CXCL12 Neutralizing Antibody Promotes Hair Growth in Androgenic Alopecia and Alopecia Areata
Abstract
1. Introduction
2. Results
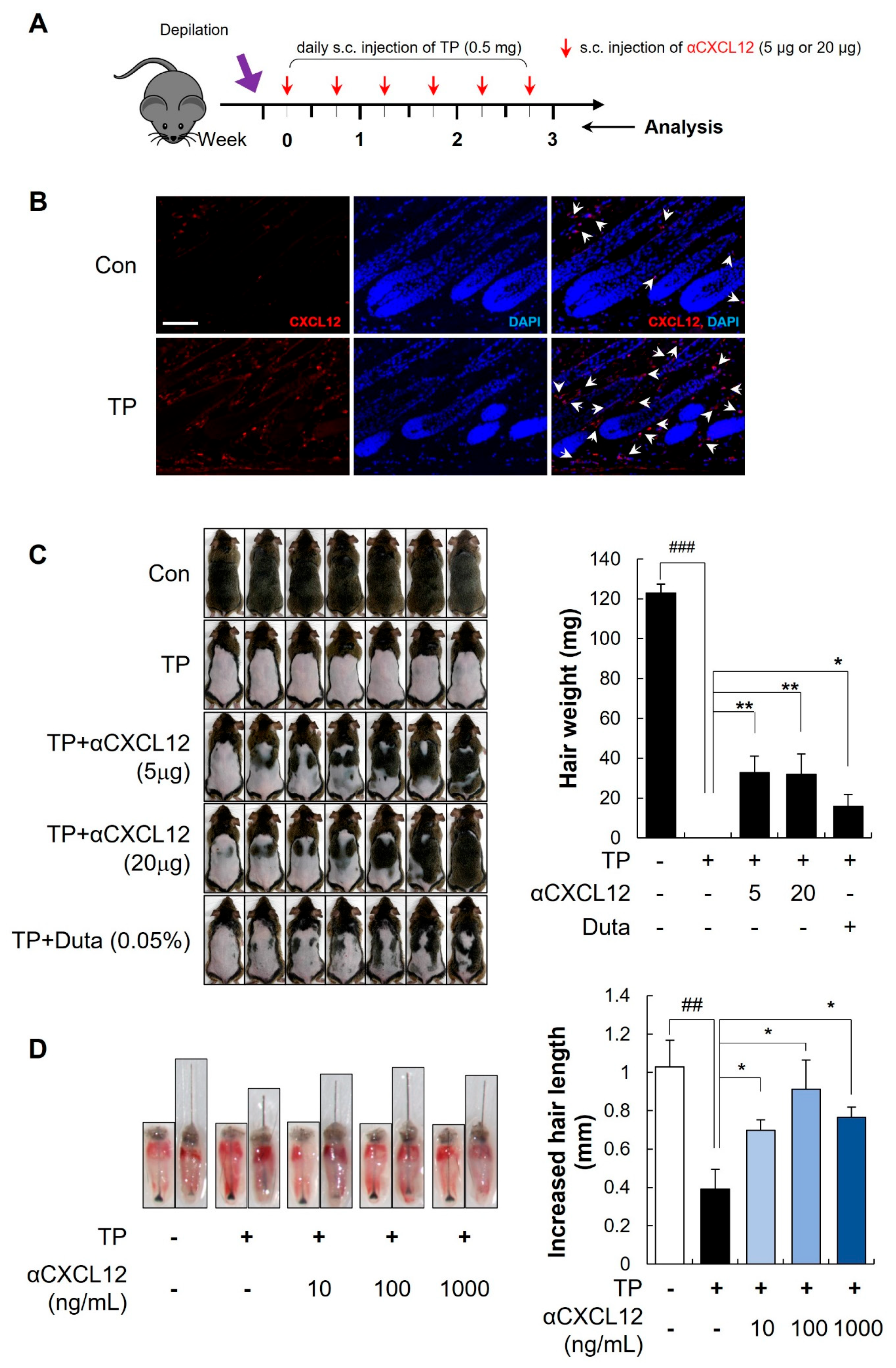
2.1. Hair Growth Promoting Effects of CXCL12 Neutralizing Antibody in Testosterone-Induced AGA Models
2.2. Hair-Growth-Promoting Effects of αCXCL12 in Dihydrotestosterone-Induced AGA Models
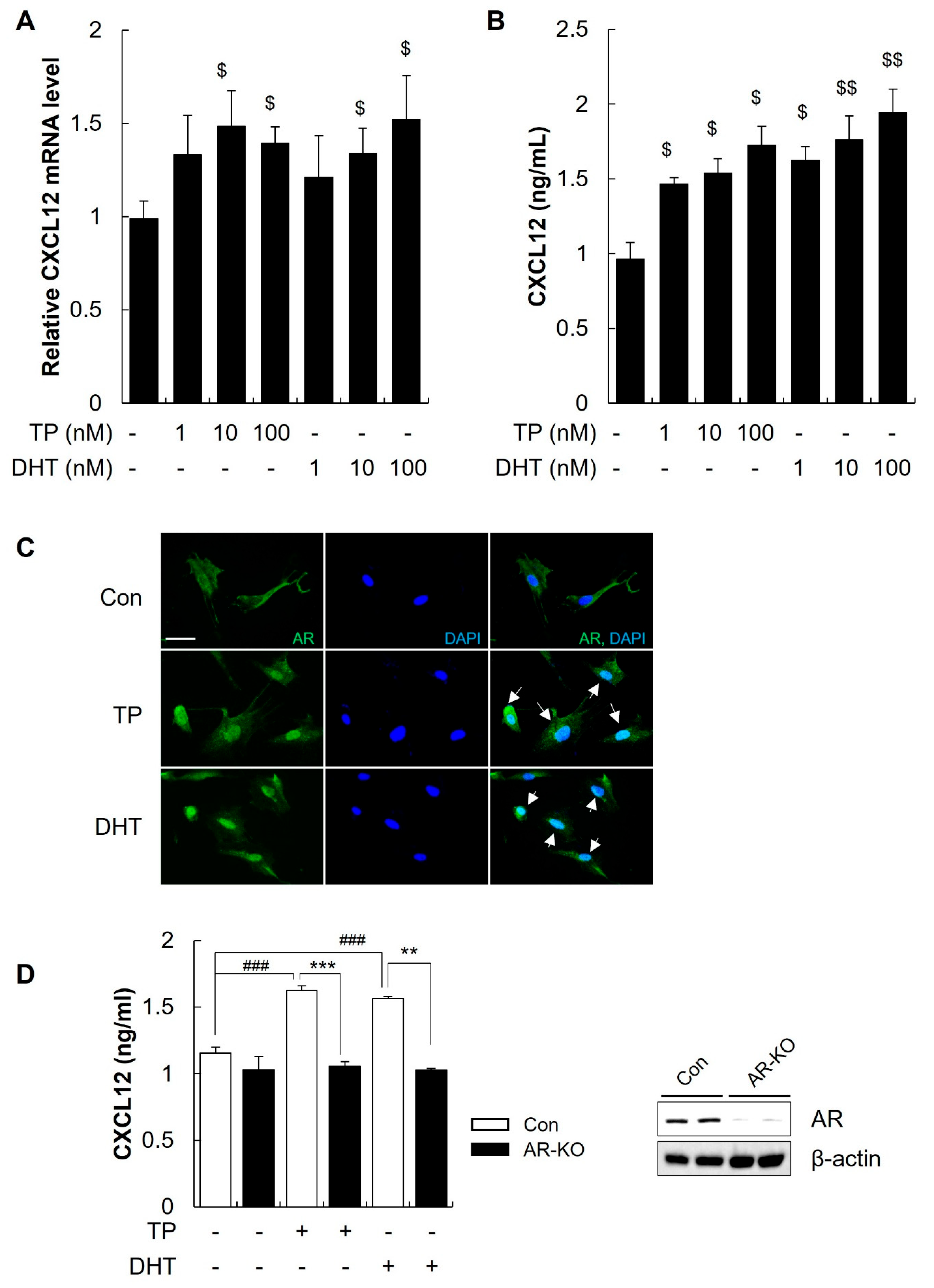
2.3. Androgens Increased the Secretion of CXCL12 from DFs through the AR
2.4. The CXCL12 Secreted from DFs Mediated the Hair Miniaturization Induced by Androgens
2.5. Increased Expression of CXCL12 in an AA Mouse Model
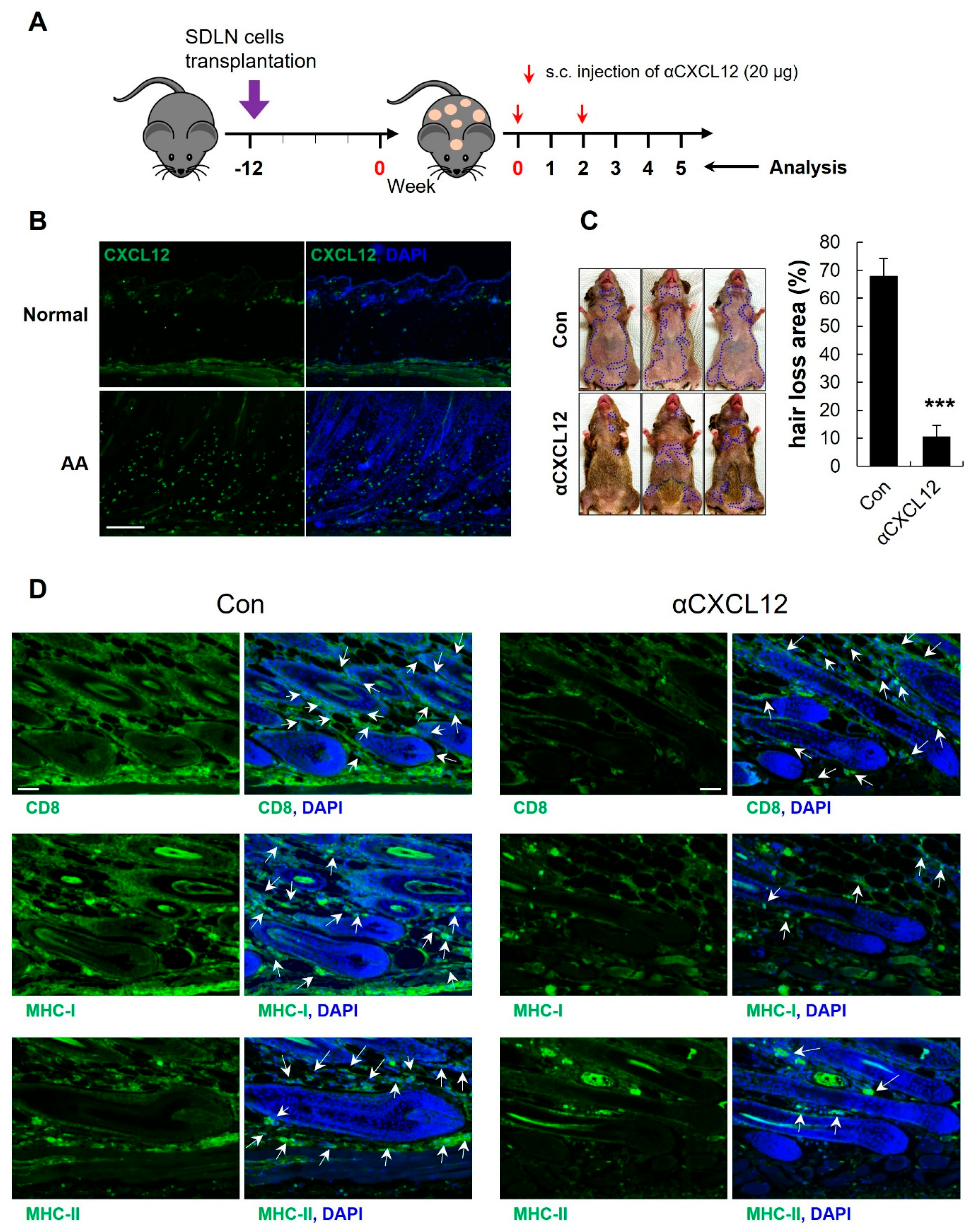
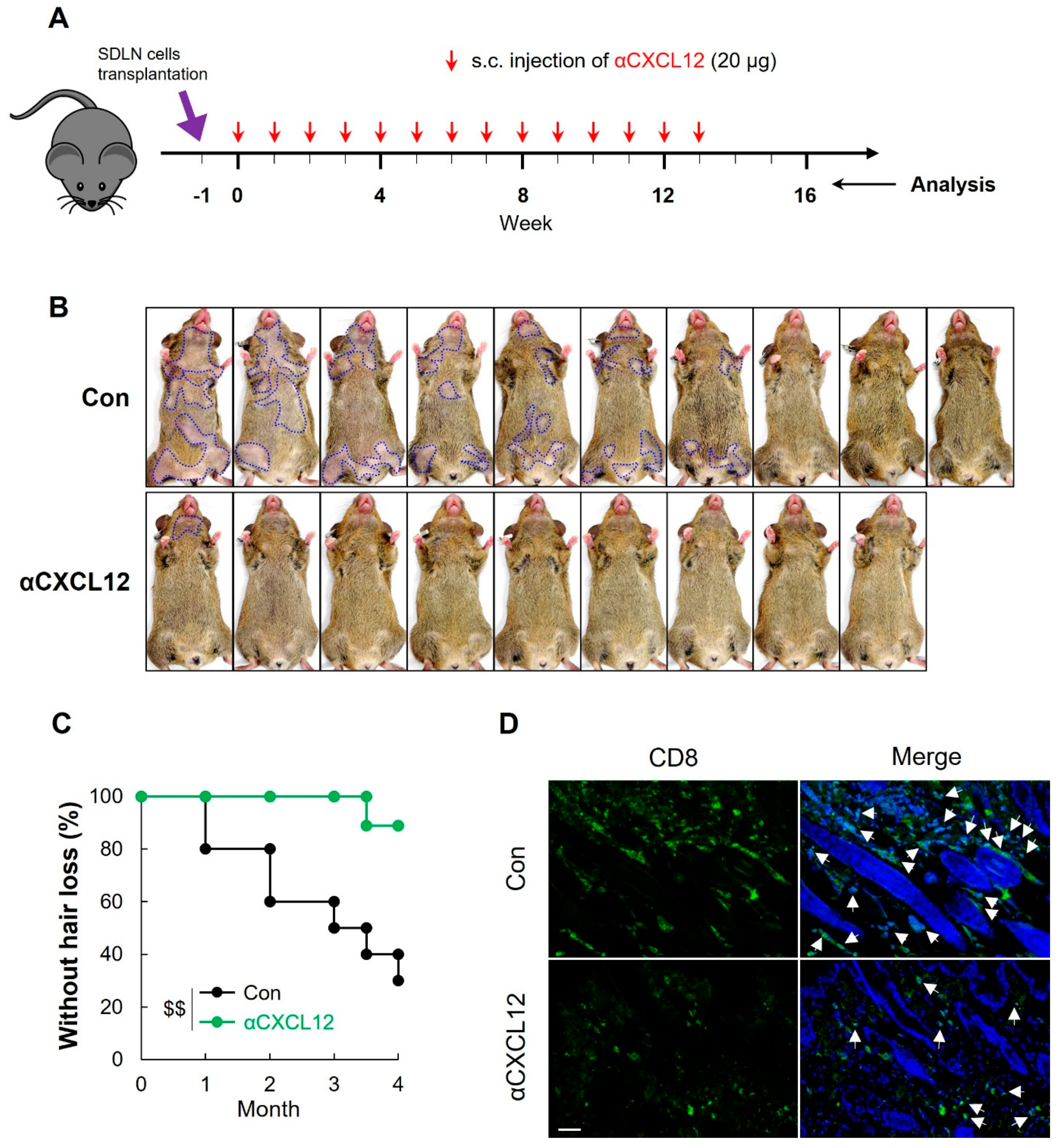
2.6. CXCL12 Neutralization Reversed AA in C3H Mice
2.7. CXCL12 Neutralization Prevented AA Onset in C3H Mice
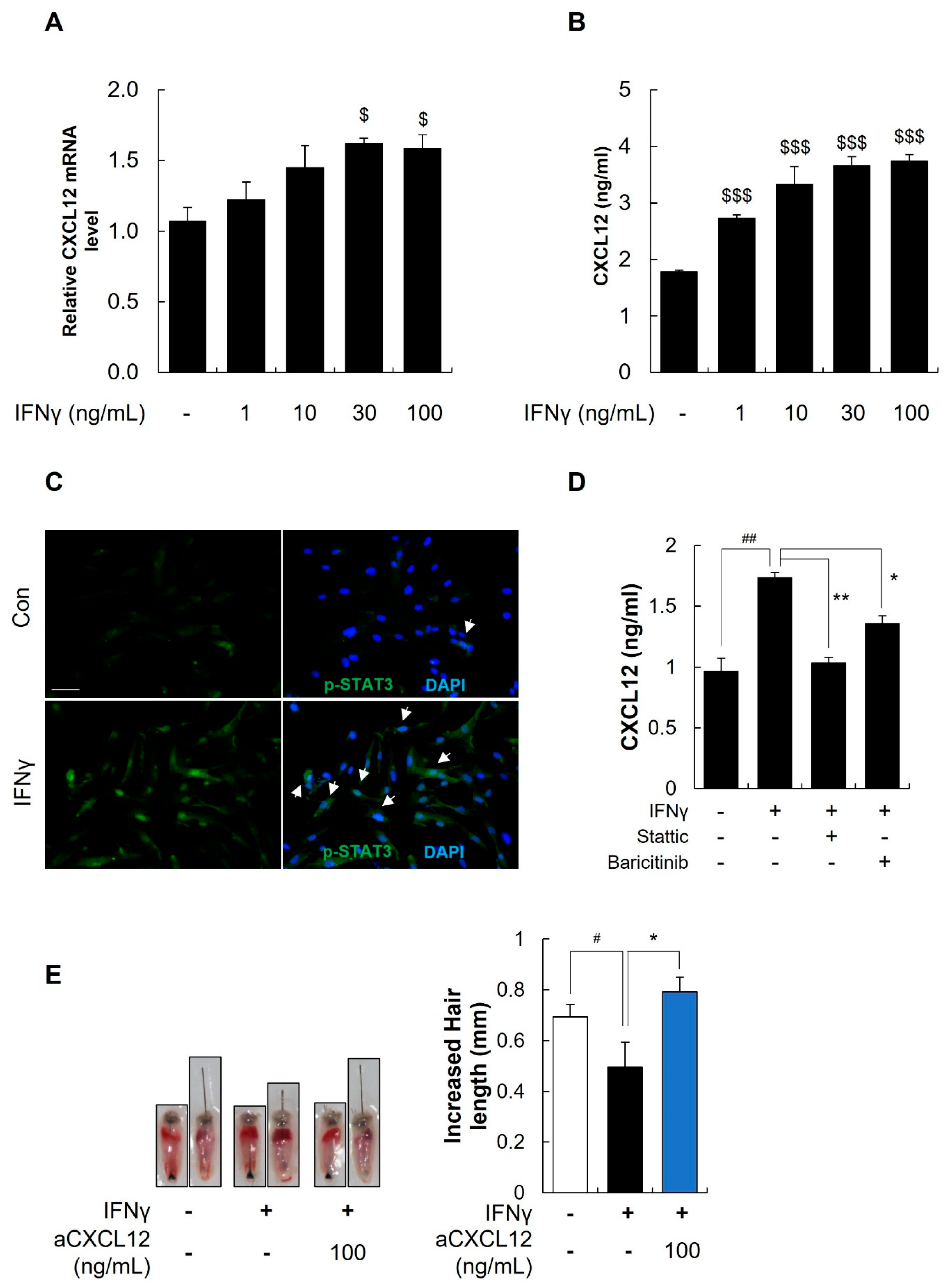
2.8. IFNγ Increased the Secretion of CXCL12 through the STAT3 Pathway
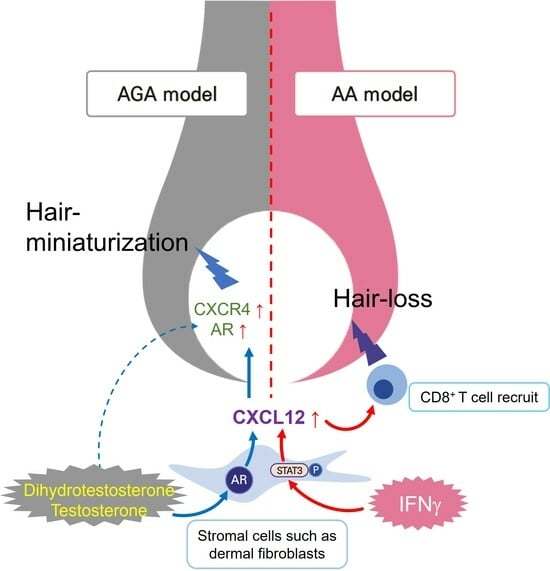
3. Discussion
4. Materials and Methods
4.1. Cell Isolation and Cell Culture
4.2. Preparation of the CXCL12 Neutralizing Antibody
4.3. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR) Assay
4.4. Western Blotting
4.5. Immunofluorescence Staining for Cells
4.6. siRNA Transfection
4.7. CRISPR/Cas9 Transfection
4.8. Measurement of CXCL12 via Enzyme-Linked Immunosorbent Assay
4.9. Animals
4.10. Hair Organ Culture
4.11. Androgenic Alopecia Animal Model
4.12. Alopecia Areata Animal Model
4.13. Immunofluorescence Staining for Paraffin Sections
4.14. Flow Cytometry Analysis
