No Interference of H9 Extract on Trastuzumab Pharmacokinetics in Their Combinations
Abstract
1. Introduction
2. Results
2.1. Dilution Linearity for Trastuzumab
2.2. ELISA Validation
2.3. Stability Test
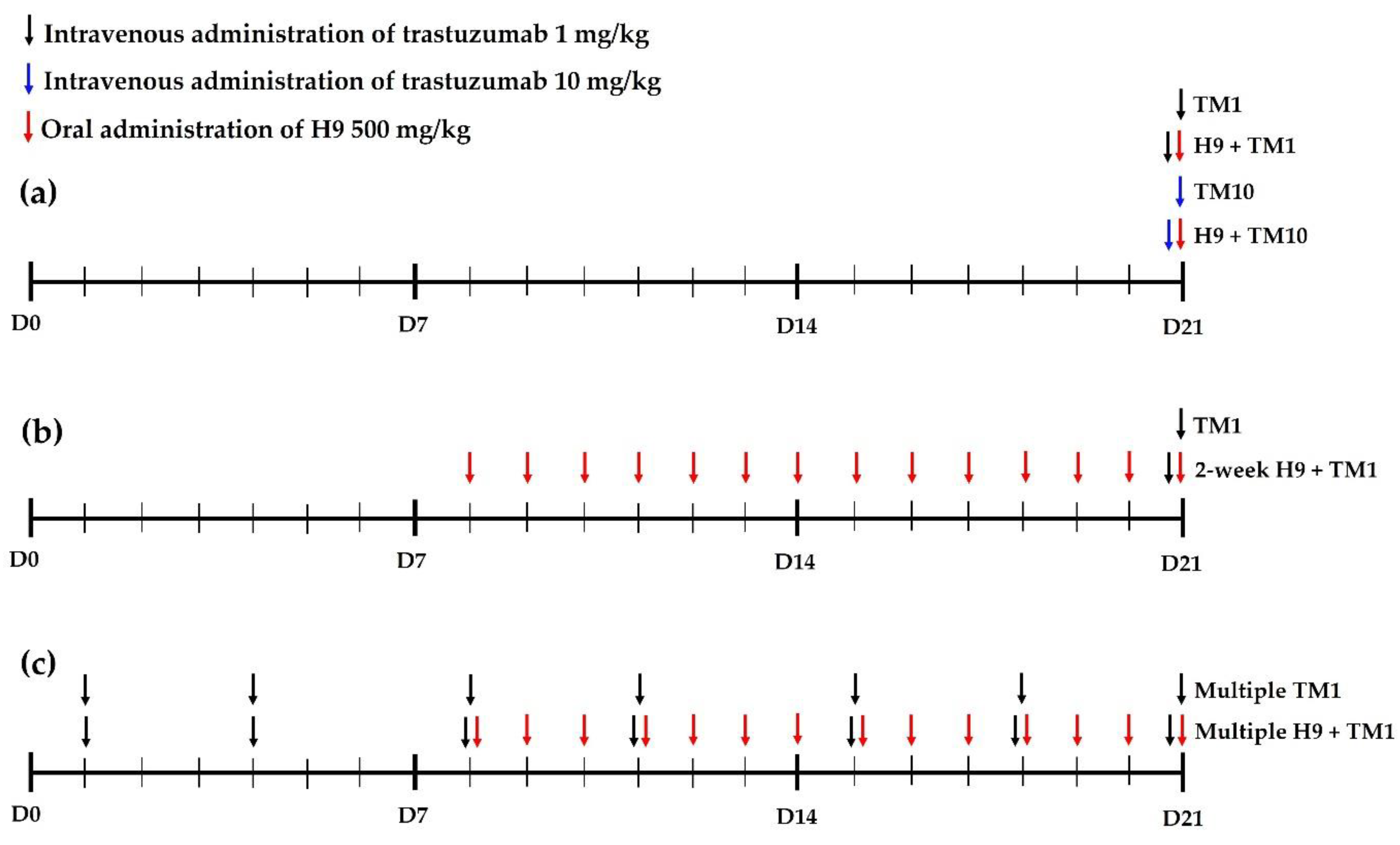
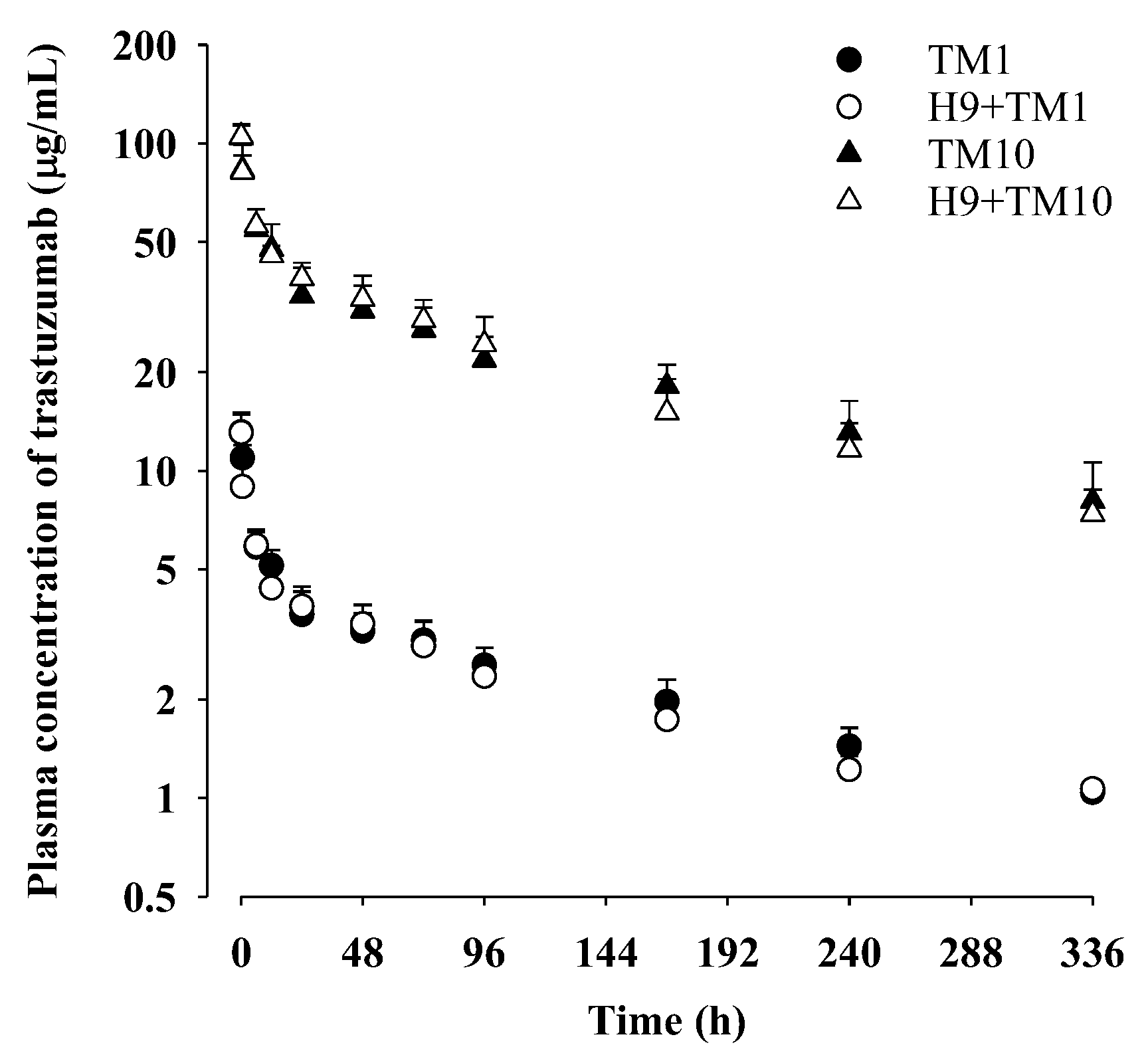
2.4. Trastuzumab Pharmacokinetics after Its Intravenous Administration (1 mg/kg) with and without Oral H9 (500 mg/kg) in Mice
2.5. Trastuzumab Pharmacokinetics after Its Intravenous Administration (10 mg/kg) with and without Oral H9 (500 mg/kg) in Mice
2.6. Trastuzumab Pharmacokinetics after Its Intravenous Administration (1 mg/kg) with and without 2-Week Treatment of Oral H9 (500 mg/kg) in Mice
2.7. Trastuzumab Pharmacokinetics after Multiple Intravenous Administration of Trastuzumab (1 mg/kg) with and without Oral H9 (500 mg/kg) in Mice
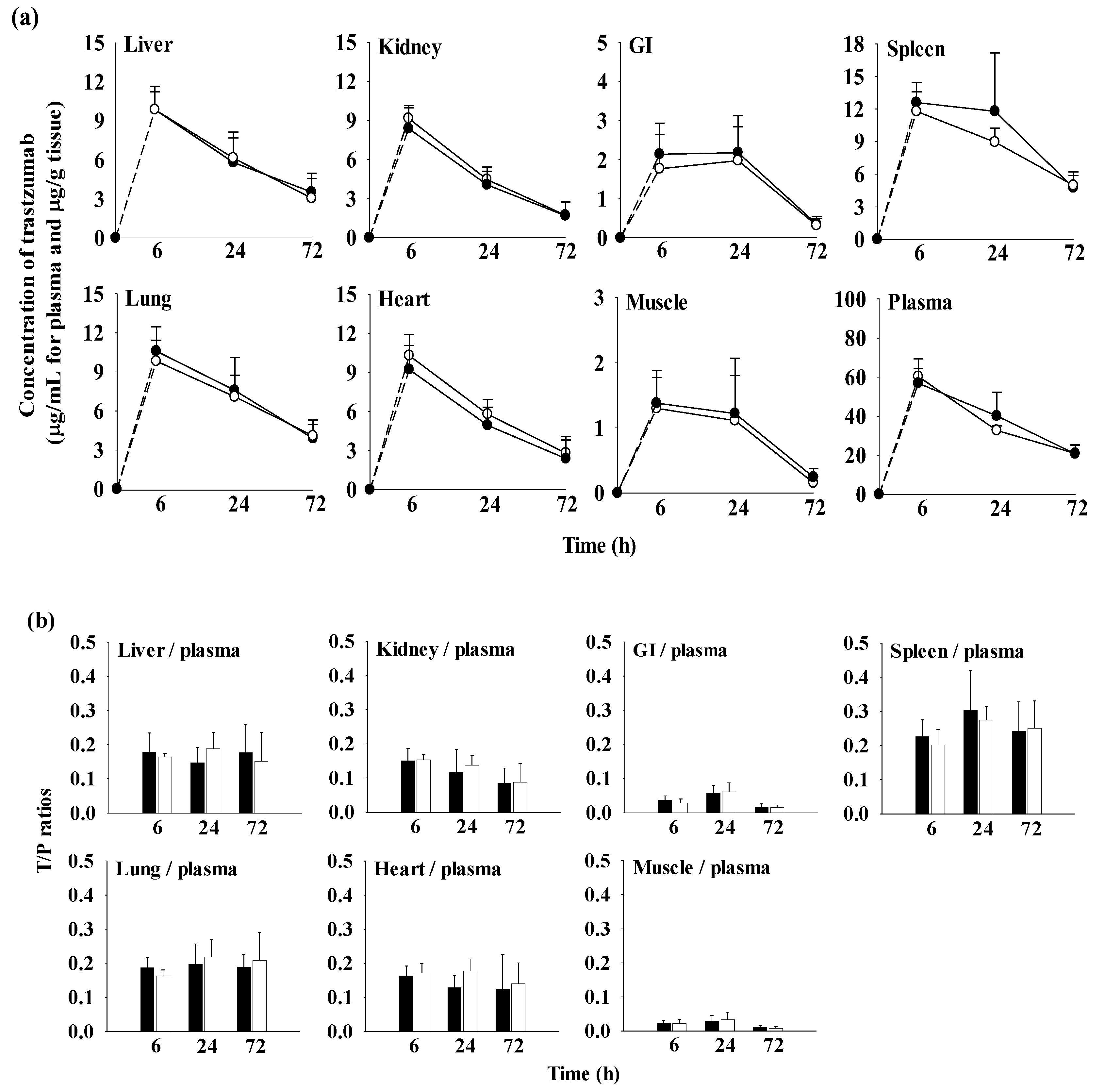
2.8. Tissue Distribution of Trastuzumab after Its Intravenous Administration (10 mg/kg) with and without Oral H9 (500 mg/kg) in Mice
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Calibration Standards and QCs
4.3. Sandwich ELISA Procedure and Assay Validation
4.4. Dilution Effect
4.5. Stability of Trastuzumab in Biological Samples
4.6. Animals
4.7. Trastuzumab Pharmacokinetics after Its Intravenous Administration (1 or 10 mg/kg) with and without Oral H9 (500 mg/kg) in Mice
4.8. Trastuzumab Pharmacokinetics after Its Intravenous Administration (1 mg/kg) with and without 2-Week Treatment of Oral H9 (500 mg/kg) in Mice
4.9. Trastuzumab Pharmacokinetics after Multiple Intravenous Administration of Trastuzumab (1 mg/kg) with and without Oral H9 (500 mg/kg) in Mice
4.10. Tissue Distribution of Trastuzumab after Its Intravenous Administration (10 mg/kg) with and without Oral H9 (500 mg/kg) in Mice
4.11. Pharmacokinetic Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeSantis, C.E.; Ma, J.; Gudet, M.M.; Newman, L.A.; Miller, K.D.; Goding, S.A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef] [PubMed]
- Seol, H.; Lee, J.H.; Choi, Y.; Lee, H.E.; Kim, Y.J.; Kim, J.H.; Kang, E.; Kim, S.W.; Park, S.Y. Intratumoral heterogeneity of HER2 gene amplification in breast cancer: Its clinicopathological significance. Mod. Pathol. 2012, 25, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Glöckner, S.; Buurman, H.; Kleeberger, W.; Lehmann, U.; Kreipe, H. Marked intratumoral heterogeneity of c-myc and CyclinD1 but not of c-erbB2 amplification in breast cancer. Lab. Investig. 2002, 82, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Baselga, J.; Gelmon, K.A.; Verma, S.; Wardley, A.; Conte, P.; Miles, D.; Bianchi, G.; Cortes, J.; McNally, V.A.; Ross, G.A.; et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J. Clin. Oncol. 2010, 28, 1138–1144. [Google Scholar] [CrossRef]
