Flavonoid-Rich Sambucus nigra Berry Extract Enhances Nrf2/HO-1 Signaling Pathway Activation and Exerts Antiulcerative Effects In Vivo
Abstract
:1. Introduction
2. Results
2.1. GC/MS Findings
2.2. Quantitative Analysis of Phenolic and Flavonoid Compounds by LC-MS/MS
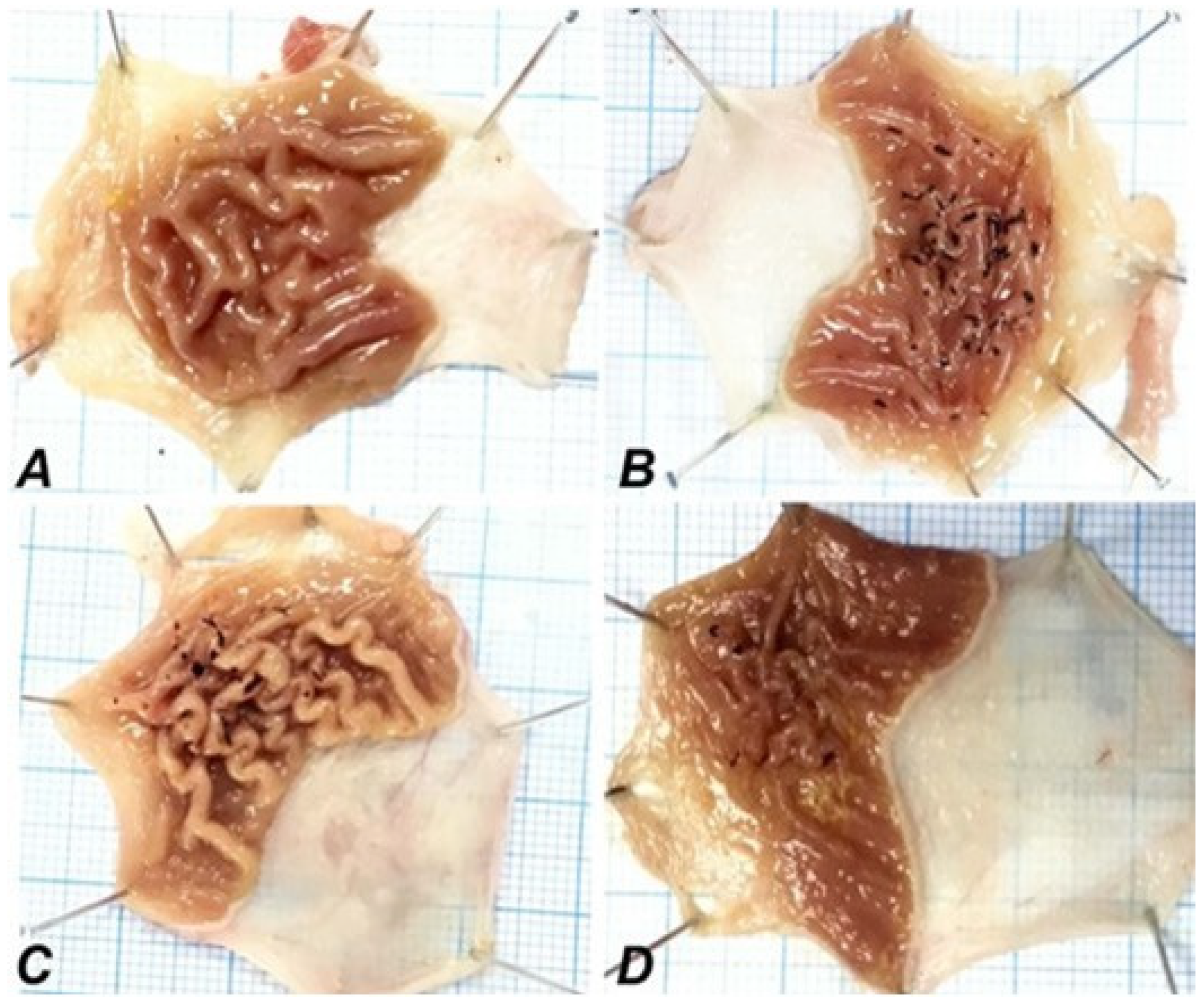
2.3. Morphological Findings
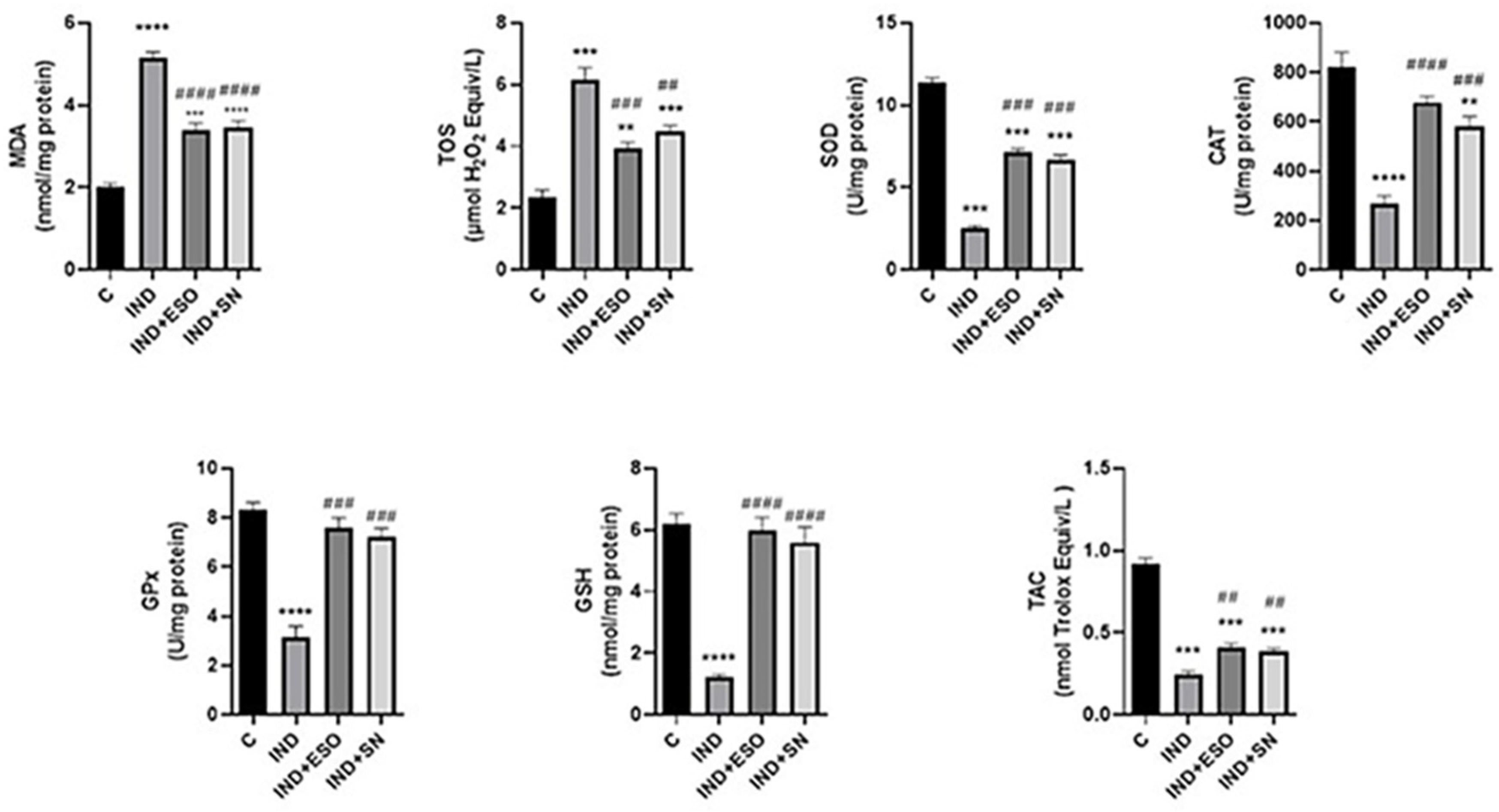
2.4. IND-Induced Oxidative Stress Was Alleviated by Pre-Treatment with SN
2.5. IND-Induced Neutrophil Infiltration Was Improved by Pre-Treatment with SN Berry Extract
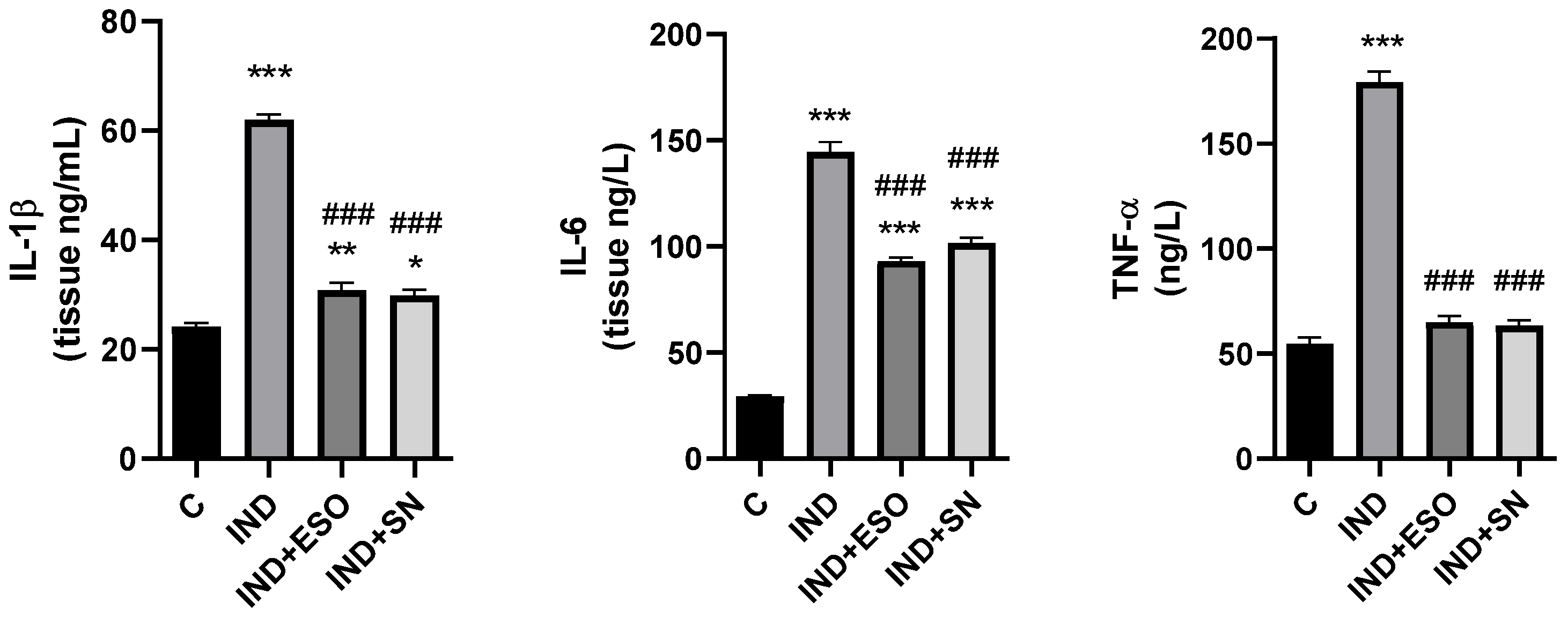
2.6. The Effects of SN Berry Extract on Inflammation Factors
2.7. Effect of SN Berry Extract on Gastric Tissue Histopathology
2.8. Correlation of Histopathological Changes and IL-33 Expression-Effect of SN
2.9. Effect of SB Berry Extract Pre-Treatment on Nrf-2 and HO-1 Expression as Analyzed by Immunofluorescence
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of SN Berry Extract
4.3. GC/MS and LC-MS/MS Analysis
4.4. Gastric Ulcer Model
4.5. Experimental Design
4.6. Morphological Examination
4.7. Measurement of Oxidative Stress and Antioxidant Enzymes
4.8. Measurement of Inflammatory Markers and Cytokines
4.9. Histopathology
4.10. Immunostaining Analyses
4.11. Double-Immunofluorescence Assays
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2012, 11, 52–64. [Google Scholar] [CrossRef]
- Tai, F.W.D.; McAlindon, M.E. Non-steroidal anti-inflammatory drugs and the gastrointestinal tract. Clin. Med. 2021, 21, 131–134. [Google Scholar] [CrossRef]
- Asali, A.M.; Alghamdi, M.A.; Fallatah, S.A.; Alholaily, W.A.; Aldandan, R.G.; Alnosair, A.H.; AlKhars, A.A.; Alreheli, M.F.; Almohaini, M.O.; Alharbi, R.A. Risk factors leading to peptic ulcer disease: Systematic review in literature. Int. J. Community Med. Public. Health 2018, 5, 4617–4624. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Lu, C.C.; Yen, G.C. Phytochemicals enhance antioxidant enzyme expression to protect against NSAID-induced oxidative damage of the gastrointestinal mucosa. Mol. Nutr. Food Res. 2017, 61, 1600659. [Google Scholar] [CrossRef]
- Almasaudi, S.B.; El-Shitany, N.A.; Abbas, A.T.; Abdel-dayem, U.A.; Ali, S.S.; Al Jaouni, S.K.; Harakeh, S. Antioxidant, Anti-inflammatory, and Antiulcer Potential of Manuka Honey against Gastric Ulcer in Rats. Oxid. Med. Cell Longev. 2016, 2016, 3643824. [Google Scholar] [CrossRef]
