Effect of Low Concentration of Nitroxides on SH-SY5Y Cells Transfected with the Tau Protein
Abstract
:1. Introduction
2. Results
2.1. Antioxidant Properties of Nitroxides
2.2. Effect of Nitroxides on the Adrenalin and Pyrogallol Autoxidation
2.3. Effect of Nitroxides on the Oxidation of Dihydrorhodamine 123
2.4. Effect of Nitroxides on the Bleaching of Fluorescein with Hypochlorite
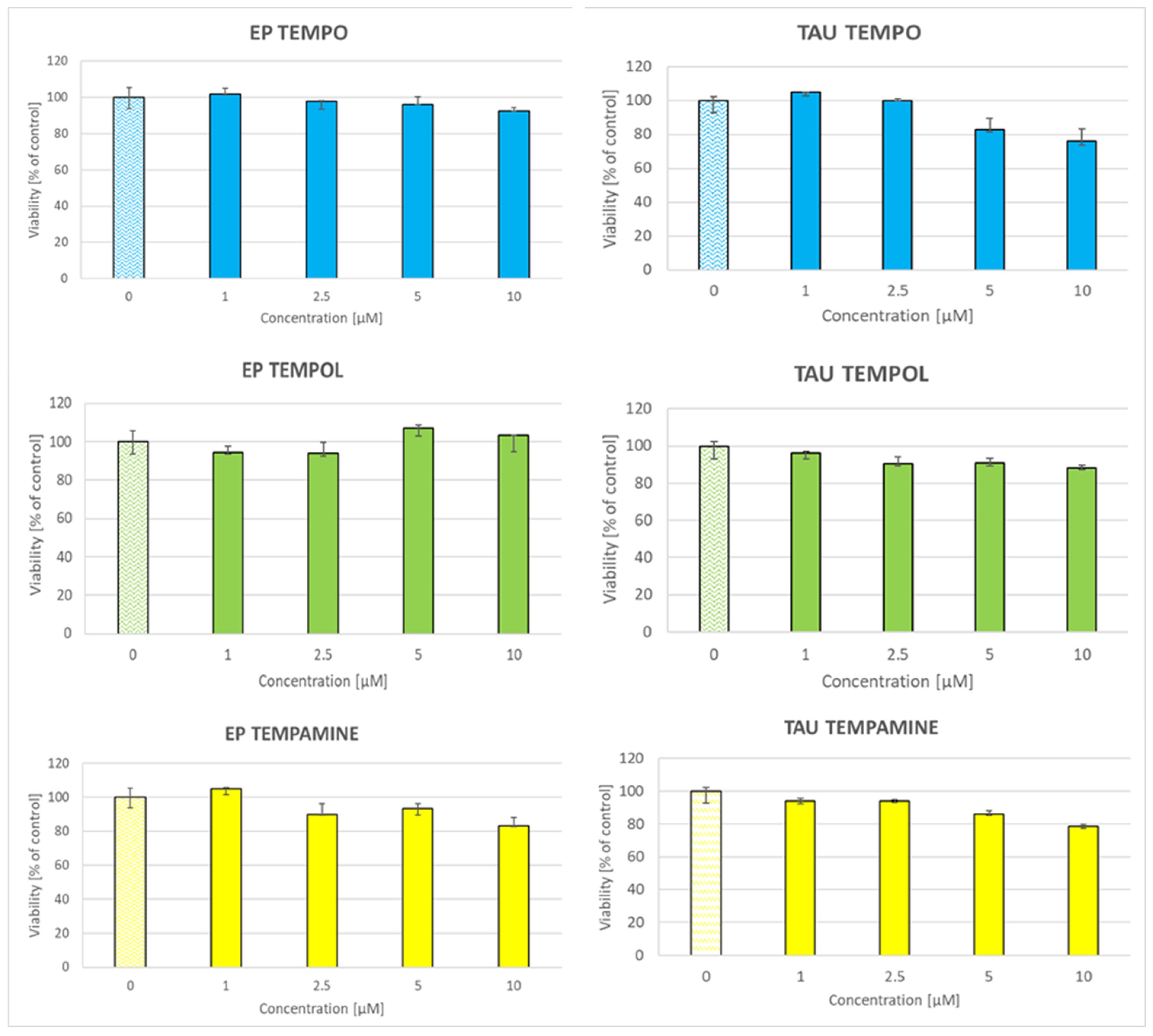
2.5. Toxicity of Nitroxides to SH-SY5Y Cells
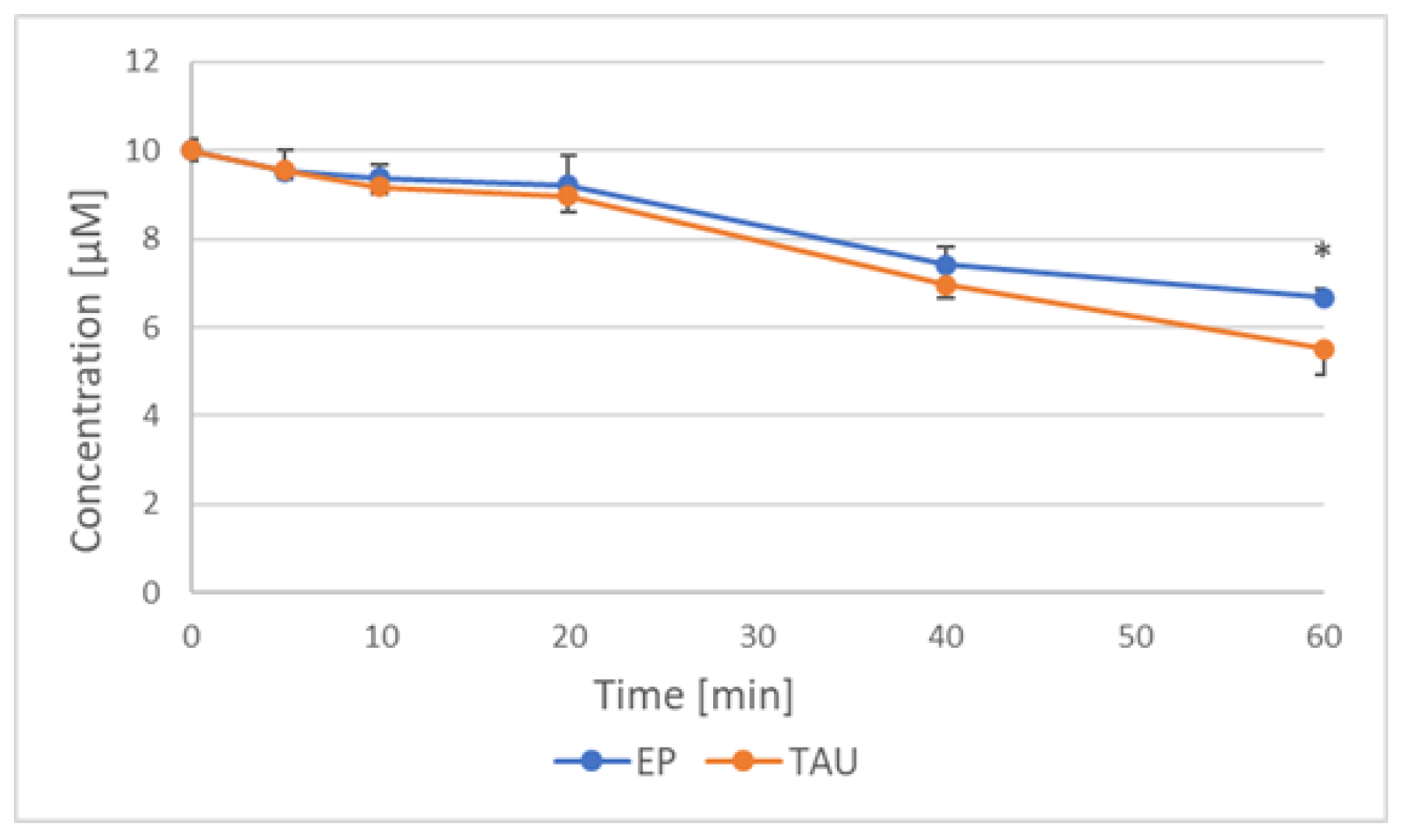
2.6. Reduction of Nitroxides by SH-SY5Y Cells
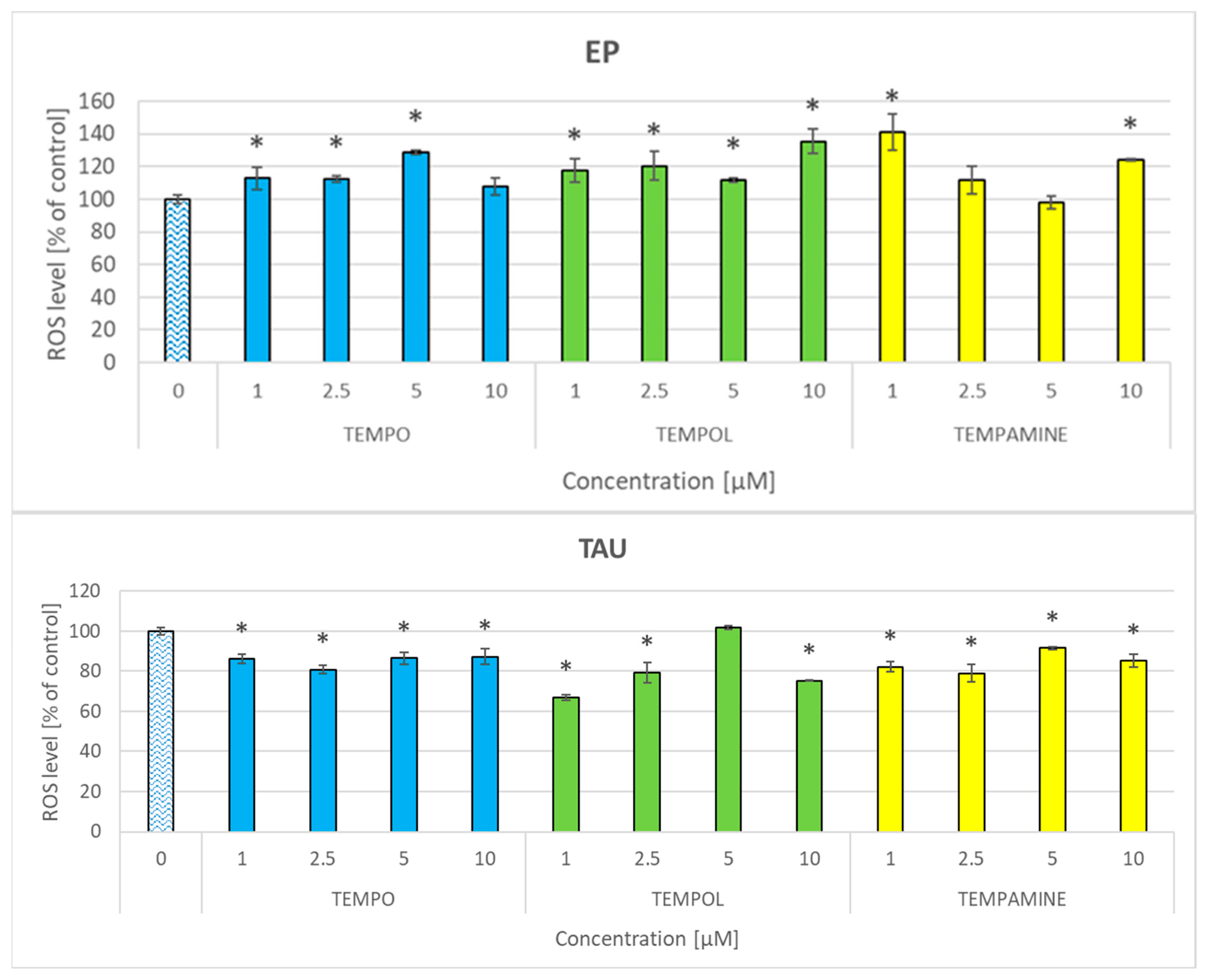
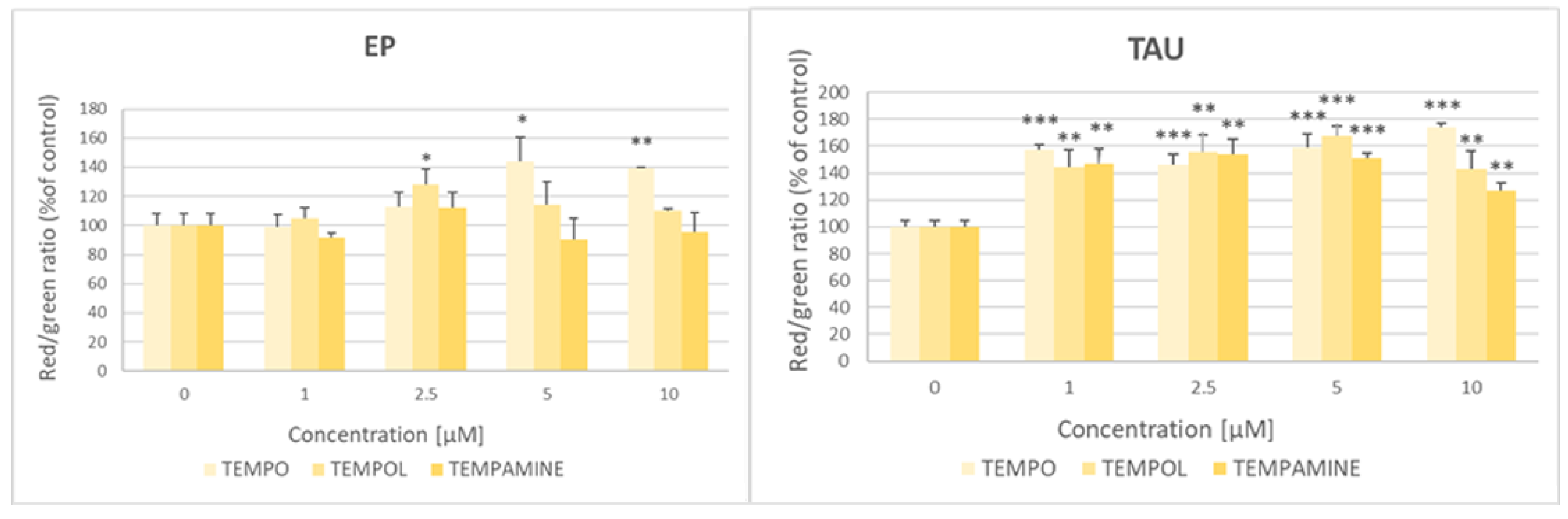
2.7. Effect of Nitroxides on the Level of Reactive Oxygen Species
2.8. Effect of Nitroxides on the Glutathione Content of the Cells
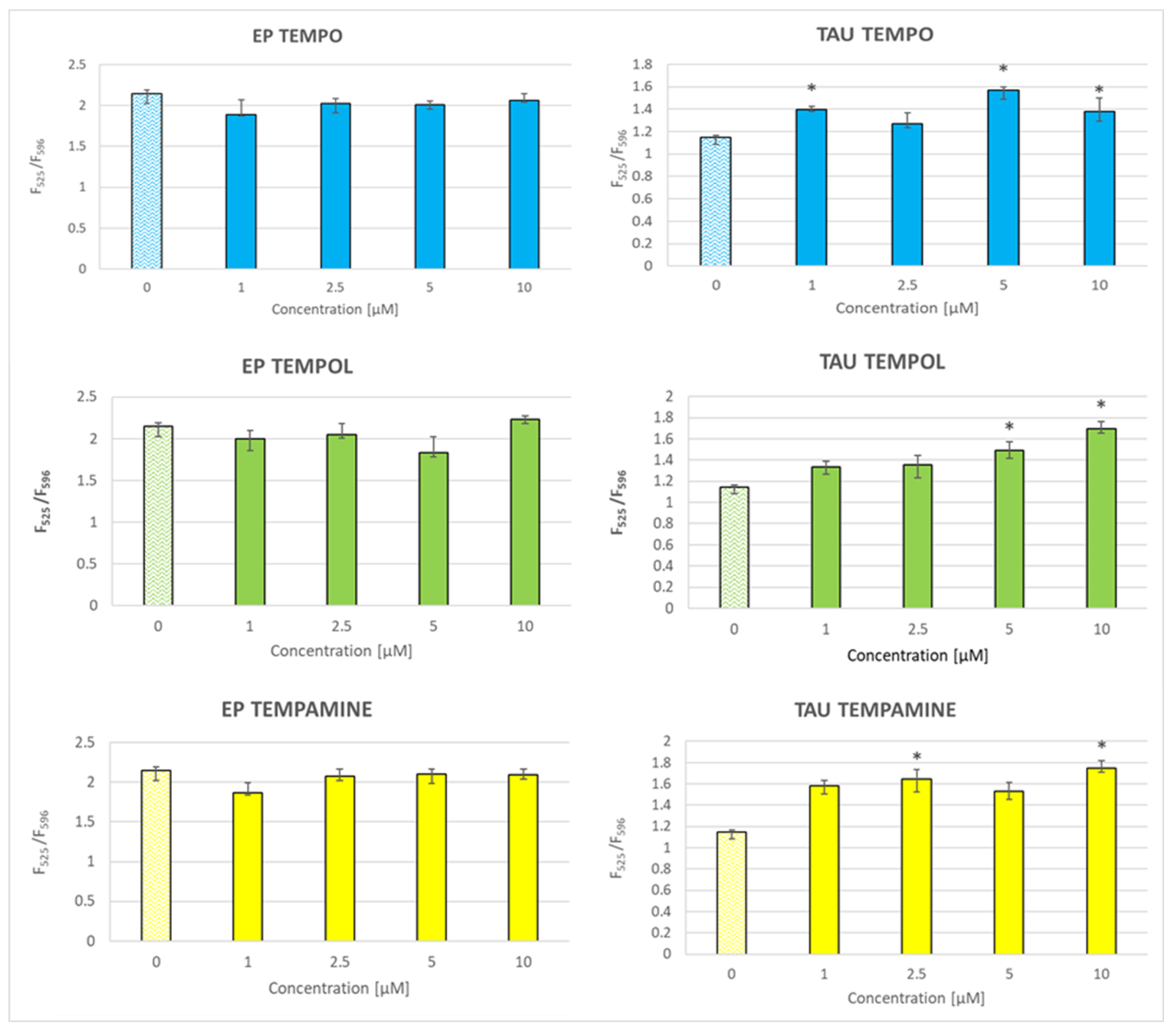
2.9. Effect of Nitroxides on Lipid Peroxidation
2.10. Effect of Nitroxides on the Mitochondrial-Membrane Potential
3. Discussion
4. Materials and Methods
4.1. Reagents, Equipment and Cells
4.2. Evaluation of the Antioxidant Activity of Nitroxides
4.2.1. ABTS• Decolorization Assay
4.2.2. DPPH• Decolorization Assay
4.2.3. FRAP Assay
4.2.4. CUPRAC Assay
4.2.5. Calculation of Antioxidant Activity
4.3. “Reversed FRAP” Assay
4.4. Cell Culture
4.5. Assessment of Nitroxide Cytotoxicity
4.6. Reduction of Nitroxides by Cells
4.7. The Level of Reactive Oxygen Species
4.8. Estimation of the Content of Reduced Glutathione
4.9. Lipid Peroxidation Assay with BODIPY™ 581/591 C11
4.10. Evaluation of Changes of the Mitochondrial-Membrane Potential (∆Ψm)
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krishna, M.C.; DeGraff, W.; Hankovszky, O.H.; Sár, C.P.; Kálai, T.; Jeko, J.; Russo, A.; Mitchell, J.B.; Hideg, K. Studies of structure-activity relationship of nitroxide free radicals and their precursors as modifiers against oxidative damage. J. Med. Chem. 1998, 41, 3477–3492. [Google Scholar] [CrossRef]
- Goldstein, S.; Merenyi, G.; Russo, A.; Samuni, A. The role of oxoammonium cation in the SOD-mimic activity of cyclic nitroxides. J. Am. Chem. Soc. 2003, 125, 789–795. [Google Scholar] [CrossRef]
