Oxygen Vacancies and Surface Wettability: Key Factors in Activating and Enhancing the Solar Photocatalytic Activity of ZnO Tetrapods
Abstract
:1. Introduction
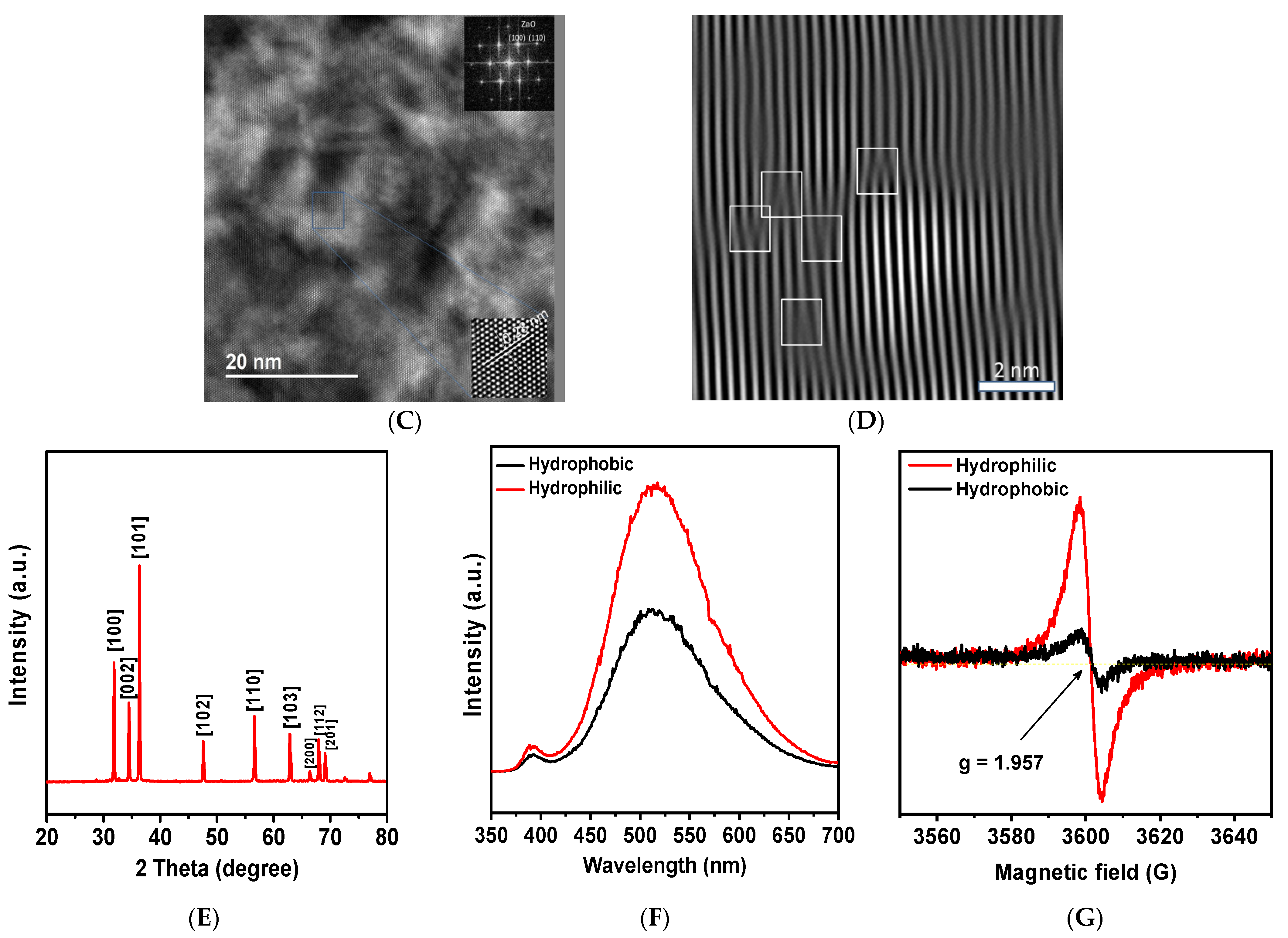
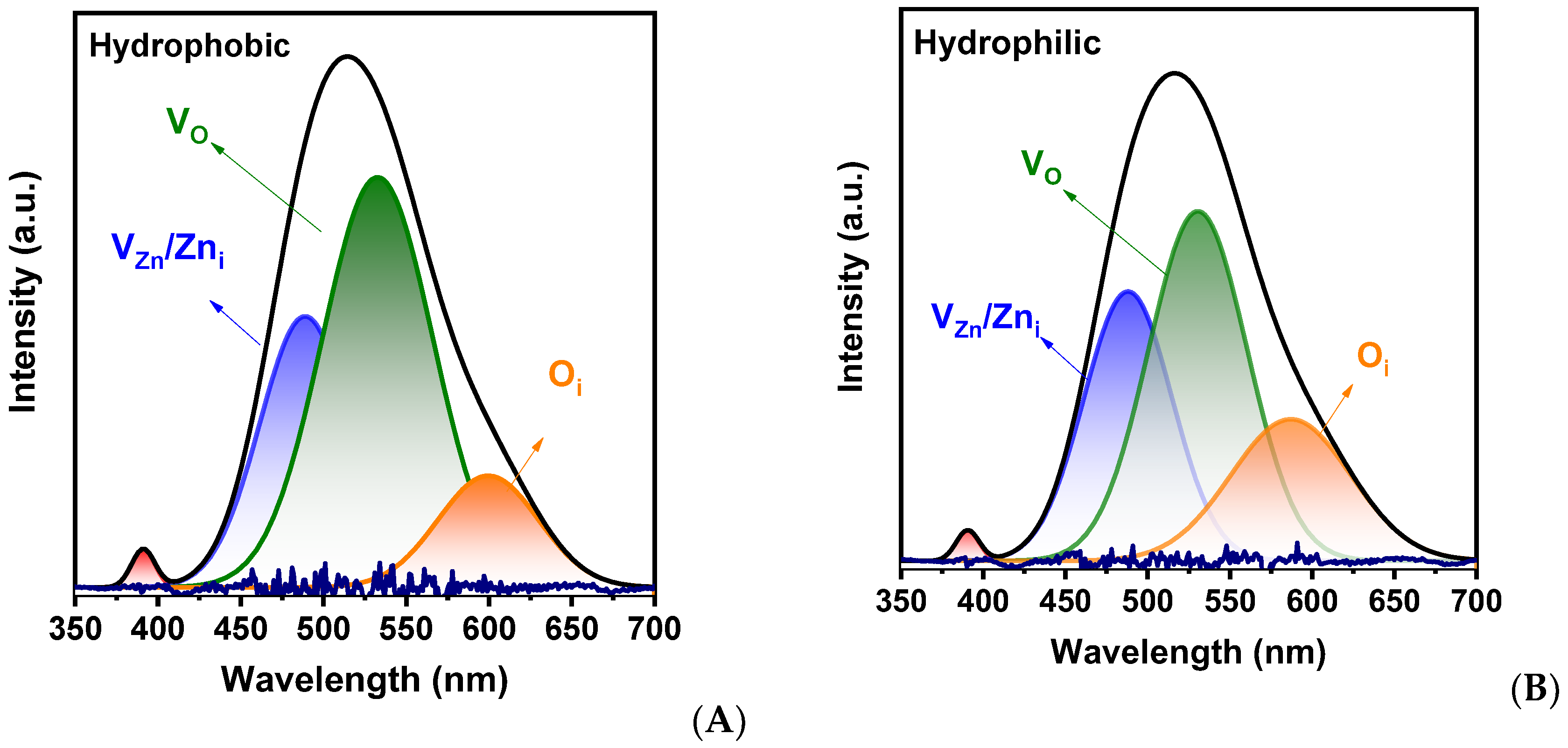
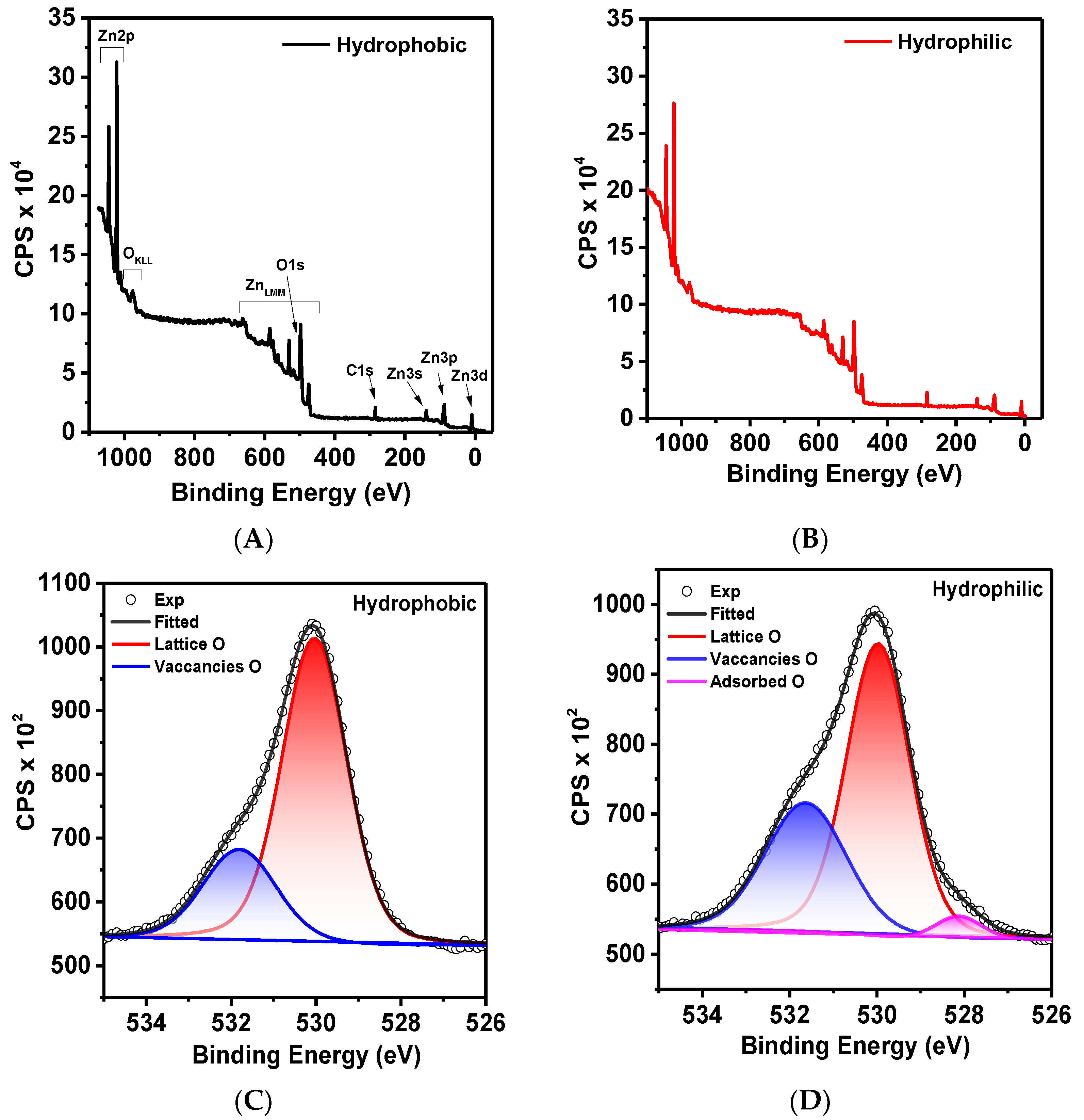
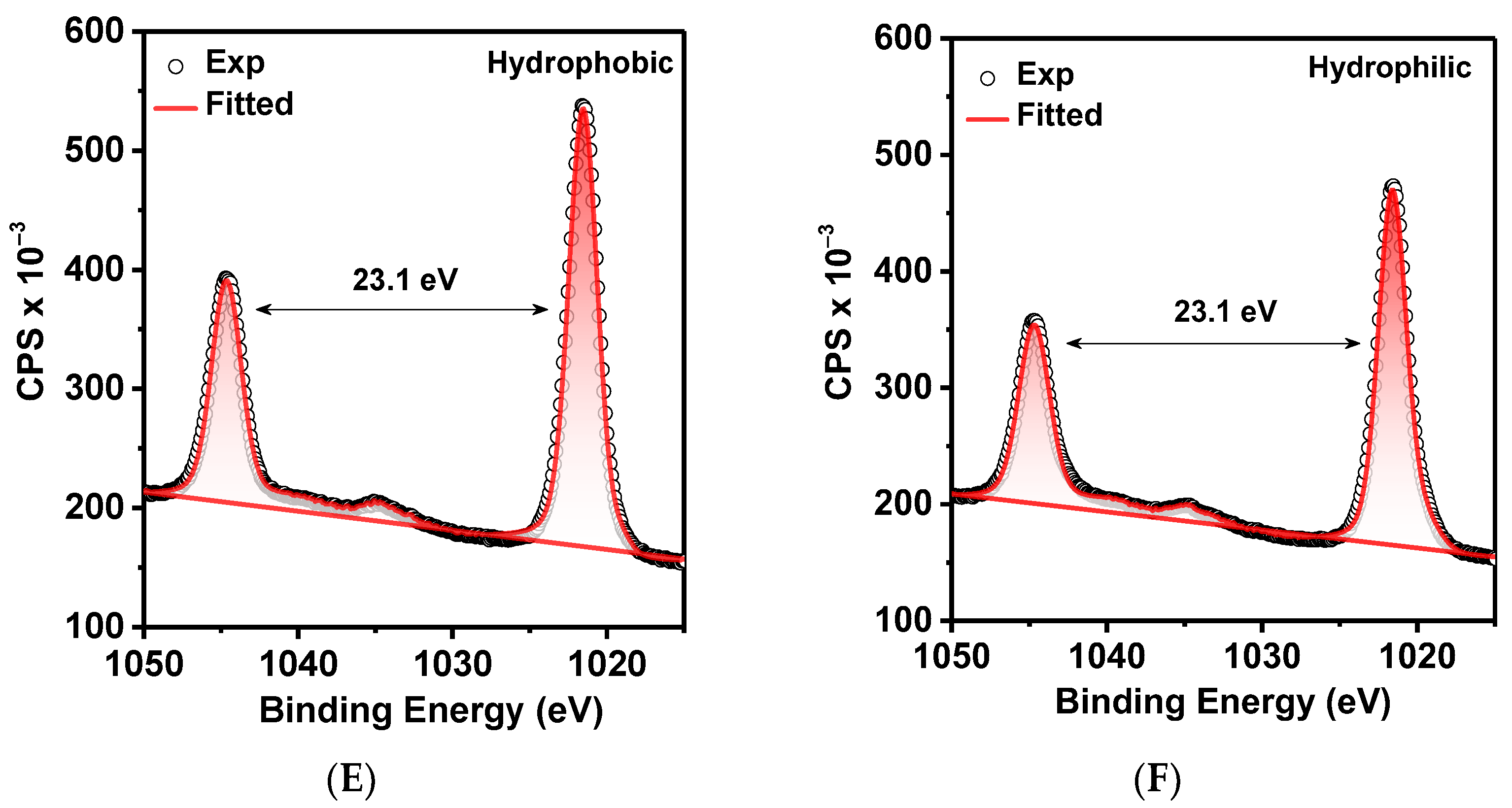
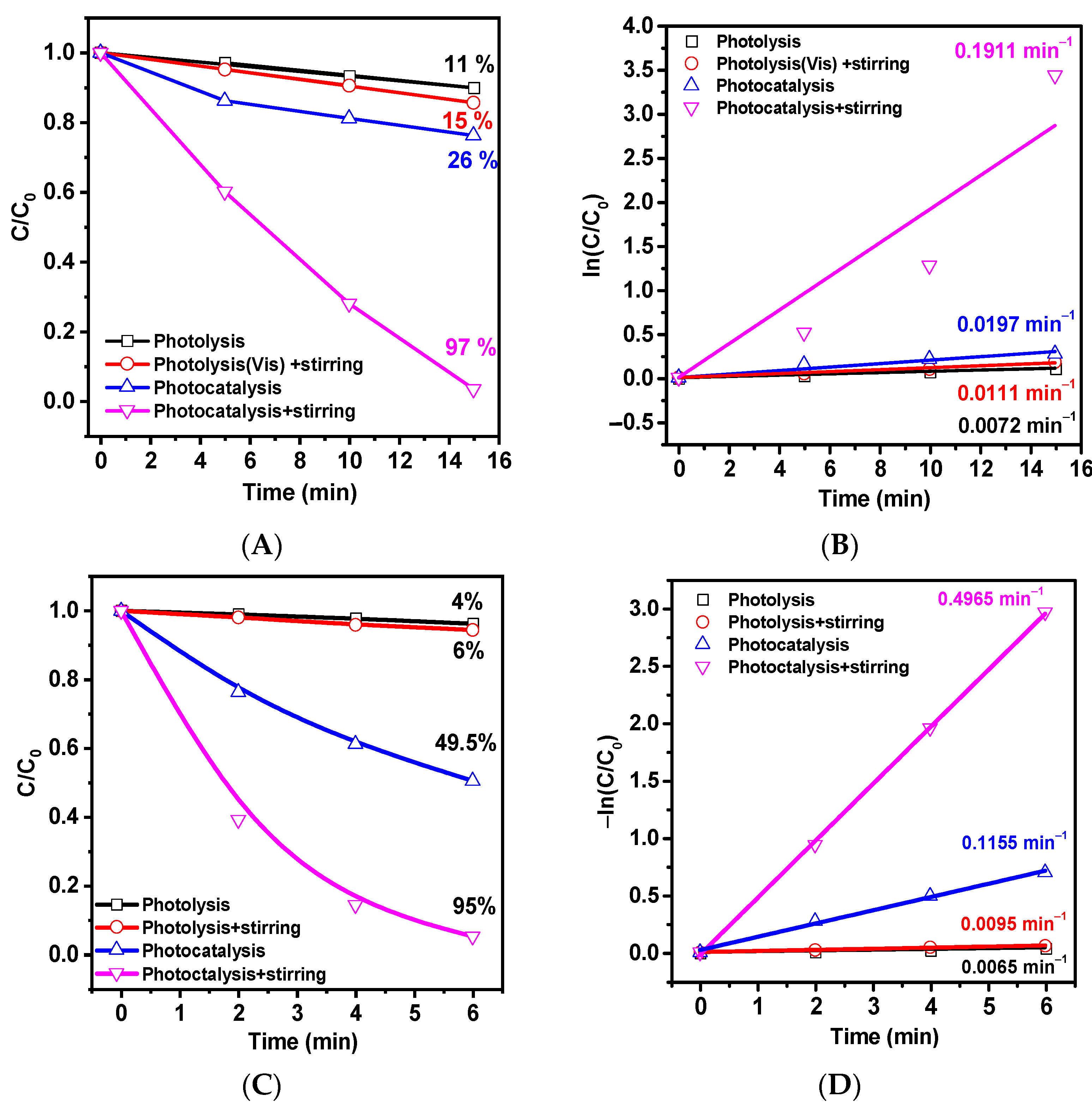
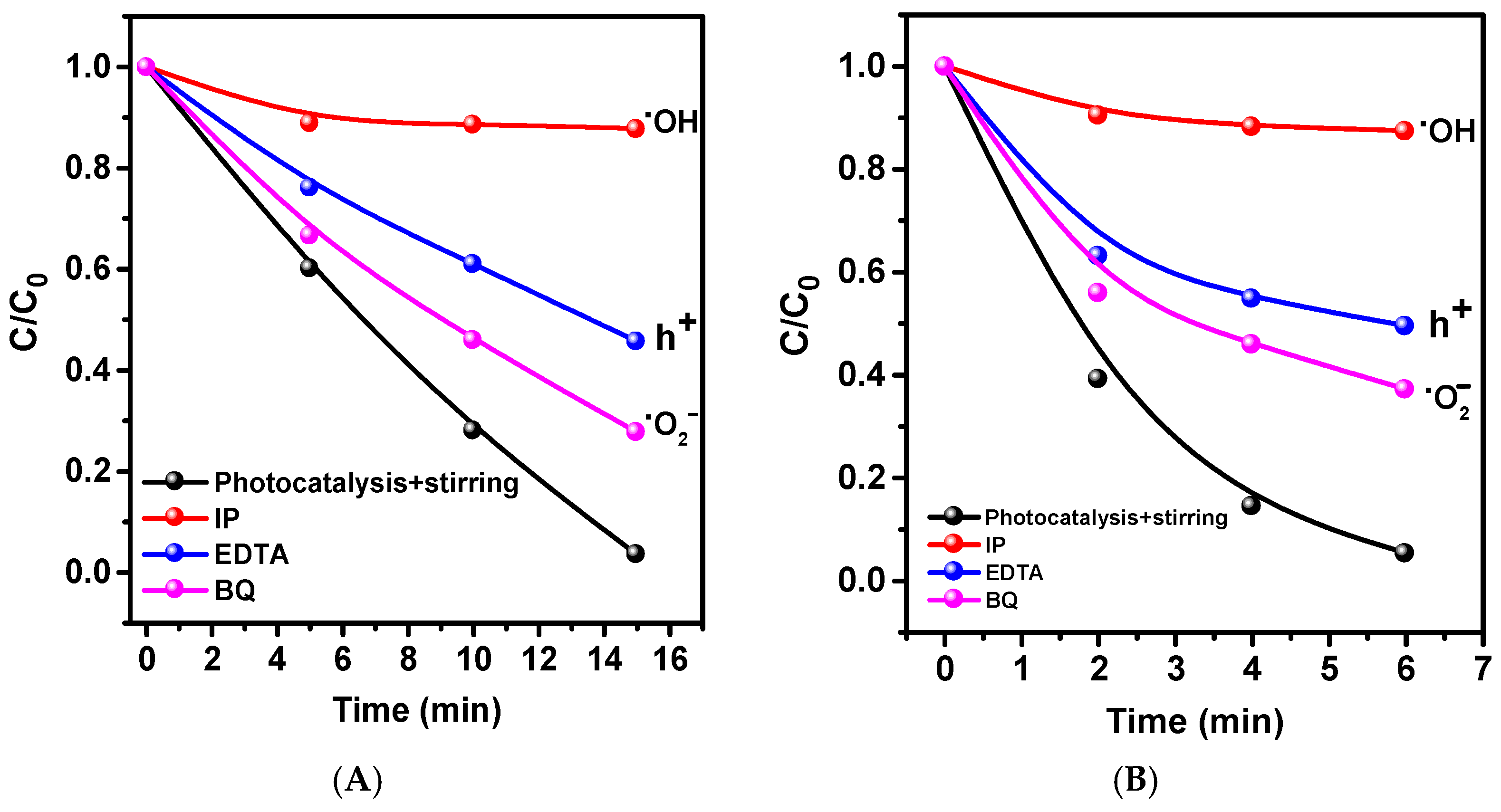
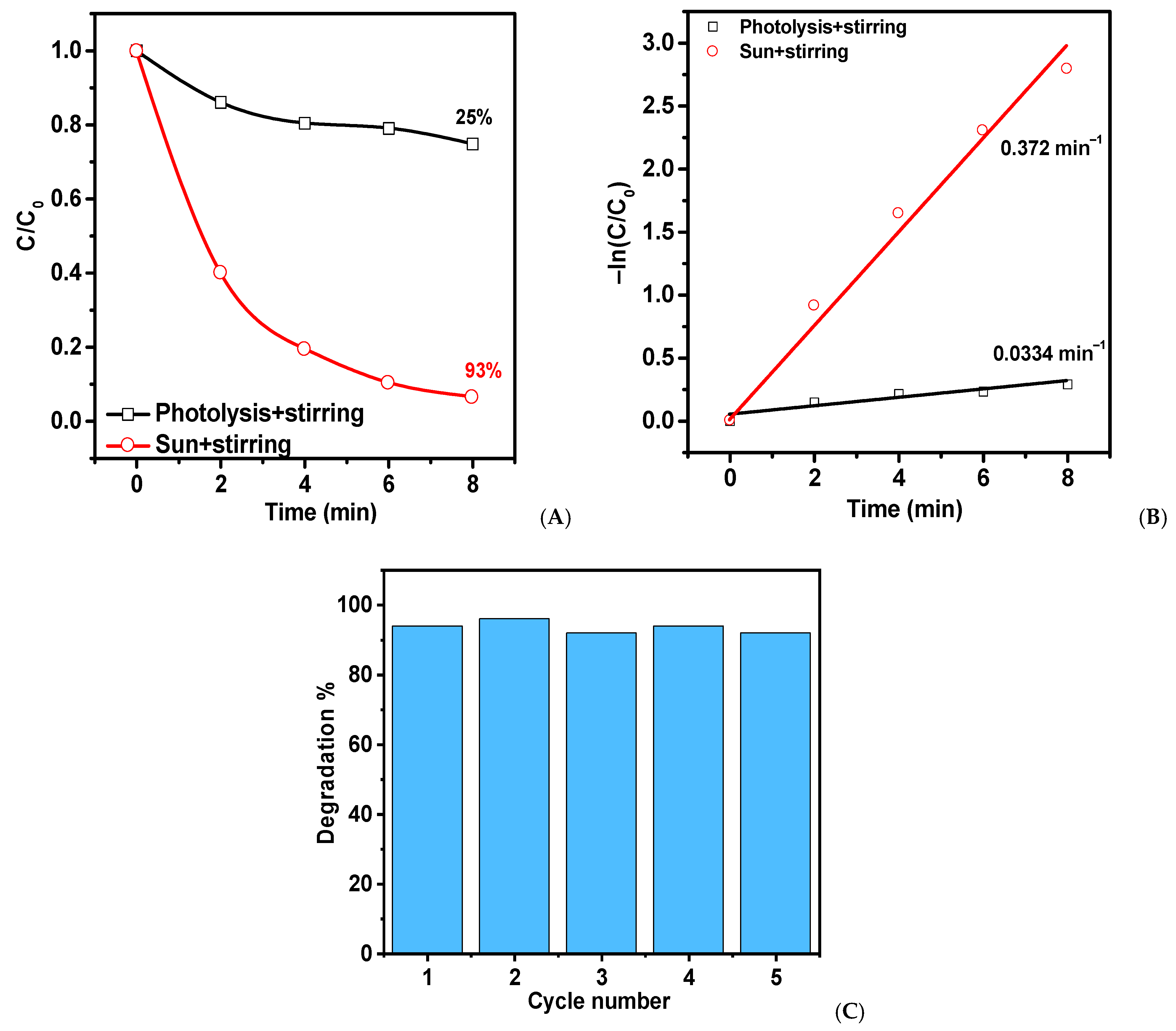
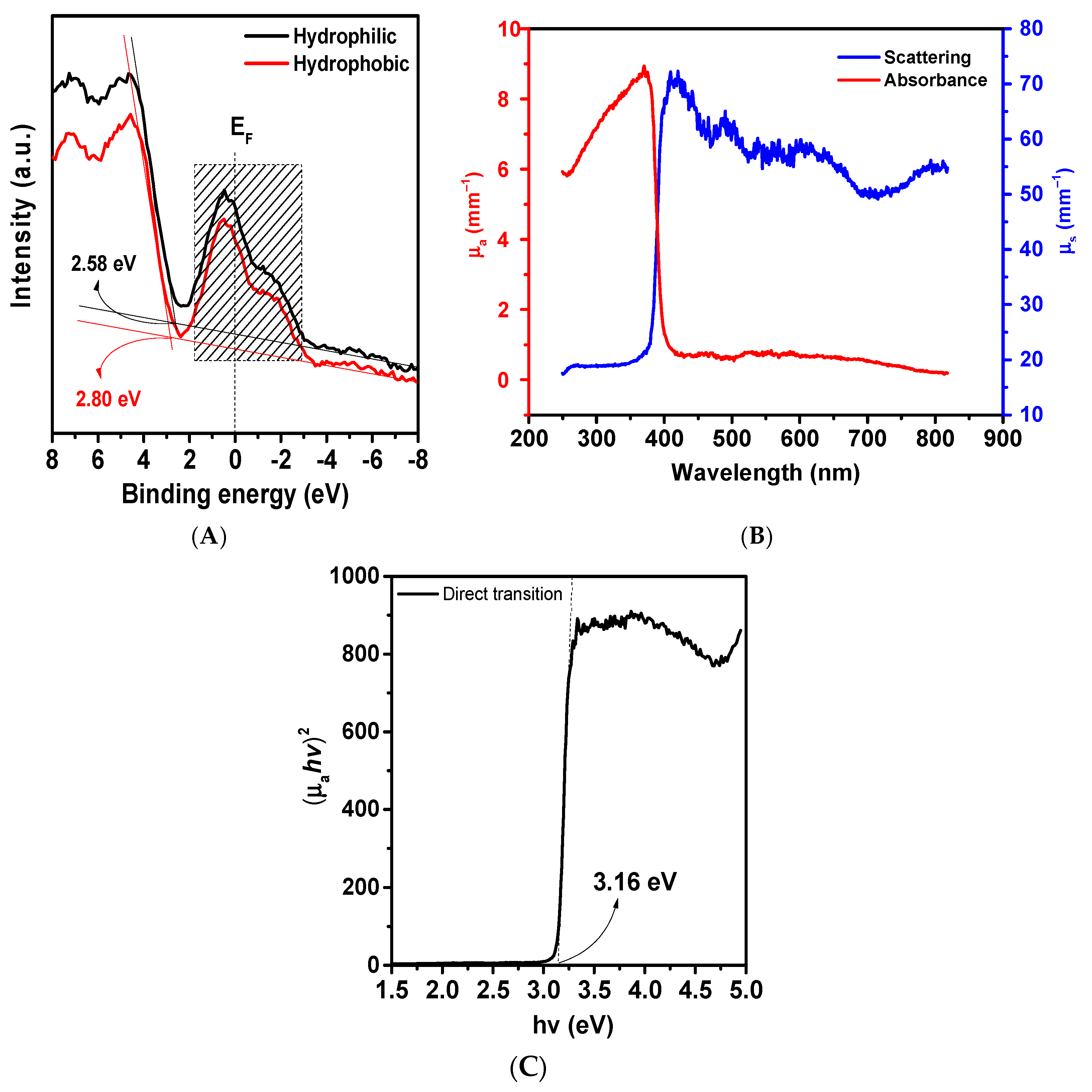
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of ZnO Tetrapods
3.2. Characterization of Samples
3.3. Photocatalytic Degradation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, X.; Yang, X.; Yu, M.; Lu, X.; Kang, H.; Yang, M.-Q.; Qian, Q.; Zhao, X.; Liang, S.; Bian, Z. 2D MXenes polar catalysts for multi-renewable energy harvesting applications. Nat. Commun. 2023, 14, 4183. [Google Scholar] [CrossRef]
- Li, J.; Lv, X.; Weng, B.; Roeffaers, M.B.; Jia, H. Engineering light propagation for synergetic photo- and thermocatalysis toward volatile organic compounds elimination. Chem. Eng. J. 2023, 461, 142022. [Google Scholar] [CrossRef]
- Feng, W.; Lei, Y.; Wu, X.; Yuan, J.; Chen, J.; Xu, D.; Zhang, X.; Zhang, S.; Liu, P.; Zhang, L.; et al. Tuning the interfacial electronic structure via Au clusters for boosting photocatalytic H2 evolution. J. Mater. Chem. A 2021, 9, 1759–1769. [Google Scholar] [CrossRef]
- Abdullah, F.H.; Bakar, N.H.H.A.; Bakar, M.A. Current advancements on the fabrication, modification, and industrial application of zinc oxide as photocatalyst in the removal of organic and inorganic contaminants in aquatic systems. J. Hazard. Mater. 2022, 424, 127416. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Meulenkamp, E.A. Synthesis and Growth of ZnO Nanoparticles. J. Phys. Chem. B 1998, 102, 5566–5572. [Google Scholar] [CrossRef]
- Gonzalez-Valls, I.; Lira-Cantu, M. Vertically-aligned nanostructures of ZnO for excitonic solar cells: A review. Energy Environ. Sci. 2008, 2, 19–34. [Google Scholar] [CrossRef]
- Tong, Y.; Liu, Y.; Shao, C.; Liu, Y.; Xu, C.; Zhang, J.; Lu, Y.; Shen, D.; Fan, X. Growth and Optical Properties of Faceted Hexagonal ZnO Nanotubes. J. Phys. Chem. B 2006, 110, 14714–14718. [Google Scholar] [CrossRef]
- Taniguchi, T.; Yamaguchi, K.; Shigeta, A.; Matsuda, Y.; Hayami, S.; Shimizu, T.; Matsui, T.; Yamazaki, T.; Funatstu, A.; Makinose, Y.; et al. Enhanced and Engineered d0Ferromagnetism in Molecularly-Thin Zinc Oxide Nanosheets. Adv. Funct. Mater. 2013, 23, 3140–3145. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, S. ZnO Nanotetrapods: Controlled Vapor-Phase Synthesis and Application for Humidity Sensing. Adv. Funct. Mater. 2007, 17, 1345–1352. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Native point defects in ZnO. Phys. Rev. B 2007, 76, 165202. [Google Scholar] [CrossRef]
