Lactoferrin-Anchored Tannylated Mesoporous Silica Nanomaterials-Induced Bone Fusion in a Rat Model of Lumbar Spinal Fusion
Abstract
:1. Introduction
2. Results
2.1. Manual Palpation
2.2. Micro-CT
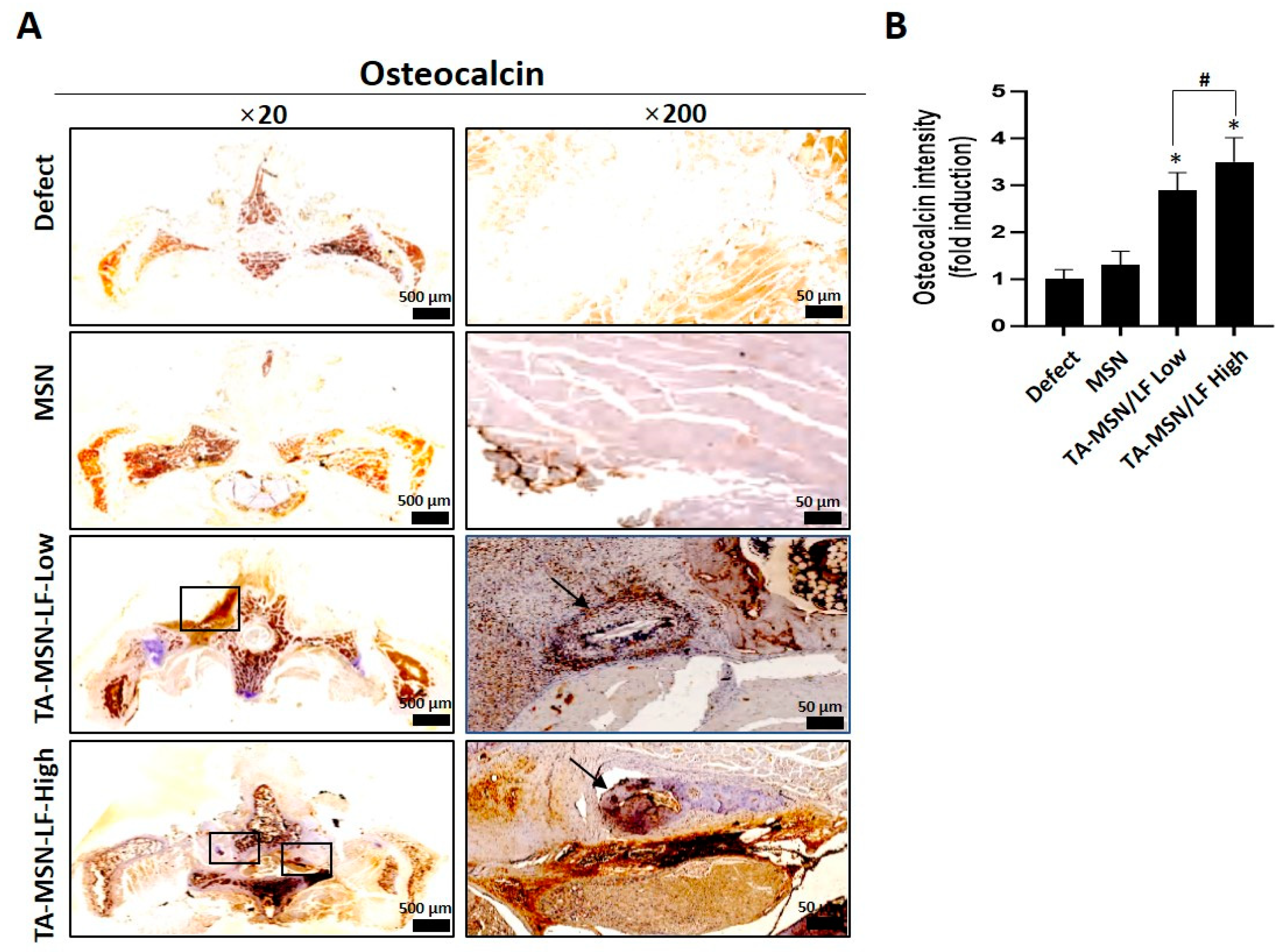
2.3. Histologic Results
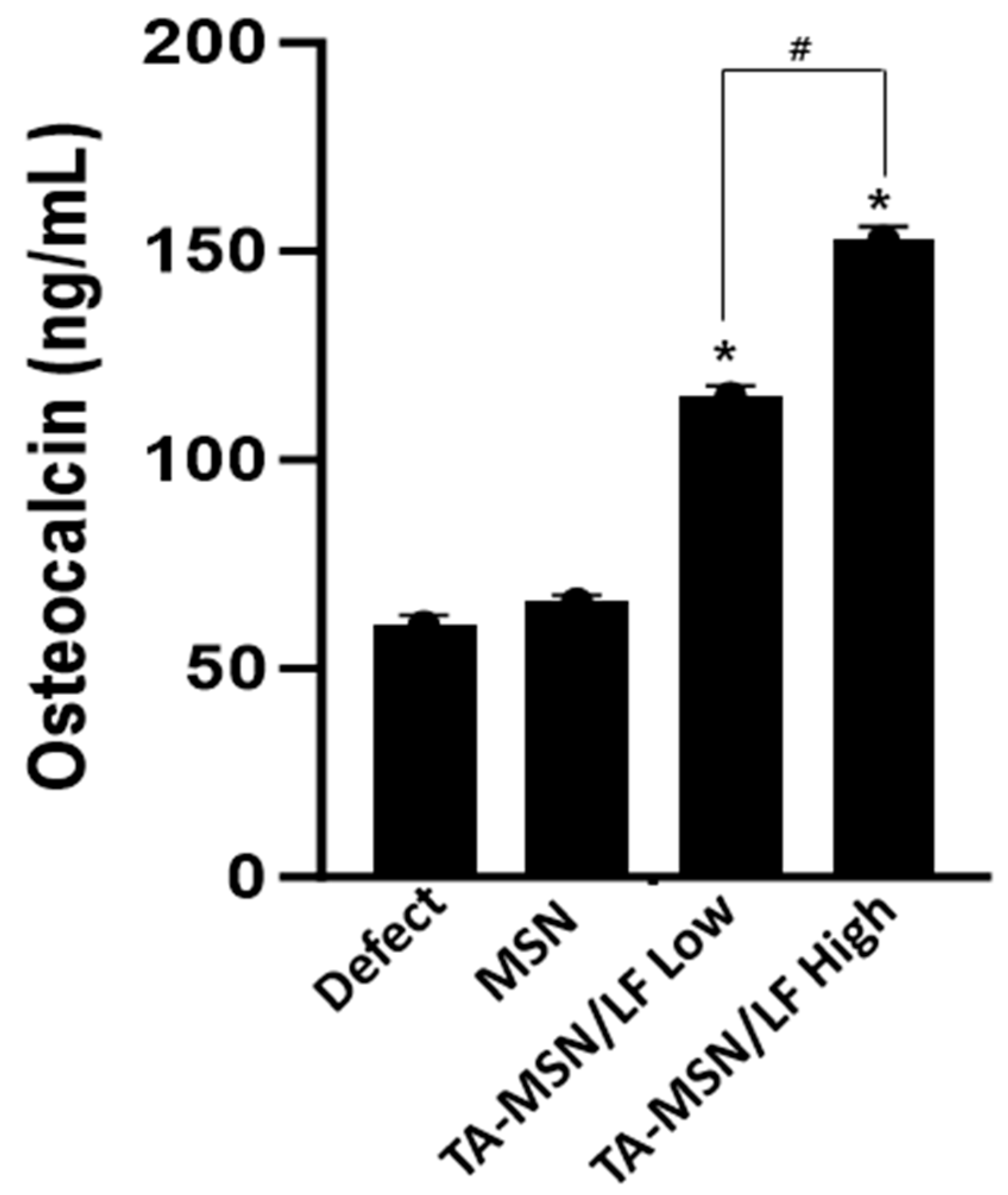
2.4. Bone Turnover Marker
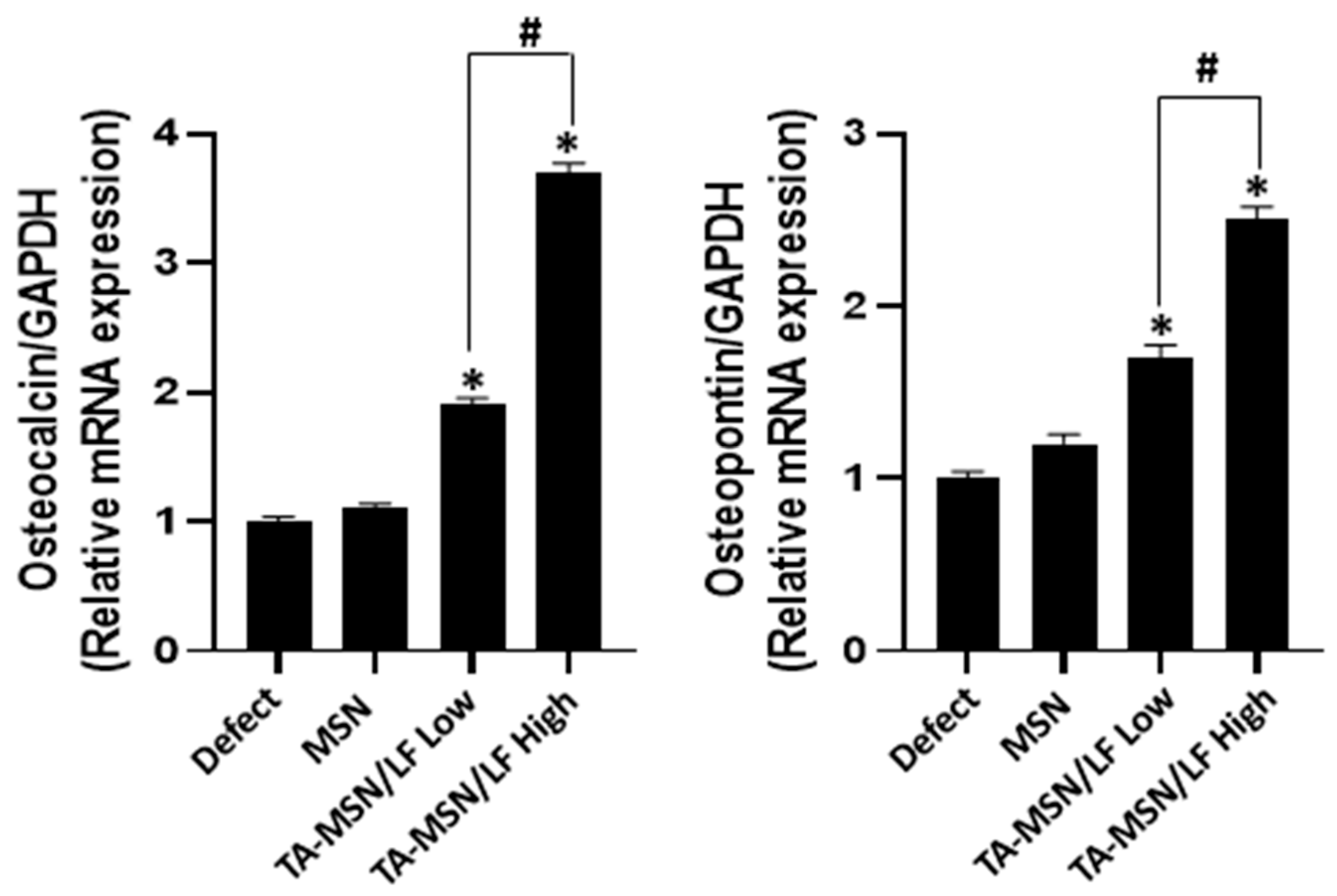
2.5. Quantification of Osteo-Differentiation-Specific Genes
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Materials
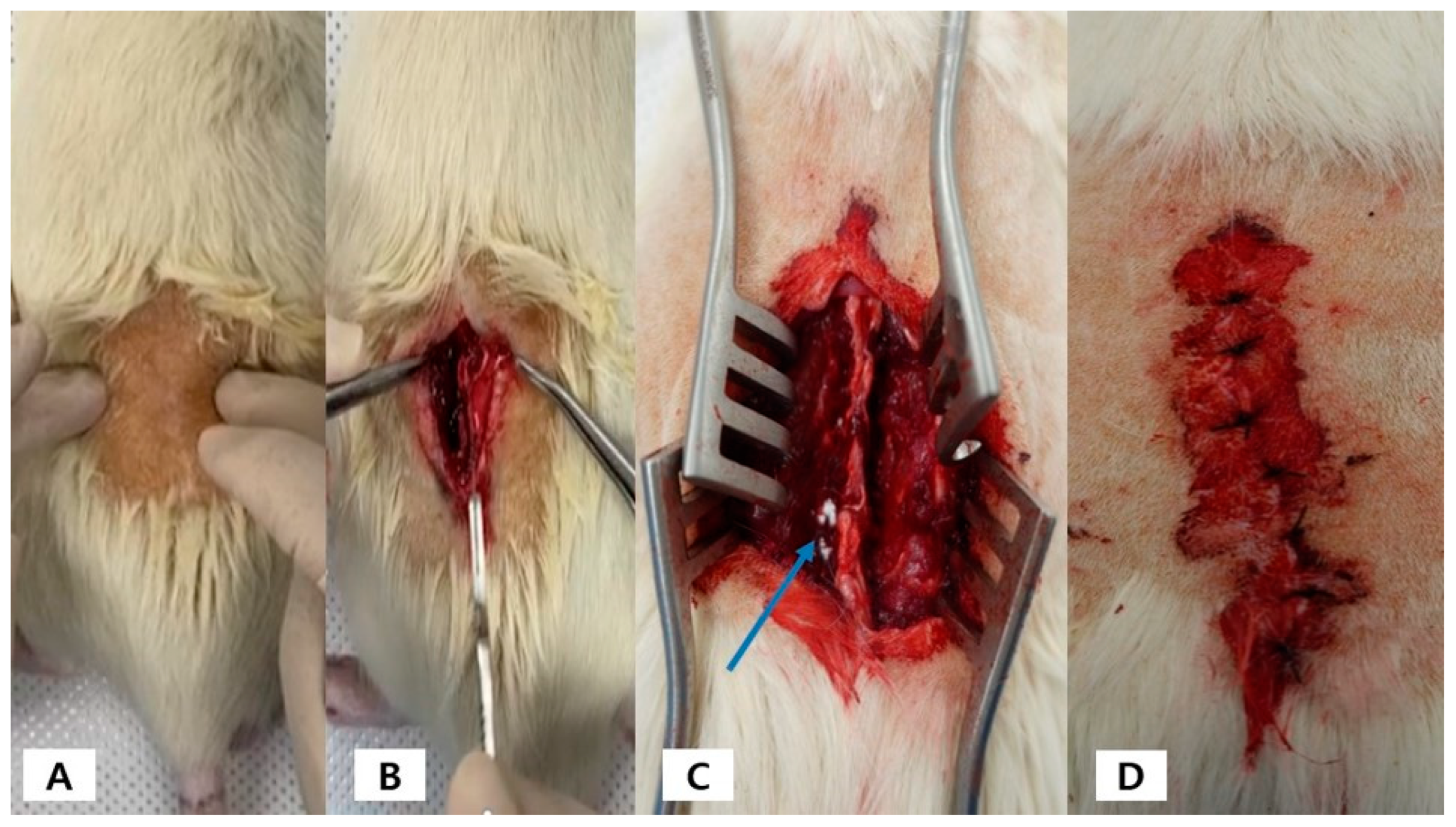
4.3. Experimental Design and Surgical Procedure
4.4. Manual Palpation
4.5. Micro CT
4.6. Histologic Evaluation
4.7. Bone Turnover Marker
4.8. Quantification of Osteo-Differentiation-Specific Genes
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reid, J.J.; Johnson, J.S.; Wang, J.C. Challenges to Bone Formation in Spinal Fusion. J. Biomech. 2011, 44, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, J.C.; Herkowitz, H.N. Pseudarthrosis of the Spine. Clin. Orthop. Relat. Res. 1992, 284, 80–90. [Google Scholar]
- Glassman, S.D.; Polly, D.W.; Bono, C.M.; Burkus, K.; Dimar, J.R. Outcome of lumbar arthrodesis in patients sixty-five years of age or older. J. Bone Jt. Surg. Am. 2009, 91, 783–790. [Google Scholar] [CrossRef]
