Some Structural Elements of Bacterial Protein MF3 That Influence Its Ability to Induce Plant Resistance to Fungi, Viruses, and Other Plant Pathogens
Abstract
1. Introduction
2. Results
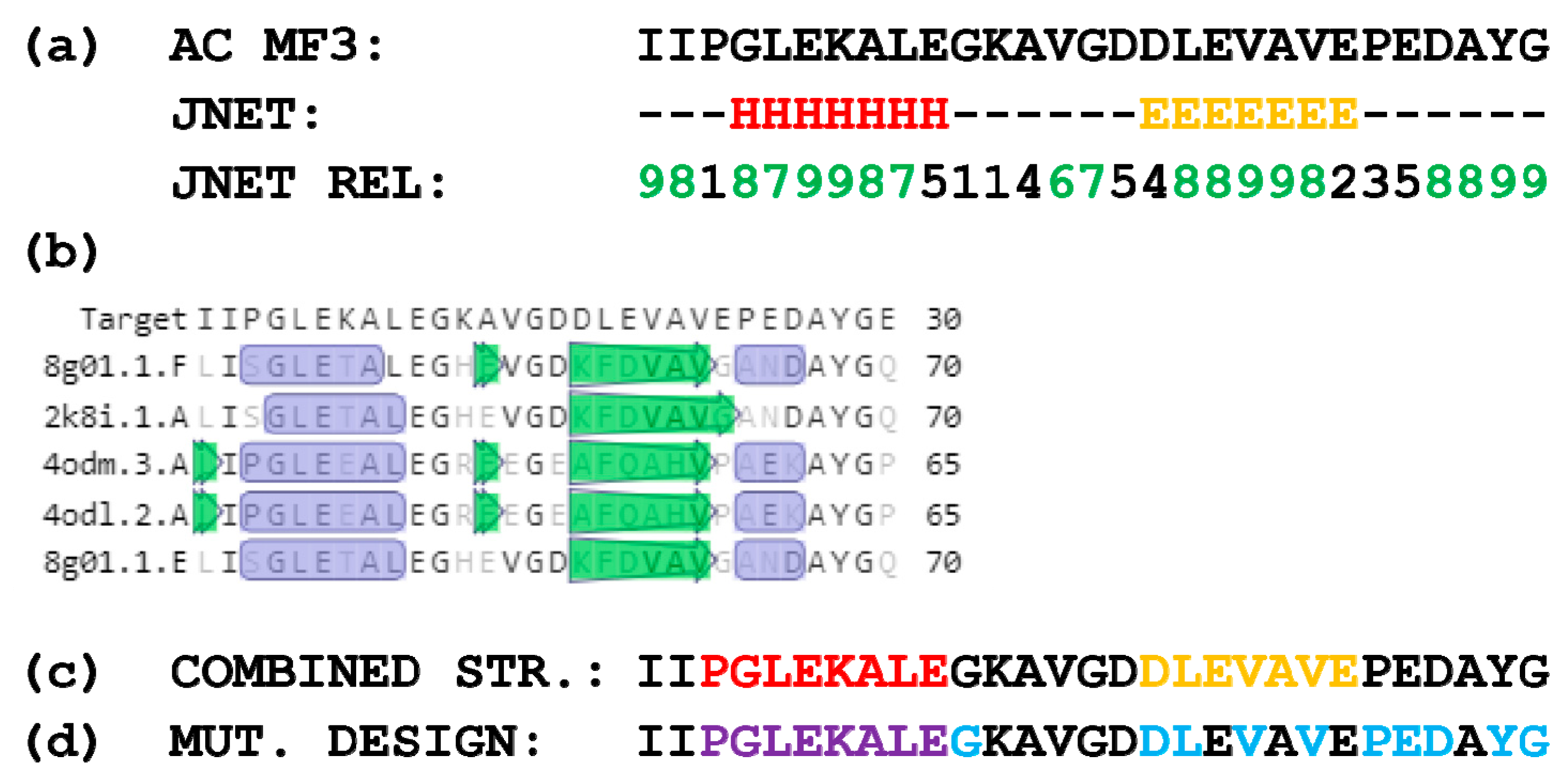
2.1. Design of AC MF3 Mutants
2.2. Inducing Activity of AC MF3 Mutants
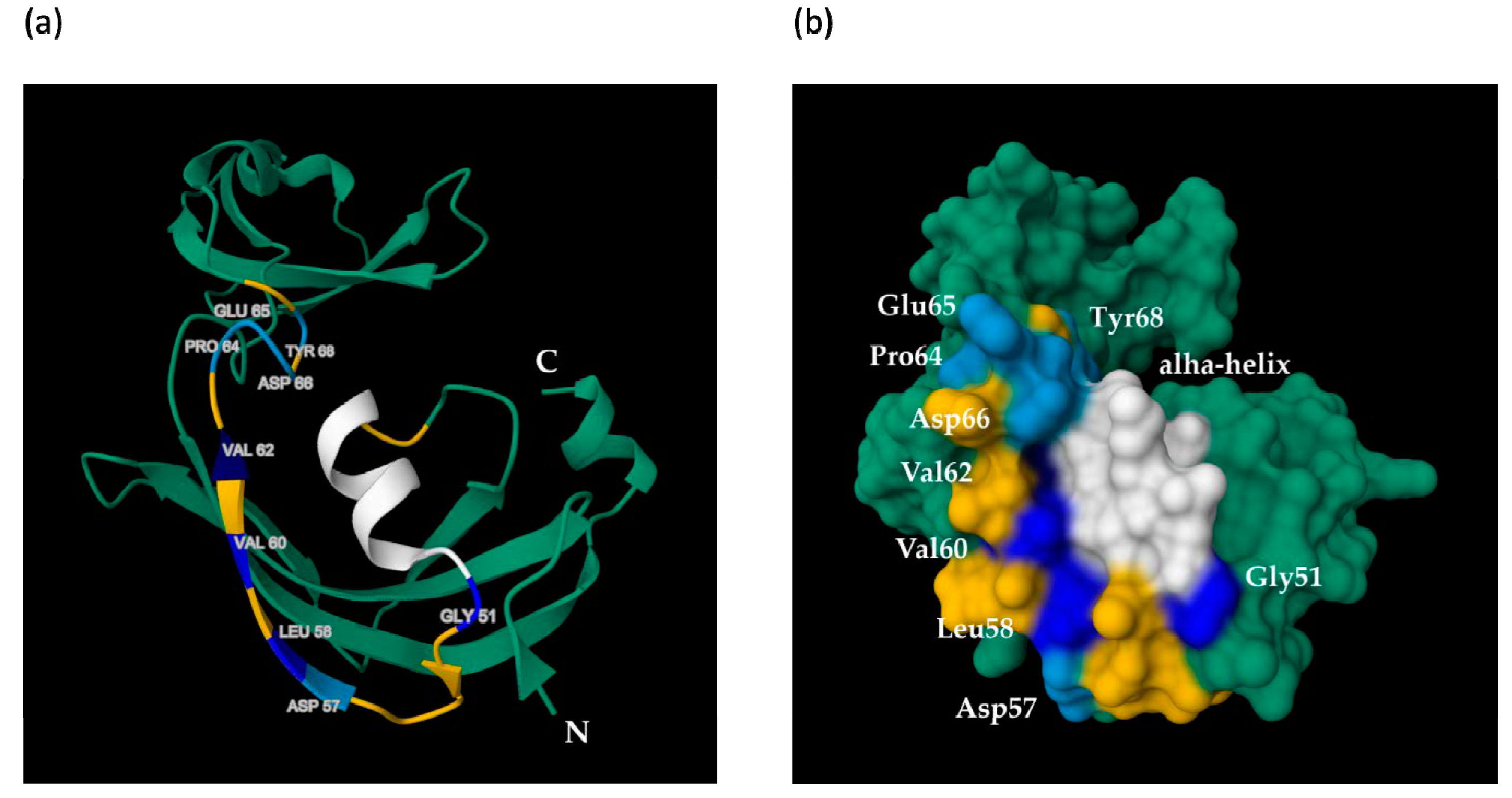
2.3. The 3D Model of MF3 and Its Analysis
3. Discussion
4. Materials and Methods
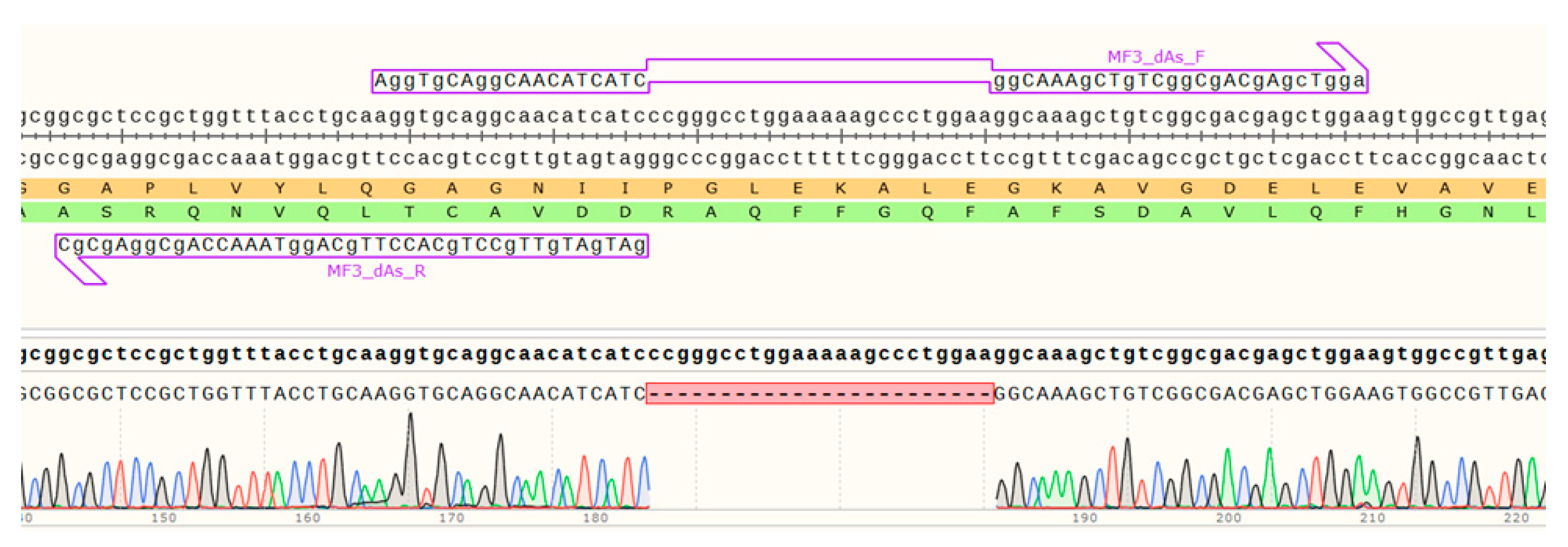
4.1. Design and Obtaining of AC MF3 and Alpha-Helix Deletion Mutants
4.2. Inducing Activity Assay
4.3. The 2D Structure Prediction and 3D Modeling
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Gleń-Karolczyk, K.; Boligłowa, E.; Antonkiewicz, J. Organic fertilization shapes the biodiversity of fungal communities associated with potato dry rot. Appl. Soil Ecol. 2018, 129, 43–51. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef]
- Millan, A.F.-S.; Larraya, L.; Farran, I.; Ancin, M.; Veramendi, J. Successful biocontrol of major postharvest and soil-borne plant pathogenic fungi by antagonistic yeasts. Biol. Control 2021, 160, 104683. [Google Scholar] [CrossRef]
- Pellegrini, M.; Ercole, C.; Di Zio, C.; Matteucci, F.; Pace, L.; Del Gallo, M. In vitro and in planta antagonistic effects of plant growth-promoting rhizobacteria consortium against soilborne plant pathogens of Solanum tuberosum and Solanum lycopersicum. FEMS Microbiol. Lett. 2020, 367, fnaa099. [Google Scholar] [CrossRef]
- Hatem, A.; Yaderets, V.; Karpova, N.; Glagoleva, E.; Ovchinnikov, A.; Petrova, K.; Shibaeva, A.; Dzhavakhiya, V. Inhibition of the growth of Botrytis cinerea by Penicillium chrysogenum VKM F-4876D combined with fludioxonil-, difenoconazole-, or tebuconazole-based fungicides. Agronomy 2023, 13, 2602. [Google Scholar] [CrossRef]
- Shcherbakova, L.; Kartashov, M.; Statsyuk, N.; Pasechnik, T.; Dzhavakhiya, V. Assessment of the sensitivity of some plant pathogenic fungi to 6-demethylmevinolin, a putative natural sensitizer able to help overcoming the fungicide resistance of plant pathogens. Antibiotics 2020, 9, 842. [Google Scholar] [CrossRef]
