Recent Advances in Biomimetic Nanocarrier-Based Photothermal Therapy for Cancer Treatment
Abstract
:1. Introduction
1.1. Photothermal Therapy for Cancer Treatment
1.2. Nanocarriers for Photothermal Therapy
2. Core: NIR-Responsive Materials
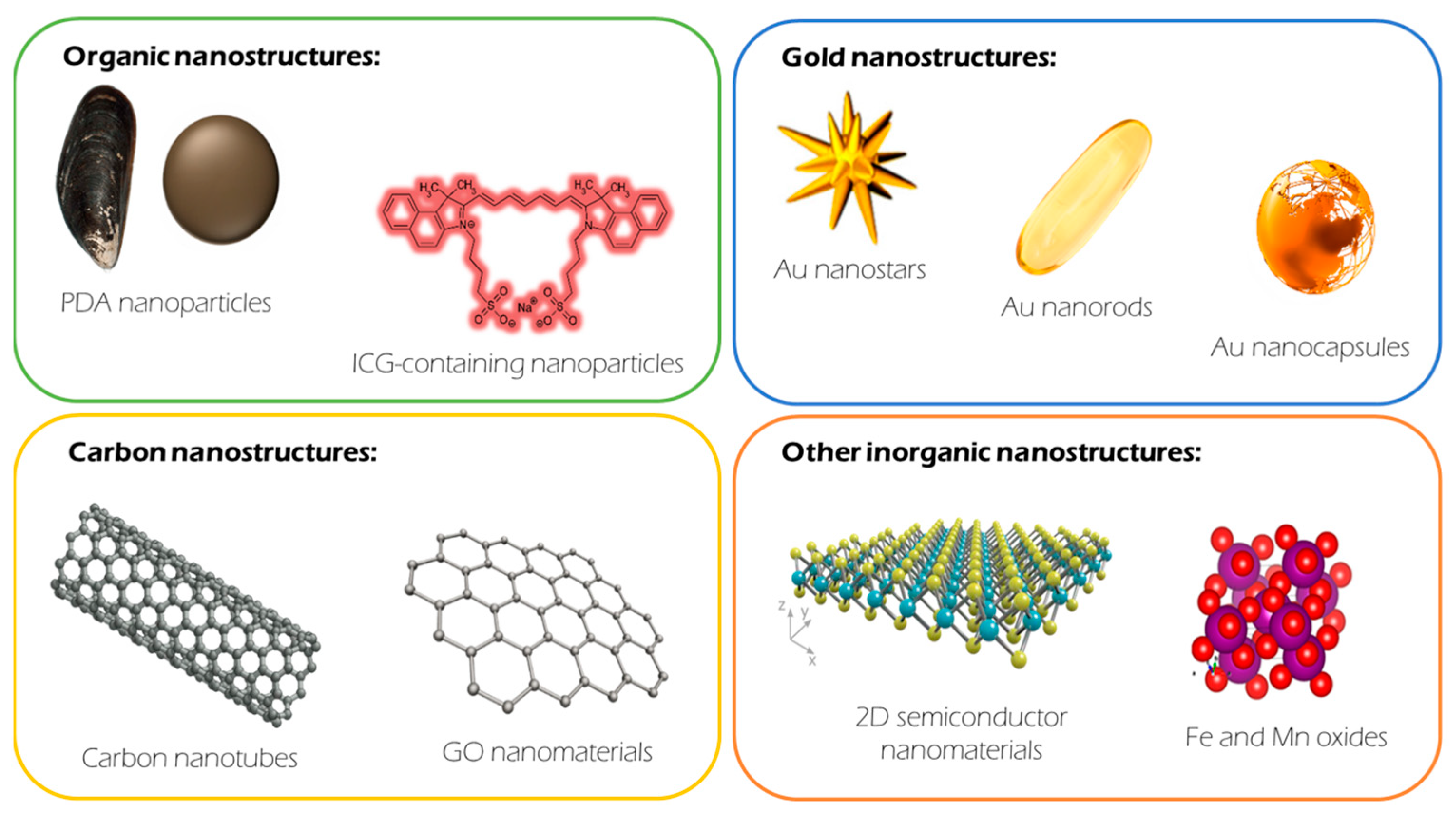
2.1. Organic Materials
2.2. Gold (Au) Nanomaterials
2.3. Carbon-Based Nanomaterials
2.3.1. Carbon Nanotubes (CNTs)
2.3.2. Graphene
2.4. Other (Inorganic) Nanomaterials
2.4.1. Semiconductor Materials
2.4.2. Oxide Materials
3. Biomimetic Shells
3.1. Membrane-Camouflaged Nanoparticles
3.1.1. Cell-Membrane-Cloaked Nanoparticles
Healthy Cell Membranes
Cancer Cell Membranes
Hybrid Cell Membranes
3.1.2. Cell-Derived Extracellular Vesicles
Healthy-Cell-Derived EVs
Cancer-Cell-Derived EVs
3.2. Nature-Inspired Nanoparticles for Photothermal Therapy
3.2.1. Virus-Based Nanoparticles
Virus-like Particles (VLPs)
Virus-Shape-Inspired Nanoparticles
3.2.2. Bacteria-Based Nanoparticles
3.2.3. Cytotoxic-Protein-Based Nanoparticles
4. Current Examples of Clinical Applications
5. Challenges in Translating from Bench to Bedside
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Raposo, L.R.; Valente, R.; Fernandes, A.R.; Baptista, P.V. Combined cancer therapeutics—Tackling the complexity of the tumor microenvironment. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1704. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H. A Review of Cancer Immunotherapy: From the Past, to the Present, to the Future. Curr. Oncol. 2020, 27, 87–97. [Google Scholar] [CrossRef]
- Habash, R.W.Y. Chapter 53—Therapeutic hyperthermia. In Handbook of Clinical Neurology; Romanovsky, A.A., Ed.; Thermoregulation: From Basic Neuroscience to Clinical Neurology, Part II; Elsevier: Amsterdam, The Netherlands, 2018; Volume 157, pp. 853–868. [Google Scholar]