- Goldstein, S.; Samuni, A. Kinetics and mechanism of peroxyl radical reactions with nitroxides. J. Phys. Chem. A 2007, 111, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- DeGraff, W.G.; Krishna, M.C.; Russo, A.; Mitchell, J.B. Antimutagenicity of a low molecular weight superoxide dismutase mimic against oxidative mutagens. Environ. Mol. Mutagen. 1992, 19, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Samuni, A.; Goldstein, S.; Russo, A.; Mitchell, J.B.; Krishna, M.C.; Neta, P. Kinetics and mechanism of hydroxyl radical and OH-adduct radical reactions with nitroxides and with their hydroxylamines. J. Am. Chem. Soc. 2002, 124, 8719–8724. [Google Scholar] [CrossRef]
- Goldstein, S.; Samuni, A.; Merenyi, G. Kinetics of the reaction between nitroxide and thiyl radicals: Nitroxides as antioxidants in the presence of thiols. J. Phys. Chem. A 2008, 112, 8600–8605. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010, 126, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Gwoździński, K.; Bartosz, G.; Leyko, W. Effect of gamma radiation on the transport of electrolyte spin labels across the human erythrocyte membrane. Studia Biophys. 1982, 89, 141–145. [Google Scholar]
- Yamasaki, T.; Mito, F.; Ito, Y.; Pandian, S.; Kinoshita, Y.; Nakano, K.; Murugesan, R.; Sakai, K.; Utsumi, H.; Yamada, K. Structure-reactivity relationship of piperidine nitroxide: Electrochemical, ESR and computational studies. J. Org. Chem. 2011, 76, 435–440. [Google Scholar] [CrossRef]
- Paleos, C.M.; Dais, P. Ready reduction of some nitroxide free radicals with ascorbic acid. J. Chem. Soc. Chem. Commun. 1977, 10, 345–346. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Yamada, K.; Yamasaki, T.; Sadasue, H.; Sakai, K.; Utsumi, H. Development of novel nitroxyl radicals for controlling reactivity with ascorbic acid. Free Radic. Res. 2009, 43, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.L.; Namazian, M.; Bottle, S.E.; Coote, M.L. One-electron oxidation and reduction potentials of nitroxide antioxidants: A theoretical study. J. Phys. Chem. A 2007, 111, 13595–13605. [Google Scholar] [CrossRef]
- Blinco, J.P.; Hodgson, J.L.; Morrow, B.J.; Walker, J.R.; Will, G.D.; Coote, M.L.; Bottle, S.E. Experimental and theoretical studies of the redox potentials of cyclic nitroxides. J. Org. Chem. 2008, 73, 6763–6771. [Google Scholar] [CrossRef] [PubMed]