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Janssens, R.; Struyf, S.; Proost, P. Pathological roles of the homeostatic chemokine CXCL12. Cytokine Growth Factor Rev. 2018, 44, 51–68. [Google Scholar] [CrossRef]
- Zheng, M.; Oh, S.H.; Choi, N.; Choi, Y.J.; Kim, J.; Sung, J.-H. CXCL12 inhibits hair growth through CXCR4. Biomed. Pharmacother. 2022, 150, 112996. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.; Reygagne, P.; Benech, P.; Jean-Louis, F.; Scalvino, S.; Ly Ka So, S.; Hamidou, Z.; Bianovici, S.; Pouch, J.; Ducos, B.; et al. Study of gene expression alteration in male androgenetic alopecia: Evidence of predominant molecular signalling pathways. Br. J. Dermatol. 2017, 177, 1322–1336. [Google Scholar] [CrossRef] [PubMed]
- Chodari, L.; Mohammadi, M.; Ghorbanzadeh, V.; Dariushnejad, H.; Mohaddes, G. Testosterone and Voluntary Exercise Promote Angiogenesis in Hearts of Rats with Diabetes by Enhancing Expression of VEGF-A and SDF-1a. Can. J. Diabetes 2016, 40, 436–441. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, L.; Han, Y.; Teng, Y.; Sun, J.; Xie, R.; Cao, J. Testosterone replacement therapy promotes angiogenesis after acute myocardial infarction by enhancing expression of cytokines HIF-1a, SDF-1a and VEGF. Eur. J. Pharmacol. 2012, 684, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Azariadis, K.; Kiagiadaki, F.; Pelekanou, V.; Bempi, V.; Alexakis, K.; Kampa, M.; Tsapis, A.; Castanas, E.; Notas, G. Androgen Triggers the Pro-Migratory CXCL12/CXCR4 Axis in AR-Positive Breast Cancer Cell Lines: Underlying Mechanism and Possible Implications for the Use of Aromatase Inhibitors in Breast Cancer. Cell. Physiol. Biochem. 2017, 44, 66–84. [Google Scholar] [CrossRef]