- Devynck, F.; Alkauskas, A.; Broqvist, P.; Pasquarello, A. Charge transition levels of carbon-, oxygen-, and hydrogen-related defects at the SiC/SiO2interface through hybrid functionals. Phys. Rev. B 2011, 84, 235320. [Google Scholar] [CrossRef]
- Vanheusden, K.; Warren, W.L.; Seager, C.H.; Tallant, D.R.; Voigt, J.A.; Gnade, B.E. Mechanisms behind green photoluminescence in ZnO phosphor powders. J. Appl. Phys. 1996, 79, 7983–7990. [Google Scholar] [CrossRef]
- Kurtz, M.; Strunk, J.; Hinrichsen, O.; Muhler, M.; Fink, K.; Meyer, B.; Wöll, C. Active Sites on Oxide Surfaces: ZnO-Catalyzed Synthesis of Methanol from CO and H2. Angew. Chem. Int. Ed. 2005, 44, 2790–2794. [Google Scholar] [CrossRef]
- Liu, K.; Yao, X.; Jiang, L. Recent developments in bio-inspired special wettability. Chem. Soc. Rev. 2010, 39, 3240–3255. [Google Scholar] [CrossRef]
- Li, J.; Sun, Q.; Han, S.; Wang, J.; Wang, Z.; Jin, C. Reversibly light-switchable wettability between superhydrophobicity and superhydrophilicity of hybrid ZnO/bamboo surfaces via alternation of UV irradiation and dark storage. Prog. Org. Coatings 2015, 87, 155–160. [Google Scholar] [CrossRef]
- Miyauchi, M.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Photocatalysis and Photoinduced Hydrophilicity of Various Metal Oxide Thin Films. Chem. Mater. 2002, 14, 2812–2816. [Google Scholar] [CrossRef]
- Watanabe, T.; Yoshida, N. Wettability control of a solid surface by utilizing photocatalysis. Chem. Rec. 2008, 8, 279–290. [Google Scholar] [CrossRef]
- Liu, H.; Feng, L.; Zhai, J.; Jiang, L.; Zhu, D. Reversible wettability of a chemical vapor deposition prepared ZnO film between superhydrophobicity and superhydro-philicity. Langmuir 2004, 20, 5659–5661. [Google Scholar] [CrossRef]
- Feng, X.; Feng, L.; Jin, M.; Zhai, J.; Jiang, L.; Zhu, D. Reversible Super-hydrophobicity to Super-hydrophilicity Transition of Aligned ZnO Nanorod Films. J. Am. Chem. Soc. 2004, 126, 62–63. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Wu, S.; Long, Y.; Li, L.; Zheng, Z.; Hei, Y.; Zhou, L.; Luo, L.; Jiang, F. Controlling wettability of AgI/BiVO4 composite photocatalyst and its effect on photocatalytic performance. J. Alloy. Compd. 2020, 835, 155367. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Pi, X.; Ma, M.; Jiang, X.; Ji, S.; Jiang, F.; Luo, L. Effect of the wettability of Ag2MoO4/BiVO4 {010} composite on the photocatalytic degradation for 17α-ethinyl estradiol. J. Alloy. Compd. 2022, 899, 163295. [Google Scholar] [CrossRef]
