Longitudinal Follow-Up of Serum and Urine Biomarkers Indicative of COVID-19-Associated Acute Kidney Injury: Diagnostic and Prognostic Impacts
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics of Patients at Inclusion
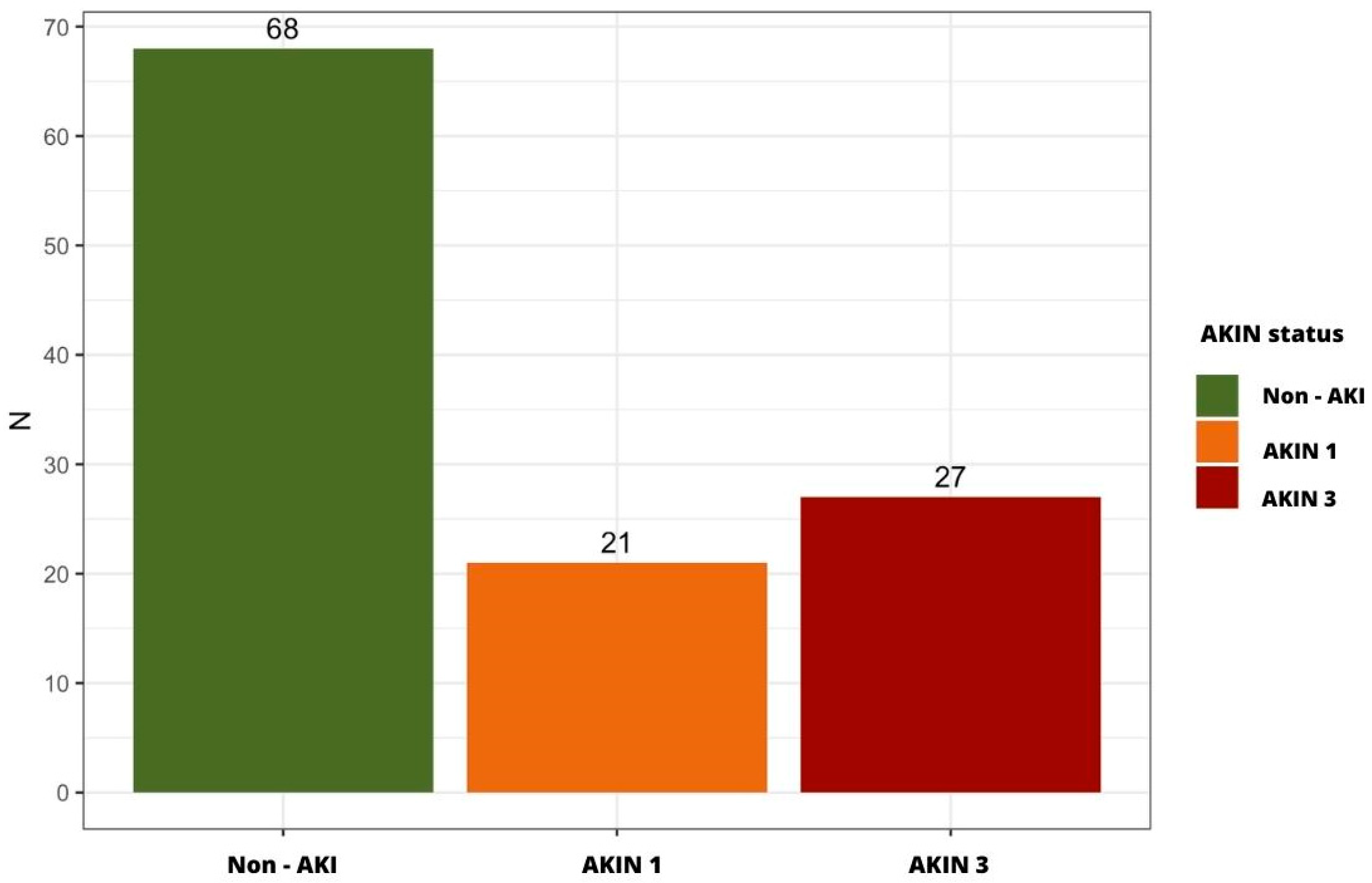
2.2. Incidence of AKI in the Study Cohort
2.3. Clinical Factors and Biological Parameters Associated with Early-Onset AKI in COVID-19 Patients
2.4. AKI Predictive Biomarkers
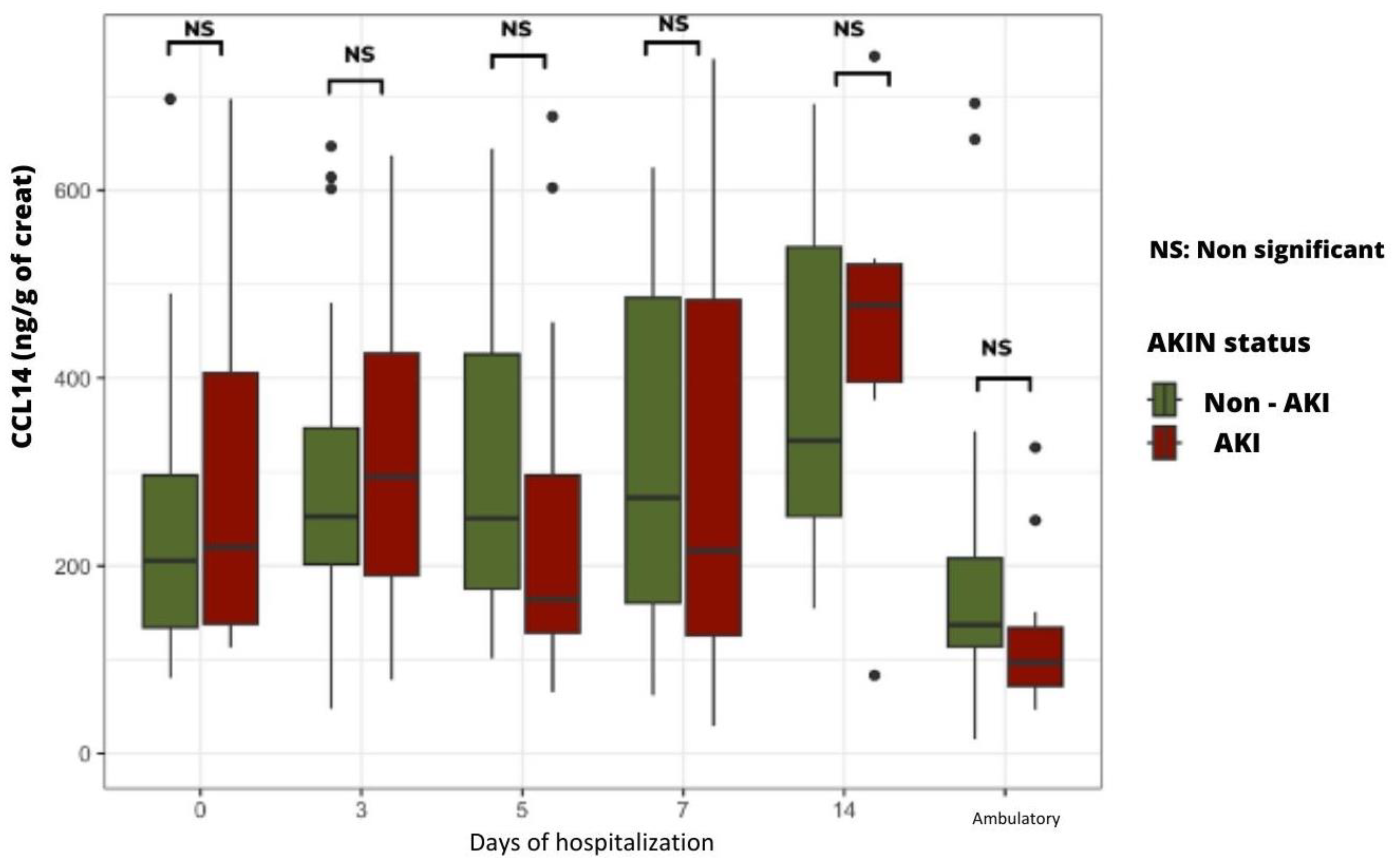
2.4.1. Time Course of Urinary LAP and CCL14 According to the Presence or Absence of AKI
2.4.2. Time Course of Plasma and Urinary NGAL Levels According to the Presence or Absence of AKI
2.5. Morbi-Mortality Prognosis Biomarkers
2.5.1. Time Course of Cystatin C Plasma Levels
2.5.2. Time Course of Plasma suPAR Levels According to the Severity of Clinical Condition
2.5.3. Time Course of suPAR Plasma Levels According to Patients’ Life Prognosis
3. Discussion
4. Materials and Methods
4.1. Data Collection
4.2. Study Variables
4.3. Selection and Assay of Specific Biomarkers
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Prac. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.S.; Ng, J.H.; Ross, D.W.; Sharma, P.; Shah, H.H.; Barnett, R.L.; Hazzan, A.D.; Fishbane, S.; Jhaveri, K.D.; on behalf of the Northwell COVID-19 Research Consortium and theNorthwell Nephrology COVID-19 Research Consortium. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020, 98, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Chaudhary, K.; Saha, A.; Chauhan, K.; Vaid, A.; Baweja, M.; Campbell, K.; Chun, N.; Chung, M.; Deshpande, P.; et al. Acute Kidney Injury in Hospitalized Patients with COVID-19. medRxiv 2020. Available online: https://www.medrxiv.org/content/10.1101/2020.05.04.20090944v1 (accessed on 8 May 2023).