- Garcia-Cuesta, E.M.; Santiago, C.A.; Vallejo-Diaz, J.; Juarranz, Y.; Rodriguez-Frade, J.M.; Mellado, M. The Role of the CXCL12/CXCR4/ACKR3 Axis in Autoimmune Diseases. Front. Endocrinol. 2019, 10, 585. [Google Scholar] [CrossRef]
- Uchida, Y.; Gherardini, J.; Schulte-Mecklenbeck, A.; Alam, M.; Cheret, J.; Rossi, A.; Kanekura, T.; Gross, C.C.; Arakawa, A.; Gilhar, A.; et al. Pro-inflammatory Vdelta1+T-cells infiltrates are present in and around the hair bulbs of non-lesional and lesional alopecia areata hair follicles. J. Dermatol. Sci. 2020, 100, 129–138. [Google Scholar] [CrossRef]
- Uchida, Y.; Gherardini, J.; Pappelbaum, K.; Cheret, J.; Schulte-Mecklenbeck, A.; Gross, C.C.; Strbo, N.; Gilhar, A.; Rossi, A.; Funk, W.; et al. Resident human dermal gammadeltaT-cells operate as stress-sentinels: Lessons from the hair follicle. J. Autoimmun. 2021, 124, 102711. [Google Scholar] [CrossRef]
- Kumar, A.; Humphreys, T.D.; Kremer, K.N.; Bramati, P.S.; Bradfield, L.; Edgar, C.E.; Hedin, K.E. CXCR4 Physically Associates with the T Cell Receptor to Signal in T Cells. Immunity 2006, 25, 213–224. [Google Scholar] [CrossRef]
- Suzuki, Y.; Rahman, M.; Mitsuya, H. Diverse transcriptional response of CD4+ T cells to stromal cell-derived factor SDF-1: Cell survival promotion and priming effects of SDF-1 on CD4+ T cells. J. Immunol. 2001, 167, 3064–3073. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Na, J.; Bak, D.H.; Lee, B.C.; Lee, E.; Choi, M.J.; Ryu, C.H.; Lee, S.; Mun, S.K.; Park, B.C.; et al. Development of finasteride polymer microspheres for systemic application in androgenic alopecia. Int. J. Mol. Med. 2019, 43, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Huang, J.; Li, K.; Chen, Y.; He, Y.; Sun, Y.; Guo, Y.; Du, L.; Qu, Q.; Miao, Y.; et al. Dihydrotestosterone-induced hair regrowth inhibition by activating androgen receptor in C57BL6 mice simulates androgenetic alopecia. Biomed. Pharmacother. 2021, 137, 111247. [Google Scholar] [CrossRef] [PubMed]
- Itami, S.; Sonoda, T.; Kurata, S.; Takayasu, S. Mechanism of action of androgen in hair follicles. J. Dermatol. Sci. 1994, 7 (Suppl. 1), S98–S103. [Google Scholar] [CrossRef] [PubMed]
- Gregoriou, S.; Papafragkaki, D.; Kontochristopoulos, G.; Rallis, E.; Kalogeromitros, D.; Rigopoulos, D. Cytokines and other mediators in alopecia areata. Mediat. Inflamm. 2010, 2010, 928030. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kageyama, R.; Nakazawa, S.; Honda, T. Understanding the significance of cytokines and chemokines in the pathogenesis of alopecia areata. Exp. Dermatol. 2020, 29, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Dai, Z.; Jabbari, A.; Cerise, J.E.; Higgins, C.A.; Gong, W.; de Jong, A.; Harel, S.; DeStefano, G.M.; Rothman, L.; et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 2014, 20, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Zgraggen, S.; Huggenberger, R.; Kerl, K.; Detmar, M. An important role of the SDF-1/CXCR4 axis in chronic skin inflammation. PLoS ONE 2014, 9, e93665. [Google Scholar] [CrossRef]
