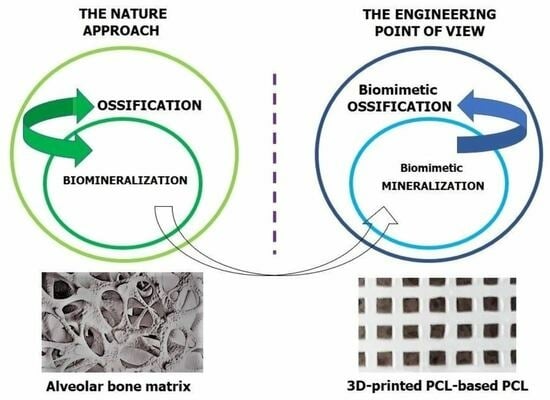
Towards Polycaprolactone-Based Scaffolds for Alveolar Bone Tissue Engineering: A Biomimetic Approach in a 3D Printing Technique
Abstract
1. Introduction
1.1. Bone Tissue Engineering (BTE)
1.2. Fused Filament Fabrication (FFF) vs. Other Three-Dimensional (3D) Printing Techniques
1.3. Polycaprolactone (PCL) vs. Other Polyesters
1.4. Hydroxyapatite (HA), Type I Collagen (CGI), and Chitosan (CS)
- It exhibits superior mechanical properties and a more streamlined purification process than silk fibroin [42].
- Its production is less costly and arises from more diverse sources than hyaluronic acid [43].
- CS surpasses bacterial cellulose in biodegradation within physiological environments and demonstrates enhanced osseointegration [44].
2. Alveolar Bone
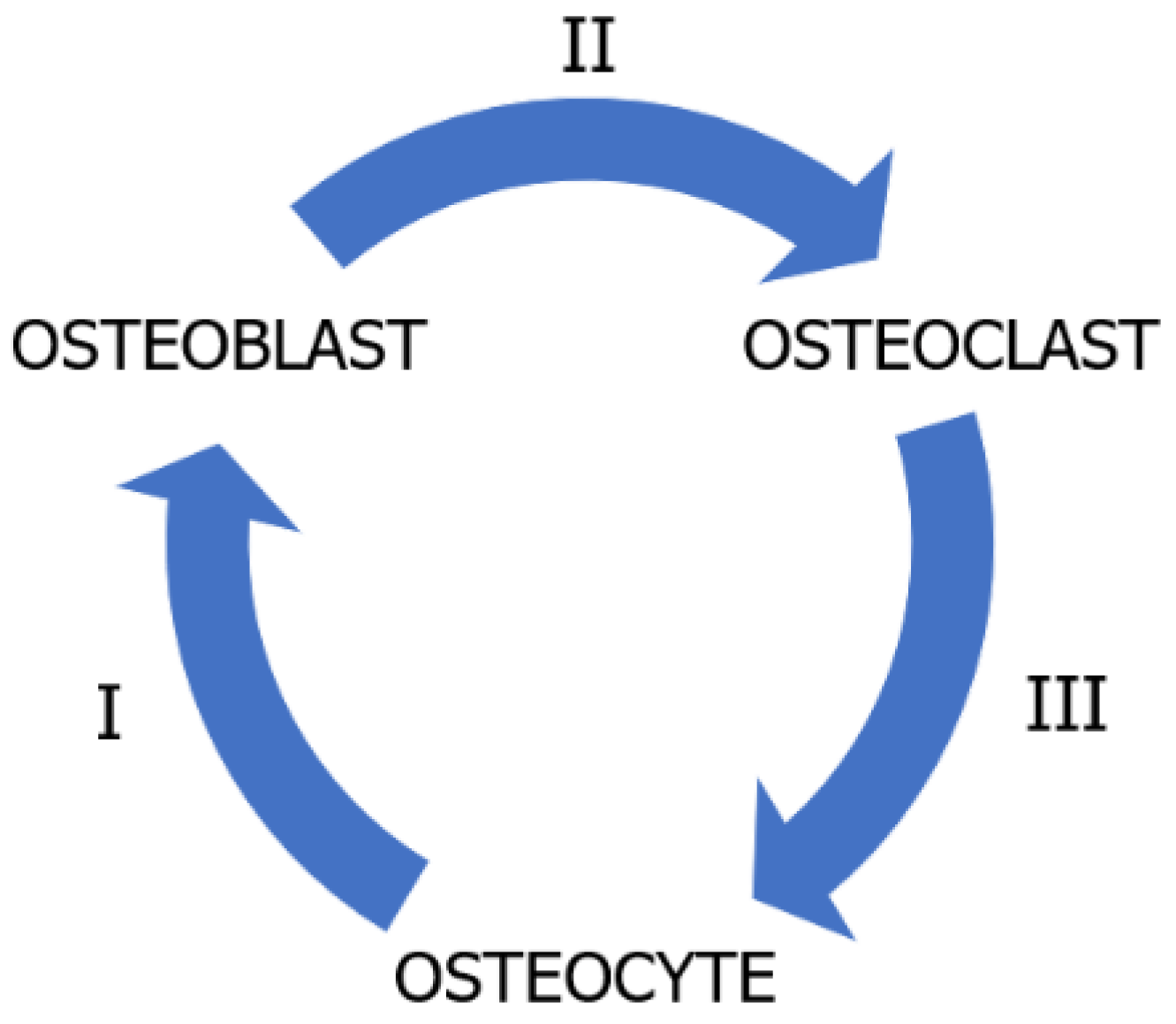
2.1. Ossification and Biomimetic Ossification
Mechanisms of Ossification
2.2. Biomineralisation and Biomimetic Mineralisation
2.2.1. Stages of Biomineralisation
2.2.2. Theories of Biomineralisation Mechanism
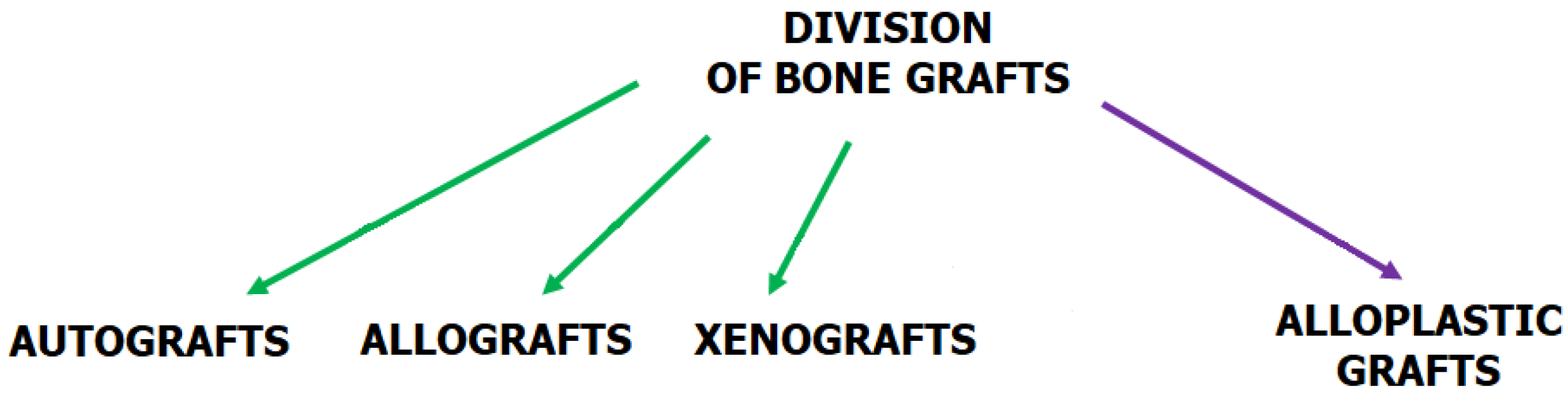
2.3. Requirements for Bone Tissue Engineering (BTE)
3. Material Candidates for Scaffolds
3.1. Polycaprolactone (PCL)
3.2. Hydroxyapatite (HA)
3.3. Natural Polymers
3.3.1. Type I Collagen (CGI)
3.3.2. Chitosan (CS)
4. Three-Dimensional (3D)-Printed PCL-Based Scaffold
4.1. Processing Conditions
4.1.1. Filament Processing
4.1.2. Three-Dimensional (3D) Printing
4.1.3. Hybrid Techniques
4.2. Structural and Mechanical Properties
4.2.1. Size and Geometry of Interconnected Pores
4.2.2. Mechanical Compressive Strength and Elastic Modulus
4.2.3. Mechanotransduction
4.3. Degradation Properties
- Material characteristics (degree of crystallinity, polydispersity and MW of PCL, amount and type of components with osteoinductive potential in the PCL matrix).
- Topological features (porosity, size and shape of pores, thickness of solid material).
4.3.1. Degradation Mechanism
4.3.2. Structural and Mechanical Integrity
4.4. Surface Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, H.J.; Jeon, J.Y.; Ahn, S.J.; Lee, S.W.; Chung, J.R.; Park, C.J.; Hwang, K.G. The preliminary study for three-dimensional alveolar bone morphologic characteristics for alveolar bone restoration. Maxillofac. Plast. Reconstr. Surg. 2019, 41, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Li, Q.; Zhang, D.; Zhang, X.; Qi, X.; Wang, Q.; Chen, Y.; Liu, C.; Li, H.; Zhang, S.; et al. Mapping the immune microenvironment for mandibular alveolar bone homeostasis at single-cell resolution. Bone Res. 2021, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology—Is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int. J. Implant Dent. 2017, 3, 23. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, H.-J.; Kim, K.-S.; Lee, S.J.; Lee, J.-T.; Kim, S.-Y.; Chang, N.-H.; Park, S.-Y. In Vivo Evaluation of 3D-Printed Polycaprolactone Scaffold Implantation Combined with β-TCP Powder for Alveolar Bone Augmentation in a Beagle Defect Model. Materials 2018, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, K.; Zhang, H. Biomimetic Mineralization: From Microscopic to Macroscopic Materials and Their Biomedical Applications. ACS Appl. Bio Mater. 2023, 6, 3516–3531. [Google Scholar] [CrossRef] [PubMed]
- Omi, M.; Mishina, Y. Roles of osteoclasts in alveolar bone remodeling. Genes. J. Genet. Dev. 2022, 60, e23490. [Google Scholar] [CrossRef]
- Guo, R.; Liwen, Z.; Menglong, H.; Huang, Y.; Weiran, L. Alveolar bone changes in maxillary and mandibular anterior teeth during orthodontic treatment: A systematic review and meta-analysis. Orthod. Craniofac. Res. 2021, 24, 165–179. [Google Scholar] [CrossRef]
- Lin, S.; Li, X.; Liu, H.; Wu, F.; Yang, L.; Su, Y.; Li, J.; Duan, S. Clinical applications of concentrated growth factors combined with bone substitutes for alveolar ridge preservation in maxillary molar area: A randomized controlled trial. Int. J. Implant Dent. 2021, 7, 115. [Google Scholar] [CrossRef]
- Hollý, D.; Klein, M.; Mazreku, M.; Zamborsk, R.; Polak, Š.; Danišovič, L. Stem Cells and Their DerivativesImplications for Alveolar Bone Regeneration: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 11746. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, X.; Guo, Q.; Wu, T.; Wang, Y. 3D Bioprinted Scaffolds for Tissue Repair and Regeneration. Front. Mater. 2022, 9, 925321. [Google Scholar] [CrossRef]
- Tejo-Otero, A.; Buj-Corral, I.; Fenollosa-Artes, F. 3D Printing in Medicine for Preoperative Surgical Planning: A Review. Ann. Biomed. Eng. 2020, 48, 536–555. [Google Scholar] [CrossRef] [PubMed]
- Tojeira, A.; Biscaia, S.S.; Viana, T.Q.; Sousa, I.S.; Mitchell, G.R. Controlling Morphology in 3D Printing. In Controlling the Morphology of Polymers: Multiple Scales of Structure and Processing; Springer: Cham, Switzerland, 2016; pp. 181–207. ISBN 9783319393223. [Google Scholar]
- Haleem, A.; Javaid, M.; Hasan, R.; Suman, R. 3D printing applications in bone tissue engineering. J. Clin. Orthop. Trauma 2020, 11, S118–S124. [Google Scholar] [CrossRef] [PubMed]
- Pitjamit, S.; Thunsiri, K.; Nakkiew, W.; Wongwichai, T.; Pothacharoen, P.; Wattanutchariya, W. The Possibility of Interlocking Nail Fabrication from FFF 3D Printing PLA/PCL/HA Composites Coated by Local Silk Fibroin for Canine Bone Fracture Treatment. Materials 2020, 13, 1564. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.G.; Han, K.S.; Lee, S.; Kim, M.C.; Kim, S.Y.; Nah, J. Fabrication of Biocompatible Polycaprolactone—Hydroxyapatite Composite Filaments for the FDM 3D Printing of Bone Scaffolds. Appl. Sci. 2021, 11, 6351. [Google Scholar] [CrossRef]
- Wang, F.; Tankus, E.B.; Santarella, F.; Rohr, N.; Sharma, N.; Märtin, S.; Michalscheck, M.; Maintz, M.; Cao, S.; Thieringer, F.M. Fabrication and Characterization of PCL/HA Filament as a 3D Printing Material Using Thermal Extrusion Technology for Bone Tissue Engineering. Polymers 2022, 14, 669. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Lee, M.; Tsai, W.; Wang, H.; Lu, W. Osteogenesis of adipose-derived stem cells on polycaprolactone—β-tricalcium phosphate scaffold fabricated via selective laser sintering and surface coating with collagen type I. J. Tissue Eng. Regen. Med. 2016, 10, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Janmohammadi, M.; Nourbakhsh, M.S.; Bahraminasab, M.; Tayebi, L. Effect of Pore Characteristics and Alkali Treatment on the Physicochemical and Biological Properties of a 3D-Printed Polycaprolactone Bone Scaffold. ACS Omega 2023, 8, 7378–7394. [Google Scholar] [CrossRef]
- Lin, Y.M.; Chen, H.; Lin, C.H.; Huang, P.J.; Lee, S.Y. Development of polycaprolactone/hydroxyapatite composite resin for 405 nm digital light projection 3D printing. Rapid Prototyp. J. 2020, 26, 951–958. [Google Scholar] [CrossRef]
- Joseph, B.; James, J.; Grohens, Y.; Kalarikkal, N.; Thomas, S. Additive Manufacturing of Poly (ε-Caprolactone) for Tissue Engineering. JOM 2020, 72, 4127–4138. [Google Scholar] [CrossRef]
- Freeman, F.E.; Pitacco, P.; Van Dommelen, L.H.A.; Nulty, J.; Browe, D.C.; Shin, J.; Alsberg, E.; Kelly, D.J. 3D bioprinting spatiotemporally defined patterns of growth factors to tightly control tissue regeneration. Sci. Adv. 2020, 6, eabb5093. [Google Scholar] [CrossRef]
- Rezania, N.; Asadi-Eydivand, M.; Abolfathi, N.; Bonakdar, S.; Mehrjoo, M.; Solati-Hashjin, M. Three-dimensional printing of polycaprolactone/hydroxyapatite bone tissue engineering scaffolds mechanical properties and biological behavior. J. Mater. Sci. Mater. Med. 2022, 33, 31. [Google Scholar] [CrossRef] [PubMed]
- Bayart, M.; Dubus, M.; Charlon, S.; Kerdjoudj, H.; Baleine, N.; Benali, S.; Raquez, J.M.; Soulestin, J. Pellet-Based Fused Filament Fabrication (FFF)-Derived Process for the Development of Polylactic Acid/Hydroxyapatite Scaffolds Dedicated to Bone Regeneration. Materials 2022, 15, 5615. [Google Scholar] [CrossRef] [PubMed]
- Åkerlund, E.; Diez-Escudero, A.; Grzeszczak, A.; Persson, C. The Effect of PCL Addition on 3D-Printable PLA/HA Composite Filaments for the Treatment of Bone Defects. Polymers 2022, 14, 3305. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.S.; Cabral, J.M.S. Bone Matrix Non-Collagenous Proteins in Tissue Engineering: Creating New Bone by Mimicking the Extracellular Matrix. Polymers 2021, 13, 1095. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Nguyen, T.; Cho, Y.D.; Kavanagh, N.M.; Ghassib, I.; Giannobile, W.V. Personalized scaffolding technologies for alveolar bone regenerative medicine. Orthod. Craniofac. Res. 2019, 22, 69–75. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Journal of Oral Biology and Craniofacial Research Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofac. Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Antheunis, H.; Van Der Meer, J.C.; De Geus, M.; Heise, A.; Koning, C.E. Autocatalytic equation describing the change in molecular weight during hydrolytic degradation of aliphatic polyesters. Biomacromolecules 2010, 11, 1118–1124. [Google Scholar] [CrossRef]
- Yeo, T.; Ko, Y.G.; Kim, E.J.; Kwon, O.K.; Chung, H.Y.; Kwon, O.H. Promoting bone regeneration by 3D-printed poly(glycolic acid)/hydroxyapatite composite scaffolds. J. Ind. Eng. Chem. 2021, 94, 343–351. [Google Scholar] [CrossRef]
- Mondal, S.; Nguyen, T.P.; Pham, V.H.; Hoang, G.; Manivasagan, P.; Kim, M.H.; Nam, S.Y.; Oh, J. Hydroxyapatite nano bioceramics optimized 3D printed poly lactic acid scaffold for bone tissue engineering application. Ceram. Int. 2020, 46, 3443–3455. [Google Scholar] [CrossRef]
- Sung, H.J.; Meredith, C.; Johnson, C.; Galis, Z.S. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials 2004, 25, 5735–5742. [Google Scholar] [CrossRef]
- Murphy, S.H.; Leeke, G.A.; Jenkins, M.J. A Comparison of the use of FTIR spectroscopy with DSC in the characterisation of melting and crystallisation in polycaprolactone. J. Therm. Anal. Calorim. 2012, 107, 669–674. [Google Scholar] [CrossRef]
- Daskalakis, E.; Hassan, M.H.; Omar, A.M.; Acar, A.A.; Fallah, A.; Cooper, G.; Weightman, A.; Blunn, G.; Koc, B.; Bartolo, P. Accelerated Degradation of Poly-ε-caprolactone Composite Scaffolds for Large Bone Defects. Polymers 2023, 15, 670. [Google Scholar] [CrossRef]
- Li, M.; Jia, W.; Zhang, X.; Weng, H.; Gu, G.; Chen, Z. Hyaluronic acid oligosaccharides modified mineralized collagen and chitosan with enhanced osteoinductive properties for bone tissue engineering. Carbohydr. Polym. 2021, 260, 117780. [Google Scholar] [CrossRef] [PubMed]
