J.Y.; Lee, J.; Lee, P.A.; Choi, S.Y.; Seo, M.; Lee, H.J.; et al. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat. Biotechnol. 2018, 36, 1100–1109. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, Y.; Liu, Q.; Yang, H.; Wang, Z.; Zhang, M. Effects of drought-tolerant Ea-DREB2B transgenic sugarcane on bacterial communities in soil. Front. Microbiol. 2020, 11, 704. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, Y.; Zhang, Y.; Song, B.; Li, H.; Chen, Z. Effects of root organic exudates on rhizosphere microbes and nutrient removal in the constructed wetlands. Ecol. Eng. 2016, 92, 243–250. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Lanoue, A.; Strecker, T.; Scheu, S.; Steinauer, K.; Thakur, M.P.; Mommer, L. Root biomass and exudates link plant diversity with soil bacterial and fungal biomass. Sci. Rep. 2017, 7, srep44641. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, C.; Zheng, Y.; Wang, J.; Wang, F. Root exudates metabolic profiling suggests distinct defense mechanisms between resistant and susceptible tobacco cultivars against blacksShank disease. Front. Plant Sci. 2020, 11, 559775. [Google Scholar] [CrossRef] [PubMed]
- Lekota, M.; Modisane, K.J.; Apostolides, Z.; van der Waals, J.E. Metabolomic fingerprinting of potato cultivars differing in susceptibility to spongospora subterranea f. sp. subterranea rootiInfection. Int. J. Mol. Sci. 2020, 21, 3788. [Google Scholar] [CrossRef] [PubMed]
- Boer, W.D.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a fungal world: Impact of fungi on soil bacterial niche development. FEMS Microbiol. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef] [PubMed]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef]
- Qu, Z.; Braima, A.; Liu, B.; Ma, Y.; Sun, H. Soil fungal community structure and function shift during a disease-driven forest succession. Microbiol. Spectr. 2022, 10, e0079522. [Google Scholar] [CrossRef]
- Tian, X.; Wang, D.; Mao, Z.; Pan, L.; Liao, J.; Cai, Z. Infection of Plasmodiophora brassicae changes the fungal endophyte community of tumourous stem mustard roots as revealed by high-throughput sequencing and culture-dependent methods. PLoS ONE 2019, 14, e214975. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Jin, Z.; Wang, Z.; Liao, Y.; Yu, L.; Li, X. Host plant selection imprints structure and assembly of fungal community along the soil-root continuum. mSystems 2022, 7, e0036122. [Google Scholar] [CrossRef]