- Badr, A.M.; El-Orabi, N.F.; Ali, R.A. The implication of the crosstalk of Nrf2 with NOXs, and HMGB1 in ethanol-induced gastric ulcer: Potential protective effect is afforded by Raspberries Ketone. PLoS ONE 2019, 14, e0220548. [Google Scholar] [CrossRef]
- Puentes-Pardo, J.D.; Moreno-SanJuan, S.; Carazo, A.; Leon, J. Heme Oxygenase-1 in Gastrointestinal Tract Health and Disease. Antioxidants 2020, 9, 1214. [Google Scholar] [CrossRef]
- Onyiah, J.C.; Schaefer, R.E.M.; Colgan, S.P. A Central Role for Heme Oxygenase-1 in the Control of Intestinal Epithelial Chemokine Expression. J. Innate Immun. 2018, 10, 228–238. [Google Scholar] [CrossRef]
- Sallam, A.M.; Darwish, S.F.; El-Dakroury, W.A.; Radwan, E. Olmesartan niosomes ameliorates the Indomethacin-induced gastric ulcer in rats: Insights on MAPK and Nrf2/HO-1 signaling pathway. Pharm. Res. 2021, 38, 1821–1838. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Fagundes, C.T.; Amaral, F.A.; Souza, A.L.; Vieira, A.T.; Xu, D.; Liew, F.Y.; Souza, D.G.; Teixeira, M.M. ST2, an IL-1R family member, attenuates inflammation and lethality after intestinal ischemia and reperfusion. J. Leukoc. Biol. 2007, 81, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-S.; Park, J.A.; Kim, J.; Rho, S.-S.; Park, H.; Kim, Y.-M.; Kwon, Y.-G. Nuclear IL-33 is a transcriptional regulator of NF-κB p65 and induces endothelial cell activation. Biochem. Biophys. Res. Commun. 2012, 421, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Ameri, A.H.; Dempsey, K.E.; Conrad, D.N.; Kem, M.; Mino-Kenudson, M.; Demehri, S. Nuclear IL-33/SMAD signaling axis promotes cancer development in chronic inflammation. EMBO J. 2021, 40, e106151. [Google Scholar] [CrossRef] [PubMed]
- Buzzelli, J.N.; Chalinor, H.V.; Pavlic, D.I.; Sutton, P.; Menheniott, T.R.; Giraud, A.S.; Judd, L.M. IL33 is a stomach alarmin that initiates a skewed Th2 response to injury and infection. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 203–221.e3. [Google Scholar] [CrossRef]
- Pisani, L.F.; Tontini, G.E.; Gentile, C.; Marinoni, B.; Teani, I.; Nandi, N.; Creo, P.; Asti, E.; Bonavina, L.; Vecchi, M.; et al. Proinflammatory Interleukin-33 Induces Dichotomic Effects on Cell Proliferation in Normal Gastric Epithelium and Gastric Cancer. Int. J. Mol. Sci. 2021, 22, 5792. [Google Scholar] [CrossRef]
- Chmiela, M.; Gonciarz, W.; Krupa, A. Proregenerative Activity of IL-33 in Gastric Tissue Cells Undergoing Helicobacter Pylori-Induced Apoptosis. Int. J. Mol. Sci. 2020, 21, 1801. [Google Scholar]
