Kaempferol: A Review of Current Evidence of Its Antiviral Potential
Abstract
1. Introduction
2. Antiviral Activity against DNA Viruses
2.1. Antiviral Activity against Hepatitis B Virus (HBV)
2.2. Antiviral Activity against Alphaherpesvirinae
2.3. Antiviral Activity against African Swine Fever Virus
2.4. Antiviral Activity against Pseudorabies Virus
3. Antiviral Activity against RNA Viruses
3.1. Antiviral Activity against Severe Acute Respiratory Syndrome-Related Coronaviruses
3.2. Antiviral Activity against Respiratory Syncytial Virus (RSV)
3.3. Antiviral Activity against Influenza Virus
3.4. Antiviral Activity against Human Immunodeficiency Virus (HIV)
3.5. Antiviral Activity against Dengue Fever Virus (DFV)
3.6. Antiviral Activity against Japanese Encephalitis Virus (JEV)
3.7. Antiviral Activity against Enterovirus 71 (EV71)
3.8. Antiviral Activity against Hepatitis A Virus (HAV)
3.9. Antiviral Activity against Poliovirus
3.10. Antiviral Activity against Chikungunya Virus (CHIKV)
3.11. Antiviral Activity against Feline Calicivirus (FCV)
3.12. Antiviral Activity against Murine Norovirus (MNV)
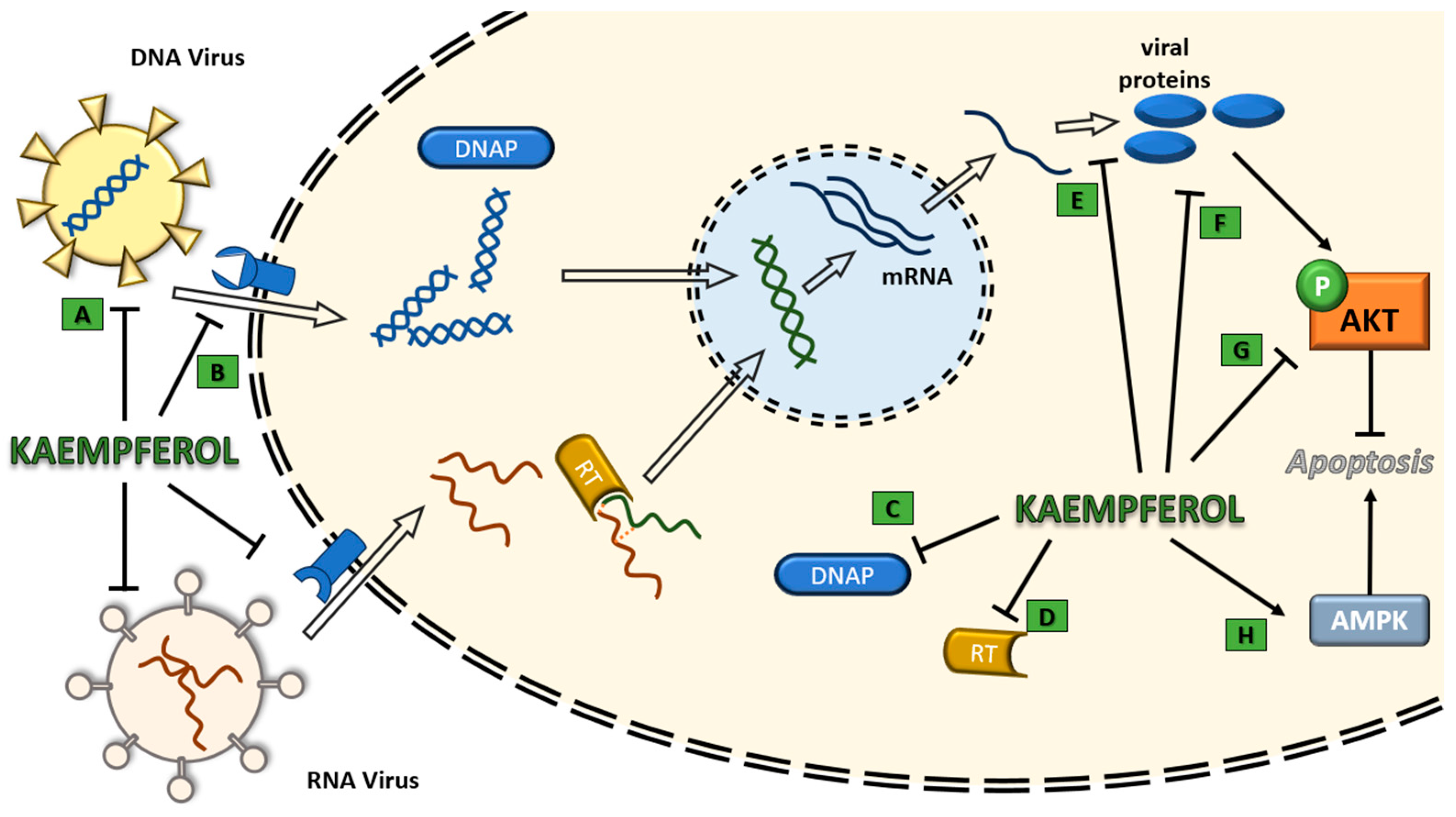
4. Effectiveness of Kaempferol Antiviral Activity
5. General Antiviral Activity and Natural Kaempferol Sources with Antiviral Effects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Periferakis, A.; Periferakis, K. On the Dissemination of Acupuncture to Europe. JournalNX 2020, 6, 201–209. [Google Scholar]
- Farombi, E.O.; Akinmoladun, A.C.; Owumi, S.E. Anti-cancer Foods: Flavonoids. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 224–236. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 5054. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L. Dietary flavonoids and cancer risk: Evidence from human population studies. Nutr. Cancer 2004, 50, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Scheau, C.; Mihai, L.; Bădărău, I.; Caruntu, C. Emerging applications of some important natural compounds in the field of oncology. Farmacia 2020, 68, 984–991. [Google Scholar] [CrossRef]
- Weng, C.J.; Yen, G.C. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 2012, 31, 323–351. [Google Scholar] [CrossRef]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef] [PubMed]
- Park, U.H.; Hwang, J.T.; Youn, H.; Kim, E.J.; Um, S.J. Kaempferol antagonizes adipogenesis by repressing histone H3K4 methylation at PPARγ target genes. Biochem. Biophys. Res. Commun. 2022, 617, 48–54. [Google Scholar] [CrossRef]
- Alkandahri, M.Y.; Pamungkas, B.T.; Oktoba, Z.; Shafirany, M.Z.; Sulastri, L.; Arfania, M.; Anggraeny, E.N.; Pratiwi, A.; Astuti, F.D.; Dewi, S.Y.; et al. Hepatoprotective Effect of Kaempferol: A Review of the Dietary Sources, Bioavailability, Mechanisms of Action, and Safety. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 1387665. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, G.; Chen, S.; He, Y.; Peng, F.; Yuan, C. Kaempferol ameliorated alcoholic liver disease through inhibiting hepatic bile acid synthesis by targeting intestinal FXR-FGF15 signaling. Phytomedicine 2023, 120, 155055. [Google Scholar] [CrossRef]
- Baňas, Š.; Benko, F.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. Kaempferol Enhances Sperm Post-Thaw Survival by Its Cryoprotective and Antioxidant Behavior. Stresses 2023, 3, 687–700. [Google Scholar] [CrossRef]
- Ajisebiola, B.S.; Oladele, J.O.; Adeyi, A.O. Kaempferol from Moringa oleifera demonstrated potent antivenom activities via inhibition of metalloproteinase and attenuation of Bitis arietans venom–induced toxicities. Toxicon 2023, 233, 107242. [Google Scholar] [CrossRef] [PubMed]
- Nwankwo, J.O.; Tahnteng, J.G.; Emerole, G.O. Inhibition of aflatoxin B1 genotoxicity in human liver-derived HepG2 cells by kolaviron biflavonoids and molecular mechanisms of action. Eur. J. Cancer Prev. 2000, 9, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Snijman, P.W.; Swanevelder, S.; Joubert, E.; Green, I.R.; Gelderblom, W.C.A. The antimutagenic activity of the major flavonoids of rooibos (Aspalathus linearis): Some dose–response effects on mutagen activation–flavonoid interactions. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2007, 631, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Caruntu, C.; Negrei, C.; Ilie Ghita, M.; Caruntu, A.; Bădărău, A.I.; Ioan, B.; Boda, D.; Albu, A.; Brănişteanu, D. Capsaicin, a hot topic in skin pharmacology and physiology. Farmacia 2015, 63, 487–491. [Google Scholar]
- Farombi, E.O.; Shrotriya, S.; Surh, Y.-J. Kolaviron inhibits dimethyl nitrosamine-induced liver injury by suppressing COX-2 and iNOS expression via NF-κB and AP-1. Life Sci. 2009, 84, 149–155. [Google Scholar] [CrossRef]
- Scheau, C.; Caruntu, C.; Badarau, I.A.; Scheau, A.-E.; Docea, A.O.; Calina, D.; Caruntu, A. Cannabinoids and Inflammations of the Gut-Lung-Skin Barrier. J. Pers. Med. 2021, 11, 494. [Google Scholar] [CrossRef]
- Choudhary, N.; Bijjem, K.R.V.; Kalia, A.N. Antiepileptic potential of flavonoids fraction from the leaves of Anisomeles malabarica. J. Ethnopharmacol. 2011, 135, 238–242. [Google Scholar] [CrossRef]
- Olaleye, M.T.; Amobonye, A.E.; Komolafe, K.; Akinmoladun, A.C. Protective effects of Parinari curatellifolia flavonoids against acetaminophen-induced hepatic necrosis in rats. Saudi J. Biol. Sci. 2014, 21, 486–492. [Google Scholar] [CrossRef]
- Ghita, M.A.; Caruntu, C.; Rosca, A.E.; Caruntu, A.; Moraru, L.; Constantin, C.; Neagu, M.; Boda, D. Real-Time Investigation of Skin Blood Flow Changes Induced by Topical Capsaicin. Acta Dermatovenerol. Croat. 2017, 25, 223–227. [Google Scholar]
- Athira, K.V.; Madhana, R.M.; Lahkar, M. Flavonoids, the emerging dietary supplement against cisplatin-induced nephrotoxicity. Chem.-Biol. Interact. 2016, 248, 18–20. [Google Scholar] [CrossRef]
- Dumitrache, M.D.; Jieanu, A.S.; Scheau, C.; Badarau, I.A.; Popescu, G.D.A.; Caruntu, A.; Costache, D.O.; Costache, R.S.; Constantin, C.; Neagu, M.; et al. Comparative effects of capsaicin in chronic obstructive pulmonary disease and asthma (Review). Exp. Ther. Med. 2021, 22, 917. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Flores, L.F.; Casas-Grajales, S.; Hernández-Aquino, E.; Vargas-Pozada, E.E.; Muriel, P. Chapter 47—Antioxidant, Antiinflammatory, and Antifibrotic Properties of Quercetin in the Liver. In Liver Pathophysiology; Muriel, P., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 653–674. [Google Scholar] [CrossRef]
- Popescu, G.D.A.; Scheau, C.; Badarau, I.A.; Dumitrache, M.D.; Caruntu, A.; Scheau, A.E.; Costache, D.O.; Costache, R.S.; Constantin, C.; Neagu, M.; et al. The Effects of Capsaicin on Gastrointestinal Cancers. Molecules 2020, 26, 94. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.-D.; Su, S.-L.; Qian, D.-W.; Guo, S.; Tao, W.-W.; Cong, X.D.; Tang, R.; Duan, J.-A. Renal protective effect and action mechanism of Huangkui capsule and its main five flavonoids. J. Ethnopharmacol. 2017, 206, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.-S. Regulatory Roles of Flavonoids in Caspase-11 Non-Canonical Inflammasome-Mediated Inflammatory Responses and Diseases. Int. J. Mol. Sci. 2023, 24, 10402. [Google Scholar] [CrossRef]
- Sitarek, P.; Kowalczyk, T.; Śliwiński, T.; Hatziantoniou, S.; Soulintzi, N.; Pawliczak, R.; Wieczfinska, J. Leonotis nepetifolia Transformed Root Extract Reduces Pro-Inflammatory Cytokines and Promotes Tissue Repair In Vitro. Int. J. Environ. Res. Public Health 2023, 20, 4706. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Mehmood, A.; Javid, S.; Khan, M.F.; Ahmad, K.S.; Mustafa, A. In vitro total phenolics, total flavonoids, antioxidant and antibacterial activities of selected medicinal plants using different solvent systems. BMC Chem. 2022, 16, 64. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Islam, S.; Salekeen, R.; Ashraf, A. Computational screening of natural MtbDXR inhibitors for novel anti-tuberculosis compound discovery. J. Biomol. Struct. Dyn. 2023, 1–11. [Google Scholar] [CrossRef]
- Mejía-Méndez, J.L.; Bach, H.; Lorenzo-Leal, A.C.; Navarro-López, D.E.; López-Mena, E.R.; Hernández, L.R.; Sánchez-Arreola, E. Biological Activities and Chemical Profiles of Kalanchoe fedtschenkoi Extracts. Plants 2023, 12, 1943. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Sharma, B.; Kanwar, S. Antiviral phytochemicals: An overview. Biochem. Physiol. 2017, 6, 7. [Google Scholar] [CrossRef]
- Biswas, D.; Nandy, S.; Mukherjee, A.; Pandey, D.K.; Dey, A. Moringa oleifera Lam. and derived phytochemicals as promising antiviral agents: A review. S. Afr. J. Bot. 2020, 129, 272–282. [Google Scholar] [CrossRef]
- Todorov, D.; Hinkov, A.; Shishkova, K.; Shishkov, S. Antiviral potential of Bulgarian medicinal plants. Phytochem. Rev. 2014, 13, 525–538. [Google Scholar] [CrossRef]
