Generation and Characterization of SORT1-Targeted Antibody–Drug Conjugate for the Treatment of SORT1-Positive Breast Tumor
Abstract
:1. Introduction
2. Results
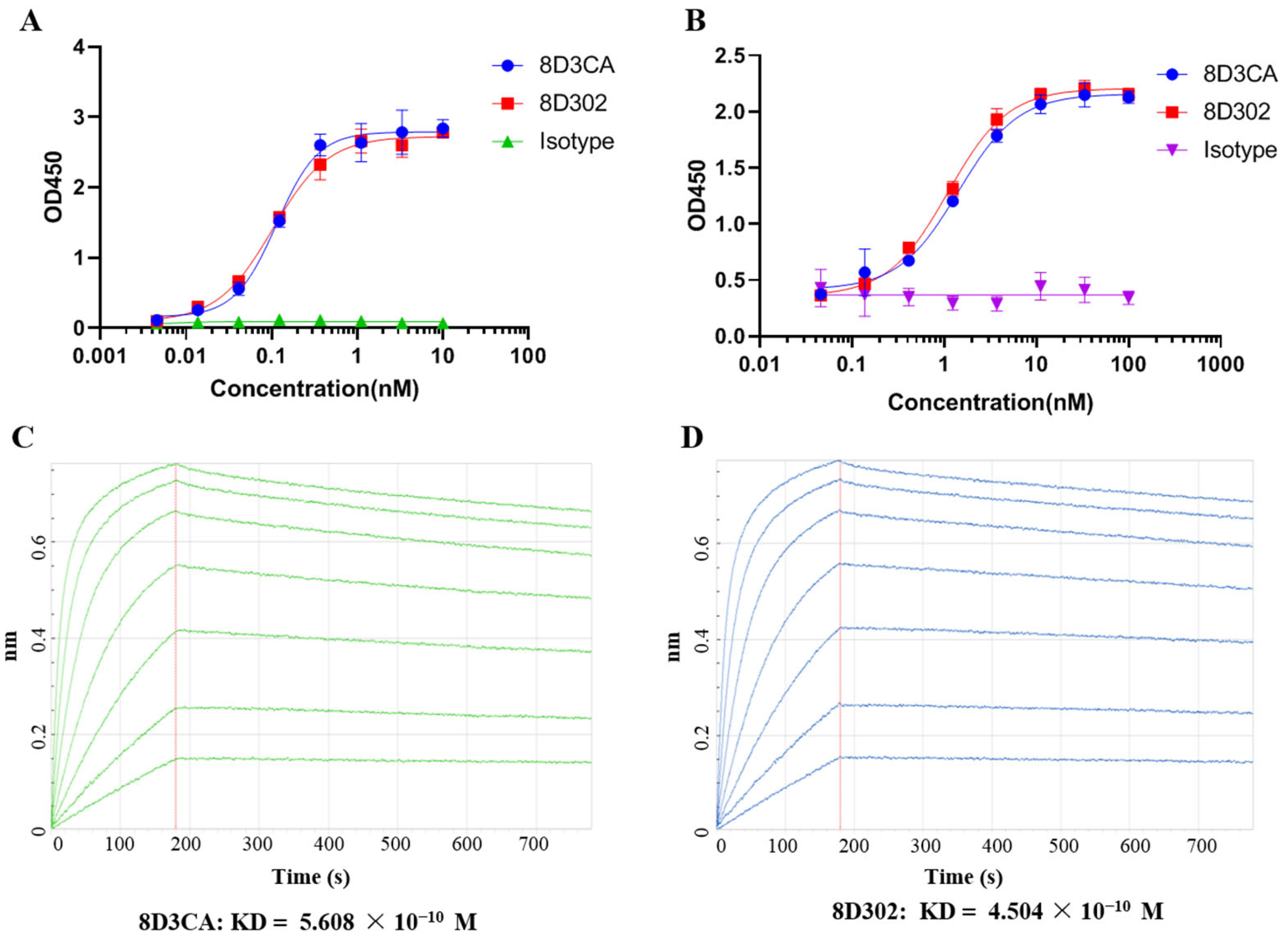
2.1. Generation and Identification of Humanized Anti-SORT1 Antibody
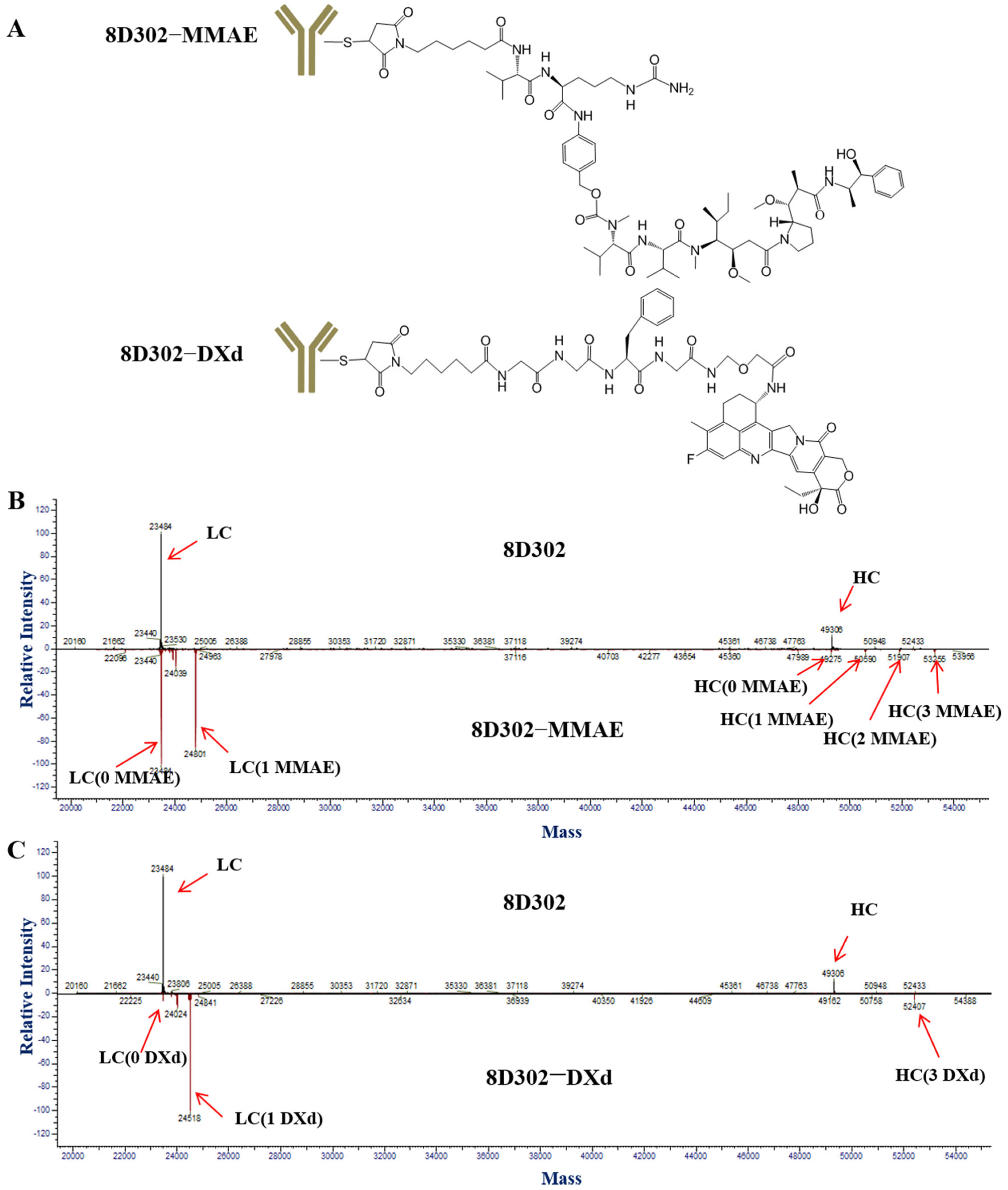
2.2. Conjugation of 8D302
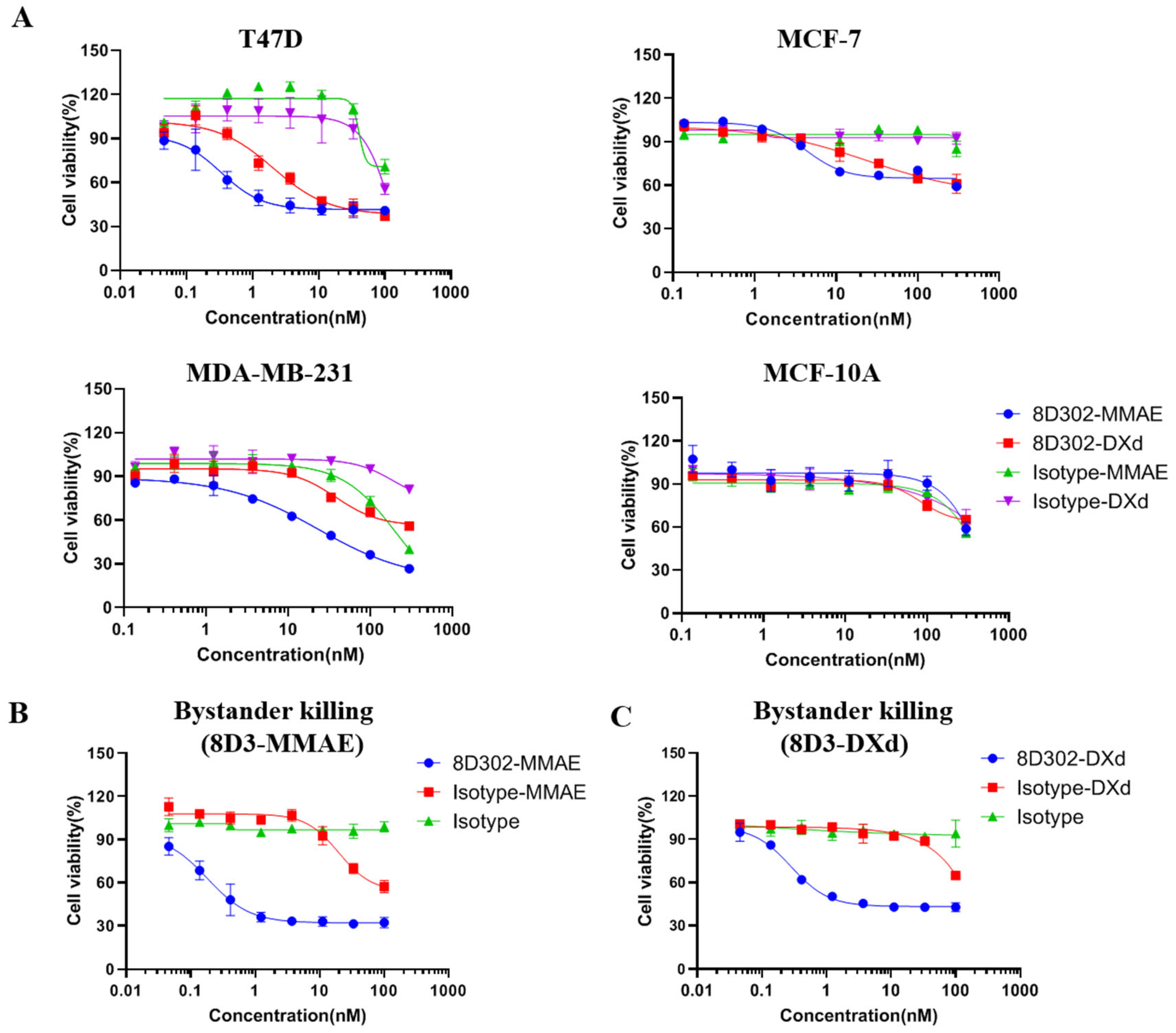
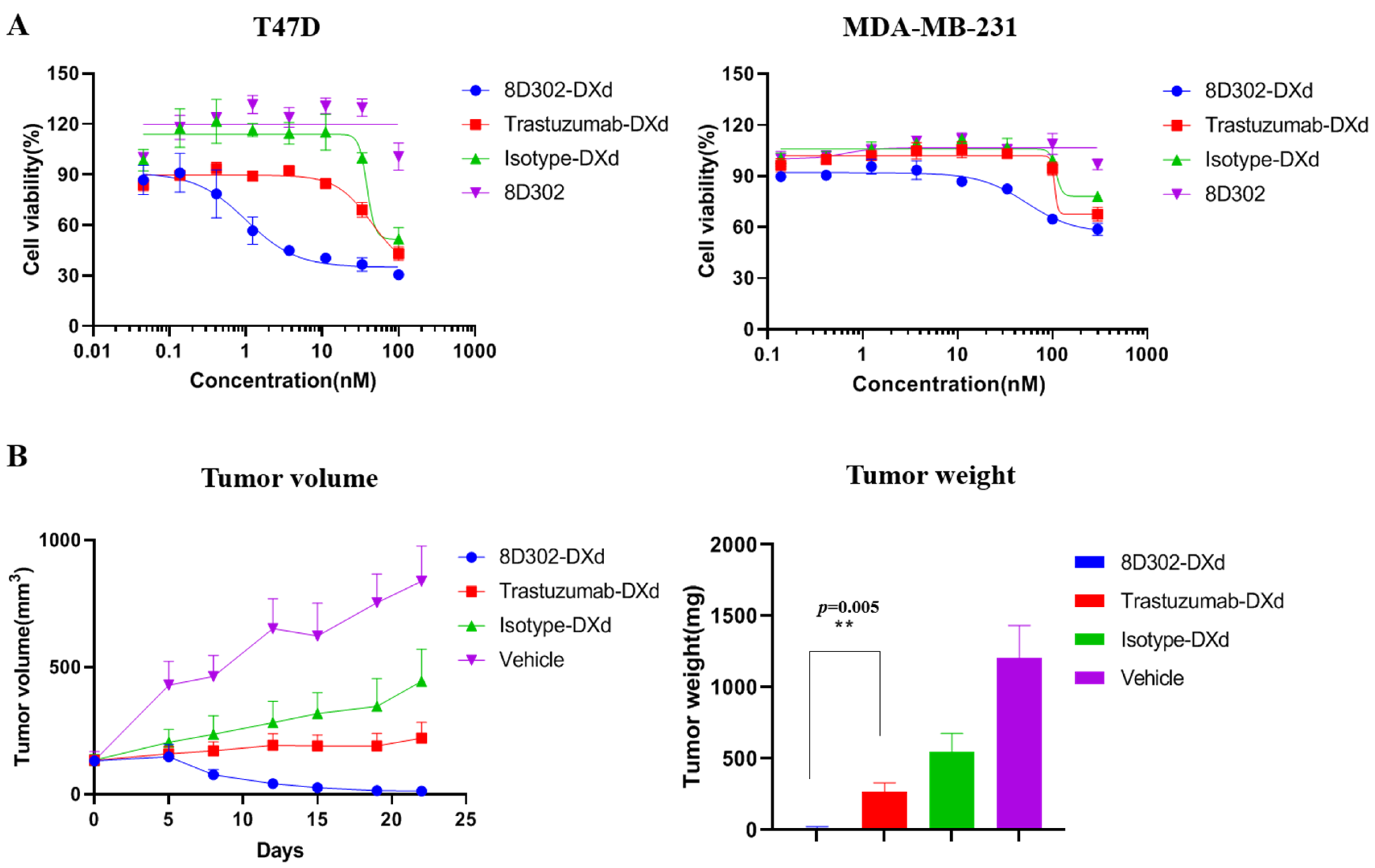
2.3. Evaluation of 8D302-ADCs Anti-Tumor Effects In Vitro
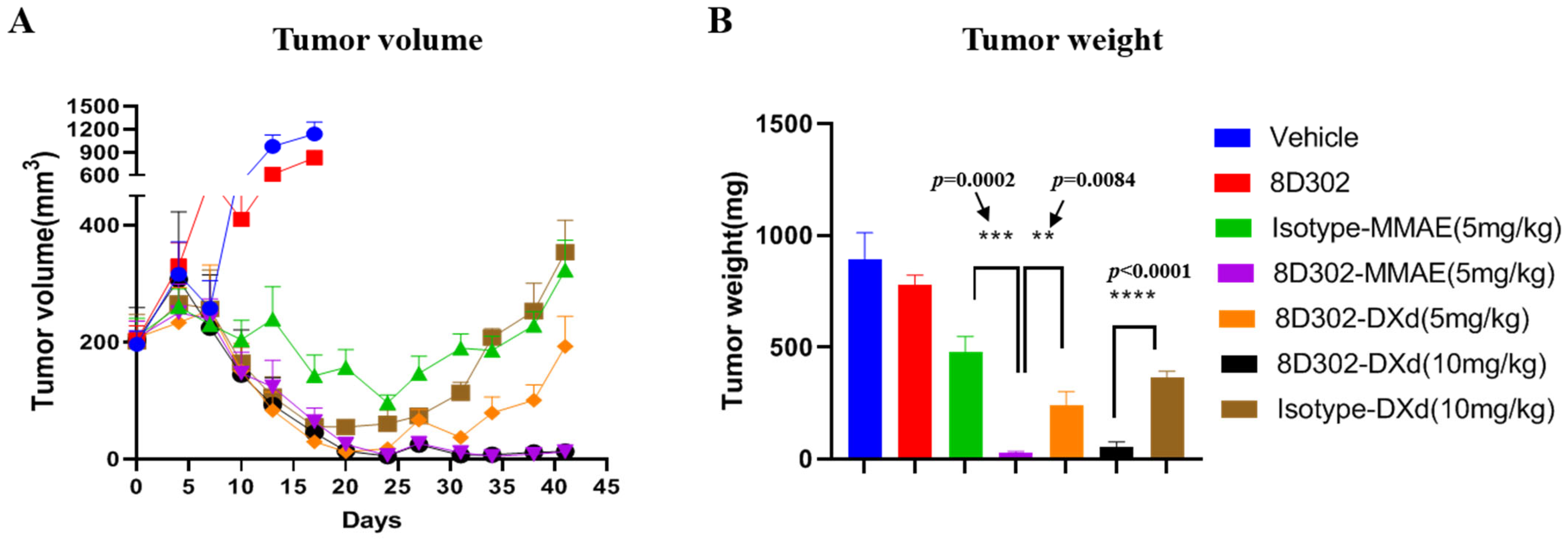
2.4. Anti-Tumor Efficacy of 8D302-ADCs In Vivo
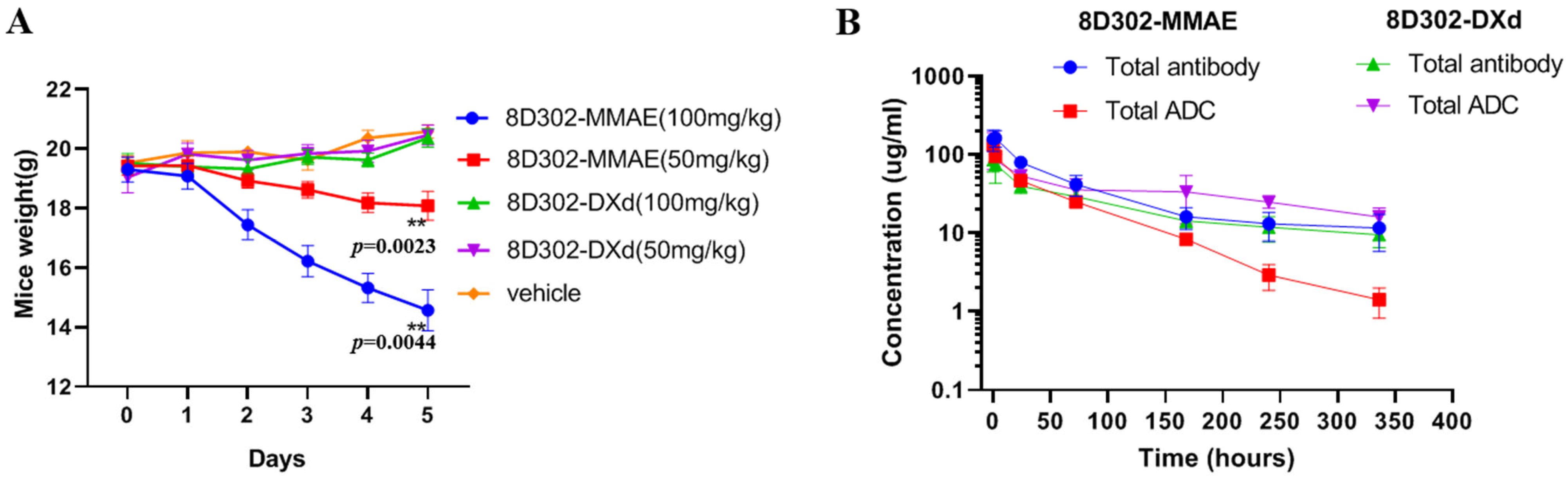
2.5. Safety and Pharmacokinetics of 8D302-ADCs in Mice
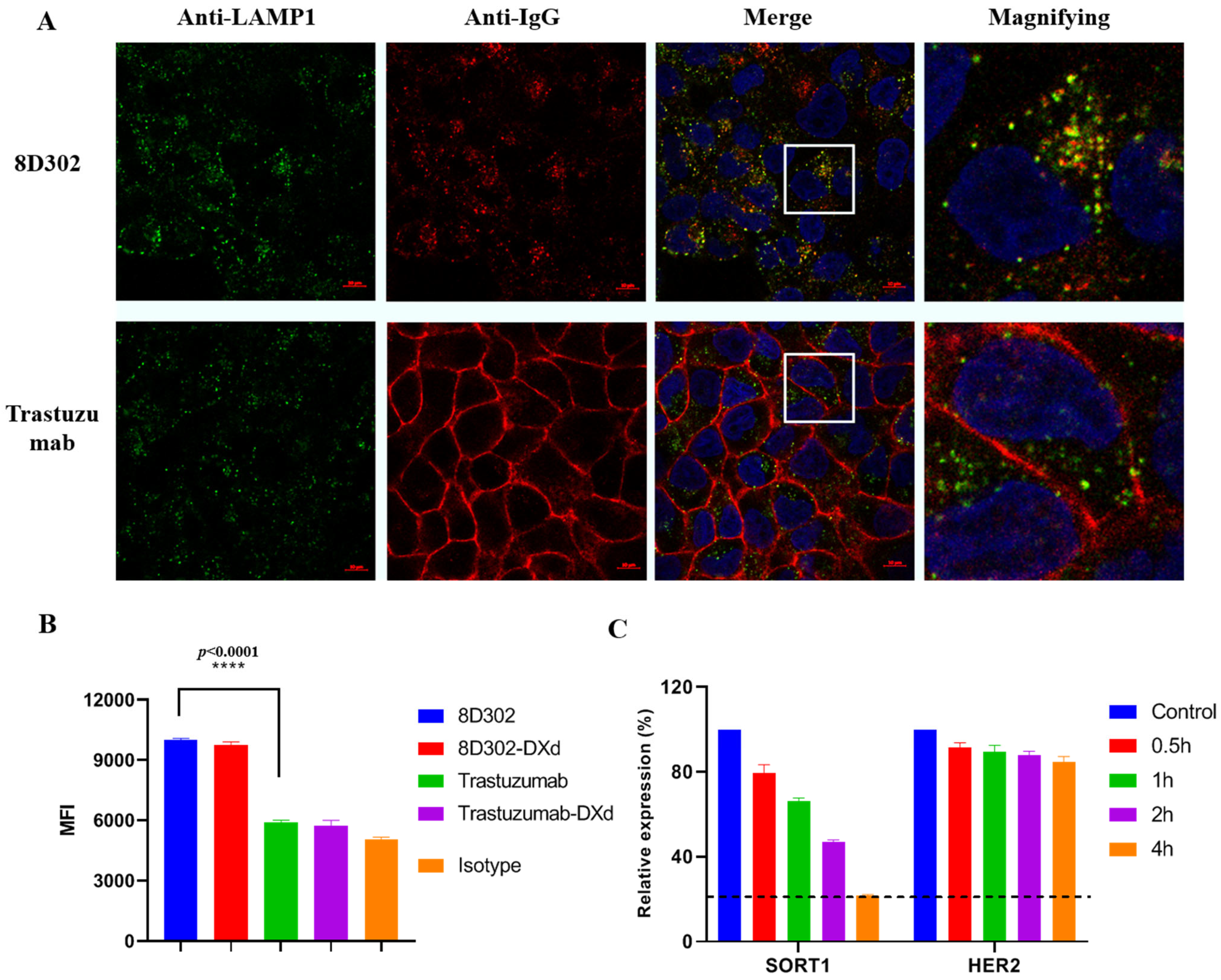
2.6. Comparison of Internalization Profile between SORT1 and HER2
2.7. Comparison of Anti-Tumor Effects between SORT1-Targeted ADC and HER2-Targeted ADC
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Antibodies and Conjugates
4.3. Binding Assay
4.4. Affinity Measurement
4.5. Confocal Microscopy
4.6. DAR Value Detection of ADC
4.7. Cytotoxicity Assay
4.8. Flow Cytometry
4.9. Xenograft Tumor Growth Model
4.10. Acute Toxicity
4.11. Pharmacokinetics Study
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | Antibody–drug conjugate |
| PDC | Peptide–drug conjugate |
| FBS | fetal bovine serum |
| FDA | U.S. Food and Drug Administration |
| MTD | maximal toxic dose |
| CDR | complementary determining region |
| MMAE | monomethylauristatin E |
| LC–MS | Liquid chromatography–tandem mass spectrometry |
| MFI | mean fluorescence intensity |
References