- Gonciarz, W.; Krupa, A.; Moran, A.P.; Tomaszewska, A.; Chmiela, M. Interference of LPS, H. pylori with IL-33-Driven Regeneration of Caviae porcellus Primary Gastric Epithelial Cells and Fibroblasts. Cells 2021, 10, 1385. [Google Scholar] [CrossRef]
- Kuo, C.J.; Chen, C.Y.; Lo, H.R.; Feng, C.L.; Wu, H.Y.; Huang, M.Z.; Liao, T.N.; Lai, C.H. Helicobacter pylori Induces IL-33 Production and Recruits ST-2 to Lipid Rafts to Exacerbate Inflammation. Cells 2019, 8, 1290. [Google Scholar] [CrossRef]
- De Salvo, C.; Pastorelli, L.; Petersen, C.P.; Butto, L.F.; Buela, K.A.; Omenetti, S.; Locovei, S.A.; Ray, S.; Friedman, H.R.; Duijser, J.; et al. Interleukin 33 Triggers Early Eosinophil-Dependent Events Leading to Metaplasia in a Chronic Model of Gastritis-Prone Mice. Gastroenterology 2021, 160, 302–316.e7. [Google Scholar] [CrossRef]
- Petersen, C.P.; Meyer, A.R.; De Salvo, C.; Choi, E.; Schlegel, C.; Petersen, A.; Engevik, A.C.; Prasad, N.; Levy, S.E.; Peebles, R.S.; et al. A signalling cascade of IL-33 to IL-13 regulates metaplasia in the mouse stomach. Gut 2018, 67, 805–817. [Google Scholar] [CrossRef]
- Meyer, A.R.; Goldenring, J.R. Injury, repair, inflammation and metaplasia in the stomach. J. Physiol. 2018, 596, 3861–3867. [Google Scholar] [CrossRef] [PubMed]
- Sabiu, S.; Garuba, T.; Sunmonu, T.; Ajani, E.; Sulyman, A.; Nurain, I.; Balogun, A. Indomethacin-induced gastric ulceration in rats: Protective roles of Spondias mombin and Ficus exasperata. Toxicol. Rep. 2015, 2, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Yibirin, M.; De Oliveira, D.; Valera, R.; Plitt, A.E.; Lutgen, S. Adverse Effects Associated with Proton Pump Inhibitor Use. Cureus 2021, 13, e12759. [Google Scholar] [CrossRef]
- Ardalani, H.; Hadipanah, A.; Sahebkar, A. Medicinal Plants in the Treatment of Peptic Ulcer Disease: A Review. Mini Rev. Med. Chem. 2020, 20, 662–702. [Google Scholar] [CrossRef] [PubMed]
- Strugała, P.; Loi, S.; Bażanów, B.; Kuropka, P.; Kucharska, A.Z.; Włoch, A.; Gabrielska, J. A comprehensive study on the biological activity of elderberries extract and cyanidin 3-O-glucoside and their interactions with membranes and human serum albumin. Molecules 2018, 23, 2566. [Google Scholar] [CrossRef] [PubMed]