- Ulbasheva, S.; Vorobyev, D.; Statsyuk, N.; Kuznetsova, M. Pre-planting and post-harvest treatment of potato with low-frequency pulse electric field suppresses the development of the leaf and tuber blight. In Agriculture Digitalization and Organic Production; Ronzhin, A., Kostyaev, A., Eds.; Smart Innovation, Systems and Technologies; Springer: Singapore, 2023; Volume 362, pp. 279–291. [Google Scholar] [CrossRef]
- Ebrahimi-Zarandi, M.; Saberi Riseh, R.; Tarkka, M.T. Actinobacteria as effective biocontrol agents against plant pathogens, an overview on their role in eliciting plant defense. Microorganisms 2022, 10, 1739. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Cao, M.Y.; Zhang, Q.P.; Mohan, R.; Schar, J.; Mitchell, M.; Chen, H.; Liu, F.; Wang, D.; Fu, Z.Q. Join the green team: Inducers of plant immunity in the plant disease sustainable control toolbox. J. Adv. Res. 2023. advance online publication. [Google Scholar] [CrossRef]
- Reglinski, T.; Havis, N.; Rees, H.J.; de Jong, H. The practical role of induced resistance for crop protection. Phytopathology 2023, 113, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Ann. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced systemic resistance for improving plant immunity by beneficial microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Raikhy, G.; Kumar, D. Chemical elicitors of systemic acquired resistance—Salicylic acid and its functional analogs. Curr. Plant Biol. 2019, 17, 48–59. [Google Scholar] [CrossRef]
- Bektas, Y.; Eulgem, T. Synthetic plant defense elicitors. Front. Plant Sci. 2015, 5, 804. [Google Scholar] [CrossRef]
- Faize, L.; Faize, M. Functional analogues of salicylic acid and their use in crop protection. Agronomy 2018, 8, 5. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Ann. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef]
- Van der Ent, S.; Van Wees, S.C.; Pieterse, C.M. Jasmonate signaling in plant interactions with resistance-inducing beneficial microbes. Phytochemistry 2009, 70, 1581–1588. [Google Scholar] [CrossRef]
- Boubakri, H.; Gargouri, M.; Mliki, A.; Brini, F.; Chong, J.; Jbara, M. Vitamins for enhancing plant resistance. Planta 2016, 244, 529–543. [Google Scholar] [CrossRef]