- Castagna, R.; Davis, P.A.; Vasu, V.T.; Soucek, K.; Cross, C.E.; Greci, L.; Valacchi, G. Nitroxide radical TEMPO reduces ozone-induced chemokine IL-8 production in lung epithelial cells. Toxicol. Vitr. 2009, 23, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; Grahame, D.A.; Samuni, A.; Mitchell, J.B.; Russo, A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc. Natl. Acad. Sci. USA 1992, 89, 5537–5541. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Pearlman, A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol. Rev. 2008, 60, 418–469. [Google Scholar] [CrossRef] [PubMed]
- Dijksman, A.; Marino-González, A.; Mairata, I.; Payeras, A.; Arends, I.W.; Sheldon, R.A. Efficient and selective aerobic oxidation of alcohols into aldehydes and ketones using ruthenium/TEMPO as the catalytic system. J. Am. Chem. Soc. 2001, 123, 6826–6833. [Google Scholar] [CrossRef] [PubMed]
- Hoover, J.M.; Stahl, S.S. Highly practical copper(I)/TEMPO catalyst system for chemoselective aerobic oxidation of primary alcohols. J. Am. Chem. Soc. 2011, 133, 16901–16910. [Google Scholar] [CrossRef]
- Pérez, M.J.; Cederbaum, A.I. Spin trapping agents (Tempol and POBN) protect HepG2 cells overexpressing CYP2E1 against arachidonic acid toxicity. Free Radic. Biol. Med. 2001, 30, 734–746. [Google Scholar] [CrossRef]
- Karbowski, M.; Kurono, C.; Wozniak, M.; Ostrowski, M.; Teranishi, M.; Soji, T.; Wakabayashi, T. Cycloheximide and 4-OH-TEMPO suppress chloramphenicol-induced apoptosis in RL-34 cells via the suppression of the formation of megamitochondria. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1999, 1449, 25–40. [Google Scholar] [CrossRef]
- Szpilewska, H.; Czyz, A.; Wegrzyn, G. Experimental evidence for the physiological role of bacterial luciferase in the protection of cells against oxidative stress. Curr. Microbiol. 2003, 47, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; DeGraff, W.; Kaufman, D.; Krishna, M.C.; Samuni, A.; Finkelstein, E.; Ahn, M.S.; Hahn, S.M.; Gamson, J.; Russo, A. Inhibition of oxygen-dependent radiation-induced damage by the nitroxide superoxide dismutase mimic, tempol. Arch. Biochem. Biophys. 1991, 289, 62–70. [Google Scholar] [CrossRef]
- Salvi, A.; Patki, G.; Khan, E.; Asghar, M.; Salim, S. Protective effect of tempol on buthionine sulfoximine-induced mitochondrial impairment in hippocampal derived HT22 cells. Oxid. Med. Cell. Longev. 2016, 2016, 5059043. [Google Scholar] [CrossRef]