- Zhu, H.; Cai, S.; Liao, G.; Gao, Z.F.; Min, X.; Huang, Y.; Jin, S.; Xia, F. Recent Advances in Photocatalysis Based on Bioinspired Superwettabilities. ACS Catal. 2021, 11, 14751–14771. [Google Scholar] [CrossRef]
- Oba, F.; Togo, A.; Tanaka, I.; Paier, J.; Kresse, G. Defect energetics in ZnO: A hybrid Hartree-Fock density functional study. Phys. Rev. B 2008, 77, 245202. [Google Scholar] [CrossRef]
- Zheng, J.; Jiang, Q.; Lian, J. Synthesis and optical properties of flower-like ZnO nanorods by thermal evaporation method. Appl. Surf. Sci. 2011, 257, 5083–5087. [Google Scholar] [CrossRef]
- Tam, K.H.; Cheung, C.K.; Leung, Y.H.; Djurišić, A.B.; Ling, C.C.; Beling, C.D.; Fung, S.; Kwok, W.M.; Chan, W.K.; Phillips, D.L.; et al. Defects in ZnO nanorods prepared by a hydrothermal method. J. Phys. Chem. B 2006, 110, 20865–20871. [Google Scholar] [CrossRef]
- Bukhtiyarov, V.I.; Hävecker, M.; Kaichev, V.V.; Knop-Gericke, A.; Mayer, R.W.; Schlögl, R. Atomic oxygen species on silver: Photoelectron spectroscopy and X-ray absorption studies. Phys. Rev. B 2003, 67, 235422. [Google Scholar] [CrossRef]
- Lee, K.; Sahu, M.; Hajra, S.; Abolhassani, R.; Mistewicz, K.; Toroń, B.; Rubahn, H.-G.; Mishra, Y.K.; Kim, H.J. Zinc oxide tetrapod sponges for environmental pollutant monitoring and degradation. J. Mater. Res. Technol. 2023, 22, 811–824. [Google Scholar] [CrossRef]
- Chamoli, P.; Shukla, R.K.; Bezbaruah, A.N.; Kar, K.K.; Raina, K. Microwave-assisted rapid synthesis of honeycomb core-ZnO tetrapods nanocomposites for excellent photocatalytic activity against different organic dyes. Appl. Surf. Sci. 2021, 555, 149663. [Google Scholar] [CrossRef]
- Heo, S.-G.; Jo, S.-I.; Jeong, G.-H. Revealing the enhanced photocatalytic properties of ZnO tetrapods produced by atmospheric-pressure microwave plasma jet system. Curr. Appl. Phys. 2023, 46, 46–54. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, R.; Purohit, L. Novel ZnO tetrapod-reduced graphene oxide nanocomposites for enhanced photocatalytic degradation of phenolic compounds and MB dye. J. Mol. Liq. 2021, 327, 114814. [Google Scholar] [CrossRef]
- Mourya, A.K.; Singh, R.P.; Kumar, T.; Talmale, A.S.; Gaikwad, G.S.; Wankhade, A.V. Tuning the morphologies of ZnO for enhanced photocatalytic activity. Inorg. Chem. Commun. 2023, 154, 110850. [Google Scholar] [CrossRef]
- Qi, F.; Gao, X.; Wang, C.; Shuai, Y.; Yang, L.; Liao, R.; Xin, J.; Peng, S.; Shuai, C. In situ grown silver nanoparticles on tetrapod-like zinc oxide whisker for photocatalytic antibacterial in scaffolds. Mater. Today Sustain. 2022, 19, 100210. [Google Scholar] [CrossRef]