- Ahn, I.P.; Kim, S.; Lee, Y.H. Vitamin B1 functions as an activator of plant disease resistance. Plant Physiol. 2005, 138, 1505–1515. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B.; Chapman, L.M. The importance of thiamine (vitamin B1) in plant health: From crop yield to biofortification. J. Biol. Chem. 2020, 295, 12002–12013. [Google Scholar] [CrossRef]
- Cohen, Y.; Vaknin, M.; Mauch-Mani, B. BABA-induced resistance: Milestones along a 55-year journey. Phytoparasitica 2016, 44, 513–538. [Google Scholar] [CrossRef]
- Návarová, H.; Bernsdorff, F.; Döring, A.C.; Zeier, J. Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 2012, 24, 5123–5141. [Google Scholar] [CrossRef] [PubMed]
- Mucharromah, E.; Kuc, J. Oxalate and phosphates induce systemic resistance against diseases caused by fungi, bacteria and viruses in cucumber. Crop Prot. 1991, 10, 265–270. [Google Scholar] [CrossRef]
- Deliopoulos, T.; Kettlewell, P.; Hare, M. Fungal disease suppression by inorganic salts: A review. Crop Prot. 2010, 29, 1059–1075. [Google Scholar] [CrossRef]
- Fesel, P.H.; Zuccaro, A. β-glucan: Crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet. Biol. 2016, 90, 53–60. [Google Scholar] [CrossRef]
- Klarzynski, O.; Plesse, B.; Joubert, J.M.; Yvin, J.C.; Kopp, M.; Kloareg, B.; Fritig, B. Linear beta-1,3 glucans are elicitors of defense responses in tobacco. Plant Physiol. 2000, 124, 1027–1038. [Google Scholar] [CrossRef]
- Gauthier, A.; Trouvelot, S.; Kelloniemi, J.; Frettinger, P.; Wendehenne, D.; Daire, X.; Joubert, J.M.; Ferrarini, A.; Delledonne, M.; Flors, V.; et al. The sulfated laminarin triggers a stress transcriptome before priming the SA- and ROS-dependent defenses during grapevine’s induced resistance against Plasmopara viticola. PLoS ONE 2014, 9, e88145. [Google Scholar] [CrossRef]
- Pusztahelyi, T. Chitin and chitin-related compounds in plant-fungal interactions. Mycology 2018, 9, 189–201. [Google Scholar] [CrossRef]
- Gong, B.Q.; Wang, F.Z.; Li, J.F. Hide-and-seek: Chitin-triggered plant immunity and fungal counterstrategies. Trends Plant Sci. 2020, 25, 805–816. [Google Scholar] [CrossRef]