- McKeage, K.; Perry, C.M. Trastuzumab: A review of its use in the treatment of metastatic breast cancer overexpressing HER2. Drugs 2002, 62, 209–243. [Google Scholar] [CrossRef]
- Orman, J.S.; Perry, C.M. Trastuzumab: In HER2 and hormone receptor co-positive metastatic breast cancer. Drugs 2007, 67, 2781–2789. [Google Scholar] [CrossRef]
- Lin, M.; Xiong, W.; Wang, S.; Li, Y.; Hou, C.; Li, C.; Li, G. The research progress of trastuzumab-induced cardiotoxicity in HER-2-positive breast cancer treatment. Front. Cardiovasc. Med. 2022, 8, 821663. [Google Scholar] [CrossRef]
- Riccio, G.; Coppla, C.; Piscopo, G.; Capasso, I.; Maurea, C.; Esposito, E.; Lorenzo, C.D.; Maurea, N. Trastuzumab and target-therapy side effects: Is still valid to differentiate anthracycline Type I from Type II cardiomyopathies? Hum. Vaccin. Immunother. 2016, 12, 1124–1131. [Google Scholar] [CrossRef]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef]
- Chan, L.J.; Bulitta, J.B.; Ascher, D.B.; Haynes, J.M.; Mcleod, V.M.; Porter, C.J.; Wiliams, C.C.; Kaminskas, L.M. PEGylation does not significantly change the initial intravenous or subcutaneous pharmacokinetics or lymphatic exposure of trastuzumab in rats but increases plasma clearance after subcutaneous administration. Mol. Pharm. 2015, 12, 794–809. [Google Scholar] [CrossRef]
- Boekhout, A.H.; Beijnen, J.H.; Schellens Jan, H.M. Trastuzumab. Oncologist 2011, 16, 800–810. [Google Scholar] [CrossRef]
- Mohammadi, M.; Jeddi-Tehrani, M.; Golsaz-Shiraz, F.; Arjmand, M.; Bahadori, T.; Judaki, M.A.; Shiravi, F.; Zare, H.A.; Haghighat, F.N.; Mobini, M.; et al. A Novel anti-HER2 bispecific antibody with potent tumor inhibitory effects In Vitro and In Vivo. Front. Immunol. 2021, 17, 600883. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wu, J.; Chen, Y.; Nie, J.; Chen, C. Activation of PI3K/AKT/mTOR pathway causes drug resistance in breast cancer. Front. Pharmacol. 2021, 12, 628690. [Google Scholar] [CrossRef] [PubMed]
- Pernas, S.; Tolaney, S.M. HER2-positive breast cancer: New therapeutic frontiers and overcoming resistance. Ther. Adv. Med. Oncol. 2019, 11, 1758835919833519. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.; Schuller, K.; Tzeng, C.W.; Cannon, E.E.; Ku, B.C.; Howard, J.H.; Vickers, S.M.; Heslin, M.J.; Buchsbaum, D.J.; Arnoletti, J.P. ErbB3 expression and dimerization with EGFR influence pancreatic cancer cell sensitivity to erlotinib. Cancer Biol. Ther. 2007, 6, 548–554. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Xu, Y.; Ding, Y.; Li, C.; Zhao, H.; Wang, J. Posttranscriptional upregulation of HER3 by HER2 mRNA induces trastuzumab resistance in breast cancer. Mol. Cancer 2018, 17, 113. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wu, L.; Liang, K.; Liu, B.; Lu, Y.; Fan, Z. Roles of the PI-3K and MEK pathways in Ras-mediated chemoresistance in breast cancer cells. Br. J. Cancer 2003, 89, 185–191. [Google Scholar] [CrossRef]