- Geherin, S.A.; Wilson, R.P.; Jennrich, S.; Debes, G.F. CXCR4 is dispensable for T cell egress from chronically inflamed skin via the afferent lymph. PLoS ONE 2014, 9, e95626. [Google Scholar] [CrossRef]
- Oliveira, C.; Coelho, C.; Teixeira, J.A.; Ferreira-Santos, P.; Botelho, C.M. Nanocarriers as Active Ingredients Enhancers in the Cosmetic Industry-The European and North America Regulation Challenges. Molecules 2022, 27, 1669. [Google Scholar] [CrossRef]
- Xu, S. Internalization, Trafficking, Intracellular Processing and Actions of Antibody-Drug Conjugates. Pharm. Res. 2015, 32, 3577–3583. [Google Scholar] [CrossRef]
- Liu, L. Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins. Protein Cell 2018, 9, 15–32. [Google Scholar] [CrossRef]
- Avniel, S.; Arik, Z.; Maly, A.; Sagie, A.; Basst, H.B.; Yahana, M.D.; Weiss, I.D.; Pal, B.; Wald, O.; Ad-El, D.; et al. Involvement of the CXCL12/CXCR4 pathway in the recovery of skin following burns. J. Investig. Dermatol. 2006, 126, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Chen, M.; Liu, F.; Wang, Y.; Xu, S.; Sha, K.; Peng, Q.; Wu, Z.; Xiao, W.; Liu, T.; et al. Androgen Receptor-Mediated Paracrine Signaling Induces Regression of Blood Vessels in the Dermal Papilla in Androgenetic Alopecia. J. Investig. Dermatol. 2022, 142, 2088–2099.e9. [Google Scholar] [CrossRef] [PubMed]
- Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Premature senescence of balding dermal papilla cells in vitro is associated with p16(INK4a) expression. J. Investig. Dermatol. 2008, 128, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Schwinge, D.; Carambia, A.; Quaas, A.; Krech, T.; Wegscheid, C.; Tiegs, G.; Prinz, I.; Lohse, A.W.; Herkel, J.; Schramm, C. Testosterone suppresses hepatic inflammation by the downregulation of IL-17, CXCL-9, and CXCL-10 in a mouse model of experimental acute cholangitis. J. Immunol. 2015, 194, 2522–2530. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Polesso, F.; Wang, C.; Sehrawat, A.; Hawkins, R.M.; Murray, S.E.; Thomas, G.V.; Caruso, B.; Thompson, R.F.; Wood, M.A.; et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature 2022, 606, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Plante, J.; Valdebran, M.; Forcucci, J.; Lucas, O.; Elston, D. Perifollicular inflammation and follicular spongiosis in androgenetic alopecia. J. Am. Acad. Dermatol. 2022, 86, 437–438. [Google Scholar] [CrossRef]
- Clocchiatti, A.; Ghosh, S.; Procopio, M.G.; Mazzeo, L.; Bordignon, P.; Ostano, P.; Goruppi, S.; Bottoni, G.; Katarkar, A.; Levesque, M.; et al. Androgen receptor functions as transcriptional repressor of cancer-associated fibroblast activation. J. Clin. Investig. 2018, 128, 5531–5548. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Hosokawa, I.; Ozaki, K.; Nakae, H.; Murakami, K.; Miyake, Y.; Matsuo, T. CXCL12 and CXCR4 expression by human gingival fibroblasts in periodontal disease. Clin. Exp. Immunol. 2005, 141, 467–474. [Google Scholar] [CrossRef]