- Moyankova, D.; Hinkov, A.; Georgieva, D.; Shishkov, S.; Djilianov, D. Inhibitory effect of extracts from haberlea rhodopensis Friv. Against herpes simplex virus. Comptes Rendus L’académie Bulg. Sci. 2014, 67, 1369–1376. [Google Scholar]
- Todorov, D.; Shishkova, K.; Dragolova, D.; Hinkov, A.; Kapchina-Toteva, V.; Shishkov, S. Antiviral activity of medicinal plant Nepeta nuda. Biotechnol. Biotechnol. Equip. 2015, 29, S39–S43. [Google Scholar] [CrossRef]
- Angelova, P.; Tsvetkov, V.; Hinkov, A.; Todorov, D.; Shishkova, K.; Yordanova, Z.; Kapchina-Toteva, V.; Shishkov, S. Antiviral activity of Stachys Thracica Dav. extracts against Human Herpes virus type 1 and 2. BioDiscovery 2017, 20, e15022. [Google Scholar] [CrossRef]
- Ghildiyal, R.; Prakash, V.; Chaudhary, V.K.; Gupta, V.; Gabrani, R. Phytochemicals as Antiviral Agents: Recent Updates. In Plant-Derived Bioactives: Production, Properties and Therapeutic Applications; Swamy, M.K., Ed.; Springer: Singapore, 2020; pp. 279–295. [Google Scholar] [CrossRef]
- Petran, M.; Dragos, D.; Gilca, M. Historical ethnobotanical review of medicinal plants used to treat children diseases in Romania (1860s–1970s). J. Ethnobiol. Ethnomed. 2020, 16, 15. [Google Scholar] [CrossRef]
- Hinkov, A.; Angelova, P.; Marchev, A.; Hodzhev, Y.; Tsvetkov, V.; Dragolova, D.; Todorov, D.; Shishkova, K.; Kapchina-Toteva, V.; Blundell, R.; et al. Nepeta nuda ssp. nuda L. water extract: Inhibition of replication of some strains of human alpha herpes virus (genus simplex virus) in vitro, mode of action and NMR-based metabolomics. J. Herb. Med. 2020, 21, 100334. [Google Scholar] [CrossRef]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Oo, T.; Saiboonjan, B.; Srijampa, S.; Srisrattakarn, A.; Sutthanut, K.; Tavichakorntrakool, R.; Chanawong, A.; Lulitanond, A.; Tippayawat, P. Inhibition of Bacterial Efflux Pumps by Crude Extracts and Essential Oil from Myristica fragrans Houtt. (Nutmeg) Seeds against Methicillin-Resistant Staphylococcus aureus. Molecules 2021, 26, 4662. [Google Scholar] [CrossRef] [PubMed]
- Dragoș, D.; Petran, M.; Gradinaru, T.-C.; Gilca, M. Phytochemicals and Inflammation: Is Bitter Better? Plants 2022, 11, 2991. [Google Scholar] [CrossRef] [PubMed]
- Gilca, M.; Dragos, D.; Madalina, P.; Gradinaru, T.-C. PlantMolecularTasteDB: A Database of Taste Active Phytochemicals. Front. Pharmacol. 2022, 12, 751712. [Google Scholar] [CrossRef]
- Quenon, C.; Hennebelle, T.; Butaud, J.-F.; Ho, R.; Samaillie, J.; Neut, C.; Lehartel, T.; Rivière, C.; Siah, A.; Bonneau, N.; et al. Antimicrobial Properties of Compounds Isolated from Syzygium malaccense (L.) Merr. and L.M. Perry and Medicinal Plants Used in French Polynesia. Life 2022, 12, 733. [Google Scholar] [CrossRef]
- Jacquin, J.; Moureu, S.; Deweer, C.; Hakem, A.; Paguet, A.-S.; Bonneau, N.; Bordage, S.; Dermont, C.; Sahpaz, S.; Muchembled, J.; et al. Hop (Humulus lupulus L.) Specialized Metabolites: Extraction, Purification, Characterization in Different Plant Parts and In Vitro Evaluation of Anti-Oomycete Activities against Phytophthora infestans. Agronomy 2022, 12, 2826. [Google Scholar] [CrossRef]
- Ellatif, S.A.; Abdel Razik, E.S.; Abu-Serie, M.M.; Mahfouz, A.; Shater, A.F.; Saleh, F.M.; Hassan, M.M.; Alsanie, W.F.; Altalhi, A.; Daigham, G.E.; et al. Immunomodulatory Efficacy-Mediated Anti-HCV and Anti-HBV Potential of Kefir Grains; Unveiling the In Vitro Antibacterial, Antifungal, and Wound Healing Activities. Molecules 2022, 27, 2016. [Google Scholar] [CrossRef]
- Vuković, S.; Popović-Djordjević, J.; Kostić, A.; Pantelić, N.; Srećković, N.; Akram, M.; Laila, U.; Katanić Stanković, J. Allium Species in the Balkan Region-Major Metabolites, Antioxidant and Antimicrobial Properties. Horticulturae 2023, 9, 408. [Google Scholar] [CrossRef]
- Srivastava, A.; Jit, B.P.; Dash, R.; Srivastava, R.; Srivastava, S. Thuja occidentalis: An Unexplored Phytomedicine with Therapeutic Applications. Comb. Chem. High Throughput Screen. 2023, 26, 3–13. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Sun, Y.L.; Ruan, X.F. Bornyl acetate: A promising agent in phytomedicine for inflammation and immune modulation. Phytomedicine 2023, 114, 154781. [Google Scholar] [CrossRef]
- Ross, S.M. Ashwagandha: An Effective Phytomedicine for Reducing Stress and Anxiety. Holist. Nurs. Pract. 2023, 37, 298–300. [Google Scholar] [CrossRef]
- Pfaar, O.; Beule, A.G.; Jobst, D.; Kraft, K.; Stammer, H.; Röschmann-Doose, K.I.L.; Wittig, T.; Stuck, B.A. Phytomedicine ELOM-080 in Acute Viral Rhinosinusitis: A Randomized, Placebo-Controlled, Blinded Clinical Trial. Laryngoscope 2023, 133, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.-T.; Periferakis, A.; Periferakis, K.; Caruntu, A.; Badarau, I.A.; Savulescu-Fiedler, I.; Scheau, C.; Caruntu, C. Antimicrobial Properties of Capsaicin: Available Data and Future Research Perspectives. Nutrients 2023, 15, 4097. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Jain, V. Kaempferol: A Key Emphasis on its Counter-Wired Potential. Int. J. Innov. Sci. Res. Technol. 2023, 8, 2534–2540. [Google Scholar]
- Hakem, A.; Desmarets, L.; Sahli, R.; Malek, R.B.; Camuzet, C.; François, N.; Lefèvre, G.; Samaillie, J.; Moureu, S.; Sahpaz, S.; et al. Luteolin Isolated from Juncus acutus L., a Potential Remedy for Human Coronavirus 229E. Molecules 2023, 28, 4263. [Google Scholar] [CrossRef]
- Moureu, S.; Jacquin, J.; Samaillie, J.; Deweer, C.; Rivière, C.; Muchembled, J. Antifungal Activity of Hop Leaf Extracts and Xanthohumol on Two Strains of Venturia inaequalis with Different Sensitivities to Triazoles. Microorganisms 2023, 11, 1605. [Google Scholar] [CrossRef]
- Shkondrov, A.; Hinkov, A.; Cvetkov, V.; Shishkova, K.; Todorov, D.; Shishkov, S.; Stambolov, I.; Yoncheva, K.; Krasteva, I. Astragalus glycyphyllos L.: Antiviral activity and tablet dosage formulation of a standardized dry extract. Biotechnol. Biotechnol. Equip. 2023, 37, 2221752. [Google Scholar] [CrossRef]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Szopa, D.; Witek-Krowiak, A. Antiviral Properties of Polyphenols from Plants. Foods 2021, 10, 2277. [Google Scholar] [CrossRef]
- Simmonds, P. Reconstructing the origins of human hepatitis viruses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 1013–1026. [Google Scholar] [CrossRef]
- Woolhouse, M.E.J.; Adair, K. The diversity of human RNA viruses. Future Virol. 2013, 8, 159–171. [Google Scholar] [CrossRef]
- Periferakis, A.; Bolocan, A.; Ion, D. A Review of Innovation in Medicine. Technol. Innov. Life Sci. 2022, 1, 42–48. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Feng, M.; Zhou, W.; Shi, X.; Zhou, P. In vitro and in vivo anti-hepatitis B virus activities of a plant extract from Geranium carolinianum L. Antivir. Res. 2008, 79, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Parvez, M.K.; Al-dosari, M.S.; Basudan, O.A.; Herqash, R.N. The anti-hepatitis B virus activity of sea buckthorn is attributed to quercetin, kaempferol and isorhamnetin. Biomed. Rep. 2022, 17, 89. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, L.; Huleihel, M.; Zaccai, M.; Ben-Shabat, S. Potent antiviral flavone glycosides from Ficus benjamina leaves. Fitoterapia 2012, 83, 362–367. [Google Scholar] [CrossRef]
- Behbahani, M.; Shanehsazzadeh, M.; Shokoohinia, Y.; Soltani, M. Evaluation of anti-herpetic activity of methanol seed extract and fractions of Securigera securidaca in vitro. J. Antivir. Antiretrovir. 2013, 5, 72–76. [Google Scholar]
- Park, S.; Kim, N.E.; Park, B.J.; Kwon, H.C.; Song, Y.J. Kaempferol Interferes with Varicella-Zoster Virus Replication in Human Foreskin Fibroblasts. Pharmaceuticals 2022, 15, 1582. [Google Scholar] [CrossRef]
- Arabyan, E.; Hakobyan, A.; Hakobyan, T.; Grigoryan, R.; Izmailyan, R.; Avetisyan, A.; Karalyan, Z.; Jackman, J.A.; Ferreira, F.; Elrod, C.C.; et al. Flavonoid Library Screening Reveals Kaempferol as a Potential Antiviral Agent Against African Swine Fever Virus. Front. Microbiol. 2021, 12, 736780. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.-Q.; Yin, Z.-Q.; Wang, R.; Hu, H.-Y.; Liang, X.-X.; He, C.-L.; Yin, L.-Z.; Ye, G.; Zou, Y.-F.; et al. Kaempferol inhibits Pseudorabies virus replication in vitro through regulation of MAPKs and NF-κB signaling pathways. J. Integr. Agric. 2021, 20, 2227–2239. [Google Scholar] [CrossRef]
- Safioleas, M.; Lygidakis, N.J.; Manti, C. Hepatitis B today. Hepatogastroenterology 2007, 54, 545–548. [Google Scholar]
- Aspinall, E.J.; Hawkins, G.; Fraser, A.; Hutchinson, S.J.; Goldberg, D. Hepatitis B prevention, diagnosis, treatment and care: A review. Occup. Med. 2011, 61, 531–540. [Google Scholar] [CrossRef]
- Trépo, C.; Chan, H.L.; Lok, A. Hepatitis B virus infection. Lancet 2014, 384, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, T.; Sams, R.; Carpenter, M. Hepatitis B: Screening, Prevention, Diagnosis, and Treatment. Am. Fam. Physician 2019, 99, 314–323. [Google Scholar] [PubMed]