- Ghayor, C.; Bhattacharya, I.; Weber, F.E. The optimal microarchitecture of 3D-printed β-TCP bone substitutes for vertical bone augmentation differs from that for osteoconduction. Mater. Des. 2021, 204, 109650. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.; Ran, J.; Shen, X.; Tong, H. Synthesis and Cytocompatibility of Collagen/Hydroxyapatite Nanocomposite Scaffold for Bone Tissue Engineering. Polym. Compos. 2014, 37, 81–90. [Google Scholar] [CrossRef]
- Alkhursani, S.A.; Ghobashy, M.M.; Al-gahtany, S.A.; Meganid, A.S.; El-halim, S.M.A.; Ahmad, Z.; Khan, F.S.; Abdel, G.; Atia, N.; Cavalu, S. Application of Nano-Inspired Scaffolds-Based Biopolymer Hydrogel for Bone and Periodontal Tissue Regeneration. Polymers 2022, 14, 3791. [Google Scholar] [CrossRef]
- Neves, M.I.; Ara, M.; Moroni, L.; Silva, R.M.P.; Barrias, C.C. Glycosaminoglycan-Inspired Biomaterials for the Development of Bioactive Hydrogel Networks. Molecules 2020, 25, 978. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications—A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Fan, T.; Zhang, Y.; Zhao, Y.; Shi, X.; Zhang, Q. Biomimetic mineralized hierarchical hybrid scaffolds based on in situ synthesis of nano-hydroxyapatite/chitosan/chondroitin sulfate/hyaluronic acid for bone tissue engineering. Colloids Surf. B Biointerfaces 2017, 157, 93–100. [Google Scholar] [CrossRef]
- Deshpande, R.; Shukla, S.; Sayyad, R.; Salunke, S.; Nisal, A.; Venugopalan, P. Silk fibroin and ceramic scaffolds: Comparative in vitro studies for bone regeneration. Bioeng. Transl. Med. 2021, 6, e10221. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Rodríguez-Amado, I.; Montemayor, M.I.; Fraguas, J.; González, M.D.P.; Murado, M.A. Chondroitin Sulfate, Hyaluronic Acid and Chitin/Chitosan Production Using Marine Waste Sources: Characteristics, Applications and Eco-Friendly Processes: A Review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef] [PubMed]
- Swanson, T.E.; Cheng, X.; Friedrich, C. Development of chitosan—Vancomycin antimicrobial coatings on titanium implants. J. Biomed. Mater. Res. Part A 2011, 97A, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xia, L.; Leng, X.; Chen, Y.; Zhang, L.; Ni, X.; Luo, J.; Leng, W. Improved repair of rabbit calvarial defects with hydroxyapatite/chitosan/polycaprolactone composite scaffold-engrafted EPCs and BMSCs. Front. Bioeng. Biotechnol. 2022, 10, 928041. [Google Scholar] [CrossRef]
- Hokmabad, V.R.; Davaran, S.; Aghazadeh, M.; Alizadeh, E.; Salehi, R.; Ramazani, A. A Comparison of the Effects of Silica and Hydroxyapatite Nanoparticles on Poly(ε-caprolactone)-Poly(ethylene glycol)-Poly(ε-caprolactone)/Chitosan Nanofibrous Scaffolds for Bone Tissue Engineering. Tissue Eng. Regen. Med. 2018, 15, 735–750. [Google Scholar] [CrossRef]
- Ressler, A. Chitosan-Based Biomaterials for Bone Tissue Engineering Applications: A Short Review. Polymers 2022, 14, 3430. [Google Scholar] [CrossRef]
- Guo, L.; Liang, Z.; Yang, L.; Du, W.; Yu, T.; Tang, H.; Li, C. The role of natural polymers in bone tissue engineering. J. Control. Release 2021, 338, 571–582. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhou, Y.; Chen, J. The Application of Polycaprolactone in Three-Dimensional Printing Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 2754. [Google Scholar] [CrossRef]
- Tsuchida, S.; Nakayama, T. Recent Clinical Treatment and Basic Research on the Alveolar Bone. Biomedicines 2023, 11, 843. [Google Scholar] [CrossRef]
- Wang, H.; Li, T.; Wang, X.; Guan, Y.; Jiang, Y.; Chen, S.; Zou, S.; Duan, P. Mechanisms of sphingosine-1-phosphate (S1P) signaling on excessive stress-induced root resorption during orthodontic molar intrusion. Clin. Oral Investig. 2022, 26, 1003–1016. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kaito, T.; Furuya, M.; Seno, S. In vivo dynamic analysis of BMP-2-induced ectopic bone formation. Sci. Rep. 2020, 10, 4751. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-Q.; Yong, X.-K.W.; Wang, Z.Z.; Wang, Z.; Jin, L.; Yin, H.; Xia, K.; Feng, Y.T.S.; Tang, P.X.S.; Jia, C.F.; et al. H-type blood vessels participate in alveolar bone remodeling during murine tooth extraction healing. Oral Dis. 2020, 26, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, W.; Qian, C.; Xiao, W.; Zhu, H.; Guo, J. Advanced biomaterials for repairing and reconstruction of mandibular defects. Mater. Sci. Eng. C 2019, 103, 109858. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nakayamada, S.; Okada, Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Proff, P.; Römer, P. The molecular mechanism behind bone remodelling: A review. Clin. Oral Investig. 2009, 13, 355–362. [Google Scholar] [CrossRef]
- Xiong, J.; O’Brien, C.A. Osteocyte RANKL: New Insights into the Control of Bone Remodeling. J. Bone Miner. Res. 2012, 27, 499–505. [Google Scholar] [CrossRef]
- Bernhard, J.C.; Marolt Presen, D.; Li, M.; Monforte, X.; Ferguson, J.; Leinfellner, G.; Heimel, P.; Betti, S.L.; Shu, S.; Teuschl-Woller, A.H.; et al. Effects of Endochondral and Intramembranous Ossification Pathways on Bone Tissue Formation and Vascularization in Human Tissue-Engineered Grafts. Cells 2022, 11, 3070. [Google Scholar] [CrossRef]
- Rodrigues, G.; Florencio-Silva, R.; Sasso-Cerri, E.; Paulo, S.; Damas, C.; Jesus, M. De Spatio-temporal immunolocalization of VEGF-A, Runx2, and osterix during the early steps of intramembranous ossification of the alveolar process in rat embryos. Dev. Biol. 2021, 478, 133–143. [Google Scholar] [CrossRef]
- Mackie, E.J.; Ahmed, Y.A.; Tatarczuch, L.; Chen, K.; Mirams, M. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 2008, 40, 46–62. [Google Scholar] [CrossRef]
- Isaksson, H.; Wilson, W.; van Donkelaar, C.C.; Huiskes, R.; Ito, K. Comparison of biophysical stimuli for mechano-regulation of tissue differentiation during fracture healing. J. Biomech. 2006, 39, 1507–1516. [Google Scholar] [CrossRef]
- Shu, H.S.; Liu, Y.L.; Tang, X.T.; Zhang, X.S.; Zhou, B.; Zou, W.; Zhou, B.O.; Shu, H.S.; Liu, Y.L.; Tang, X.T.; et al. Tracing the skeletal progenitor transition during postnatal bone formation. Cell Stem Cell 2021, 28, 2122–2136.e3. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.W.; Tan, Z.; To, M.K.T.; Chan, D. Regulation and Role of Transcription Factors in Osteogenesis. Int. J. Mol. Sci. 2021, 22, 5445. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, S.; Liu, H.; Ru, K.; Jia, Y.; Wu, Z. Piezo Channels: Awesome Mechanosensitive Structures in Cellular Mechanotransduction and Their Role in Bone. Int. J. Mol. Sci. 2021, 22, 6429. [Google Scholar] [CrossRef] [PubMed]
- Foley, B.; Selmane, M.; Mezzetti, A.; Lefebvre, C.; El Kirat, K.; Landoulsi, J. Tunable Enzyme-Assisted Mineralization of Apatitic Calcium Phosphate by Homogeneous Catalysis. Int. J. Mol. Sci. 2023, 24, 43. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chemie Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Dong, L.; Wang, S.-J.; Zhao, X.-R.; Zhu, Y.-F.; Yu, J.-K. 3D-Printed Poly(ε-caprolactone) Scaffold Integrated with Cell-laden Chitosan Hydrogels for Bone Tissue Engineering. Sci. Rep. 2017, 7, 13412. [Google Scholar] [CrossRef]
- Cai, X.; Tong, H.; Shen, X.; Chen, W.; Yan, J.; Hu, J. Preparation and characterization of homogeneous chitosan—Polylactic acid/hydroxyapatite nanocomposite for bone tissue engineering and evaluation of its mechanical properties. Acta Biomater. 2009, 5, 2693–2703. [Google Scholar] [CrossRef]
- Kunitomi, Y.; Hara, E.S.; Okada, M.; Nagaoka, N.; Kuboki, T.; Nakano, T.; Kamioka, H.; Matsumoto, T. Biomimetic mineralization using matrix vesicle nanofragments. J. Biomed. Mater. Res. Part A 2019, 107A, 1021–1030. [Google Scholar] [CrossRef]
- Moradian-Oldak, J.; George, A. Biomineralization of Enamel and Dentin Mediated by Matrix Proteins. J. Dent. Res. 2021, 100, 1020–1029. [Google Scholar] [CrossRef]
- Bozycki, L.; Mroczek, J.; Bessueille, L.; Mebarek, S.; Buchet, R.; Pikula, S.; Strzelecka-Kiliszek, A. Annexins A2, A6 and Fetuin—A Affect the Process of Mineralization in Vesicles Derived from Human Osteoblastic. Int. J. Mol. Sci. 2021, 22, 3993. [Google Scholar] [CrossRef]
- Anderson, H.C. Matrix Vesicles and Calcification. Curr. Rheumatol. Rep. 2017, 5, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T. Ultrastructure and biological function of matrix vesicles in bone mineralization. Histochem. Cell Biol. 2018, 149, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wei, M. Biomineralization of Collagen-Based Materials for Hard Tissue Repair. Int. J. Mol. Sci. 2021, 22, 944. [Google Scholar] [CrossRef]
- De-Leon, S.B.-T. The Evolution of Biomineralization through the Co-Option of Organic Scaffold Forming Networks. Cells 2022, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Thang, L.H.; Bang, L.T.; Long, B.D.; Son, N.A.; Ramesh, S. Effect of Carbonate Contents on the Thermal Stability and Mechanical Properties of Carbonated Apatite Artificial Bone Substitute with Different Carbonate Ion Sources. J. Mater. Eng. Perform. 2023, 32, 1006–1016. [Google Scholar] [CrossRef]
- Olszta, M.J.; Cheng, X.; Soo, S.; Kumar, R.; Kim, Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Le Cann, S.; Törnquist, E.; Silva, I.; Fraulob, M.; Albini, H.; Verezhak, M.; Guizar-sicairos, M.; Isaksson, H.; Haïat, G. Acta Biomaterialia Spatio-temporal evolution of hydroxyapatite crystal thickness at the bone-implant interface. Acta Biomater. 2020, 116, 391–399. [Google Scholar] [CrossRef]
- Wang, L.; Ciani, C.; Doty, S.B.; Fritton, S.P. Delineating bone’s interstitial fluid pathway in vivo. Bone 2004, 34, 499–509. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Best, S.M.; Duer, M.J.; Reid, D.G.; Wise, R. Towards a model of the mineral—Organic interface in bone: NMR of the structure of synthetic glycosaminoglycan- and polyaspartate-calcium phosphate composites. Magn. Reson. Chem. 2008, 46, 323–329. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A. In fluence of glycosaminoglycans on the properties of thin films based on chitosan/collagen blends. J. Mech. Behav. Biomed. Mater. 2018, 80, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Menezes, R.; Vincent, R.; Osorno, L.; Hu, P.; Livingston, T. Biomaterials and tissue engineering approaches using glycosaminoglycans for tissue repair: Lessons learned from the native extracellular matrix. Acta Biomater. 2023, 163, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Wojtas, M.; Lausch, A.J.; Sone, E.D. Glycosaminoglycans accelerate biomimetic collagen mineralization in a tissue-based in vitro model. Proc. Natl. Acad. Sci. USA 2020, 117, 12636–12642. [Google Scholar] [CrossRef]