- Marțiș, G.S.; Mureșan, V.; Marc, R.M.; Mureșan, C.C.; Pop, C.R.; Buzgău, G.; Mureșan, A.E.; Ungur, R.A.; Muste, S. The Physicochemical and Antioxidant Properties of Sambucus nigra, L. and Sambucus nigra Haschberg during Growth Phases: From Buds to Ripening. Antioxidants 2021, 10, 1093. [Google Scholar] [CrossRef]
- Milbury, P.E.; Cao, G.; Prior, R.L.; Blumberg, J. Bioavailablility of elderberries anthocyanins. Mech. Ageing Dev. 2002, 123, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Murkovic, M.; Mülleder, U.; Adam, U.; Pfannhauser, W. Detection of anthocyanins from elderberries juice in human urine. J. Sci. Food Agric. 2001, 81, 934–937. [Google Scholar] [CrossRef]
- Salvador, A.C.; Krol, E.; Lemos, V.C.; Santos, S.A.; Bento, F.P.; Costa, C.P.; Almeida, A.; Szczepankiewicz, D.; Kulczyński, B.; Krejpcio, Z.; et al. Effect of Elderberries (Sambucus nigra L.) Extract Supplementation in STZ-Induced Diabetic Rats Fed with a High-Fat Diet. Int. J. Mol. Sci. 2016, 18, 13. [Google Scholar] [CrossRef]
- Kuskov, A.; Nikitovic, D.; Berdiaki, A.; Shtilman, M.; Tsatsakis, A. Amphiphilic poly-N-vinylpyrrolidone nanoparticles as carriers for nonsteroidal, anti-inflammatory drugs: Pharmacokinetic, anti-inflammatory, and ulcerogenic activity study. Pharmaceutics 2022, 14, 925. [Google Scholar] [CrossRef]
- Ruiz-Hurtado, P.A.; Garduno-Siciliano, L.; Dominguez-Verano, P.; Balderas-Cordero, D.; Gorgua-Jimenez, G.; Canales-Alvarez, O.; Canales-Martínez, M.M.; Rodríguez-Monroy, M.A. Propolis and Its Gastroprotective Effects on NSAID-Induced Gastric Ulcer Disease: A Systematic Review. Nutrients 2021, 13, 3169. [Google Scholar] [CrossRef]
- Kangwan, N.; Park, J.M.; Kim, E.H.; Hahm, K.B. Quality of healing of gastric ulcers: Natural products beyond acid suppression. World J. Gastrointest. Pathophysiol. 2014, 5, 40–47. [Google Scholar] [CrossRef]
- Zielinska-Wasielica, J.; Olejnik, A.; Kowalska, K.; Olkowicz, M.; Dembczynski, R. Elderberries (Sambucus nigra L.) Fruit Extract Alleviates Oxidative Stress, Insulin Resistance, and Inflammation in Hypertrophied 3T3-L1 Adipocytes and Activated RAW 264.7 Macrophages. Foods 2019, 8, 326. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Mazumder, S.; De, R.; Debsharma, S.; Bindu, S.; Maity, P.; Sarkar, S.; Saha, S.J.; Siddiqui, A.A.; Banerjee, C.; Nag, S.; et al. Indomethacin impairs mitochondrial dynamics by activating the PKCzeta-p38-DRP1 pathway and inducing apoptosis in gastric cancer and normal mucosal cells. J. Biol. Chem. 2019, 294, 8238–8258. [Google Scholar] [CrossRef]
