The Wheat Annexin TaAnn12 Plays Positive Roles in Plant Disease Resistance by Regulating the Accumulation of Reactive Oxygen Species and Callose
Abstract
:1. Introduction
2. Results
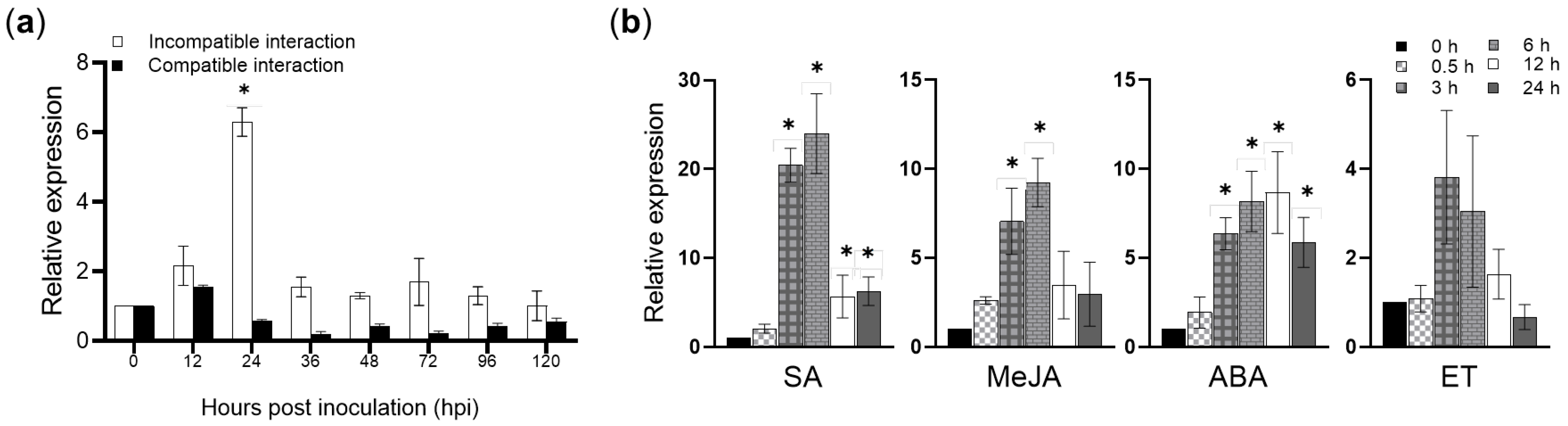
2.1. The Expression of TaAnn12 Is Differently Induced during Pst Infection, Hormone Treatments, and Abiotic Stresses
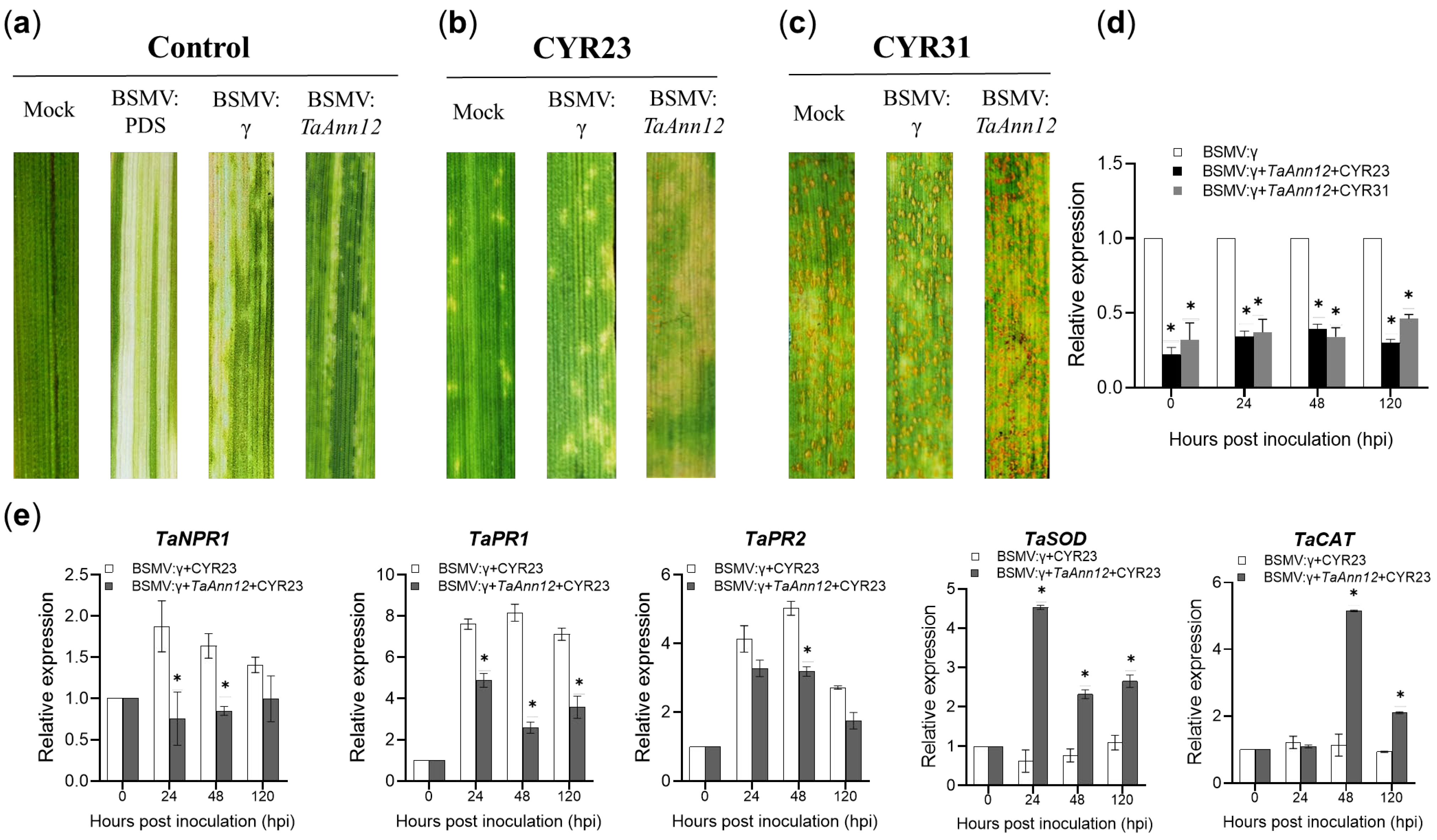
2.2. Suppression of TaAnn12 Reduces Wheat Resistance to Pst
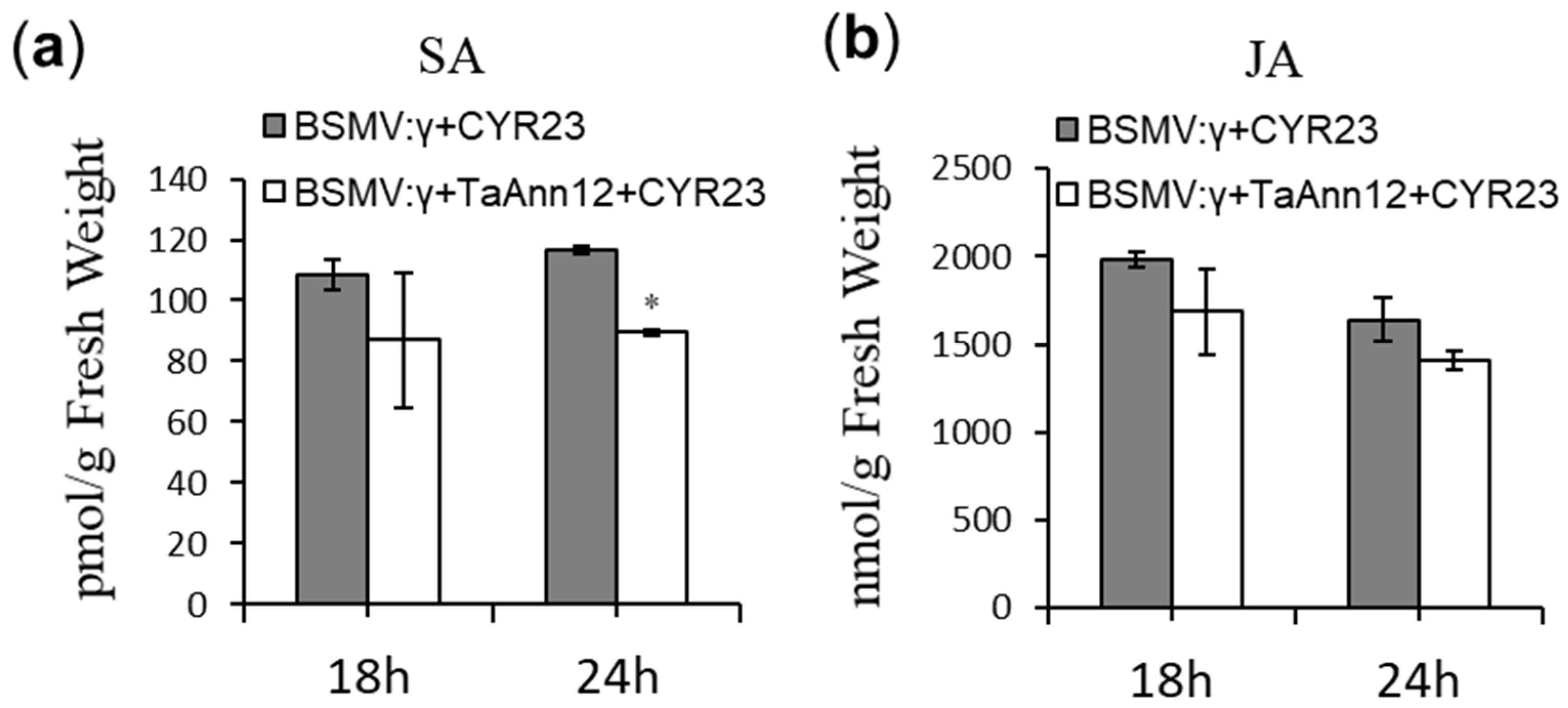
2.3. Reduced Accumulation of SA in TaAnn12-Silenced Plants
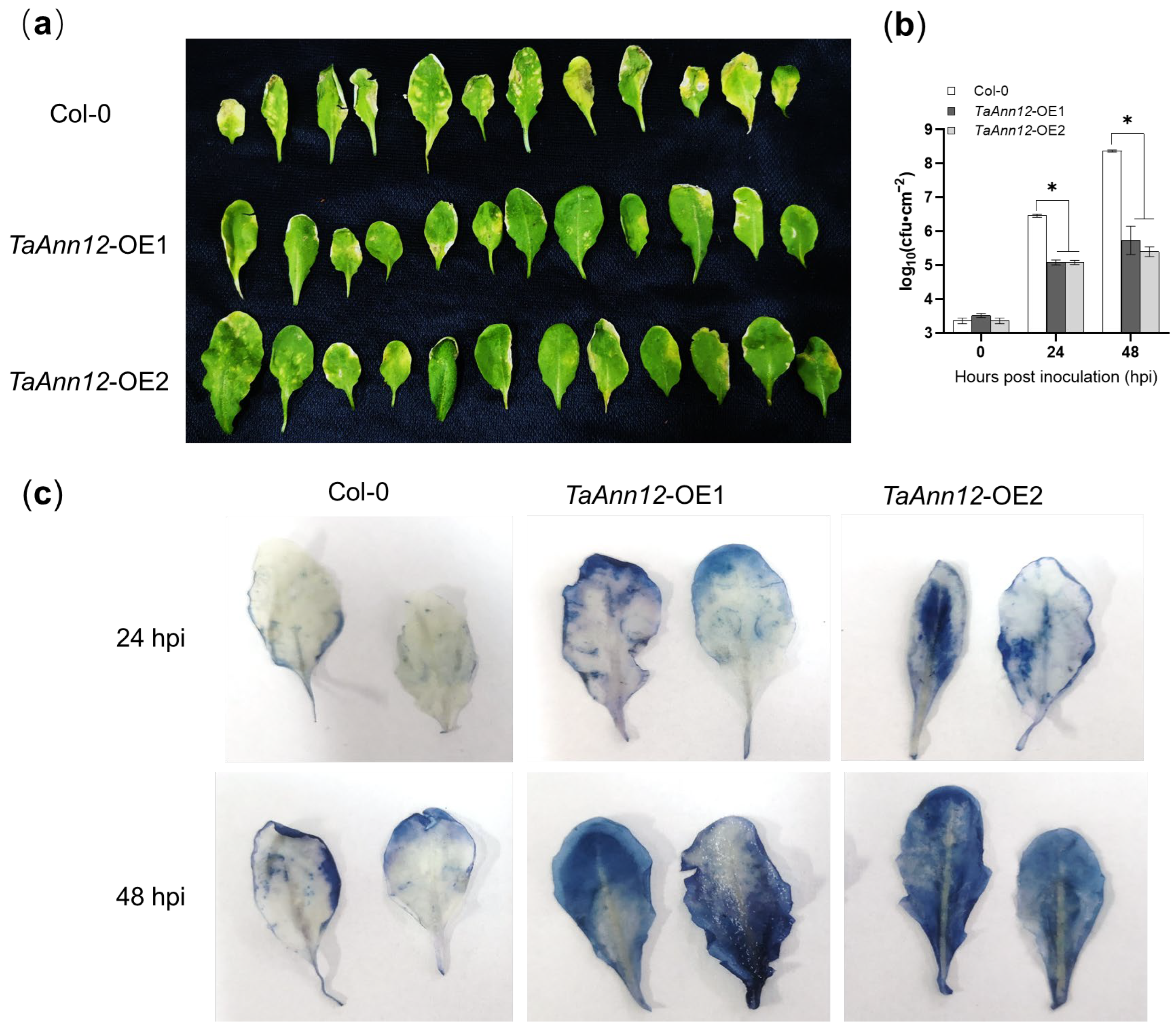
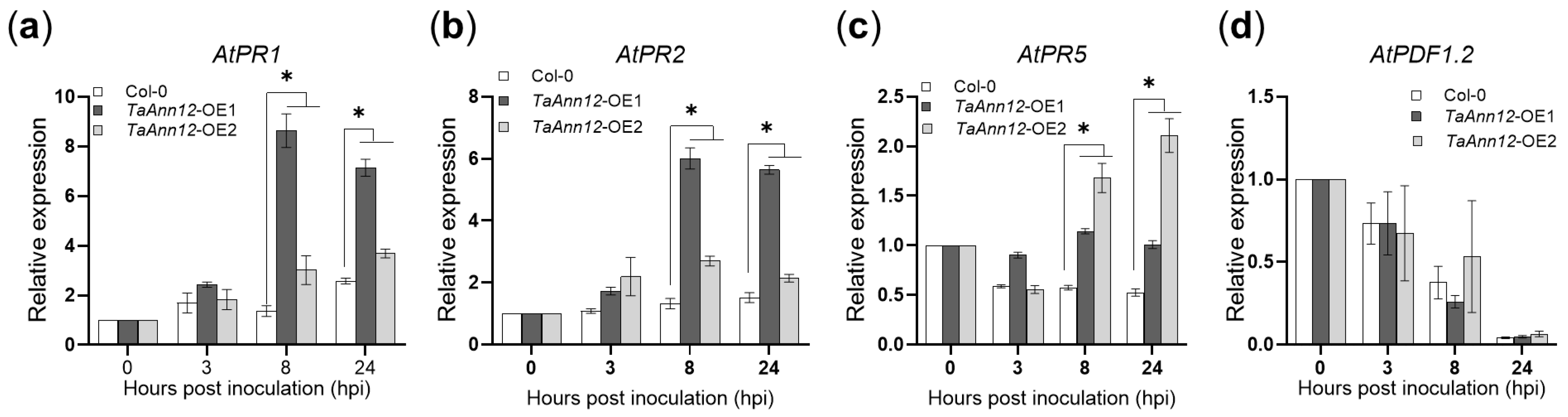
2.4. Overexpressing TaAnn12 in Arabidopsis Enhances Plant Defense
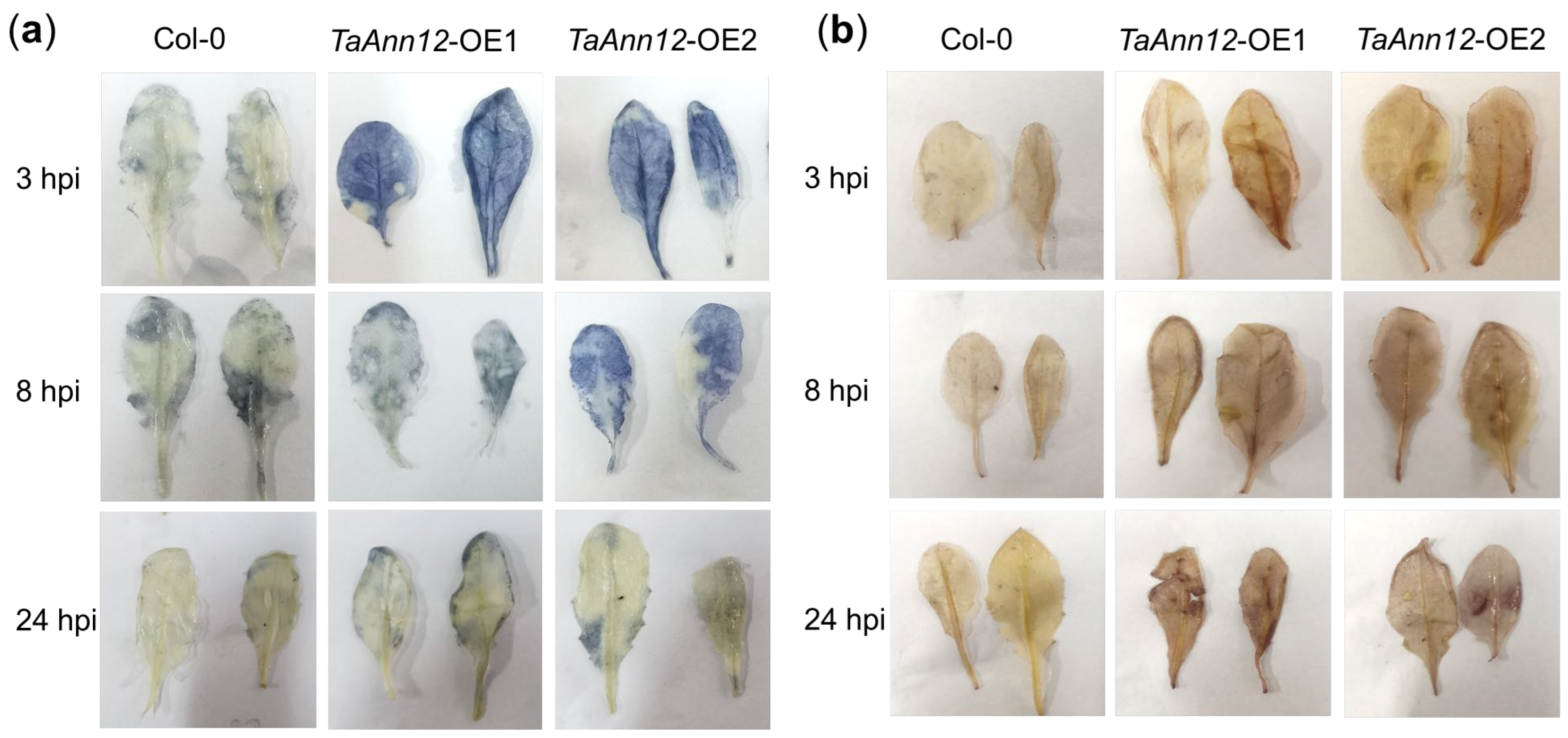
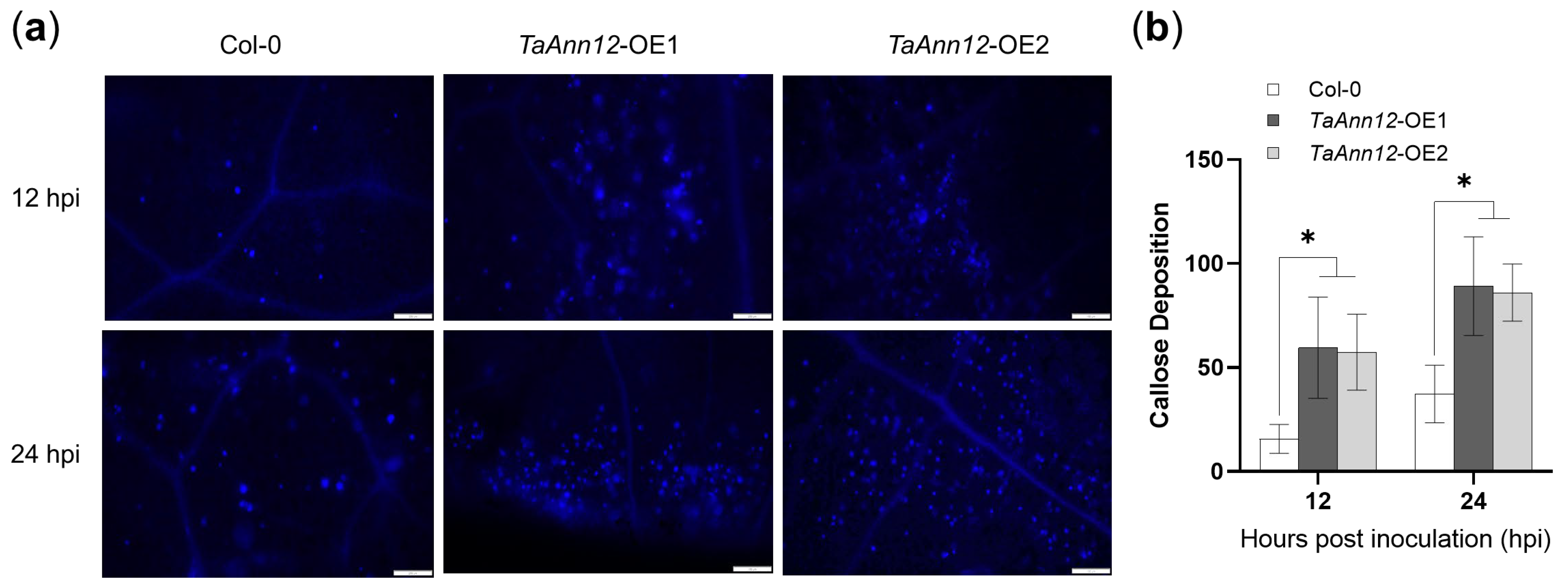
2.5. TaAnn12 Affects Plant Resistance by Regulating ROS and Callose Accumulation
3. Discussion
4. Materials and Methods
4.1. Plants, Pathogen Material, and Treatment
4.2. Real-Time Quantitative PCR (RT-qPCR) Analysis and Statistical Analysis
4.3. Barley Stripe Mosaic Virus (BSMV)-Mediated Gene Silencing
4.4. Detection of the Accumulation of Plant Hormones in TaAnn12-Silenced Plants
4.5. Production of Transgenic Arabidopsis
4.6. Identification of Disease Resistance in Transgenic TaAnn12 Arabidopsis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laohavisit, A.; Davies, J.M. Annexins. New Phytol. 2011, 189, 40–53. [Google Scholar] [CrossRef]
- Clark, G.B.; Reginald, O.M.; Maria-Pilar, F.; Roux, S.J. Evolutionary Adaptation of Plant Annexins Has Diversified Their Molecular Structures, Interactions and Functional Roles. New Phytol. 2012, 196, 695–712. [Google Scholar] [CrossRef] [PubMed]
