Genome-Wide Identification and Analysis of the ABCF Gene Family in Triticum aestivum
Abstract
1. Introduction
2. Results
2.1. Identification and Analysis of ABCF in Triticum aestivum
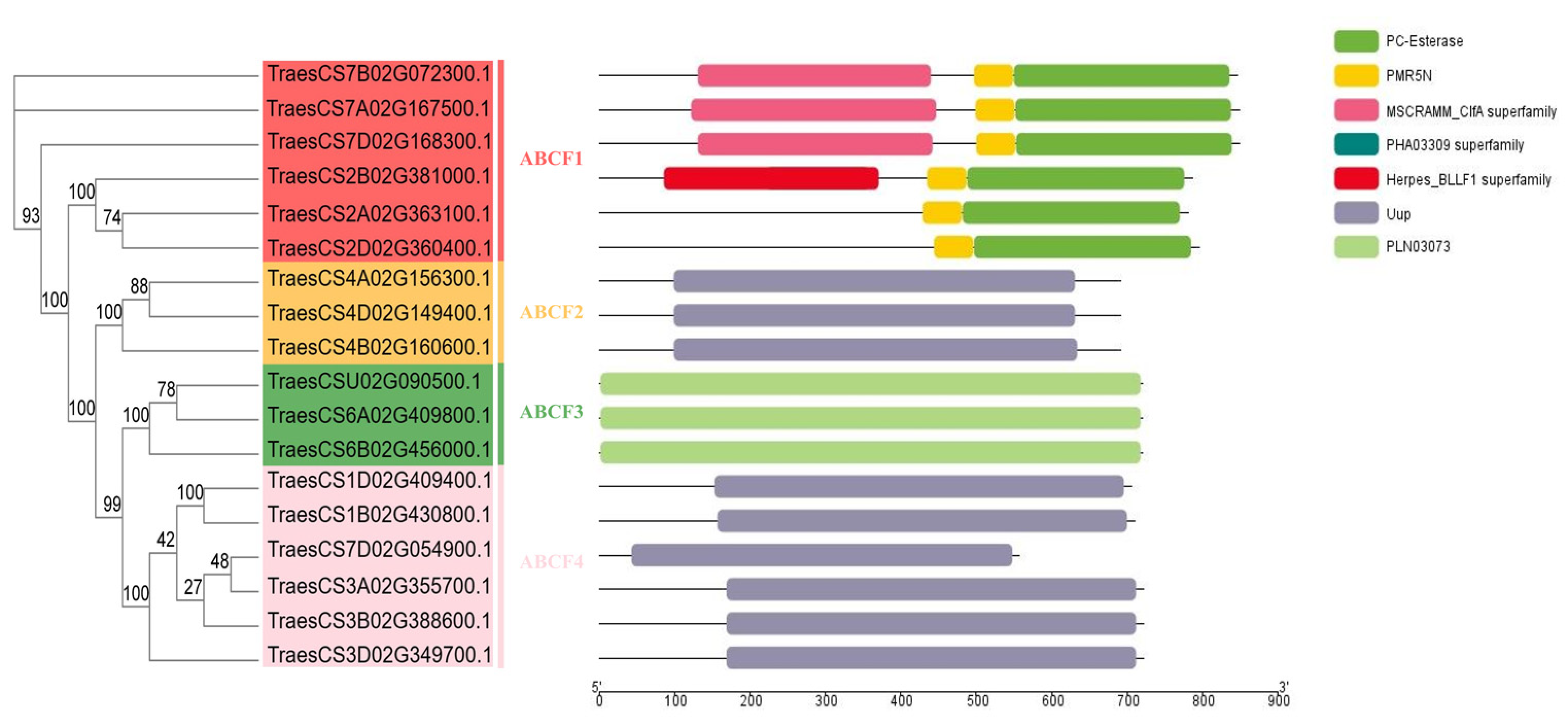
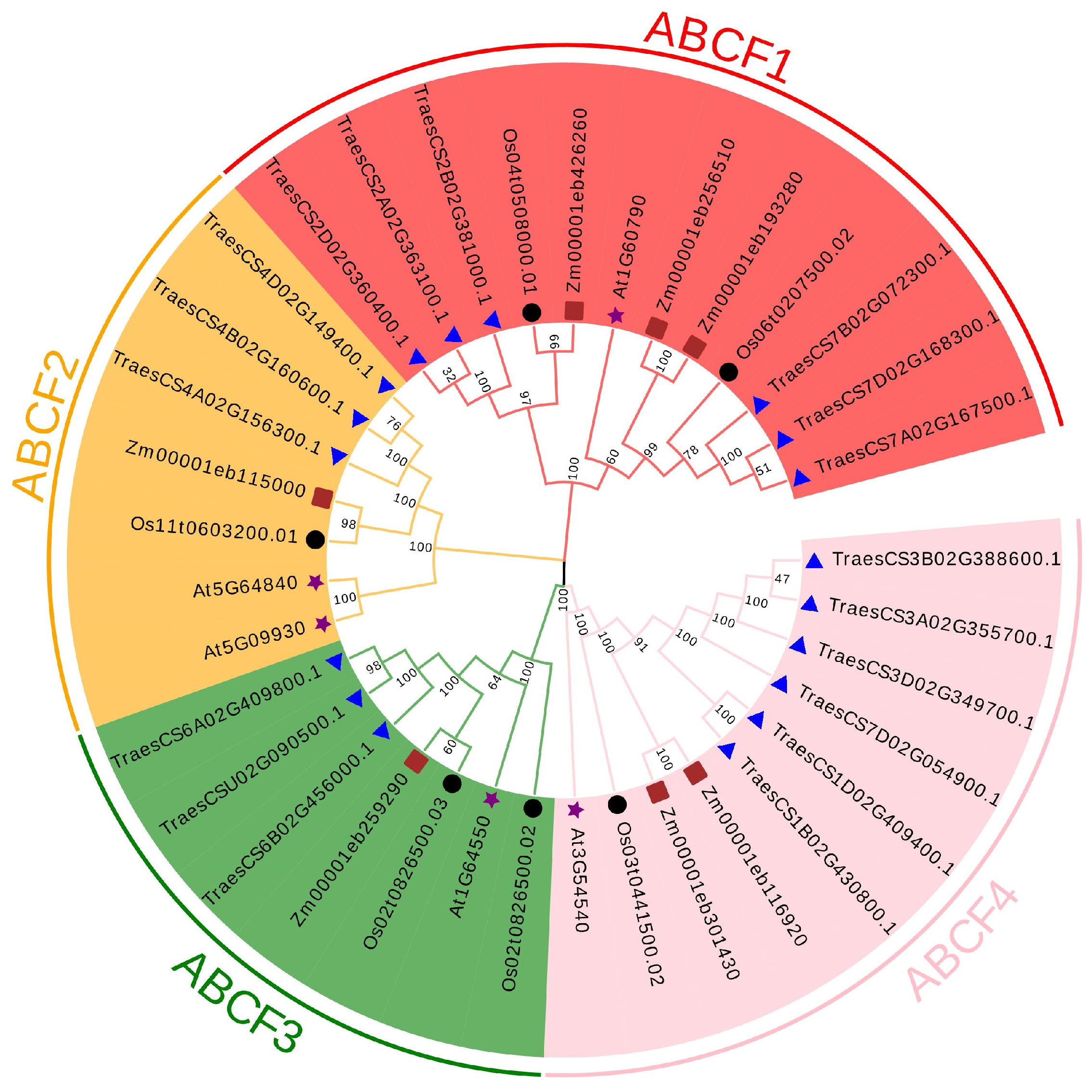
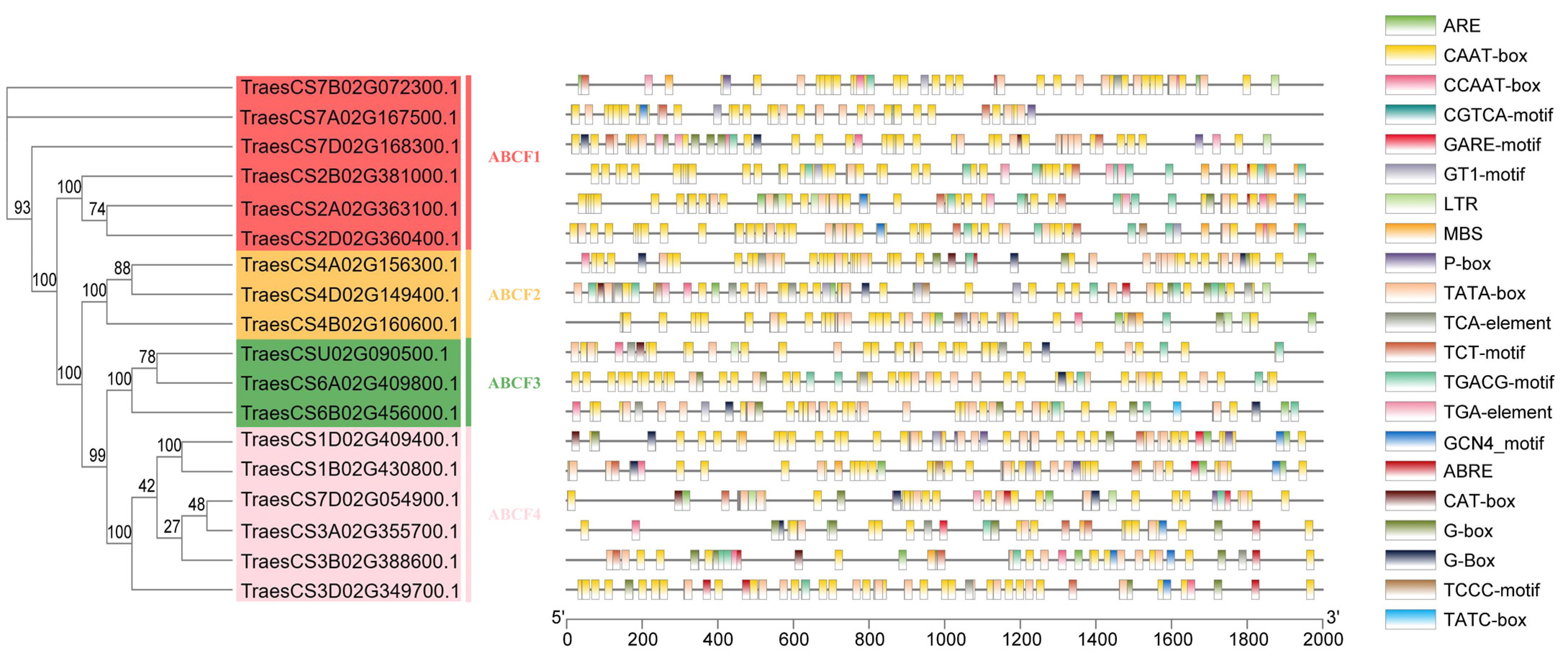
2.2. Phylogenetic Analysis of the ABCF Proteins
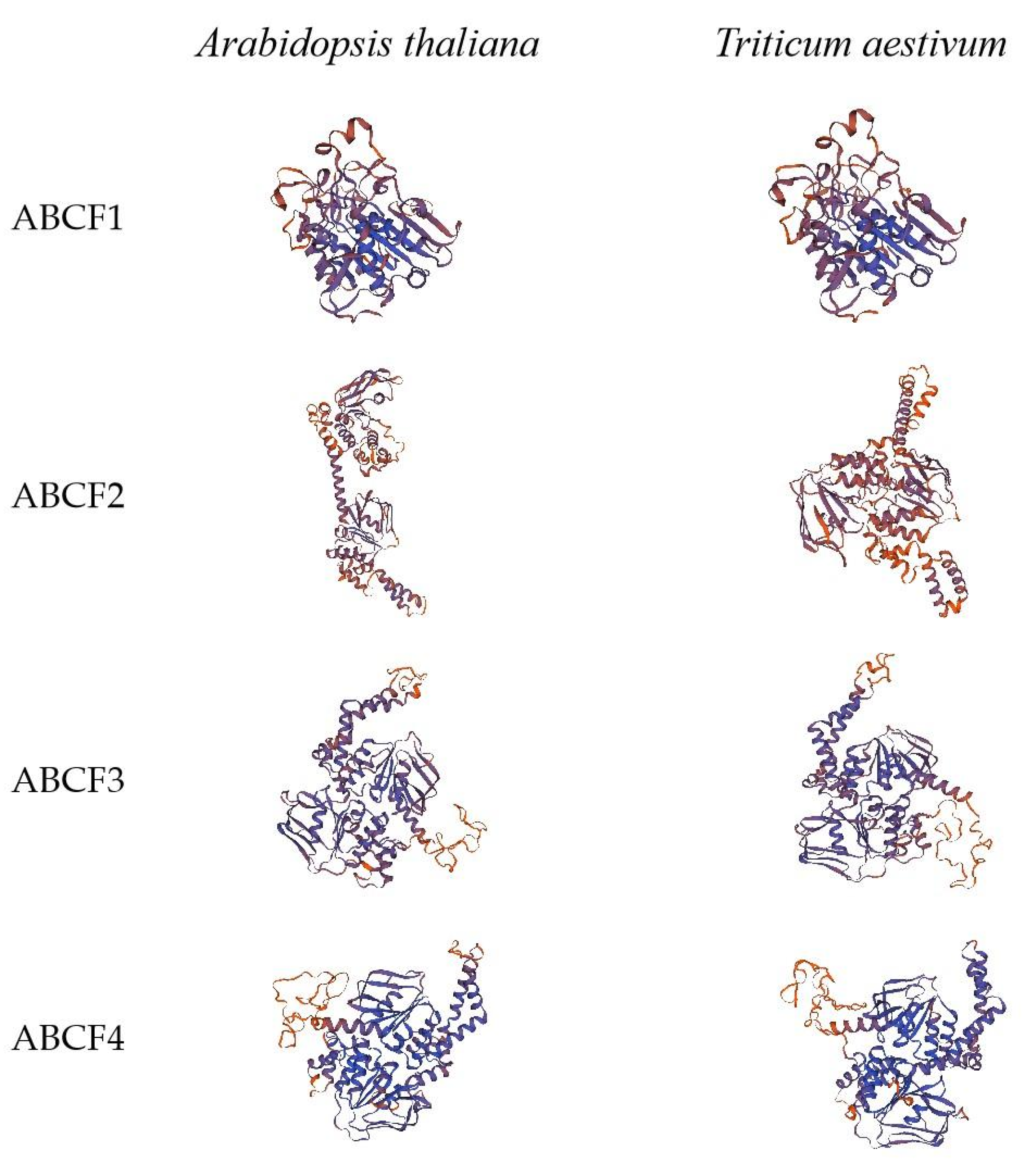
2.3. Tertiary Structure Models of ABCF Protein
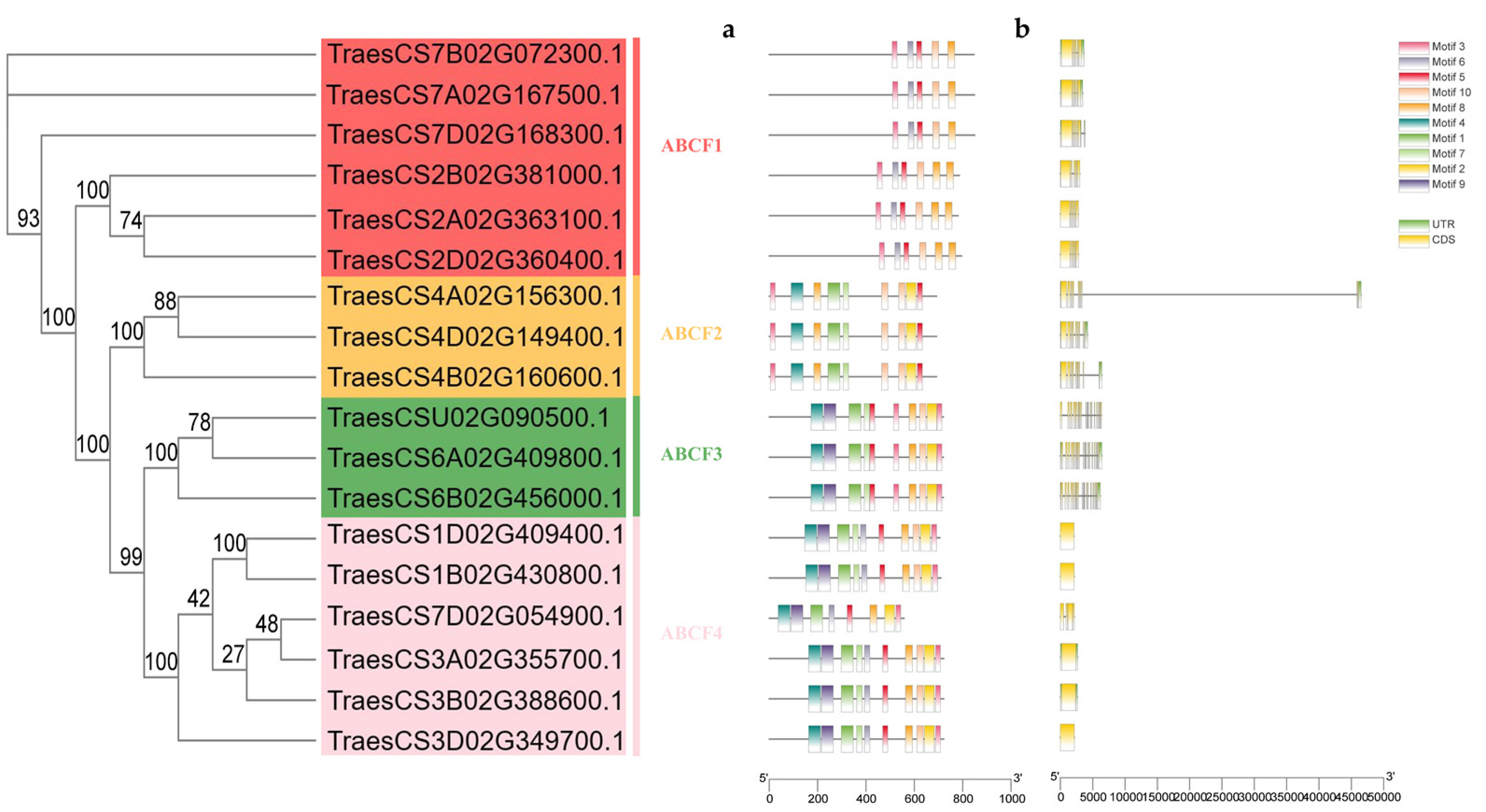
2.4. Analysis of TaABCF Structure and Conserved Motifs
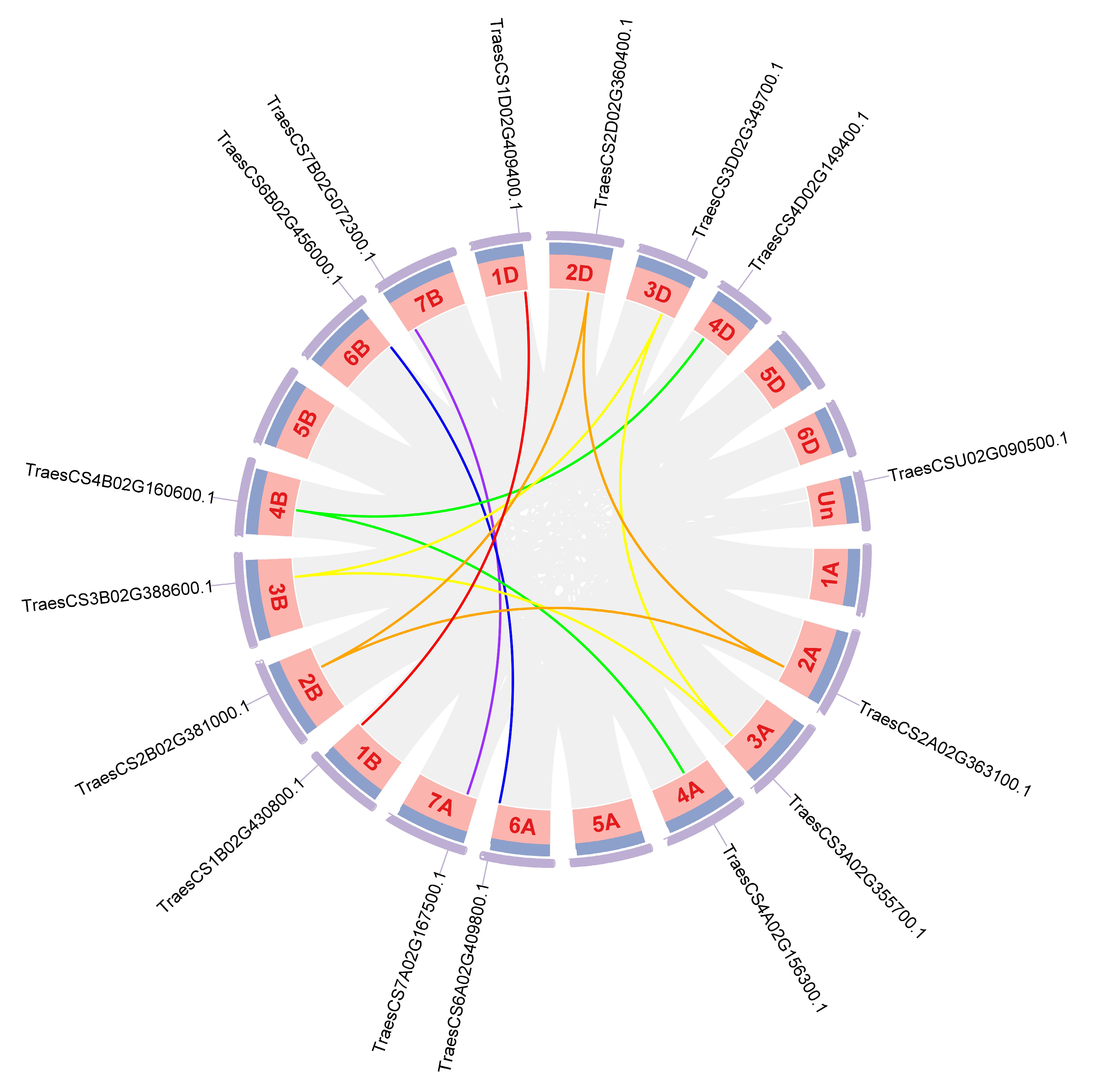
2.5. Chromosomal Location, Synteny Analysis, and Duplication Events of TaABCF
2.6. Prediction and Analysis of Cis-Acting Elements in the Promoter Regions of TaABCF Genes
2.7. Tissue-Specific Analysis of TaABCF Expression
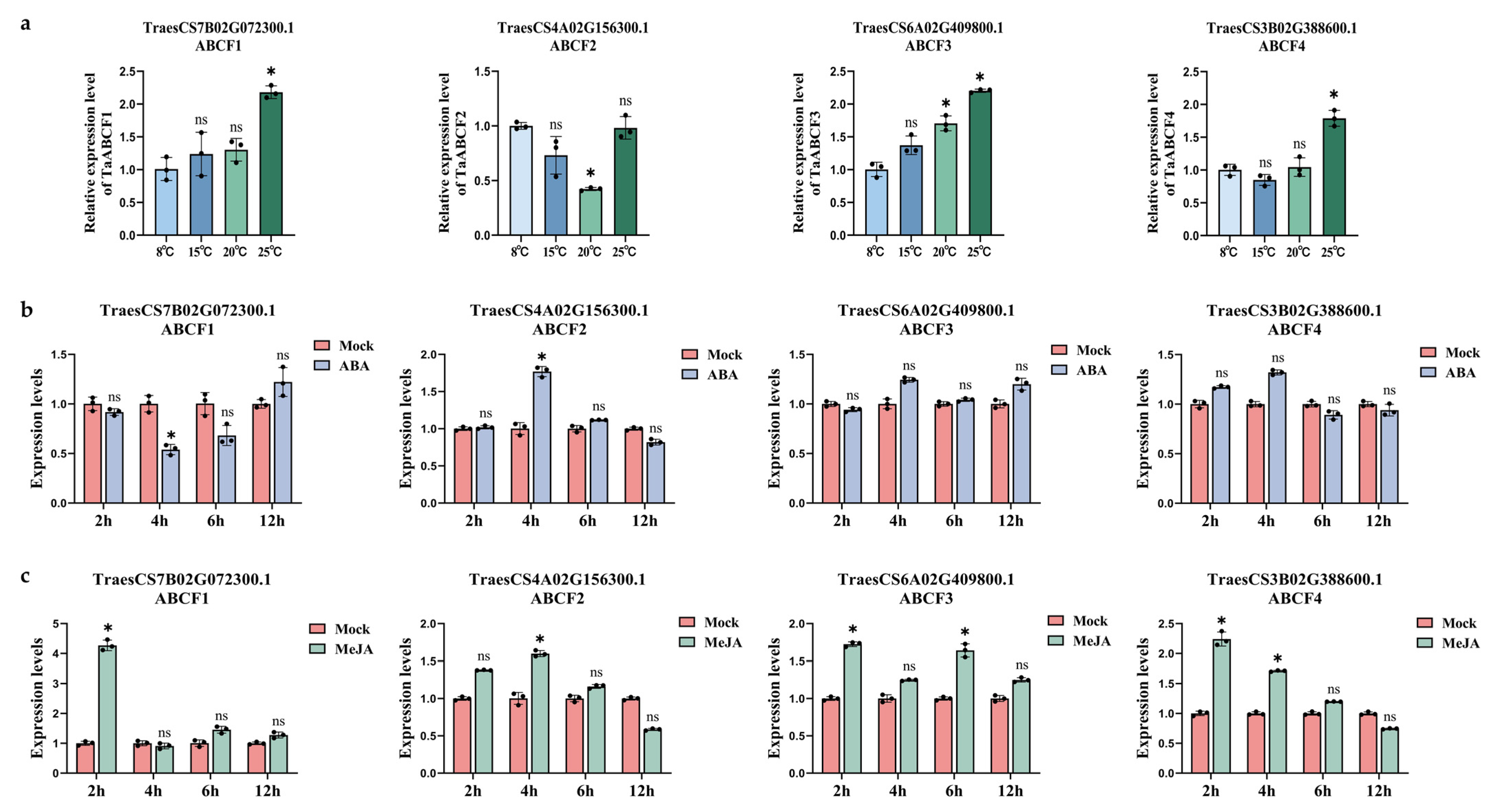
2.8. Expression Patterns of TaABCFs under Different Stresses
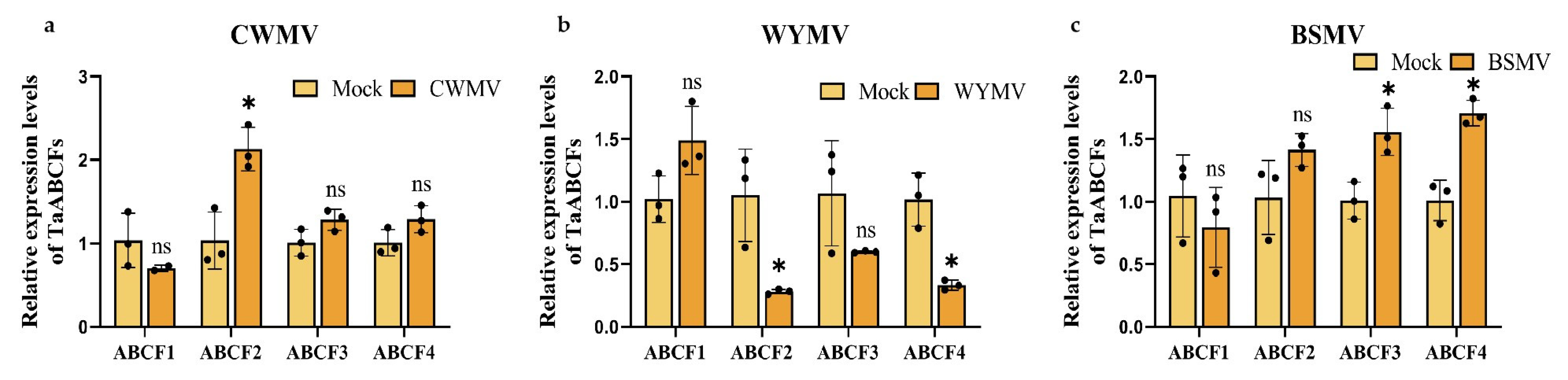
2.9. Analysis of TaABCF Expression after Different Viral Infections
2.10. Functional Analysis of TaABCF2 in Wheat Resistance to CWMV or WYMV Infection
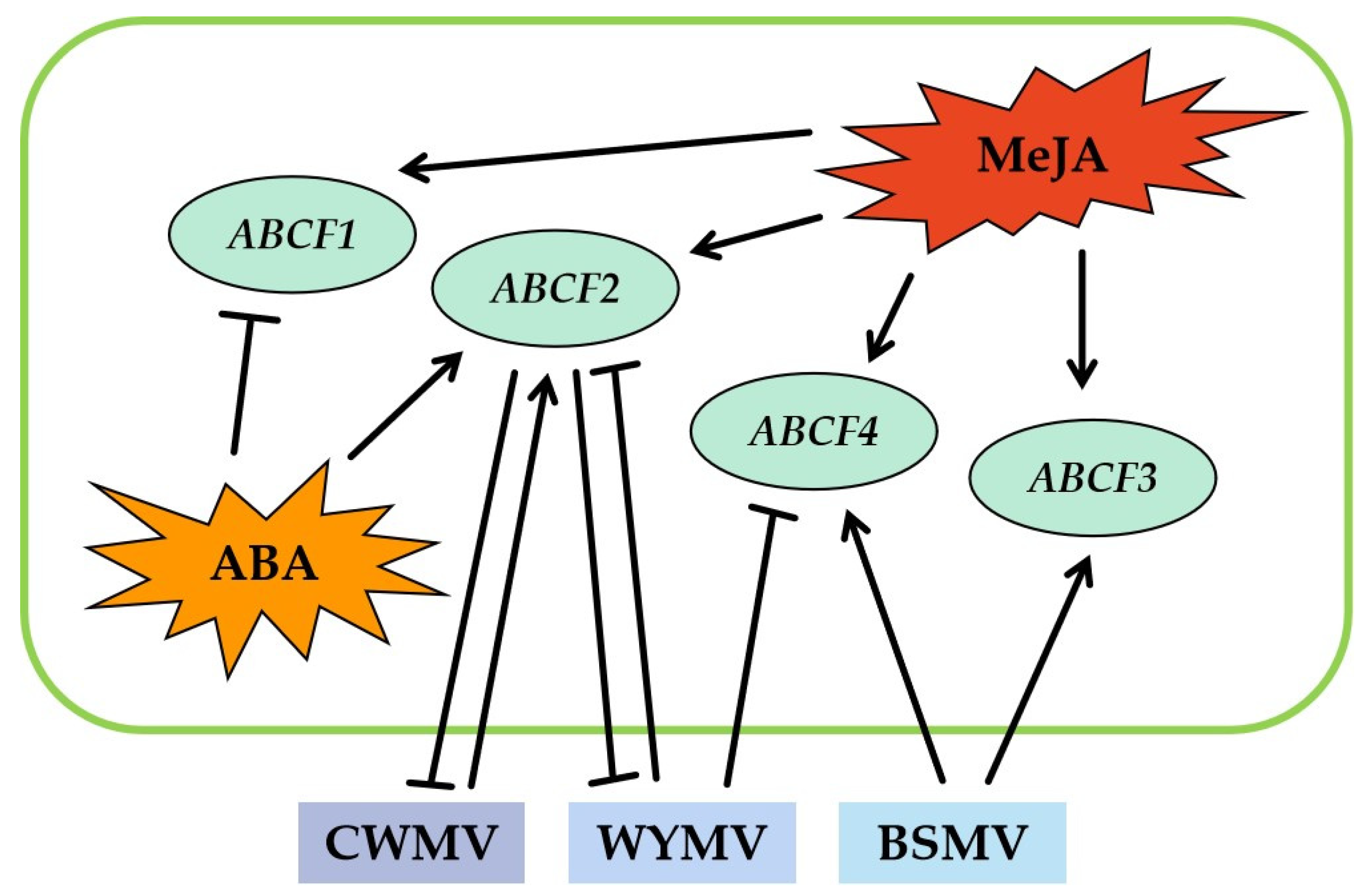
2.11. A Model Summarising the Function of TaABCFs in the Response of Wheat to Virus Infection
3. Discussion
4. Materials and Methods
4.1. Genome-Wide Identification of TaABCF Family Genes
4.2. Multiple Sequence Alignment and Phylogenetic Tree Construction
4.3. Structural Prediction of TaABCF Protein
4.4. Gene Structural Domain, Gene Structure, and Motif Analysis of TaABCF
4.5. Chromosome Localisation and Gene Duplication
4.6. Calculation of Ka/Ks Values
4.7. Prediction of Cis-Acting Elements in the Promoter Region
4.8. Plant Material, Growth Conditions, and Virus Inoculation
4.9. Plant RNA Isolation and RT-qPCR Assay
4.10. Tissue-Specific Expression of TaABCF
4.11. Expression of TaABCF under ABA and MeJA Treatments
4.12. Virus-Induced Gene Silencing (VIGS) in Wheat
4.13. Yeast Two-Hybrid (Y2H) Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caetano-Anollés, D.; Kim, K.M.; Mittenthal, J.E.; Caetano-Anollés, G. Proteome evolution and the metabolic origins of translation and cellular life. J. Mol. Evol. 2011, 72, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Raichaudhuri, A.; Peng, M.; Naponelli, V.; Chen, S.; Sánchez-Fernández, R.; Gu, H.; Gregory JF 3rd Hanson, A.D.; Rea, P.A. Plant Vacuolar ATP-binding Cassette Transporters That Translocate Folates and Antifolates in Vitro and Contribute to Antifolate Tolerance in Vivo. J. Biol. Chem. 2009, 284, 8449–8460. [Google Scholar] [CrossRef]
- Do, T.H.T.; Martinoia, E.; Lee, Y.; Hwang, J.U. 2021 update on ATP-binding cassette (ABC) transporters: How they meet the needs of plants. Plant Physiol. 2021, 187, 1876–1892. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.U.; Song, W.Y.; Hong, D.; Ko, D.; Yamaoka, Y.; Jang, S.; Yim, S.; Lee, E.; Khare, D.; Kim, K.; et al. Plant ABC Transporters Enable Many Unique Aspects of a Terrestrial Plant’s Lifestyle. Mol. Plant 2016, 9, 338–355. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.N.; Moon, S.; Jung, K.H. Genome-wide expression analysis of rice ABC transporter family across spatio-temporal samples and in response to abiotic stresses. J. Plant Physiol. 2014, 171, 1276–1288. [Google Scholar] [CrossRef]