- Duff, P. Hepatitis in pregnancy. Semin. Perinatol. 1998, 22, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-F.; Bai, L.-P.; Huang, W.-B.; Li, X.-Z.; Zhao, S.-S.; Zhong, N.-S.; Jiang, Z.-H. Comparison of in vitro antiviral activity of tea polyphenols against influenza A and B viruses and structure–activity relationship analysis. Fitoterapia 2014, 93, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Whitley, R.J.; Roizman, B. Herpes simplex virus infections. Lancet 2001, 357, 1513–1518. [Google Scholar] [CrossRef]
- Omarova, S.; Cannon, A.; Weiss, W.; Bruccoleri, A.; Puccio, J. Genital Herpes Simplex Virus-An Updated Review. Adv. Pediatr. 2022, 69, 149–162. [Google Scholar] [CrossRef]
- Landy, H.J.; Grossman, J.H., 3rd. Herpes simplex virus. Obstet. Gynecol. Clin. N. Am. 1989, 16, 495–515. [Google Scholar] [CrossRef]
- Fatahzadeh, M.; Schwartz, R.A. Human herpes simplex virus infections: Epidemiology, pathogenesis, symptomatology, diagnosis, and management. J. Am. Acad. Dermatol. 2007, 57, 737–763. [Google Scholar] [CrossRef]
- Madavaraju, K.; Koganti, R.; Volety, I.; Yadavalli, T.; Shukla, D. Herpes Simplex Virus Cell Entry Mechanisms: An Update. Front. Cell. Infect. Microbiol. 2020, 10, 617578. [Google Scholar] [CrossRef]
- James, S.H.; Kimberlin, D.W. Neonatal Herpes Simplex Virus Infection. Infect. Dis. Clin. N. Am. 2015, 29, 391–400. [Google Scholar] [CrossRef]
- Samies, N.L.; James, S.H.; Kimberlin, D.W. Neonatal Herpes Simplex Virus Disease: Updates and Continued Challenges. Clin. Perinatol. 2021, 48, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Sibley, D.; Larkin, D.F.P. Update on Herpes simplex keratitis management. Eye 2020, 34, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Agelidis, A.; Koujah, L.; Suryawanshi, R.; Yadavalli, T.; Mishra, Y.K.; Adelung, R.; Shukla, D. An Intra-Vaginal Zinc Oxide Tetrapod Nanoparticles (ZOTEN) and Genital Herpesvirus Cocktail Can Provide a Novel Platform for Live Virus Vaccine. Front. Immunol. 2019, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M.A.; Gilden, D. Neurological complications of varicella zoster virus reactivation. Curr. Opin. Neurol. 2014, 27, 356–360. [Google Scholar] [CrossRef]
- Kennedy, P.G.E.; Gershon, A.A. Clinical Features of Varicella-Zoster Virus Infection. Viruses 2018, 10, 609. [Google Scholar] [CrossRef]
- Kennedy, P.G.E.; Mogensen, T.H.; Cohrs, R.J. Recent Issues in Varicella-Zoster Virus Latency. Viruses 2021, 13, 2018. [Google Scholar] [CrossRef]
- Grahn, A.; Studahl, M. Varicella-zoster virus infections of the central nervous system—Prognosis, diagnostics and treatment. J. Infect. 2015, 71, 281–293. [Google Scholar] [CrossRef]
- Garrett, R.; Romanos, M.T.V.; Borges, R.M.; Santos, M.G.; Rocha, L.; Silva, A.J.R.d. Antiherpetic activity of a flavonoid fraction from Ocotea notata leaves. Rev. Bras. Farmacogn. 2012, 22, 306–313. [Google Scholar] [CrossRef]
- Duan, X.; Ru, Y.; Yang, W.; Ren, J.; Hao, R.; Qin, X.; Li, D.; Zheng, H. Research progress on the proteins involved in African swine fever virus infection and replication. Front. Immunol. 2022, 13, 947180. [Google Scholar] [CrossRef]
- Njau, E.P.; Machuka, E.M.; Cleaveland, S.; Shirima, G.M.; Kusiluka, L.J.; Okoth, E.A.; Pelle, R. African Swine Fever Virus (ASFV): Biology, Genomics and Genotypes Circulating in Sub-Saharan Africa. Viruses 2021, 13, 2285. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; Hou, L.; Yang, C.; Wen, Y. Advance of African swine fever virus in recent years. Res. Vet. Sci. 2021, 136, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biology of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 2005, 69, 462–500. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Hahn, E.C.; Tottewitz, F.; Kramer, M.; Klupp, B.G.; Mettenleiter, T.C.; Freuling, C. Pseudorabies virus in wild swine: A global perspective. Arch. Virol. 2011, 156, 1691–1705. [Google Scholar] [CrossRef] [PubMed]
- Bo, Z.; Li, X. A Review of Pseudorabies Virus Variants: Genomics, Vaccination, Transmission, and Zoonotic Potential. Viruses 2022, 14, 1003. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.W.; Weng, S.S.; Cheng, Q.; Cui, P.; Li, Y.J.; Wu, H.L.; Zhu, Y.M.; Xu, B.; Zhang, W.H. Human Endophthalmitis Caused By Pseudorabies Virus Infection, China, 2017. Emerg. Infect. Dis. 2018, 24, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Lu, J.; Zhang, W.; Gao, G.F. Pseudorabies virus: A neglected zoonotic pathogen in humans? Emerg. Microbes Infect. 2019, 8, 150–154. [Google Scholar] [CrossRef]
- Pasick, J. Application of DIVA vaccines and their companion diagnostic tests to foreign animal disease eradication. Anim. Health Res. Rev. 2004, 5, 257–262. [Google Scholar] [CrossRef]
- Card, J.P. Pseudorabies virus neuroinvasiveness: A window into the functional organization of the brain. Adv. Virus Res. 2001, 56, 39–71. [Google Scholar] [CrossRef]
- Parvez, M.K.; Parveen, S. Evolution and Emergence of Pathogenic Viruses: Past, Present, and Future. Intervirology 2017, 60, 1–7. [Google Scholar] [CrossRef]
- Schwarz, S.; Sauter, D.; Wang, K.; Zhang, R.; Sun, B.; Karioti, A.; Bilia, A.R.; Efferth, T.; Schwarz, W. Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Med. 2014, 80, 177–182. [Google Scholar] [CrossRef]
- Zhou, Z.-L.; Yin, W.-Q.; Zou, X.-P.; Huang, D.-Y.; Zhou, C.-L.; Li, L.-M.; Chen, K.-C.; Guo, Z.-Y.; Lin, S.-Q. Flavonoid glycosides and potential antivirus activity of isolated compounds from the leaves of Eucalyptus citriodora. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 813–817. [Google Scholar] [CrossRef]
- Jeong, H.J.; Ryu, Y.B.; Park, S.J.; Kim, J.H.; Kwon, H.J.; Kim, J.H.; Park, K.H.; Rho, M.C.; Lee, W.S. Neuraminidase inhibitory activities of flavonols isolated from Rhodiola rosea roots and their in vitro anti-influenza viral activities. Bioorg. Med. Chem. 2009, 17, 6816–6823. [Google Scholar] [CrossRef] [PubMed]
- Kai, H.; Obuchi, M.; Yoshida, H.; Watanabe, W.; Tsutsumi, S.; Park, Y.K.; Matsuno, K.; Yasukawa, K.; Kurokawa, M. In vitro and in vivo anti-influenza virus activities of flavonoids and related compounds as components of Brazilian propolis (AF-08). J. Funct. Foods 2014, 8, 214–223. [Google Scholar] [CrossRef]
- Derksen, A.; Kühn, J.; Hafezi, W.; Sendker, J.; Ehrhardt, C.; Ludwig, S.; Hensel, A. Antiviral activity of hydroalcoholic extract from Eupatorium perfoliatum L. against the attachment of influenza A virus. J. Ethnopharmacol. 2016, 188, 144–152. [Google Scholar] [CrossRef]
- Behbahani, M.; Sayedipour, S.; Pourazar, A.; Shanehsazzadeh, M. In Vitro anti-HIV-1 activities of kaempferol and kaempferol-7-O-glucoside isolated from Securigera securidaca. Res. Pharm. Sci. 2014, 9, 463–469. [Google Scholar]
- Care, C.; Sornjai, W.; Jaratsittisin, J.; Hitakarun, A.; Wikan, N.; Triwitayakorn, K.; Smith, D. Discordant Activity of Kaempferol Towards Dengue Virus and Japanese Encephalitis Virus. Molecules 2020, 25, 1246. [Google Scholar] [CrossRef]
- Dwivedi, V.D.; Bharadwaj, S.; Afroz, S.; Khan, N.; Ansari, M.A.; Yadava, U.; Tripathi, R.C.; Tripathi, I.P.; Mishra, S.K.; Kang, S.G. Anti-dengue infectivity evaluation of bioflavonoid from Azadirachta indica by dengue virus serine protease inhibition. J. Biomol. Struct. Dyn. 2021, 39, 1417–1430. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, Z.; Du, J.; Hu, Y.; Liu, L.; Yang, F.; Jin, Q. Anti-Japanese-Encephalitis-Viral Effects of Kaempferol and Daidzin and Their RNA-Binding Characteristics. PLoS ONE 2012, 7, e30259. [Google Scholar] [CrossRef]
- Tsai, F.J.; Lin, C.W.; Lai, C.C.; Lan, Y.C.; Lai, C.H.; Hung, C.H.; Hsueh, K.C.; Lin, T.H.; Chang, H.C.; Wan, L.; et al. Kaempferol inhibits enterovirus 71 replication and internal ribosome entry site (IRES) activity through FUBP and HNRP proteins. Food Chem. 2011, 128, 312–322. [Google Scholar] [CrossRef]
- Sauter, D.; Schwarz, S.; Wang, K.; Zhang, R.; Sun, B.; Schwarz, W. Genistein as antiviral drug against HIV ion channel. Planta Med. 2014, 80, 682–687. [Google Scholar] [CrossRef]
- Orabi, M.A.; Orabi, E.A. Antiviral and antioxidant activities of flavonoids of Ficus virens: Experimental and theoretical investigations. J. Pharmacogn. Phytochem. 2016, 5, 120–128. [Google Scholar]
- Robin, V.; Irurzun, A.; Amoros, M.; Boustie, J.; Carrasco, L. Antipoliovirus flavonoids from Psiadia dentata. Antivir. Chem. Chemother. 2001, 12, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Lani, R.; Hassandarvish, P.; Chiam, C.W.; Moghaddam, E.; Chu, J.J.; Rausalu, K.; Merits, A.; Higgs, S.; Vanlandingham, D.; Abu Bakar, S.; et al. Antiviral activity of silymarin against chikungunya virus. Sci. Rep. 2015, 5, 11421. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.J.; Jeon, S.B.; Oh, H.; Lee, B.-H.; Lee, S.-Y.; Oh, S.H.; Jung, J.Y.; Choi, C. Comparison of the antiviral activity of flavonoids against murine norovirus and feline calicivirus. Food Control 2016, 60, 25–30. [Google Scholar] [CrossRef]
