CRISPR/Cas9 Mutagenesis through Introducing a Nanoparticle Complex Made of a Cationic Polymer and Nucleic Acids into Maize Protoplasts
Abstract
:1. Introduction
2. Results and Discussion
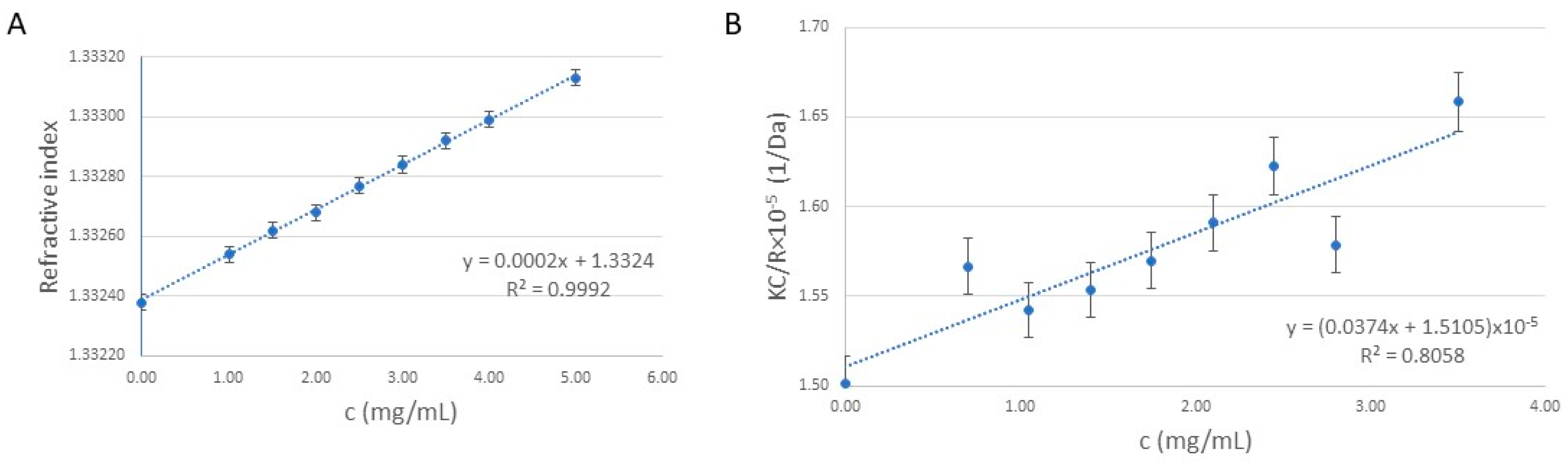
2.1. Synthesis and Molecular Weight Determination of the Cationic Polymer (Poly(2-hydroxy-propylene Imine), PHPI)
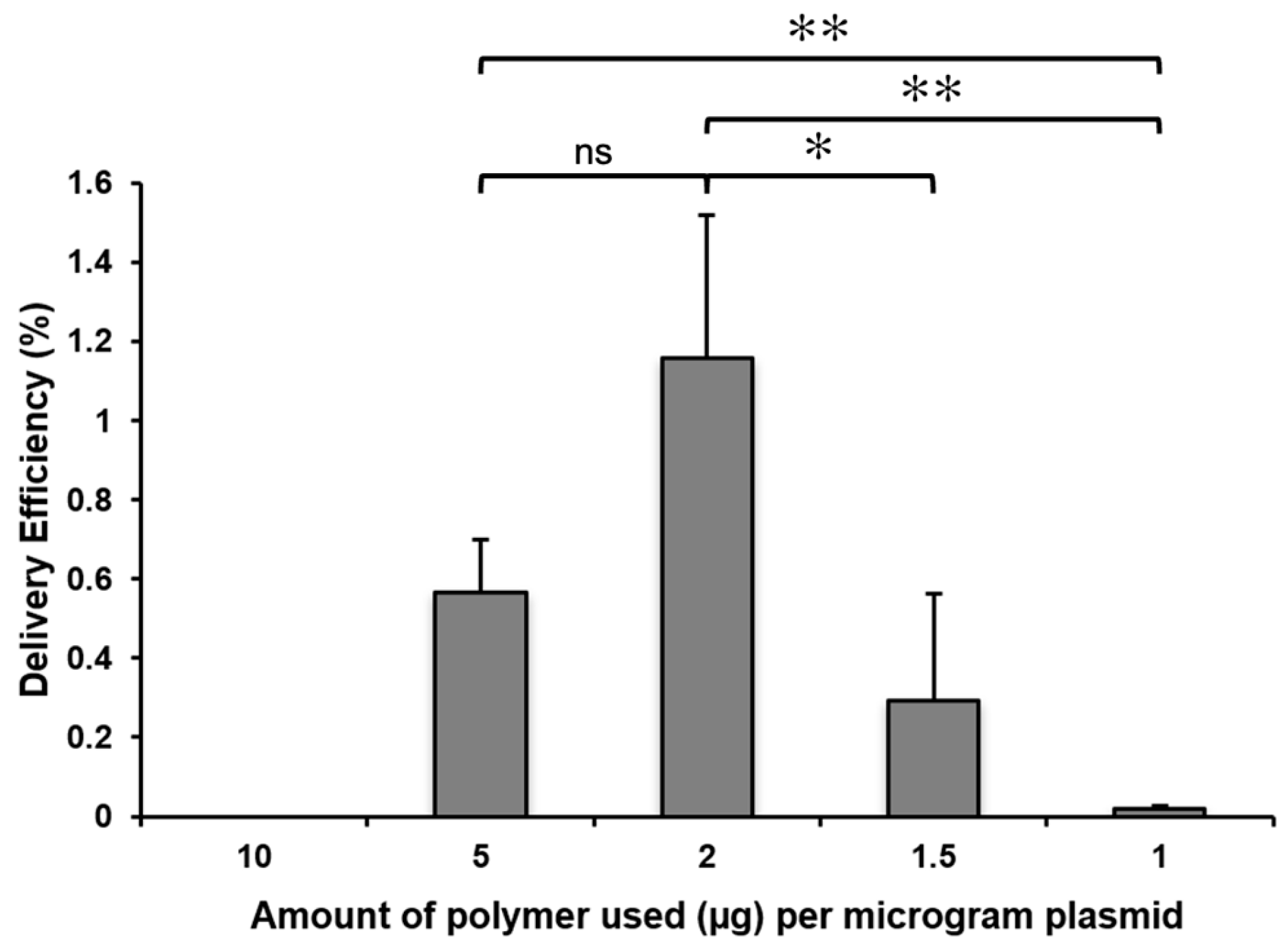
2.2. The Concentration of the Cationic Polymer and the Plasmid/Polymer Ratio Influence the Efficiency of the Delivery of GFP Plasmids
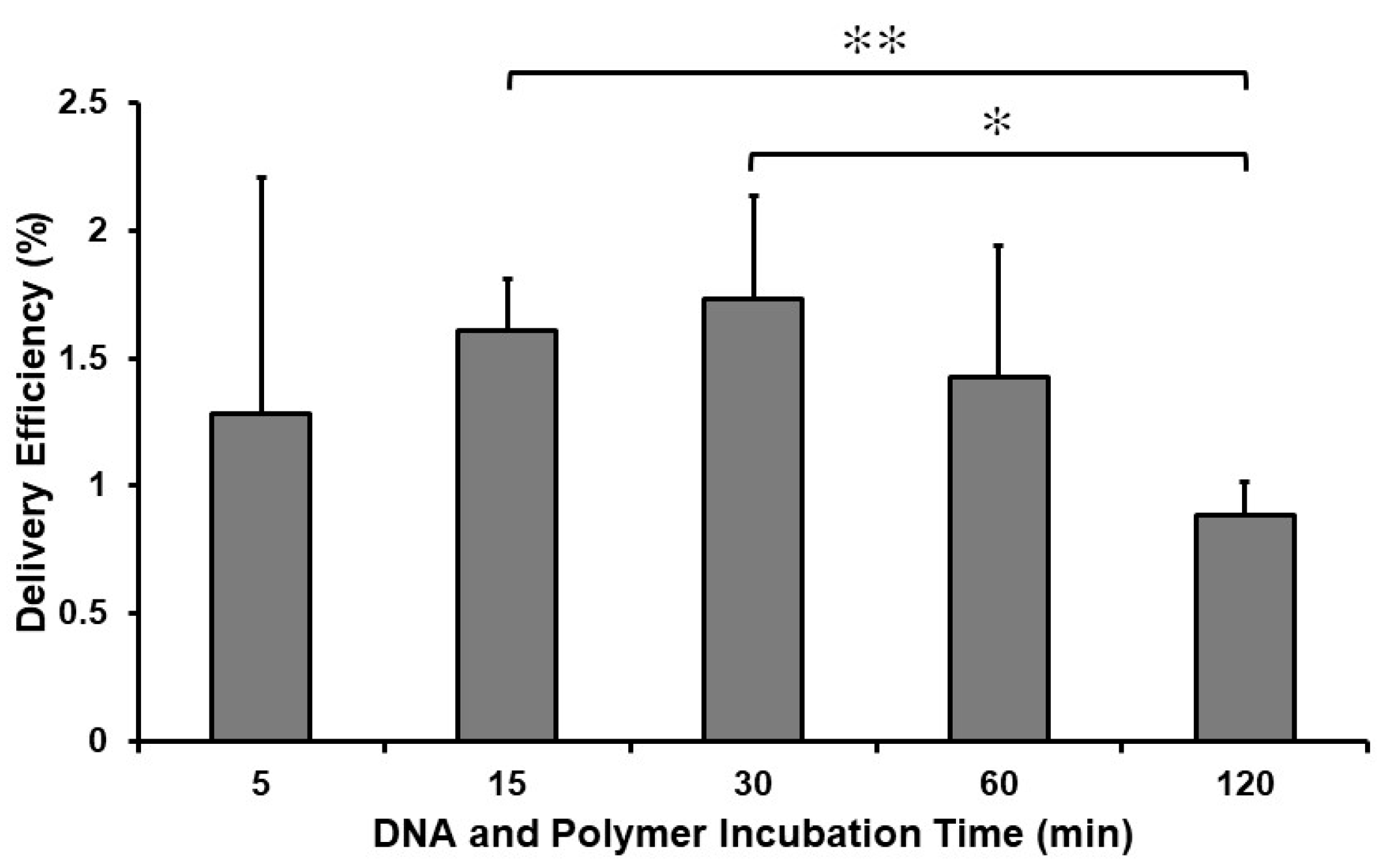
2.3. Incubation Time with the Complex Determines the Efficiency of the Delivery of GFP Plasmids
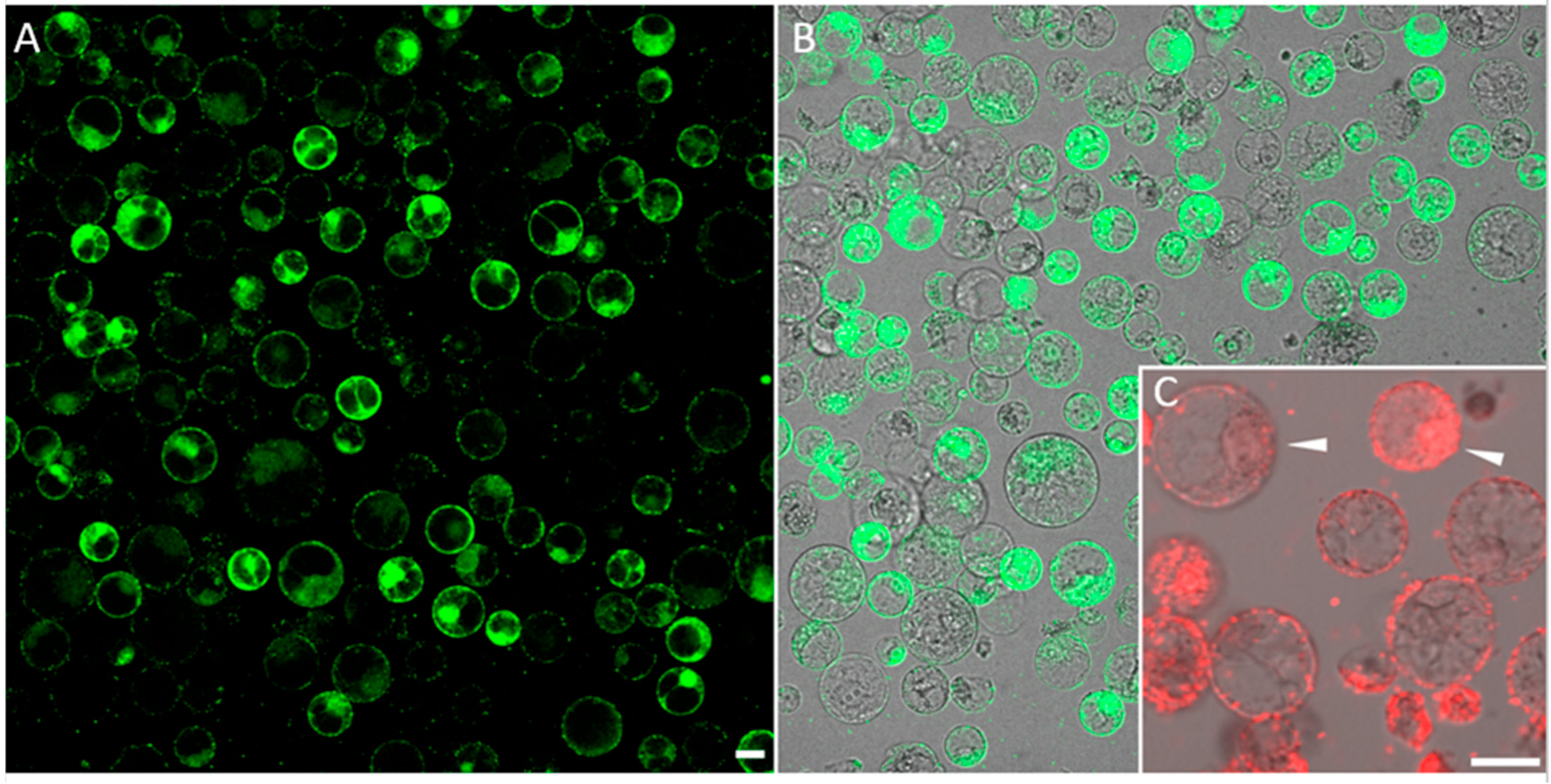
2.4. Cationic Polymer Poly (2-hydroxypropylene Imine) (PHPI) Can Be Complexed with Short Synthetic DNA Molecules and Used for the Introduction of Oligonucleotides into the Membranes and Nuclei of Maize Protoplasts
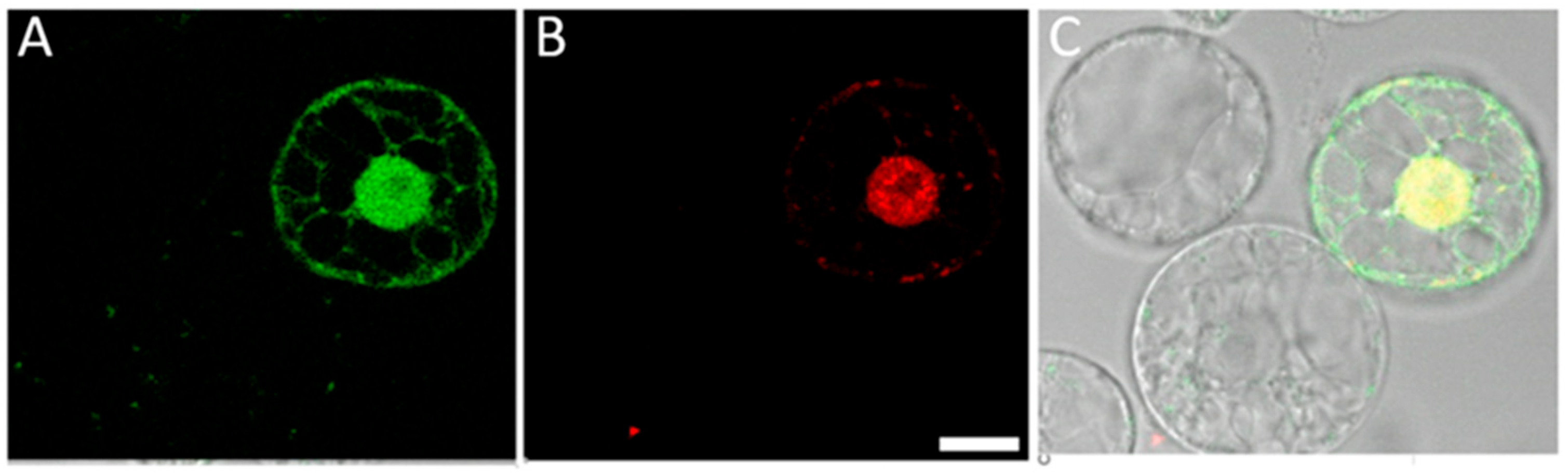
2.5. Co-Delivery of Synthetic Oligonucleotides (ssODNs) and Plasmids into Maize Protoplasts through a PHPI Cationic Polymer
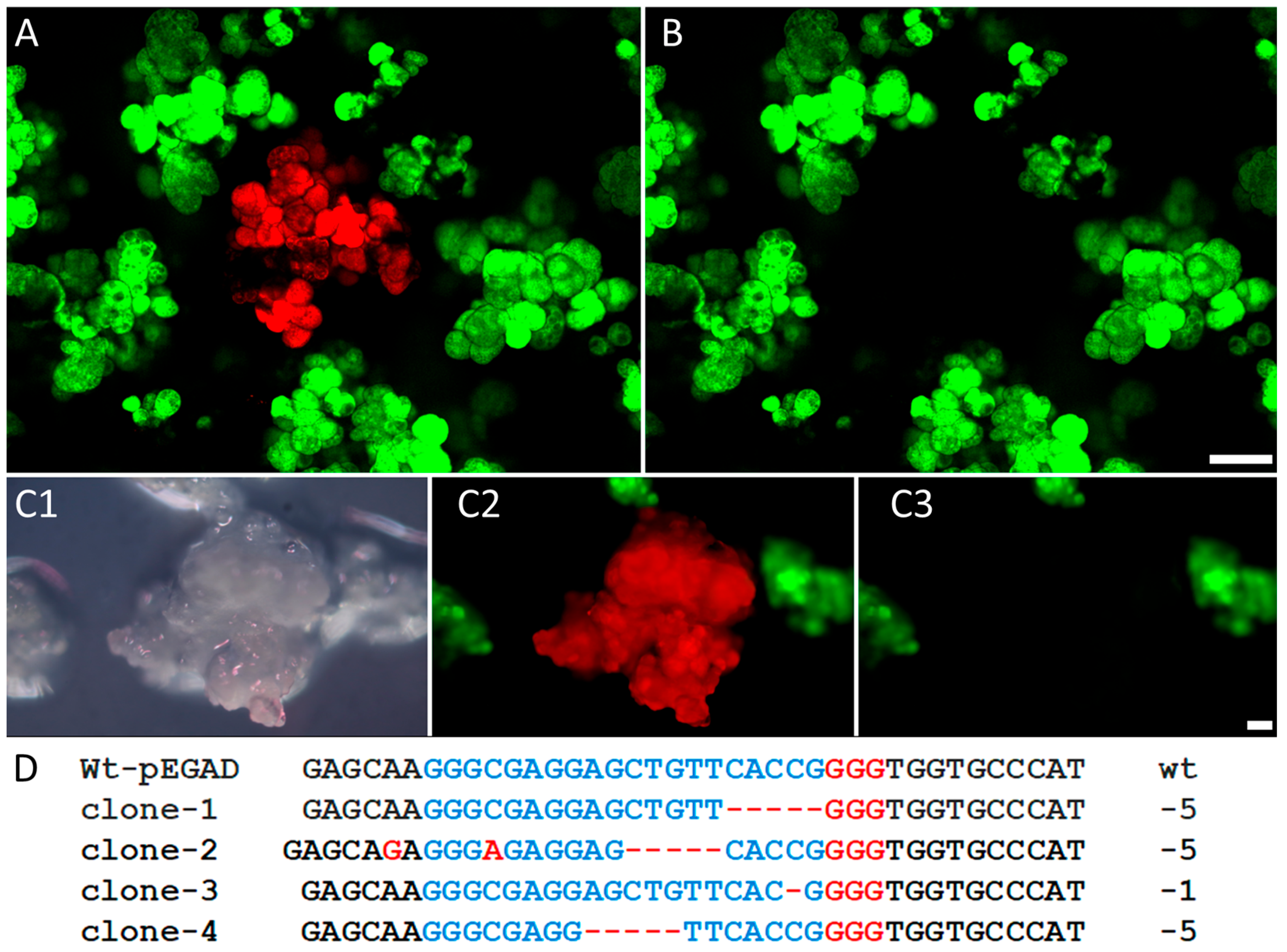
2.6. The PHPI Cationic Polymer-Induced Uptake of CRISPR/Cas9 Plasmids Can Generate Deletion and Insertion Mutations in the Marker GFP Transgene
3. Materials and Methods