- Laohavisit, A.; Brown, A.T.; Cicuta, P.; Davies, J.M. Annexins: Components of the Calcium and Reactive Oxygen Signaling Network1. Plant Physiol. 2010, 152, 1824–1829. [Google Scholar] [CrossRef]
- Konopka-Postupolska, D.; Clark, G.; Goch, G.; Debski, J.; Floras, K.; Cantero, A.; Fijolek, B.; Roux, S.; Hennig, J. The Role of Annexin 1 in Drought Stress in Arabidopsis. Plant Physiol. 2009, 150, 1394–1410. [Google Scholar] [CrossRef]
- Anuphon, L.; Mortimer, J.C.; Demidchik, V.; Coxon, K.M.; Stancombe, M.A.; Macpherson, N.; Brownlee, C.; Hofmann, A.; Webb, A.A.; Miedema, H.; et al. Zea mays Annexins Modulate Cytosolic Free Ca2+ and Generate a Ca2+-Permeable Conductance. Plant Cell 2009, 21, 479–493. [Google Scholar]
- Zhou, M.; Yang, X.; Zhang, Q.; Zhou, M.; Zhao, E.; Tang, Y.; Zhu, X.; Shao, J.; Wu, Y. Induction of Annexin by Heavy Metals and Jasmonic Acid in Zea mays. Funct. Integr. Genom. 2013, 13, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Stes, E.; Holsters, M.; Vereecke, D. Phytopathogenic Strategies of Rhodococcus fascians. In Biology of Rhodococcus; Springer: Berlin/Heidelberg, Germany, 2010; pp. 315–329. [Google Scholar]
- Mafra, V.; Martins, P.K.; Francisco, C.S.; Ribeiro-Alves, M.; Freitas-Astúa, J.; Machado, M.A. Candidatusliberibacter Americanus Induces Significant Reprogramming of the Transcriptome of the Susceptible Citrus Genotype. BMC Genom. 2013, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Boyidi, P.; Ahmed, I.; Kirti, P.B. Plant Annexins and Their Involvement in Stress Responses. Environ. Exp. Bot. 2018, 155, 293–306. [Google Scholar] [CrossRef]