- Patel, V.V.; Wuthrich, Z.R.; Ortega, A.; Ferguson, V.L.; Lindley, E.M. Recombinant Human Bone Morphogenetic Protein–2 Improves Spine Fusion in a Vitamin D–Deficient Rat Model. Int. J. Spine Surg. 2020, 14, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Benzel, E.; Ferrara, L.; Roy, S.; Fleischman, A. Micromachines in spine surgery. Spine 2004, 29, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lim, H.; Ahn, J.W.; Jang, D.; Lee, S.H.; Park, K.; Kim, S.E. Design of a 3D BMP-2-delivering tannylated PCL scaffold and its anti-oxidant, anti-inflammatory, and osteogenic effects in vitro. Int. J. Mol. Sci. 2018, 19, 3602. [Google Scholar] [CrossRef]
- Hettiaratchi, M.H.; Krishnan, L.; Rouse, T.; Chou, C.; McDevitt, T.C.; Guldberg, R.E. Heparin-mediated delivery of bone morphogenetic protein-2 improves spatial localization of bone regeneration. Sci. Adv. 2020, 6, eaay1240. [Google Scholar] [CrossRef] [PubMed]
- Cornish, J.; Naot, D. Lactoferrin as an Effector Molecule in the Skeleton. Biometals 2010, 23, 425–430. [Google Scholar] [CrossRef]
- Brock, J.H.; Takayama, Y.; Aoki, R.; León-Sicairos, N.; Martínez-Pardo, L.; Sánchez-Hernández, B.; de la Garza, M.; Carrero, J.C.; Ochoa, T.J.; Pezo, A.; et al. The Physiology of Lactoferrin. Biochem. Cell Biol. 2002, 80, 1–6. [Google Scholar] [CrossRef]
- Yanagisawa, S.; Nagasaki, K.; Chea, C.; Ando, T.; Ayuningtyas, N.F.; Inubushi, T.; Ishikado, A.; Imanaka, H.; Sugiyama, E.; Takahashi, I.; et al. Oral Administration of Bovine Lactoferrin Suppresses the Progression of Rheumatoid Arthritis in an SKG Mouse Model. PLoS ONE 2022, 17, e0263254. [Google Scholar] [CrossRef]