3.1. Synthesis and Quantification of the Cationic Polymer Poly(2-hydroxypropylene Imine) (PHPI)
3.2. Theoretical Background of the Molecular Weight Measurement
3.3. Isolation and Culture of Maize Protoplasts
3.4. Introduction of Plasmid DNAs and Synthetic Oligonucleotides into Maize Protoplasts through a Cationic Polymer
3.5. Cationic Polymer Complexed with CRISPR/Cas9 Editing Plasmids for Targeted Mutagenesis of the Transgenic EGFP Gene in Cultured Maize Cells
3.6. PCR Amplification and Sequencing of the Green Enhanced Fluorescence Transgene
3.7. Confocal Fluorescence Microscopy
3.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, Y.; Sun, K.; An, X. CRISPR/Cas System: Applications and Prospects for Maize Improvement. ACS Agric. Sci. Technol. 2022, 2, 174–183. [Google Scholar] [CrossRef]
- Gordon-Kamm, W.; Barone, P.; Svitashev, S.; Sander, J.D.; Kumar, S.; Jones, T. Strategies for CRISPR/Cas9-Mediated Genome Editing: From Delivery to Production of Modified Plants; Burleigh Dodds Science Publishing: Sawston, UK, 2021. [Google Scholar]
- Rivera-Torres, N.; Kmiec, E.B. Genetic spell-checking: Gene editing using single-stranded DNA oligonucleotides. Plant Biotechnol. J. 2016, 14, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Sauer, N.J.; Mozoruk, J.; Miller, R.B.; Warburg, Z.J.; Walker, K.A.; Beetham, P.R.; Schöpke, C.R.; Gocal, G.F.W. Oligonucleotide-directed mutagenesis for precision gene editing. Plant Biotechnol. J. 2016, 14, 496–502. [Google Scholar] [CrossRef]