- Janthur, W.D.; Cantoni, N.; Mamot, C. Drug conjugates such as Antibody Drug Conjugates (ADCs), immunotoxins and immunoliposomes challenge daily clinical practice. Int. J. Mol. Sci. 2012, 13, 16020–16045. [Google Scholar] [CrossRef] [PubMed]
- Najjar, M.K.; Manore, S.G.; Regua, A.T.; Lo, H.W. Antibody-Drug Conjugates for the Treatment of HER2-Positive Breast Cancer. Genes 2022, 13, 2065. [Google Scholar] [CrossRef] [PubMed]
- Matusz-Fisher, A.; Tan, A.R. Sacituzumab govitecan and other antibody-drug conjugates targeting trophoblast cell-surface antigen 2 (Trop-2) in breast cancer. Ann. Transl. Med. 2022, 10, 1031. [Google Scholar] [CrossRef] [PubMed]
- Caldarella, A.; Crocetti, E.; Bianchi, S.; Vezzosi, V.; Urso, C.; Biancalani, M.; Zappa, M. Female breast cancer status according to ER, PR and HER2 expression: A population based analysis. Pathol. Oncol. Res. 2011, 17, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Sakach, E.; Sacks, R.; Kalinsky, K. Trop-2 as a Therapeutic Target in Breast Cancer. Cancers 2022, 14, 5936. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Kim, S.B.; Chung, W.P.; Im, S.A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.M.; Petry, V.; Chung, C.F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Tolaney, S.M.; Punie, K.; Loirat, D.; Oliveira, M.; Kalinsky, K.; Zelnak, A.; Aftimos, P.; Dalenc, F.; Sardesai, S.; et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 1148–1156. [Google Scholar] [CrossRef]
- Chen, Y.F.; Xu, Y.Y.; Shao, Z.M.; Yu, K.D. Resistance to antibody-drug conjugates in breast cancer: Mechanisms and solutions. Cancer Commun. 2023, 43, 297–337. [Google Scholar] [CrossRef]
- Corti, C.; Boscolo Bielo, L.; Schianca, A.C.; Salimbeni, B.T.; Criscitiello, C.; Curigliano, G. Future potential targets of antibody-drug conjugates in breast cancer. Breast 2023, 69, 312–322. [Google Scholar] [CrossRef]