- Santos-López, G.; Cortés-Hernández, P.; Vallejo-Ruiz, V.; Reyes-Leyva, J. SARS-CoV-2: Basic concepts, origin and treatment advances. Gac. Medica Mex. 2021, 157, 84–89. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Kirtipal, N.; Bharadwaj, S.; Kang, S.G. From SARS to SARS-CoV-2, insights on structure, pathogenicity and immunity aspects of pandemic human coronaviruses. Infect. Genet. Evol. 2020, 85, 104502. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Al-Ahmed, S.H.; Haque, S.; Sah, R.; Tiwari, R.; Malik, Y.S.; Dhama, K.; Yatoo, M.I.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. SARS-CoV-2, SARS-CoV, and MERS-COV: A comparative overview. Le Infez. Med. 2020, 28, 174–184. [Google Scholar]
- Yang, H.; Rao, Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat. Rev. Microbiol. 2021, 19, 685–700. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.-Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Daia, C.; Scheau, C.; Neagu, G.; Andone, I.; Spanu, A.; Popescu, C.; Stoica, S.I.; Verenca, M.C.; Onose, G. Nerve conduction study and electromyography findings in patients recovering from COVID-19—Case report. Int. J. Infect. Dis. 2020, 103, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Cines, D.B.; Bussel, J.B. SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 384, 2254–2256. [Google Scholar] [CrossRef]
- Zamfir, M.-A.; Moraru, L.; Dobrea, C.; Scheau, A.-E.; Iacob, S.; Moldovan, C.; Scheau, C.; Caruntu, C.; Caruntu, A. Hematologic Malignancies Diagnosed in the Context of the mRNA COVID-19 Vaccination Campaign: A Report of Two Cases. Medicina 2022, 58, 874. [Google Scholar] [CrossRef]
- Goldman, S.; Bron, D.; Tousseyn, T.; Vierasu, I.; Dewispelaere, L.; Heimann, P.; Cogan, E.; Goldman, M. Rapid Progression of Angioimmunoblastic T Cell Lymphoma Following BNT162b2 mRNA Vaccine Booster Shot: A Case Report. Front. Med. 2021, 8, 798095. [Google Scholar] [CrossRef]
- Brumfiel, C.M.; Patel, M.H.; DiCaudo, D.J.; Rosenthal, A.C.; Pittelkow, M.R.; Mangold, A.R. Recurrence of primary cutaneous CD30-positive lymphoproliferative disorder following COVID-19 vaccination. Leuk. Lymphoma 2021, 62, 2554–2555. [Google Scholar] [CrossRef]
- Anand, A.V.; Balamuralikrishnan, B.; Kaviya, M.; Bharathi, K.; Parithathvi, A.; Arun, M.; Senthilkumar, N.; Velayuthaprabhu, S.; Saradhadevi, M.; Al-Dhabi, N.A.; et al. Medicinal Plants, Phytochemicals, and Herbs to Combat Viral Pathogens Including SARS-CoV-2. Molecules 2021, 26, 1775. [Google Scholar] [CrossRef]
- Khazdair, M.; Anaeigoudari, A.; Agbor, G. Anti-viral and anti-inflammatory effects of kaempferol and quercetin and COVID-2019: A scoping review. Asian Pac. J. Trop. Biomed. 2021, 11, 327–334. [Google Scholar] [CrossRef]
- Johnson, A.P. Methicillin-resistant Staphylococcus aureus: The European landscape. J. Antimicrob. Chemother. 2011, 66 (Suppl. S4), iv43–iv48. [Google Scholar] [CrossRef] [PubMed]
- Owis, A.I.; El-Hawary, M.S.; El Amir, D.; Aly, O.M.; Abdelmohsen, U.R.; Kamel, M.S. Molecular docking reveals the potential of Salvadora persica flavonoids to inhibit COVID-19 virus main protease. RSC Adv. 2020, 10, 19570–19575. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, R. Polyphenolics Screening in Mollungo Nudicaulis using UPLC-ESI-MS and Its Active Compound Kaempferol against SARs CoV2 Receptor. M.Sc. Thesis, JKK Nattaraja College of Pharmacy, Kumarapalayam, India, 2021. [Google Scholar]
- Qiu, X.; Xu, S.; Lu, Y.; Luo, Z.; Yan, Y.; Wang, C.; Ji, J. Development of mRNA vaccines against respiratory syncytial virus (RSV). Cytokine Growth Factor Rev. 2022, 68, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Chang, C.; Gershwin, M.E.; Gershwin, L.J. Respiratory syncytial virus—A comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 331–379. [Google Scholar] [CrossRef] [PubMed]
- Gatt, D.; Martin, I.; AlFouzan, R.; Moraes, T.J. Prevention and Treatment Strategies for Respiratory Syncytial Virus (RSV). Pathogens 2023, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Priante, E.; Cavicchiolo, M.E.; Baraldi, E. RSV infection and respiratory sequelae. Minerva Pediatr. 2018, 70, 623–633. [Google Scholar] [CrossRef]
- Jenkins, V.A.; Hoet, B.; Hochrein, H.; De Moerlooze, L. The Quest for a Respiratory Syncytial Virus Vaccine for Older Adults: Thinking beyond the F Protein. Vaccines 2023, 11, 382. [Google Scholar] [CrossRef]
- Labella, A.M.; Merel, S.E. Influenza. Med. Clin. N. Am. 2013, 97, 621–645. [Google Scholar] [CrossRef]
- Gaitonde, D.Y.; Moore, F.C.; Morgan, M.K. Influenza: Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 751–758. [Google Scholar]
- Wu, N.C.; Wilson, I.A. Influenza Hemagglutinin Structures and Antibody Recognition. Cold Spring Harb. Perspect. Med. 2020, 10, a038778. [Google Scholar] [CrossRef]
- Webster, R.G.; Govorkova, E.A. Continuing challenges in influenza. Ann. N. Y. Acad. Sci. 2014, 1323, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Świerczyńska, M.; Mirowska-Guzel, D.M.; Pindelska, E. Antiviral Drugs in Influenza. Int. J. Environ. Res. Public Health 2022, 19, 3018. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.-L.; Wang, H.-D.; Lee, S.M.; Wang, Y.-T.; Du, G.-H. Structure–activity relationship of flavonoids as influenza virus neuraminidase inhibitors and their in vitro anti-viral activities. Bioorg. Med. Chem. 2008, 16, 7141–7147. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.I.; Lee, I.; Lee, S.; Hwang, M.-W.; Bae, J.-Y.; Heo, J.; Kim, D.; Han, S.-Z.; Park, M.-S. Aronia melanocarpa and its components demonstrate antiviral activity against influenza viruses. Biochem. Biophys. Res. Commun. 2013, 440, 14–19. [Google Scholar] [CrossRef]
- World Health Organization. Global Situation and Trends of HIV. Available online: https://www.who.int/data/gho/data/themes/hiv-aids (accessed on 24 September 2023).
- Fanales-Belasio, E.; Raimondo, M.; Suligoi, B.; Buttò, S. HIV virology and pathogenetic mechanisms of infection: A brief overview. Ann. Ist. Super. Sanita 2010, 46, 5–14. [Google Scholar] [CrossRef]
- Lucas, S.; Nelson, A.M. HIV and the spectrum of human disease. J. Pathol. 2015, 235, 229–241. [Google Scholar] [CrossRef]
- Hu, W.S.; Hughes, S.H. HIV-1 reverse transcription. Cold Spring Harb. Perspect. Med. 2012, 2, a006882. [Google Scholar] [CrossRef]
- Sabin, C.A.; Lundgren, J.D. The natural history of HIV infection. Curr. Opin. HIV AIDS 2013, 8, 311–317. [Google Scholar] [CrossRef]
- Phanuphak, N.; Gulick, R.M. HIV treatment and prevention 2019: Current standards of care. Curr. Opin. HIV AIDS 2020, 15, 4–12. [Google Scholar] [CrossRef]
- Tsukada, K. HIV infection/AIDS. Nihon Rinsho 2016, 74, 1992–1997. [Google Scholar]
- Achappa, B.; Madi, D.; Bhaskaran, U.; Ramapuram, J.T.; Rao, S.; Mahalingam, S. Adherence to Antiretroviral Therapy Among People Living with HIV. N. Am. J. Med. Sci. 2013, 5, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Dorcélus, L.; Bernard, J.; Georgery, C.; Vanessa, C. Factors associated with antiretroviral therapy adherence among people living with HIV in Haiti: A cross-sectional study. AIDS Res. Ther. 2021, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, E.; Park, B.-J.; Bang, J.H.; Lee, J.Y. Adherence to antiretroviral therapy and factors affecting low medication adherence among incident HIV-infected individuals during 2009–2016: A nationwide study. Sci. Rep. 2018, 8, 3133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, K.; Kalichman, S.C. Barriers to HIV Medication Adherence as a Function of Regimen Simplification. Ann. Behav. Med. 2017, 51, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Jilg, N.; Li, J.Z. On the Road to a HIV Cure: Moving Beyond Berlin and London. Infect. Dis. Clin. N. Am. 2019, 33, 857–868. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, R.; Cheng, G. Progress towards understanding the pathogenesis of dengue hemorrhagic fever. Virol. Sin. 2017, 32, 16–22. [Google Scholar] [CrossRef]
- Martina, B.E.; Koraka, P.; Osterhaus, A.D. Dengue virus pathogenesis: An integrated view. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef]
- Kariyawasam, R.; Lachman, M.; Mansuri, S.; Chakrabarti, S.; Boggild, A.K. A dengue vaccine whirlwind update. Ther. Adv. Infect. Dis. 2023, 10, 20499361231167274. [Google Scholar] [CrossRef]
- López-Medina, E.; Biswal, S.; Saez-Llorens, X.; Borja-Tabora, C.; Bravo, L.; Sirivichayakul, C.; Vargas, L.M.; Alera, M.T.; Velásquez, H.; Reynales, H.; et al. Efficacy of a Dengue Vaccine Candidate (TAK-003) in Healthy Children and Adolescents 2 Years after Vaccination. J. Infect. Dis. 2022, 225, 1521–1532. [Google Scholar] [CrossRef]
- Troost, B.; Smit, J.M. Recent advances in antiviral drug development towards dengue virus. Curr. Opin. Virol. 2020, 43, 9–21. [Google Scholar] [CrossRef]