- Guo, H.Y.; Jiang, L.; Ibrahim, S.A.; Zhang, L.; Zhang, H.; Zhang, M.; Ren, F.Z. Orally Administered Lactoferrin Preserves Bone Mass and Microarchitecture in Ovariectomized Rats12. J. Nutr. 2009, 139, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Cornish, J.; Callon, K.E.; Naot, D.; Palmano, K.P.; Banovic, T.; Bava, U.; Watson, M.; Lin, J.-M.; Tong, P.C.; Chen, Q.; et al. Lactoferrin is a potent regulator of bone cell activity and increases bone formation in vivo. Endocrinology 2004, 145, 4366–4374. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.H.; Jo, H.; Choi, S.; Song, H.G.; Kim, H.; Kim, K.N.; Kim, S.E.; Park, K. Lactoferrin-Anchored Tannylated Mesoporous Silica Nanomaterials for Enhanced Osteo-Differentiation Ability. Pharmaceutics 2020, 13, 30. [Google Scholar] [CrossRef]
- Choi, H.J.; Choi, S.; Kim, J.G.; Song, M.H.; Shim, K.S.; Lim, Y.M.; Kim, H.J.; Park, K.; Kim, S.E. Enhanced tendon restoration effects of anti-inflammatory, lactoferrin-immobilized, heparin-polymeric nanoparticles in an Achilles tendinitis rat model. Carbohydr. Polym. 2020, 241, 116284. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Karambelkar, A.; Gu, L.; Lin, K.; Miller, J.S.; Chen, C.S.; Sailor, M.J.; Bhatia, S.N. Bioresponsive mesoporous silica nanoparticles for triggered drug release. J. Am. Chem. Soc. 2011, 133, 19582–19585. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Wang, C.C. Surface modification with peptide for enhancing chondrocyte adhesion and cartilage regeneration in porous scaffolds. Colloids Surf. B Biointerfaces 2011, 84, 63–70. [Google Scholar] [CrossRef]
- Abouelmagd, S.A.; Meng, F.; Kim, B.K.; Hyun, H.; Yeo, Y. Tannic acid-mediated surface functionalization of polymeric nanoparticles. ACS Biomater. Sci. Eng. 2016, 2, 2294–2303. [Google Scholar] [CrossRef]
- Liang, H.; Zhou, B.; Wu, D.; Li, J.; Li, B. Supramolecular design and applications of polyphenol-based architecture: A review. Adv. Colloid. Interface Sci. 2019, 272, 102019. [Google Scholar] [CrossRef]
- Yagi, M.; Suzuki, N.; Takayama, T.; Arisue, M.; Kodama, T.; Yoda, Y.; Otsuka, K.; Ito, K. Effects of Lactoferrin on the Differentiation of Pluripotent Mesenchymal Cells. Cell Biol. Int. 2009, 33, 283–289. [Google Scholar] [CrossRef]
- Balasubramanian, S.A.; Pye, D.C.; Willcox, M.D.P. Levels of Lactoferrin, Secretory IgA and Serum Albumin in the Tear Film of People with Keratoconus. Exp. Eye Res. 2012, 96, 132–137. [Google Scholar] [CrossRef]
- Montagne, P.; Cuillière, M.L.; Molé, C.; Béné, M.C.; Faure, G. Changes in Lactoferrin and Lysozyme Levels in Human Milk During the First Twelve Weeks of Lactation. In Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2001; Volume 501, pp. 241–247. [Google Scholar] [CrossRef]