- Mishra, A.K.; Choi, J.; Rabbee, M.F.; Baek, K.H. Corrigendum to “In Silico Genome-Wide Analysis of the ATP-Binding Cassette Transporter Gene Family in Soybean (Glycine max L.) and Their Expression Profiling”. Biomed. Res. Int. 2019, 2019, 1289174. [Google Scholar] [CrossRef]
- Schulz, B.; Kolukisaoglu, H.U. Genomics of plant ABC transporters: The alphabet of photosynthetic life forms or just holes in membranes? FEBS Lett. 2006, 580, 1010–1016. [Google Scholar] [CrossRef]
- Shin, S.; Chairattanawat, C.; Yamaoka, Y.; Yang, Q.; Lee, Y.; Hwang, J.U. Early seed development requires the A-type ATP-binding cassette protein ABCA10. Plant Physiol. 2022, 189, 360–374. [Google Scholar] [CrossRef]
- Kaneda, M.; Schuetz, M.; Lin, B.S.; Chanis, C.; Hamberger, B.; Western, T.L.; Ehlting, J.; Samuels, A.L. ABC transporters coordinately expressed during lignification of Arabidopsis stems include a set of ABCBs associated with auxin transport. J. Exp. Bot. 2011, 62, 2063–2077. [Google Scholar] [CrossRef]
- Sidler, M.; Hassa, P.; Hasan, S.; Ringli, C.; Dudler, R. Involvement of an ABC transporter in a developmental pathway regulating hypocotyl cell elongation in the light. Plant Cell 1998, 10, 1623–1636. [Google Scholar] [CrossRef]
- Park, J.; Song, W.Y.; Ko, D.; Eom, Y.; Hansen, T.H.; Schiller, M.; Lee, T.G.; Martinoia, E.; Lee, Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012, 69, 278–288. [Google Scholar] [CrossRef]
- Francisco, R.M.; Regalado, A.; Ageorges, A.; Burla, B.J.; Bassin, B.; Eisenach, C.; Zarrouk, O.; Vialet, S.; Marlin, T.; Chaves, M.M.; et al. ABCC1, an ATP binding cassette protein from grape berry, transports anthocyanidin 3-O-Glucosides. Plant Cell 2013, 25, 1840–1854. [Google Scholar] [CrossRef] [PubMed]
- Badone, F.C.; Cassani, E.; Landoni, M.; Doria, E.; Panzeri, D.; Lago, C.; Mesiti, F.; Nielsen, E.; Pilu, R. The low phytic acid1-241 (lpa1-241) maize mutation alters the accumulation of anthocyanin pigment in the kernel. Planta 2010, 231, 1189–1199. [Google Scholar] [CrossRef]
- Bussell, J.D.; Reichelt, M.; Wiszniewski, A.A.; Gershenzon, J.; Smith, S.M. Peroxisomal ATP-binding cassette transporter COMATOSE and the multifunctional protein abnormal INFLORESCENCE MERISTEM are required for the production of benzoylated metabolites in Arabidopsis seeds. Plant Physiol. 2014, 164, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Komatsuda, T.; Ma, J.F.; Nawrath, C.; Pourkheirandish, M.; Tagiri, A.; Hu, Y.G.; Sameri, M.; Li, X.; Zhao, X.; et al. An ATP-binding cassette subfamily G full transporter is essential for the retention of leaf water in both wild barley and rice. Proc. Natl. Acad. Sci. USA 2011, 108, 12354–12359. [Google Scholar] [CrossRef]