- Hadwiger, L.A. Multiple effects of chitosan on plant systems: Solid science or hype. Plant Sci. 2013, 208, 42–49. [Google Scholar] [CrossRef]
- Mukarram, M.; Ali, J.; Dadkhah-Aghdash, H.; Kurjak, D.; Kačík, F.; Ďurkovič, J. Chitosan-induced biotic stress tolerance and crosstalk with phytohormones, antioxidants, and other signalling molecules. Front. Plant Sci. 2023, 14, 1217822. [Google Scholar] [CrossRef]
- Felix, G.; Duran, J.D.; Volko, S.; Boller, T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999, 18, 265–276. [Google Scholar] [CrossRef]
- Sun, W.; Dunning, F.M.; Pfund, C.; Weingarten, R.; Bent, A.F. Within-species flagellin polymorphism in Xanthomonas campestris pv. campestris and its impact on elicitation of Arabidopsis FLAGELLIN SENSING2-dependent defenses. Plant Cell 2006, 18, 764–779. [Google Scholar] [CrossRef]
- Sanguankiattichai, N.; Buscaill, P.; Preston, G.M. How bacteria overcome flagellin pattern recognition in plants. Curr. Opin. Plant Biol. 2022, 67, 102224. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gomez, M.; Sandal, N.; Stougaard, J.; Boller, T. Interplay of flg22-induced defence responses and nodulation in Lotus japonicus. J. Exp. Bot. 2012, 63, 393–401. [Google Scholar] [CrossRef]
- Czékus, Z.; Martics, A.; Pollák, B.; Kukri, A.; Tari, I.; Ördög, A.; Poór, P. The local and systemic accumulation of ethylene determines the rapid defence responses induced by flg22 in tomato (Solanum lycopersicum L.). J. Plant Physiol. 2023, 287, 154041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Qiu, D.; Zeng, H.; Guo, L.; Yang, X. BcGs1, a glycoprotein from Botrytis cinerea, elicits defence response and improves disease resistance in host plants. Biochem. Biophys. Res. Commun. 2015, 457, 627–634. [Google Scholar] [CrossRef]
- Wei, Z.M.; Laby, R.J.; Zumoff, C.H.; Bauer, D.W.; He, S.Y.; Collmer, A.; Beer, S.V. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science 1992, 257, 85–88. [Google Scholar] [CrossRef]
- Choi, M.S.; Kim, W.; Lee, C.; Oh, C.S. Harpins, multifunctional proteins secreted by gram-negative plant-pathogenic bacteria. Mol. Plant Microbe Interact. 2013, 26, 1115–1122. [Google Scholar] [CrossRef]