- Guo, M.Y.; Ng, A.M.C.; Liu, F.; Djurišić, A.B.; Chan, W.K.; Su, H.; Wong, K.S. Effect of Native Defects on Photocatalytic Properties of ZnO. J. Phys. Chem. C 2011, 115, 11095–11101. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Sasaki, J.M.; Silva, R.S., Jr.; Attah-Baah, J.M.; Macêdo, M.A. Visible-Light-Responsive Photocatalytic Activity Significantly Enhanced by Active [VZn+VO+] Defects in Self-Assembled ZnO Nanoparticles. Inorg. Chem. 2021, 60, 4475–4496. [Google Scholar] [CrossRef]
- Park, S.J.; Das, G.S.; Schütt, F.; Adelung, R.; Mishra, Y.K.; Tripathi, K.; Kim, T.M. Visible-light photocatalysis by carbon-nano-onion-functionalized ZnO tetrapods: Degradation of 2,4-dinitrophenol and a plant-model-based ecological assessment. NPG Asia Mater. 2019, 11, 8. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Modi, G.; Cretu, V.; Postica, V.; Lupan, O.; Reimer, T.; Paulowicz, I.; Hrkac, V.; Benecke, W.; Kienle, L.; et al. Direct Growth of Freestanding ZnO Tetrapod Networks for Multifunctional Applications in Photocatalysis, UV Photodetection, and Gas Sensing. ACS Appl. Mater. Interfaces 2015, 7, 14303–14316. [Google Scholar] [CrossRef]
- Ghosh, A.; Guha, P.; Samantara, A.K.; Jena, B.K.; Bar, R.; Ray, S.; Satyam, P.V. Simple Growth of Faceted Au–ZnO Hetero-nanostructures on Silicon Substrates (Nanowires and Triangular Nanoflakes): A Shape and Defect Driven Enhanced Photocatalytic Performance under Visible Light. ACS Appl. Mater. Interfaces 2015, 7, 9486–9496. [Google Scholar] [CrossRef]
- Sun, C.; Fu, Y.; Wang, Q.; Xing, L.; Liu, B.; Xue, X. Ultrafast piezo-photocatalytic degradation of organic pollutions by Ag2O/tetrapod-ZnO nanostructures under ultrasonic/UV exposure. RSC Adv. 2016, 6, 87446–87453. [Google Scholar] [CrossRef]
- Lv, Y.; Yao, W.; Ma, X.; Pan, C.; Zong, R.; Zhu, Y. The surface oxygen vacancy induced visible activity and enhanced UV activity of a ZnO1−x photocatalyst. Catal. Sci. Technol. 2013, 3, 3136–3146. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, C.; Xie, K.; Li, X.; Liao, G. Photocatalytic degradation of organic pollutants over MoS2/Ag-ZnFe2O4 Z-scheme heterojunction: Revealing the synergistic effects of exposed crystal facets, defect engineering, and Z-scheme mechanism. Chem. Eng. J. 2023, 453, 139775. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Z.; Zhao, J.; Sun, X.; Wang, R.; Liao, G.; Jia, X. 1D/2D carbon-doped nanowire/ultra-thin nanosheet g-C3N4 isotype heterojunction for effective and durable photocatalytic H2 evolution. Int. J. Hydrogen Energy 2021, 46, 25436–25447. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, Z.; Zhu, W.-B.; Coker, E.N.; Li, B.; Zheng, M.; Yu, W.; Fan, H.; Sun, Z. Oxygen Vacancy Enhanced Photocatalytic Activity of Pervoskite SrTiO3. ACS Appl. Mater. Interfaces 2014, 6, 19184–19190. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhu, Y.; Zhu, Y. Enhanced photocatalytic performance for the BiPO4-x nanorod induced by surface oxygen vacancy. J. Phys. Chem. C 2013, 117, 18520–18528. [Google Scholar] [CrossRef]