- Hua, R.; Ni, Q.; Eliason, T.D.; Han, Y.; Gu, S. Biglycan and chondroitin sulfate play pivotal roles in bone toughness via retaining bound water in bone mineral matrix. Matrix Biol. 2020, 94, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Melbouci, M.; Mason, R.W.; Suzuki, Y.; Fukao, T.; Orii, T.; Tomatsu, S. Review: Growth Impairment in Mucopolysaccharidoses. Mol. Genet. Metab. 2018, 124, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nakamura-utsunomiya, A. Bone Biomarkers in Mucopolysaccharidoses. Int. J. Mol. Sci. 2021, 22, 12651. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, A.; Hu, G.; Bian, M.; Chen, L.; Zhao, Q.; Sun, W.; Wu, Y. Potential of an Aligned Porous Hydrogel Scaffold Combined with Periodontal Ligament Stem Cells or Gingival Mesenchymal Stem Cells to Promote Tissue Regeneration in Rat Periodontal Defects. ACS Biomater. Sci. Eng. 2023, 9, 1961–1975. [Google Scholar] [CrossRef]
- Waddington, R.J.; Embery, G.; Last, K. Glycosaminoglycans of human alveolar bone. Archs Oral Biol. 1989, 34, 587–589. [Google Scholar] [CrossRef]
- He, H.; Shao, C.; Mu, Z.; Mao, C.; Sun, J.; Chen, C.; Tang, R.; Gu, X. Promotion effect of immobilized chondroitin sulfate on intra fibrillar mineralization of collagen. Carbohydr. Polym. 2020, 229, 115547. [Google Scholar] [CrossRef]
- Hao, J.; Wan, Q.; Mu, Z.; Gu, J.; Yu, W.; Qin, W.; Li, Y.; Wang, C.; Ma, Y.; Jiao, K.; et al. A seminal perspective on the role of chondroitin sulfate in biomineralization. Carbohydr. Polym. 2023, 310, 120738. [Google Scholar] [CrossRef]
- Tatara, Y.; Suto, S.; Itoh, K. Novel roles of glycosaminoglycans in the degradation of type I collagen by cathepsin K. Glycobiology 2017, 27, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.R.; Evans, E.B.; Matuszewski, P.E.; Chen, Y.; Satchel, L.N.; Elliott, D.M.; Soslowsky, L.J.; Dodge, G.R. Distributions of types I, II and III collagen by region in the human supraspinatus supraspinatus tendon. Connect. Tissue Res. 2013, 54, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Sorvina, A.; Antoniou, M.; Esmaeili, Z.; Kochetkova, M. Unusual Suspects: Bone and Cartilage ECM Proteins as Carcinoma Facilitators. Cancers 2023, 15, 791. [Google Scholar] [CrossRef]
- Holwerda, A.M.; van Loon, L.J.C. The impact of collagen protein ingestion on musculoskeletal connective tissue remodeling: A narrative review. Nutr. Rev. 2021, 80, 1497–1514. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.M.; Li, W.T.; Xia, X.M.; Wang, F.; Macdougall, M.; Chen, S. Dentine Sialophosphoprotein Signal in Dentineogenesis and Dentine Regeneration. Eur. Cell Mater. 2021, 42, 43–62. [Google Scholar] [CrossRef]
- Yang, H.; Fan, Z. The functional extracellular matrix on the regulation of odontogenic differentiation of stem cells. Curr. Med. 2022, 1, 10. [Google Scholar] [CrossRef]
- Combes, C.; Cazalbou, S.; Rey, C. Apatite Biominerals. Minerals 2016, 6, 34. [Google Scholar] [CrossRef]
- Lawrence, L.M.; Salary, R.R.; Miller, V.; Valluri, A.; Denning, K.L.; Case-perry, S.; Abdelgaber, K.; Smith, S.; Claudio, P.P.; Day, J.B. Osteoregenerative Potential of 3D-Printed Poly ε-Caprolactone Tissue Scaffolds In Vitro Using Minimally Manipulative Expansion of Primary Human Bone Marrow Stem Cells. Int. J. Mol. Sci. 2023, 24, 4940. [Google Scholar] [CrossRef]
- Moghaddaszadeh, A.; Seddiqi, H.; Najmoddin, N.; Ravasjani, S.A.; Klein-Nulend, J. Biomimetic 3D-printed PCL scaffold containing a high concentration carbonated-nanohydroxyapatite with immobilized-collagen for bone tissue engineering: Enhanced bioactivity and physicomechanical characteristics. Biomed. Mater. 2021, 16, 65029. [Google Scholar] [CrossRef]
- Yadav, N.; Mudgal, D.; Anand, R.; Jindal, S.; Mishra, V. Recent development in nanoencapsulation and delivery of natural bioactives through chitosan scaffolds for various biological applications. Int. J. Biol. Macromol. 2022, 220, 537–572. [Google Scholar] [CrossRef]
- Egorov, A.; Riedel, B.; Vinke, J.; Schmal, H.; Thomann, R.; Thomann, Y.; Seidenstuecker, M. The Mineralization of Various 3D-Printed PCL Composites. J. Funct. Biomater. 2022, 13, 238. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, Z.; Irani, S.; Ardeshirylajimi, A.; Seyedjafari, E. Enhanced osteogenic differentiation of stem cells by 3D printed PCL scaffolds coated with collagen and hydroxyapatite. Sci. Rep. 2022, 12, 12359. [Google Scholar] [CrossRef] [PubMed]
- Daculsi, G.; Fellah, B.H.; Miramond, T.; Durand, M. Osteoconduction, Osteogenicity, Osteoinduction, what are the fundamental properties for a smart bone substitutes. IRBM 2013, 34, 346–348. [Google Scholar] [CrossRef]
- Gomes-Araújo, N.; Gavilán-Romero, F.; Arnáez-García, I.; Ramos-Martínez, C.; Pérez-Sánchez, A.M. Osseointegration mechanisms: A proteomic approach. JBIC J. Biol. Inorg. Chem. 2018, 23, 459–470. [Google Scholar] [CrossRef]
- Rather, H.A.; Jhala, D.; Vasita, R. Dual functional approaches for osteogenesis coupled angiogenesis in bone tissue engineering. Mater. Sci. Eng. C 2019, 103, 109761. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zhang, J.; Zhang, C.; Barbieri, D.; Yuan, H.; Moroni, L.; Feng, G. The role of calcium phosphate surface structure in osteogenesis and the mechanisms involved. Acta Biomater. 2020, 106, 22–33. [Google Scholar] [CrossRef]
- Ghayor, C.; Weber, F.E. Osteoconductive microarchitecture of bone substitutes for bone regeneration revisited. Front. Physiol. 2018, 9, 960. [Google Scholar] [CrossRef]
- Weber, F.E. Reconsidering Osteoconduction in the Era of Additive Manufacturing. Tissue Eng. Part B Rev. 2019, 25, 375–386. [Google Scholar] [CrossRef]
- Barrere, F.; van der Valk, C.M.; Dalmeijer, R.A.J.; Meijer, G.; Van Blitterswijk, C.A.; De Groot, K.; Layrolle, P. Osteogenecity of octacalcium phosphate coatings applied on porous metal implants. J. Biomed. Mater. Res. Part A 2002, 66A, 792–889. [Google Scholar] [CrossRef]
- Yousefiasl, S.; Sharifi, E.; Salahinejad, E.; Makvandi, P.; Irani, S.; Cam, C.A.D. Bioactive 3D-printed chitosan-based scaffolds for personalized craniofacial bone tissue engineering. Eng. Regen. 2023, 4, 1–11. [Google Scholar] [CrossRef]
- Srividya, S.; Sridevi, G.; Kumar, B.S.; Sastry, T.P. Biocatalysis and Agricultural Biotechnology Fabrication, characterization and osseointegration of bonegraft incorporated with leaf extracts of Ormocarpum Sennoides and biocompatible polymers. Biocatal. Agric. Biotechnol. 2018, 15, 92–102. [Google Scholar] [CrossRef]
- Chen, X.; Fan, H.; Deng, X.; Wu, L.; Yi, T.; Gu, L.; Zhou, C.; Fan, Y.; Zhang, X. Scaffold Structural Microenvironmental Cues to Guide Tissue Regeneration in Bone Tissue Applications. Nanomaterials 2018, 8, 960. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Zhu, J.; Deng, D.; Jin, S.; Li, J.; Man, Y. Enhanced osteogenesis and angiogenesis by PCL/chitosan/Sr-doped calcium phosphate electrospun nanocomposite membrane for guided bone regeneration. J. Biomater. Sci. 2019, 30, 1505–1522. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, O.; Hamai, R.; Sakai, S. The material design of octacalcium phosphate bone substitute: Increased dissolution and osteogenecity. Acta Biomater. 2023, 158, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Rios, H.F.; Giannobile, W.V.; Oh, T. Periodontal Regeneration: Current Therapies; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780123971579. [Google Scholar]
- Oryan, A.; Hassanajili, S.; Sahvieh, S. Effectiveness of a biodegradable 3D polylactic acid/poly(ε-caprolactone)/hydroxyapatite scaffold loaded by differentiated osteogenic cells in a critical-sized radius bone defect in rat. J. Tissue Eng. Regen. Med. 2021, 15, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.; Xu, H.H.K. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells 2019, 8, 537. [Google Scholar] [CrossRef]
- Naik, C.; Srinath, N.; Ranganath, M.K.; Umashankar, D.N.; Gupta, H. Evaluation of polycaprolactone scaffold for guided bone regeneration in maxillary and mandibular defects: A clinical study. Natl. J. Maxillofac. Surg. 2020, 11, 207–212. [Google Scholar] [CrossRef]
- Fan, Y.; Cui, C.; Rosen, C.J.; Sato, T.; Xu, R.; Li, P.; Wei, X.; Bi, R.; Yuan, Q. Klotho in Osx+-mesenchymal progenitors exerts pro-osteogenic and anti-inflammatory effects during mandibular alveolar bone formation and repair. Signal Transduct. Target. Ther. 2022, 7, 155. [Google Scholar] [CrossRef]
- Yoshida, M.; Turner, P.R.; John, C.; Azam, M.; Jaydee, A. A comparison between β-tricalcium phosphate and chitosan poly-caprolactone-based 3D melt extruded composite scaffolds. Biopolymers 2022, 113, e23482. [Google Scholar] [CrossRef]
- Gerdes, S.; Mostafavi, A.; Ramesh, S.; Memic, A.; Rivero, I.V.; Rao, P.; Tamayol, A. Bone Process–Structure–Quality Relationships of Three-Dimensional Printed Poly(Caprolactone)-Hydroxyapatite Scaffolds. Tissue Eng. Part A 2020, 26, 279–291. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, Y. Preparation and characterization of a novel polylactic acid/hydroxyapatite composite scaffold with biomimetic micro-nano fibrous porous structure. J. Mater. Sci. Mater. Med. 2020, 31, 74. [Google Scholar] [CrossRef] [PubMed]
- Banimohamad-shotorbani, B.; Rahmani, A.; Bakhshayesh, D.; Mehdipour, A.; Jarolmasjed, S.; Shafaei, H. The efficiency of PCL/HAp electrospun nanofibers in bone regeneration: A review. J. Med. Eng. Technol. 2021, 45, 511–531. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A.A.; Hameed, P.; Whenish, R.; Elsen, R.S.; Aswin, G.; Jaiswal, A.K.; Prashanth, K.G.; Manivasagam, G. A review on development of bio-inspired implants using 3d printing. Biomimetics 2021, 6, 65. [Google Scholar] [CrossRef]
- Hong, J.K.; Cooke, S.L.; Whittington, A.R.; Roman, M. Bioactive Cellulose Nanocomposites for Bone Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2021, 9, 605924. [Google Scholar] [CrossRef] [PubMed]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V. PCL and PCL-Based Materials in Biomedical Applications; Taylor & Francis: Abingdon, UK, 2017; Volume 11, ISBN 9031221031. [Google Scholar]