- Shen, F.; Ying, J.; Xu, L.; Sun, X.; Wang, J.; Wang, Y.; Mei, Y.; Zhu, Y.; Liu, L. Characterization of Annexin Gene Family and Functional Analysis of RsANN1a Involved in Heat Tolerance in Radish (Raphanus sativus L.). Physiol. Mol. Biol. Plants 2021, 27, 2027–2041. [Google Scholar] [CrossRef]
- Loukehaich, R.; Wang, T.; Ouyang, B.; Ziaf, K.; Li, H.; Zhang, J.; Lu, Y.; Ye, Z. SpUSP, an Annexin-Interacting Universal Stress Protein, Enhances Drought Tolerance in Tomato. J. Exp. Bot. 2012, 63, 5593–5606. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Yang, X.; Han, J.; Zhu, Z. OsANN3, a Calcium-Dependent Lipid Binding Annexin is a Positive Regulator of ABA-Dependent Stress Tolerance in Rice. Plant Sci. 2019, 284, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Jami, S.K.; Clark, G.B.; Ayele, B.T.; Roux, S.J.; Kirti, P.B. Identification and Characterization of Annexin Gene Family in Rice. Plant Cell Rep. 2012, 31, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Zheng, Y.; Guo, Y. MYB30 Transcription Factor Regulates Oxidative and Heat Stress Responses through Annexin-Ediated Cytosolic Calcium Signaling in Arabidopsis. New Phytol. 2017, 216, 163. [Google Scholar] [CrossRef]
- Qiao, B.; Zhang, Q.; Liu, D.; Wang, H.; Yin, J.R.; He, M.; Cui, M.; Shang, Z.; Wang, D. A Calcium-Binding Protein, Rice Annexin OsANN1, Enhances Heat Stress Tolerance by Modulating the Production of H2O2. J. Exp. Bot. 2015, 66, 5853–5866. [Google Scholar] [CrossRef]