- Afara, I.O.; Shaikh, R.; Nippolainen, E.; Querido, W.; Torniainen, J.; Sarin, J.K.; Kandel, S.; Pleshko, N.; Töyräs, J. Characterization of connective tissues using near-infrared spectroscopy and imaging. Nat. Protoc. 2021, 16, 1297–1329. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Du, B.; Ma, C.; Ding, G.; Han, X.; Li, D.; Wang, E.; Wang, J. Cooperative Strategies for Enhancing Performance of Photothermal Therapy (PTT) Agent: Optimizing Its Photothermal Conversion and Cell Internalization Ability. Small 2017, 13, 1603275. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Ming, T.; Sun, Z.; Zhao, C.; Yang, B.; Wang, J. Understanding the Photothermal Conversion Efficiency of Gold Nanocrystals. Small 2010, 6, 2272–2280. [Google Scholar] [CrossRef]
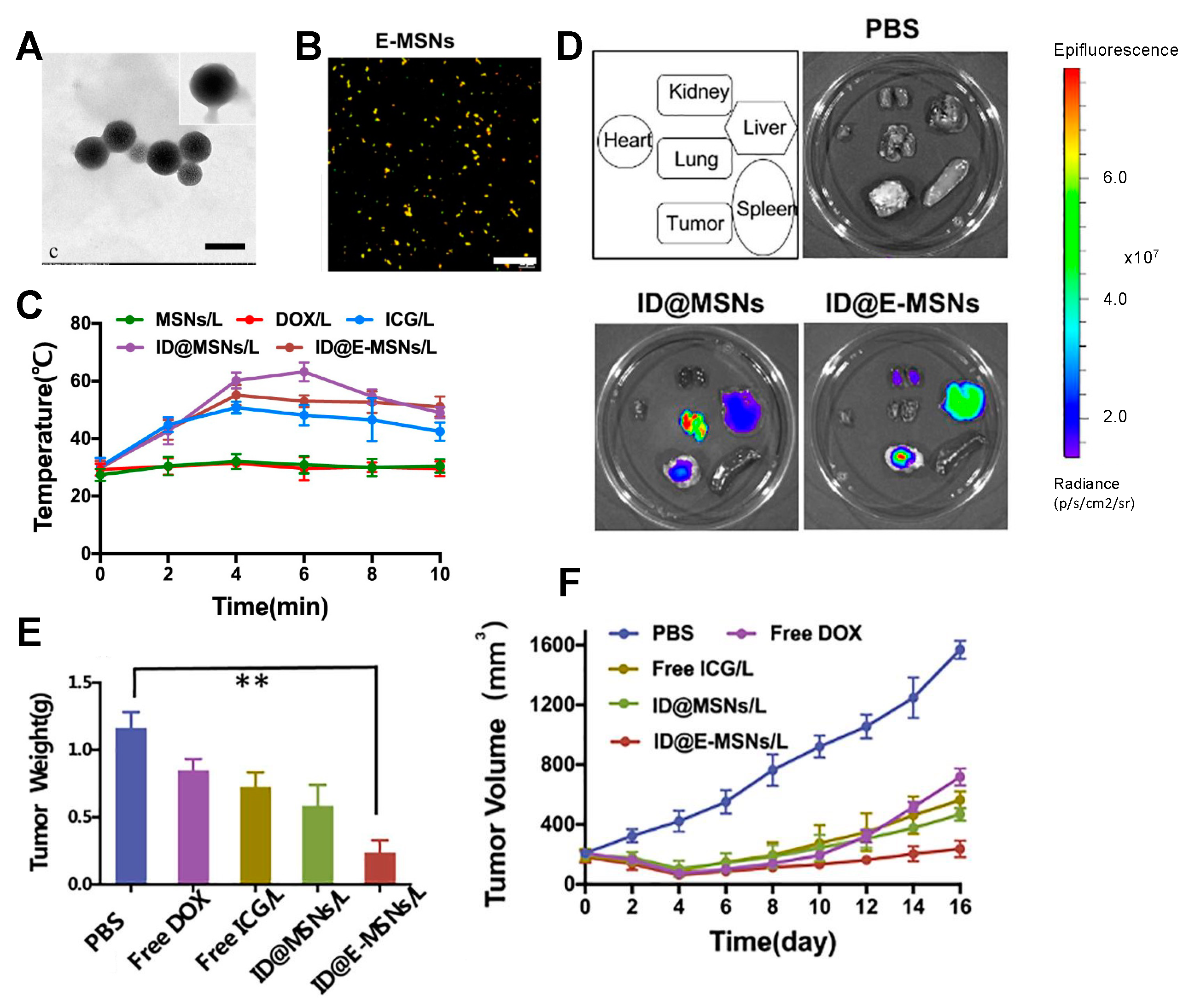
- Farokhi, M.; Mottaghitalab, F.; Saeb, M.R.; Thomas, S. Functionalized theranostic nanocarriers with bio-inspired polydopamine for tumor imaging and chemo-photothermal therapy. J. Control. Release 2019, 309, 203–219. [Google Scholar] [CrossRef]
- Jin, A.; Wang, Y.; Lin, K.; Jiang, L. Nanoparticles modified by polydopamine: Working as “drug” carriers. Bioact. Mater. 2020, 5, 522–541. [Google Scholar] [CrossRef]
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef] [PubMed]
- Shramova, E.I.; Kotlyar, A.B.; Lebedenko, E.N.; Deyev, S.M.; Proshkina, G.M. Near-Infrared Activated Cyanine Dyes As Agents for Photothermal Therapy and Diagnosis of Tumors. Acta Naturae 2020, 12, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Definition of Indocyanine Green Solution—NCI Drug Dictionary—NCI. 2011. Available online: https://www.cancer.gov/publications/dictionaries/cancer-drug/def/indocyanine-green-solution (accessed on 25 September 2023).