- Kumar, A.; Mohammad, S.; Ibrahim, M.; Agrim, A.; Anwar, A.; Leon, C.H.; Terracciano, A.; Zhao, X.; Su, T.; Kalyon, D.M. Load-bearing biodegradable PCL-PGA-beta TCP scaffolds for bone tissue regeneration. J. Biomed. Mater. Res. 2021, 109, 193–200. [Google Scholar] [CrossRef]
- Bruyas, A.; Lou, F.; Stahl, A.M.; Gardner, M.; Maloney, W.; Goodman, S.; Yang, Y.P. Systematic characterization of 3D-printed PCL/β-TCP scaffolds for biomedical devices and bone tissue engineering: In fluence of composition and porosity. J. Mater. Res. 2018, 33, 1948–1959. [Google Scholar] [CrossRef]
- Wang, W.; Caetano, G.; Ambler, W.S.; Blaker, J.J.; Frade, M.A.; Mandal, P.; Diver, C.; Bártolo, P. Enhancing the Hydrophilicity and Cell Attachment of 3D Printed PCL/Graphene Scaffolds for Bone Tissue Engineering. Materials 2016, 9, 992. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Nagarajan, S.; Belaid, H.; Farha, C.; Iatsunskyi, I.; Coy, E.; Soussan, L.; Huon, V.; Bares, J.; Belkacemi, K.; et al. Fabrication of 3D printed antimicrobial polycaprolactone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2021, 118, 111525. [Google Scholar] [CrossRef]
- Zimmerling, A.; Yazdanpanah, Z.; Cooper, D.M.L.; Johnston, J.D.; Chen, X. 3D printing PCL/nHA bone scaffolds: Exploring the influence of material synthesis techniques. Biomater. Res. 2021, 25, 3. [Google Scholar] [CrossRef]
- López-Castejón, M.L.; Reviriego, M.L.; Álvarez-Castillo, E.; Aguilar, J.M.; Bengoechea, C. Eco-Composites from Silkworm Meal and Polycaprolactone: Effect of Formulation and Processing Conditions. Polymers 2022, 14, 2342. [Google Scholar] [CrossRef]
- Fairag, R.; Li, L.; Ramirez-GarcialunaLuna, J.L.; Taylor, M.S.; Gaerke, B.; Weber, M.H.; Rosenzweig, D.H.; Haglund, L. A Composite Lactide-Mineral 3D-Printed Scaffold for Bone Repair and Regeneration. Front. Cell Dev. Biol. 2021, 9, 654518. [Google Scholar] [CrossRef] [PubMed]
- Averianov, I.; Stepanova, M.; Solomakha, O.; Gofman, I.; Serdobintsev, M.; Blum, N.; Kaftuirev, A.; Baulin, I.; Nashchekina, J.; Lavrentieva, A.; et al. 3D-Printed composite scaffolds based on poly(ε-caprolactone) filled with poly(glutamic acid)-modified cellulose nanocrystals for improved bone tissue regeneration. J. Biomed. Rsearch 2022, 110, 2422–2437. [Google Scholar] [CrossRef] [PubMed]
- Krobot, Š.; Melcová, V.; Mencík, P.; Kontárová, S.; Rampichová, M.; Hedvičáková, V.; Mojžišová, E.; Baco, A.; Prikryl, R. Based Blends for Tissue Engineering and Bone Medical Applications Processed by FDM 3D Printing. Polymers 2023, 15, 2404. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, E.; Shah, L.; Jindal, S.; Serenelli, C.; Michail, Z.; Khanbareh, H.; Tirella, A. Materials Science & Engineering C Additively manufactured BaTiO3 composite scaffolds: A novel strategy for load bearing bone tissue engineering applications. Mater. Sci. Eng. C 2021, 126, 112192. [Google Scholar] [CrossRef]
- Kao, C.-Y.; LinY, T.-L.; Lin, Y.-H.; Lee, A.K.-X.; Ng, S.Y.; Huang, T.-H.; Hsu, T.-T. Synergistic Effect of Static Magnetic Fields and 3D-Printed Iron-Oxide-Nanoparticle-Containing Calcium Silicate/Poly-ε-Caprolactone Scaffolds for Bone Tissue Engineering. Cells 2022, 11, 3967. [Google Scholar] [CrossRef]
- Cao, C.; Huang, P.; Prasopthum, A.; Parsons, A.J.; Ai, F.; Yang, J. Characterisation of bone regeneration in 3D printed ductile PCL/PEG/hydroxyapatite scaffolds with high ceramic microparticle concentrations. Biomater. Sci. 2022, 10, 138–152. [Google Scholar] [CrossRef]
- Christen, M.; Vercesi, F. Polycaprolactone: How a Well-Known and Futuristic Polymer Has Become an Innovative Collagen-Stimulator in Esthetics. Clin. Cosmet. Investig. Dermatol. ISSN 2022, 13, 31–48. [Google Scholar] [CrossRef]
- She, Y.; Fan, Z.; Wang, L.; Li, Y.; Sun, W.; Tang, H.; Zhang, L. 3D Printed Biomimetic PCL Scaffold as Framework Interspersed With Collagen for Long Segment Tracheal Replacement. Front. Cell Dev. Biol. 2021, 9, 629796. [Google Scholar] [CrossRef]
- Kim, S.; Heo, S.; Oh, G.; Yi, M. A 3D-Printed Polycaprolactone/Marine Collagen Scaffold Reinforced with Carbonated Hydroxyapatite from Fish Bones for Bone Regeneration. Mar. Drugs 2022, 20, 344. [Google Scholar] [CrossRef]
- Li, P.; Fu, L.; Liao, Z.; Peng, Y.; Ning, C.; Gao, C. Biomaterials containing synovial mesenchymal stem cells for cartilage regeneration based on tetrahedral framework nucleic acid recruitment. Biomaterials 2021, 278, 121131. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, H.; Fu, Q.; Xie, X.; Song, Y.; Xu, M.; Li, J. Materials & Design 3D-printed polycaprolactone-chitosan based drug delivery implants for personalized administration. Mater. Des. 2022, 214, 110394. [Google Scholar] [CrossRef]
- Shirzaei Sani, I.; Rezaei, M.; Baradar Khoshfetrat, A.; Razzaghi, D. Preparation and characterization of polycaprolactone/chitosan-g-polycaprolactone/hydroxyapatite electrospun nanocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2021, 182, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Ressler, A.; Bauer, L.; Prebeg, T.; Ledinski, M.; Hussainova, I.; Urli, I. PCL/Si-Doped Multi-Phase Calcium Phosphate Scaffolds Derived from Cuttlefish Bone. Materials 2022, 15, 3348. [Google Scholar] [CrossRef] [PubMed]
- Stulajterova, R.; Medvecky, L. Effect of calcium ions on transformation brushite to hydroxyapatite in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2008, 316, 104–109. [Google Scholar] [CrossRef]
- Alghazwani, Y.; Venkatesan, K.; Prabahar, K.; El-sherbiny, M. The Combined Anti-Tumor Efficacy of Bioactive Hydroxyapatite Nanoparticles Loaded with Altretamine. Pharmaceutics 2023, 15, 302. [Google Scholar] [CrossRef]
- Wang, R.; Liu, W.; Wang, Q.; Li, G.; Wan, B.; Sun, Y.; Niu, X.; Chen, D.; Tian, W. Anti-osteosarcoma effect of hydroxyapatite nanoparticles both in vitro and in vivo by downregulating the FAK/PI3K/Akt signaling pathway. Biomater. Sci. 2020, 8, 4426–4437. [Google Scholar] [CrossRef]
- Kargozar, S.; Mollazadeh, S.; Kermani, F.; Webster, T.J.; Nazarnezhad, S.; Hamzehlou, S.; Baino, F. Hydroxyapatite Nanoparticles for Improved Cancer Theranostics. J. Funct. Biomater. 2022, 13, 100. [Google Scholar] [CrossRef]
- Du, M.; Chen, J.; Liu, K.; Xing, H.; Song, C. Recent advances in biomedical engineering of nano-hydroxyapatite including dentistry, cancer treatment and bone repair. Compos. Part B Eng. 2021, 215, 108790. [Google Scholar] [CrossRef]
- Cestari, F.; Petretta, M.; Yang, Y.; Motta, A.; Grigolo, B.; Sglavo, V.M. 3D printing of PCL/nano-hydroxyapatite scaffolds derived from biogenic sources for bone tissue engineering. Sustain. Mater. Technol. 2021, 29, e00318. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.; Dinpanah-khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Qi, M.; Huang, Z.; Phakatkar, A.; Yao, W.; Yuan, Y.; Foroozan, T.; Xiao, G.; Shahbazian-Yassar, R.; Lua, Y.; Shokuhfar, T. Facile hydrothermal synthesis of antibacterial multi-layered hydroxyapatite nanostructures with superior flexibility. CrystEngComm 2018, 20, 1304–1312. [Google Scholar] [CrossRef]
- Lakrat, M.; Jodati, H.; Mejdoubi, E.M.; Evis, Z. Synthesis and characterization of pure and Mg, Cu, Ag, and Sr doped calcium-deficient hydroxyapatite from brushite as precursor using the dissolution-precipitation method. Powder Technol. 2023, 413, 118026. [Google Scholar] [CrossRef]
- Li, X.; Zou, Q.; Man, Y.; Li, W. Synergistic Effects of Novel Superparamagnetic/Upconversion HA Material and Ti/Magnet Implant on Biological Performance and Long-Term In Vivo Tracking. Small 2019, 15, 1901617. [Google Scholar] [CrossRef]
- Schwartz, K.; Milne, D. Growth-promoting Effects of Silicon in Rats. Nature 1972, 239, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Srinath, P.; Azeem, P.A.; Reddy, K.V. Review on calcium silicate-based bioceramics in bone tissue engineering. International Journal of Applied Ceramic Technology. Int. J. Appl. Ceram. Technol. 2020, 17, 2450–2464. [Google Scholar] [CrossRef]
- Gomez-Vazquez, O.M.; Correa-Piña, B.A.; Zubieta-Otero, L.F.; Castillo-Paz, A.M.; Londoño-Restrepo, S.M.; Rodriguez-García, M.E. Synthesis and characterization of bioinspired nano-hydroxyapatite by wet chemical precipitation. Ceram. Int. 2021, 47, 32775–32785. [Google Scholar] [CrossRef]
- Tarafder, S.; Davies, N.M.; Bandyopadhyay, A.; Bose, S. 3D printed tricalcium phosphate bone tissue engineering scaffolds: Effect of SrO and MgO doping on in vivo osteogenesis in a rat distal femoral defect model. Biomater. Sci. 2013, 1, 1250–1259. [Google Scholar] [CrossRef]
- Campodoni, E.; Montanari, M.; Artusi, C.; Bergamini, L.; Bassi, G.; Destro, E.; Fenoglio, I.; Panseri, S.; Tampieri, A.; Sanson, A.; et al. Biomineralization: A new tool for developing eco-sustainable Ti-doped hydroxyapatite-based hybrid UV filters. Biomater. Adv. 2023, 151, 213474. [Google Scholar] [CrossRef]
- Li, J.; Zhang, T.; Liao, Z.; Wei, Y.; Hang, R.; Huang, D. Engineered functional doped hydroxyapatite coating on titanium implants for osseointegration. J. Mater. Res. Technol. 2023, 27, 122–152. [Google Scholar] [CrossRef]
- Li, X.; Zou, Q.; Chen, H.; Li, W. In vivo changes of nanoapatite crystals during bone reconstruction and the differences with native bone apatite. Sci. Adv. 2019, 5, eaay6484. [Google Scholar] [CrossRef]
- Ottensmeyer, P.F.; Witzler, M.; Schulze, M. Small Molecules Enhance Scaffold-Based Bone Grafts via Purinergic Receptor Signaling in Stem Cells. Int. J. Mol. Sci. 2018, 19, 3601. [Google Scholar] [CrossRef]
- Noorzai, S.; Johannes, C.; Verbeek, R.; Christopher, M.; Janis, L. Collagen Extraction from Various Waste Bovine Hide Sources. Waste Biomass Valorization 2020, 11, 5687–5698. [Google Scholar] [CrossRef]
- Brooker, C.; Tronci, G. Effect of Mammalian Tissue Source on the Molecular and Macroscopic Characteristics of UV-Cured Type I Collagen Hydrogel Networks. Prosthesis 2022, 4, 1. [Google Scholar] [CrossRef]
- Acharya, P.P.; Kupendra, M.H.; Fasim, A.; Shivajirao, S.; Krishna, M.V. A comparative assessment of collagen type 1 from silver carp (fresh water) and milk shark (marine) fish waste. 3 Biotech 2022, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Binlateh, T.; Thammanichanon, P.; Rittipakorn, P.; Thinsathid, N.; Jitprasertwong, P. Collagen-Based Biomaterials in Periodontal Regeneration: Current Applications and Future Perspectives of. Biomimetics 2022, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, D.; Macedo, M.H.; Cui, W.; Sarmento, B. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2019, 29, 1804943. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Leary, L.E.R.O.; Fallas, J.A.; Bakota, E.L.; Kang, M.K.; Hartgerink, J.D. Multi-hierarchical self-assembly of a collagen mimetic peptide from triple helix to nanofibre and hydrogel. Nat. Chem. 2011, 3, 821–828. [Google Scholar] [CrossRef]