- Lee, J.H.; Son, Y.H.; Kwon, Y.W.; Park, S.Y.; Koo, B.S.; Jung, S.H. Anticancer effects of a Korean herbal medicine formula (H9) via AMPK and HER2-PI3K/Akt signaling in breast cancer cells. Phytother. Res. 2017, 31, 1765–1775. [Google Scholar] [CrossRef]
- Bergamini, C.; Torelli, F.; Ghiselli, L.; Rossi, A.; Trevisani, L.; Vinco, G. Left ventricular end-diastolic volume as early indicator of trastuzumab-related cardiotoxicity in HER2+ breast cancer patients: Results from a single-center retrospective study. Minerva. Cardioangiol. 2017, 65, 278–287. [Google Scholar] [CrossRef]
- Marquette, C.; Nabell, L. Chemotherapy-resistant metastatic breast cancer. Curr. Treat. Options. Oncol. 2012, 2, 263–275. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y.; Wang, H.C. Adjuvant concomitant treatment with traditional Chinese medicines in patients receiving chemotherapy for HER2-positive breast cancer: A pilot randomized controlled trial. Complement. Ther. Clin. Pract. 2021, 43, 101373. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, M.; Du, K.; Ma, C.; Cheng, Y.; Wang, S.; Nie, X.; Fu, C.; He, Y. Traditional Chinese medicine for adjuvant treatment of breast cancer: Taohong siwu decoction. Chin. Med. 2021, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.M.; Hsu, H.Y. Fucose-containing fraction of Ling-Zhi enhances lipid rafts dependent ubiquitination of TGFβ receptor degradation and attenuates breast cancer tumorigenesis. Sci. Rep. 2016, 6, 36563. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Song, A.N.; An, J.H.; Kim, E.H.; Park, S.J.; Kim, K.S.; Lee, S. A Case Study of Soeumin with peripheral T-cell lymphoma who showed symptomatic improvement including fever, myalgia, performance status, and headache after treated with Osuyubujaijung-tang and Geopoong-san. J. Sasang Constitut. Med. 2012, 24, 100–108. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, K.Y.; Won, K.S. Study in the hepatoprotective effect of Sipymiguanjung-tang and Osuyubujaijung-tang. J. Sasang Constitut. Med. 2003, 15, 90–108. [Google Scholar]
- Kwon, S.; Kim, M.; Ham, S.Y.; Park, G.W.; Choi, K.; Jung, S.H.; Yoon, D. H9 induces apoptosis via the intrinsic pathway in non-small-cell lung cancer A549 cells. J. Microbiol. Biotechnol. 2015, 25, 343–352. [Google Scholar] [CrossRef]
- Guo, J.; Wu, H.; Weng, X.; Yan, J.; Bi, K. Studies on extraction and isolation of active constituents from Psoralen corylifolia L. and the antitumor effect of the constituents in vitro. Zhong Yao Cai 2003, 26, 185–187. [Google Scholar] [PubMed]