- Espinoza, C.; Liang, Y.; Stacey, G. Chitin Receptor Cerk 1 Links Salt Stress and Chitin-Triggered Innate Immunity in Arabidopsis. Plant J. 2017, 89, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Jami, S.K.; Clark, G.B.; Turlapati, S.A.; Handley, C.; Roux, S.J.; Kirti, P.B. Ectopic Expression of an Annexin from Brassica juncea Confers Tolerance to Abiotic and Biotic Stress Treatments in Transgenic Tobacco. Plant Physiol. Biochem. 2008, 46, 1019–1030. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Zhang, X.; Liu, X.; Wang, P.; Hou, Y. Cloning and Characterization of an Annexin Gene from Cynanchum komarovii that Enhances Tolerance to Drought and Fusarium oxysporum in Transgenic Cotton. J. Plant Biol. 2011, 54, 303–313. [Google Scholar] [CrossRef]
- Chandran, D.; Inada, N.; Hather, G.; Kleindt, C.K.; Wildermuth, M.C. Laser Microdissection of Arabidopsis Cells at the Powdery Mildew Infection Site Reveals Site-Specific Processes and Regulators. Proc. Natl. Acad. Sci. USA 2010, 107, 460–465. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, Y.; Li, Q.; Zhao, J.; Li, Y.; Fan, J.; Xiao, S.; Wang, W. Annexin 8 Negatively Regulates RPW8. 1-Mediated Cell Death and Disease Resistance in Arabidopsis. J. Integr. Plant Biol. 2021, 63, 378–392. [Google Scholar] [CrossRef]
- Laohavisit, A.; Davies, J.M. Multifunctional Annexins. Plant Sci. 2009, 177, 532–539. [Google Scholar] [CrossRef]
- Lo, L.; Daniel, P.; Gabriel, L.; Shigeyuki, S.; Liang, T.; Marie, T.; Alga, Z.; Stefanie, R.; Regine, K. Fungal Effectors and Plant Susceptibility. Annu. Rev. Plant Biol. 2015, 66, 513–545. [Google Scholar]
