ZNF643/ZFP69B Exerts Oncogenic Properties and Associates with Cell Adhesion and Immune Processes
Abstract
:1. Introduction
2. Results
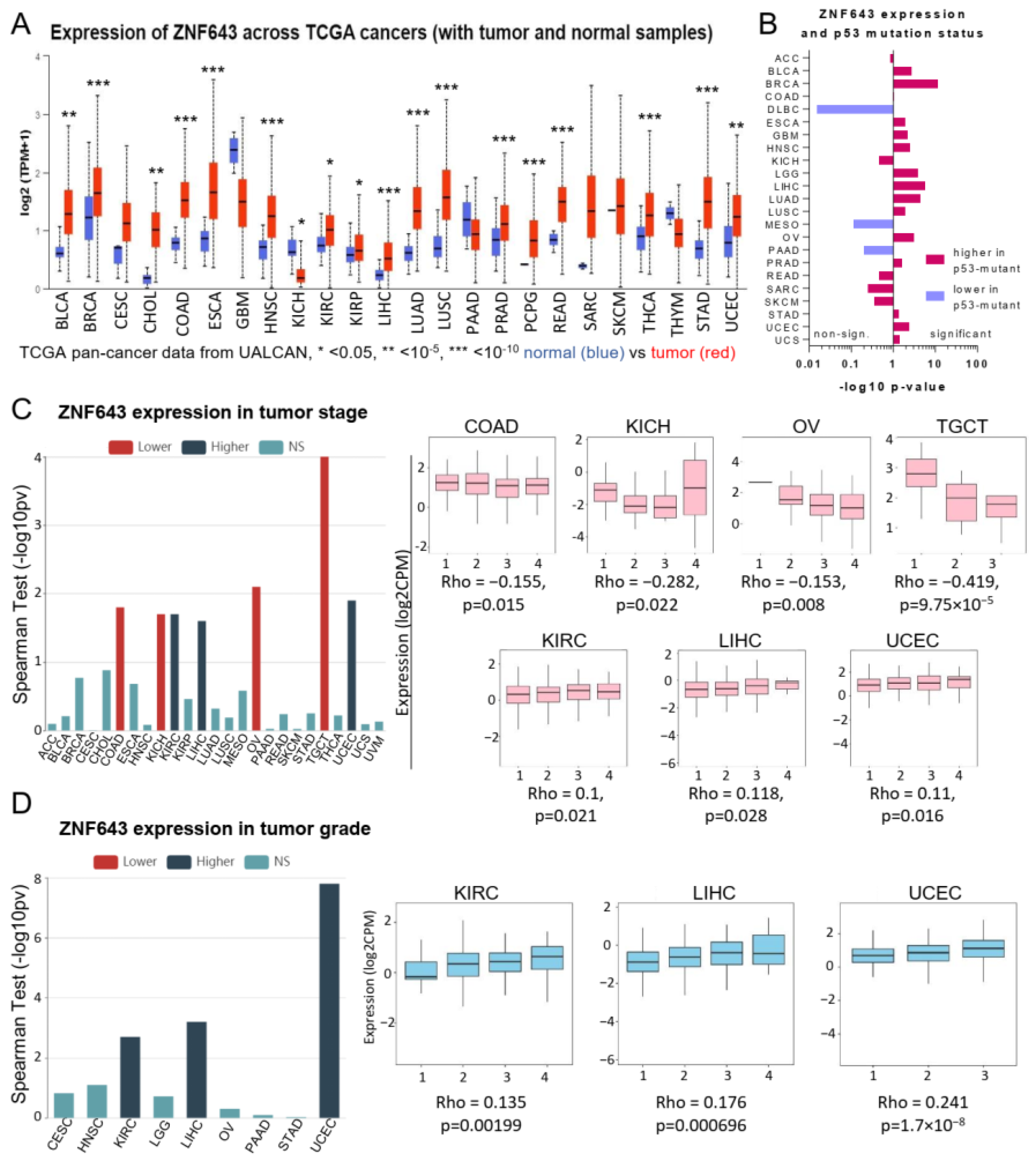
2.1. Association between ZNF643 Expression and Clinical Parameters in a Pan-Cancer Setting
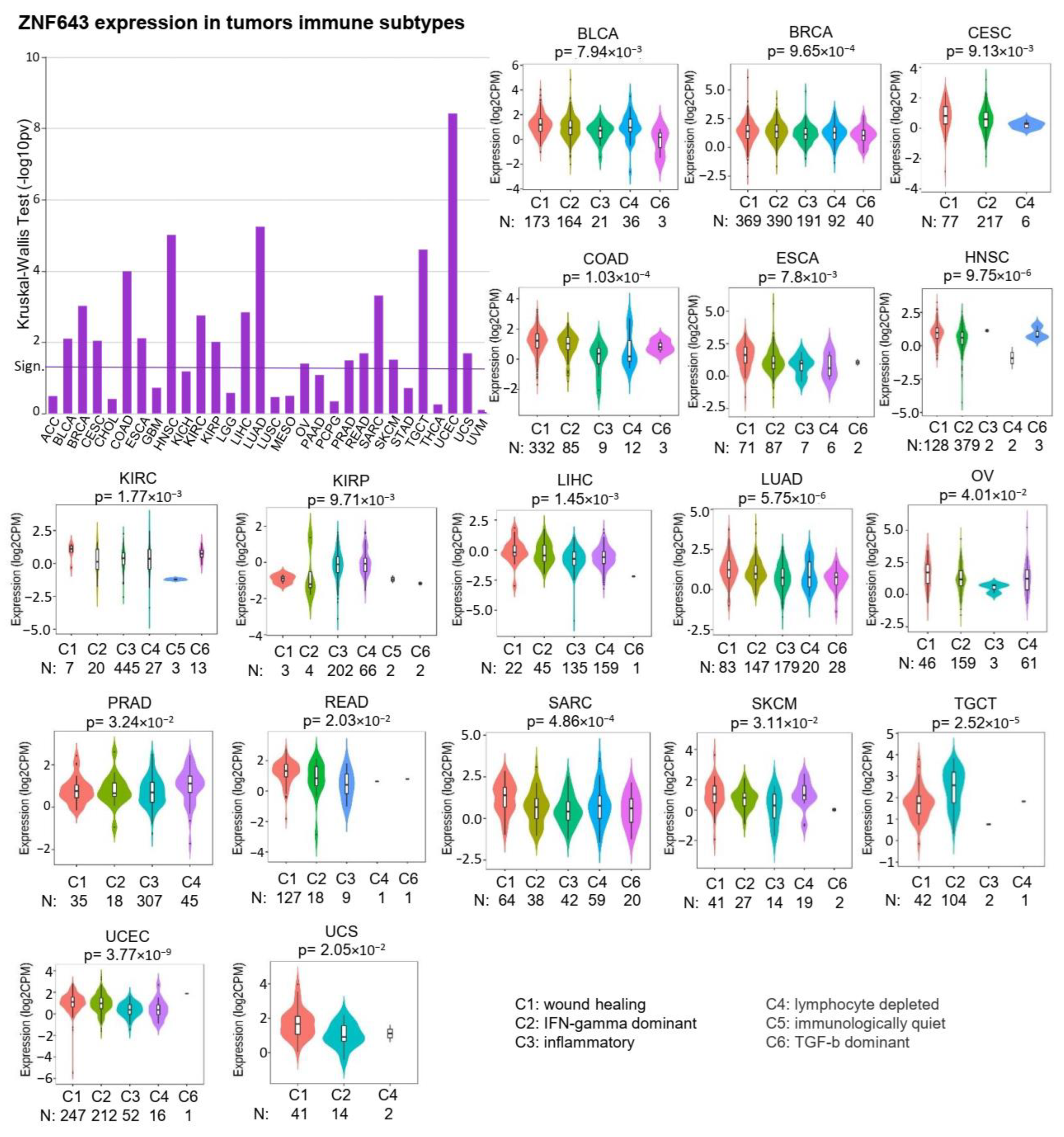
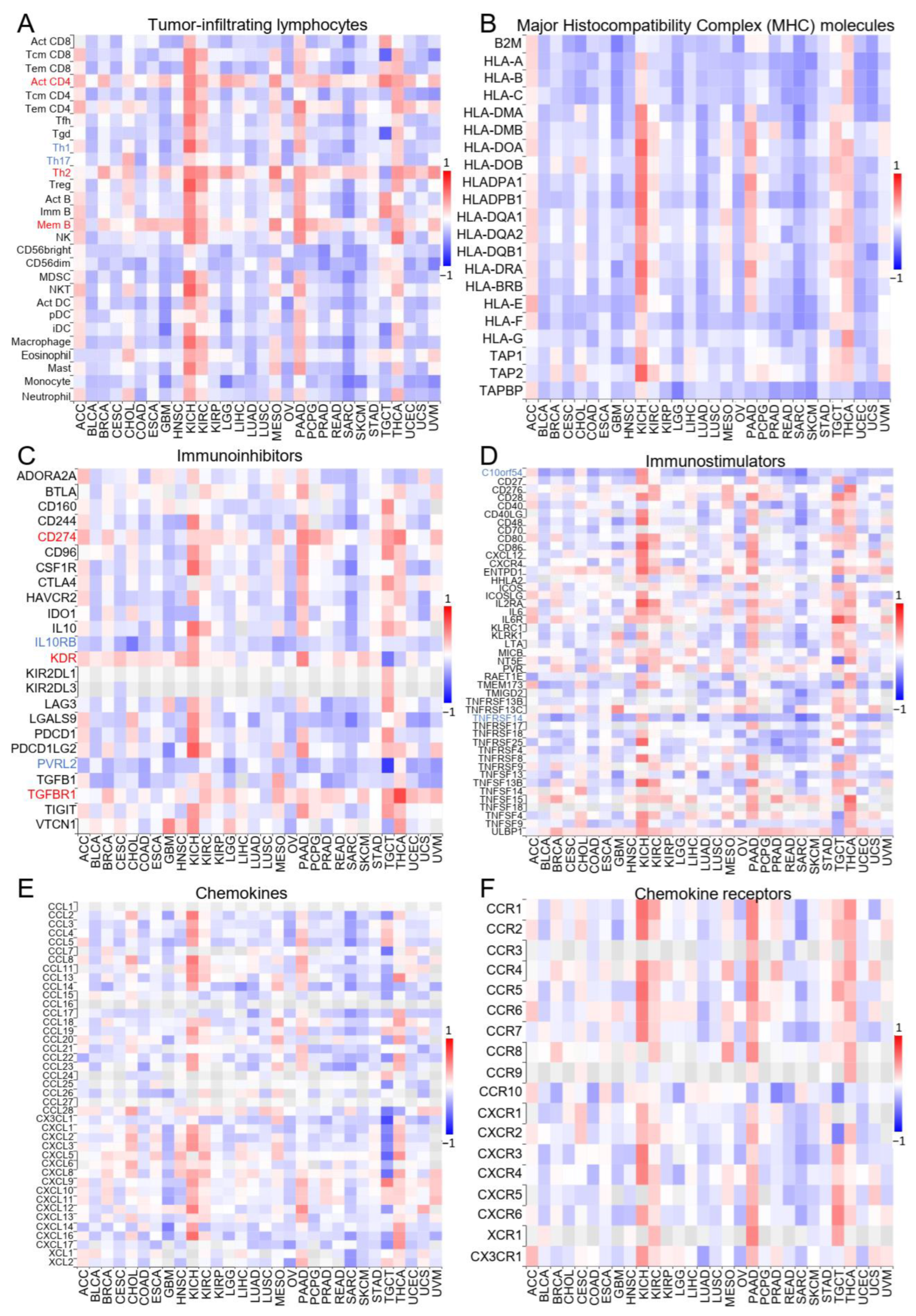
2.2. ZNF643 Expression and Tumor Immune Status
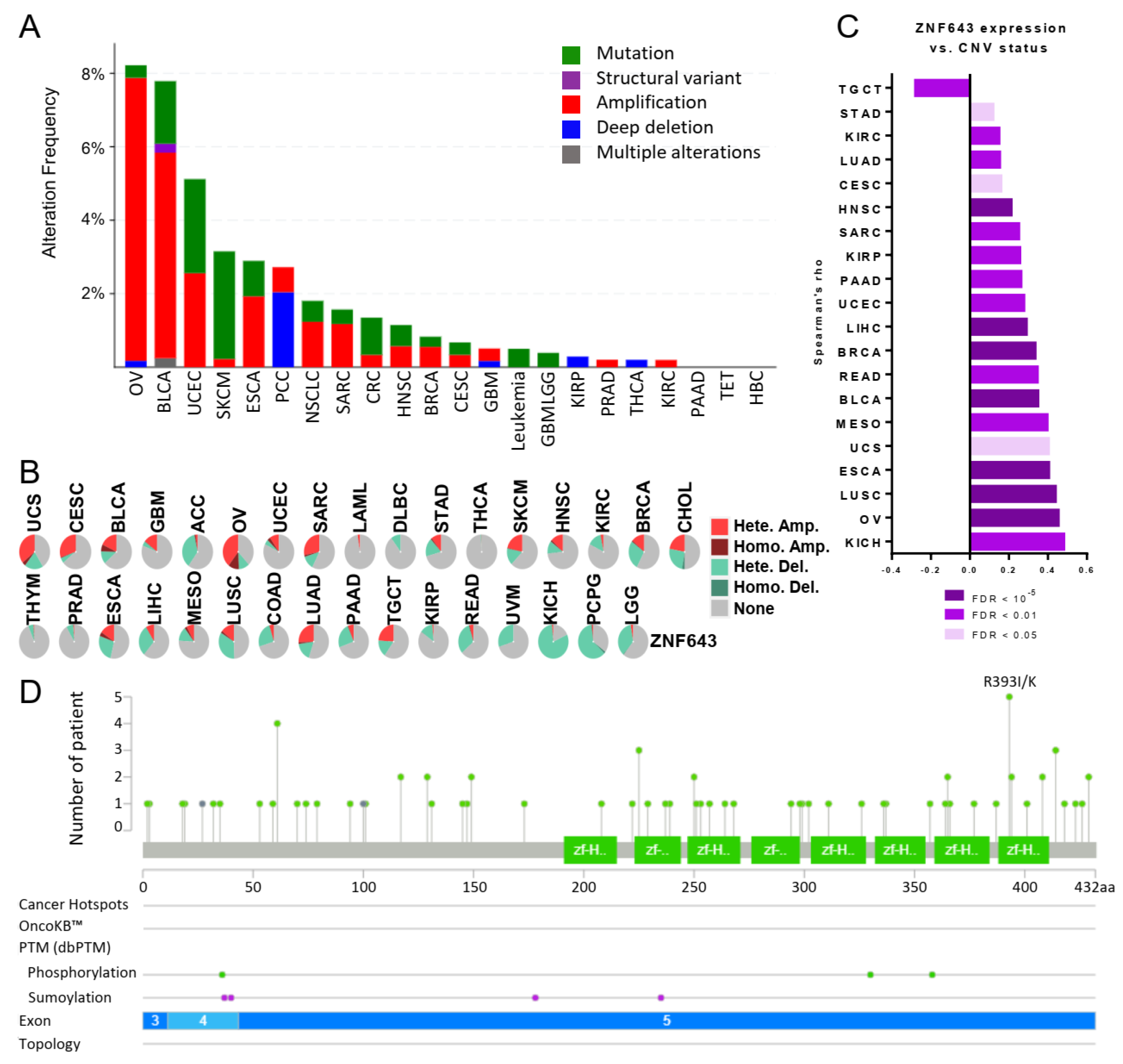
2.3. ZNF643 Expression Is Associated with Copy Number Variations (CNVs) in Many Tumors
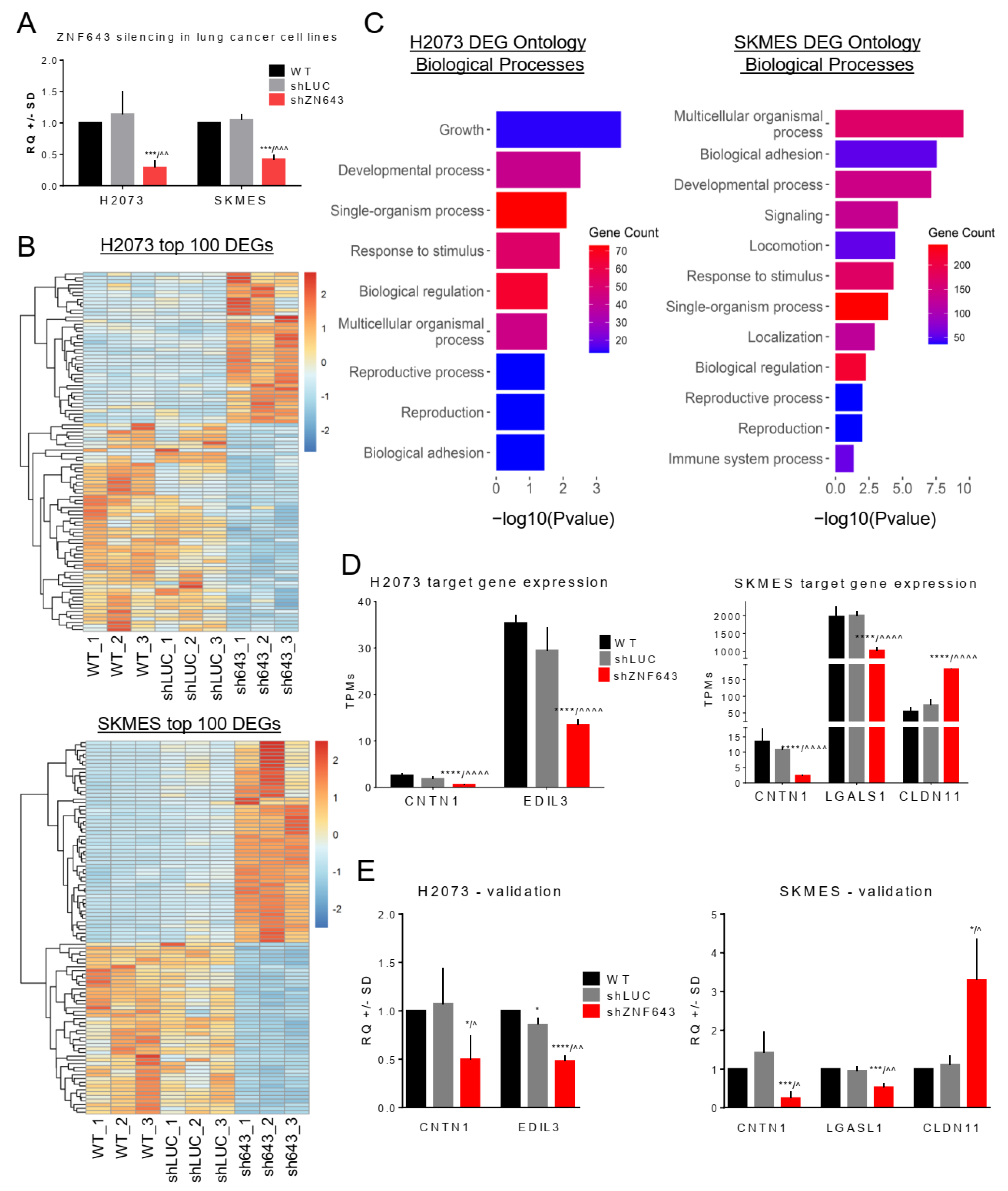
2.4. Transcriptomic Profiling of the Cells with Silenced Expression of ZNF643
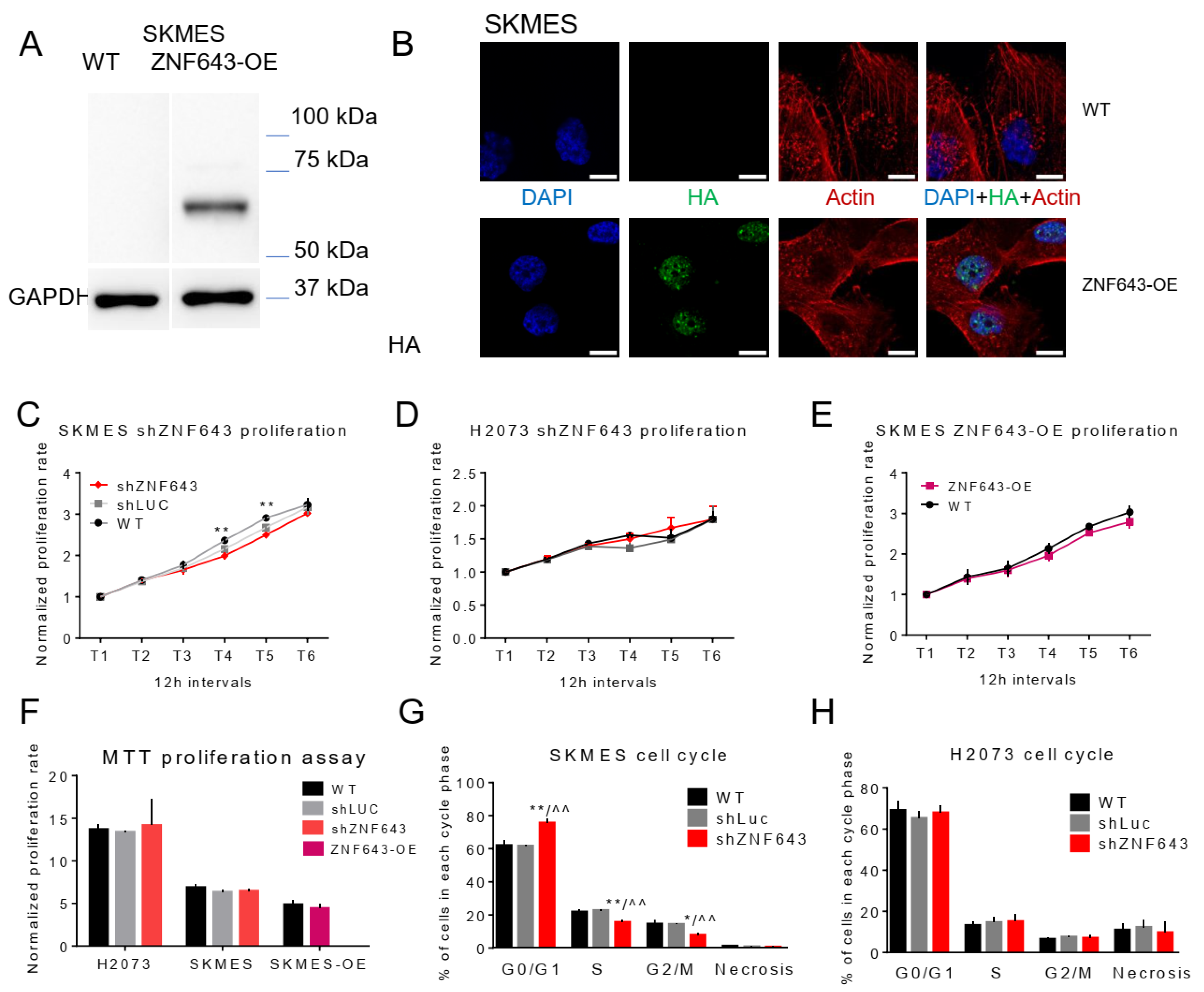
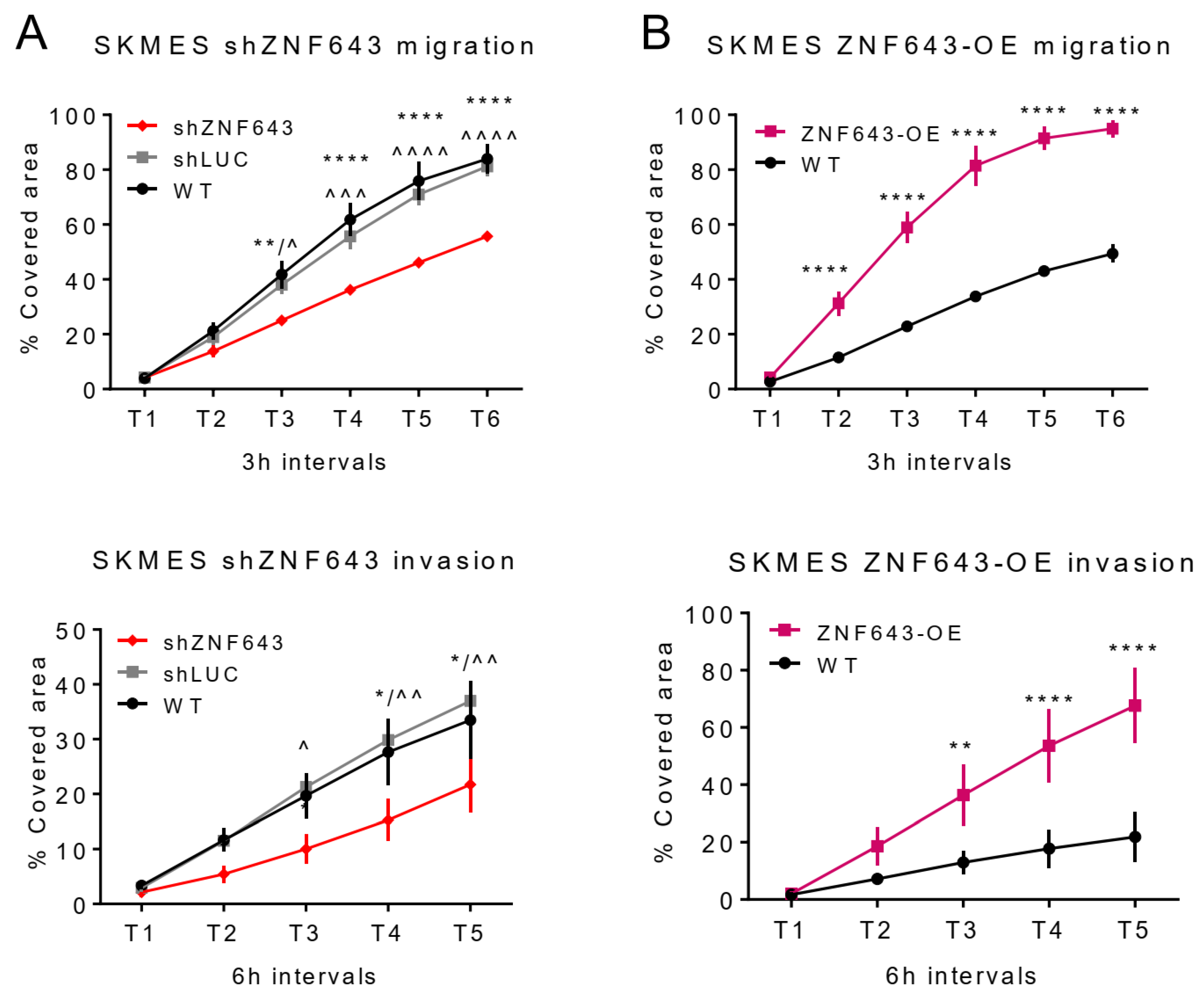
2.5. In Vitro Studies on ZNF643 Function in Cancer Cells
2.6. Identification, Characterization, and Single-Gene Validation of the Targets Bound by ZNF643
3. Discussion
4. Materials and Methods
4.1. Databases Used for the Analysis of Pan-Cancer Omics Data Related to ZNF643
4.2. ZNF643 Gene Expression Knock-Down and Overexpression in a Cell-Line Model
4.3. RNA-seq and Differential Gene Expression Analysis
4.4. Western Blot
4.5. Immunofluorescence Cell Staining
4.6. Assessment of Cell Proliferation
4.7. Analysis of Cell Cycle and Apoptosis
4.8. Migration and Invasion
4.9. Chromatin Immunoprecipitation Coupled with Next-Generation Sequencing (ChIP-seq) Analysis
4.10. RT-qPCR and ChIP-qPCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Llinas-Arias, P.; Esteller, M. Epigenetic inactivation of tumour suppressor coding and non-coding genes in human cancer: An update. Open Biol. 2017, 7, 170152. [Google Scholar] [CrossRef] [PubMed]