- Taslem Mourosi, J.; Awe, A.; Jain, S.; Batra, H. Nucleic Acid Vaccine Platform for DENGUE and ZIKA Flaviviruses. Vaccines 2022, 10, 834. [Google Scholar] [CrossRef] [PubMed]
- Le Flohic, G.; Porphyre, V.; Barbazan, P.; Gonzalez, J.P. Review of climate, landscape, and viral genetics as drivers of the Japanese encephalitis virus ecology. PLoS Negl. Trop. Dis. 2013, 7, e2208. [Google Scholar] [CrossRef] [PubMed]
- Ravanini, P.; Huhtamo, E.; Ilaria, V.; Crobu, M.G.; Nicosia, A.M.; Servino, L.; Rivasi, F.; Allegrini, S.; Miglio, U.; Magri, A.; et al. Japanese encephalitis virus RNA detected in Culex pipiens mosquitoes in Italy. Eurosurveillance 2012, 17, 20221. [Google Scholar] [CrossRef]
- Yasui, K. Neuropathogenesis of Japanese encephalitis virus. J. Neurovirol. 2002, 8 (Suppl. S2), 112–114. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.B.; Vrati, S.; Kalia, M. Pathobiology of Japanese encephalitis virus infection. Mol. Asp. Med. 2021, 81, 100994. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.I.; Lee, Y.M. Japanese encephalitis: The virus and vaccines. Hum. Vaccines Immunother. 2014, 10, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Johari, J.; Kianmehr, A.; Mustafa, M.R.; Abubakar, S.; Zandi, K. Antiviral Activity of Baicalein and Quercetin against the Japanese encephalitis Virus. Int. J. Mol. Sci. 2012, 13, 16785–16795. [Google Scholar] [CrossRef]
- Solomon, T.; Lewthwaite, P.; Perera, D.; Cardosa, M.J.; McMinn, P.; Ooi, M.H. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis. 2010, 10, 778–790. [Google Scholar] [CrossRef]
- Chong, P.; Liu, C.C.; Chow, Y.H.; Chou, A.H.; Klein, M. Review of enterovirus 71 vaccines. Clin. Infect. Dis. 2015, 60, 797–803. [Google Scholar] [CrossRef]
- Nayak, G.; Bhuyan, S.K.; Bhuyan, R.; Sahu, A.; Kar, D.; Kuanar, A. Global emergence of Enterovirus 71: A systematic review. Beni Suef Univ. J. Basic Appl. Sci. 2022, 11, 78. [Google Scholar] [CrossRef]
- de Crom, S.C.; Rossen, J.W.; van Furth, A.M.; Obihara, C.C. Enterovirus and parechovirus infection in children: A brief overview. Eur. J. Pediatr. 2016, 175, 1023–1029. [Google Scholar] [CrossRef]
- Cao, G.; Jing, W.; Liu, J.; Liu, M. The global trends and regional differences in incidence and mortality of hepatitis A from 1990 to 2019 and implications for its prevention. Hepatol. Int. 2021, 15, 1068–1082. [Google Scholar] [CrossRef]
- Abutaleb, A.; Kottilil, S. Hepatitis A: Epidemiology, Natural History, Unusual Clinical Manifestations, and Prevention. Gastroenterol. Clin. N. Am. 2020, 49, 191–199. [Google Scholar] [CrossRef]
- Pereira, F.E.; Gonçalves, C.S. Hepatitis A. Rev. Soc. Bras. Med. Trop. 2003, 36, 387–400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jeong, S.H.; Lee, H.S. Hepatitis A: Clinical manifestations and management. Intervirology 2010, 53, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Ohemu, T.; Agunu, A.; Chollom, S.; Okwori, V.; Dafam, D.; Olotu, P. Preliminary Phytochemical Screening and Antiviral Potential of Methanol Stem Bark Extract of Enantia chlorantha Oliver (Annonaceae) and Boswellia dalzielii Hutch (Burseraceae) against Newcastle Disease In Ovo. Eur. J. Med. Plants 2018, 25, 1–8. [Google Scholar] [CrossRef]
- Kidd, D.; Williams, A.J.; Howard, R.S. Poliomyelitis. Postgrad. Med. J. 1996, 72, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.K.; Robinson, L.R. Post-polio syndrome and the late effects of poliomyelitis: Part 2. treatment, management, and prognosis. Muscle Nerve 2018, 58, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.T.; Sherman, A.L. Medical Comorbidities and Complications Associated with Poliomyelitis and Its Sequelae. Phys. Med. Rehabil. Clin. N. Am. 2021, 32, 591–600. [Google Scholar] [CrossRef]
- Goodrick, S. Preventing polio. Lancet Neurol. 2014, 13, 653. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.S.; Garon, J.; Seib, K.; Orenstein, W.A. Polio vaccination: Past, present and future. Future Microbiol. 2015, 10, 791–808. [Google Scholar] [CrossRef]
- Suhrbier, A.; Jaffar-Bandjee, M.C.; Gasque, P. Arthritogenic alphaviruses—An overview. Nat. Rev. Rheumatol. 2012, 8, 420–429. [Google Scholar] [CrossRef]
- Caglioti, C.; Lalle, E.; Castilletti, C.; Carletti, F.; Capobianchi, M.R.; Bordi, L. Chikungunya virus infection: An overview. New Microbiol. 2013, 36, 211–227. [Google Scholar]
- Burt, F.J.; Chen, W.; Miner, J.J.; Lenschow, D.J.; Merits, A.; Schnettler, E.; Kohl, A.; Rudd, P.A.; Taylor, A.; Herrero, L.J.; et al. Chikungunya virus: An update on the biology and pathogenesis of this emerging pathogen. Lancet Infect. Dis. 2017, 17, e107–e117. [Google Scholar] [CrossRef]
- Martins, D.O.S.; Santos, I.A.; de Oliveira, D.M.; Grosche, V.R.; Jardim, A.C.G. Antivirals against Chikungunya Virus: Is the Solution in Nature? Viruses 2020, 12, 272. [Google Scholar] [CrossRef]
- Ghildiyal, R.; Gabrani, R. Antiviral therapeutics for chikungunya virus. Expert. Opin. Ther. Pat. 2020, 30, 467–480. [Google Scholar] [CrossRef]
- Radford, A.D.; Coyne, K.P.; Dawson, S.; Porter, C.J.; Gaskell, R.M. Feline calicivirus. Vet. Res. 2007, 38, 319–335. [Google Scholar] [CrossRef]
- Spiri, A.M. An Update on Feline Calicivirus. Schweiz Arch. Tierheilkd. 2022, 164, 225–241. [Google Scholar] [CrossRef]
- Bordicchia, M.; Fumian, T.M.; Van Brussel, K.; Russo, A.G.; Carrai, M.; Le, S.J.; Pesavento, P.A.; Holmes, E.C.; Martella, V.; White, P.; et al. Feline Calicivirus Virulent Systemic Disease: Clinical Epidemiology, Analysis of Viral Isolates and In Vitro Efficacy of Novel Antivirals in Australian Outbreaks. Viruses 2021, 13, 2040. [Google Scholar] [CrossRef]
- Radford, A.D.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; et al. Feline calicivirus infection. ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 556–564. [Google Scholar] [CrossRef]
- Hofmann-Lehmann, R.; Hosie, M.J.; Hartmann, K.; Egberink, H.; Truyen, U.; Tasker, S.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Lloret, A.; et al. Calicivirus Infection in Cats. Viruses 2022, 14, 937. [Google Scholar] [CrossRef]
- Henderson, K.S. Murine norovirus, a recently discovered and highly prevalent viral agent of mice. Lab Anim. 2008, 37, 314–320. [Google Scholar] [CrossRef]
- Karst, S.M.; Tibbetts, S.A. Recent advances in understanding norovirus pathogenesis. J. Med. Virol. 2016, 88, 1837–1843. [Google Scholar] [CrossRef]
- Graziano, V.R.; Wei, J.; Wilen, C.B. Norovirus Attachment and Entry. Viruses 2019, 11, 495. [Google Scholar] [CrossRef]
- MacGregor, J.T.; Jurd, L. Mutagenicity of plant flavonoids: Structural requirements for mutagenic activity in Salmonella typhimurium. Mutat. Res. 1978, 54, 297–309. [Google Scholar] [CrossRef]
- Francis, A.R.; Shetty, T.K.; Bhattacharya, R.K. Modifying role of dietary factors on the mutagenicity of aflatoxin B1: In Vitro effect of plant flavonoids. Mutat. Res. 1989, 222, 393–401. [Google Scholar] [CrossRef]
- Niering, P.; Michels, G.; Wätjen, W.; Ohler, S.; Steffan, B.; Chovolou, Y.; Kampkötter, A.; Proksch, P.; Kahl, R. Protective and detrimental effects of kaempferol in rat H4IIE cells: Implication of oxidative stress and apoptosis. Toxicol. Appl. Pharmacol. 2005, 209, 114–122. [Google Scholar] [CrossRef]
- Takanashi, H.; Aiso, S.; Hirono, I.; Matsushima, T.; Sugimura, T. Carcinogenicity test of quercetin and kaempferol in rats by oral administration. J. Food Saf. 1983, 5, 55–60. [Google Scholar] [CrossRef]
- Halstead, S.B. Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine 2017, 35, 6355–6358. [Google Scholar] [CrossRef]
- Song, Y.; Mugavero, J.; Stauft, C.B.; Wimmer, E. Dengue and Zika Virus 5′ Untranslated Regions Harbor Internal Ribosomal Entry Site Functions. mBio 2019, 10, 1110–1128. [Google Scholar] [CrossRef]
- Calzada, F.; Cedillo-Rivera, R.; Mata, R. Antiprotozoal activity of the constituents of Conyza filaginoides. J. Nat. Prod. 2001, 64, 671–673. [Google Scholar] [CrossRef]
- Arot Manguro, L.O.; Ugi, I.; Hermann, R.; Lemmen, P. Flavonol and drimane-type sesquiterpene glycosides of Warburgia stuhlmannii leaves. Phytochemistry 2003, 63, 497–502. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M. Flavonoids from the genus Taxus. Z. Naturforschung C J. Biosci. 2004, 59, 43–47. [Google Scholar] [CrossRef]
- Nguemeving, J.R.; Azebaze, A.G.B.; Kuete, V.; Eric Carly, N.N.; Beng, V.P.; Meyer, M.; Blond, A.; Bodo, B.; Nkengfack, A.E. Laurentixanthones A and B, antimicrobial xanthones from Vismia laurentii. Phytochemistry 2006, 67, 1341–1346. [Google Scholar] [CrossRef]
- Jiang, R.-W.; Zhou, J.-R.; Hon, P.-M.; Li, S.-L.; Zhou, Y.; Li, L.-L.; Ye, W.-C.; Xu, H.-X.; Shaw, P.-C.; But, P.P.-H. Lignans from Dysosma versipellis with Inhibitory Effects on Prostate Cancer Cell Lines. J. Nat. Prod. 2007, 70, 283–286. [Google Scholar] [CrossRef]