- Ichino, T.; Yazaki, K. Modes of secretion of plant lipophilic metabolites via ABCG transporter-dependent transport and vesicle-mediated trafficking. Curr. Opin. Plant Biol. 2022, 66, 102184. [Google Scholar] [CrossRef]
- Elejalde-Palmett, C.; Martinez San Segundo, I.; Garroum, I.; Charrier, L.; De Bellis, D.; Mucciolo, A.; Guerault, A.; Liu, J.; Zeisler-Diehl, V.; Aharoni, A.; et al. ABCG transporters export cutin precursors for the formation of the plant cuticle. Curr. Biol. 2021, 31, 2111–2123.e9. [Google Scholar] [CrossRef]
- Li, S.; Li, D.; Zhang, P.; Wang, R.; Sun, L.; Wan, J.; Xu, J. ABCF3 regulates the expression of aquaporin genes and endoplasmic reticulum stress-related genes in Arabidopsis. Theor. Exp. Plant Physiol. 2018, 30, 215–222. [Google Scholar] [CrossRef]
- Vazquez de Aldana, C.R.; Marton, M.J.; Hinnebusch, A.G. GCN20, a novel ATP binding cassette protein, and GCN1 reside in a complex that mediates activation of the eIF-2 alpha kinase GCN2 in amino acid-starved cells. EMBO J. 1995, 14, 3184–3199. [Google Scholar] [CrossRef]
- Tyzack, J.K.; Wang, X.; Belsham, G.J.; Proud, C.G. ABC50 interacts with eukaryotic initiation factor 2 and associates with the ribosome in an ATP-dependent manner. J. Biol. Chem. 2000, 275, 34131–34139. [Google Scholar] [CrossRef]
- Byrne, S.L.; Durandeau, K.; Nagy, I.; Barth, S. Identification of ABC transporters from Lolium perenne L. that are regulated by toxic levels of selenium. Planta 2010, 231, 901–911. [Google Scholar] [CrossRef]
- Krattinger, S.G.; Lagudah, E.S.; Spielmeyer, W.; Singh, R.P.; Huerta-Espino, J.; McFadden, H.; Bossolini, E.; Selter, L.L.; Keller, B. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 2009, 323, 1360–1363. [Google Scholar] [CrossRef]
- Kato, T.; Tabata, S.; Sato, S. Analyses of expression and phenotypes of knockout lines for Arabidopsis ABCF subfamily members. Plant Biotechnol. 2009, 26, 409–414. [Google Scholar] [CrossRef]
- Khan, N.; You, F.M.; Datla, R.; Ravichandran, S.; Jia, B.; Cloutier, S. Genome-wide identification of ATP binding cassette (ABC) transporter and heavy metal associated (HMA) gene families in flax (Linum usitatissimum L.). BMC Genom. 2020, 21, 722. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Dhar, J.; Chakrabarti, P. Structural motif, topi and its role in protein function and fibrillation. Mol. Omics. 2018, 14, 247–256. [Google Scholar] [CrossRef]
- Yan, J.; Ma, Z.; Xu, X.; Guo, A.Y. Evolution, functional divergence and conserved exon-intron structure of bHLH/PAS gene family. Mol. Genet. Genom. 2014, 289, 25–36. [Google Scholar] [CrossRef]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.H. Evolution of Gene Duplication in Plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef] [PubMed]