- Xu, Y.; An, J.; Tabys, D.; Xie, Y.; Zhao, T.; Ren, H.; Liu, N. Effect of Lactoferrin on the Expression Profiles of Long Non-Coding RNA during Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 4834. [Google Scholar] [CrossRef]
- Kim, S.E.; Lee, D.; Yun, Y.; Shim, K.; Jeon, D.I.; Rhee, J.; Kim, H.; Park, K. Heparin-Immobilized Hydroxyapatite Nanoparticles as a Lactoferrin Delivery System for Improving Osteogenic Differentiation of Adipose-Derived Stem Cells. Biomed. Mater. 2016, 11, 025004. [Google Scholar] [CrossRef] [PubMed]
- Amirthalingam, S.; Lee, S.S.; Rajendran, A.K.; Kim, I.; Hwang, N.S.; Rangasamy, J. Addition of Lactoferrin and Substance P in a Chitin/PLGA-CaSO4 Hydrogel for Regeneration of Calvarial Bone Defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112172. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, S.; Hu, J. Bone Regeneration is Promoted by Orally Administered Bovine Lactoferrin in a Rabbit Tibial Distraction Osteogenesis Model. Clin. Orthop. Relat. Res. 2015, 473, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, J.; Ji, P.; Zhu, S.; Zhu, Y. Oral Administration of Bovine Lactoferrin Accelerates the Healing of Fracture in Ovariectomized Rats. J. Bone Miner. Metab. 2020, 38, 648–657. [Google Scholar] [CrossRef] [PubMed]