- Tiricz, H.; Nagy, B.; Ferenc, G.; Török, K.; Nagy, I.; Dudits, D.; Ayaydin, F. Relaxed chromatin induced by histone deacetylase inhibitors improves the oligonucleotide-directed gene editing in plant cells. J. Plant Res. 2018, 131, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Rádi, F.; Nagy, B.; Ferenc, G.; Török, K.; Nagy, I.; Zombori, Z.; Dudits, D.; Ayaydin, F. In planta test system for targeted cellular mutagenesis by injection of oligonucleotides to apical meristem of maize seedlings. Acta Physiol. Plant. 2021, 43, 79. [Google Scholar] [CrossRef]
- Fierlej, Y.; Jacquier, N.M.A.; Guille, L.; Just, J.; Montes, E.; Richard, C.; Loue-Manifel, J.; Depège-Fargeix, N.; Gaillard, A.; Widiez, T.; et al. Evaluation of genome and base editing tools in maize protoplasts. Front. Plant Sci. 2022, 13, 1010030. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Hsu, C.T.; Yang, L.H.; Lee, L.Y.; Fu, J.Y.; Cheng, Q.W.; Wu, F.H.; Hsiao, H.C.W.; Zhang, Y.; Zhang, R.; et al. Application of protoplast technology to CRISPR/Cas9 mutagenesis: From single-cell mutation detection to mutant plant regeneration. Plant Biotechnol. J. 2018, 16, 1295–1310. [Google Scholar] [CrossRef]