- Pieńkowska, N.; Bartosz, G.; Pichla, M.; Grzesik-Pietrasiewicz, M.; Gruchala, M.; Sadowska-Bartosz, I. Effect of antioxidants on the H2O2-induced premature senescence of human fibroblasts. Aging 2020, 12, 1910–1927. [Google Scholar] [CrossRef]
- Guo, X.; Seo, J.E.; Bryce, S.M.; Tan, J.A.; Wu, Q.; Dial, S.L.; Moore, M.M.; Mei, N. Comparative genotoxicity of TEMPO and 3 of its derivatives in mouse lymphoma cells. Toxicol. Sci. 2018, 163, 214–225. [Google Scholar] [CrossRef]
- Pattison, D.I.; Lam, M.; Shinde, S.S.; Anderson, R.F.; Davies, M.J. The nitroxide TEMPO is an efficient scavenger of protein radicals: Cellular and kinetic studies. Free Radic. Biol. Med. 2012, 53, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Mołoń, M.; Szlachcikowska, D.; Stępień, K.; Kielar, P.; Galiniak, S. Two faces of TEMPO (2,2,6,6-tetramethylpiperidinyl-1-oxyl)—An antioxidant or a toxin? Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2023, 1870, 119412. [Google Scholar] [CrossRef]
- Israeli, A.; Patt, M.; Oron, M.; Samuni, A.; Kohen, R.; Goldstein, S. Kinetics and mechanism of the comproportionation reaction between oxoammonium cation and hydroxylamine derived from cyclic nitroxides. Free Radic. Biol. Med. 2005, 38, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Mehlhorn, R. Mutagenicity of nitroxide-free radicals. Arch. Biochem. Biophys. 1986, 251, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Gallez, B.; De Meester, C.; Debuyst, R.; Dejehet, F.; Dumont, P. Mutagenicity of nitroxyl compounds: Structure-activity relationships. Toxicol. Lett. 1992, 63, 35–45. [Google Scholar] [CrossRef]
- Voest, E.E.; van Faassen, E.; van Asbeck, B.S.; Neijt, J.P.; Marx, J.J. Increased hydrogen peroxide concentration in human tumor cells due to a nitroxide free radical. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1992, 1136, 113–118. [Google Scholar] [CrossRef]
- Offer, T.; Russo, A.; Samuni, A. The pro-oxidative activity of SOD and nitroxide SOD mimics. FASEB J. 2000, 14, 1215–1223. [Google Scholar] [CrossRef]
- Guo, X.; Mittelstaedt, R.A.; Guo, L.; Shaddock, J.G.; Heflich, R.H.; Bigger, A.H.; Moore, M.M.; Mei, N. Nitroxide TEMPO: A genotoxic and oxidative stress inducer in cultured cells. Toxicol. In Vitro 2013, 27, 1496–1502. [Google Scholar] [CrossRef]