- McRae, J.M.; Kennedy, J.A. Wine and Grape Tannin Interactions with Salivary Proteins and their Impact on Astringency: A Review of Current Research. Molecules 2011, 16, 2348–2364. [Google Scholar] [CrossRef]
- Park, Y.E.; Chandramouli, K.; Watson, M.; Zhu, M.; Callon, K.E.; Tuari, D.; Abdeltawab, H.; Svirskis, D.; Musson, D.S.; Sharma, M.; et al. Sustained Delivery of Lactoferrin using Poloxamer Gels for Local Bone Regeneration in a Rat Calvarial Defect Model. Materials 2021, 15, 212. [Google Scholar] [CrossRef]
- Koca, C.G.; Yıldırım, B.; Ozmen, O.; Dikilitas, A.; Cicek, M.F.; Simsek, A.T.; Gungor, M.A.; Tuncay, E. Effect of Single-Dose Locally Applied Lactoferrin on Autograft Healing in Peri-Implant Bone in Rat Models. Injury 2022, 53, 858–867. [Google Scholar] [CrossRef]
- Icriverzi, M.; Dinca, V.; Moisei, M.; Evans, R.W.; Trif, M.; Roseanu, A. Lactoferrin in Bone Tissue Regeneration. Curr. Med. Chem. 2020, 27, 838–853. [Google Scholar] [CrossRef]
- Chang, Y.; Ping, A.; Chang, C.; Betz, V.M.; Cai, L.; Ren, B. Lactoferrin Mediates Enhanced Osteogenesis of Adipose-Derived Stem Cells: Innovative Molecular and Cellular Therapy for Bone Repair. Int. J. Mol. Sci. 2023, 24, 1749. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Ren, F.; Zhang, W.; Zhang, H.; Zhao, L.; Zhang, M.; Cui, W.; Wang, X.; Guo, H. Lactoferrin Promotes Osteogenesis through TGF-β Receptor II Binding in Osteoblasts and Activation of Canonical TGF-β Signaling in MC3T3-E1 Cells and