- Li, H.; Yin, D.; Li, W.; Tang, Q.; Zou, L.; Peng, Q. Polydopamine-based nanomaterials and their potentials in advanced drug delivery and therapy. Colloids Surf. B Biointerfaces 2021, 199, 111502. [Google Scholar] [CrossRef]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-Covalent Self-Assembly and Covalent Polymerization Co-Contribute to Polydopamine Formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Xiong, Y.; Xu, Z.; Li, Z. Polydopamine-Based Nanocarriers for Photosensitizer Delivery. Front. Chem. 2019, 7, 471. [Google Scholar] [CrossRef] [PubMed]
- Yun, W.S.; Park, J.-H.; Lim, D.-K.; Ahn, C.-H.; Sun, I.-C.; Kim, K. How Did Conventional Nanoparticle-Mediated Photothermal Therapy Become “Hot” in Combination with Cancer Immunotherapy? Cancers 2022, 14, 2044. [Google Scholar] [CrossRef]
- Abadeer, N.S.; Murphy, C.J. Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Mackey, M.A.; Ali, M.R.K.; Austin, L.A.; Near, R.D.; El-Sayed, M.A. The Most Effective Gold Nanorod Size for Plasmonic Photothermal Therapy: Theory and In Vitro Experiments. J. Phys. Chem. B 2014, 118, 1319–1326. [Google Scholar] [CrossRef]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold nanoparticle based photothermal therapy: Development and application for effective cancer treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- Tabish, T.A.; Dey, P.; Mosca, S.; Salimi, M.; Palombo, F.; Matousek, P.; Stone, N. Smart Gold Nanostructures for Light Mediated Cancer Theranostics: Combining Optical Diagnostics with Photothermal Therapy. Adv. Sci. 2020, 7, 1903441. [Google Scholar] [CrossRef]
- Wang, F.; Dukovic, G.; Brus, L.E.; Heinz, T.F. The Optical Resonances in Carbon Nanotubes Arise from Excitons. Science 2005, 308, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Doughty, A.C.V.; Hoover, A.R.; Layton, E.; Murray, C.K.; Howard, E.W.; Chen, W.R. Nanomaterial Applications in Photothermal Therapy for Cancer. Materials 2019, 12, 779. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.; Rodrigues, C.F.; Moreira, A.F.; Correia, I.J. Overview of the application of inorganic nanomaterials in cancer photothermal therapy. Biomater. Sci. 2020, 8, 2990–3020. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wan, J.; Zhang, S.; Tian, B.; Zhang, Y.; Liu, Z. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials 2012, 33, 2206–2214. [Google Scholar] [CrossRef]
- de Melo-Diogo, D.; Lima-Sousa, R.; Alves, C.G.; Correia, I.J. Graphene family nanomaterials for application in cancer combination photothermal therapy. Biomater. Sci. 2019, 7, 3534–3551. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sui, L.; Huang, J.; Miao, L.; Nie, Y.; Wang, K.; Yang, Z.; Huang, Q.; Gong, X.; Nan, Y.; et al. MoS2-based nanocomposites for cancer diagnosis and therapy. Bioact. Mater. 2021, 6, 4209–4242. [Google Scholar] [CrossRef]
- Fu, C.; Tan, L.; Ren, X.; Wu, Q.; Shao, H.; Ren, J.; Zhao, Y.; Meng, X. Interlayer expansion of 2D MoS2 nanosheets for highly improved photothermal therapy of tumors in vitro and in vivo. Chem. Commun. 2018, 54, 13989–13992. [Google Scholar] [CrossRef]