- Lin, C.-S.; Hsu, C.-T.; Yuan, Y.-H.; Zheng, P.-X.; Wu, F.-H.; Cheng, Q.-W.; Wu, Y.-L.; Wu, T.-L.; Lin, S.; Yue, J.-J.; et al. DNA-free CRISPR-Cas9 gene editing of wild tetraploid tomato Solanum peruvianum using protoplast regeneration. Plant Physiol. 2022, 188, 1917–1930. [Google Scholar] [CrossRef]
- González, M.N.; Massa, G.A.; Andersson, M.; Décima Oneto, C.A.; Turesson, H.; Storani, L.; Olsson, N.; Fält, A.-S.; Hofvander, P.; Feingold, S.E. Comparative potato genome editing: Agrobacterium tumefaciens-mediated transformation and protoplasts transfection delivery of CRISPR/Cas9 components directed to StPPO2 gene. Plant Cell Tissue Organ Cult. 2021, 145, 291–305. [Google Scholar] [CrossRef]
- Liu, Y.; Andersson, M.; Granell, A.; Cardi, T.; Hofvander, P.; Nicolia, A. Establishment of a DNA-free genome editing and protoplast regeneration method in cultivated tomato (Solanum lycopersicum). Plant Cell Rep. 2022, 41, 1843–1852. [Google Scholar] [CrossRef]
- Najafi, S.; Bertini, E.; D’incà, E.; Fasoli, M.; Zenoni, S. DNA-free genome editing in grapevine using CRISPR/Cas9 ribonucleoprotein complexes followed by protoplast regeneration. Hortic. Res. 2023, 10, uhac240. [Google Scholar] [CrossRef] [PubMed]