- Tan, X.; Xue, Z.; Zhu, H.; Wang, X.; Xu, D. How Charged Amino Acids Regulate Nucleation of Biomimetic Hydroxyapatite Nanoparticles on the Surface of Collagen Mimetic Peptides: Molecular Dynamics and Free Energy Investigations. Cryst. Growth Des. 2020, 20, 4561–4572. [Google Scholar] [CrossRef]
- Duanis-Assaf, T.; Hu, T.; Lavie, M.; Zhang, Z.; Reches, M. Understanding the Adhesion Mechanism of Hydroxyapatite-Binding Peptide. Langmuir 2022, 38, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Umrath, F.; Cen, W.; Reinert, S.; Alexander, D. Angiogenic Potential of VEGF Mimetic Peptides for the Biofunctionalization of Collagen/Hydroxyapatite Composites. Biomolecules 2021, 11, 1538. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Zhao, W.; Wang, Z.; Chen, J.; Ustriyana, P.; Gao, M.; Sahai, N. Structure–Activity Relationships of Hydroxyapatite-Binding Peptides. Langmuir 2020, 36, 2729–2739. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Wang, X.; Xu, D. Molecular dynamic simulation of prenucleation of apatite at a type I collagen template: Ion association and mineralization control. Phys. Chem. Chem. Phys. 2022, 24, 11370–11381. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wong, H.M.; Zhang, Y.Y.; Li, Q.L. Constructing an Antibiofouling and Mineralizing Bioactive Tooth Surface to Protect against Decay and Promote Self-Healing. ACS Appl. Mater. Interfaces 2020, 12, 3021–3031. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Q.L.; Wong, H.M. A Novel Strategy for Caries Management: Constructing an Antibiofouling and Mineralizing Dual-Bioactive Tooth Surface. ACS Appl. Mater. Interfaces 2021, 13, 31140–31152. [Google Scholar] [CrossRef]
- Zhou, Z.; Ge, X.; Bian, M.; Xu, T.; Li, N.; Lu, J.; Yu, J. Remineralization of dentin slices using casein phosphopeptide—Amorphous calcium phosphate combined with sodium tripolyphosphate. Biomed. Eng. 2020, 19, 18. [Google Scholar] [CrossRef]
- Buckle, E.L.; Prakash, A.; Bonomi, M.; Sampath, J.; Pfaendtner, J.; Drobny, G.P. Solid-State NMR and MD Study of the Structure of the Statherin Mutant SNa15 on Mineral Surfaces. J. Am. Chem. Soc. 2019, 141, 1998–2011. [Google Scholar] [CrossRef]
- Zeng, J.; Yang, S.; Yu, H.; Xu, Z.; Quan, X.; Zhou, J. Simulation Insight into the Synergic Role of Citrate and Polyaspartic Peptide in Biomineralization. Langmuir 2021, 37, 3410–3419. [Google Scholar] [CrossRef]
- Basu, S.; Basu, B.; Maiti, P.K. A computational study on strontium ion modified hydroxyapatite–fibronectin interactions. Phys. Chem. Chem. Phys. 2023, 24, 27989–28002. [Google Scholar] [CrossRef]
- Khor, E.; Yong, L. Implantable applications of chitin and chitosan. Biomaterials 2003, 24, 2339–2349. [Google Scholar] [CrossRef]
- Korpayev, S.; Kaygusuz, G.; Murat, Ş.; Orhan, K. International Journal of Biological Macromolecules Chitosan/collagen based biomimetic osteochondral tissue constructs: A growth factor-free approach. Int. J. Biol. Macromol. 2020, 156, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Kim, J.; Rolandi, M.; Duong, T. Natural biopolymers as proton conductors in bioelectronics. Biopolymers 2021, 112, e23433. [Google Scholar] [CrossRef]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Al Sagheer, F.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Bajaj, M.; Winter, J.; Gallert, C. Effect of deproteination and deacetylation conditions on viscosity of chitin and chitosan extracted from Crangon crangon shrimp waste. Biochem. Eng. J. 2011, 56, 51–62. [Google Scholar] [CrossRef]
- Tian, X.; Hua, T.; Poon, T.; Yang, Y.; Hu, H.; Fu, J.; Li, J.; Niu, B. Study on Effects of Blending Fiber Type and Ratio on Antibacterial Properties of Chitosan Blended Yarns and Fabrics. Fibers Polym. 2022, 23, 2565–2576. [Google Scholar] [CrossRef]
- Wu, P.; Xi, X.; Li, R.; Sun, G. Engineering Polysaccharides for Tissue Repair and Regeneration. Macromol. Biosci. 2021, 21, 2100141. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. International Journal of Biological Macromolecules Chitosan as an environment friendly biomaterial—A review on recent modi fi cations and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, D.; Wu, D.; Cui, Y.; Ren, G.; Wang, Y.; Webster, T. Chitosan-Based Biomaterial Scaffolds for the Repair of Infected Bone Defects. Front. Bioeng. Biotechnol. 2022, 10, 899760. [Google Scholar] [CrossRef]
- Patel, A.; Zaky, S.H.; Schoedel, K.; Li, H.; Sant, V.; Beniash, E.; Sfeir, C.; Stolz, D.B.; Sant, S. Acta Biomaterialia Design and evaluation of collagen-inspired mineral-hydrogel nanocomposites for bone regeneration. Acta Biomater. 2020, 112, 262–273. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, G.; Ma, W.; Song, Y.; Huang, C.; Xie, C. The root-like chitosan nanofiber porous scaffold cross-linked by genipin with type I collagen and its osteoblast compatibility. Carbohydr. Polym. 2022, 285, 119255. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nano fibers in bone regenerative engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xu, Y.; Yi, B.; Wang, X.; Tang, H.; Chen, C.; Zhang, Y. Engineering a Highly Biomimetic Chitosan-Based Cartilage Scaffold by Using Short Fibers and a Cartilage-Decellularized Matrix. Biomacromolecules 2021, 22, 2284–2297. [Google Scholar] [CrossRef] [PubMed]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-based scaffold for mineralized tissues regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef] [PubMed]
- Emilia, S.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Progress in Polymer Science Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.T.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Levengood, S.K.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef]
- Mogosanu, G.D.; Grumezescu, M.A. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef]
- Lim, C.; Hwang, S.D.; Lee, W.D. Intermolecular interactions of chitosan: Degree of acetylation and molecular weight. Carbohydr. Polym. 2021, 259, 117782. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-H.; Whu, S.W.; Hsieh, S.-C.; Tsai, C.-L.; Chen, D.C.; Tan, T. Evaluation of Chitosan-alginate-hyaluronate Complexes Modified by an RGD-containing Protein as Tissue-engineering Scaffolds for Cartilage Regeneration. Artif. Organs 2004, 28, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Whu, S.W.; Tsai, C.; Wu, Y.; Chen, H.; Hsieh, K. Chitosan as Scaffold Materials: Effects of Molecular Weight and Degree of Deacetylation. J. Polym. Res. 2004, 11, 141–147. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X. Calcium Phosphate-Based Biomaterials for Bone Repair. Funct. Mater. 2022, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.P.; Lu, X.; Fang, L.M.; Qu, S.X.; Feng, B.; Weng, J. Atomic-scale interactions at the interface of biopolymer/hydroxyapatite. Biomed. Mater. 2008, 3, 44110. [Google Scholar] [CrossRef]
- Mathesan, S.; Rath, A.; Ghosh, P. Molecular mechanisms in deformation of cross-linked hydrogel nanocomposite. Mater. Sci. Eng. C 2016, 59, 157–167. [Google Scholar] [CrossRef]
- Snyder, A.D.; Salehinia, I. Study of nanoscale deformation mechanisms in bulk hexagonal hydroxyapatite under uniaxial loading using molecular dynamics. J. Mech. Behav. Biomed. Mater. 2020, 110, 103894. [Google Scholar] [CrossRef]
- Ji, C.; He, B.; Yun, S.; Bai, X.; Lin, B. Journal of the Mechanical Behavior of Biomedical Materials The fracture mechanical behavior simulation of calcium-deficient hydroxyapatite crystals by molecular dynamics and first-principles calculation. J. Mech. Behav. Biomed. Mater. 2023, 137, 105526. [Google Scholar] [CrossRef]
- Lew, A.J.; Beniash, E.; Gilbert, P.U.P.A.; Buehler, M.J. Role of the Mineral in the Self-Healing of Cracks in Human Enamel. ASC Nano 2022, 16, 10273–10280. [Google Scholar] [CrossRef]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. European Journal of Pharmaceutics and Biopharmaceutics Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S. Chitosan Composites for Bone Tissue Engineering—An Overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Holme, K.R.; Perlin, A.S. Chitosan N-sulfate. A water-soluble polyelectrolyte. Carbohydr. Res. 1997, 302, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Kai, H.; Shinada, K.; Yoshidaa, T.; Tokurab, S.; Kurita, K.; Nakashimad, H.; Yamamotod, N.; Uryue, T. Regioselective syntheses of sulfated polysaccharides: Specific anti-HIV-1 activity of novel chitin sulfates. Carbohydr. Res. 1998, 306, 427–433. [Google Scholar] [CrossRef]
- Budiraharjo, R.; Neoh, K.G.; Kang, E.T.; Kishen, A. Bioactivity of novel carboxymethyl chitosan scaffold incorporating MTA in a tooth model. Int. Endod. J. 2010, 43, 930–939. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Mano, J.F.; Reis, R.L. Nature-inspired calcium phosphate coatings: Present status and novel advances in the science of mimicry. Curr. Opin. Solid State Mater. Sci. 2003, 7, 309–318. [Google Scholar] [CrossRef]
- Sagar, N.; Pandey, A.K.; Gurbani, D.; Khan, K.; Singh, D.; Chaudhari, B.P.; Soni, V.P.; Chattopadhyay, N.; Dhawan, A.; Bellare, J.R. In-Vivo Efficacy of Compliant 3D Nano-Composite in Critical-Size Bone Defect Repair: A Six Month Preclinical Study in Rabbit. PLoS ONE 2013, 8, e77578. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, W.; Sun, W. Influence of the carboxymethyl chitosan anti-adhesion solution on the TGF-β1 in a postoperative peritoneal adhesion rat. J. Mater. Sci. Mater. Med. 2013, 24, 2549–2559. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Y.; Jiang, Z.; Yang, Y.; Liu, W.; Han, B. Preparation and renoprotective effects of carboxymethyl chitosan oligosaccharide on adriamycin nephropathy. Carbohydr. Polym. 2018, 201, 347–356. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Zhao, X.; Wang, Z. Fabrication and applications of bioactive chitosan-based organic-inorganic hybrid materials: A review. Carbohydr. Polym. 2021, 267, 118179. [Google Scholar] [CrossRef] [PubMed]
- Ghilan, A.; Chiriac, A.P.; Nita, L.E.; Rusu, A.G.; Neamtu, I.; Chiriac, V.M. Trends in 3D Printing Processes for Biomedical Field: Opportunities and Challenges. J. Polym. Environ. 2020, 28, 1345–1367. [Google Scholar] [CrossRef] [PubMed]