- Wang, S.; Li, K.; Chen, Y.; Chen, H.; Ma, M.; Feng, J.; Zhao, Q.; Shi, J. Biocompatible PEGylated MoS2 nanosheets: Controllable bottom-up synthesis and highly efficient photothermal regression of tumor. Biomaterials 2015, 39, 206–217. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Q.; Liu, M.; Chen, Q.; Ai, K. Emerging Bismuth Chalcogenides Based Nanodrugs for Cancer Radiotherapy. Front. Pharmacol. 2022, 13, 844037. [Google Scholar] [CrossRef]
- Vaishnav Pavan Kumar, A.; Dubey, S.K.; Tiwari, S.; Puri, A.; Hejmady, S.; Goraine, B.; Kesharwani, P. Recent advances in nanoparticles mediated photothermal therapy induced tumor regression. Int. J. Pharm. 2021, 606, 120848. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wang, D.; Zhang, T.; Ghosal, A.; Guo, Z.; Miao, Y.; Li, G.; Liu, X.; Lu, J.; Yu, J.; et al. Facile synthesis of Bi2S3-MoS2 heterogeneous nanoagent as dual functional radiosensitizer for triple negative breast cancer theranostics. Chem. Eng. J. 2020, 395, 125032. [Google Scholar] [CrossRef]
- Bañobre-López, M.; García-Hevia, L.; Cerqueira, M.F.; Rivadulla, F.; Gallo, J. Tunable Performance of Manganese Oxide Nanostructures as MRI Contrast Agents. Chem. Eur. J. 2018, 24, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- García-Hevia, L.; Bañobre-López, M.; Gallo, J. Recent Progress on Manganese-Based Nanostructures as Responsive MRI Contrast Agents. Chem. Eur. J. 2019, 25, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.; Degl’Innocenti, A.; Gümüş, M.B.; Ciofani, G. Superparamagnetic iron oxide nanoparticles for magnetic hyperthermia: Recent advancements, molecular effects, and future directions in the omics era. Biomater. Sci. 2022, 10, 2103–2121. [Google Scholar] [CrossRef]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef]
- Ranji-Burachaloo, H.; Gurr, P.A.; Dunstan, D.E.; Qiao, G.G. Cancer Treatment through Nanoparticle-Facilitated Fenton Reaction. ACS Nano 2018, 12, 11819–11837. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Wang, Y.; Gong, X.; Xu, X.; Wang, J.; Li, Y.; Sha, X.; Zhang, Z. Nanoparticles-mediated reoxygenation strategy relieves tumor hypoxia for enhanced cancer therapy. J. Control. Release 2020, 319, 25–45. [Google Scholar] [CrossRef]
- Gallo, J.; Long, N.J.; Aboagye, E.O. Magnetic nanoparticles as contrast agents in the diagnosis and treatment of cancer. Chem. Soc. Rev. 2013, 42, 7816–7833. [Google Scholar] [CrossRef]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L. Erythrocyte membrane nano-capsules: Biomimetic delivery and controlled release of photothermal–photochemical coupling agents for cancer cell therapy. Dalton Trans. 2020, 49, 2645–2651. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.-G.; Wang, L.; Gao, F.; You, Y.-Z.; Xiong, Y.; Yang, L. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano 2014, 8, 10414–10425. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zheng, R.; Fang, X.; Wang, X.; Zhang, X.; Yang, W.; Sha, X. Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy. Biomaterials 2016, 92, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yin, Y.; Song, W.; Zhang, Q.; Yang, Z.; Dong, Z.; Xu, Y.; Cai, S.; Wang, K.; Yang, W.; et al. Red-blood-cell-membrane-enveloped magnetic nanoclusters as a biomimetic theranostic nanoplatform for bimodal imaging-guided cancer photothermal therapy. J. Mater. Chem. B 2020, 8, 803–812. [Google Scholar] [CrossRef]