C57BL/6J Mice. J. Nutr. 2018, 148, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, X.; Shan, Y.; Zhang, L.; Du, M.; Liu, M.; Yi, H.; Ma, Y. Effect of bovine lactoferrin and human lactoferrin on the proliferative activity of the osteoblast cell line MC3T3-E1 in vitro. J. Dairy Sci. 2018, 101, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
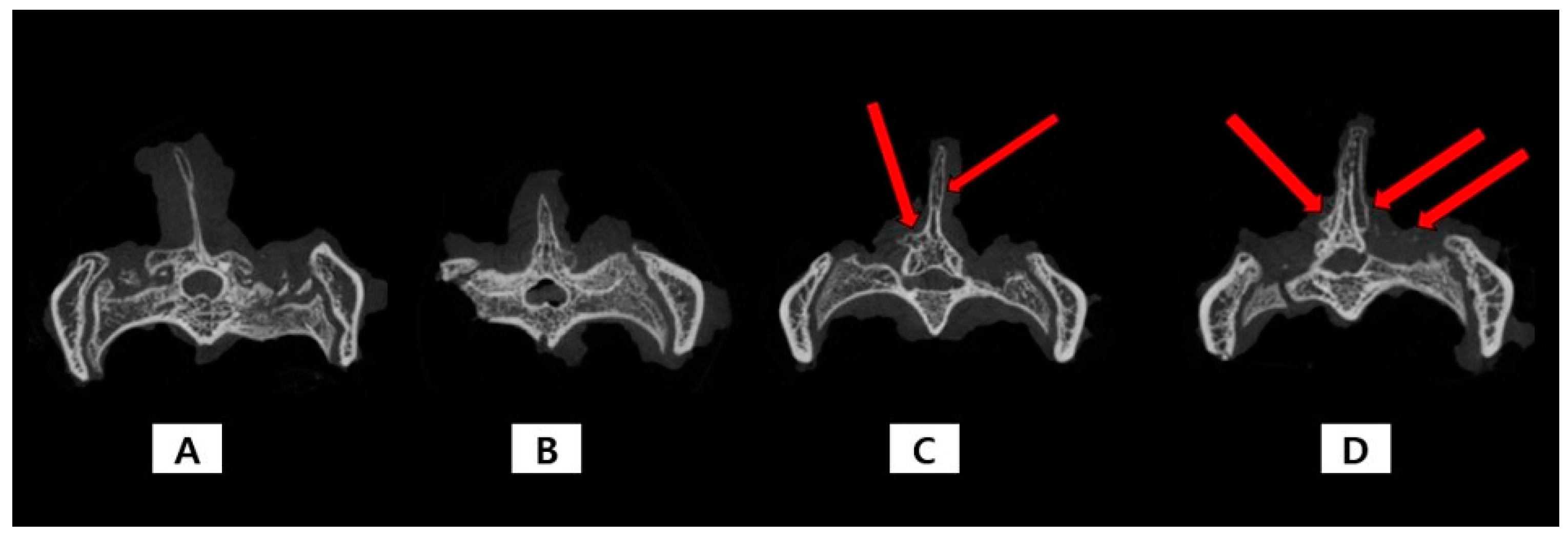

| Characteristics | MSN Group (n = 8) | TA-MSN-LF Low Group (n = 8) | TA-MSN-LF High Group (n = 8) | p |
|---|---|---|---|---|
| Fusion grade | ||||
| 1 | 2 | 0 | 0 | |
| 2 | 6 | 3 | 1 | |
| 3 | 0 | 5 | 7 | <0.001 ** |
| Degree of firmness | ||||
| 1 | 5 | 0 | 0 | |
| 2 | 3 | 4 | 0 | |
| 3 | 0 | 4 | 8 | <0.001 ** |
| Volume of bridging bone | ||||
| 1 | 2 | 0 | 0 | |
| 2 | 5 | 2 | 0 | |
| 3 | 1 | 6 | 8 | <0.001 ** |
| Defect Group (n = 6) | MSN Group (n = 8) | TA-MSN-LF Low Group (n = 8) | TA-MSN-LF High Group (n = 8) | p | |
|---|---|---|---|---|---|
| Bone volume (mm3) | 84.761 ± 8.507 | 99.697 ± 14.234 | 114.701 ± 15.703 | 121.335 ± 4.204 | <0.05 * |
| Trabecular thickness (mm) | 0.485 ± 0.058 | 0.400 ± 0.029 | 0.416 ± 0.046 | 0.412 ± 0.047 | 0.348 |
| Trabecular number (1/mm) | 0.152 ± 0.022 | 0.179 ± 0.030 | 0.211 ± 0.015 | 0.212 ± 0.020 | <0.05 * |
| Trabecular separation (mm) | 4.500 ± 0.343 | 4.409 ± 0.246 | 4.277 ± 0.238 | 4.307 ± 0.425 | 0.637 |