- Wang, D.; Wang, B.; Wang, J.; Wang, S.; Wang, W.; Niu, Y. Exogenous application of harpin protein Hpa1 onto Pinellia ternata induces systemic resistance against tobacco mosaic virus. Phytopathology 2020, 110, 1189–1198. [Google Scholar] [CrossRef]
- Chen, L.; Qian, J.; Qu, S.; Long, J.; Yin, Q.; Zhang, C.; Wu, X.; Sun, F.; Wu, T.; Hayes, M.; et al. Identification of specific fragments of HpaG Xooc, a harpin from Xanthomonas oryzae pv. oryzicola, that induce disease resistance and enhance growth in plants. Phytopathology 2008, 98, 781–791. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, L.; Zhang, S.J.; Zhang, S.S.; Qu, S.; Ren, X.; Long, J.; Yin, Q.; Qian, J.; Sun, F.; Zhang, C.; et al. A fragment of the Xanthomonas oryzae pv. oryzicola harpin HpaG Xooc reduces disease and increases yield of rice in extensive grower plantings. Phytopathology 2008, 98, 792–802. [Google Scholar] [CrossRef]
- Oliveira, C.; Domingues, L. Guidelines to reach high-quality purified recombinant proteins. Appl. Microbiol. Biotechnol. 2018, 102, 81–92. [Google Scholar] [CrossRef]
- Shumilina, D.; Krämer, R.; Klocke, E.; Dzhavakhiya, V. MF3 (peptidyl-prolyl cis-trans isomerase of FKBP type from Pseudomonas fluorescens)—An elicitor of non-specific plant resistance against pathogens. Phytopathol. Pol. 2006, 41, 39–49. [Google Scholar]
- Shumilina, D.; Dzhavakhiya, V. A new approach to development and practical application of bio-pesticides based on the Pseudomonas fluorescens protein—Inductor of non-specific plant resistance to fungal, viral and bacterial diseases. In Proceedings of the 10th ISTC/Korea Workshop “Industrial Applications of Russia’s New Biotechnologies”, Mokpo, Republic of Korea, 14–15 March 2006. [Google Scholar]
- Dzhavakhiya, V.G.; Voinova, T.M.; Shumilina, D.V. Search for the active center pf peptidyl-prolyl cys/trans isomerase from Pseudomonas fuorescens responsible for the induction of tobacco (Nicotiana tabacum L.) plant resistance to tobacco mosaic virus. Sel’skokhozyaistvennaya Biol. Agric. Biol. 2016, 51, 392–400. [Google Scholar] [CrossRef]