- Hermey, G. The Vps10p-domain receptor family. Cell. Mol. Life Sci. 2009, 66, 2677–2689. [Google Scholar] [CrossRef]
- Mazella, J.; Zsürger, N.; Navarro, V.; Chabry, J.; Kaghad, M.; Caput, D.; Ferrara, P.; Vita, N.; Gully, D.; Maffrand, J.P.; et al. The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J. Biol. Chem. 1998, 273, 26273–26276. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Padukkavidana, T.; Vægter, C.B.; Brady, O.A.; Zheng, Y.; Mackenzie, I.R.; Feldman, H.H.; Nykjaer, A.; Strittmatter, S.M. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron 2010, 68, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.; Guo, L.; Strong, A.; Madrigal-Matute, J.; Wang, H.; Kaushik, S.; Brodsky, J.L.; Rader, D.J.; Cuervo, A.M.; Fisher, E.A. Autophagy Is Required for Sortilin-Mediated Degradation of Apolipoprotein B100. Circ. Res. 2018, 122, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Carlo, A.S. Sortilin, a novel APOE receptor implicated in Alzheimer disease. Prion 2013, 7, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Goettsch, C.; Kjolby, M.; Aikawa, E. Sortilin and Its Multiple Roles in Cardiovascular and Metabolic Diseases. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Rabinowich, L.; Fishman, S.; Hubel, E.; Thurm, T.; Park, W.J.; Pewzner-Jung, Y.; Saroha, A.; Erez, N.; Halpern, Z.; Futerman, A.H.; et al. Sortilin deficiency improves the metabolic phenotype and reduces hepatic steatosis of mice subjected to diet-induced obesity. J. Hepatol. 