| Defect Group (n = 6) | MSN Group (n = 8) | TA-MSN-LF Low Group (n = 8) | TA-MSN-LF High Group (n = 8) | p | |
|---|---|---|---|---|---|
| Frequency of blood vessels | 1.00 ± 0.00 | 1.37 ± 0.50 | 1.51 ± 0.50 | 1.96 ± 0.50 | <0.05 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, S.H.; Sung, K.; Byeon, H.E.; Kim, S.E.; Kim, K.N. Lactoferrin-Anchored Tannylated Mesoporous Silica Nanomaterials-Induced Bone Fusion in a Rat Model of Lumbar Spinal Fusion. Int. J. Mol. Sci. 2023, 24, 15782. https://doi.org/10.3390/ijms242115782
Noh SH, Sung K, Byeon HE, Kim SE, Kim KN. Lactoferrin-Anchored Tannylated Mesoporous Silica Nanomaterials-Induced Bone Fusion in a Rat Model of Lumbar Spinal Fusion. International Journal of Molecular Sciences. 2023; 24(21):15782. https://doi.org/10.3390/ijms242115782
Chicago/Turabian StyleNoh, Sung Hyun, Kanghyon Sung, Hye Eun Byeon, Sung Eun Kim, and Keung Nyun Kim. 2023. "Lactoferrin-Anchored Tannylated Mesoporous Silica Nanomaterials-Induced Bone Fusion in a Rat Model of Lumbar Spinal Fusion" International Journal of Molecular Sciences 24, no. 21: 15782. https://doi.org/10.3390/ijms242115782
APA StyleNoh, S. H., Sung, K., Byeon, H. E., Kim, S. E., & Kim, K. N. (2023). Lactoferrin-Anchored Tannylated Mesoporous Silica Nanomaterials-Induced Bone Fusion in a Rat Model of Lumbar Spinal Fusion. International Journal of Molecular Sciences, 24(21), 15782. https://doi.org/10.3390/ijms242115782