- Sobocińska, J.; Molenda, S.; Machnik, M.; Oleksiewicz, U. KRAB-ZFP Transcriptional Regulators Acting as Oncogenes and Tumor Suppressors: An Overview. Int. J. Mol. Sci. 2021, 22, 2212. [Google Scholar] [CrossRef]
- Sun, M.; Ju, J.; Ding, Y.; Zhao, C.; Tian, C. The signaling pathways regulated by KRAB zinc-finger proteins in cancer. Biochim. Biophys. Acta BBA Rev. Cancer 2022, 1877, 188731. [Google Scholar] [CrossRef]
- Lupo, A.; Cesaro, E.; Montano, G.; Zurlo, D.; Izzo, P.; Costanzo, P. KRAB-Zinc Finger Proteins: A Repressor Family Displaying Multiple Biological Functions. Curr. Genom. 2013, 14, 268–278. [Google Scholar] [CrossRef]
- Machnik, M.; Cylwa, R.; Kielczewski, K.; Biecek, P.; Liloglou, T.; Mackiewicz, A.; Oleksiewicz, U. The expression signature of cancer-associated KRAB-ZNF factors identified in TCGA pan-cancer transcriptomic data. Mol. Oncol. 2019, 13, 701–724. [Google Scholar] [CrossRef]
- Dixon, S.J.; Patel, D.N.; Welsch, M.; Skouta, R.; Lee, E.D.; Hayano, M.; Thomas, A.G.; Gleason, C.E.; Tatonetti, N.P.; Slusher, B.S.; et al. Pharmacological inhibition of cystine–glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 2014, 3, e02523. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, B.; Ma, X.; Zhuang, H.; Xie, Z.; Tang, C.; Tan, W.; Yang, L.; Shang, C.; Chen, Y. Identification of a Novel Ferroptosis-Related Gene Signature for Predicting Prognosis and Responsiveness to Immunotherapy in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2023, 10, 1–16. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Z.; Jing, T. A novel signature of combing cuproptosis- with ferroptosis-related genes for prediction of prognosis, immunologic therapy responses and drug sensitivity in hepatocellular carcinoma. Front. Oncol. 2022, 12, 1000993. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Li, B.; Liu, Y.; Yang, C.; Tang, S.; Cho, W.C.; Huang, Z. Ferroptosis and triple-negative breast cancer: Potential therapeutic targets. Front. Oncol. 2022, 12, 1017041. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wang, R.; Lin, Y.; Yan, D.; Zuo, J.; Chen, J.; Shen, B. A Ferroptosis-Related Gene Signature Identified as a Novel Prognostic Biomarker for Colon Cancer. Front. Genet. 2021, 12, 692426. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.-H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Su, J.L.; Yang, P.C.; Shih, J.Y.; Yang, C.Y.; Wei, L.H.; Hsieh, C.Y.; Chou, C.H.; Jeng, Y.M.; Wang, M.Y.; Chang, K.J.; et al. The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell 2006, 9, 209–223. [Google Scholar] [CrossRef]
- Yan, J.; Wong, N.; Hung, C.; Chen, W.X.; Tang, D. Contactin-1 reduces E-cadherin expression via activating AKT in lung cancer. PLoS ONE 2013, 8, e65463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Sun, S.; Ji, F.; Liu, C.; Lin, H.; Xie, L.; Yang, H.; Tang, W.; Zhou, Y.; Xu, J.; et al. CNTN-1 Enhances Chemoresistance in Human Lung Adenocarcinoma through Induction of Epithelial-Mesenchymal Transition by Targeting the PI3K/Akt Pathway. Cell. Physiol. Biochem. 2017, 43, 465–480. [Google Scholar] [CrossRef]
- Chetry, M.; Song, Y.; Pan, C.; Li, R.; Zhang, J.; Zhu, X. Effects of Galectin-1 on Biological Behavior in Cervical Cancer. J. Cancer 2020, 11, 1584–1595. [Google Scholar] [CrossRef]
- Li, J.M.; Tseng, C.W.; Lin, C.C.; Law, C.H.; Chien, Y.A.; Kuo, W.H.; Chou, H.C.; Wang, W.C.; Chan, H.L. Upregulation of LGALS1 is associated with oral cancer metastasis. Ther. Adv. Med. Oncol. 2018, 10, 1758835918794622. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Jia, A.; Cao, Y.; Yang, L.; Jia, Z. LGALS1 regulates cell adhesion to promote the progression of ovarian cancer. Oncol. Lett. 2023, 26, 326. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, H.; Yan, Y.; Li, Y.; Che, G.; Zhou, C.; Nicot, C.; Ma, H. NCAPG promotes the oncogenesis and progression of non-small cell lung cancer cells through upregulating LGALS1 expression. Mol. Cancer 2022, 21, 55. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Chen, J.; Shi, M.; Gao, H.; Sekar, K.; Seshachalam, V.P.; Ooi, L.L.; Hui, K.M. EDIL3 is a novel regulator of epithelial-mesenchymal transition controlling early recurrence of hepatocellular carcinoma. J. Hepatol. 2015, 63, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Peng, K.W.; Wang, B.; Yang, X.F.; Zhang, Z.M. EDIL3 regulates gastric cancer cell migration, invasion and epithelial-mesenchymal transition via TGF-β1/XIST/miR-137 feedback loop. Transl. Cancer Res. 2020, 9, 6313–6330. [Google Scholar] [CrossRef]
- Agarwal, R.; Mori, Y.; Cheng, Y.; Jin, Z.; Olaru, A.V.; Hamilton, J.P.; David, S.; Selaru, F.M.; Yang, J.; Abraham, J.M.; et al. Silencing of claudin-11 is associated with increased invasiveness of gastric cancer cells. PLoS ONE 2009, 4, e8002. [Google Scholar] [CrossRef]
- Li, H.P.; Peng, C.C.; Wu, C.C.; Chen, C.H.; Shih, M.J.; Huang, M.Y.; Lai, Y.R.; Chen, Y.L.; Chen, T.W.; Tang, P.; et al. Inactivation of the tight junction gene CLDN11 by aberrant hypermethylation modulates tubulins polymerization and promotes cell migration in nasopharyngeal carcinoma. J. Exp. Clin. Cancer Res. CR 2018, 37, 102. [Google Scholar] [CrossRef]
- Walesch, S.K.; Richter, A.M.; Helmbold, P.; Dammann, R.H. Claudin11 Promoter Hypermethylation Is Frequent in Malignant Melanoma of the Skin, but Uncommon in Nevus Cell Nevi. Cancers 2015, 7, 1233–1243. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, X.; Zheng, L.; Zhao, H.; Li, W.; Wang, B.; Xue, L.; Tian, Y.; Xie, Y. Construction and Analysis of the Tumor-Specific mRNA-miRNA-lncRNA Network in Gastric Cancer. Front. Pharmacol. 2020, 11, 1112. [Google Scholar] [CrossRef]