- Gariboldi, M.B.; Lucchi, S.; Caserini, C.; Supino, R.; Oliva, C.; Monti, E. Antiproliferative effect of the piperidine nitroxide TEMPOL on neoplastic and nonneoplastic mammalian cell lines. Free Radic. Biol. Med. 1998, 24, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Bujak-Pietrek, S.; Pieniazek, A.; Gwozdzinski, K.; Gwozdzinski, L. The effect of piperidine nitroxides on the properties of metalloproteins in human red blood cells. Molecules 2023, 28, 6174. [Google Scholar] [CrossRef] [PubMed]
- Kroll, C.; Borchert, H.H. Metabolism of the stable nitroxyl radical 4-oxo-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPONE). Eur. J. Pharm. Sci. 1999, 8, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R.; Erker, L.; Barlow, C.; Yakushiji, H.; Larson, D.; Russo, A.; Mitchell, J.B.; Wynshaw-Boris, A. Cancer chemoprevention by the antioxidant tempol in Atm-deficient mice. Hum. Mol. Genet. 2004, 13, 1793–1802. [Google Scholar] [CrossRef]
- Sodagam, L.; Lewinska, A.; Kwasniewicz, E.; Kokhanovska, S.; Wnuk, M.; Siems, K.; Rattan, S.I.S. Phytochemicals rosmarinic acid, ampelopsin, and amorfrutin-a can modulate age-related phenotype of serially passaged human skin fibroblasts in vitro. Front. Genet. 2019, 10, 81. [Google Scholar] [CrossRef]
- Daudt, D.R., 3rd; Mueller, B.; Park, Y.H.; Wen, Y.; Yorio, T. Methylene blue protects primary rat retinal ganglion cells from cellular senescence. Invest. Ophthalmol. Vis. Sci. 2012, 53, 4657–4667. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.M.; O’Donovan, M.; Sun, L.; Choi, J.Y.; Ren, M.; Cao, K. Anti-aging potentials of methylene blue for human skin longevity. Sci. Rep. 2017, 7, 2475. [Google Scholar] [CrossRef]
- Bertolo, A.; Capossela, S.; Fränkl, G.; Baur, M.; Pötzel, T.; Stoyanov, J. Oxidative status predicts quality in human mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 3. [Google Scholar] [CrossRef]
- Pieńkowska, N.; Fahnestock, M.; Mahadeo, C.; Zaborniak, I.; Chmielarz, P.; Bartosz, G.; Sadowska-Bartosz, I. Induction of oxidative stress in SH-SY5Y cells by overexpression of htau40 and its mitigation by redox-active nanoparticles. Int. J. Mol. Sci. 2022, 24, 359. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Apak, R. The main and modified CUPRAC methods of antioxidant measurement. TrAC Trends Anal. Chem. 2011, 30, 652–664. [Google Scholar] [CrossRef]