- Zhang, W.; Li, Z.; Zhou, M.; Wu, F.; Hou, X.; Luo, H.; Liu, H.; Han, X.; Yan, G.; Ding, Z.; et al. Synthesis and biological evaluation of 4-(1,2,3-triazol-1-yl)coumarin derivatives as potential antitumor agents. Bioorg. Med. Chem. Lett. 2014, 24, 799–807. [Google Scholar] [CrossRef]
- Kim, M.J.; Kwon, S.B.; Ham, S.H.; Jeong, E.S.; Choi, Y.K.; Choi, K.D.; Hong, J.T.; Jung, S.H.; Yoon, D.Y. H9 inhibits tumor growth and induces apoptosis via intrinsic and extrinsic signaling pathway in human non-small cell lung cancer xenografts. J. Microbiol. Biotechnol. 2015, 25, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Han, S.; Jeong, A.L.; Park, J.S.; Jung, S.H.; Choi, K.D.; Yang, Y. Combined treatment of herbal mixture extract H9 with trastuzumab enhances anti-tumor growth effect. J. Microbiol. Biotechnol. 2015, 25, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, T.Z.; Woo, J.; Shang, X.; Park, B.H.; Gabrielson, E. AMP-activated kinase (AMPK) regulates activity of HER2 and EGFR in breast cancer. Oncotarget 2015, 6, 14754–14765. [Google Scholar] [CrossRef]
- Liu, W.; Pan, H.; Yang, L.; Zhao, Z.; Yuan, D.; Liu, Y.; Lin, L. Panax ginseng C.A. Meyer (Rg3) Ameliorates Gastric Precancerous Lesions in Atp4a-/- Mice via inhibition of glycolysis through PI3K/AKT/miRNA-21 pathway. Evid.-Based Complement. Altern. Med. 2020, 31, 2672648. [Google Scholar] [CrossRef] [PubMed]
- Gundewar, S.; Calvert, J.W.; Jha, S.; Toedt-Pingel, I.; Ji, S.Y.; Nunez, D. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ. Res. 2009, 104, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Kitani, T.; Ong, S.G.; Lam, C.K.; Rhee, J.W.; Zhang, J.Z.; Oikonomopoulos, A. Human-induced pluripotent stem cell model of trastuzumab-induced cardiac dysfunction in patients with breast cancer. Circulation 2019, 139, 2451–2465. [Google Scholar] [CrossRef]
- Choi, Y.H.; Zhang, C.; Liu, Z.; Tu, M.J.; Yu, A.X.; Yu, A.M. A novel integrated pharmacokinetic-pharmacodynamic model to evaluate combination therapy and determine in vivo synergisms. J. Pharmacol. Exp. Ther. 2021, 377, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. The combination index (CI < 1) as the definition of synergism and of synergy claims. Synergy 2018, 7, 49–50. [Google Scholar]
- Choi, Y.H. Interpretation of drug interaction using systemic and local tissue exposure changes. Pharmaceutics 2020, 12, 417. [Google Scholar] [CrossRef]
- Lee, C.W.; You, B.H.; Yim, S.; Han, S.Y.; Chae, H.S.; Bae, M.; Kim, S.Y.; Yu, J.E.; Jung, J.; Nhoek, P.; et al. Change of metformin concentrations in the liver as a pharmacological target site of metformin after long-term combined treatment with ginseng berry extract. Front. Pharmacol. 2023, 14, 1148155. [Google Scholar] [CrossRef]
- US FDA. Guidance for Industry: Bioanalytical Method Validation. May 2018. Available online: https://www.fda.gov/media/70858/download (accessed on 8 June 2023).