- Moh, M.C.; Lorenzini, P.A.; Gullo, C.; Schwarz, H. Tumor necrosis factor receptor 1 associates with CD137 ligand and mediates its reverse signaling. FASEB J. 2013, 27, 2957–2966. [Google Scholar] [CrossRef]
- Pennycuick, A.; Teixeira, V.H.; AbdulJabbar, K.; Raza, S.E.A.; Lund, T.; Akarca, A.U.; Rosenthal, R.; Kalinke, L.; Chandrasekharan, D.P.; Pipinikas, C.P.; et al. Immune Surveillance in Clinical Regression of Preinvasive Squamous Cell Lung Cancer. Cancer Discov. 2020, 10, 1489–1499. [Google Scholar] [CrossRef]
- Bartkowiak, T.; Curran, M.A. 4-1BB Agonists: Multi-Potent Potentiators of Tumor Immunity. Front. Oncol. 2015, 5, 117. [Google Scholar] [CrossRef] [PubMed]
- Segal, N.H.; He, A.R.; Doi, T.; Levy, R.; Bhatia, S.; Pishvaian, M.J.; Cesari, R.; Chen, Y.; Davis, C.B.; Huang, B.; et al. Phase I Study of Single-Agent Utomilumab (PF-05082566), a 4-1BB/CD137 Agonist, in Patients with Advanced Cancer. Clin. Cancer Res. 2018, 24, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Pei, D.; Cheng, T.; Wu, C.; Pu, X.; Chen, X.; Liu, Y.; Shen, H.; Zhang, W.; Shu, Y. CD137 ligand-mediated reverse signaling inhibits proliferation and induces apoptosis in non-small cell lung cancer. Med. Oncol. 2015, 32, 44. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.L.; Gan, Y.; Gao, H.F.; Fan, Y.C.; Wang, Q.; Yuan, H.; Song, Y.F.; Wang, J.D.; Tu, H. TNFSF9 exerts an inhibitory effect on hepatocellular carcinoma. J. Dig. Dis. 2017, 18, 395–403. [Google Scholar] [CrossRef]