- Suleyman, H.; Albayrak, A.; Bilici, M.; Cadirci, E.; Halici, Z. Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation 2010, 33, 224–234. [Google Scholar] [CrossRef]
- Alkushi, A.G.R.; Elsawy, N.A.M. Quercetin attenuates, indomethacin-induced acute gastric ulcer in rats. Folia Morphol. 2017, 76, 252–261. [Google Scholar] [CrossRef]
- Simoes, S.; Lopes, R.; Campos, M.C.D.; Marruz, M.J.; da Cruz, M.E.M.; Corvo, L. Animal models of acute gastric mucosal injury: Macroscopic and microscopic evaluation. Animal Model. Exp. Med. 2019, 2, 121–126. [Google Scholar] [CrossRef]
- Kucukler, S.; Kandemir, F.M.; Yildirim, S. Protective effect of chrysin on indomethacin induced gastric ulcer in rats: Role of multi-pathway regulation. Biotech. Histochem. 2022, 97, 490–503. [Google Scholar] [CrossRef]
- Ciocoiu, M.; Miron, A.; Mares, L.; Tutunaru, D.; Pohaci, C.; Groza, M.; Badescu, M. The effects of Sambucus nigra polyphenols on oxidative stress and metabolic disorders in experimental diabetes mellitus. J. Physiol. Biochem. 2009, 65, 297–304. [Google Scholar] [CrossRef]
- Balaha, M.F.; Almalki, Z.S.; Alahmari, A.K.; Ahmed, N.J.; Balaha, M.F. AMPK/mTOR-driven autophagy & Nrf2/HO-1 cascade modulation by amentoflavone ameliorates indomethacin-induced gastric ulcer. Biomed. Pharmacother. 2022, 151, 113200. [Google Scholar] [CrossRef]
- Lin, P.; Hwang, E.; Ngo, H.T.T.; Seo, S.A.; Yi, T.H. Sambucus nigra L. ameliorates UVB-induced photoaging and inflammatory response in human skin keratinocytes. Cytotechnology 2019, 71, 1003–1017. [Google Scholar] [CrossRef]
- Salem, N.A.; Wahba, M.A.; Eisa, W.H.; El-Shamarka, M.; Khalil, W. Silver oxide nanoparticles alleviate indomethacin-induced gastric injury: A novel antiulcer agent. Inflammopharmacology 2018, 26, 1025–1035. [Google Scholar] [CrossRef]
- Santin, J.R.; Benvenutti, L.; Broering, M.F.; Nunes, R.; Goldoni, F.C.; Patel, Y.B.K.; de Souza, J.A.; Kopp, M.A.T.; de Souza, P.; da Silva, R.D.C.V.; et al. Sambucus nigra: A traditional medicine effective in reducing inflammation in mice. J. Ethnopharmacol. 2022, 283, 114736. [Google Scholar] [CrossRef]
- Farrell, N.J.; Norris, G.H.; Ryan, J.; Porter, C.M.; Jiang, C.; Blesso, C.N. Black elderberries extract attenuates inflammation and metabolic dysfunction in diet-induced obese mice. Br. J. Nutr. 2015, 114, 1123–1131. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Khedr, E.G.; El-Bahrawy, H.A.; Selim, H.M. Gastroprotective effect of garlic in indomethacin induced gastric ulcer in rats. Nutrition 2016, 32, 849–854. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Placer, Z.A.; Cushman, L.L.; Johnson, B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef]