- Wang, P.; Jiang, F.; Chen, B.; Tang, H.; Zeng, X.; Cai, D.; Zhu, M.; Long, R.; Yang, D.; Kankala, R.K.; et al. Bioinspired red blood cell membrane-encapsulated biomimetic nanoconstructs for synergistic and efficacious chemo-photothermal therapy. Colloids Surf. B Biointerfaces 2020, 189, 110842. [Google Scholar] [CrossRef]
- Zhu, D.M.; Xie, W.; Xiao, Y.S.; Suo, M.; Zan, M.H.; Liao, Q.Q.; Hu, X.J.; Chen, L.B.; Chen, B.; Wu, W.T.; et al. Erythrocyte membrane-coated gold nanocages for targeted photothermal and chemical cancer therapy. Nanotechnology 2018, 29, 084002. [Google Scholar] [CrossRef]
- Luo, L.; Zeng, F.; Xie, J.; Fan, J.; Xiao, S.; Wang, Z.; Xie, H.; Liu, B. A RBC membrane-camouflaged biomimetic nanoplatform for enhanced chemo-photothermal therapy of cervical cancer. J. Mater. Chem. B 2020, 8, 4080–4092. [Google Scholar] [CrossRef]
- Liu, B.; Wang, W.; Fan, J.; Long, Y.; Xiao, F.; Daniyal, M.; Tong, C.; Xie, Q.; Jian, Y.; Li, B.; et al. RBC membrane camouflaged prussian blue nanoparticles for gamabutolin loading and combined chemo/photothermal therapy of breast cancer. Biomaterials 2019, 217, 119301. [Google Scholar] [CrossRef]
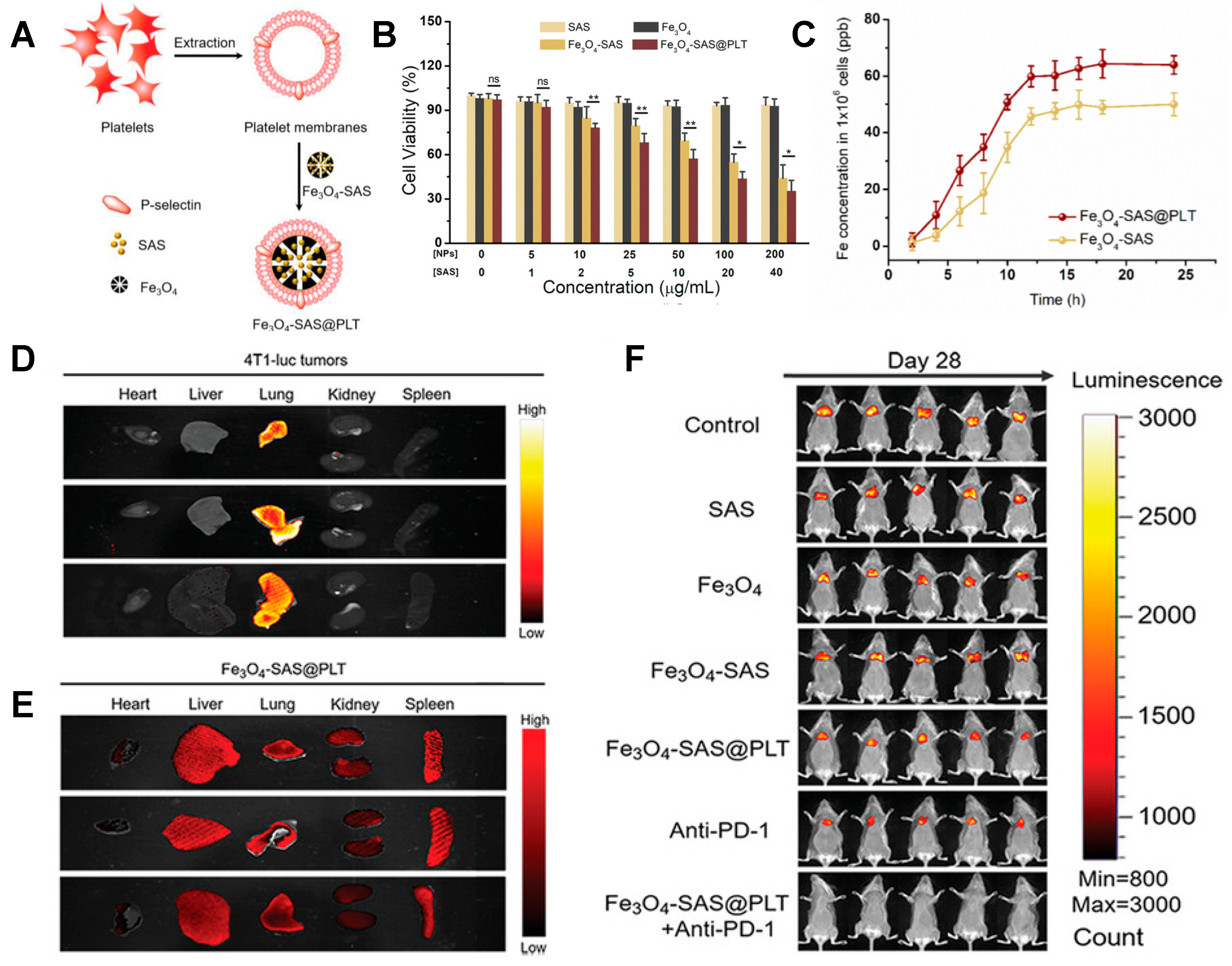
- Chen, Y.; Zhao, G.; Wang, S.; He, Y.; Han, S.; Du, C.; Li, S.; Fan, Z.; Wang, C.; Wang, J. Platelet-membrane-camouflaged bismuth sulfide nanorods for synergistic radio-photothermal therapy against cancer. Biomater. Sci. 2019, 7, 3450–3459. [Google Scholar] [CrossRef]
- Wu, L.; Xie, W.; Zan, H.-M.; Liu, Z.; Wang, G.; Wang, Y.; Liu, W.; Dong, W. Platelet membrane-coated nanoparticles for targeted drug delivery and local chemo-photothermal therapy of orthotopic hepatocellular carcinoma. J. Mater. Chem. B 2020, 8, 4648–4659. [Google Scholar] [CrossRef]
- Pei, W.; Huang, B.; Chen, S.; Wang, L.; Xu, Y.; Niu, C. Platelet-Mimicking Drug Delivery Nanoparticles for Enhanced Chemo-Photothermal Therapy of Breast Cancer. Int. J. Nanomed. 2020, 15, 10151–10167. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, K.; Zhang, X.; Ouyang, B.; Liu, H.; Pang, Z.; Yang, W. Platelet Membrane-Camouflaged Magnetic Nanoparticles for Ferroptosis-Enhanced Cancer Immunotherapy. Small 2020, 16, 2001704. [Google Scholar] [CrossRef]
- Meng, Q.-F.; Rao, L.; Zan, M.; Chen, M.; Yu, G.-T.; Wei, X.; Wu, Z.; Sun, Y.; Guo, S.-S.; Zhao, X.-Z.; et al. Macrophage membrane-coated iron oxide nanoparticles for enhanced photothermal tumor therapy. Nanotechnology 2018, 29, 134004. [Google Scholar] [CrossRef]
- Xuan, M.; Shao, J.; Dai, L.; Li, J.; He, Q. Macrophage Cell Membrane Camouflaged Au Nanoshells for in Vivo Prolonged Circulation Life and Enhanced Cancer Photothermal Therapy. ACS Appl. Mater. Interfaces 2016, 8, 9610–9618. [Google Scholar] [CrossRef]