2015, 62, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Roselli, S.; Pundavela, J.; Demont, Y.; Faulkner, S.; Keene, S.; Attia, J.; Jiang, C.C.; Zhang, X.D.; Walker, M.M.; Hondermarck, H. Sortilin is associated with breast cancer aggressiveness and contributes to tumor cell adhesion and invasion. Oncotarget 2015, 6, 10473–10486. [Google Scholar] [CrossRef]
- Ghaemimanesh, F.; Ahmadian, G.; Talebi, S.; Zarnani, A.H.; Behmanesh, M.; Hemmati, S.; Hadavi, R.; Jeddi-Tehrani, M.; Farzi, M.; Akhondi, M.M.; et al. The effect of sortilin silencing on ovarian carcinoma cells. Avicenna J. Med. Biotechnol. 2014, 6, 169–177. [Google Scholar]
- Demeule, M.; Charfi, C.; Currie, J.C.; Larocque, A.; Zgheib, A.; Kozelko, S.; Béliveau, R.; Marsolais, C.; Annabi, B. TH1902, a new docetaxel-peptide conjugate for the treatment of sortilin-positive triple-negative breast cancer. Cancer Sci. 2021, 112, 4317–4334. [Google Scholar] [CrossRef]
- Safdari, Y.; Farajnia, S.; Asgharzadeh, M.; Khalili, M. Antibody humanization methods—A review and update. Biotechnol. Genet. Eng. Rev. 2013, 29, 175–186. [Google Scholar] [CrossRef]
- Morinville, A.; Martin, S.; Lavallée, M.; Vincent, J.P.; Beaudet, A.; Mazella, J. Internalization and trafficking of neurotensin via NTS3 receptors in HT29 cells. Int. J. Biochem. Cell Biol. 2004, 36, 2153–2168. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Gou, L.; Li, W.; Wang, Y. Antibody-drug conjugates: Recent advances in payloads. Acta Pharm. Sin. B 2023, 13, 4025–4059. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016, 107, 1039–1046. [Google Scholar] [CrossRef]