- Xu, X.; Ding, X.; Yang, X.; Wang, P.; Li, S.; Lu, Z.; Chen, H. Oxygen vacancy boosted photocatalytic decomposition of ciprofloxacin over Bi2MoO6: Oxygen vacancy engineering, biotoxicity evaluation and mechanism study. J. Hazard. Mater. 2019, 364, 691–699. [Google Scholar] [CrossRef]
- Liu, J.; Xie, F.; Li, R.; Li, T.; Jia, Z.; Wang, Y.; Wang, Y.; Zhang, X.; Fan, C. TiO2-x/Ag3PO4 photocatalyst: Oxygen vacancy dependent visible light photocatalytic performance and BPA degradative pathway. Mater. Sci. Semicond. Process. 2019, 97, 1–10. [Google Scholar] [CrossRef]
- Bettini, S.; Pagano, R.; Valli, D.; Ingrosso, C.; Roeffaers, M.; Hofkens, J.; Giancane, G.; Valli, L. ZnO nanostructures based piezo-photocatalytic degradation enhancement of steroid hormones. Surfaces Interfaces 2023, 36, 102581. [Google Scholar] [CrossRef]
- Xue, X.; Zang, W.; Deng, P.; Wang, Q.; Xing, L.; Zhang, Y.; Wang, Z.L. Piezo-potential enhanced photocatalytic degradation of organic dye using ZnO nanowires. Nano Energy 2015, 13, 414–422. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, K.; Huang, X.; Lei, R.; Zhao, Y.; Liu, P. Integration of piezoelectric effect into a Au/ZnO photocatalyst for efficient charge separation. Catal. Sci. Technol. 2019, 9, 3771–3778. [Google Scholar] [CrossRef]
- Chimupala, Y.; Phromma, C.; Yimklan, S.; Semakul, N.; Ruankham, P. Dye wastewater treatment enabled by piezo-enhanced photocatalysis of single-component ZnO nanoparticles. RSC Adv. 2020, 10, 28567–28575. [Google Scholar] [CrossRef]
- Yu, C.; Yu, X.-X.; Zheng, D.-S.; Yin, H. Piezoelectric potential enhanced photocatalytic performance based on ZnO with different nanostructures. Nanotechnology 2021, 32, 135703. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Vaish, R.; Elqahtani, Z.M.; Kebaili, I.; Al-Buriahi, M.; Sung, T.H.; Hwang, W.; Kumar, A. Piezo-photocatalytic activity of Bi2VO5.5 for methylene blue dye degradation. J. Mater. Res. Technol. 2022, 21, 1998–2012. [Google Scholar] [CrossRef]
- Masekela, D.; Masekela, D.; Hintsho-Mbita, N.C.; Hintsho-Mbita, N.C.; Ntsendwana, B.; Ntsendwana, B.; Mabuba, N.; Mabuba, N. Thin Films (FTO/BaTiO3/AgNPs) for Enhanced Piezo-Photocatalytic Degradation of Methylene Blue and Ciprofloxacin in Wastewater. ACS Omega 2022, 7, 24329–24343. [Google Scholar] [CrossRef] [PubMed]