- Ding, Y.; Wang, X.; Wang, D.; Jiang, L.; Xie, J.; Wang, T.; Song, L.; Zhao, X. Identification of CmBHlh Transcription Factor Family and Excavation of CmBHlhs Resistant to Necrotrophic Fungus Alternaria in Chrysanthemum. Genes 2023, 14, 275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, L.; Liu, Q.; Liu, P.; Li, S.; Yang, D.; Chen, Y.; Pagnotta, S.; Favery, B.; Abad, P. A MIF-Like Effector Suppresses Plant Immunity and Facilitates Nematode Parasitism by Interacting with Plant Annexins. J. Exp. Bot. 2019, 70, 5943–5958. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Hamamouch, N.; Li, C.; Hewezi, T.; Hussey, R.S.; Baum, T.J.; Mitchum, M.G.; Davis, E.L. A Nematode Effector Protein Similar to Annexins in Host Plants. J. Exp. Bot. 2010, 61, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Tang, Y.; Gao, S.; Su, S.; Hong, L.; Wang, W.; Fang, Z.; Li, X.; Ma, J.; Quan, W. Comprehensive Analyses of the Annexin Gene Family in Wheat. BMC Genom. 2016, 17, 415. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jørgensen, L.N.; Hovmøller, M.S.; Huerta-Espino, J. Disease Impact on Wheat Yield Potential and Prospects of Genetic Control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Mccallum, B.; Hiebert, C.; Huerta-Espino, J.; Cloutier, S. Wheat Leaf Rust. Dis. Resist. Wheat 2012, 85, 33–62. [Google Scholar]
- Caarls, L.; Pieterse, C.M.J.; Van Wees, S.C.M. How Salicylic Acid Takes Transcriptional Control over Jasmonic Acid Signaling. Front. Plant Sci. 2015, 6, 170. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Signaling in Plants; Mary Ann Liebert, Inc.: New Rochelle, NY, USA, 2013. [Google Scholar]