- Davies, B.; Morris, T. Physiological parameters in laboratory animals and humans. Pharm. Res. 1993, 10, 1093–1095. [Google Scholar] [CrossRef]
- Altman, P.L.; Katz, D.D. Blood and Other Body Fluids, 1st ed.; Federation of American Societies for Experimental Biology: Washington, DC, USA, 1961. [Google Scholar]
- Carling, D. AMPK signaling in health and disease. Curr. Opin. Cell. Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef]
- Grimstein, M.; Huang, S. A regulatory science viewpoint on botanical-drug interactions. J. Food Drug Anal. 2018, 2, S12–S25. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, Z.; Tay-Sontheimer, J.; Levy, L.H.; Ragueneau-Majlessi, I. Intestinal Drug Interactions Mediated by OATPs: A Systematic Review of Preclinical and Clinical Findings. J. Pharm. Sci. 2017, 106, 2312–2325. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Chae, H.; You, B.H.; Chin, Y.; Kim, H.; Choi, H.S.; Choi, Y.H. Lonicera japonica extract increases metformin distribution in the liver without change of systemic exposed metformin in rats. J. Ethnopharmacol. 2019, 238, 111892. [Google Scholar] [CrossRef] [PubMed]
- You, B.H.; Chin, Y.; Kim, H.; Choi, H.S.; Choi, Y.H. Houttuynia cordata extract increased systemic exposure and liver concentrations of metformin through OCTs and MATEs in rats. Phytother Res. 2018, 32, 1004–1013. [Google Scholar] [CrossRef]
- Yim, S.; You, B.H.; Chae, H.; Chin, Y.; Kim, H.; Choi, H.S.; Choi, Y.H. Multidrug and toxin extrusion protein 1-mediated interaction of metformin and Scutellariae radix in rats. Xenobiotica 2017, 47, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Damen, C.W.N.; de Groot, E.R.; Heij, M.; Boss, D.S.; Schellens Jan, H.M.; Rosing, H.; Beijnen, J.H.; Aarden, L.A. Development and validation of an enzyme-linked immunosorbent assay for the quantification of trastuzumab in human serum and plasma. Anal. Biochem. 2009, 391, 114–120. [Google Scholar] [CrossRef]
- Maple, L.; Lathrop, R.; Bozich, S.; Harman, W.; Tacey, R.; Kelley, M.; Danilkovitch Miagkova, A. Development and validation of ELISA for herceptin detection in human serum. J. Immunol. Methods. 2004, 295, 169–182. [Google Scholar] [CrossRef]
- Sakamoto, S.; Putalun, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. J. Nat. Med. 2018, 72, 32–42. [Google Scholar] [CrossRef]
- Tran, T.N.; Schimenti, J.C. A putative human infertility allele of the meiotic recombinase DMC1 does not affect fertility in mice. Hum. Mol. Genet. 2018, 27, 3911–3918. [Google Scholar] [CrossRef]
- Bryda, E.C. The mighty mouse: The impact of rodents on advances in biomedical research. Mo. Med. 2013, 110, 207–211. [Google Scholar]
- Phillips, G.L.; Guo, J.; Kiefer, J.R.; Proctor, W.; Yadav, D.B.; Dybdl, N.; Shen, B.Q. Trastuzumab does not bind rat or mouse ErbB2/neu: Implications for selection of non-clinical safety models for trastuzumab-based therapeutics. Breast. Cancer Res. Treat. 2022, 191, 303–317. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Morris, M.E. A Physiologically based pharmacokinetic model of mitoxantrone in mice and scale-up to humans: A semi-mechanistic model incorporating DNA and protein binding. AAPS. J. 2012, 14, 352–364. [Google Scholar] [CrossRef]
- Choi, Y.H.; Han, S.Y.; Kim, Y.J.; Kim, Y.M.; Chin, Y.W. Absorption, tissue distribution, tissue metabolism and safety of α-mangostin in mangosteen extract using mouse models. Food. Chem. Toxicol. 2014, 66, 140–146. [Google Scholar] [CrossRef]
- Bialer, M. Pharmacodynamic and pharmacokinetic characteristics of intravenous drugs in status epilepticus. Epilepsia 2009, 12, 44–48. [Google Scholar] [CrossRef]
- Levêque, D.; Gigou, L.; Bergerat, J.P. Clinical pharmacology of trastuzumab. Curr. Clin. Pharmacol. 2008, 3, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.; Washington, C.B.; Lu, J.F.; Lieberman, G.; Banken, L.; Klein, P. Population pharmacokinetics of trastuzumab in patients with HER2+ metastatic breast cancer. Cancer Chemother. Pharmacol. 2005, 56, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Levêque, D.; Wisniewski, S.; Jehl, F. Pharmacokinetics of therapeutic monoclonal antibodies used in oncology. Anticancer Res. 2005, 25, 2327–2344. [Google Scholar]
- Less, J.R.; Posner, M.C.; Boucher, Y.; Borochovitz, D.; Wolmark, N.; Jain, R.K. Interstitial hypertension in human breast and colorectal tumors. Cancer Res. 1992, 52, 6371–6374. [Google Scholar]
- Juweid, M.; Neumann, R.; Paik, C.; Perez-Bacete, M.J.; Sato, J.; Osdol, W.V.; Weinstein, J.N. Micropharmacology of monoclonal antibodies in solid tumors: Direct experimental evidence for a binding site barrier. Cancer Res. 1992, 52, 5144–5153. [Google Scholar]