- Marín, C.; Boutaleb-Charki, S.; Díaz, J.G.; Huertas, O.; Rosales, M.J.; Pérez-Cordon, G.; Guitierrez-Sánchez, R.; Sánchez-Moreno, M. Antileishmaniasis activity of flavonoids from Consolida oliveriana. J. Nat. Prod. 2009, 72, 1069–1074. [Google Scholar] [CrossRef]
- Calzada, F.; Correa-Basurto, J.; Barbosa, E.; Mendez-Luna, D.; Yepez-Mulia, L. Antiprotozoal Constituents from Annona cherimola Miller, a Plant Used in Mexican Traditional Medicine for the Treatment of Diarrhea and Dysentery. Pharmacogn. Mag. 2017, 13, 148–152. [Google Scholar]
- Maas, M.; Hensel, A.; Costa, F.B.; Brun, R.; Kaiser, M.; Schmidt, T.J. An unusual dimeric guaianolide with antiprotozoal activity and further sesquiterpene lactones from Eupatoriumperfoliatum. Phytochemistry 2011, 72, 635–644. [Google Scholar] [CrossRef]
- Prasasty, V.D.; Cindana, S.; Ivan, F.X.; Zahroh, H.; Sinaga, E. Structure-based discovery of novel inhibitors of Mycobacterium tuberculosis CYP121 from Indonesian natural products. Comput. Biol. Chem. 2020, 85, 107205. [Google Scholar] [CrossRef]
- Tagne, M.; Nougang, M.; Metsopkeng, C.; Bricheux, G.; Donnadieu, F.; Nana, P.-A.; Ripoche, I.; Donfagsiteli Tchinda, N.; Agbor, G.; Chalard, P.; et al. Effects of aqueous and hydro-ethanolic Moringa oleifera Lam leaf extracts on the cultivability of 2 Bacillus strains isolated from rainwater. J. Food Stab. 2023, 6, 1–19. [Google Scholar]
- Khan, M.; Khan, T.; Wahab, S.; Aasim, M.; Sherazi, T.A.; Zahoor, M.; Yun, S.-I. Solvent based fractional biosynthesis, phytochemical analysis, and biological activity of silver nanoparticles obtained from the extract of Salvia moorcroftiana. PLoS ONE 2023, 18, e0287080. [Google Scholar] [CrossRef] [PubMed]
- Youl, O.; Moné-Bassavé, B.R.H.; Yougbaré, S.; Yaro, B.; Traoré, T.K.; Boly, R.; Yaméogo, J.B.G.; Koala, M.; Ouedraogo, N.; Kabré, E.; et al. Phytochemical Screening, Polyphenol and Flavonoid Contents, and Antioxidant and Antimicrobial Activities of Opilia amentacea Roxb. (Opiliaceae) Extracts. Appl. Biosci. 2023, 2, 493–512. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Feliciano, A.S. Flavonoids: From Structure to Health Issues. Molecules 2017, 22, 477. [Google Scholar] [CrossRef]
- Wu, S.; Chen, W.; Lu, S.; Zhang, H.; Yin, L. Metabolic Engineering of Shikimic Acid Biosynthesis Pathway for the Production of Shikimic Acid and Its Branched Products in Microorganisms: Advances and Prospects. Molecules 2022, 27, 4779. [Google Scholar] [CrossRef]
- Duan, L.; Ding, W.; Liu, X.; Cheng, X.; Cai, J.; Hua, E.; Jiang, H. Biosynthesis and engineering of kaempferol in Saccharomyces cerevisiae. Microb. Cell Factories 2017, 16, 165. [Google Scholar] [CrossRef]
- Viskupicova, J.; Ondrejovič, M.; Sturdik, E. Bioavailability and metabolism of flavonoids. J. Food Nutr. Res. 2008, 47, 151–162. [Google Scholar]
- Nielsen, S.E.; Kall, M.; Justesen, U.; Schou, A.; Dragsted, L.O. Human absorption and excretion of flavonoids after broccoli consumption. Cancer Lett. 1997, 114, 173–174. [Google Scholar] [CrossRef]
- de Vries, J.H.; Hollman, P.C.; Meyboom, S.; Buysman, M.N.; Zock, P.L.; van Staveren, W.A.; Katan, M.B. Plasma concentrations and urinary excretion of the antioxidant flavonols quercetin and kaempferol as biomarkers for dietary intake. Am. J. Clin. Nutr. 1998, 68, 60–65. [Google Scholar] [CrossRef]
- Németh, K.; Plumb, G.W.; Berrin, J.G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef]
- Terao, J. Dietary flavonoids as antioxidants. Forum. Nutr. 2009, 61, 87–94. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Williamson, G.; Kay, C.D.; Crozier, A. The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2022, 63, 9580–9604. [Google Scholar] [CrossRef]
- Ćavar, S.; Maksimović, M.; Vidic, D.; Parić, A. Chemical composition and antioxidant and antimicrobial activity of essential oil of Artemisia annua L. from Bosnia. Ind. Crops Prod. 2012, 37, 479–485. [Google Scholar] [CrossRef]
- Mueller, M.S.; Karhagomba, I.B.; Hirt, H.M.; Wemakor, E. The potential of Artemisia annua L. as a locally produced remedy for malaria in the tropics: Agricultural, chemical and clinical aspects. J. Ethnopharmacol. 2000, 73, 487–493. [Google Scholar] [CrossRef]
- Kumar, V.S.; Navaratnam, V. Neem (Azadirachta indica): Prehistory to contemporary medicinal uses to humankind. Asian Pac. J. Trop. Biomed. 2013, 3, 505–514. [Google Scholar] [CrossRef]
- Alzohairy, M.A. Therapeutics Role of Azadirachta indica (Neem) and Their Active Constituents in Diseases Prevention and Treatment. Evid. Based Complement. Altern. Med. 2016, 2016, 7382506. [Google Scholar] [CrossRef]
- Islas, J.F.; Acosta, E.; G-Buentello, Z.; Delgado-Gallegos, J.L.; Moreno-Treviño, M.G.; Escalante, B.; Moreno-Cuevas, J.E. An overview of Neem (Azadirachta indica) and its potential impact on health. J. Funct. Foods 2020, 74, 104171. [Google Scholar] [CrossRef]
- Gbekley, H.E.; Katawa, G.; Karou, S.D.; Anani, S.; Tchadjobo, T.; Ameyapoh, Y.; Batawila, K.; Simpore, J. Ethnobotanical Study of Plants Used to Treat Asthma in the Maritime Region in Togo. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Tolba, H.; Moghrani, H.; Benelmouffok, A.; Kellou, D.; Maachi, R. Essential oil of Algerian Eucalyptus citriodora: Chemical composition, antifungal activity. J. Mycol. Méd. 2015, 25, e128–e133. [Google Scholar] [CrossRef] [PubMed]
- Seyoum, A.; Pålsson, K.; Kung’a, S.; Kabiru, E.W.; Lwande, W.; Killeen, G.F.; Hassanali, A.; Knots, B.G.J. Traditional use of mosquito-repellent plants in western Kenya and their evaluation in semi-field experimental huts against Anopheles gambiae: Ethnobotanical studies and application by thermal expulsion and direct burning. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Muanza, D.; Kim, B.; Euler, K.; Williams, L. Antibacterial and antifungal activities of nine medicinal plants from Zaire. Int. J. Pharmacogn. 1994, 32, 337–345. [Google Scholar] [CrossRef]
- Stammel, H.J. Die Apotheke Manitous—Das Medizinische Wissen der Indianer und Ihre Heilpflanzen; Rowolth Verlag GmbH: Reinbek bei Hamburg, Germany, 1986. [Google Scholar]
- Moerman, D.A. Native American Ethnobotany; Timber Press: Portland, OR, USA, 1998. [Google Scholar]
- Hensel, A.; Maas, M.; Sendker, J.; Lechtenberg, M.; Petereit, F.; Deters, A.; Schmidt, T.; Stark, T. Eupatorium perfoliatum L.: Phytochemistry, traditional use and current applications. J. Ethnopharmacol. 2011, 138, 641–651. [Google Scholar] [CrossRef]
- Imran, M.; Rasool, N.; Rizwan, K.; Zubair, M.; Riaz, M.; Zia-Ul-Haq, M.; Rana, U.A.; Nafady, A.; Jaafar, H.Z. Chemical composition and Biological studies of Ficus benjamina. Chem. Cent. J. 2014, 8, 12. [Google Scholar] [CrossRef]
- Parajuli, S.P. Ethnobotanical study at Khandbari Municipality of Sankhuwasabha District, Nepal. Banko Janakari 2000, 10, 29–34. [Google Scholar] [CrossRef]
- Kanaujia, V.K.; Rirchhaiya, H.; Kailasiya, S.; Verma, M.; Yadav, R.; Shivhare, D. Evaluation of hepatoprotective activity on the leaves of Ficus benjamina Linn. J. Nat. Prod. Plant 2011, 1, 59–69. [Google Scholar]
- Sirisha, N.; Sreenivasulu, M.; Sangeeta, K.; Chetty, C.M. Antioxidant properties of Ficus species-a review. Int. J. PharmTech Res. 2010, 2, 2174–2182. [Google Scholar]
- Patel, M.H.; Desai, B.; Jha, S.; Patel, D.; Mehta, A.; Garde, Y. Phytochemical, pharmacognosy and ethnobotanical importance of the Ficus virens Aiton. Pharma Innov. J. 2023, 12, 4017–4021. [Google Scholar]
- Li, Y.; Ye, Y.; Wang, S.-J.; Xia, W.; Rahman, K.; Yue, W.; Zhang, H. Analgesic, anti-inflammatory and antipyretic activities of the aqueous extract of Geranium carolinianum L. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 105–113. [Google Scholar] [CrossRef]
- Bal, L.M.; Meda, V.; Naik, S.N.; Satya, S. Sea buckthorn berries: A potential source of valuable nutrients for nutraceuticals and cosmoceuticals. Food Res. Int. 2011, 44, 1718–1727. [Google Scholar] [CrossRef]
- Guliyev, V.B.; Gul, M.; Yildirim, A. Hippophae rhamnoides L.: Chromatographic methods to determine chemical composition, use in traditional medicine and pharmacological effects. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 812, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Vogl, S.; Picker, P.; Mihaly-Bison, J.; Fakhrudin, N.; Atanasov, A.G.; Heiss, E.H.; Wawrosch, C.; Reznicek, G.; Dirsch, V.M.; Saukel, J.; et al. Ethnopharmacological in vitro studies on Austria’s folk medicine--an unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs. J. Ethnopharmacol. 2013, 149, 750–771. [Google Scholar] [CrossRef]
- Pundir, S.; Garg, P.; Dviwedi, A.; Ali, A.; Kapoor, V.K.; Kapoor, D.; Kulshrestha, S.; Lal, U.R.; Negi, P. Ethnomedicinal uses, phytochemistry and dermatological effects of Hippophae rhamnoides L.: A review. J. Ethnopharmacol. 2021, 266, 113434. [Google Scholar] [CrossRef]