- Yang, W.; Söderberg, M.; Eriksson, A.I.; Boschloo, G. Efficient aqueous dye-sensitized solar cell electrolytes based on a TEMPO/TEMPO+ redox couple. RSC Adv. 2015, 5, 26706–26709. [Google Scholar] [CrossRef]
- Ilbert, M.; Bonnefoy, V. Insight into the evolution of the iron oxidation pathways. Biochim. Biophys. Acta (BBA) Bioenerg. 2013, 1827, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Samuni, A.; Krishna, C.M.; Mitchell, J.B.; Collins, C.R.; Russo, A. Superoxide reaction with nitroxides. Free Radic. Res. Commun. 1990, 9, 241–249. [Google Scholar] [CrossRef]
- Samuni, A.; Mitchell, J.B.; DeGraff, W.; Krishna, C.M.; Samuni, U.; Russo, A. Nitroxide SOD-mimics: Modes of action. Free Radic. Res. Commun. 1991, 12–13 Pt 1, 187–194. [Google Scholar] [CrossRef]
- Singh, R.J.; Hogg, N.; Joseph, J.; Konorev, E.; Kalyanaraman, B. The peroxynitrite generator, SIN-1, becomes a nitric oxide donor in the presence of electron acceptors. Arch. Biochem. Biophys. 1999, 361, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Gajewska, A.; Skolimowski, J.; Szewczyk, R.; Bartosz, G. Nitroxides protect against peroxynitrite-induced nitration and oxidation. Free Radic. Biol. Med. 2015, 89, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Hazell, L.J.; Davies, M.J.; Stocker, R. Secondary radicals derived from chloramines of apolipoprotein B-100 contribute to HOCl-induced lipid peroxidation of low-density lipoproteins. Biochem. J. 1999, 339 Pt 3, 489–495. [Google Scholar] [CrossRef]
- Pattison, D.I.; Hawkins, C.L.; Davies, M.J. Hypochlorous acid-mediated protein oxidation: How important are chloramine transfer reactions and protein tertiary structure? Biochemistry 2007, 46, 9853–9864. [Google Scholar] [CrossRef]
- Swartz, H.M.; Sentjurc, M.; Morse, P.D., II. Cellular metabolism of water-soluble nitroxides: Effect on rate of reduction of cell/nitroxide ratio, oxygen concentrations and permeability of nitroxides. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1986, 888, 82–90. [Google Scholar] [CrossRef]