- Madsen, S.J.; Christie, C.; Hong, S.J.; Trinidad, A.; Peng, Q.; Uzal, F.A.; Hirschberg, H. Nanoparticle-loaded macrophage-mediated photothermal therapy: Potential for glioma treatment. Lasers Med. Sci. 2015, 30, 1357–1365. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, F.; Liu, T.; Shao, P.; Duan, L.; Yan, J.; Mu, X.; Jiang, J. Polydopamine Nanoparticles Camouflaged by Stem Cell Membranes for Synergistic Chemo-Photothermal Therapy of Malignant Bone Tumors. Int. J. Nanomed. 2020, 15, 10183–10197. [Google Scholar] [CrossRef]
- Mu, X.; Li, J.; Yan, S.; Zhang, H.; Zhang, W.; Zhang, F.; Jiang, J. siRNA Delivery with Stem Cell Membrane-Coated Magnetic Nanoparticles for Imaging-Guided Photothermal Therapy and Gene Therapy. ACS Biomater. Sci. Eng. 2018, 4, 3895–3905. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Hu, C.-M.J.; Fang, R.H.; Luk, B.T.; Su, J.; Zhang, L. Surface functionalization of gold nanoparticles with red blood cell membranes. Adv. Mater. Deerfield Beach Fla 2013, 25, 3549–3553. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Cheng, P.; Pu, K. Recent Advances in Cell Membrane-Camouflaged Nanoparticles for Cancer Phototherapy. Small Weinh. Bergstr. Ger. 2019, 15, e1804105. [Google Scholar] [CrossRef]
- Stegner, D.; Dütting, S.; Nieswandt, B. Mechanistic explanation for platelet contribution to cancer metastasis. Thromb. Res. 2014, 133 (Suppl. S2), S149–S157. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.-L.; Huang, Z.-J.; Lin, Z.-T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Zmerli, I.; Michel, J.-P.; Makky, A. Bioinspired polydopamine nanoparticles: Synthesis, nanomechanical properties, and efficient PEGylation strategy. J. Mater. Chem. B 2020, 8, 4489–4504. [Google Scholar] [CrossRef]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. J. Biol. Chem. 2020, 295, 2495–2505. [Google Scholar] [CrossRef]
- Wu, D.; Shou, X.; Zhang, Y.; Li, Z.; Wu, G.; Wu, D.; Wu, J.; Shi, S.; Wang, S. Cell membrane-encapsulated magnetic nanoparticles for enhancing natural killer cell-mediated cancer immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102333. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, W.; Yuan, Y.; Sui, M.; Yang, Y.; Huang, L.; Jiang, L.; Liu, M.; Chen, S.; Zhou, X. Delicately Designed Cancer Cell Membrane-Camouflaged Nanoparticles for Targeted 19F MR/PA/FL Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 12, 57290–57301. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liu, Y.; Guo, R.; Yao, X.; Sung, S.; Pang, Z.; Yang, W. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials 2019, 192, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhai, W.; Liu, X.; Song, X.; Gao, X.; Xu, K.; Tang, B. Homotypic cell membrane-cloaked biomimetic nanocarrier for the accurate photothermal-chemotherapy treatment of recurrent hepatocellular carcinoma. J. Nanobiotechnol. 2020, 18, 60. [Google Scholar] [CrossRef]