- Oweis, A.O.; A Alshelleh, S.; Hawasly, L.; Alsabbagh, G.; Alzoubi, K.H. Acute Kidney Injury among Hospital-Admitted COVID-19 Patients: A Study from Jordan. Int. J. Gen. Med. 2022, 15, 4475–4482. [Google Scholar] [CrossRef] [PubMed]
- Burtey, S.; Sallée, M. Les atteintes rénales de la COVID-19. Néphrologie & Thérapeutique. Nation Libr. Med. 2021, 17, 203–207. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8221000/ (accessed on 22 August 2022).
- Ricci, Z.; Cruz, D.; Ronco, C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008, 73, 538–546. [Google Scholar] [CrossRef]
- Silver, S.A.; Long, J.; Zheng, Y.; Chertow, G.M. Cost of Acute Kidney Injury in Hospitalized Patients. J. Hosp. Med. 2017, 12, 70–76. [Google Scholar] [CrossRef]
- Coca, S.G.; Yusuf, B.; Shlipak, M.G.; Garg, A.X.; Parikh, C.R. Long-term Risk of Mortality and Other Adverse Outcomes after Acute Kidney Injury: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2009, 53, 961–973. [Google Scholar] [CrossRef]
- Ahmadian, E.; Khatibi, S.M.H.; Soofiyani, S.R.; Abediazar, S.; Shoja, M.M.; Ardalan, M.; Vahed, S.Z. COVID-19 and kidney injury: Pathophysiology and molecular mechanisms. Rev. Med. Virol. 2020, 31, e2176. [Google Scholar] [CrossRef]
- Buonaguro, F.M.; Ascierto, P.A.; Morse, G.D.; Buonaguro, L.; Puzanov, I.; Tornesello, M.L.; Bréchot, C.; Gallo, R.C. COVID-19: Time for a paradigm change. Rev. Med. Virol. 2020, 3, 30. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 1–8. Available online: https://www.nature.com/articles/s41577-020-0331-4 (accessed on 29 September 2023). [CrossRef]
- Yalameha, B.; Roshan, B.; Bhaskar, L.V.; Mohmoodnia, L. Perspectives on the relationship of renal disease and coronavirus disease 2019. J. Nephropharmacology 2020, 9, e22. [Google Scholar] [CrossRef]
- Pan, X.W.; Xu, D.; Zhang, H.; Zhou, W.; Wang, L.-H.; Cui, X.G. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: A study based on single-cell transcriptome analysis. Intensive Care Med. 2020, 46, 1114–1116. [Google Scholar] [CrossRef]
- Coca, S.; Yalavarthy, R.; Concato, J.; Parikh, C. Biomarkers for the diagnosis and risk stratification of acute kidney injury: A systematic review. Kidney Int. 2008, 73, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.; the RUBY Investigators; Bihorac, A.; Al-Khafaji, A.; Ortega, L.M.; Ostermann, M.; Haase, M.; Zacharowski, K.; Wunderink, R.; Heung, M.; et al. Identification and validation of biomarkers of persistent acute kidney injury: The RUBY study. Intensiv. Care Med. 2020, 46, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Chaudhary, K.; Saha, A.; Chauhan, K.; Vaid, A.; Zhao, S.; Paranjpe, I.; Somani, S.; Richter, F.; Miotto, R.; et al. AKI in Hospitalized Patients with COVID-19. J. Am. Soc. Nephrol. 2020, 32, 151. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Lukitsch, I.; Torres-Ortiz, A.E.; Walker, J.B.; Varghese, V.; Hernandez-Arroyo, C.F.; Alqudsi, M.; LeDoux, J.R.; Velez, J.C.Q. Acute Kidney Injury Associated with Coronavirus Disease 2019 in Urban New Orleans. Kidney360 2020, 1, 614–622. [Google Scholar] [CrossRef]
- Soto, G.J.; Frank, A.; Christiani, D.C.; Gong, M.N. Body mass index and acute kidney injury in the acute respiratory distress syndrome. Crit. Care Med. 2012, 40, 2601–2608. [Google Scholar] [CrossRef]