- Bobko, A.A.; Kirilyuk, I.A.; Grigor’ev, I.A.; Zweier, J.L.; Khramtsov, V.V. Reversible reduction of nitroxides to hydroxylamines: Roles for ascorbate and glutathione. Free Radic. Biol. Med. 2007, 42, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Tachibana, C.; Rahr Winther, J.; Appenzeller-Herzog, C. Intracellular glutathione pools are heterogeneously concentrated. Redox Biol. 2013, 1, 508–513. [Google Scholar] [CrossRef]
- Brand, M.D.; Buckingham, J.A.; Esteves, T.C.; Green, K.; Lambert, A.J.; Miwa, S.; Murphy, M.P.; Pakay, J.L.; Talbot, D.A.; Echtay, K.S. Mitochondrial superoxide and aging: Uncoupling-protein activity and superoxide production. Biochem. Soc. Symp. 2004, 71, 203–213. [Google Scholar]
- Nagasaki, Y. Design and application of redox polymers for nanomedicine. Polym. J. 2018, 50, 821–836. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Redox nanoparticles: Synthesis, properties and perspectives of use for treatment of neurodegenerative diseases. J. Nanobiotechnol. 2018, 16, 1–16. [Google Scholar] [CrossRef]
- Vong, L.B.; Yoshitomi, T.; Matsui, H.; Nagasaki, Y. Development of an oral nanotherapeutics using redox nanoparticles for treatment of colitis-associated colon cancer. Biomaterials 2015, 55, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Mei, T.; Shashni, B.; Maeda, H.; Nagasaki, Y. Fibrinolytic tissue plasminogen activator installed redox-active nanoparticles (t-PA@iRNP) for cancer therapy. Biomaterials 2020, 259, 120290. [Google Scholar] [CrossRef]
- Kut, K.; Cieniek, B.; Stefaniuk, I.; Bartosz, G.; Sadowska-Bartosz, I. A modification of the ABTS• decolorization method and an insight into its mechanism. Processes 2022, 10, 1288. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kuczera, K.; Naparło, K.; Soszyński, M.; Bartosz, G.; Sadowska-Bartosz, I. Capsaicin toxicity to the yeast Saccharomyces cerevisiae is not due to oxidative stress but to disruption of membrane structure. Chem. Biol. Interact. 2023, 374, 110407. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Özyürek, M.; Güçlü, K.; Tütem, E.; Başkan, K.S.; Erçağ, E.; Çelik, S.E.; Baki, S.; Yıldız, L.; Karaman, Ş.; Apak, R. A comprehensive review of CUPRAC methodology. Anal. Meth. 2011, 3, 2439–2453. [Google Scholar] [CrossRef]
- Senft, A.P.; Dalton, T.P.; Shertzer, H.G. Determining glutathione and glutathione disulfide using the fluorescence probe o-phthalaldehyde. Anal. Biochem. 2000, 280, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]