- Li, L.; Yang, M.; Yu, J.; Cheng, S.; Ahmad, M.; Wu, C.; Wan, X.; Xu, B.; Ben-David, Y.; Luo, H. A Novel L-Phenylalanine Dipeptide Inhibits the Growth and Metastasis of Prostate Cancer Cells via Targeting DUSP1 and TNFSF9. Int. J. Mol. Sci. 2022, 23, 10916. [Google Scholar] [CrossRef]
- Ji, Y.; Jiang, J.; Huang, L.; Feng, W.; Zhang, Z.; Jin, L.; Xing, X. Sperm-associated antigen 4 (SPAG4) as a new cancer marker interacts with Nesprin3 to regulate cell migration in lung carcinoma. Oncol. Rep. 2018, 40, 783–792. [Google Scholar] [CrossRef]
- Morgan, J.T.; Pfeiffer, E.R.; Thirkill, T.L.; Kumar, P.; Peng, G.; Fridolfsson, H.N.; Douglas, G.C.; Starr, D.A.; Barakat, A.I. Nesprin-3 regulates endothelial cell morphology, perinuclear cytoskeletal architecture, and flow-induced polarization. Mol. Biol. Cell 2011, 22, 4324–4334. [Google Scholar] [CrossRef]
- Stewart-Hutchinson, P.J.; Hale, C.M.; Wirtz, D.; Hodzic, D. Structural requirements for the assembly of LINC complexes and their function in cellular mechanical stiffness. Exp. Cell Res. 2008, 314, 1892–1905. [Google Scholar] [CrossRef]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. J. Biol. Chem. 2020, 295, 2495–2505. [Google Scholar] [CrossRef]
- Xu, S.; Lam, S.K.; Cheng, P.N.; Ho, J.C. Contactin 1 modulates pegylated arginase resistance in small cell lung cancer through induction of epithelial-mesenchymal transition. Sci. Rep. 2019, 9, 12030. [Google Scholar] [CrossRef]
- Zhang, R.; Yao, W.; Qian, P.; Li, Y.; Jiang, C.; Ao, Z.; Qian, G.; Wang, C.; Wu, G.; Li, J.; et al. Increased sensitivity of human lung adenocarcinoma cells to cisplatin associated with downregulated contactin-1. Biomed. Pharmacother. 