- Naeimi, R.A.; Talebpour Amiri, F.; Khalatbary, A.R.; Ghasemi, A.; Zargari, M.; Ghesemi, M.; Hosseinimehr, S.J. Atorvastatin mitigates testicular injuries induced by ionizing radiation in mice. Reprod. Toxicol. 2017, 72, 115–121. [Google Scholar] [CrossRef]
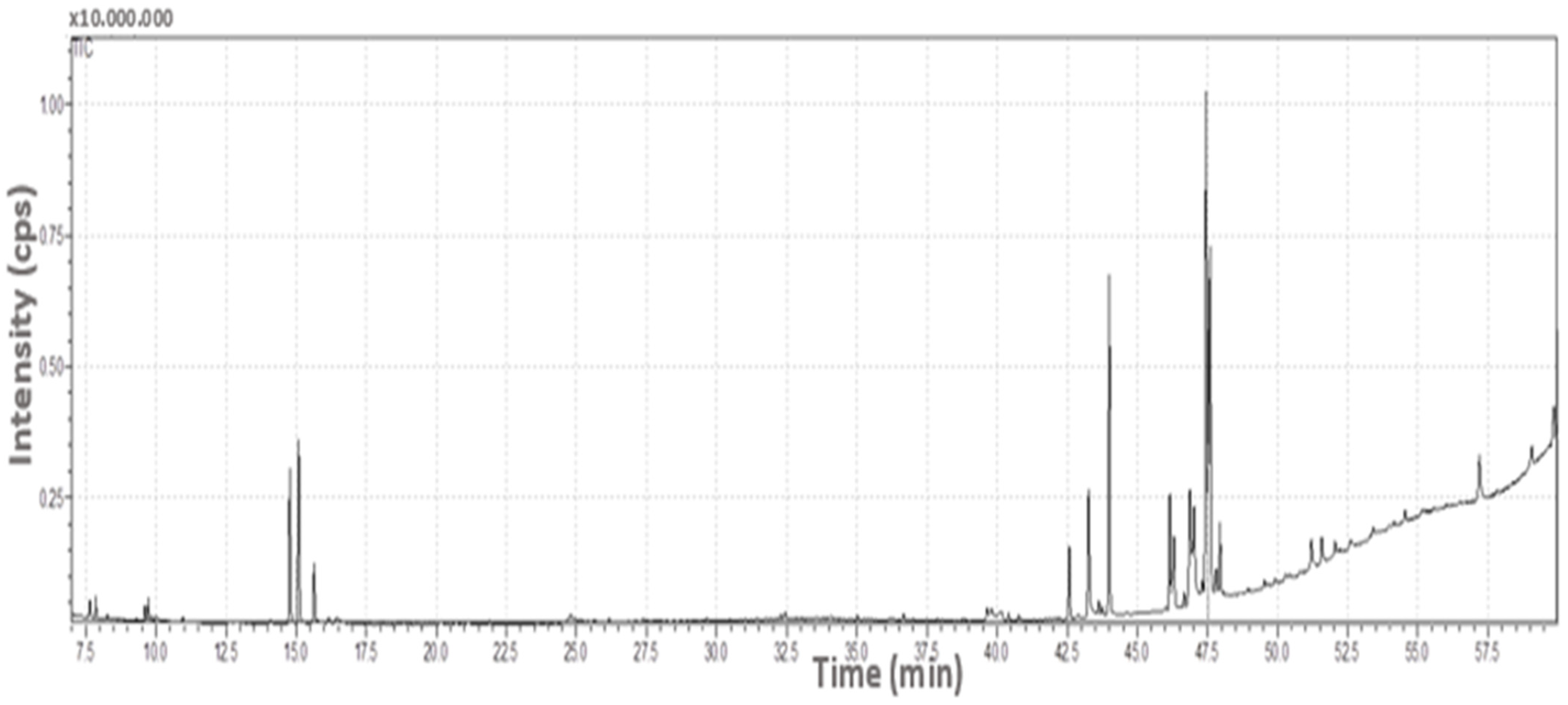
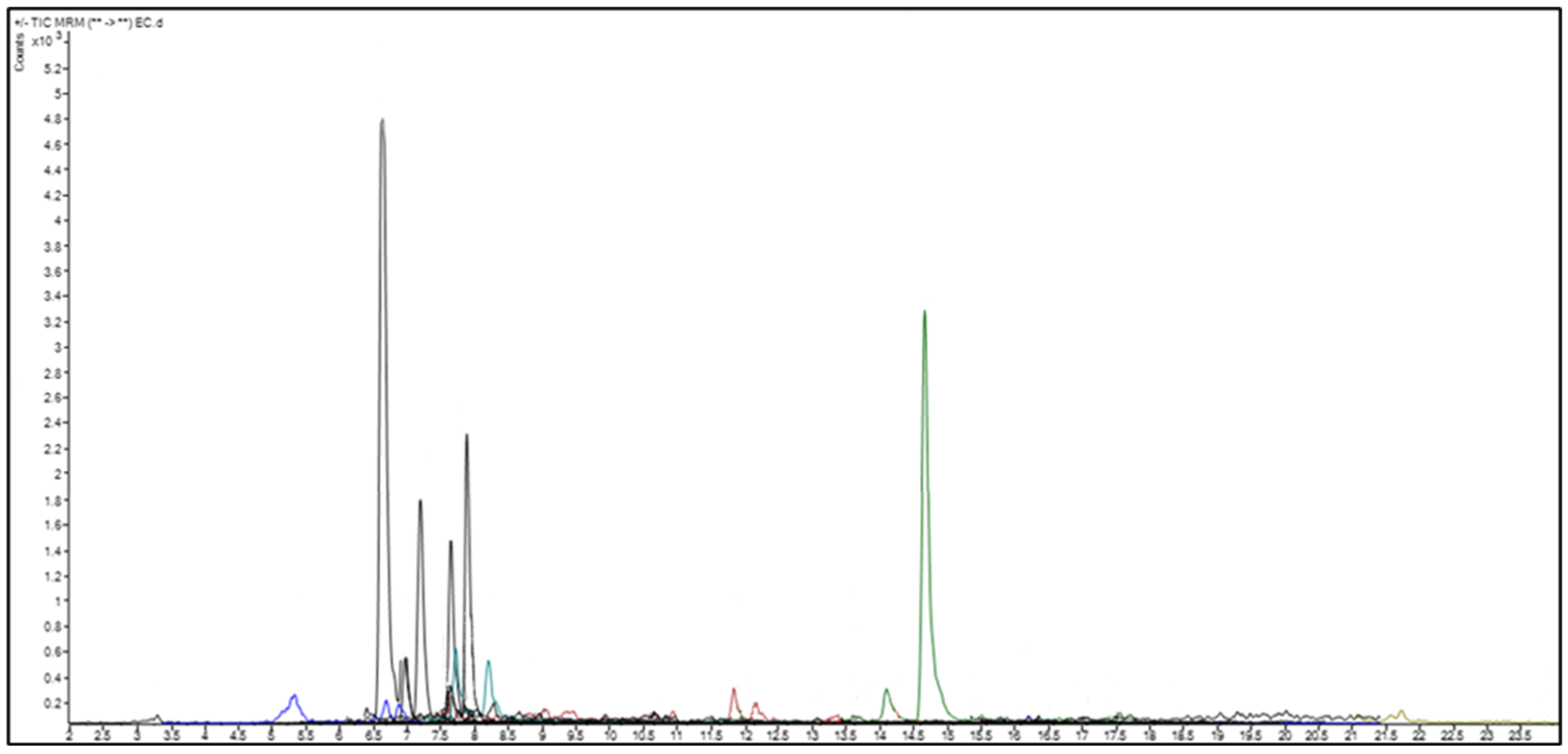




| R.T. a | Area b | R.A. (%) c | Compound Name | CAS d | SI (%) e | |
|---|---|---|---|---|---|---|
| 1 | 7.853 | 909,102 | 0.45 | 2,5-Dimethyltetrahydrofuran | 1003-38-9 | 94 |
| 2 | 9.611 | 743,461 | 0.37 | 3-Hexanone | 589-38-8 | 96 |
| 3 | 9.728 | 1,084,622 | 0.53 | 2-Hexanone | 591-78-6 | 97 |
| 4 | 9.882 | 230,105 | 0.11 | 3-Hexanol | 623-37-0 | 94 |
| 5 | 10.963 | 208,713 | 0.1 | Isovaleric acid | 503-74-2 | 95 |
| 6 | 14.770 | 9,605,684 | 4.72 | Dimethipin | 55290-64-7 | 96 |
| 7 | 15.089 | 11,505,459 | 5.66 | Aldicarb | 116-06-3 | 96 |
| 8 | 15.636 | 3,910,438 | 1.92 | 1-Butenyl methyl ketone | 763-93-9 | 91 |
| 9 | 39.642 | 754,109 | 0.37 | Ethyl myristate | 124-06-1 | 94 |
| 10 | 39.807 | 950,844 | 0.47 | n-Tetracosane | 646-31-1 | 93 |
| 11 | 40.762 | 282,753 | 0.14 | Neophytadiene | 504-96-1 | 93 |
| 12 | 42.572 | 4,416,769 | 2.17 | Methyl palmitate | 112-39-0 | 95 |
| 13 | 43.263 | 9,498,091 | 4.67 | L-(+)-Ascorbyl dipalmitate | 28474-90-0 | 91 |
| 14 | 43.990 | 20,970,704 | 10.31 | Ethyl palmitate | 628-97-7 | 94 |
| 15 | 46.150 | 7,407,006 | 3.64 | Methyl linoleate | 112-63-0 | 94 |
| 16 | 46.669 | 874,377 | 0.43 | Methyl stearate | 112-61-8 | 94 |
| 17 | 47.446 | 34,862,172 | 17.14 | Ethyl linoleate | 544-35-4 | 92 |
| 18 | 47.594 | 24,967,197 | 12.27 | Ethyl linolenate | 1191-41-9 | 95 |
| 19 | 47.804 | 2,458,265 | 1.21 | Octadecanamide | 124-26-5 | 92 |
| 20 | 47.942 | 5,129,745 | 2.52 | Ethyl stearate | 111-61-5 | 91 |
| 21 | 49.900 | 276,963 | 0.14 | n-Dotriacontane | 544-85-4 | 91 |
| 22 | 51.210 | 2,094,842 | 1.03 | Oleoamide | 301-02-0 | 93 |
| 23 | 57.200 | 4,562,804 | 2.24 | Tetrapentacosan | 5856-66-6 | 92 |
| No | Analytes | R.T. a (min) | Parent Ion b | Fragment Ion c (m/z) | CE d (eV) | Polarity | Quantification (µg Analyte/G Extract) |