- Netti, P.A.; Hamberg, L.M.; Babich, J.W.; Kierstead, D.; Graham, W.; Wolf, G.L.; Fischman, A.; Boucher, Y.; Jain, R.K. Enhancement of fluid filtration across tumor vessels: Implication for delivery of macromolecules. Proc. Natl. Acad. Sci. USA 1999, 96, 3137–3142. [Google Scholar] [CrossRef]
- Dellian, M.; Yuan, F.; Trubetskoy, V.S.; Torchilin, V.P.; Jain, R.K. Vascular permeability in a human tumour xenograft: Molecular charge dependence. Br. J. Cancer 2000, 82, 1513–1518. [Google Scholar] [PubMed]
- Padera, T.P.; Stoll, B.R.; Tooredman, J.B.; Capen, D.; Tomaso, E.D.; Kain, R.K. Pathology: Cancer cells compress intratumoral vessels. Nature 2004, 427, 695. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, J.; Rajasekaran, A.K. Biological impediments to monoclonal antibody-based cancer immunotherapy. Mol. Cancer Ther. 2004, 3, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Wiig, H.; Gyenge, C.C.; Tenstad, O. The interstitial distribution of macromolecules in rat tumours is influenced by the negatively charged matrix components. J. Physiol. 2005, 567, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Jeong, Y.H.; Hwang, J.A.; Lee, J.J.; Yu, A.R.; Kang, J.A.; Raza, A.M.; Park, S.H.; Park, Y.H.; Kim, D.H. Radioisotope-ADME studies of Trastuzumab-monomethyl auristatin F in tumor bearing mice and healthy marmosets. J. Biomed. Transl. Res. 2020, 21, 79–90. [Google Scholar] [CrossRef]
- Lencer, W.I.; Blumberg, R.S. A passionate kiss, then run: Exocytosis and recycling of IgG by FcRn. Trends. Cell. Biol. 2005, 15, 5–9. [Google Scholar] [CrossRef]
- Wani, M.A.; Haynes, L.D.; Kim, J.; Bronson, C.L.; Chaudhury, C.; Mohanty, S.; Waldmann, T.A.; Robinsom, J.M.; Anderson, C.L. Familial hypercatabolic hypoproteinemia caused by deficiency of the neonatal Fc receptor, FcRn, due to a mutant beta2-microglobulin gene. Proc. Natl. Acad. Sci. USA 2006, 103, 5084–5089. [Google Scholar] [CrossRef]
- Kim, J.; Hayton, W.L.; Robinson, J.M.; Anderson, C.L. Kinetics of FcRn mediated recycling of IgG and albumin in human: Pathophysiology and therapeutic implications using a simplified mechanism-based model. Clin. Immunol. 2007, 122, 146–155. [Google Scholar] [CrossRef]
- Ryman, J.T.; Meibohm, B. Pharmacokinetics of Monoclonal Antibodies. CPT Pharmacometrics Syst Pharmacol. 2017, 6, 576–588. [Google Scholar] [CrossRef]
- Burnett, J.P.; Korkaya, H.; Ouzounova, M.D.; Jiang, H.; Conley, S.J.; Newman, B.W.; Sun, L.; Connarn, J.N.; Chen, C.S.; Zhang, N.; et al. Trastuzumab resistance induces EMT to transform HER2(+) PTEN(-) to a triple negative breast cancer that requires unique treatment options. Sci. Rep. 2015, 2, 15821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hop, C.E.; Patilea-Vrana, G.; Gampa, G.; Seneviratne, H.K.; Unadkat, J.D.; Kenny, J.R.; Nagapudi, K.; Di, L.; Zhou, L.; et al. Drug concentration asymmetry in tissues and plasma for small molecule–related therapeutic modalities. Drug. Metab. Dispos. 2019, 47, 1122–1135. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Benet, L.Z. The operational multiple dosing half-life: A key to defining drug accumulation in patients and to designing extended release dosage forms. Pharm. Res. 2008, 25, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Rossum, J.M.V. Pharmacokinetics of accumulation. J. Pharm. Sci. 1968, 57, 2162–2165. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.E.; Byon, W.; Song, Y.; Wang, J.; Schuster, A.E.; Boyd, R.A.; Zhang, D.; Yu, Z.; Dias, C.; Shenker, A.; et al. Effect of ketoconazole and diltiazem on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor. Br. J. Clin. Pharmacol. 2015, 79, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Rengelshausen, J.; Göggelmann, C.; Burhenne, J.; Riedel, K.; Ludwig, J.; Weiss, J.; Mikus, G.; Walter-Sack, I.; Haefeli, W.E. Contribution of increased oral bioavailability and reduced nonglomerular renal clearance of digoxin to the digoxin-clarithromycin interaction. Br. J. Clin. Pharmacol. 2003, 56, 32–38. [Google Scholar] [CrossRef]
- Asberg, A.; Hartmann, A.; Fjeldså, E.; Bergan, S.; Holdaas, H. Bilateral pharmacokinetic interaction between cyclosporine A and atorvastatin in renal transplant recipients. Am. J. Transplant. 2001, 1, 382–386. [Google Scholar] [CrossRef]
- Eisenmann, E.D.; Talebi, Z.; Sparreboom, A.; Baker, S.D. Boosting the oral bioavailability of anticancer drugs through intentional drug-drug interactions. Basic Clin. Pharmacol. Toxicol. 2022, 130 (Suppl. S1), 23–35. [Google Scholar] [CrossRef]
- Ismail, M.; Khan, S.; Khan, F.; Noor, S.; Sajid, H.; Yar, S.; Rasheed, I. Prevalence and significance of potential drug-drug interactions among cancer patients receiving chemotherapy. BMC Cancer 2020, 20, 335. [Google Scholar] [CrossRef]