- Hurst, L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002, 18, 486. [Google Scholar] [CrossRef]
- Hernandez-Garcia, C.M.; Finer, J.J. Identification and validation of promoters and cis-acting regulatory elements. Plant Sci. 2014, 217–218, 109–119. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Hormone Profiling in Plant Tissues. Methods Mol. Biol. 2017, 1497, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Boël, G.; Smith, P.C.; Ning, W.; Englander, M.T.; Chen, B.; Hashem, Y.; Testa, A.J.; Fischer, J.J.; Wieden, H.-J.; Frank, J.; et al. The ABC-F protein EttA gates ribosome entry into the translation elongation cycle. Nat. Struct. Mol. Biol. 2014, 21, 143–151. [Google Scholar] [CrossRef]
- Su, W.; Kumar, V.; Ding, Y.; Ero, R.; Serra, A.; Lee, B.S.T.; Wong, A.S.W.; Shi, J.; Sze, S.K.; Yang, L.; et al. Ribosome protection by antibiotic resistance ATPbinding cassette protein. Proc. Natl. Acad. Sci. USA 2018, 115, 5157–5162. [Google Scholar] [CrossRef]
- Crowe-McAuliffe, C.; Graf, M.; Huter, P.; Takada, H.; Abdelshahid, M.; Nováček, J.; Murina, V.; Atkinson, G.C.; Hauryliuk, V.; Wilson, D.N. Structural basis for antibiotic resistance mediated by the Bacillus subtilis ABCF ATPase VmlR. Proc. Natl. Acad. Sci. USA 2018, 115, 8978–8983. [Google Scholar] [CrossRef]
- Su, T.; Izawa, T.; Thoms, M.; Yamashita, Y.; Cheng, J.; Berninghausen, O.; Hartl, F.U.; Inada, T.; Neupert, W.; Beckmann, R. Structure and function of Vms1 and Arb1 in RQC and mitochondrial proteome homeostasis. Nature 2019, 570, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yuan, X.; Li, L.; Zeng, M.; Yang, J.; Tang, H.; Duan, C. Genome-Wide Analysis of the ATP-Binding Cassette (ABC) Transporter Family in Zea mays L. and Its Response to Heavy Metal Stresses. Int. J. Mol. Sci. 2022, 23, 2109. [Google Scholar] [CrossRef]
- Chen, P.; Li, Y.; Zhao, L.; Hou, Z.; Yan, M.; Hu, B.; Liu, Y.; Azam, S.M.; Zhang, Z.; Rahman, Z.U.; et al. Genome-Wide Identification and Expression Profiling of ATP-Binding Cassette (ABC) Transporter Gene Family in Pineapple (Ananas comosus (L.) Merr.) Reveal the Role of AcABCG38 in Pollen Development. Front. Plant Sci. 2017, 8, 2150. [Google Scholar] [CrossRef]
- Zhang, Z.; Tong, T.; Fang, Y.; Zheng, J.; Zhang, X.; Niu, C.; Li, J.; Zhang, X.; Xue, D. Genome-Wide Identification of Barley ABC Genes and Their Expression in Response to Abiotic Stress Treatment. Plants 2020, 9, 1281. [Google Scholar] [CrossRef]
- Lopez-Ortiz, C.; Dutta, S.K.; Natarajan, P.; Peña-Garcia, Y.; Abburi, V.; Saminathan, T.; Nimmakayala, P.; Reddy, U.K. Genome-wide identification and gene expression pattern of ABC transporter gene family in Capsicum spp. PLoS ONE 2019, 14, e0215901. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Chen, X.; Guo, Y.; Liang, W.; Wang, H. Genome-Wide Identification of Soybean ABC Transporters Relate to Aluminum Toxicity. Int. J. Mol. Sci. 2021, 22, 6556. [Google Scholar] [CrossRef]