- Costa, I.F.d.J.B.; Simão, T.L.B.V.; Calixto, S.D.; Pereira, R.V.; Konno, T.U.P.; Pinto, S.C.; Tinoco, L.W.; Lasunskaia, E.; Leal, I.C.R.; Muzitano, M.F. Anti-mycobacterial and immunomodulatory activity of n-hexane fraction and spathulenol from Ocotea notata leaves. Rodriguésia 2021, 72. [Google Scholar] [CrossRef]
- Fortin, H.; Tomasi, S.; Jaccard, P.; Robin, V.; Boustie, J. A Prenyloxycoumarin from Psiadia dentata. Chem. Pharm. Bull. 2001, 49, 619–621. [Google Scholar] [CrossRef][Green Version]
- Mahadeo, K.; Grondin, I.; Kodja, H.; Soulange Govinden, J.; Jhaumeer Laulloo, S.; Frederich, M.; Gauvin-Bialecki, A. The genus Psiadia: Review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 210, 48–68. [Google Scholar] [CrossRef]
- Ivanova Stojcheva, E.; Quintela, J.C. The Effectiveness of Rhodiola rosea L. Preparations in Alleviating Various Aspects of Life-Stress Symptoms and Stress-Induced Conditions-Encouraging Clinical Evidence. Molecules 2022, 27, 3902. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, M.; Yuan, S.; Fu, S.; Li, Y.; Li, Y.; Wang, Q.; Cao, Y.; Liu, L.; Zhang, Q. Rhodiola rosea: A Therapeutic Candidate on Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 1348795. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Khatak, M.; Khatak, S.; Siddqui, A.A.; Vasudeva, N.; Aggarwal, A.; Aggarwal, P. Salvadora persica. Pharmacogn. Rev. 2010, 4, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Aljarbou, F.; Almobarak, A.; Binrayes, A.; Alamri, H.M. Salvadora persica’s Biological Properties and Applications in Different Dental Specialties: A Narrative Review. Evid. Based Complement. Alternat. Med. 2022, 2022, 8667687. [Google Scholar] [CrossRef] [PubMed]
- Abdelbagi, M.E.M.; Al-Mazaideh, G.M.; Ahmed, A.E.; Al-Rimawi, F.; Ayyal Salman, H.; Almutairi, A.; Abuilaiwi, F.A.; Wedian, F. Drug Formulation of Securigera securidaca Seed Extracts. Processes 2023, 11, 1955. [Google Scholar] [CrossRef]
- Nasehi, Z.; Kheiripour, N.; Taheri, M.A.; Ardjmand, A.; Jozi, F.; Aghadavod, E.; Doustimotlagh, A.H.; Shahaboddin, M.E. The Protective Effects of Securigera securidaca Seed Extract on Liver Injury Induced by Bile Duct Ligation in Rats. BioMed Res. Int. 2022, 2022, 6989963. [Google Scholar] [CrossRef]
- He, X.; Bai, Y.; Zhao, Z.; Wang, X.; Fang, J.; Huang, L.; Zeng, M.; Zhang, Q.; Zhang, Y.; Zheng, X. Local and traditional uses, phytochemistry, and pharmacology of Sophora japonica L.: A review. J. Ethnopharmacol. 2016, 187, 160–182. [Google Scholar] [CrossRef]
- Chen, H.-N.; Hsieh, C.-L. Effects of Sophora japonica flowers (Huaihua) on cerebral infarction. Chin. Med. 2010, 5, 34. [Google Scholar] [CrossRef]
- Matei, A.-M.; Caruntu, C.; Tampa, M.; Georgescu, S.R.; Matei, C.; Constantin, M.M.; Constantin, T.V.; Calina, D.; Ciubotaru, D.A.; Badarau, I.A.; et al. Applications of Nanosized-Lipid-Based Drug Delivery Systems in Wound Care. Appl. Sci. 2021, 11, 4915. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Liu, R.; Luo, C.; Pang, Z.; Zhang, J.; Ruan, S.; Wu, M.; Wang, L.; Sun, T.; Li, N.; Han, L. Advances of nanoparticles as drug delivery systems for disease diagnosis and treatment. Chin. Chem. Lett. 2023, 34, 107518. [Google Scholar] [CrossRef]
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 2005, 68, 66–74. [Google Scholar] [CrossRef]
- Isaacs, C.E.; Wen, G.Y.; Xu, W.; Jia, J.H.; Rohan, L.; Corbo, C.; Di Maggio, V.; Jenkins, E.C., Jr.; Hillier, S. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob. Agents Chemother. 2008, 52, 962–970. [Google Scholar] [CrossRef]
- Panara, A.; Gikas, E.; Tzavellas, I.; Thomaidis, N.S. Comprehensive HRMS Chemical Characterization of Pomegranate-Based Antioxidant Drinks via a Newly Developed Suspect and Target Screening Workflow. Molecules 2023, 28, 4986. [Google Scholar] [CrossRef]
- Zandi, K.; Teoh, B.-T.; Sam, S.-S.; Wong, P.-F.; Mustafa, M.R.; AbuBakar, S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011, 8, 560. [Google Scholar] [CrossRef]
- Bachmetov, L.; Gal-Tanamy, M.; Shapira, A.; Vorobeychik, M.; Giterman-Galam, T.; Sathiyamoorthy, P.; Golan-Goldhirsh, A.; Benhar, I.; Tur-Kaspa, R.; Zemel, R. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J. Viral Hepat. 2012, 19, e81–e88. [Google Scholar] [CrossRef]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Wang, Q.; Nanua, S.; Hershenson, M.B.; Sajjan, U.S. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antivir. Res. 2012, 94, 258–271. [Google Scholar] [CrossRef]
- Ieven, M.; Vlietinck, A.J.; Vanden Berghe, D.A.; Totte, J.; Dommisse, R.; Esmans, E.; Alderweireldt, F. Plant antiviral agents. III. Isolation of alkaloids from Clivia miniata Regel (Amaryllidaceae). J. Nat. Prod. 1982, 45, 564–573. [Google Scholar] [CrossRef]
- Ren, G.; Ding, G.; Zhang, H.; Wang, H.; Jin, Z.; Yang, G.; Han, Y.; Zhang, X.; Li, G.; Li, W. Antiviral activity of sophoridine against enterovirus 71 in vitro. J. Ethnopharmacol. 2019, 236, 124–128. [Google Scholar] [CrossRef]
- Zhang, X.; Hung, T.M.; Phuong, P.T.; Ngoc, T.M.; Min, B.S.; Song, K.S.; Seong, Y.H.; Bae, K. Anti-inflammatory activity of flavonoids from Populus davidiana. Arch. Pharm. Res. 2006, 29, 1102–1108. [Google Scholar] [CrossRef]
- Periferakis, A.; Caruntu, A.; Periferakis, A.T.; Scheau, A.E.; Badarau, I.A.; Caruntu, C.; Scheau, C. Availability, Toxicology and Medical Significance of Antimony. Int. J. Environ. Res. Public Health 2022, 19, 4669. [Google Scholar] [CrossRef]
- Thomas, E.; Stewart, L.E.; Darley, B.A.; Pham, A.M.; Esteban, I.; Panda, S.S. Plant-Based Natural Products and Extracts: Potential Source to Develop New Antiviral Drug Candidates. Molecules 2021, 26, 6197. [Google Scholar] [CrossRef] [PubMed]
- Frediansyah, A.; Sofyantoro, F.; Alhumaid, S.; Al Mutair, A.; Albayat, H.; Altaweil, H.I.; Al-Afghani, H.M.; AlRamadhan, A.A.; AlGhazal, M.R.; Turkistani, S.A.; et al. Microbial Natural Products with Antiviral Activities, Including Anti-SARS-CoV-2: A Review. Molecules 2022, 27, 4305. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, M.E.; Brierley, L.; McCaffery, C.; Lycett, S. Assessing the Epidemic Potential of RNA and DNA Viruses. Emerg. Infect. Dis. 2016, 22, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
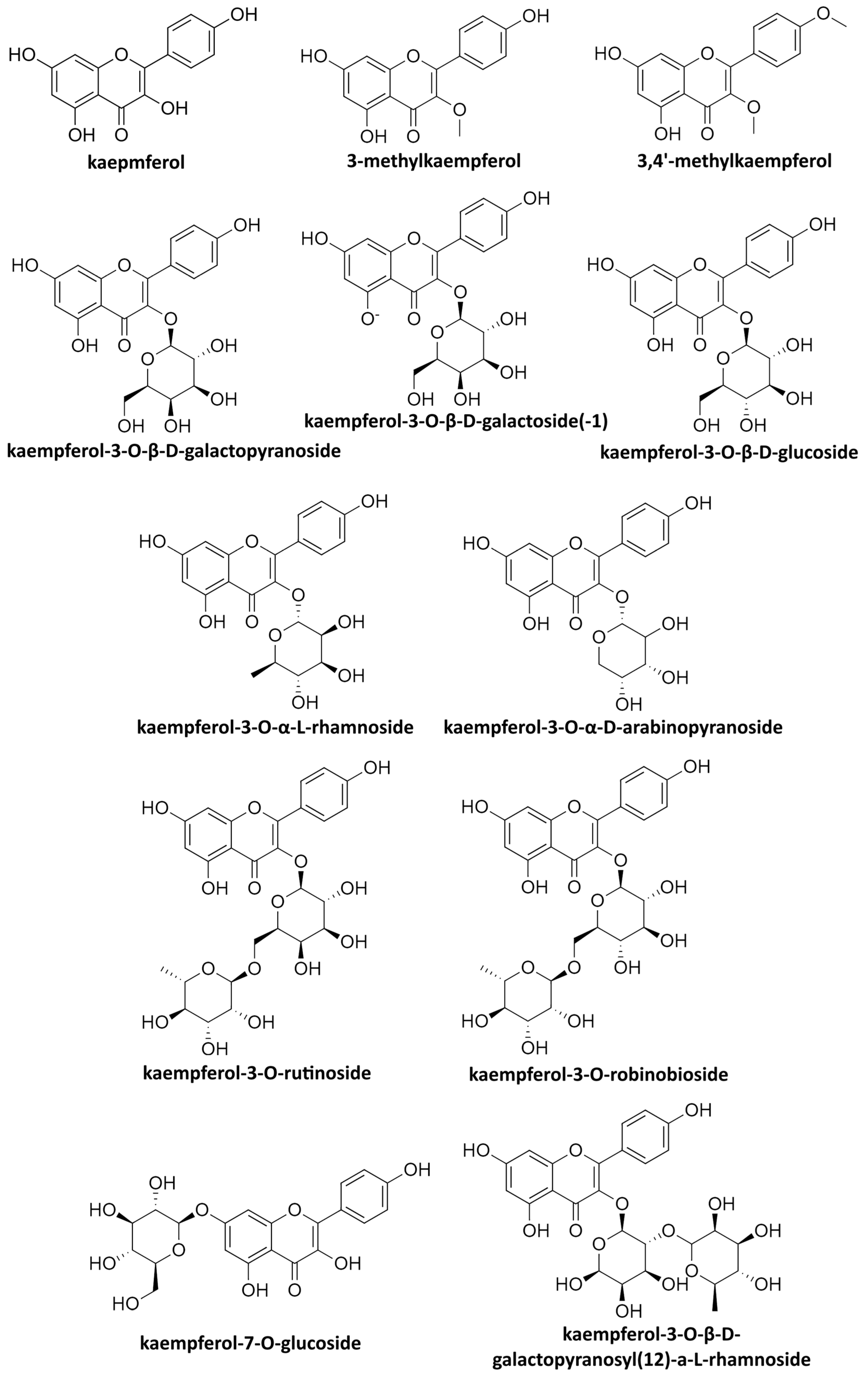

| Family | Genus | Extract from | Compound Tested | Toxicity Limit | Concentration (Type of Effect) | Mechanism | Year of Research | Reference |
|---|---|---|---|---|---|---|---|---|
| Hepadnaviridae | Hepatitis B virus (HBV) | Extract of Geranium carolianum L. | kaempferol | 160.79 μg/mL | 47.54 mg/kg (ED50) | Decrease in HBsAg and HBeAg and of viral DNA synthesis | 2008 | [65] |
| Leaf ethanol extract of Hippophae rhamnoides | kaempferol | 32.58 μg/mL (CC50 for SB-Chl) 150 μg/mL (SB-Eac, SB-But and SB-Aqu) | 10 μg/mL (stable concentration used in the experiment) | Inhibition of HBsAg and HBeAg expression | 2022 | [66] | ||
| Orthoherpesviridae (Alphaherpesvirinae) | Human herpesvirus 1virus 1 (HHV-1) | Extract from Ficus benjamina | kaempferol 3-O-rutinoside | 300 ± 2.79 μmol/L | 3.00 ± 0.97 μmol/L (EC50) | Unknown | 2012 | [67] |
| kaempferol 3-O-robinobioside | 600 ± 10.45 μmol/L | 0.90 ± 0.23 μmol/L (EC50) | ||||||
| Extracts of dried and powdered Securigera securidaca seeds | kaempferol | 60.0 ± 5.0 μg/mL (CC50) | 0.20 ± 0.12 μg/mL (EC50) | Inhibition of viral attachment and entry into cells; inhibition of viral polymerase | 2014 | [68] | ||
| kaempferol-7-O-glycoside | 250 ± 1.7 μg/mL (CC50) | 0.20 ± 0.01 μg/mL (EC50) | ||||||
| Human herpesvirus 2 (HHV-2) | Extract from Ficus benjamina | kaempferol 3-O-rutinoside | Unknown | n/a | n/a | 2012 | [67] | |
| kaempferol 3-O-robinobioside | Unknown | |||||||
| Varicella-Zoster Virus (VZV) | Pure compound | kaempferol | No cytotoxicity detected | 6.36 ± 0.73 μg/m (IC50) | Blockade of viral DNA synthesis and viral replication | 2022 | [69] | |
| Asfarviridae | African Swine Fever Virus (ASFV) | Pure compound | kaempferol | 93.10 μg/mL (CC50) | 2.20 μg/mL (IC50) | Unknown | 2021 | [70] |
| Orthoherpesviridae | Pseudorabies Virus (PRV) | Pure compound | kaempferol | 254.97 ± 1.86 μmol/L (CC50) | 25.57 ± 0.74 μmol/L (IC50) | Regulation of the MAPK and NF-κB pathways | 2021 | [71] |
| Family | Genus | Extract From | Compound Tested | Toxicity Limit | Concentration (Type) | Mechanism | Year of Research | Reference |
|---|---|---|---|---|---|---|---|---|
| Coronaviridae | SARS coronavirus (SARS-COV-2) | Pure compounds | Numerous kaempferol glycosides | Not calculated | 20 μΜ (minimum effective concentration) | Inhibition of the 3a membrane channel | 2014 | [103] |
| Pneumoviridae | Respiratory syncytial virus (RSV) | Extract from Eucalyptus citriodora | Kaempferol-3-O-β-D-glucopyranosyl (12)-α-L-rhamnoside | 137.60 μg/mL (CC50) | 57.30 μg/mL (IC50) | Reduction in virus multiplication | 2014 | [104] |
| Kaempferol-3-O-α-L-rhamnoside | 258.1 μg/mL (CC50) | 56.90 μg/mL (IC50) | ||||||