- Vats, S.; Kumawat, S.; Brar, J.; Kaur, S.; Yadav, K.; Magar, S.G.; Jadhav, P.V.; Salvi, P.; Sonah, H.; Sharma, S.; et al. Opportunity and challenges for nanotechnology application for genome editing in plants. Plant Nano Biol. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Kaur, P.; Stanton, D.; Grosser, J.W.; Dutt, M. A cationic lipid mediated CRISPR/Cas9 technique for the production of stable genome edited citrus plants. Plant Methods 2022, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Olden, B.R.; Cheng, Y.; Yu, J.L.; Pun, S.H. Cationic polymers for non-viral gene delivery to human T cells. J. Control. Release 2018, 282, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Boussif, O.; Lezoualc’H, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.A.; Strong, D.D.; Zimmer, W.E. Nuclear entry of nonviral vectors. Gene Ther. 2005, 12, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Wirtz, D.; Hanes, J. Efficient active transport of gene nanocarriers to the cell nucleus. Proc. Natl. Acad. Sci. USA 2003, 100, 3878–3882. [Google Scholar] [CrossRef]
- Pollard, H.; Remy, J.-S.; Loussouarn, G.; Demolombe, S.; Behr, J.-P.; Escande, D. Polyethylenimine but Not Cationic Lipids Promotes Transgene Delivery to the Nucleus in Mammalian Cells. J. Biol. Chem. 1998, 273, 7507–7511. [Google Scholar] [CrossRef]