- Choi, W.K.; Yoon, K.D.; Lee, J.K.; Park, J.B.; Heo, T.H.; Lee, C.; Bae, S.K. Development and validation of a liquid chromatography with tandem mass spectrometry method for the quantification of vitisin B in rat plasma and urine. J. Sep. Sci. 2015, 38, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Diehl, K.H.; Hull, R.; Morton, D.; Pfister, R.; Rabemampianina, Y.; Smith, D.; Vidal, J.M.; Vorstenbosch, C.V.D. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 2001, 21, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Florida Atlantic University Institutional Animal Care and Use Committee (FAU IACUC). Guidelines for Rodent Survival Blood Collection; Florida Atlantic University: Boca Raton, FL, USA, 2021. [Google Scholar]
- Golde, W.T.; Gollobin, P.; Rodriguez, L.L. A rapid, simple, and human method for submandibular bleeding of mice using a lancet. Lab. Anim. 2005, 34, 39–43. [Google Scholar] [CrossRef] [PubMed]
- You, B.H.; BasavanaGowda, M.K.; Lee, J.U.; Chin, Y.; Choi, W.J.; Choi, Y.H. Pharmacokinetic properties of moracin C in mice. Planta Med. 2021, 87, 642–651. [Google Scholar] [CrossRef]


| Dilution Factors | 1:40 | 1:20 | 1:10 | 1:2 | ||||
|---|---|---|---|---|---|---|---|---|
| Nominal Concentration (μg/mL) | 0.100 | 1.00 | 0.100 | 1.00 | 0.100 | 1.00 | 0.100 | 1.00 |
| Plasma | ||||||||
| Measured concentration (μg/mL) | 0.0992 | 1.05 | 0.104 | 1.036 | 0.100 | 0.972 | 0.103 | 0.970 |
| SD (μg/mL) | 0.00273 | 0.0938 | 0.00547 | 0.0751 | 0.00718 | 0.0615 | 0.00789 | 0.519 |
| Accuracy (bias %) | −0.81 | 4.87 | 3.96 | 3.59 | −0.155 | −2.78 | 3.22 | −2.99 |
| Precision (CV %) | 2.75 | 8.94 | 5.26 | 7.25 | 7.19 | 6.32 | 7.64 | 5.35 |
| GI | ||||||||
| Measured concentration (μg/mL) | 0.100 | 1.05 | 0.0962 | 1.02 | 0.101 | 1.03 | 0.104 | 1.03 |
| SD (μg/mL) | 0.00714 | 0.0522 | 0.0115 | 0.0833 | 0.00730 | 0.0673 | 0.00536 | 0.0542 |
| Accuracy (bias %) | 0.0211 | 5.09 | −3.79 | 1.80 | 0.570 | 2.59 | 3.51 | 2.68 |
| Precision (CV %) | 7.14 | 4.96 | 12.0 | 8.18 | 7.26 | 6.56 | 5.18 | 5.28 |
| Lung | ||||||||
| Measured concentration (μg/mL) | 0.0993 | 0.968 | 0.104 | 1.02 | 0.0952 | 1.02 | ||
| SD (μg/mL) | 0.00869 | 0.0615 | 0.00632 | 0.0798 | 0.00566 | 0.0516 | ||
| Accuracy (bias %) | −0.720 | −3.19 | 3.65 | 2.31 | −4.78 | 1.78 | ||
| Precision (CV %) | 8.76 | 6.35 | 6.10 | 7.80 | 5.94 | 5.07 | ||
| Liver | ||||||||
| Measured concentration (μg/mL) | 0.0974 | 1.03 | 0.101 | 1.041 | 0.0970 | 1.01 | ||
| SD (μg/mL) | 0.00744 | 0.0872 | 0.00724 | 0.0594 | 0.00359 | 0.0810 | ||
| Accuracy (bias %) | −2.56 | 3.30 | 0.924 | 4.10 | −3.04 | 0.801 | ||
| Precision (CV %) | 7.63 | 8.44 | 7.18 | 5.71 | 3.71 | 8.04 | ||
| Spleen | ||||||||
| Measured concentration (μg/mL) | 0.103 | 1.00 | 0.103 | 0.972 | 0.109 | 1.04 | ||
| SD (μg/mL) | 0.00622 | 0.0519 | 0.00655 | 0.0801 | 0.00541 | 0.0480 | ||
| Accuracy (bias %) | 2.96 | 0.156 | 2.85 | −2.79 | 9.23 | 3.58 | ||
| Precision (CV %) | 6.04 | 5.18 | 6.37 | 8.24 | 4.95 | 4.63 | ||
| Muscle | ||||||||
| Measured concentration (μg/mL) | 0.0987 | 0.100 | 0.105 | 1.04 | 0.106 | 1.05 | ||
| SD (μg/mL) | 0.00606 | 0.0711 | 0.00354 | 0.0703 | 0.00646 | 0.0715 | ||
| Accuracy (bias %) | −1.28 | −0.0202 | 4.65 | 3.94 | 5.69 | 5.31 | ||
| Precision (CV %) | 6.14 | 7.11 | 3.38 | 6.77 | 6.11 | 6.79 | ||
| Heart | ||||||||
| Measured concentration (μg/mL) | 0.100 | 1.02 | 0.100 | 1.01 | 0.107 | 1.04 | ||
| SD (μg/mL) | 0.00463 | 0.0791 | 0.00602 | 0.0986 | 0.0106 | 0.0599 | ||
| Accuracy (bias %) | 0.206 | 2.24 | 0.167 | 0.845 | 6.88 | 3.71 | ||
| Precision (CV %) | 4.62 | 7.74 | 6.01 | 9.78 | 9.96 | 5.78 | ||
| Kidney | ||||||||
| Measured concentration (μg/mL) | 0.101 | 1.03 | 0.103 | 1.03 | 0.100 | 1.04 | ||
| SD (μg/mL) | 0.00644 | 0.0774 | 0.00623 | 0.0521 | 0.00869 | 0.0639 | ||
| Accuracy (bias %) | 1.06 | 3.03 | 2.98 | 2.78 | −0.230 | 3.63 | ||
| Precision (CV %) | 6.37 | 7.51 | 6.05 | 5.07 | 8.71 | 6.17 | ||
| Nominal Concentration (μg/mL) | Measured Concentration (μg/mL) | Accuracy (Bias %) | Precision (CV %) | |||||
|---|---|---|---|---|---|---|---|---|
| Inter-Day | Intra-Day | Inter-Day | Intra-Day | Inter-Day | Intra-Day | |||
| Mean | SD | Mean | SD | |||||
| Plasma | ||||||||
| 0.1 | 0.101 | 0.00631 | 0.0997 | 0.00675 | 1.22 | −0.274 | 6.23 | 6.77 |
| 0.3 | 0.308 | 0.0160 | 0.310 | 0.0224 | 2.50 | 3.44 | 5.19 | 7.23 |
| 1 | 1.01 | 0.0639 | 0.977 | 0.0526 | 1.01 | −2.30 | 6.32 | 5.38 |
| 3 | 3.01 | 0.141 | 3.03 | 0.156 | 0.339 | 0.875 | 4.68 | 5.15 |
| GI | ||||||||
| 0.1 | 0.0978 | 0.00773 | 0.0996 | 0.00634 | −2.18 | −0.445 | 7.91 | 6.37 |
| 0.3 | 0.305 | 0.0154 | 0.308 | 0.0173 | 1.54 | 2.73 | 5.07 | 5.62 |
| 1 | 1.01 | 0.0695 | 1.04 | 0.0779 | 1.47 | 3.58 | 6.85 | 7.52 |
| 3 | 3.09 | 0.185 | 3.19 | 0.0642 | 3.06 | 6.31 | 6.00 | 2.01 |
| Lung | ||||||||
| 0.1 | 0.101 | 0.00641 | 0.102 | 0.00402 | 1.02 | 2.17 | 6.34 | 3.94 |