- Li, Y.Y.; Li, H.; Liu, Z.L.; Li, Q.; Qiu, H.W.; Zeng, L.J.; Yang, W.; Zhang, X.Z.; Li, Z.Y. Activation of STAT3-mediated CXCL12 up-regulation in the dorsal root ganglion contributes to oxaliplatin-induced chronic pain. Mol. Pain 2017, 13, 1744806917747425. [Google Scholar] [CrossRef]
- Shen, H.B.; Gu, Z.Q.; Jian, K.; Qi, J. CXCR4-mediated Stat3 activation is essential for CXCL12-induced cell invasion in bladder cancer. Tumour Biol. 2013, 34, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Jindo, T.; Imai, R.; Takamori, K.; Ogawa, H. Organ culture of mouse vibrissal hair follicles in serum-free medium. J. Dermatol. 1993, 20, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Hye Kim, J.; Gyu Park, S.; Kim, W.K.; Song, S.U.; Sung, J.H. Functional regulation of adipose-derived stem cells by PDGF-D. Stem Cells 2015, 33, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Stenn, K.S.; Link, R.E. Telogen skin contains an inhibitor of hair growth. Br. J. Dermatol. 1990, 122, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.H.C.; Khosravi-Maharlooei, M.; Jalili, R.B.; Yu, R.; Ghahary, A.; Shapiro, J.; McElwee, K.J. Transfer of Alopecia Areata to C3H/HeJ Mice Using Cultured Lymph Node-Derived Cells. J. Investig. Dermatol. 2015, 135, 2530–2532. [Google Scholar] [CrossRef]
- Wang, E.H.C.; McElwee, K.J. Nonsurgical Induction of Alopecia Areata in C3H/HeJ Mice via Adoptive Transfer of Cultured Lymphoid Cells. Mol. Dermatol. Methods Protoc. 2020, 2154, 121–131. [Google Scholar]

| Antibodies | Source | Dilution (IF) | Dilution (WB) | Used to Identify |