- Kunze, G.; Zipfel, C.; Robatzek, S.; Niehaus, K.; Boller, T.; Felix, G. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 2004, 16, 3496–3507. [Google Scholar] [CrossRef]
- Noda, J.; Brito, N.; González, C. The Botrytis cinerea xylanase Xyn11A contributes to virulence with its necrotizing activity, not with its catalytic activity. BMC Plant Biol. 2010, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Frías, M.; González, M.; González, C.; Brito, N. A 25-residue peptide from Botrytis cinerea xylanase BcXyn11A elicits plant defenses. Front. Plant Sci. 2019, 10, 474. [Google Scholar] [CrossRef]
- Yu, T.Y.; Sun, M.K.; Liang, L.K. Receptors in the induction of the plant innate immunity. Mol. Plant Microbe Interact. 2021, 34, 587–601. [Google Scholar] [CrossRef]
- Cuff, J.A.; Clamp, M.E.; Siddiqui, A.S.; Finlay, M.; Barton, G.J. JPred: A consensus secondary structure prediction server. Bioinformatics 1998, 14, 892–893. [Google Scholar] [CrossRef] [PubMed]
- Cuff, J.A.; Barton, G.J. Application of multiple sequence alignment profiles to improve protein secondary structure prediction. Proteins 2000, 40, 502–511. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef] [PubMed]
- Morrison, K.L.; Weiss, G.A. Combinatorial alanine-scanning. Curr. Opin. Chem. Biol. 2001, 5, 302–307. [Google Scholar] [CrossRef]
- Edwards, T.A.; Wilson, A.J. Helix-mediated protein--protein interactions as targets for intervention using foldamers. Amino Acids 2011, 41, 743–754. [Google Scholar] [CrossRef]
- Popletaeva, S.B.; Statsyuk, N.V.; Voinova, T.M.; Arslanova, L.R.; Zernov, A.L.; Bonartsev, A.P.; Bonartseva, G.A.; Dzhavakhiya, V.G. Evaluation of eliciting activity of peptidil prolyl cys/trans isomerase from Pseudonomas fluorescens encapsulated in sodium alginate regarding plant resistance to viral and fungal pahogens. AIMS Microbiol. 2018, 12, 192–208. [Google Scholar] [CrossRef]
- Voinova, T.; Kartashov, M.; Pasechnik, T.; Shcherbakova, L.; Statsyuk, N.; Dzhavakhiya, V. Peptidyl prolyl cis/trans isomerase from Pseudomonas fluorescens encapsulated into biodegradable natural polymers: A potential plant protection agent inducing plant resistance to fungal pathogens. Biocatal. Agric. Biotechnol. 2021, 36, 102112. [Google Scholar] [CrossRef]