- Finiuk, N.; Buziashvili, A.; Burlaka, O.; Zaichenko, A.; Mitina, N.; Miagkota, O.; Lobachevska, O.; Stoika, R.; Blume, Y.; Yemets, A. Investigation of novel oligoelectrolyte polymer carriers for their capacity of DNA delivery into plant cells. Plant Cell Tissue Organ Cult. 2017, 131, 27–39. [Google Scholar] [CrossRef]
- An, Z.; Cao, B.; Zhang, J.; Zhang, B.; Zhou, C.; Hu, X.; Chen, W. Efficient Transient Expression of Plasmid DNA Using Poly (2-(N,N-Dimethylamino) Ethyl Methacrylate) in Plant Cells. Front. Bioeng. Biotechnol. 2022, 10, 805996. [Google Scholar] [CrossRef]
- Zaliauskiene, L.; Bernadisiute, U.; Vareikis, A.; Makuska, R.; Volungeviciene, I.; Petuskaite, A.; Riauba, L.; Lagunavicius, A.; Zigmantas, S. Efficient Gene Transfection Using Novel Cationic Polymers Poly(hydroxyalkylene imines). Bioconjug. Chem. 2010, 21, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Bono, N.; Ponti, F.; Mantovani, D.; Candiani, G. Non-Viral in Vitro Gene Delivery: It Is Now Time to Set the Bar! Pharmaceutics 2020, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Gary, D.J.; Min, J.; Kim, Y.; Park, K.; Won, Y.-Y. The Effect of N/P Ratio on the In Vitro and In Vivo Interaction Properties of Pegylated Poly[2-(Dimethylamino)Ethyl Methacrylate]-Based siRNA Complexes. Macromol. Biosci. 2013, 13, 1059–1071. [Google Scholar] [CrossRef]