- Adiba, A.; Waris; Munjal, S.; Khan, M.Z.; Ahmad, T. Piezo-photocatalytic degradation of organic pollutant by a novel BaTiO3–NiO composite. Eur. Phys. J. Plus 2023, 138, 408. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Zhao, H.; Zhang, Z.; An, B.; Bai, C.; Ren, Z.; Wu, J.; Li, Y.; Liu, W.; et al. Synthesis of ternary ZnO/ZnS/MoS2 piezoelectric nanoarrays for enhanced photocatalytic performance by conversion of dual heterojunctions. Appl. Surf. Sci. 2021, 556, 149695. [Google Scholar] [CrossRef]
- Kumar, M.; Vaish, R.; ben Ahmed, S. Piezo-photocatalytic activity of mechanochemically synthesized BiVO4 for dye cleaning. J. Am. Ceram. Soc. 2022, 105, 2309–2322. [Google Scholar] [CrossRef]
- Hong, D.; Zang, W.; Guo, X.; Fu, Y.; He, H.; Sun, J.; Xing, L.; Liu, B.; Xue, X. High Piezo-photocatalytic Efficiency of CuS/ZnO Nanowires Using Both Solar and Mechanical Energy for Degrading Organic Dye. ACS Appl. Mater. Interfaces 2016, 8, 21302–21314. [Google Scholar] [CrossRef]
- Yan, S.; Chen, Z.; Lu, Z.; Wang, X. Piezoelectric-Enhanced Photocatalytic Performance of BaTi2O5 Nanorods for Degradation of Organic Pollutants. ACS Appl. Nano Mater. 2023, 6, 15721–15733. [Google Scholar] [CrossRef]
- Ren, Z.; Li, X.; Guo, L.; Wu, J.; Li, Y.; Liu, W.; Li, P.; Fu, Y.; Ma, J. Facile synthesis of ZnO/ZnS heterojunction nanoarrays for enhanced piezo-photocatalytic performance. Mater. Lett. 2021, 292, 129635. [Google Scholar] [CrossRef]



| Materials | Pollutants | Time (min) | Light Source | Degradation, % | Rate Constant (min−1) | References |
|---|---|---|---|---|---|---|
| ZnO tetrapod1 ZnO tetrapod2 | methyl orange methylene blue methyl orange methylene blue | 130 | UV lamp 100 W λ = 254 nm | 50.8 85.7 61.6 96.4 | 1.6 × 10−4 2.9 × 10−4 1.7 × 10−4 3.6 × 10−4 | [28] |
| GNs-ZnO 20 mg | MB (1 mg/L) | 40 | UV (125 W) Vis (125 W) | 70.87 17.26 | 4.5 × 10−2 5 × 10−3 | [29] |
| ZnO tetrapods 1 mg | MB (10 ppm) | 70 | UV light (Philips, 350–400 nm wavelength, 60 W) | 94.5 | 1.02 × 10−1 | [30] |
| ZTPG | Methylene blue (20 ppm) | 90 | UV light (60 W, 365 nm) | 98.05 | 0.03 | [31] |
| ZnO sample 25 mg | V = 100 mL Rhodamine B (20 ppm) | 110 | UV irradiation (8 W) | 98.86 | 0.036 | [32] |
| T-ZnOw/PLLA | V = 50 mL MB (3 × 10−4 M) | 60 | Visible light | 30 | 0.0065 | [33] |
| T-ZnO 50 mg | V = 50 mL MB (5 mg/L) | 8 | UV illumination (365 nm, 66.2 mW/cm2, Blak-Ray B-100 AP lamp) | 100 | - | [34] |
| Rod-like ZnO nanoparticles 10 mg | V = 50 mL MB (50 mg/L) | 120 | 100 W halogen lamp (with λ > 420 nm and a light intensity of 2.87 W m–2) | 30.67 | 2.9 × 10−3 | [35] |
| T-ZnO T-ZnO-CNO 100 mg | V = 50 mL DNP (0.1 mM) | 140 | 60 W tungsten bulb | 30 92 | 0.00274 0.01834 | [36] |