- Wu, M.; Mei, T.; Lin, C.; Wang, Y.; Chen, J.; Le, W.; Sun, M.; Xu, J.; Dai, H.; Zhang, Y.; et al. Melanoma Cell Membrane Biomimetic Versatile CuS Nanoprobes for Homologous Targeting Photoacoustic Imaging and Photothermal Chemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 16031–16039. [Google Scholar] [CrossRef]
- Ji, B.; Cai, H.; Yang, Y.; Peng, F.; Song, M.; Sun, K.; Yan, F.; Liu, Y. Hybrid membrane camouflaged copper sulfide nanoparticles for photothermal-chemotherapy of hepatocellular carcinoma. Acta Biomater. 2020, 111, 363–372. [Google Scholar] [CrossRef] [PubMed]
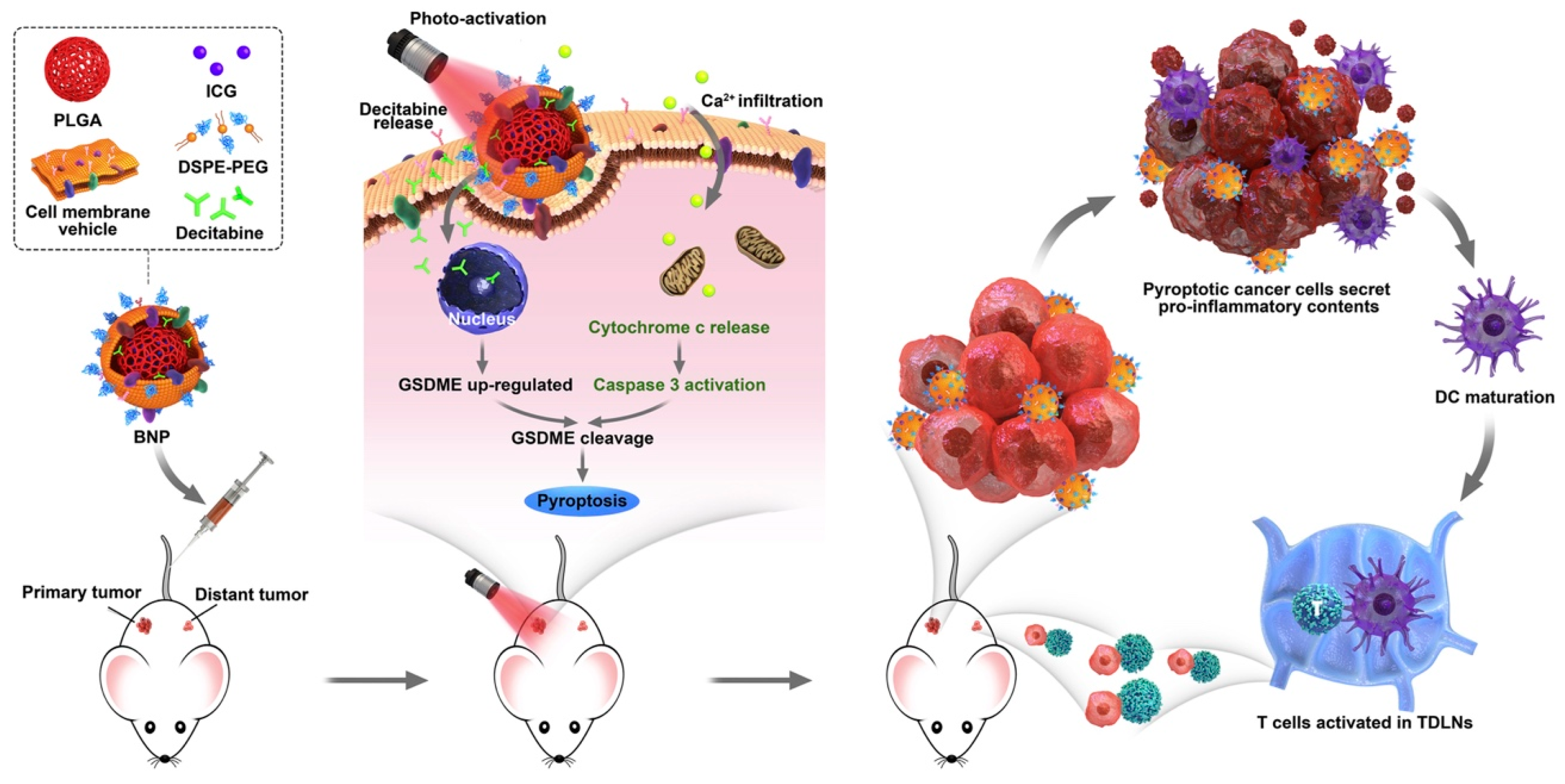
- Zhao, P.; Wang, M.; Chen, M.; Chen, Z.; Peng, X.; Zhou, F.; Song, J.; Qu, J. Programming cell pyroptosis with biomimetic nanoparticles for solid tumor immunotherapy. Biomaterials 2020, 254, 120142. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, C.; You, S.; Zhang, K.; Li, M.; Cao, Y.; Wang, C.; Dong, H.; Zhang, X. Bacterial Vesicle-Cancer Cell Hybrid Membrane-Coated Nanoparticles for Tumor Specific Immune Activation and Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 12, 41138–41147. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wang, X.; Cheng, Z.; Qin, W.; Lei, L.; Jiang, J.; Hu, J. The role of pyroptosis in cancer: Pro-cancer or pro-“host”? Cell Death Dis. 2019, 10, 650. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef]
- Busato, A.; Bonafede, R.; Bontempi, P.; Scambi, I.; Schiaffino, L.; Benati, D.; Malatesta, M.; Sbarbati, A.; Marzola, P.; Mariotti, R. Magnetic resonance imaging of ultrasmall superparamagnetic iron oxide-labeled exosomes from stem cells: A new method to obtain labeled exosomes. Int. J. Nanomed. 2016, 11, 2481–2490. [Google Scholar]
- Huang, L.; Xu, C.; Xu, P.; Qin, Y.; Chen, M.; Feng, Q.; Pan, J.; Cheng, Q.; Liang, F.; Wen, X.; et al. Intelligent Photosensitive Mesenchymal Stem Cells and Cell-Derived Microvesicles for Photothermal Therapy of Prostate Cancer. Nanotheranostics 2019, 3, 41–53. [Google Scholar] [CrossRef]
- Altanerova, U.; Babincova, M.; Babinec, P.; Benejova, K.; Jakubechova, J.; Altanerova, V.; Zduriencikova, M.; Repiska, V.; Altaner, C. Human mesenchymal stem cell-derived iron oxide exosomes allow targeted ablation of tumor cells via magnetic hyperthermia. Int. J. Nanomed. 2017, 12, 7923–7936. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, H.; Xiong, X.; Wei, X.; Guo, X.; Zhao, J.; Zhou, S. A near-infrared light-responsive extracellular vesicle as a “Trojan horse” for tumor deep penetration and imaging-guided therapy. Biomaterials 2021, 269, 120647. [Google Scholar] [CrossRef]
- Tian, R.; Wang, Z.; Niu, R.; Wang, H.; Guan, W.; Chang, J. Tumor Exosome Mimicking Nanoparticles for Tumor Combinatorial Chemo-Photothermal Therapy. Front. Bioeng. Biotechnol. 2020, 8, 1010. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, X.; Tang, J.; Lv, Q.; Liu, J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials 2021, 275, 120964. [Google Scholar] [CrossRef] [PubMed]
- Fathi, P.; Rao, L.; Chen, X. Extracellular vesicle-coated nanoparticles. View 2021, 2, 20200187. [Google Scholar] [CrossRef]