- Kantaros, A. 3D Printing in Regenerative Medicine: Technologies and Resources Utilized. Int. J. Mol. Sci. 2022, 23, 14621. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, N.H.M.; Teo, L.-R.I.; Phang, S.-J.; Wong, V.-L.; Cheah, K.-H.; Siew-Shee, L. Recent Advances in Polymer-based 3D Printing for Wastewater Treatment Application: An Overview. Chem. Eng. J. 2021, 429, 132311. [Google Scholar] [CrossRef]
- Nyberg, E.; Rindone, A.; Dorafshar, A.; Grayson, W.L. Comparison of 3D-Printed Poly-e-Caprolactone Scaffolds Functionalized with Tricalcium Phosphate, Hydroxyapatite, Bio-Oss, or Decellularized Bone Matrix. Tissue Eng. Part A 2017, 23, 503–514. [Google Scholar] [CrossRef]
- Wu, Y.; Sriram, G.; Fawzy, A.S.; Fuh, J.Y.H.; Rosa, V.; Cao, T.; Wong, Y.S. Fabrication and evaluation of electrohydrodynamic jet 3D printed polycaprolactone/chitosan cell carriers using human embryonic stem cell-derived fibroblasts. J. Biomater. Appl. 2016, 31, 181–192. [Google Scholar] [CrossRef]
- Hernandez, I.; Kumar, A. A Bioactive Hydrogel and 3D Printed Polycaprolactone System for Bone Tissue Engineering. Gels 2017, 3, 26. [Google Scholar] [CrossRef]
- Kim, J.; Ahn, G.; Kim, C.; Lee, J.; Lee, I.; An, S.; Yun, W.; Kim, S.; Shim, J. Synergistic Effects of Beta Tri-Calcium Phosphate and Porcine-Derived Decellularized Bone Extracellular Matrix in 3D-Printed Polycaprolactone Scaffold on Bone Regeneration. Macromol. Biosci. 2018, 18, 1800025. [Google Scholar] [CrossRef]
- Cho, S.Y.; Choi, S.; Lee, S.-H.; Kim, K.K.; Cho, Y. Assessments of polycaprolactone/hydroxyapatite composite scaffold with enhanced biomimetic mineralization by exposure to hydroxyapatite via a 3D-printing system and alkaline erosion. Eur. Polym. J. 2019, 113, 340–348. [Google Scholar] [CrossRef]
- Kurzyk, A.; Ostrowska, B.; Wojciech, Ś.; Pojda, Z. Characterization and Optimization of the Seeding Process of Adipose Stem Cells on the Polycaprolactone Scaffolds. Stem Cells Int. 2019, 2019, 1201927. [Google Scholar] [CrossRef]
- Cakmak, A.M.; Unal, S.; Sahin, A.; Oktar, F.N.; Sengor, M.; Ekren, N.; Gunduz, O.; Kalaskar, D.M. 3D Printed Polycaprolactone/Gelatin/Bacterial Cellulose/Hydroxyapatite Composite Scaffold for Bone Tissue Engineering. Polymers 2020, 12, 1962. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, S.K.; Behravesh, A.H.; Hasannia, S.; Bagheri Saed, A.; Akhoundi, B. 3D printed PCL scaffold reinforced with continuous biodegradable fiber yarn: A study on mechanical and cell viability properties. Polym. Test. 2020, 83, 106347. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Gwak, S.-J.; Seo, K.D.; Lee, S.; Yun, J.-H.; Cho, Y.-S.; Lee, S.-J. Fabrication of Three-Dimensional Composite Scaffold for Simultaneous Alveolar Bone Regeneration in Dental Implant Installation. Int. J. Mol. Sci. 2020, 21, 1863. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, R.; Chen, S.; Xu, Z.; Wang, Q.; Yuan, P.; Zhou, Y.; Zhang, Y.; Chen, J. Heparan sulfate loaded polycaprolactone-hydroxyapatite scaffolds with 3D printing for bone defect repair. Int. J. Biol. Macromol. 2020, 148, 153–162. [Google Scholar] [CrossRef]
- Moura, C.; Trindade, D.; Vieira, M.; Francisco, L.; Ângelo, D.F.; Alves, N. Multi-Material Implants for Temporomandibular Joint Disc Repair: Tailored Additive Manufacturing Production. Front. Bioeng. Biotechnol. 2020, 8, 342. [Google Scholar] [CrossRef] [PubMed]
- Thunsiri, K.; Pitjamit, S.; Pothacharoen, P.; Pruksakorn, D.; Nakkiew, W.; Wattanutchariya, W. The 3D-Printed Bilayer’s Bioactive-Biomaterials Scaffold for Full-Thickness Articular Cartilage Defects Treatment. Materials 2020, 13, 3417. [Google Scholar] [CrossRef]
- El-Habashy, S.E.; El-Kamel, A.H.; Essawy, M.M.; Abdelfattah, E.A.; Eltaher, H.M. Engineering 3D-printed core–shell hydrogel scaffolds reinforced with hybrid hydroxyapatite/polycaprolactone nanoparticles for in vivo bone regeneration. Biomater. Sci. 2021, 9, 4019–4039. [Google Scholar] [CrossRef]
- Kim, Y.; Son, K.; Lee, J. Auxetic structures for tissue engineering scaffolds and biomedical devices. Materials 2021, 14, 6821. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Z.; Ai, F.; Wu, C.; Zhou, K.; Cao, C.; Li, W. Characterization and evaluation of polycaprolactone/hydroxyapatite composite scaffolds with extra surface morphology by cryogenic printing for bone tissue engineering. Mater. Des. 2021, 205, 109712. [Google Scholar] [CrossRef]
- Petretta, M.; Gambardella, A.; Desando, G.; Cavallo, C.; Bartolotti, I.; Shelyakova, T.; Goranov, V.; Brucale, M.; Dediu, V.A.; Fini, M.; et al. Multifunctional 3D-Printed Magnetic Polycaprolactone/Hydroxyapatite Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 3825. [Google Scholar] [CrossRef]
- Rezaei, F.S.; Khorshidian, A.; Beram, M. 3D printed chitosan/polycaprolactone scaffold for lung tissue engineering: Hope to be useful for. RSC Adv. 2021, 11, 19508–19520. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Omar, A.M.; Bartolo, P. Experimental and Numerical Simulations of 3D-Printed Polycaprolactone Scaffolds for Bone Tissue Engineering Applications. Materials 2021, 14, 3546. [Google Scholar] [CrossRef] [PubMed]
- Biscaia, S.; Branquinho, M.V.; Alvites, R.D.; Fonseca, R.; Sousa, A.C.; Pedrosa, S.; Caseiro, A.R.; Guedes, F.; Patr, T.; Viana, T.; et al. 3D Printed Poly(ε-caprolactone)/Hydroxyapatite Scaffolds for Bone Tissue Engineering: A Comparative Study on a Composite Preparation by Melt Blending or Solvent Casting Techniques and the Influence of Bioceramic Content on Scaffold Properties. Int. J. Mol. Sci. 2022, 23, 2318. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pujana, A.; Carranza, T.; Santos-vizcaino, E.; Igartua, M. Hybrid 3D Printed and Electrospun Multi-Scale Hierarchical Polycaprolactone Scaffolds to Induce Bone Differentiation. Parmaceutics 2022, 14, 2843. [Google Scholar] [CrossRef]
- Sousa, A.C.; Biscaia, S.; Alvites, R.; Branquinho, M.; Lopes, B.; Valente, J.; Franco, M.; Santos, D.; Mendonça, C.; Alves, N.; et al. Assessment of 3D-Printed Polycaprolactone, Hydroxyapatite Nanoparticles and Diacrylate Poly(ethylene glycol) Scaffolds for Bone Regeneration. Pharmaceutics 2022, 14, 2643. [Google Scholar] [CrossRef]
- López-González, I.; Hernández-Heredia, A.B.; Rodríguez-López, M.I.; Auñón-Calles, D.; Boudifa, M.; Gabaldón, J.A.; Meseguer-Olmo, L. Evaluation of the In Vitro Antimicrobial Efficacy against Staphylococcus Aureus and Epidermidis of a Novel 3D-Printed Degradable Drug Delivery System Based on Polycaprolactone/Chitosan/Vancomycin—Preclinical Study. Pharmaceutics 2023, 15, 1763. [Google Scholar] [CrossRef]
- Lee, S.J.; Heo, D.N.; Park, J.S.; Kwon, S.K.; Lee, J.H.; Lee, J.H.; Kim, W.D.; Kwon, I.K.; Park, S.A. Characterization and preparation of bio-tubular scaffolds for fabricating artificial vascular grafts by combining electrospinning and a 3D printing system. Phys. Chem. Chem. Phys. 2014, 17, 2996–2999. [Google Scholar] [CrossRef]
- Najafabadi, F.M.; Karbasi, S.; Benisi, S.Z.; Shojaei, S. Physical, mechanical, and biological performance of chitosan-based nanocomposite coating deposited on the polycaprolactone-based 3D printed scaffold: Potential application in bone tissue engineering. Int. J. Biol. Macromol. 2023, 243, 125218. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Huang, B.; Bártolo, P.J. Rheological characterization of polymer/ceramic blends for 3D printing of bone scaffolds. Polym. Test. 2018, 68, 365–378. [Google Scholar] [CrossRef]
- Babilotte, J.; Guduric, V.; Le Nihouannen, D.; Naveau, A.; Fricain, J.C.; Catros, S. 3D printed polymer–mineral composite biomaterials for bone tissue engineering: Fabrication and characterization. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2579–2595. [Google Scholar] [CrossRef] [PubMed]
- Šupová, M. Problem of hydroxyapatite dispersion in polymer matrices: A review. J. Mater. Sci. Mater. Med. 2009, 20, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Mohd Pu’Ad, N.A.S.; Abdul Haq, R.H.; Mohd Noh, H.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis method of hydroxyapatite: A review. Mater. Today Proc. 2019, 29, 233–239. [Google Scholar] [CrossRef]
- Seyedsalehi, A.; Daneshmandi, L.; Barajaa, M.; Riordan, J.; Laurencin, C.T. Fabrication and characterization of mechanically competent 3D printed polycaprolactone-reduced graphene oxide scaffolds. Sci. Rep. 2020, 10, 22210. [Google Scholar] [CrossRef]
- Ogata, N.; Yamaguchi, S.; Shimada, N.; Lu, G.; Iwata, T.; Nakane, K.; Ogihara, T. Poly(lactide) Nanofibers Produced by a Melt-Electrospinning System with a Laser Melting Device. J. Appl. Polym. Sci. 2007, 104, 1641–1645. [Google Scholar] [CrossRef]
- Wissamitanan, T.; Dechwayukul, C.; Kalkornsurapranee, E.; Thongruang, W. Proper blends of biodegradable polycaprolactone and natural rubber for 3D printing. Polymers 2020, 12, 2416. [Google Scholar] [CrossRef]
- Ghorbani, F.M.; Kaffashi, B.; Shokrollahi, P.; Akhlaghi, S.; Hedenqvist, M. Effect of hydroxyapatite nano-particles on morphology, rheology and thermal behavior of poly(caprolactone)/chitosan blends. Mater. Sci. Eng. C 2016, 59, 980–989. [Google Scholar] [CrossRef]
- Dubinenko, G.; Zinoviev, A.; Bolbasov, E.; Kozelskaya, A.; Shesterikov, E.; Novikov, V.; Tverdokhlebov, S. Highly filled poly(l-lactic acid)/hydroxyapatite composite for 3D printing of personalized bone tissue engineering scaffolds. J. Appl. Polym. Sci. 2021, 138, 49662. [Google Scholar] [CrossRef]
- Honarvar, A.; Karbasi, S.; Hashemibeni, B.; Setayeshmehr, M.; Kazemi, M.; Valiani, A. Effects of cartilage acellular solubilised ECM on physicomechanical and biological properties of polycaprolactone/fibrin hybrid scaffold fabricated by 3D-printing and salt-leaching methods. Mater. Technol. 2022, 37, 204–212. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef]
- Yoshida, M.; Turner, P.R.; Ali, M.A.; Cabral, J.D. Three-Dimensional Melt-Electrowritten Polycaprolactone/Chitosan Scaffolds Enhance Mesenchymal Stem Cell Behavior. ACS Appl. Bio Mater. 2021, 4, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Nawrotek, K.; Mąkiewicz, M.; Zawadzki, D. Fabrication and Characterization of Polycaprolactone/Chitosan—Hydroxyapatite Hybrid Implants for Peripheral. Polymers 2021, 13, 775. [Google Scholar] [CrossRef] [PubMed]