|---|---|---|---|---|---|---|---|
| 1 | Gallic acid | 3.387 | 169.0 [M−H]− | 125.1 | 12 | Negative | 32.82 |
| 2 | Protocatechuic acid | 5.411 | 153.0 [M−H]− | 109.0 | 14 | Negative | 21.12 |
| 3 | Chlorogenic acid | 6.818 | 353.1 [M−H]− | 191.0 | 12 | Negative | 1783.38 |
| 4 | Caffeic acid | 6.964 | 178.9 [M−H]− | 135.1 | 14 | Negative | 89.30 |
| 5 | Hydroxybenzaldehyde | 7.412 | 121.0 [M−H]− | 92.0 | 24 | Negative | 166.09 |
| 6 | Epigallocatechin | 7.769 | 307.0 [M+H]+ | 139.0 | 8 | Positive | 2.53 |
| 7 | Catechin | 7.852 | 288.9 [M−H]− | 245.1 | 12 | Negative | 7.87 |
| 8 | o-coumaric acid | 7.945 | 163.0 [M−H]− | 119.1 | 10 | Negative | 145.02 |
| 9 | Taxifolin | 8.191 | 304.8 [M] | 258.9 | 12 | Positive | 746.71 |
| 10 | Trans-ferulic acid | 8.441 | 193.1 [M−H]− | 133.9 | 12 | Negative | 217.21 |
| 11 | Vanillic acid | 8.758 | 167.0 [M−H]− | 151.8 | 12 | Negative | 364.94 |
| 12 | Salicylic acid | 9.479 | 137.0 [M−H]− | 93.1 | 18 | Negative | 211.07 |
| 13 | Syringic acid | 9.581 | 197.1 [M−H]− | 181.8 | 10 | Negative | 383.32 |
| 14 | Vanillin | 9.687 | 153.0 [M+H]+ | 125.0 | 6 | Positive | 25.90 |
| 15 | Sinapic acid | 10.425 | 223.1 [M−H]− | 208.0 | 10 | Negative | 45.12 |
| 16 | Protocatechuic acid ethyl ester | 11.324 | 181.0 [M−H]− | 107.9 | 20 | Negative | 9.83 |
| 17 | p-coumaric acid | 11.513 | 163.0 [M−H]− | 119.0 | 10 | Negative | 11.10 |
| 18 | Quercetin-3-xyloside | 12.363 | 432.7 [M−H]− | 299.5 | 24 | Negative | 7.98 |
| 19 | Kaempferol-3-glucoside | 13.055 | 448.8 [M] | 286.9 | 10 | Positive | 5.03 |
| 20 | Fisetin | 13.217 | 287.0 [M+H]+ | 212.9 | 32 | Positive | 16.77 |
| 21 | Baicalin | 13.653 | 446.8 [M] | 270.9 | 16 | Positive | 6.01 |
| 22 | Chrysin | 14.169 | 254.9 [M+H]+ | 153.0 | 32 | Positive | 16.93 |
| 23 | Quercetin | 14.847 | 300.8 [M−H]− | 179.0 | 20 | Negative | 55.18 |
| 24 | Naringenin | 14.986 | 270.9 [M−H]− | 119.1 | 24 | Negative | 35.28 |
| 25 | Biochanin A | 15.718 | 284.9 [M+H]+ | 151.9 | 28 | Positive | 1.16 |
| 26 | Hesperetin | 16.599 | 300.9 [M−H]− | 164.0 | 20 | Negative | 22.93 |
| 27 | Kaempferol | 17.173 | 284.9 [M−H]− | 116.9 | 44 | Negative | 105.52 |
| 28 | Diosgenin | 23.382 | 415.0 [M+H]+ | 271.0 | 18 | Positive | 37.54 |
| Ulcer Score | Ulcer Index | Preventive Index | |
|---|---|---|---|
| Control | - | - | - |
| IND | 25 ± 1.09 a | 2450 | - |
| IND + ESO | 1.42 ± 0.74 b | 145 | %92.28 |
| IND + SN | 1.75 ± 0.93 b | 160 | %90.14 |