- He, B.; Cai, Q.; Qiao, L.; Huang, C.; Wang, S.; Miao, W.; Ha, T.; Wang, Y.; Jin, H. RNA-Binding Proteins Contribute to Small Rna Loading in Plant Extracellular Vesicles. Nat. Plants 2021, 7, 342–352. [Google Scholar] [CrossRef]
- Cantero, A.; Barthakur, S.; Bushart, T.J.; Chou, S.; Morgan, R.O.; Fernandez, M.P.; Clark, G.B.; Roux, S.J. Expression Profiling of the Arabidopsis Annexin Gene Family During Germination, De-Etiolation and Abiotic Stress. Plant Physiol. Biochem. 2006, 44, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, E.J.; Yang, E.J.; Lee, J.E.; Park, O.K. Proteomic Identification of Annexins, Calcium-Dependent Membrane Binding Proteins that Mediate Osmotic Stress and Abscisic Acid Signal Transduction in Arabidopsis. Plant Cell 2004, 16, 1378–1391. [Google Scholar] [CrossRef]
- Zebell, S.G.; Dong, X. Cell-Cycle Regulators and Cell Death in Immunity. Cell Host Microbe 2015, 18, 402–407. [Google Scholar] [CrossRef]
- Coll, N.S.; Epple, P.; Dangl, J.L. Programmed Cell Death in the Plant Immune System. Cell Death Differ. 2011, 18, 1247–1256. [Google Scholar] [CrossRef]
- Bouzenzana, J.; Pelosi, L.; Briolay, A.; Briolay, J.; Bulone, V. Identification of the First Oomycete Annexin as a (1→3)-Β-D-Glucan Synthase Activator. Mol. Microbiol. 2006, 62, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Dong, X. Systemic Acquired Resistance: Turning Local Infection into Global Defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hua, Y.; Wang, J.; Huo, Y.; Shimono, M.; Day, B.; Ma, Q. TaADF4, an Actin-Depolymerizing Factor from Wheat, is Required for Resistance to the Stripe Rust Pathogen Puccinia striiformis f. sp. tritici. Plant J. 2017, 89, 1210–1224. [Google Scholar] [CrossRef]