- Lim, L.W.K.; Chung, H.H.; Gan, H.M. Genome-Wide Identification, Characterisation and Phylogenetic Analysis of 52 Striped Catfish (Pangasianodon hypophthalmus) ATP-Binding Cassette (ABC) Transporter Genes. Trop. Life Sci. Res. 2022, 33, 257–293. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Peng, W.; Feng, S.; Feng, J.; Mahboob, S.; Al-Ghanim, K.A.; Xu, P. Genome-Wide Identification, Characterization and Phylogenetic Analysis of ATP-Binding Cassette (ABC) Transporter Genes in Common Carp (Cyprinus carpio). PLoS ONE 2016, 11, e0153246. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.B.; Kim, H.S.; Kang, H.M.; Lee, Y.H.; Zhou, B.; Choe, J.; Lee, J.S. Genome-wide identification of ATP-binding cassette (ABC) transporters and conservation of their xenobiotic transporter function in the monogonont rotifer (Brachionus koreanus). Comp. Biochem. Physiol. Part. D Genom. Proteom. 2017, 21, 17–26. [Google Scholar] [CrossRef]
- Kaur, A.; Sharma, A.; Madhu Dixit, S.; Singh, K.; Upadhyay, S.K. OSCA Genes in Bread Wheat: Molecular Characterization, Expression Profiling, and Interaction Analyses Indicated Their Diverse Roles during Development and Stress Response. Int. J. Mol. Sci. 2022, 23, 14867. [Google Scholar] [CrossRef]
- Sun, N.; Xie, Y.F.; Wu, Y.; Guo, N.; Li, D.H.; Gao, J.S. Genome-wide identification of ABCC gene family and their expression analysis in pigment deposition of fiber in brown cotton (Gossypium hirsutum). PLoS ONE 2021, 16, e0246649. [Google Scholar] [CrossRef]
- Zhang, J.Z. Evolution by gene duplication: An update. Trends Ecol. Evol. 2003, 18, 292–298. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Moore, R.C.; Purugganan, M.D. The evolutionary dynamics of plant duplicate genes. Curr. Opin. Plant Biol. 2005, 8, 122–128. [Google Scholar] [CrossRef]
- Howe, G.A.; Yoshida, Y. Evolutionary Origin of JAZ Proteins and Jasmonate Signaling. Mol. Plant 2019, 12, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Sharma, A.; Rajput, R.; Sidhu, S.; Dhillon, H.; Verma, P.C.; Pandey, A.; Upadhyay, S.K. Molecular Characterization, Evolutionary Analysis, and Expression Profiling of BOR Genes in Important Cereals. Plants 2022, 11, 911. [Google Scholar] [CrossRef]
- Zan, Y.; Ji, Y.; Zhang, Y.; Yang, S.; Song, Y.; Wang, J. Genome-wide identification, characterization and expression analysis of populusleucine-rich repeat receptor-like protein kinase genes. BMC Genom. 2013, 14, 318. [Google Scholar] [CrossRef]
- Islam, W.; Naveed, H.; Zaynab, M.; Huang, Z.; Chen, H.Y.H. Plant defense against virus diseases; growth hormones in highlights. Plant Signal Behav. 2019, 14, 1596719. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef] [PubMed]
- Himmelbach, A.; Liu, L.; Zierold, U.; Altschmied, L.; Maucher, H.; Beier, F.; Müller, D.; Hensel, G.; Heise, A.; Schützendübel, A.; et al. Promoters of the barley germin-like GER4 gene cluster enable strong transgene expression in response to pathogen attack. Plant Cell 2010, 22, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Rea, P.A. Plant ATP-binding cassette transporters. Annu. Rev. Plant Biol. 2007, 58, 347–375. [Google Scholar] [CrossRef]