|---|---|---|---|---|
| p-STAT3Tyr705 | Cell signaling; #9145T | 1:100 | DF cell | |
| AR | Santa cruz; sc-7305 | 1:100 | 1:1000 | DP, DF cell |
| CXCR4 | NOVUS; NB100-56437 | 1:1000 | DP cell | |
| CD8 | Santa cruz; sc-1177 | 1:100 | Mouse skin tissue | |
| MHC-I | Santa cruz; sc-55582 | 1:100 | Mouse skin tissue | |
| MHC-II | Santa cruz; sc-32247 | 1:100 | Mouse skin tissue | |
| CXCL12 | R&D systems; MAB350-SP | 1:100 | Mouse skin tissue |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Kim, M.-H.; Park, S.-G.; Kim, W.-S.; Oh, S.-H.; Sung, J.-H. CXCL12 Neutralizing Antibody Promotes Hair Growth in Androgenic Alopecia and Alopecia Areata. Int. J. Mol. Sci. 2024, 25, 1705. https://doi.org/10.3390/ijms25031705
Zheng M, Kim M-H, Park S-G, Kim W-S, Oh S-H, Sung J-H. CXCL12 Neutralizing Antibody Promotes Hair Growth in Androgenic Alopecia and Alopecia Areata. International Journal of Molecular Sciences. 2024; 25(3):1705. https://doi.org/10.3390/ijms25031705
Chicago/Turabian StyleZheng, Mei, Min-Ho Kim, Sang-Gyu Park, Won-Serk Kim, Sang-Ho Oh, and Jong-Hyuk Sung. 2024. "CXCL12 Neutralizing Antibody Promotes Hair Growth in Androgenic Alopecia and Alopecia Areata" International Journal of Molecular Sciences 25, no. 3: 1705. https://doi.org/10.3390/ijms25031705
APA StyleZheng, M., Kim, M.-H., Park, S.-G., Kim, W.-S., Oh, S.-H., & Sung, J.-H. (2024). CXCL12 Neutralizing Antibody Promotes Hair Growth in Androgenic Alopecia and Alopecia Areata. International Journal of Molecular Sciences, 25(3), 1705. https://doi.org/10.3390/ijms25031705