- Bai, X.; Huang, X.; Tian, S.; Peng, H.; Guo, J. RNAi-Ediated Stable Silencing of TaCSN5 Confers Broad: Pectrum Resistance to Puccinia striiformis f. sp. tritici. Mol. Plant Pathol. 2021, 22, 410–421. [Google Scholar] [CrossRef]
- Lu, P.; Tai, Y.; Zheng, W.; Chen, M.; Zhou, Y.; Chen, J.; Ma, Y.; Xi, Y.; Xu, Z. The Wheat Bax Inhibitor-1 Protein Interacts with an Aquaporin TaPIP1 and Enhances Disease Resistance in Arabidopsis. Front. Plant Sci. 2018, 9, 20. [Google Scholar] [CrossRef]
- Shi, B.; Zhao, X.; Li, M.; Dong, Z.; Yang, Q.; Wang, Y.; Gao, H.; Day, B.; Ma, Q. Wheat Thioredoxin (TaTRXh1) Associates with RD19-like Cysteine Protease TaCP1 to Defend against Stripe Rust Fungus through Modulation of Programmed Cell Death. Mol. Plant-Microbe Interact. 2021, 34, 426–438. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−Δδct Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Feng, H.; Tang, C.; Bai, P.; Wei, G.; Huang, L.; Kang, Z. TaMCA4, a Novel Wheat Metacaspase Gene Functions in Programmed Cell Death Induced by the Fungal Pathogen Puccinia striiformis f. sp. tritici. Mol. Plant-Microbe Interact. 2012, 25, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Ayliffe, M.; Devilla, R.; Mago, R.; White, R.; Talbot, M.; Pryor, A.; Leung, H. Nonhost Resistance of Rice to Rust Pathogens. Mol. Plant-Microbe Interact. 