| Control | IND | IND + ESO | IND + SN | |
|---|---|---|---|---|
| Bleeding in mucosa | - | +++ | + | + |
| Erosion of mucosa epithelium | - | +++ | + | + |
| Necrosis of mucosa epithelium | - | +++ | + | + |
| Inflammation | - | +++ | + | + |
| Hyperemia | - | +++ | + | + |
| Edema in submucosa | - | +++ | - | + |
| Control | IND | IND + ESO | IND + SN | |
|---|---|---|---|---|
| IL-33 | 21.48 ± 1.18 a | 96.52 ± 3.16 b | 38.76 ± 2.28 c | 41.15 ± 2.12 c |
| Control | IND | IND + ESO | IND + SN | |
|---|---|---|---|---|
| Nrf-2 | 98.64 ± 4.26 b | 41.16 ± 1.17 a | 65.35 ± 3.78 c | 62.63 ± 2.06 c |
| HO-1 | 92.66 ± 3.61 b | 35.24 ± 1.58 a | 58.74 ± 2.44 c | 55.12 ± 2.15 c |
| Rate (°C/min) | Temperature (°C) | Hold (min) |
|---|---|---|
| - | 50.0 | 2.0 |
| 10.0 | 300.0 | 5.0 |
| 3.0 | 320.0 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicek, B.; Danısman, B.; Yildirim, S.; Yuce, N.; Nikitovic, D.; Bolat, I.; Kuzucu, M.; Ceyran, E.; Bardas, E.; Golokhvast, K.S.; et al. Flavonoid-Rich Sambucus nigra Berry Extract Enhances Nrf2/HO-1 Signaling Pathway Activation and Exerts Antiulcerative Effects In Vivo. Int. J. Mol. Sci. 2023, 24, 15486. https://doi.org/10.3390/ijms242015486
Cicek B, Danısman B, Yildirim S, Yuce N, Nikitovic D, Bolat I, Kuzucu M, Ceyran E, Bardas E, Golokhvast KS, et al. Flavonoid-Rich Sambucus nigra Berry Extract Enhances Nrf2/HO-1 Signaling Pathway Activation and Exerts Antiulcerative Effects In Vivo. International Journal of Molecular Sciences. 2023; 24(20):15486. https://doi.org/10.3390/ijms242015486
Chicago/Turabian StyleCicek, Betul, Betul Danısman, Serkan Yildirim, Neslihan Yuce, Dragana Nikitovic, Ismail Bolat, Mehmet Kuzucu, Ertuğrul Ceyran, Ebru Bardas, Kirill S. Golokhvast, and et al. 2023. "Flavonoid-Rich Sambucus nigra Berry Extract Enhances Nrf2/HO-1 Signaling Pathway Activation and Exerts Antiulcerative Effects In Vivo" International Journal of Molecular Sciences 24, no. 20: 15486. https://doi.org/10.3390/ijms242015486
APA StyleCicek, B., Danısman, B., Yildirim, S., Yuce, N., Nikitovic, D., Bolat, I., Kuzucu, M., Ceyran, E., Bardas, E., Golokhvast, K. S., Tsatsakis, A., & Taghizadehghalehjoughi, A. (2023). Flavonoid-Rich Sambucus nigra Berry Extract Enhances Nrf2/HO-1 Signaling Pathway Activation and Exerts Antiulcerative Effects In Vivo. International Journal of Molecular Sciences, 24(20), 15486. https://doi.org/10.3390/ijms242015486