- Theodoulou, F.L. Plant ABC transporters. Biochim. Biophys. Acta. 2000, 1465, 79–103. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, E.J. Vernalization. Curr. Biol. 2012, 22, R471–R472. [Google Scholar] [CrossRef][Green Version]
- Adams, M.J.; Antoniw, J.F.; Kreuze, J. Virgaviridae: A new family of rod-shaped plant viruses. Arch. Virol. 2009, 154, 1967–1972. [Google Scholar] [CrossRef] [PubMed]
- Andika, I.B.; Zheng, S.; Tan, Z.; Sun, L.; Kondo, H.; Zhou, X.; Chen, J. Endoplasmic reticulum export and vesicle formation of the movement protein of Chinese wheat mosaic virus are regulated by two transmembrane domains and depend on the secretory pathway. Virology 2013, 435, 493–503. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, F.; Xie, L.; Song, X.J.; Li, J.; Chen, J.P.; Zhang, H.M. Functional identification of two minor capsid proteins from Chinese wheat mosaic virus using its infectious full-length cDNA clones. J. Gen. Virol. 2016, 97, 2441–2450. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, P.; Zhong, K.; Zhang, F.; Xu, M.; He, L.; Jin, P.; Chen, J.; Yang, J. Wheat Yellow Mosaic Virus NIb Interacting with Host Light Induced Protein (LIP) Facilitates Its Infection through Perturbing the Abscisic Acid Pathway in Wheat. Biology 2019, 8, 80. [Google Scholar] [CrossRef]
- Cheuk, A.; Houde, M. A New Barley Stripe Mosaic Virus Allows Large Protein Overexpression for Rapid Function Analysis. Plant Physiol. 2018, 176, 1919–1931. [Google Scholar] [CrossRef]
- Bennypaul, H.; Gill, U.S. Barley Stripe Mosaic Virus (BSMV)-Based Virus-Induced Gene Silencing to Functionally Characterize Genes in Wheat and Barley. Methods Mol. Biol. 2022, 2408, 85–93. [Google Scholar] [CrossRef]
- Andika, I.B.; Sun, L.; Xiang, R.; Li, J.; Chen, J. Root-specific role for Nicotiana benthamiana RDR6 in the inhibition of Chinese wheat mosaic virus accumulation at higher temperatures. Mol. Plant Microbe Interact. 2013, 26, 1165–1175. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef]
- Ziemann, S.; van der Linde, K.; Lahrmann, U.; Acar, B.; Kaschani, F.; Colby, T.; Kaiser, M.; Ding, Y.; Schmelz, E.; Huffaker, A.; et al. An apoplastic peptide activates salicylic acid signalling in maize. Nat. Plants 2018, 4, 172–180. [Google Scholar] [CrossRef]
- Stegmann, M.; Monaghan, J.; Smakowska-Luzan, E.; Rovenich, H.; Lehner, A.; Holton, N.; Belkhadir, Y.; Zipfel, C. The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science 2017, 355, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Schurr, U.; Davies, W. Control of stomatal behaviour by abscisic acid which apparently originates in the roots. J. Exp. Bot. 1987, 38, 1174–1181. [Google Scholar] [CrossRef]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J.; et al. Pfam: The protein families database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Bryant, S.H. CD-Search: Protein domain annotations on the fly. Nucleic Acids Res. 2004, 32, W327–W331. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Bolser, D.M.; Kerhornou, A.; Walts, B.; Kersey, P. Triticeae resources in Ensembl Plants. Plant Cell Physiol. 2015, 56, e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, F.; Chen, D.; Chu, W.; Liu, H.; Xiang, Y. Genome-wide identification and analysis of the Populus trichocarpa TIFY gene family. Plant Physiol. Biochem. 2017, 115, 360–371. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Kong, J.; Chen, W.; Shen, J.; Qin, C.; Lai, T.; Zhang, P.; Wang, Y.; Wu, C.; Yang, X.; Hong, Y. Virus-induced gene complementation in tomato. Plant Signal Behav. 2013, 8, e27142. [Google Scholar] [CrossRef][Green Version]
- Yang, J.; Wei, H.; Hou, M.; Chen, L.; Zou, T.; Ding, H.; Jing, Y.; Zhang, X.; Zhao, Y.; Liu, Q.; et al. ZmSPL13 and ZmSPL29 act together to promote vegetative and reproductive transition in maize. New Phytol. 2023, 239, 1505–1520. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yu, X.; Han, J.; Wang, E.; Xiao, J.; Hu, R.; Yang, G.; He, G. Genome-Wide Identification and Homoeologous Expression Analysis of PP2C Genes in Wheat (Triticum aestivum L.). Front. Genet. 2019, 10, 561. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, T.; Li, J.; Wu, N.; Wu, G.; Yang, J.; Chen, X.; He, L.; Chen, J. Chinese wheat mosaic virus-derived vsiRNA-20 can regulate virus infection in wheat through inhibition of vacuolar- (H+)-PPase induced cell death. New Phytol. 2020, 226, 205–220. [Google Scholar] [CrossRef] [PubMed]


| Gene ID | Location | CDS Length (bp) | Size (aa) | MW (kDa) | PI | Exons | Group |
|---|---|---|---|---|---|---|---|
| TraesCS7B02G072300.1 | 7B: 79,386,663–79,390,314 | 2544 | 1027 | 106.42 | 8.48 | 5 | ABCF1 |
| TraesCS7A02G167500.1 | 7A: 122,759,312–122,762,749 | 2550 | 849 | 88.69 | 6.29 | 5 | ABCF1 |
| TraesCS7D02G168300.1 | 7D: 118,824,218–118,828,023 | 2553 | 850 | 88.59 | 6.67 | 5 | ABCF1 |
| TraesCS2B02G381000.1 | 2B: 545,154,783–545,157,775 | 2364 | 787 | 85.31 | 9.45 | 5 | ABCF1 |
| TraesCS2A02G363100.1 | 2A: 607,514,687–607,517,465 | 2346 | 781 | 85.01 | 9.28 | 5 | ABCF1 |
| TraesCS2D02G360400.1 | 2D: 462,242,110–462,244,920 | 2391 | 796 | 86.55 | 9.41 | 5 | ABCF1 |
| TraesCS4A02G156300.1 | 4A: 311,618,303–311,664,820 | 2079 | 692 | 77.69 | 7.09 | 9 | ABCF2 |
| TraesCS4D02G149400.1 | 4D: 155,574,671–155,578,885 | 2079 | 692 | 77.57 | 7.9 | 9 | ABCF2 |
| TraesCS4B02G160600.1 | 4B: 315,061,175–315,067,581 | 2079 | 692 | 77.53 | 6.78 | 9 | ABCF2 |
| TraesCSU02G090500.1 | Un: 81,296,469–81,302,814 | 2163 | 720 | 80.13 | 6 | 15 | ABCF3 |
| TraesCS6A02G409800.1 | 6A: 613,481,932–613,488,341 | 2163 | 720 | 80.14 | 5.95 | 16 | ABCF3 |