- de Goeij, B.E.; Satijn, D.; Freitag, C.M.; Wubbolts, R.; Bleeker, W.K.; Khasanov, A.; Zhu, T.; Chen, G.; Miao, D.; van Berkel, P.H.; et al. High turnover of tissue factor enables efficient intracellular delivery of antibody-drug conjugates. Mol. Cancer Ther. 2015, 14, 1130–1140. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Koyama, K.; Kamai, Y.; Hirotani, K.; Ogitani, Y.; Zembutsu, A.; Abe, M.; Kaneda, Y.; Maeda, N.; Shiose, Y.; et al. A Novel HER3-Targeting Antibody-Drug Conjugate, U3-1402, Exhibits Potent Therapeutic Efficacy through the Delivery of Cytotoxic Payload by Efficient Internalization. Clin. Cancer Res. 2019, 25, 7151–7161. [Google Scholar] [CrossRef]
- Andreev, J.; Thambi, N.; Perez Bay, A.E.; Delfino, F.; Martin, J.; Kelly, M.P.; Kirshner, J.R.; Rafique, A.; Kunz, A.; Nittoli, T.; et al. Bispecific Antibodies and Antibody-Drug Conjugates (ADCs) Bridging HER2 and Prolactin Receptor Improve Efficacy of HER2 ADCs. Mol. Cancer Ther. 2017, 16, 681–693. [Google Scholar] [CrossRef]
- Austin, C.D.; De Mazière, A.M.; Pisacane, P.I.; van Dijk, S.M.; Eigenbrot, C.; Sliwkowski, M.X.; Klumperman, J.; Scheller, R.H. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol. Biol. Cell 2004, 15, 5268–5282. [Google Scholar] [CrossRef]
- Takegawa, N.; Nonagase, Y.; Yonesaka, K.; Sakai, K.; Maenishi, O.; Ogitani, Y.; Tamura, T.; Nishio, K.; Nakagawa, K.; Tsurutani, J. DS-8201a, a new HER2-targeting antibody-drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int. J. Cancer 2017, 141, 1682–1689. [Google Scholar] [CrossRef]