| Extract from Sophora japonica flowers | Kaempferol | 143.79 μg/mL (TC50) | 4.84 μg/mL (IC50) | Reduction in viral cytopathic effects | 2014 | [77] | ||
| Orthomyxoviridae | Influenza virus | Extract from Rhodiola rosea roots | Kaempferol | >300 μΜ | 18.50–30.20 μM (EC50 depending on viral strain) | Inhibition of neuraminidase | 2009 | [105] |
| Extract from Brazilian propolis | Kaempferol | >100 μg/mL | 21.70–38.20 μM (depending on viral strain) | Limitation of infection symptoms | 2014 | [106] | ||
| Extract from Eupatorium perfoliatum L. | Kaempferol-3-O-β-D-galactoside (trifolin) | Not calculated for individual compounds | Various effective concentrations | Prevention of viral attachment and entry into the cells | 2016 | [107] | ||
| Kaempferol-3-O-β-D-glucoside (astragalin) | ||||||||
| Retroviridae | Human immunodeficiency virus (HIV) | Extract from Securigera securidaca | Kaempferol | 320 μg/mL | 50 μg/mL (IC50) | Inhibition of reverse transcriptase | 2014 | [108] |
| Kaempferol-7-O-glycoside | 2500 μg/mL | 32 μg/mL (IC50) | ||||||
| Flaviviridae | Dengue fever virus (DFV) | Pure compound | Kaempferol | 228.50 Μμ (HEK293Τ/17 cells); 139.70 Μμ (BHK-21 cells) | None (not effective at tested concentration) | - | 2020 | [109] |
| Extract from Azadirachta indica | Kaempferol-3-O-rutinoside | Not significant | 10 μΜ (minimum tested concentration) | Inhibition of viral protease | 2021 | [110] | ||
| Japanese encephalitis virus | Pure compound | Kaempferol | 230 μΜ | 12.6–21.5 μM (depending on experimental conditions) | Inhibition of viral protein expression | 2012 | [111] | |
| Pure compound | Kaempferol | 228.50 Μμ (HEK293Τ/17 cells); 139.70 Μμ (BHK-21 cells) | 66.33 μM (EC50) | Probably inhibition of cap-dependent translation | 2020 | [109] | ||
| Picornaviridae | Enterovirus 71 | Pure compound | Kaempferol | 50 μΜ< | >35 μM (standard concentration used) | Inhibition of translation and replication | 2011 | [112] |
| Hepatitis A virus (HAV) | Pure compound | Kaempferol | Not determined | None (not effective at tested concentrations) | - | 2014 | [113] | |
| Extract from Ficus virens | Kaempferol-3-O-α-D-arabinopyranoside | 329.9 ± 5.3 μg/mL | None (not effective at tested concentrations) | - | 2016 | [114] | ||
| Kaempferol-3-O-β-D-galactopyranoside | 313.3 ± 1.19 μg/mL | None (not effective at tested concentrations) | ||||||
| Poliovirus | Extract from Psiadia dentata | 3-Methylkaempferol | 107 μΜ | Various tested concentration under different settings | Inhibition of the replication | 2001 | [115] | |
| 3,4′-Dimethylkaempferol | 197 μΜ | |||||||
| Togaviridae | Chikungunya virus (CHIKV) | Pure compound | Kaempferol | >1000 μg/mL (CC50 Vero cells); 537.30 μg/mL (CC50 BHK-21 cells) | 400 μΜ (concentration necessary for a degree of inhibition) | Inhibition of post-entry replication | 2015 | [116] |
| Caliciviridae | Feline calicivirus (FCV) | Pure compound | Kaempferol | >300 μΜ | 50 μΜ (minimum effective concentration) | Unknown | 2016 | [117] |
| Murine norovirus (MNV) | None (not effective at tested concentrations) | - |
| Virus | Compound Tested | Compound Effectiveness (Concentration) | Reference Drug | Drug Effectiveness (Concentration) | Reference |
|---|---|---|---|---|---|
| Hepatitis B virus | Kaempferol | 10 μg/mL (62.3% viral inhibition of HBsAg synthesis) | Lamivudine | 2 μΜ (87.4% viral inhibiton of HBsAg synthesis) | [66] |
| Human herpesvirus 1 | Kaempferol | 0.20 ± 0.01 μg/mL (EC50) | Acyclovir | 0.10 ± 0.01 μg/mL (EC50) | [68] |
| Kaempferol-7-O glycoside | 0.10 ± 0.01 μg/mL (EC50) | ||||
| Varicella-zoster | Kaempferol | 6.36 ± 0.73 µg/mL (IC50) | Acyclovir | 0.54 ± 0.12 µM (IC50) | [69] |
| Pseudorabies virus | Kaempferol | 25.57 μg/mL (IC50) | Acyclovir | 54.97 μg/mL (IC50) | [71] |
| Feline calicivirus | Kaempferol | 50–100 μΜ (tested concentrations) | Ribavirin | Higher effectiveness at the same concentrations | [117] |
| Influenza virus | Kaempferol | 21.70–38.20 μg/mL (EC50) | Ribavirin | 19.20 ± 7.5 μg/mL (EC50) | [106] |
| Respiratory syncytial virus | Kaempferol-3-O-β-D-glucopyranosyl (12)-α-L-rhamnoside | 57.30 μg/mL (IC50) | Ribavirin | 2.60 μg/mL (IC50) | [104] |
| Kaempferol-3-O-α-L-rhamnoside | 56.90 μg/mL (IC50) | Ribavirin | 2.60 μg/mL (IC50) | ||
| HIV | Kaempferol | 50 μg/mL (IC50) | Zidovudine | 1 μg/mL (IC50) | [108] |
| Kaempferol-7-O-glycoside | 32 μg/mL (IC50) |
| Plant | Medical Tradition | Traditional/Ethnobotanical Uses | References |
|---|---|---|---|
| Atermisia annua | Traditional Chinese medicine | Anti-hyperlipidaemic, anti-plasmodial, anti-convulsant, anti-inflammatory, antimicrobial, anti-cholesterolaemic and antiviral properties | [231,232] |
| Azadirachta indica | Traditional Chinese medicine, Ayurvedic medicine, Unani medicine | Antimicrobial and anti-inflammatory uses | [233,234,235] |
| Eucalyptus citriodora | African folk medicine (various geographical areas) | Anti-asthmatic, antifungal, general antimicrobial | [236,237,238,239] |
| Eupatorium perfoliatum | Native American folk medicine, European medical traditions | Antipyretic, antirheumatic agent and treatment of colds, anti-malarial agent and use as an antiviral agent | [240,241,242] |
| Ficus benjamina | Numerous local remedies in Asia, Africa, the Pacific islands and the Americas | Antimicrobial, antinociceptive, antipyretic and hypotensive uses; anti-dysentery remedy | [243,244,245,246] |
| Ficus virens | Traditional Indian medicine and Ayurveda | Prevention and treatment of diseases and various other reported medicinal effects | [247] |
| Geranium carolinianum L. | Traditional Chinese medicine | Antimicrobial, anti-inflammatory, and antipyretic uses | [248] |
| Hippophae rhamnoides | Local medical traditions in Russia and Asia, Austrian folk medicine | Treatment of hypertension, oedema, inflammation; tissue regeneration; treatment of burns, wounds, and ulcers | [249,250,251,252] |
| Ocotea notata | Folk medicine of South America | Treatment of chest pain, rheumatism wounds and viral infections | [253] |
| Psiadia dentata | Local African medical traditions, folk medicine of the Mascarene islands | Treatment of abdominal pains, colds, fevers, bronchitis, asthma, rheumatoid arthritis, skin infections and liver disorders | [254,255] |
| Rhodiola rosea | Traditional Chinese medicine, Viking folk medicine, various local medical traditions of Asian and European countries | Nervous system stimulation, stress and fatigue alleviation, treatment for gastrointestinal complaints, anaemia, infections, and impotence | [256,257,258] |
| Salvadora persica | Traditional Indian medicine, African folk medicine, medical tradition of Saudi Arabia | Antidote to poison, prevention of scurvy, treatment of rheumatism, anti-inflammatory use, treatment of skin conditions, purgative, treatment of gastrointestinal disorders, antimicrobial properties | [259,260] |
| Securigera securidaca | Traditional Iranian medicine, traditional Egyptian medicine, traditional Indian medicine | Anti-epileptic, anticonvulsant, and blood lipid-lowering actions | [261,262] |
| Sophora japonica | Traditional Chinese medicine, traditional Japanese medicine, traditional Korean medicine | Treatment of haemorrhoids, haematochezia, haematuria, hematemesis, haemorrhinia, uterine or intestinal haemorrhage, arteriosclerosis, headache, hypertension, dysentery, dizziness, and pyoderma | [263,264] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Periferakis, A.; Periferakis, A.-T.; Troumpata, L.; Periferakis, K.; Scheau, A.-E.; Savulescu-Fiedler, I.; Caruntu, A.; Badarau, I.A.; Caruntu, C.; Scheau, C. Kaempferol: A Review of Current Evidence of Its Antiviral Potential. Int. J. Mol. Sci. 2023, 24, 16299. https://doi.org/10.3390/ijms242216299
Periferakis A, Periferakis A-T, Troumpata L, Periferakis K, Scheau A-E, Savulescu-Fiedler I, Caruntu A, Badarau IA, Caruntu C, Scheau C. Kaempferol: A Review of Current Evidence of Its Antiviral Potential. International Journal of Molecular Sciences. 2023; 24(22):16299. https://doi.org/10.3390/ijms242216299
Chicago/Turabian StylePeriferakis, Argyrios, Aristodemos-Theodoros Periferakis, Lamprini Troumpata, Konstantinos Periferakis, Andreea-Elena Scheau, Ilinca Savulescu-Fiedler, Ana Caruntu, Ioana Anca Badarau, Constantin Caruntu, and Cristian Scheau. 2023. "Kaempferol: A Review of Current Evidence of Its Antiviral Potential" International Journal of Molecular Sciences 24, no. 22: 16299. https://doi.org/10.3390/ijms242216299
APA StylePeriferakis, A., Periferakis, A.-T., Troumpata, L., Periferakis, K., Scheau, A.-E., Savulescu-Fiedler, I., Caruntu, A., Badarau, I. A., Caruntu, C., & Scheau, C. (2023). Kaempferol: A Review of Current Evidence of Its Antiviral Potential. International Journal of Molecular Sciences, 24(22), 16299. https://doi.org/10.3390/ijms242216299