| 0.3 | 0.303 | 0.0171 | 0.309 | 0.0272 | 0.979 | 3.05 | 5.66 | 8.80 |
| 1 | 1.01 | 0.0637 | 0.999 | 0.0588 | 0.935 | −0.113 | 6.31 | 5.89 |
| 3 | 3.07 | 0.111 | 3.08 | 0.145 | 2.34 | 2.59 | 3.63 | 4.72 |
| Liver | ||||||||
| 0.1 | 0.0979 | 0.00499 | 0.0979 | 0.00347 | −2.10 | −2.08 | 5.10 | 3.54 |
| 0.3 | 0.303 | 0.0117 | 0.305 | 0.0129 | 1.12 | 1.64 | 3.85 | 4.25 |
| 1 | 1.01 | 0.0631 | 1.02 | 0.0452 | 1.29 | 2.11 | 6.23 | 4.43 |
| 3 | 3.01 | 0.120 | 3.12 | 0.0864 | 0.207 | 3.84 | 3.99 | 2.77 |
| Spleen | ||||||||
| 0.1 | 0.102 | 0.00583 | 0.104 | 0.00453 | 1.74 | 4.12 | 5.73 | 4.35 |
| 0.3 | 0.299 | 0.0163 | 0.300 | 0.0196 | −0.260 | 0.151 | 5.44 | 6.51 |
| 1 | 0.983 | 0.0711 | 1.01 | 0.0609 | −1.66 | 0.878 | 7.23 | 6.04 |
| 3 | 2.97 | 0.138 | 2.93 | 0.179 | −1.09 | −2.20 | 4.65 | 6.09 |
| Muscle | ||||||||
| 0.1 | 0.103 | 0.00562 | 0.0993 | 0.00442 | 3.07 | −0.701 | 5.46 | 4.45 |
| 0.3 | 0.311 | 0.0139 | 0.305 | 0.0130 | 3.54 | 1.82 | 4.49 | 4.26 |
| 1 | 1.02 | 0.0797 | 0.992 | 0.0775 | 1.69 | −0.762 | 7.84 | 7.80 |
| 3 | 3.02 | 0.122 | 3.10 | 0.0858 | 0.759 | 3.18 | 4.03 | 2.77 |
| Heart | ||||||||
| 0.1 | 0.100 | 0.00584 | 0.0985 | 0.00565 | −0.0134 | −1.49 | 5.84 | 5.74 |
| 0.3 | 0.302 | 0.0150 | 0.295 | 0.0127 | 0.682 | −1.79 | 4.98 | 4.31 |
| 1 | 1.02 | 0.0819 | 1.03 | 0.0712 | 2.13 | 3.06 | 8.02 | 6.91 |
| 3 | 3.01 | 0.125 | 3.13 | 0.0760 | 0.476 | 4.21 | 4.13 | 2.43 |
| Kidney | ||||||||
| 0.1 | 0.100 | 0.00688 | 0.104 | 0.00770 | 0.190 | 3.96 | 6.86 | 7.40 |
| 0.3 | 0.312 | 0.0140 | 0.310 | 0.0182 | 4.16 | 3.40 | 4.47 | 5.87 |
| 1 | 1.01 | 0.0492 | 0.985 | 0.0583 | 1.03 | −1.45 | 4.87 | 5.91 |
| 3 | 2.99 | 0.120 | 2.93 | 0.124 | −0.376 | −2.19 | 4.02 | 4.23 |
| Parameters | H9+TM1 | TM1 | H9+TM10 | TM10 |
|---|---|---|---|---|
| (n = 6) | (n = 7) | (n = 6) | (n = 6) | |
| Body weight (g) | 38.2 ± 1.17 | 37.7 ± 0.756 | 36.8 ± 1.83 | 36.2 ± 1.17 |
| AUC0–336 h (μg h/mL) | 656 ± 115 | 715 ± 109 | 6847 ± 713 | 6885 ± 1166 |
| AUC0–∞ (μg h/mL) | 873 ± 117 | 983 ± 105 | 8245 ± 941 | 8835 ± 1936 |
| Terminal half-life (h) | 139 ± 17.3 | 164 ± 32.9 | 134 ± 14.6 | 156 ± 32.6 |
| CL (mL/h/kg) | 1.16 ± 0.159 | 1.03 ± 0.111 | 1.23 ± 0.157 | 1.18 ± 0.284 |
| MRT (h) | 193 ± 24.6 | 227 ± 44.8 | 185 ± 21.5 | 215 ± 44.8 |
| Vss (mL/kg) | 223 ± 23.0 | 231 ± 39.7 | 226 ± 23.6 | 247 ± 38.9 |
| Parameters | 2-Week H9+TM1 | TM1 |
|---|---|---|
| (n = 6) | (n = 6) | |
| Body weight (g) | 45.2 ± 3.19 | 44.7 ± 2.88 |
| AUC0–336 h (μg h/mL) | 758 ± 154 | 795 ± 208 |
| AUC0–∞ (μg h/mL) | 1012 ± 141 | 1095 ± 221 |
| Terminal half-life (h) | 150 ± 24.4 | 163 ± 37.3 |
| CL (mL/h/kg) | 1.00 ± 0.136 | 0.947 ± 0.203 |
| MRT (h) | 208 ± 35.2 | 226 ± 51.3 |
| Vss (mL/kg) | 208 ± 40.2 | 213 ± 58.0 |
| Parameters | Multiple H9+TM1 | Multiple TM1 |
|---|---|---|
| (n = 5) | (n = 6) | |
| Body weight (g) | 47.0 ± 1.58 | 47.3 ± 2.80 |
| AUC0–336 h (μg h/mL) | 897 ± 101 | 770 ± 214 |
| AUC0–∞ (μg h/mL) | 1156 ± 138 | 1073 ± 187 |
| Terminal half-life (h) | 141 ± 40.3 | 159 ± 35.1 |
| CL (mL/h/kg) | 0.876 ± 0.110 | 0.963 ± 0.213 |
| MRT (h) | 199 ± 54.2 | 219 ± 45.2 |
| Vss (mL/kg) | 170 ± 32.3 | 208 ± 45.9 |
| Tissue | H9+TM10 | TM10 |
|---|---|---|
| (n = 5) | (n = 5) | |
| Liver | 379 ± 67.0 | 381 ± 88.6 |
| Heart | 366 ± 40.5 | 315 ± 90.1 |
| Lung | 441 ± 87.4 | 451 ± 34.6 |
| Spleen | 545 ± 51.7 | 623 ± 158 |
| Kidney | 279 ± 53.1 | 260 ± 53.5 |
| GI | 90.8 ± 18.5 | 80.9 ± 18.4 |
| Muscle | 47.2 ± 13.5 | 54.6 ± 20.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.Y.; Yu, J.-E.; You, B.H.; Kim, S.-Y.; Bae, M.; Chae, H.-S.; Chin, Y.-W.; Hong, S.-H.; Lee, J.-H.; Jung, S.H.; et al. No Interference of H9 Extract on Trastuzumab Pharmacokinetics in Their Combinations. Int. J. Mol. Sci. 2023, 24, 16677. https://doi.org/10.3390/ijms242316677
Han SY, Yu J-E, You BH, Kim S-Y, Bae M, Chae H-S, Chin Y-W, Hong S-H, Lee J-H, Jung SH, et al. No Interference of H9 Extract on Trastuzumab Pharmacokinetics in Their Combinations. International Journal of Molecular Sciences. 2023; 24(23):16677. https://doi.org/10.3390/ijms242316677
Chicago/Turabian StyleHan, Seung Yon, Jeong-Eun Yu, Byoung Hoon You, Seo-Yeon Kim, Mingoo Bae, Hee-Sung Chae, Young-Won Chin, Soo-Hwa Hong, Ju-Hee Lee, Seung Hyun Jung, and et al. 2023. "No Interference of H9 Extract on Trastuzumab Pharmacokinetics in Their Combinations" International Journal of Molecular Sciences 24, no. 23: 16677. https://doi.org/10.3390/ijms242316677
APA StyleHan, S. Y., Yu, J.-E., You, B. H., Kim, S.-Y., Bae, M., Chae, H.-S., Chin, Y.-W., Hong, S.-H., Lee, J.-H., Jung, S. H., & Choi, Y. H. (2023). No Interference of H9 Extract on Trastuzumab Pharmacokinetics in Their Combinations. International Journal of Molecular Sciences, 24(23), 16677. https://doi.org/10.3390/ijms242316677