- Dong, C.; Beetham, P.; Vincent, K.; Sharp, P. Oligonucleotide-directed gene repair in wheat using a transient plasmid gene repair assay system. Plant Cell Rep. 2006, 25, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Sauer, N.J.; Narváez-Vásquez, J.; Mozoruk, J.; Miller, R.B.; Warburg, Z.J.; Woodward, M.J.; Mihiret, Y.A.; Lincoln, T.A.; Segami, R.E.; Sanders, S.L.; et al. Oligonucleotide-Mediated Genome Editing Provides Precision and Function to Engineered Nucleases and Antibiotics in Plants. Plant Physiol. 2016, 170, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, Q.; Kang, Y.; Wang, X.; Zhang, H.; Li, X. Analysis of the Utilization and Prospects of CRISPR-Cas Technology in the Annotation of Gene Function and Creation New Germplasm in Maize Based on Patent Data. Cells 2022, 11, 3471. [Google Scholar] [CrossRef] [PubMed]
- Mórocz, S.; Donn, G.; Nérneth, J.; Dudits, D. An improved system to obtain fertile regenerants via maize protoplasts isolated from a highly embryogenic suspension culture. Theor. Appl. Genet. 1990, 80, 721–726. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, B.; Öktem, A.; Ferenc, G.; Ungor, D.; Kalac, A.; Kelemen-Valkony, I.; Fodor, E.; Nagy, I.; Dudits, D.; Ayaydin, F. CRISPR/Cas9 Mutagenesis through Introducing a Nanoparticle Complex Made of a Cationic Polymer and Nucleic Acids into Maize Protoplasts. Int. J. Mol. Sci. 2023, 24, 16137. https://doi.org/10.3390/ijms242216137