2015, 71, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.H.; Wang, Y.; Yang, J.Y.; Li, J.; Feng, M.X.; Wang, Y.H.; Yang, X.M.; He, P.; Tian, G.A.; Zhang, X.X.; et al. Overexpressed EDIL3 predicts poor prognosis and promotes anchorage-independent tumor growth in human pancreatic cancer. Oncotarget 2016, 7, 4226–4240. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Ru, B.; Wong, C.N.; Tong, Y.; Zhong, J.Y.; Zhong, S.S.W.; Wu, W.C.; Chu, K.C.; Wong, C.Y.; Lau, C.Y.; Chen, I.; et al. TISIDB: An integrated repository portal for tumor-immune system interactions. Bioinformatics 2019, 35, 4200–4202. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Liu, C.-J.; Hu, F.-F.; Xia, M.-X.; Han, L.; Zhang, Q.; Guo, A.-Y. GSCALite: A web server for gene set cancer analysis. Bioinformatics 2018, 34, 3771–3772. [Google Scholar] [CrossRef]
- Liu, C.-J.; Hu, F.-F.; Xie, G.-Y.; Miao, Y.-R.; Li, X.-W.; Zeng, Y.; Guo, A.-Y. GSCA: An integrated platform for gene set cancer analysis at genomic, pharmacogenomic and immunogenomic levels. Brief. Bioinform. 2022, 24, bbac558. [Google Scholar] [CrossRef]
- Wiznerowicz, M.; Trono, D. Conditional suppression of cellular genes: Lentivirus vector-mediated drug-inducible RNA interference. J. Virol. 2003, 77, 8957–8961. [Google Scholar] [CrossRef] [PubMed]
- Imbeault, M.; Helleboid, P.-Y.; Trono, D. KRAB zinc-finger proteins contribute to the evolution of gene regulatory networks. Nature 2017, 543, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T.; Meyer, C.A.; Eeckhoute, J.; Johnson, D.S.; Bernstein, B.E.; Nusbaum, C.; Myers, R.M.; Brown, M.; Li, W.; et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008, 9, R137. [Google Scholar] [CrossRef]
- Salmon-Divon, M.; Dvinge, H.; Tammoja, K.; Bertone, P. PeakAnalyzer: Genome-wide annotation of chromatin binding and modification loci. BMC Bioinform. 2010, 11, 415. [Google Scholar] [CrossRef]