| Method | Ascorbate | TEMPO | TEMPOL | TEMPAMINE |
|---|---|---|---|---|
| Antioxidant activity [TE/mol] | ||||
| ABTS• decolorization | 0.98 ± 0.06 | 0.20 ± 0.02 | 0.03 ± 0.01 | 0.03 ± 0.002 |
| DPPH• decolorization | 0.70 ± 0.05 | 0.59 ± 0.06 | 0.53 ± 0.05 | 0.48 ± 0.06 |
| FRAP | 1.16 ± 0.08 | 0.40 ± 0.03 | 0.50 ± 0.08 | 0.30 ± 0.06 |
| CUPRAC | 0.86 ± 0.11 | 0.70 ± 0.27 | 0.44 ± 0.08 | 0.57 ± 0.09 |
| Number of moles of a radical/ion reduced by mole of a nitroxide | ||||
| ABTS• | 1.625 ± 0.031 | 0.291 ± 0.082 | 0.045 ± 0.006 | 0.044 ± 0.002 |
| DPPH• | 1.42 ± 0.07 | 1.20 ± 0.11 | 1.08 ± 0.09 | 0.88 ± 0.07 |
| Fe3+ | 2.16 ± 0.07 | 0.85 ± 0.07 | 1.06 ± 0.17 | 0.64 ± 0.13 |
| Nitroxide Concentration [μM] | TEMPO | TEMPOL | TEMPAMINE |
|---|---|---|---|
| Fe2+ oxidized [μM] | |||
| 8 | 18.4 ± 0.1 | 17.7 ± 1.2 | 14.2 ± 1.1 ** |
| 15 | 25.5 ± 0.9 | 28.1 ± 0.5 * | 33.3 ± 0.8 *** |
| Cells/Nitroxide | TEMPO | TEMPOL | TEMPAMINE |
|---|---|---|---|
| EP | 0.057 ± 0.005 | 0.049 ± 0.009 | 0.054 ± 0.006 |
| TAU | 0.075 ± 0.005 * | 0.068 ± 0.007 * | 0.071 ± 0.008 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartosz, G.; Pieńkowska, N.; Kut, K.; Cieniek, B.; Stefaniuk, I.; Sadowska-Bartosz, I. Effect of Low Concentration of Nitroxides on SH-SY5Y Cells Transfected with the Tau Protein. Int. J. Mol. Sci. 2023, 24, 16675. https://doi.org/10.3390/ijms242316675
Bartosz G, Pieńkowska N, Kut K, Cieniek B, Stefaniuk I, Sadowska-Bartosz I. Effect of Low Concentration of Nitroxides on SH-SY5Y Cells Transfected with the Tau Protein. International Journal of Molecular Sciences. 2023; 24(23):16675. https://doi.org/10.3390/ijms242316675
Chicago/Turabian StyleBartosz, Grzegorz, Natalia Pieńkowska, Kacper Kut, Bogumił Cieniek, Ireneusz Stefaniuk, and Izabela Sadowska-Bartosz. 2023. "Effect of Low Concentration of Nitroxides on SH-SY5Y Cells Transfected with the Tau Protein" International Journal of Molecular Sciences 24, no. 23: 16675. https://doi.org/10.3390/ijms242316675
APA StyleBartosz, G., Pieńkowska, N., Kut, K., Cieniek, B., Stefaniuk, I., & Sadowska-Bartosz, I. (2023). Effect of Low Concentration of Nitroxides on SH-SY5Y Cells Transfected with the Tau Protein. International Journal of Molecular Sciences, 24(23), 16675. https://doi.org/10.3390/ijms242316675