Nagy B, Öktem A, Ferenc G, Ungor D, Kalac A, Kelemen-Valkony I, Fodor E, Nagy I, Dudits D, Ayaydin F. CRISPR/Cas9 Mutagenesis through Introducing a Nanoparticle Complex Made of a Cationic Polymer and Nucleic Acids into Maize Protoplasts. International Journal of Molecular Sciences. 2023; 24(22):16137. https://doi.org/10.3390/ijms242216137
Chicago/Turabian StyleNagy, Bettina, Ayşegül Öktem, Györgyi Ferenc, Ditta Ungor, Aladina Kalac, Ildikó Kelemen-Valkony, Elfrieda Fodor, István Nagy, Dénes Dudits, and Ferhan Ayaydin. 2023. "CRISPR/Cas9 Mutagenesis through Introducing a Nanoparticle Complex Made of a Cationic Polymer and Nucleic Acids into Maize Protoplasts" International Journal of Molecular Sciences 24, no. 22: 16137. https://doi.org/10.3390/ijms242216137
APA StyleNagy, B., Öktem, A., Ferenc, G., Ungor, D., Kalac, A., Kelemen-Valkony, I., Fodor, E., Nagy, I., Dudits, D., & Ayaydin, F. (2023). CRISPR/Cas9 Mutagenesis through Introducing a Nanoparticle Complex Made of a Cationic Polymer and Nucleic Acids into Maize Protoplasts. International Journal of Molecular Sciences, 24(22), 16137. https://doi.org/10.3390/ijms242216137