- Dzhavakhiya, V.G.; Filippov, A.V.; Skryabin, K.G.; Voinova, T.M.; Kuznetsova, M.A.; Shulga, O.A.; Pridannikov, M.V.; Shumilina, D.V.; Kromina, K.A.; Battchikova, N.V.; et al. Proteins Inducing Multiple Resistance of Plants to Phytopathogens and Pests; World Intellectual Property Organization (WIPO): Geneva, Switzerland, 2005. [Google Scholar]
- Rao, G.P.; Reddy, M.G. Overview of yield losses due to plant viruses. In Applied Plant Virology. Advances, Detection, and Antiviral Strategies; Awasthi, L.P., Ed.; Academic Press: London, UK, 2020; pp. 531–562. [Google Scholar] [CrossRef]
- Nie, X.; Singh, M. Response of potato, tobacco and Physalis floridana plants to mixed infection with PVX, PVYNTN and PVYO strains. Can. J. Plant Pathol. 2013, 35, 390–401. [Google Scholar] [CrossRef]
- Tatineni, S.; Hein, G.L. Plant viruses of agricultural importance: Current and future perspectives of virus disease management strategies. Phytopathology 2023, 113, 117–141. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chu, W.; Qi, Q.; Xun, L. New insights into the QuikChange™ process guide the use of Phusion DNA polymerase for site-directed mutagenesis. Nucleic Acids Res. 2015, 43, e12. [Google Scholar] [CrossRef]
- Yip, T.T.; Hutchens, T.W. Immobilized metal ion affinity chromatography. Methods Mol. Biol. 1992, 11, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Holmes, F.O. Local lesions in tobacco mosaic. Bot. Gaz. 1929, 87, 39–55. [Google Scholar] [CrossRef]
- Nourinejhad Zarghani, S.; Ehlers, J.; Monavari, M.; von Bargen, S.; Hamacher, J.; Büttner, C.; Bandte, M. Applicability of different methods for quantifying virucidal efficacy using MENNO florades and tomato brown rugose fruit virus as an example. Plants 2023, 12, 894. [Google Scholar] [CrossRef]




| No. | Primer | 5′–3′ Sequence |
|---|---|---|
| 1 | 2 | 3 |
| 1 | 11(G_A)F | cctggaagccaaagctgtcggcg |
| 2 | 11(G_A)R | ctttggcttccagggctttttc |
| 3 | 17(D_A)F | tcggcgacgccttggaagtggc |
| 4 | 17(D_A)R | acttccaaggcgtcgccgacag |
| 5 | 18(L_A)F | gcgacgacgcggaagtggccgttg |
| 6 | 18(L_A)R | ccacttccgcgtcgtcgccgacag |
| 7 | 20(V_A)F | ttggaagcggccgttgagcc |