2011, 24, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.N.; Perfus-Barbeoch, L.; Quentin, M.; Zhao, J.; Magliano, M.; Marteu, N.; Da Rocha, M.; Nottet, N.; Abad, P.; Favery, B. A Root-Knot Nematode Small Glycine and Cysteine-Rich Secreted Effector, MiSGCR1, is Involved in Plant Parasitism. New Phytol. 2018, 217, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Liu, W.; Wang, Y. Heterologous Expression of Chinese Wild Grapevine Vqerfs in Arabidopsis thaliana Enhance Resistance to Pseudomonas syringae pv. tomato DC3000 and to Botrytis cinerea. Plant Sci. 2020, 293, 110421. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, B.; Liu, W.; Ma, Q. The Wheat Annexin TaAnn12 Plays Positive Roles in Plant Disease Resistance by Regulating the Accumulation of Reactive Oxygen Species and Callose. Int. J. Mol. Sci. 2023, 24, 16381. https://doi.org/10.3390/ijms242216381
Shi B, Liu W, Ma Q. The Wheat Annexin TaAnn12 Plays Positive Roles in Plant Disease Resistance by Regulating the Accumulation of Reactive Oxygen Species and Callose. International Journal of Molecular Sciences. 2023; 24(22):16381. https://doi.org/10.3390/ijms242216381
Chicago/Turabian StyleShi, Beibei, Weijian Liu, and Qing Ma. 2023. "The Wheat Annexin TaAnn12 Plays Positive Roles in Plant Disease Resistance by Regulating the Accumulation of Reactive Oxygen Species and Callose" International Journal of Molecular Sciences 24, no. 22: 16381. https://doi.org/10.3390/ijms242216381
APA StyleShi, B., Liu, W., & Ma, Q. (2023). The Wheat Annexin TaAnn12 Plays Positive Roles in Plant Disease Resistance by Regulating the Accumulation of Reactive Oxygen Species and Callose. International Journal of Molecular Sciences, 24(22), 16381. https://doi.org/10.3390/ijms242216381