- Melo, I.S.; Santos, S.N.; Rosa, L.H.; Parma, M.M.; Silva, L.J.; Queiroz, S.C.N.; Pellizari, V.H. Isolation and biological activities of an endophytic Mortierella alpina strain from the Antarctic moss Schistidium antarctici. Extremophiles 2014, 18, 15–23. [Google Scholar] [CrossRef]
- Xi, D.; Li, X.; Gao, L.; Zhang, Z.; Zhu, Y.; Zhu, H. Application of exogenous salicylic acid reduces disease severity of Plasmodiophora brassicae in pakchoi (Brassica campestris ssp. chinensis Makino). PLoS ONE 2021, 16, e248648. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
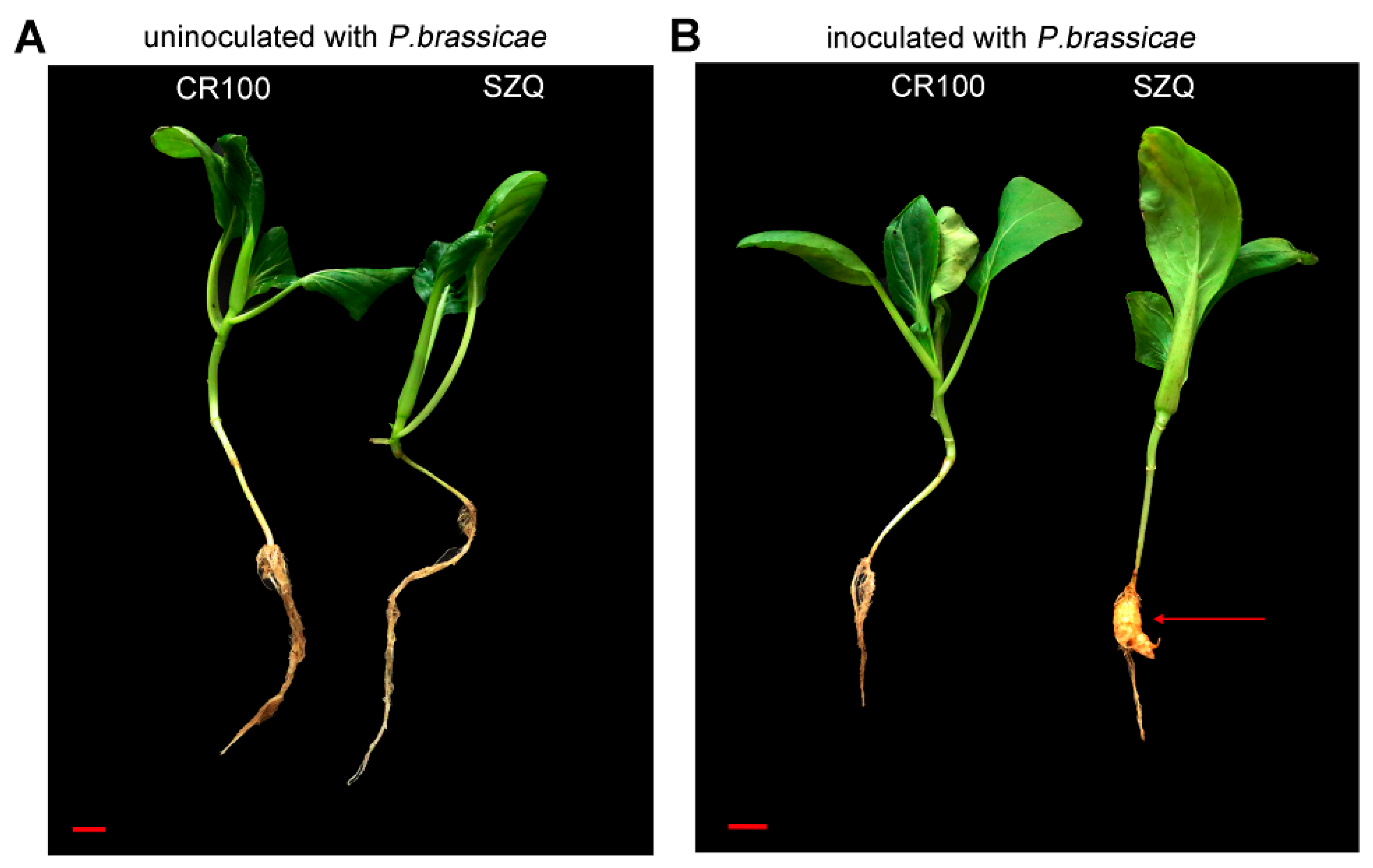
| Cultivar | Incidence | |
|---|---|---|
| Uninoculated with P. brassicae | Inoculated with P. brassicae | |
| CR100 | 0% | 0% |
| SZQ | 0% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, D.-D.; Gao, L.; Miao, L.-M.; Ge, L.-A.; Zhang, D.-Y.; Zhang, Z.-H.; Li, X.-F.; Zhu, Y.-Y.; Shen, H.-B.; Zhu, H.-F. Changes in Diversity and Composition of Rhizosphere Bacterial and Fungal Community between Resistant and Susceptible Pakchoi under Plasmodiophora brassicae. Int. J. Mol. Sci. 2023, 24, 16779. https://doi.org/10.3390/ijms242316779
Xi D-D, Gao L, Miao L-M, Ge L-A, Zhang D-Y, Zhang Z-H, Li X-F, Zhu Y-Y, Shen H-B, Zhu H-F. Changes in Diversity and Composition of Rhizosphere Bacterial and Fungal Community between Resistant and Susceptible Pakchoi under Plasmodiophora brassicae. International Journal of Molecular Sciences. 2023; 24(23):16779. https://doi.org/10.3390/ijms242316779
Chicago/Turabian StyleXi, Dan-Dan, Lu Gao, Li-Ming Miao, Li-Ao Ge, Ding-Yu Zhang, Zhao-Hui Zhang, Xiao-Feng Li, Yu-Ying Zhu, Hai-Bin Shen, and Hong-Fang Zhu. 2023. "Changes in Diversity and Composition of Rhizosphere Bacterial and Fungal Community between Resistant and Susceptible Pakchoi under Plasmodiophora brassicae" International Journal of Molecular Sciences 24, no. 23: 16779. https://doi.org/10.3390/ijms242316779
APA StyleXi, D.-D., Gao, L., Miao, L.-M., Ge, L.-A., Zhang, D.-Y., Zhang, Z.-H., Li, X.-F., Zhu, Y.-Y., Shen, H.-B., & Zhu, H.-F. (2023). Changes in Diversity and Composition of Rhizosphere Bacterial and Fungal Community between Resistant and Susceptible Pakchoi under Plasmodiophora brassicae. International Journal of Molecular Sciences, 24(23), 16779. https://doi.org/10.3390/ijms242316779