- Bailey, T.L. DREME: Motif discovery in transcription factor ChIP-seq data. Bioinformatics 2011, 27, 1653–1659. [Google Scholar] [CrossRef]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, D. The Human Genome Browser at UCSC. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oleksiewicz, U.; Machnik, M.; Sobocińska, J.; Molenda, S.; Olechnowicz, A.; Florczak, A.; Mierzejewska, J.; Adamczak, D.; Smolibowski, M.; Kaczmarek, M.; et al. ZNF643/ZFP69B Exerts Oncogenic Properties and Associates with Cell Adhesion and Immune Processes. Int. J. Mol. Sci. 2023, 24, 16380. https://doi.org/10.3390/ijms242216380
Oleksiewicz U, Machnik M, Sobocińska J, Molenda S, Olechnowicz A, Florczak A, Mierzejewska J, Adamczak D, Smolibowski M, Kaczmarek M, et al. ZNF643/ZFP69B Exerts Oncogenic Properties and Associates with Cell Adhesion and Immune Processes. International Journal of Molecular Sciences. 2023; 24(22):16380. https://doi.org/10.3390/ijms242216380
Chicago/Turabian StyleOleksiewicz, Urszula, Marta Machnik, Joanna Sobocińska, Sara Molenda, Anna Olechnowicz, Anna Florczak, Julia Mierzejewska, Dominika Adamczak, Mikołaj Smolibowski, Mariusz Kaczmarek, and et al. 2023. "ZNF643/ZFP69B Exerts Oncogenic Properties and Associates with Cell Adhesion and Immune Processes" International Journal of Molecular Sciences 24, no. 22: 16380. https://doi.org/10.3390/ijms242216380
APA StyleOleksiewicz, U., Machnik, M., Sobocińska, J., Molenda, S., Olechnowicz, A., Florczak, A., Mierzejewska, J., Adamczak, D., Smolibowski, M., Kaczmarek, M., & Mackiewicz, A. (2023). ZNF643/ZFP69B Exerts Oncogenic Properties and Associates with Cell Adhesion and Immune Processes. International Journal of Molecular Sciences, 24(22), 16380. https://doi.org/10.3390/ijms242216380