| TraesCS6B02G456000.1 | 6B: 712,345,436–712,351,600 | 2163 | 720 | 80.18 | 6.08 | 14 | ABCF3 |
| TraesCS1D02G409400.1 | 1D: 471,300,960–471,303,080 | 2121 | 706 | 77.33 | 6.51 | 1 | ABCF4 |
| TraesCS1B02G430800.1 | 1B: 655,102,218–655,104,350 | 2133 | 710 | 77.61 | 6.5 | 1 | ABCF4 |
| TraesCS7D02G054900.1 | 7D: 29,535,278–29,537,442 | 1677 | 558 | 61.55 | 6.13 | 4 | ABCF4 |
| TraesCS3A02G355700.1 | 3A: 603,789,869–603,792,554 | 2169 | 722 | 78.63 | 6.51 | 1 | ABCF4 |
| TraesCS3B02G388600.1 | 3B: 611,676,317–611,678,940 | 2169 | 722 | 78.71 | 6.5 | 1 | ABCF4 |
| TraesCS3D02G349700.1 | 3D: 461,199,491–461,201,659 | 2169 | 722 | 78.69 | 6.4 | 1 | ABCF4 |
| Paralogous | Ka | Ks | Ka/Ks | T (Mya) | |
|---|---|---|---|---|---|
| TraesCS1B02G430800.1 | TraesCS1D02G409400.1 | 0.0056 | 0.0756 | 0.0739 | 4.153 |
| TraesCS2A02G363100.1 | TraesCS2B02G381000.1 | 0.0385 | 0.0692 | 0.5566 | 3.801 |
| TraesCS2A02G363100.1 | TraesCS2D02G360400.1 | 0.0345 | 0.0947 | 0.3646 | 5.201 |
| TraesCS2B02G381000.1 | TraesCS2D02G360400.1 | 0.0353 | 0.0805 | 0.4385 | 4.424 |
| TraesCS3A02G355700.1 | TraesCS3B02G388600.1 | 0.0036 | 0.1111 | 0.0327 | 6.107 |
| TraesCS3A02G355700.1 | TraesCS3D02G349700.1 | 0.0036 | 0.0737 | 0.0494 | 4.050 |
| TraesCS3B02G388600.1 | TraesCS3D02G349700.1 | 0.0049 | 0.0910 | 0.0534 | 4.998 |
| TraesCS4A02G156300.1 | TraesCS4B02G160600.1 | 0.0110 | 0.0444 | 0.2482 | 2.438 |
| TraesCS4B02G160600.1 | TraesCS4D02G149400.1 | 0.0082 | 0.0365 | 0.2238 | 2.007 |
| TraesCS6A02G409800.1 | TraesCS6B02G456000.1 | 0.0036 | 0.0667 | 0.0543 | 3.666 |
| TraesCS7A02G167500.1 | TraesCS7B02G072300.1 | 0.0345 | 0.1057 | 0.3265 | 5.809 |
| TraesCS7A02G167500.1 | TraesCS7B02G072300.2 | 0.0327 | 0.0961 | 0.3403 | 5.281 |
| TraesCS7B02G072300.1 | TraesCS7B02G072300.3 | 0.0318 | 0.1078 | 0.2951 | 5.920 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Tu, A.; Feng, H.; Guo, Y.; Xu, G.; Shi, J.; Chen, J.; Yang, J.; Zhong, K. Genome-Wide Identification and Analysis of the ABCF Gene Family in Triticum aestivum. Int. J. Mol. Sci. 2023, 24, 16478. https://doi.org/10.3390/ijms242216478
Wu M, Tu A, Feng H, Guo Y, Xu G, Shi J, Chen J, Yang J, Zhong K. Genome-Wide Identification and Analysis of the ABCF Gene Family in Triticum aestivum. International Journal of Molecular Sciences. 2023; 24(22):16478. https://doi.org/10.3390/ijms242216478
Chicago/Turabian StyleWu, Mila, Aizhu Tu, Huimin Feng, Yunfei Guo, Gecheng Xu, Jingjing Shi, Jianping Chen, Jian Yang, and Kaili Zhong. 2023. "Genome-Wide Identification and Analysis of the ABCF Gene Family in Triticum aestivum" International Journal of Molecular Sciences 24, no. 22: 16478. https://doi.org/10.3390/ijms242216478
APA StyleWu, M., Tu, A., Feng, H., Guo, Y., Xu, G., Shi, J., Chen, J., Yang, J., & Zhong, K. (2023). Genome-Wide Identification and Analysis of the ABCF Gene Family in Triticum aestivum. International Journal of Molecular Sciences, 24(22), 16478. https://doi.org/10.3390/ijms242216478