- Sarasam, A.; Madihally, S.V. Characterization of chitosan—Polycaprolactone blends for tissue engineering applications. Biomaterials 2005, 26, 5500–5508. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, Y.; Hu, Y.; Liu, J.; Yuan, T.; Zhou, W.; Dong, X.; Wang, C.; Binks, B.P.; Yang, Z. Fabrication of Poly(ϵ-caprolactone)-embedded Lignin-Chitosan Nanocomposite Porous Scaffolds from Pickering Emulsions. Langmuir 2023, 39, 6947–6956. [Google Scholar] [CrossRef] [PubMed]
- Hassanajili, S.; Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater. Sci. Eng. C 2019, 104, 109960. [Google Scholar] [CrossRef]
- Freeman, F.E.; Brennan, M.Á.; Browe, D.C.; Renaud, A.; De Lima, J.; Kelly, D.J.; Mcnamara, L.M.; Layrolle, P. A Developmental Engineering-Based Approach to Bone Repair: Endochondral Priming Enhances Vascularization and New Bone Formation in a Critical Size Defect. Front. Bioeng. Biotechnol. 2020, 8, 230. [Google Scholar] [CrossRef]
- Mohammadi, H.; Sepantafar, M.; Muhamad, N.; Bakar Sulong, A. How Does Scaffold Porosity Conduct Bone Tissue Regeneration? Adv. Eng. Mater. 2021, 23, 2100463. [Google Scholar] [CrossRef]
- Moura, C.S.; Da Silva, C.L.; Bártolo, P.J.; Ferreira, F.C. Combination of 3D extruded-based poly(ε-caprolactone) scaffolds with mesenchymal stem/stromal cells: Strategy optimization. Procedia Eng. 2015, 110, 122–127. [Google Scholar] [CrossRef]
- Oh, S.H.; Park, I.K.; Kim, J.M.; Lee, J.H. In vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials 2007, 28, 1664–1671. [Google Scholar] [CrossRef]
- Fantini, M.; Curto, M.; De Crescenzio, F. A method to design biomimetic scaffolds for bone tissue engineering based on Voronoi lattices. Virtual Phys. Prototyp. 2016, 11, 77–90. [Google Scholar] [CrossRef]
- Kühl, J.; Gorb, S.; Kern, M.; Klüter, T.; Kühl, S.; Seekamp, A.; Fuchs, S. Extrusion-based 3D printing of osteoinductive scaffolds with a spongiosa-inspired structure. Front. Bioeng. Biotechnol. 2023, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, E.M.; Oliveira, F.J.; Silva, R.F.; Neto, M.A.; Fernandes, M.H.; Amaral, M.; Vallet-Regí, M.; Vila, M. Three-dimensional printed PCL-hydroxyapatite scaffolds filled with CNTs for bone cell growth stimulation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, S.J.; Jo, H.H.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Fabrication and characterization of 3D-printed bone-like β-tricalcium phosphate/polycaprolactone scaffolds for dental tissue engineering. J. Ind. Eng. Chem. 2017, 46, 175–181. [Google Scholar] [CrossRef]
- Cho, Y.S.; Gwak, S.J.; Cho, Y.S. Fabrication of polycaprolactone/nano hydroxyapatite (Pcl/nHA) 3D scaffold with enhanced in vitro cell response via design for additive manufacturing (dfam). Polymers 2021, 13, 1394. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Paris, M.; Götz, A.; Hettrich, I.; Bidan, C.M.; John, W.C.; Razi, H.; Zizak, I.; Hutmacher, D.W.; Fratzl, P.; Georg, N.; et al. SCaffold curvature-mediated novel biomineralization process originates a continuous soft tissue-to-bone interface. Acta Biomater. 2017, 60, 64–80. [Google Scholar] [CrossRef]
- Fonseca, D.R.; Sobreiro-Almeida, R.; Sol, P.C.; Neves, N.M. Development of non-orthogonal 3D-printed scaffolds to enhance their osteogenic performance. Biomater. Sci. 2018, 6, 1569–1579. [Google Scholar] [CrossRef]
- Zamani, Y.; Amoabediny, G.; Mohammadi, J.; Seddiqi, H.; Helder, M.N.; Zandieh-Doulabi, B.; Klein-Nulend, J.; Koolstra, J.H. 3D-printed poly(Ɛ-caprolactone) scaffold with gradient mechanical properties according to force distribution in the mandible for mandibular bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103638. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Zhu, C.F.; Lanctot, D.R.; Agrawal, C.M.; Wang, X. Fundamentals of biomechanics in tissue engineering of bone. Tissue Eng. 2000, 6, 361–381. [Google Scholar] [CrossRef]
- Yaszemski, M.J.; Payne, R.G.; Hayes, W.C.; Langer, R.; Mikos, A.G. Evolution of bone transplantation: Molecular, cellular and tissue strategies to engineer human bone. Biomaterials 1996, 17, 175–185. [Google Scholar] [CrossRef]
- Røhl, L.; Larsen, E.; Linde, F.; Odgaard, A.; Jørgensen, J. Tensile and compressive properties of cancellous bone. J. Biomech. 1991, 24, 1143–1149. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, G.; Wang, B.; Tang, Y.; Lin, Z.; Liu, J.; Wei, G.; Wang, L. Pore Strategy Design of a Novel NiTi-Nb Biomedical Porous Scaffold Based on a Triply Periodic Minimal Surface. Front. Bioeng. Biotechnol. 2022, 10, 910475. [Google Scholar] [CrossRef] [PubMed]
- Pitrolino, K.A.; Felfel, R.M.; Pellizzeri, L.M.; McLaren, J.; Popov, A.A.; Sottile, V.; Scotchford, C.A.; Scammell, B.E.; Roberts, G.A.F.; Grant, D.M. Development and in vitro assessment of a bi-layered chitosan-nano-hydroxyapatite osteochondral scaffold. Carbohydr. Polym. 2022, 282, 119126. [Google Scholar] [CrossRef] [PubMed]
- Fung, Y.C. Bone and Cartilage. In Biomechanics. Mechanical Properties of Living Tissues; Springer: New York, NY, USA, 2013; pp. 500–544. ISBN 9781441931047. [Google Scholar]
- Tanaka, E.; Van Eijden, T. Biomechanical Behavior of the Temporomandibular Joint Disc. Crit. Rev. Oral Biol. Med. 2003, 14, 138–150. [Google Scholar] [CrossRef]
- Jiang, N.; Yang, Y.; Zhang, L.; Jiang, Y.; Wang, M.; Zhu, S. 3D-Printed Polycaprolactone Reinforced Hydrogel as an Artificial TMJ Disc. Res. Rep. Biomater. Bioeng. 2021, 100, 1–8. [Google Scholar] [CrossRef]
- Jiang, Y.; Guan, Y.; Lan, Y.; Chen, S.; Li, T.; Zou, S.; Hu, Z.; Ye, Q. Mechanosensitive Piezo1 in Periodontal Ligament Cells Promotes Alveolar Bone Remodeling During Orthodontic Tooth Movement. Front. Physiol. 2021, 12, 767136. [Google Scholar] [CrossRef]
- Capponi, G.; Zambito, M.; Neri, I.; Cottone, F.; Mattarelli, M.; Vassalli, M.; Caponi, S.; Florio, T. Cellular Mechanosensitivity: Validation of an Adaptable 3D-Printed Device for Microindentation. Nanomaterials 2022, 12, 2691. [Google Scholar] [CrossRef]
- Al-Maslamani, N.A.; Khilan, A.A.; Horn, H.F. Design of a 3D printed, motorized, uniaxial cell stretcher for microscopic and biochemical analysis of mechanotransduction. Biol. Open 2021, 10, bio057778. [Google Scholar] [CrossRef]
- Guarino, V.; Lewandowska, M.; Bil, M.; Polak, B.; Ambrosio, L. Morphology and degradation properties of PCL/HYAFF11® composite scaffolds with multi-scale degradation rate. Compos. Sci. Technol. 2010, 70, 1826–1837. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Chlanda, A.; Kijeńska-Gawrońska, E.; Zdunek, J.; Swieszkowski, W. Internal nanocrystalline structure and stiffness alterations of electrospun polycaprolactone-based mats after six months of in vitro degradation. An atomic force microscopy assay. J. Mech. Behav. Biomed. Mater. 2020, 101, 103437. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Abdal-hay, A.; Bartnikowski, N.J.; Kim, K.Y. A comprehensive study of acid and base treatment of 3D printed poly(ε-caprolactone) scaffolds to tailor surface characteristics. Appl. Surf. Sci. 2021, 555, 149602. [Google Scholar] [CrossRef]
- Tsuji, H.; Saeki, T.; Tsukegi, T.; Daimon, H.; Fujie, K. Comparative study on hydrolytic degradation and monomer recovery of poly(l-lactic acid) in the solid and in the melt. Polym. Degrad. Stab. 2008, 93, 1956–1963. [Google Scholar] [CrossRef]
- Lam, C.X.F.; Teoh, S.H.; Hutmacher, D.W. Comparison of the degradation of polycaprolactone and polycaprolactone-(β-tricalcium phosphate) scaffolds in alkaline medium. Polym. Int. 2007, 56, 718–728. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Huang, M.H.; Li, S.; Hutmacher, D.W.; Coudane, J.; Vert, M. Degradation characteristics of poly(ε-caprolactone)-based copolymers and blends. J. Appl. Polym. Sci. 2006, 102, 1681–1687. [Google Scholar] [CrossRef]
- Li, F.; Yu, D.; Lin, X.; Liu, D.; Xia, H.; Chen, S. Biodegradation of poly(ε-caprolactone) (PCL) by a new Penicillium oxalicum strain DSYD05-1. World J. Microbiol. Biotechnol. 2012, 28, 2929–2935. [Google Scholar] [CrossRef]
- Li, S.M.; Espartero, J.L.; Foch, P.; Vert, M. Structural characterization and hydrolytic degradation of a Zn metal initiated copolymer of L-lactide and ε-caprolactone. J. Biomater. Sci. Polym. Ed. 1996, 8, 165–187. [Google Scholar] [CrossRef]
- Jennings, J.A.; Beenken, K.E.; Parker, A.C.; Smith, J.K.; Courtney, H.S.; Smeltzer, M.S.; Haggard, W.O. Polymicrobial Biofilm Inhibition Effects of Acetate-Buffered Chitosan Sponge Delivery Device. Macromol. Biosci. 2016, 16, 591–598. [Google Scholar] [CrossRef]
- Tatu, R.R.; Oria, M.; Rao, M.B.; Peiro, J.L.; Lin, C.Y. Biodegradation of poly(l-lactic acid) and poly(ε-caprolactone) patches by human amniotic fluid in an in-vitro simulated fetal environment. Sci. Rep. 2022, 12, 3950. [Google Scholar] [CrossRef]
- Greene, A.F.; Vaidya, A.; Collet, C.; Wade, K.R.; Patel, M.; Gaugler, M.; West, M.; Petcu, M.; Parker, K. 3D-printed enzyme-embedded plastics. Biomacromolecules 2021, 22, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Von Burkersroda, F.; Schedl, L.; Göpferich, A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials 2002, 23, 4221–4231. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Grizzi, I.; Garreau, H.; Li, S.; Vert, M. Hydrolytic degradation of devices based on poly(DL-lactic acid) size-dependence. Biomaterials 1995, 16, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.H.; Nieuwenhuis, P.; Molenaar, I.; Esselbrugge, H.; Feijen, J.; Dijkstra, P.J.; Schakenraad, J.M. Biodegradation of porous versus non-porous poly(L-lactic acid) films. J. Mater. Sci. Mater. Med. 1994, 5, 181–189. [Google Scholar] [CrossRef]
- Lam, K.H.; Schakenraad, J.M.; Groen, H.; Esselbrugge, H.; Dijkstra, P.J.; Feijen, J.; Nieuwenhuis, P. The influence of surface morphology and wettability on the inflammatory response against poly(L-lactic acid): A semi-quantitative study with monoclonal antibodies. J. Biomed. Mater. Res. 1995, 29, 929–942. [Google Scholar] [CrossRef][Green Version]
- Cama, G.; Mogosanu, D.E.; Houben, A.; Dubruel, P. Synthetic Biodegradable Medical Polyesters: Poly-σ-Caprolactone; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081003930. [Google Scholar]
- Nelson, M.T.; Pattanaik, L.; Allen, M.; Gerbich, M.; Hux, K.; Allen, M.; Lannutti, J.J. Recrystallization improves the mechanical properties of sintered electrospun polycaprolactone. J. Mech. Behav. Biomed. Mater. 2014, 30, 150–158. [Google Scholar] [CrossRef]
- Yeo, A.; Sju, E.; Rai, B.; Teoh, S.H. Customizing the degradation and load-bearing profile of 3D polycaprolactone-tricalcium phosphate scaffolds under enzymatic and hydrolytic conditions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87, 562–569. [Google Scholar] [CrossRef]