| ZnO tetrapods 60 mg | V = 60 mL MB (1 μmol·L–1) | 10 | UV diode array consisting of four diodes (central wavelength = 370 nm, 170 mW/diode) | 96 | - | [37] |
| NWs TNFs coated Si substrates of area 0.5 cm2 | V = 10 mL RhB (5 × 10–6 M) | 180 | 100 W bulb (with a luminous irradiance of 10 mW/cm2 at the sample) λ ≥ 400 nm | 95 | - | [38] |
| T-ZnO Ag2O/T-ZnO 200 mg | V = 100 mL MB (5 mg L−1) | 2 | UV lamp, 50 W | 63 85 | - - | [39] |
| ZnO1−x 50 mg | V = 100 mL MB (1 × 10−5 M) | 360 | Halogen–tungsten lamp (power = 175 W; λmain = 550 nm | 95 | 0.522 h−1 | [40] |
| MoS2/Ag-ZnFe2O4 40 mg | V = 50 mL TC = 10 mg/L | 60 | 300 W Xenon lamp with optical filter (λ ≥ 420 nm) | 95 | 0.04868 | [41] |
| 2D g-C3N4 nanosheets 20 mg | V = 100mL TEOA = 10% | 240 | 300 W Xenon lamp with optical filter (λ ≥ 420 nm) | - | 7.414 mmol g−1 h−1 | [42] |
| SrTiO3 50 mg | 120 mL of 25% aqueous methanol solution | 300 | UV–visible light 300 W Xenon lamp | - | 2.2 mmol h–1 g–1 | [43] |
| BiPO4–x 25 mg | V = 50 mL MB = 1 × 10–5 M | 30 | UV-light 300 W high-pressure mercury lamp | 89 | 0.300 | [44] |
| Bi2MoO6 20 mg | V = 50 mL CIP = 20 ppm | 40 | 300 W Xenon lamp with optical filter (λ ≥ 400 nm) | 97 | 1.7990 mg m−2 min−1 | [45] |
| TiO2-x/Ag3PO4 100 mg | V = 100 mL BPA = 10 mg/L | 16 | 500 W Xenon lamp with optical filter (λ ≥ 420 nm) | 95 | - | [46] |
| 70W metal–halogen lamp: | ||||||
| ZnO-T 20 mg | 20 mL of MB (2.5 mg/L) | 6 15 15 8 | Without cut-off λ > 400 nm λ < 400 nm Direct sunlight | 95 65 96 93 | 0.496 0.101 0.229 0.372 | This work |
| Materials | Pollutants | Time (min) | Light/Mechanical Source | Degradation, % | Rate Constant, (min−1) | References |
|---|---|---|---|---|---|---|
| ZnO NS/ 2.5 mg | V = 10 mL TST (testosterone) (5 × 10−5 M) | 45 | LOT-Oriel Solar S (140 W), 35 kHz | 50 | 1.8 × 10−2 | [47] |
| ZnO nanowires/CFs 200 mg | V = 100 mL MB (C0 = 5 mg/L) | 120 | High-pressure mercury lamp (50 W)/stirring | 96 | - | [48] |
| ZnO nanorods 20 mg | V = 50 mL RhB (10 ppm) | 20 | 300 W Xe lamp equipped with a 350 nm bandpass filter/ultrasonic frequency 27 kHz | 75 | 0.0744 | [49] |
| calcined ZnOTW-0.20 1g/L | V = 100 mL MB (5 ppm) | 120 | UVA light with peak wavelength of 365 nm and intensity of 940 μW cm−2/ultrasonic bath (120 W, 40 kHz) | 90 | - | [50] |
| T-ZnO nanostructures 200 mg | V = 100 mL MB (5 mg L−1) | 2 | UV lamp/ultrasonic probe 50 W UV, 200 W ultrasonic | 74 | - | [51] |
| Bi2VO5.5 0.25 g | V = 10 mL MB = (5 mg/L) | 240 | 15W (Havells company) 2 lamp visible light; ultrasonicator (40 kHz, 150 W). | 82 | 0.00528 | [52] |
| FTO/BaTiO3 /AgNPs 2 cm × 2 cm | V = 75 mL MB = (5 mg/L) | 180 | 70 W UV lamp; 24 kHz ultrasonic vibration 30 W | 90 | 0.02329 | [53] |