- Li, Z.; Guo, S.; Xue, H.; Li, L.; Guo, Y.; Duan, S.; Zhu, H. Efficacy and safety of trastuzumab deruxtecan in the treatment of HER2-low/positive advanced breast cancer: A single-arm meta-analysis. Front. Pharmacol. 2023, 14, 1183514. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Narayan, P.; Osgood, C.L.; Wedam, S.; Prowell, T.M.; Gao, J.J.; Shah, M.; Krol, D.; Wahby, S.; Royce, M.; et al. U.S. FDA Drug Approvals for Breast Cancer: A Decade in Review. Clin. Cancer Res. 2022, 28, 1072–1086. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, C.M.; Yamaguchi, A.; Anami, Y.; Xiong, W.; Otani, Y.; Lee, J.; Ueno, N.T.; Zhang, N.; An, Z.; Tsuchikama, K. Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nat. Commun. 2021, 12, 3528. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Shitara, K.; Naito, Y.; Shimomura, A.; Fujiwara, Y.; Yonemori, K.; Shimizu, C.; Shimoi, T.; Kuboki, Y.; Matsubara, N.; et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: A phase 1 dose-escalation study. Lancet Oncol. 2017, 18, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, X.; Sun, X.; Li, J.; Wang, W.; Zhang, L.; Gou, S. Preclinical pharmacokinetics of a novel anti-c-Met antibody-drug conjugate, SHR-A1403, in rodents and non-human primates. Xenobiotica 2019, 49, 1097–1105. [Google Scholar] [CrossRef]
- Tsumura, R.; Anzai, T.; Manabe, S.; Takashima, H.; Koga, Y.; Yasunaga, M.; Matsumura, Y. Antitumor effect of humanized anti-tissue factor antibody-drug conjugate in a model of peritoneal disseminated pancreatic cancer. Oncol. Rep. 2021, 45, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Y.; Gong, J.; Zhang, X.; Peng, Z.; Sheng, X.; Mao, C.; Fan, Q.; Bai, Y.; Ba, Y.; et al. Phase I study of the recombinant humanized anti-HER2 monoclonal antibody-MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors. Gastric Cancer 2021, 24, 913–925. [Google Scholar] [CrossRef]