- Azam, T.U.; Shadid, H.R.; Blakely, P.; O’Hayer, P.; Berlin, H.; Pan, M.; Zhao, P.; Zhao, L.; Pennathur, S.; Pop-Busui, R.; et al. Soluble Urokinase Receptor (SuPAR) in COVID-19–Related AKI. J. Am. Soc. Nephrol. 2020, 31, 2725–2735. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7608953/ (accessed on 10 April 2022). [CrossRef]
- Lee, J.D.; Silberzweig, J.; Akchurin, O.; Choi, M.E.; Srivatana, V.; Lin, J.; Liu, F.; Malha, L.; Lubetzky, M.; Dadha-nia, D.M.; et al. Characteristics of Acute Kidney Injury in Hospitalized COVID-19 Patients in an Urban Academic Medical Center. Clin. J. Am. Soc. Nephrol. 2021, 16, 284–286. [Google Scholar] [CrossRef]
- Yang, X.; Jin, Y.; Li, R.; Zhang, Z.; Sun, R.; Chen, D. Prevalence and impact of acute renal impairment on COVID-19: A systematic review and meta-analysis. Crit. Care 2020, 24, 1–8. [Google Scholar] [CrossRef]
- Wasfy, S.; Wasfey, E.; Elmaraghy, A.; AbdelFatah, E.; Tharwat, A. Cystatin C and Neutrophil Gelatin-associated Lipocalin (NGAL) Can Predict Acute Kidney Injury and In-Hospital Mortality in COVID-19 Patients. J. Cell. Mol. Anesth. 2022, 7, 32–39. Available online: https://journals.sbmu.ac.ir/jcma/article/view/36855 (accessed on 15 May 2023).
- Xu, K.; Shang, N.; Levitman, A.; Corker, A.; Kudose, S.; Yaeh, A.; Neupane, U.; Stevens, J.; Sampogna, R.; Mills, A.M.; et al. Elevated Neutrophil Gelatinase-Associated Lipocalin Is Associated With the Severity of Kidney Injury and Poor Prognosis of Patients With COVID-19. Kidney Int. Rep. 2021, 6, 2979–2992. [Google Scholar] [CrossRef] [PubMed]
- Koyner, J.L.; Chawla, L.S.; Bihorac, A.; Gunnerson, K.J.; Schroeder, R.; Demirjian, S.; Hodgson, L.; Frey, J.A.; Wilber, S.T.; Kampf, J.P.; et al. Performance of a Standardized Clinical Assay for Urinary C–C Motif Chemokine Ligand 14 (CCL14) for Persistent Severe Acute Kidney Injury. Kidney360 2022, 3, 1158–1168. [Google Scholar] [CrossRef]
- Hayek, S.S.; Sever, S.; Ko, Y.A.; Trachtman, H.; Awad, M.; Wadhwani, S.; Altintas, M.M.; Wei, C.; Hotton, A.L.; French, A.L.; et al. Soluble Urokinase Receptor and Chronic Kidney Disease. N. Engl. J. Med. 2015, 373, 1916–1925. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Sun, W.; Li, J.; Wei, B.; Liu, W.; Wang, X.; Song, F.; Chen, L.; Yang, J.; Yu, L. Serum Cystatin C and Coronavirus Disease 2019: A Potential Inflammatory Biomarker in Predicting Critical Illness and Mortality for Adult Patients. Mediat. Inflamm. 2020, 2020, 3764515. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chen, Y.-C. Acute kidney injury classification: AKIN and RIFLE criteria in critical patients. World J. Crit. Care Med. 2012, 1, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, M.; Zarbock, A.; Goldstein, S.; Kashani, K.; Macedo, E.; Murugan, R.; Bell, M.; Forni, L.; Guzzi, L.; Joannidis, M.; et al. Recommendations on Acute Kidney Injury Biomarkers From the Acute Disease Quality Initiative Consensus Conference. JAMA Netw. Open 2020, 3, e2019209. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | Data (N = 116) |
|---|---|
| Gender Female Male | 44 (37.9%) 72 (62.1%) |
| Average age (years) | 60.7 (from 18 to 85) |
| Active smoking | 36 (31%) |