| BaTiO3 –NiO 0.2 g | V = 200 mL MB = (10 mg/L) | 80 | UV lamp, 125 W; ultrasonic cleaner, ~40 kHz | 90 | 0.028 | [54] |
| ZnO/ZnS/MoS2 10 mg | V = 50 mL MB = 10 mg/L | 50 | 300 W Xenon lamp to simulate the solar source; stirring at 1000 rpm | 87 | 0.0411 | [55] |
| BiVO4 0.2 g | V = 10 mL MB = (5 mg/L) | 240 | 15W (Havells company) 2 lamp visible light; ultrasonicator (40 kHz, 70 W). | 81 | 0.00802 | [56] |
| CuS/ZnO nanowires on stainless steel mesh 6.0 × 6.0 cm, 100 mg | V = 50 mL MB = 5 mg/L | 20 | Xenon lamp, 500 W, to simulate the solar source; ultrasonic probe, 200 W | 98 | 0.18236 | [57] |
| BaTi2O5 40 mg | V = 60 mL RhB = 10 mg/L MB = 10 mg/L MO = 10 mg/L | 50 | Xenon lamp, 300 W, λ > 400 nm; ultrasonic cleaner, 53 kHz, 100 W | 82.5 - - | 0.0353 0.1775 0.0314 | [58] |
| ZnO/ZnS | V = 50 mL MB = 5 mg/L | 50 | 300 W UV irradiation; 180 W sonication, 40 kHz | 60 | 0.0154 | [59] |
| 70 W metal–halogen lamp: | ||||||
| ZnO-T 20 mg | V = 20 mL MB (2.5 mg/L) | 6 15 15 8 | Without cut-off λ > 400 nm λ < 400 nm Direct sunlight | 95 65 96 93 | 0.496 0.101 0.229 0.372 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orudzhev, F.; Muslimov, A.; Selimov, D.; Gulakhmedov, R.R.; Lavrikov, A.; Kanevsky, V.; Gasimov, R.; Krasnova, V.; Sobola, D. Oxygen Vacancies and Surface Wettability: Key Factors in Activating and Enhancing the Solar Photocatalytic Activity of ZnO Tetrapods. Int. J. Mol. Sci. 2023, 24, 16338. https://doi.org/10.3390/ijms242216338
Orudzhev F, Muslimov A, Selimov D, Gulakhmedov RR, Lavrikov A, Kanevsky V, Gasimov R, Krasnova V, Sobola D. Oxygen Vacancies and Surface Wettability: Key Factors in Activating and Enhancing the Solar Photocatalytic Activity of ZnO Tetrapods. International Journal of Molecular Sciences. 2023; 24(22):16338. https://doi.org/10.3390/ijms242216338
Chicago/Turabian StyleOrudzhev, Farid, Arsen Muslimov, Daud Selimov, Rashid R. Gulakhmedov, Alexander Lavrikov, Vladimir Kanevsky, Rashid Gasimov, Valeriya Krasnova, and Dinara Sobola. 2023. "Oxygen Vacancies and Surface Wettability: Key Factors in Activating and Enhancing the Solar Photocatalytic Activity of ZnO Tetrapods" International Journal of Molecular Sciences 24, no. 22: 16338. https://doi.org/10.3390/ijms242216338
APA StyleOrudzhev, F., Muslimov, A., Selimov, D., Gulakhmedov, R. R., Lavrikov, A., Kanevsky, V., Gasimov, R., Krasnova, V., & Sobola, D. (2023). Oxygen Vacancies and Surface Wettability: Key Factors in Activating and Enhancing the Solar Photocatalytic Activity of ZnO Tetrapods. International Journal of Molecular Sciences, 24(22), 16338. https://doi.org/10.3390/ijms242216338