- Lam, C.X.F.; Savalani, M.M.; Teoh, S.H.; Hutmacher, D.W. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: Accelerated versus simulated physiological conditions. Biomed. Mater. 2008, 3, 034108. [Google Scholar] [CrossRef]
- Meng, C.; Tang, D.; Liu, X.; Meng, J.; Wei, W.; Gong, R.H.; Li, J. Heterogeneous porous PLLA/PCL fibrous scaffold for bone tissue regeneration. Int. J. Biol. Macromol. 2023, 235, 123781. [Google Scholar] [CrossRef]
- Ge, S.; Zhu, X.; Zhang, C.; Jia, D.; Shang, W.; Ding, C.; Yang, J. Nanosilica-Anchored Polycaprolactone/Chitosan Nanofibrous Bioscaffold to Boost Osteogenesis for Bone Tissue Engineering. Molecules 2022, 27, 8832. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, X.; Liu, P.; Zhang, M.; Wang, C.; Zhang, B. Multifunctional Chitosan/Polycaprolactone Nanofiber Scaffolds with Varied Dual-Drug Release for Wound-Healing Applications. ACS Biomater. Sci. Eng. 2020, 6, 4666–4676. [Google Scholar] [CrossRef] [PubMed]
- Karakeçili, A.; Korpayev, S.; Orhan, K. Optimizing Chitosan/Collagen Type I/Nanohydroxyapatite Cross-linked Porous Scaffolds for Bone Tissue Engineering. Appl. Biochem. Biotechnol. 2022, 194, 3843–3859. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Lee, J.H. Hydrophilization of synthetic biodegradable polymer scaffolds for improved cell/tissue compatibility. Biomed. Mater. 2013, 8, 014101. [Google Scholar] [CrossRef]
- Haglund, L.; Ahangar, P.; Rosenzweig, D.H. Advancements in 3D printed scaffolds to mimic matrix complexities for musculoskeletal repair. Curr. Opin. Biomed. Eng. 2019, 10, 142–148. [Google Scholar] [CrossRef]
- Kim, J.W.; Shin, K.H.; Koh, Y.H.; Hah, M.J.; Moon, J.; Kim, H.E. Production of poly(ε-caprolactone)/hydroxyapatite composite scaffolds with a tailored macro/micro-porous structure, high mechanical properties, and excellent bioactivity. Materials 2017, 10, 1123. [Google Scholar] [CrossRef]
- Vyas, C.; Zhang, J.; Øvrebø, Ø.; Huang, B.; Roberts, I.; Setty, M.; Allardyce, B.; Haugen, H.; Rajkhowa, R.; Bartolo, P. 3D printing of silk microparticle reinforced polycaprolactone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2021, 118, 111433. [Google Scholar] [CrossRef]
- Vargas-Osorio, Z.; Ruther, F.; Chen, S.; Sengupta, S.; Liverani, L.; Michálek, M.; Galusek, D.; Boccaccini, A.R. Environmentally friendly fabrication of electrospun nanofibers made of polycaprolactone, chitosan and κ-carrageenan (PCL/CS/κ-C). Biomed. Mater. 2022, 17, 045019. [Google Scholar] [CrossRef]
- Asghari, F.; Rabiei Faradonbeh, D.; Malekshahi, Z.V.; Nekounam, H.; Ghaemi, B.; Yousefpoor, Y.; Ghanbari, H.; Faridi-Majidi, R. Hybrid PCL/chitosan-PEO nanofibrous scaffolds incorporated with A. euchroma extract for skin tissue engineering application. Carbohydr. Polym. 2022, 278, 118926. [Google Scholar] [CrossRef]
- Amornkitbamrung, U.; In, Y.; Wang, Z.; Song, J.; Oh, S.H.; Hong, M.H.; Shin, H. C-axis-oriented platelets of crystalline hydroxyapatite in biomimetic intrafibrillar mineralization of polydopamine-functionalized collagen type I. ACS Omega 2022, 7, 4821–4831. [Google Scholar] [CrossRef]
- Cheng, Z.; Teoh, S.H. Surface modification of ultra thin poly (ε-caprolactone) films using acrylic acid and collagen. Biomaterials 2004, 25, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xing, J.; Lin, M. The Biocompatibility of the Scaffolds Reinforced by Fibers or Tubes for Tissue Repair. In Tissue Repair. Reinforced Scaffolds; Li, X., Ed.; Springer: Singapore, 2017; Volume 6, pp. 225–233. ISBN 9789811035531. [Google Scholar]
- Jaidev, L.R.; Chatterjee, K. Surface functionalization of 3D printed polymer scaffolds to augment stem cell response. Mater. Des. 2019, 161, 44–54. [Google Scholar] [CrossRef]


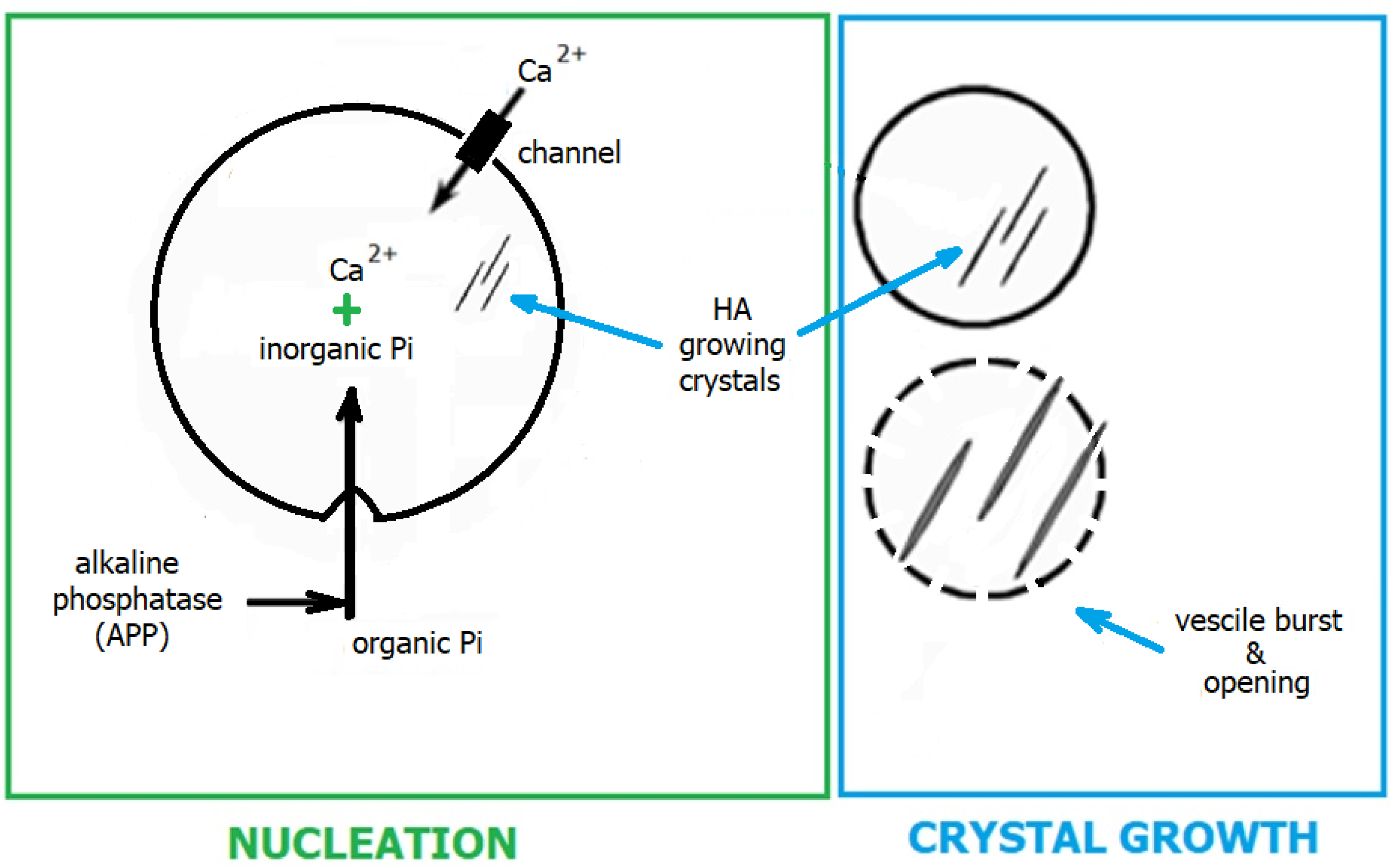
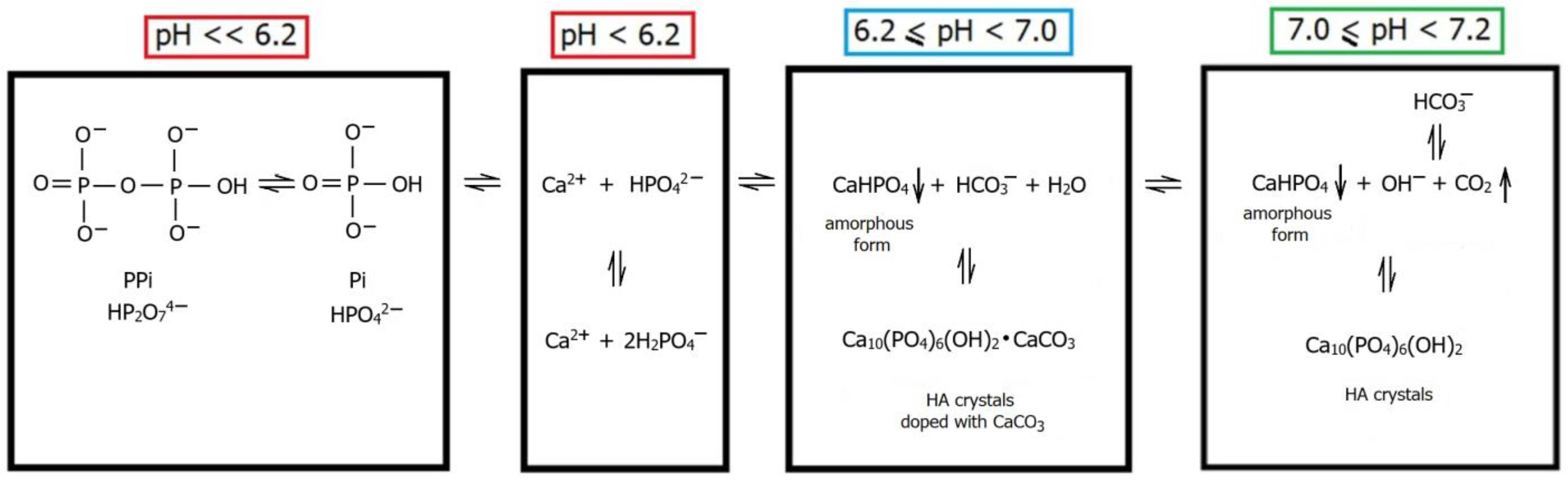
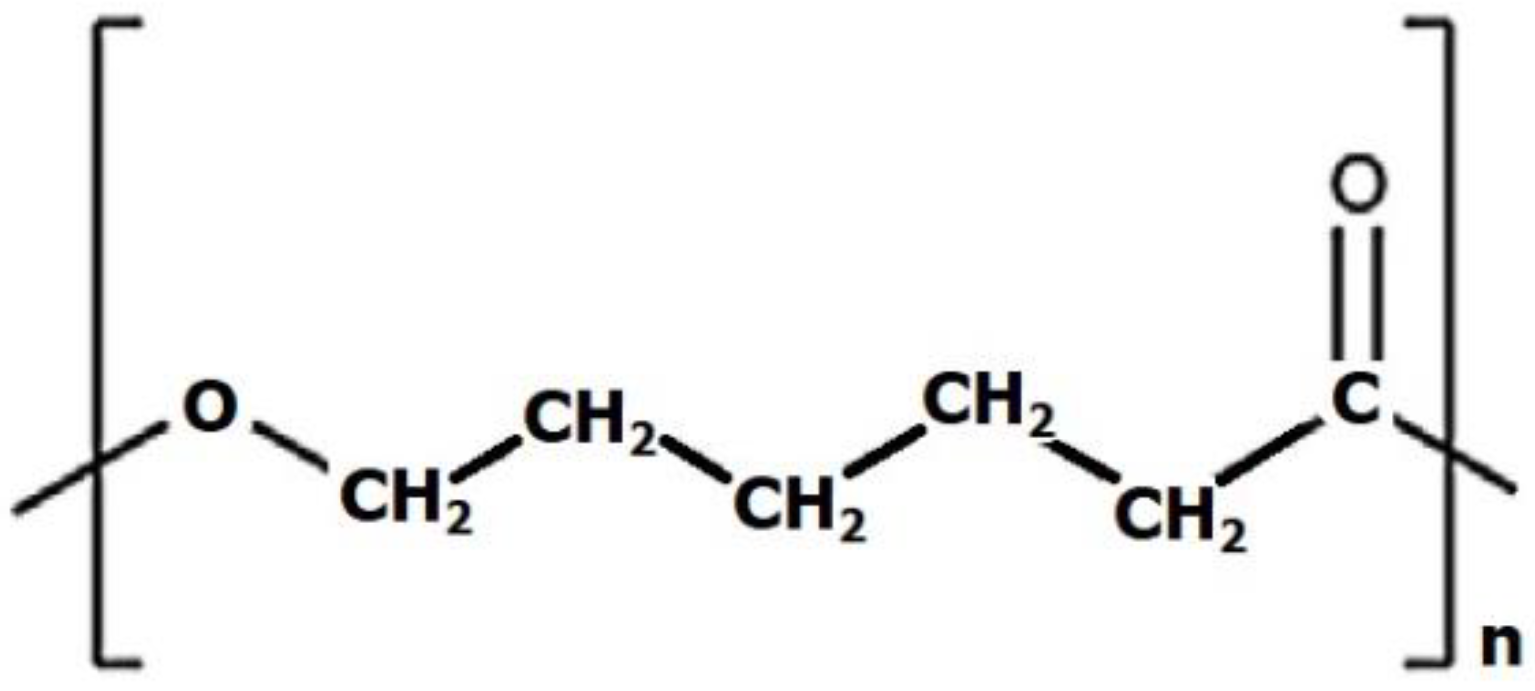
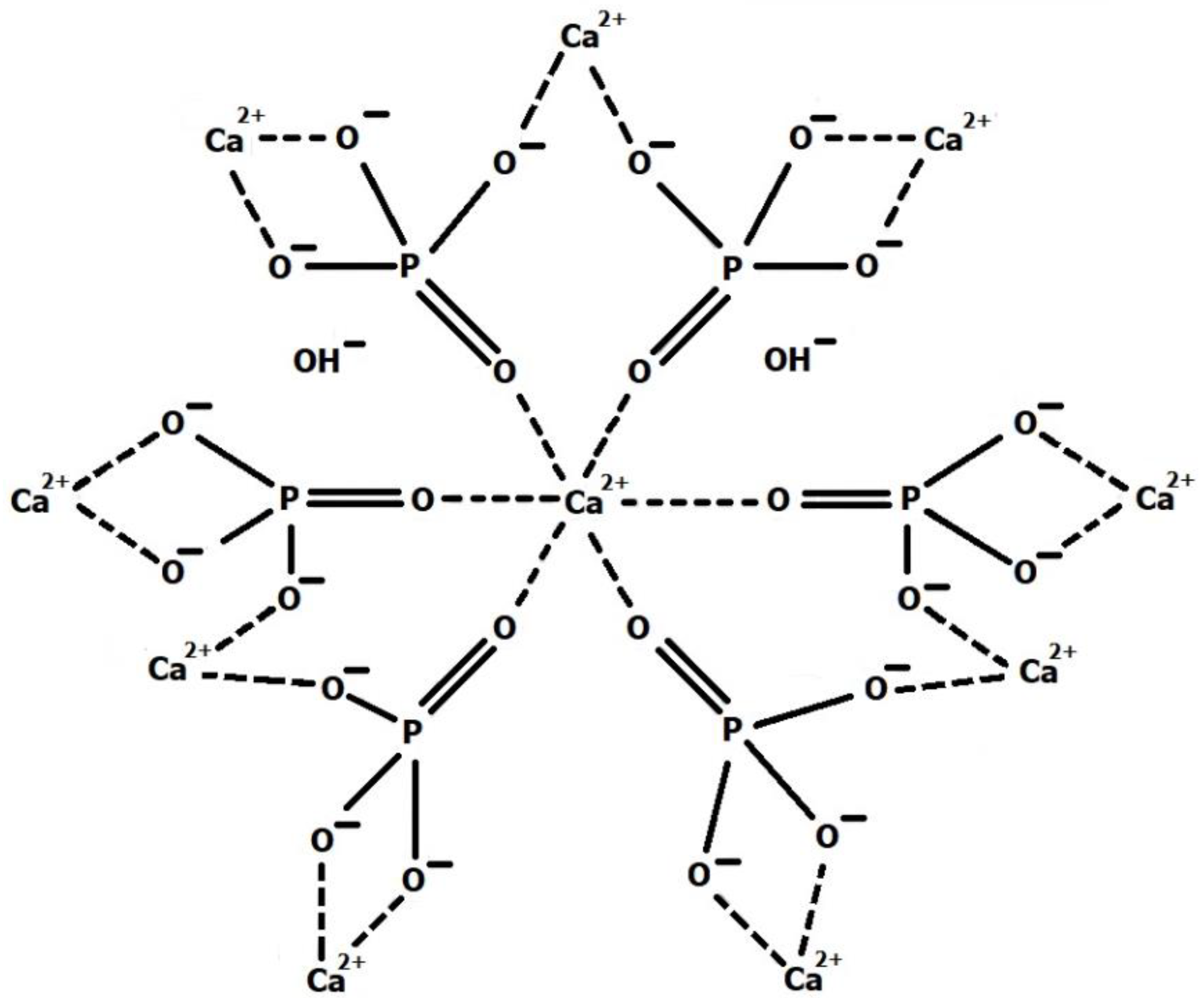
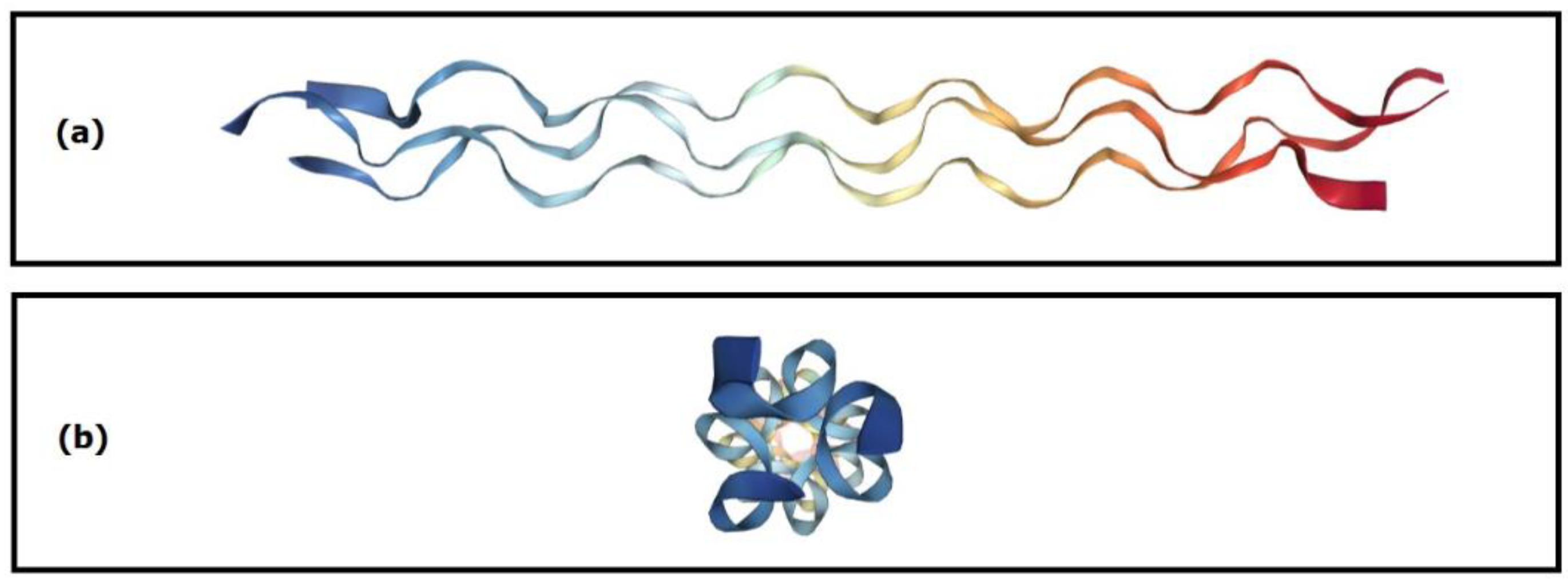
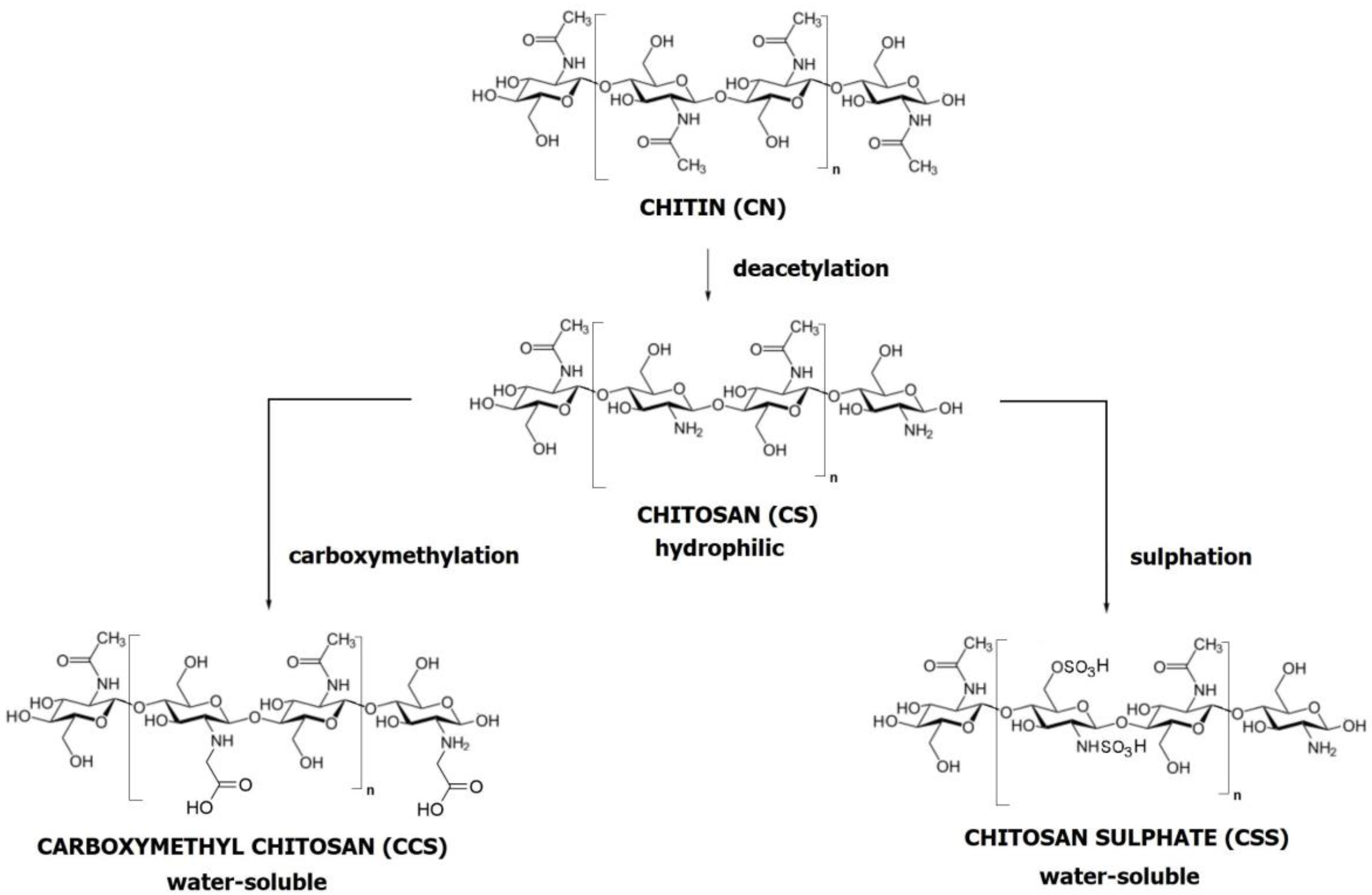
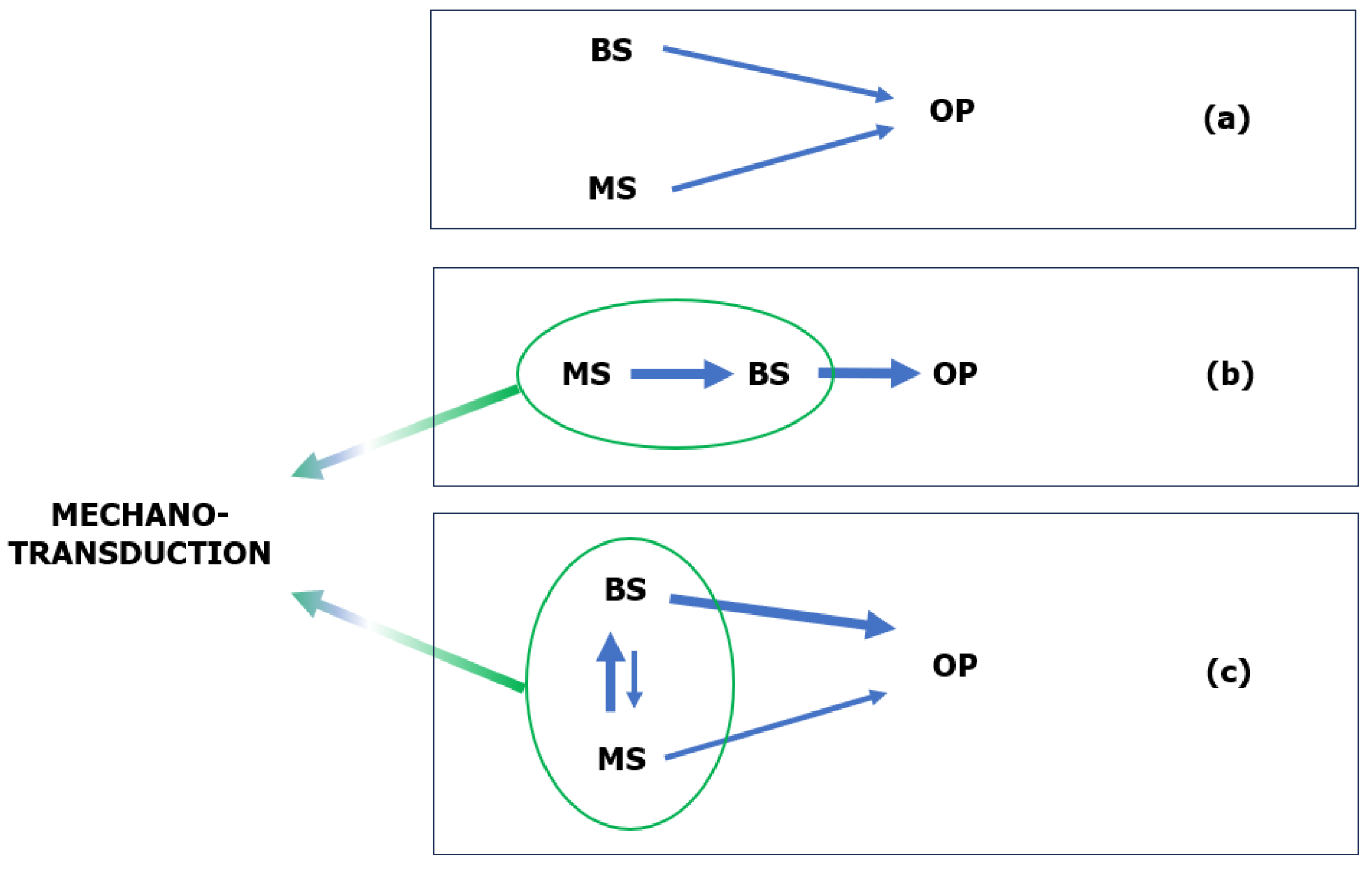

| Composite | 3D Printing Method | Scaffold Shape | Scaffold Dimensions (mm) | Interfibre Gap/Pore Size (μm) | Scaffold (Macro) Porosity (%) | Ref. |
|---|---|---|---|---|---|---|
| PCL/β-TCP/CGI | SLS | Cylindrical | 12.00(D) × 2.40(H) | 300–500 | 75–77 | Liao et al. [17] |
| PCL/HA, PCL/β-TCP | FDM | Cubic | Mechanical tests: 10.0(L) × 10.0(W)× 10.0(T); | Approx. 800 | Approx. 60 | Nyberg et al. [228] |
| Cylindrical | Other tests: 4.00(D) × 0.64(H) | |||||
| PCL/CS | E-jet 3D printing | Cuboidal | 21.0 ± 1.7(L) × 12.6 ± 0.9(W) × 123.1 ± 7.2(T) | 195.8 ± 9.1 | 74 ± 3 | Wu et al. [229] |
| PCL (subsequently modified by a CS thermogel) | FDM | Cuboidal | Mechanical tests: 10.0(L) × 10.0(W) × 2.0(T); | 325.2 ± 26.3 | 62.4 ± 0.23 | Dong et al. [67] |
| Cylindrical | 6.0(D) × 2.0(H) | |||||
| PCL (subsequently modified by a CS thermogel) | FDM | Cylindrical | 15.0(D) × 5.0(H) | Approx. 1200 | Approx. 65 | Hernandez et al. [230] |
| PCL/β-TCP | Extrusion-based 3D printing | Cylindrical | 8.0(D) × 2.0(H) | Approx. 300 | Kim et al. [231] | |
| PCL/HA | SFF | Cubic | 5.0(L) × 5.0(W) × 5.0(T) | Approx. 350 | Approx. 45 | Cho et al. [232] |
| PCL/TCP | FDM | Cylindrical | 6.0(D) × 4.0(H) | Approx. 420 | Kurzyk et al. [233] | |
| PCL/GEL/BC/HA | FDM | Rectangular strips | 10.0(L) × 5.0(W) × 3.0(T); | Approx. 300 | Max. 80 | Cakmak et al. [234] |
| PCL/HA | Extrusion-based 3D printing | Rectangular | Mechanical tests: 10.0(L) × 10.0(W) × 2.5(T); | Approx. 700 | Gerdes et al. [122] | |
| Biological tests: 10.0(L) × 10.0(W) × 2.4(T) | ||||||
| PCL/PGA | FDM | Disc | Morphological analysis and mechanical tests: 4.0(D) × 4.0(H) | 250–400, 150–350, >450 | 60, 50, 75 | Hedayati et al. [235] |
| Cuboidal | Biodegradation tests: 10(L) × 6(W) × 2(T) | |||||
| PCL/TCP PCL/HA | Laboratory-prepared 3D plotting system | Cylindrical (ring) | 3(Din) × 8(Dout) × 3(H) | >200 100–200 <100 | Approx. 80 | Jeong et al. [236] |
| Plate | 10(D) × 3(H) | |||||
| PCL/HA/HS | FDM | Disc | 6.0(D) × 6.5(H) | 400 | 70.8 | Liu et al. [237] |
| Biological tests: 6.0(D) × 1.0(H) | ||||||
| PCL (subsequently modified by PEGDA) | FDM | Anatomic | 24.7(L) × 11.8(W) × approx. 4(T) | 284 ± 31 | 63 | Moura et al. [238] |
| PCL/PLA/HA (subsequently modified by CS/HS) | FFF | Cylindrical | Mechanical specimen: 6.0(D) × 8.0(H) | 500 | Thunsiri et al. [239] | |
| Cubic | Mechanical specimen: 10.0(D) × 10.0(H) Biodegradation specimen: 5.0(L) × 5.0(W) × 5.0(T) | |||||
| PCL/HA | Dual-extruder 3D printing | Cuboidal | 1.0(L) × 5.0(W) × 5.0(T) | 77.1 ± 13.8 | El-Habashy et al. [240] | |
| PCL/HA | FFF | Cuboidal | 15(L) × 15(W) × 5(T) 15(L) × 15(W) × 3(T) 35(L) × 13(W) × 8(T) | Approx. 1200 | Kim et al. [241] | |
| PCL/HA | Cryogenic 3D printing | Orthogonal grid | 10(L) × 10(W) × 2(T) | 235 ± 43 | 42.84 ± 1.41 | Li et al. [242] |
| PLA/PCL/HA | Indirect 3D printing | Cubic | 5(L) × 5(W) × 5(T) | 141.0 ± 47.0 | 69.00–70.00 | Oryan et al. [117] |
| PCL/HA/SPION | 3D printing with a screw-based extruding system | Cubic | 5(L) × 5(W) × 5(T) | Approx. 250 | 45.81 | Petretta et al. [243] |
| Disc | 15.0(D) × 5.0(L) | |||||
| PCL/AgNps | FDM | Disc | 10.0(D) | 431.7 ± 24.6 | Radhakrishnan et al. [131] | |
| PCL/CS | 3D bioprinting | Cuboidal | 10(L) × 10(W) × 5(T) | 360 | Approx. 55 | Rezaei et al. [244] |
| PCL | 3D printing with novel plasma-assisted bioextrusion system | Cuboidal | 11(L) × 11(W) × 5(H) | 500 1000 1500 2000 | 60.7 75.5 78.6 85.7 | Xu et al. [245] |
| PCL/CS PCL/β-TPC | 3D pneumatic melt extruded scaffold generation | Cuboidal | 16.0(L) ×16.0 (W) × approx. 4.0(T) | 381 ± 6 395 ± 17 | 47.0 ± 2.0– 63.0 ± 2.0 | Yoshida et al. [121] |
| Biological tests: 4.0(L) × 4.0(W) × approx. 2.5(T) | ||||||
| PCL/HA | 3D printing with pneumatic chamber system | Cuboidal | Mechanical tests: 12(L) × 12(W) × 4(T) | Approx. 300 | Zimmerling et al. [132] | |
| PCL/HA | 3D printing with a screw-fitted chamber system | Cylindrical | 10(D) × 2.5(H) | Approx. 350 | 58.0–60.0 | Biscaia et al. [246] |
| PCL | Combination of 3D printing and electrospinning | Disc | 21(D) × 1(H) | Approx. 300 | Gonzalez-Pujana et al. [247] | |
| PCL/HA | FFF | Cylinder | Mechanical tests: Approx. 1.6(D) × 80(H) | Approx. 400 | 37.0 | Rezania et al. [22] |
| PCL/HA/PEGDA | 3D printing with extrusion system | Disc | 10(D) × 3(H) | 380 | 51.53 ± 2.00 –52.20± 1.67 | Sousa et al. [248] |
| PCL/HA | FFF | Cuboid | Morphological analysis: 15(L) × 15(D) × 2(H) Mechanical tests: 15(L) × 15(D) × 4(H) | Approx. 550 | 60.0 ± 0.9 –65.4 ± 0.3 | Wang et al. [16] |
| PCL/HA PCL/TCP | FDM | Cuboid | 31.0(L) × 26.7(D) × 10.0(H) | Gradient of approx. 200, 300 and 500, 600 | Approx. 52.0 | Daskalakis et al. [34] |
| PCL | SLS | Disc | 20(D) × 2(H) | Approx. 500, and approx. 700 | 49.7 ± 3.0–63.6 ± 3.0 | Janmohammadi et al. [18] |
| PCL/CS (subsequently loaded with VAN) | FDM | Disc-shaped | 8.00(D) × 1.50(H) | Approx. 200 | Approx. 59.0 | López-González et al. [249] |
| PCL/CS | RP | Anatomic (mandibular condyle) | 100–200 | Lee et al. [250] | ||
| PCL/MMBG/Al2O3 | LDM | Cubic | 5(L) × 5(W) × 5(T) | 0.005–0.020 (mesopores) | Above 80 | Najafabadi et al. [251] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stafin, K.; Śliwa, P.; Piątkowski, M. Towards Polycaprolactone-Based Scaffolds for Alveolar Bone Tissue Engineering: A Biomimetic Approach in a 3D Printing Technique. Int. J. Mol. Sci. 2023, 24, 16180. https://doi.org/10.3390/ijms242216180
Stafin K, Śliwa P, Piątkowski M. Towards Polycaprolactone-Based Scaffolds for Alveolar Bone Tissue Engineering: A Biomimetic Approach in a 3D Printing Technique. International Journal of Molecular Sciences. 2023; 24(22):16180. https://doi.org/10.3390/ijms242216180
Chicago/Turabian StyleStafin, Krzysztof, Paweł Śliwa, and Marek Piątkowski. 2023. "Towards Polycaprolactone-Based Scaffolds for Alveolar Bone Tissue Engineering: A Biomimetic Approach in a 3D Printing Technique" International Journal of Molecular Sciences 24, no. 22: 16180. https://doi.org/10.3390/ijms242216180
APA StyleStafin, K., Śliwa, P., & Piątkowski, M. (2023). Towards Polycaprolactone-Based Scaffolds for Alveolar Bone Tissue Engineering: A Biomimetic Approach in a 3D Printing Technique. International Journal of Molecular Sciences, 24(22), 16180. https://doi.org/10.3390/ijms242216180