- Yao, B.; Gao, X.; Dan, M.; Yuan, C.; Hu, X.; Sun, Z.; Hui, X.; Liu, B.; Ouyang, P.; Chen, G. Selection of Payloads for Antibody-Drug Conjugates Targeting Ubiquitously Expressed Tumor-Associated Antigens: A Case Study. AAPS J. 2022, 24, 70. [Google Scholar] [CrossRef]
- Anami, Y.; Yamazaki, C.M.; Xiong, W.; Gui, X.; Zhang, N.; An, Z.; Tsuchikama, K. Glutamic acid-valine-citrulline linkers ensure stability and efficacy of antibody-drug conjugates in mice. Nat. Commun. 2018, 9, 2512. [Google Scholar] [CrossRef]
- Hammood, M.; Craig, A.W.; Leyton, J.V. Impact of Endocytosis Mechanisms for the Receptors Targeted by the Currently Approved Antibody-Drug Conjugates (ADCs)-A Necessity for Future ADC Research and Development. Pharmaceuticals 2021, 14, 674. [Google Scholar] [CrossRef]
- Lemech, C.; Woodward, N.; Chan, N.; Mortimer, J.; Naumovski, L.; Nuthalapati, S.; Tong, B.; Jiang, F.; Ansell, P.; Ratajczak, C.K.; et al. A first-in-human, phase 1, dose-escalation study of ABBV-176, an antibody-drug conjugate targeting the prolactin receptor, in patients with advanced solid tumors. Investig. New Drugs 2020, 38, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Jänne, P.A.; Baik, C.; Su, W.C.; Johnson, M.L.; Hayashi, H.; Nishio, M.; Kim, D.W.; Koczywas, M.; Gold, K.A.; Steuer, C.E.; et al. Efficacy and Safety of Patritumab Deruxtecan (HER3-DXd) in EGFR Inhibitor-Resistant, EGFR-Mutated Non-Small Cell Lung Cancer. Cancer Discov. 2022, 12, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Gogia, P.; Ashraf, H.; Bhasin, S.; Xu, Y. Antibody-Drug Conjugates: A Review of Approved Drugs and Their Clinical Level of Evidence. Cancers 2023, 15, 3886. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Jiang, J.; Wang, X.; Huang, C.; Li, D.; Xie, K.; Xu, Q.; Li, H.; Li, Z.; Lou, L.; et al. A novel humanized anti-HER2 antibody conjugated with MMAE exerts potent anti-tumor activity. Breast Cancer Res. Treat. 2015, 153, 123–133. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, W.; Zhang, W.; Xie, L.; Wang, L.; Li, Y.; Wang, Z.; Zhang, A.; Qiu, H.; Feng, J.; Zhang, B.; et al. Generation and Characterization of SORT1-Targeted Antibody–Drug Conjugate for the Treatment of SORT1-Positive Breast Tumor. Int. J. Mol. Sci. 2023, 24, 17631. https://doi.org/10.3390/ijms242417631
Zhuang W, Zhang W, Xie L, Wang L, Li Y, Wang Z, Zhang A, Qiu H, Feng J, Zhang B, et al. Generation and Characterization of SORT1-Targeted Antibody–Drug Conjugate for the Treatment of SORT1-Positive Breast Tumor. International Journal of Molecular Sciences. 2023; 24(24):17631. https://doi.org/10.3390/ijms242417631
Chicago/Turabian StyleZhuang, Weiliang, Wei Zhang, Liping Xie, Lei Wang, Yuan Li, Ziyu Wang, Ao Zhang, Haitao Qiu, Jun Feng, Baohong Zhang, and et al. 2023. "Generation and Characterization of SORT1-Targeted Antibody–Drug Conjugate for the Treatment of SORT1-Positive Breast Tumor" International Journal of Molecular Sciences 24, no. 24: 17631. https://doi.org/10.3390/ijms242417631
APA StyleZhuang, W., Zhang, W., Xie, L., Wang, L., Li, Y., Wang, Z., Zhang, A., Qiu, H., Feng, J., Zhang, B., & Hu, Y. (2023). Generation and Characterization of SORT1-Targeted Antibody–Drug Conjugate for the Treatment of SORT1-Positive Breast Tumor. International Journal of Molecular Sciences, 24(24), 17631. https://doi.org/10.3390/ijms242417631