| 8 | 20(V_A)R | aacggccgcttccaagtcgtc |
| 9 | 22(V_A)F | gtggccgctgagccggaagatg |
| 10 | 22(V_A)R | tccggctcagcggccacttccaag |
| 11 | 24(P_A)F | gttgaggcggaagatgcttacg |
| 12 | 24(P_A)R | tcttccgcctcaacggccacttc |
| 13 | 25(E_A)F | ttgagccggcagatgcttacggc |
| 14 | 25(E_A)R | aagcatctgccggctcaacggcc |
| 15 | 26(D_A)F | cggaagctgcttacggcgaatac |
| 16 | 26(D_A)R | gtaagcagcttccggctcaacgg |
| 17 | 28(Y_L)R | gatgctttaggcgaatacgccg |
| 18 | 28(Y_L)F | attcgcctaaagcatcttccgg |
| 19 | 29(G_A)F | tgcttacgccgaatacgccgcc |
| 20 | 29(G_A)R | cgtattcggcgtaagcatcttcc |
| 21 | MF3_dAs_F | AggTgCAggCAACATCATCggCAAAgCTgTCggCgACgAgcTgga |
| 22 | MF3_dAs_R | gATgATgTTgCCTgCACCTTgCAggTAAACCAgCggAgCgC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erokhin, D.; Popletaeva, S.; Sinelnikov, I.; Rozhkova, A.; Shcherbakova, L.; Dzhavakhiya, V. Some Structural Elements of Bacterial Protein MF3 That Influence Its Ability to Induce Plant Resistance to Fungi, Viruses, and Other Plant Pathogens. Int. J. Mol. Sci. 2023, 24, 16374. https://doi.org/10.3390/ijms242216374
Erokhin D, Popletaeva S, Sinelnikov I, Rozhkova A, Shcherbakova L, Dzhavakhiya V. Some Structural Elements of Bacterial Protein MF3 That Influence Its Ability to Induce Plant Resistance to Fungi, Viruses, and Other Plant Pathogens. International Journal of Molecular Sciences. 2023; 24(22):16374. https://doi.org/10.3390/ijms242216374
Chicago/Turabian StyleErokhin, Denis, Sophya Popletaeva, Igor Sinelnikov, Alexandra Rozhkova, Larisa Shcherbakova, and Vitaly Dzhavakhiya. 2023. "Some Structural Elements of Bacterial Protein MF3 That Influence Its Ability to Induce Plant Resistance to Fungi, Viruses, and Other Plant Pathogens" International Journal of Molecular Sciences 24, no. 22: 16374. https://doi.org/10.3390/ijms242216374
APA StyleErokhin, D., Popletaeva, S., Sinelnikov, I., Rozhkova, A., Shcherbakova, L., & Dzhavakhiya, V. (2023). Some Structural Elements of Bacterial Protein MF3 That Influence Its Ability to Induce Plant Resistance to Fungi, Viruses, and Other Plant Pathogens. International Journal of Molecular Sciences, 24(22), 16374. https://doi.org/10.3390/ijms242216374