| Average BMI Overweight (25–29.9) Moderate obesity (30–34.9) Severe or major obesity (>35) | 30 (17 to 53) 41 (35%) 29 (25%) 20 (17%) |
| ICU admission | 54 (46.6%) |
| Deaths | 21 (18.1%) |
| Comorbidities Type 2 diabetes HTA CKD Neurological disease Rheumatological disease Neoplasia status Pulmonary disease | 36 (31%) 69 (59.5%) 15 (12.9%) 10 (8.6%) 12 (10.3%) 14 (12%) 18 (15.5%) |
| Characteristics and Parameters | Non-AKI (N = 68) | AKI (N = 48) | p-Value |
|---|---|---|---|
| Gender Female Male | 29 (43%) 39 (57%) | 15 (31%) 33 (69%) | 0.25 |
| Age <65 years ≥65 years | 38 (56%) 30 (44%) | 29 (60%) 19 (40%) | 0.7 |
| Active smoking | 19 (28%) | 16 (33%) | 0.54 |
| Deaths | 11 (16%) | 10 (21%) | 0.47 |
| BMI > 30 | 18 (26%) | 32 (67%) | <0.05 |
| ICU admission | 24 (35%) | 29 (60%) | <0.05 |
| Comorbidities Hypertension Type 2 diabetes CKD Neurological disease Rheumatology disease Neoplasia status Pulmonary disease | 33 (49%) 19 (28%) 8 (12%) 5 (7%) 9 (13%) 7 (10%) 9 (13%) | 35 (73%) 17 (35%) 6 (12%) 4 (8%) 3 (6%) 6 (12%) 9 (19%) | <0.05 0.42 1 1 0.36 0.77 0.44 |
| Day 0 Hemoglobin (g/dL) White blood cells Platelets CRP (mg/L) ASAT (UI/L) Urea (mg/dL) Creatininemia (mg/dL) Urinary creatinine (mmol/L) Proteinuria (mg/L) | 13 [11, 14] 7.0 [4.9, 10] 240 [180, 340] 70 [26, 110] 39 [26, 52] 33 [25, 44] 0.79 [0.66, 0.99] 120 [64, 160] 0.44 [0.02, 1.14] | 13 [12, 14] 6.8 [5.1, 8.3] 230 [160, 310] 110 [51, 190] 37 [27, 52] 44 [31, 63] 0.93 [0.72, 1.1] 140 [110, 200] 0.59 [0.13, 1.69] | 0.75 0.36 0.21 <0.05 0.37 <0.05 <0.05 <0.05 0.425 |
| Day 3 ASAT (UI/L) Urea (mg/dL) Creatininemia (mg/dL) Urinary creatinine (mmol/L) | 27 [21, 44] 34 [29, 49] 0.73 [0.59, 0.87] 84 [54, 140] | 37 [27, 59] 46 [35, 65] 0.73 [0.63, 1.1] 110 [86, 140] | <0.05 <0.05 0.214 0.108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lablad, Y.; Vanhomwegen, C.; De Prez, E.; Antoine, M.-H.; Hasan, S.; Baudoux, T.; Nortier, J. Longitudinal Follow-Up of Serum and Urine Biomarkers Indicative of COVID-19-Associated Acute Kidney Injury: Diagnostic and Prognostic Impacts. Int. J. Mol. Sci. 2023, 24, 16495. https://doi.org/10.3390/ijms242216495
Lablad Y, Vanhomwegen C, De Prez E, Antoine M-H, Hasan S, Baudoux T, Nortier J. Longitudinal Follow-Up of Serum and Urine Biomarkers Indicative of COVID-19-Associated Acute Kidney Injury: Diagnostic and Prognostic Impacts. International Journal of Molecular Sciences. 2023; 24(22):16495. https://doi.org/10.3390/ijms242216495
Chicago/Turabian StyleLablad, Yahya, Charlotte Vanhomwegen, Eric De Prez, Marie-Hélène Antoine, Sania Hasan, Thomas Baudoux, and Joëlle Nortier. 2023. "Longitudinal Follow-Up of Serum and Urine Biomarkers Indicative of COVID-19-Associated Acute Kidney Injury: Diagnostic and Prognostic Impacts" International Journal of Molecular Sciences 24, no. 22: 16495. https://doi.org/10.3390/ijms242216495
APA StyleLablad, Y., Vanhomwegen, C., De Prez, E., Antoine, M.-H., Hasan, S., Baudoux, T., & Nortier, J. (2023). Longitudinal Follow-Up of Serum and Urine Biomarkers Indicative of COVID-19-Associated Acute Kidney Injury: Diagnostic and Prognostic Impacts. International Journal of Molecular Sciences, 24(22), 16495. https://doi.org/10.3390/ijms242216495