- Tanziela, T.; Shaikh, S.; Jiang, H.; Lu, Z.; Wang, X. Efficient encapsulation of biocompatible nanoparticles in exosomes for cancer theranostics. Nano Today 2020, 35, 100964. [Google Scholar] [CrossRef]
- Qiao, L.; Hu, S.; Huang, K.; Su, T.; Li, Z.; Vandergriff, A.; Cores, J.; Dinh, P.-U.; Allen, T.; Shen, D.; et al. Tumor cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics 2020, 10, 3474–3487. [Google Scholar] [CrossRef]
- Takimoto, C.H.; Chao, M.P.; Gibbs, C.; McCamish, M.A.; Liu, J.; Chen, J.Y.; Majeti, R.; Weissman, I.L. The Macrophage ‘Do not eat me’ signal, CD47, is a clinically validated cancer immunotherapy target. Ann. Oncol. 2019, 30, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Bachmann, M.F. Virus-like particle vaccinology, from bench to bedside. Cell. Mol. Immunol. 2022, 19, 993–1011. [Google Scholar] [CrossRef]
- Kim, K.R.; Lee, A.S.; Kim, S.M.; Heo, H.R.; Kim, C.S. Virus-like nanoparticles as a theranostic platform for cancer. Front. Bioeng. Biotechnol. 2023, 10, 1106767. [Google Scholar] [CrossRef]
- Venkataraman, S.; Apka, P.; Shoeb, E.; Badar, U.; Hefferon, K. Plant Virus Nanoparticles for Anti-cancer Therapy. Front. Bioeng. Biotechnol. 2021, 9, 642794. [Google Scholar] [CrossRef]
- Shan, W.; Chen, R.; Zhang, Q.; Zhao, J.; Chen, B.; Zhou, X.; Ye, S.; Bi, S.; Nie, L.; Ren, L. Improved Stable Indocyanine Green (ICG)-Mediated Cancer Optotheranostics with Naturalized Hepatitis B Core Particles. Adv. Mater. 2018, 30, 1707567. [Google Scholar] [CrossRef] [PubMed]
- Shahrivarkevishahi, A.; Luzuriaga, M.A.; Herbert, F.C.; Tumac, A.C.; Brohlin, O.R.; Wijesundara, Y.H.; Adlooru, A.V.; Benjamin, C.; Lee, H.; Parsamian, P.; et al. PhotothermalPhage: A Virus-Based Photothermal Therapeutic Agent. J. Am. Chem. Soc. 2021, 143, 16428–16438. [Google Scholar] [CrossRef]
- Nkanga, C.I.; Ortega-Rivera, O.A.; Steinmetz, N.F. Photothermal immunotherapy of melanoma using TLR-7 agonist laden tobacco mosaic virus with polydopamine coat. Nanomed. Nanotechnol. Biol. Med. 2022, 44, 102573. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Q.; Li, J.; Yang, M.; Zhao, N.; Xu, F.-J. Rattle-Structured Rough Nanocapsules with in-Situ-Formed Gold Nanorod Cores for Complementary Gene/Chemo/Photothermal Therapy. ACS Nano 2018, 12, 5646–5656. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, P.; Tang, X.; Elzatahry, A.A.; Wang, S.; Al-Dahyan, D.; Zhao, M.; Yao, C.; Hung, C.-T.; Zhu, X.; et al. Facile Synthesis of Uniform Virus-like Mesoporous Silica Nanoparticles for Enhanced Cellular Internalization. ACS Cent. Sci. 2017, 3, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, J.; Tang, S.; Zhou, L.; Yang, W. Polypyrrole Composite Nanoparticles with Morphology-Dependent Photothermal Effect and Immunological Responses. Small 2016, 12, 721–726. [Google Scholar] [CrossRef]
- Zhong, R.; Wang, R.; Hou, X.; Song, L.; Zhang, Y. Polydopamine-doped virus-like structured nanoparticles for photoacoustic imaging guided synergistic chemo-/photothermal therapy. RSC Adv. 2020, 10, 18016–18024. [Google Scholar] [CrossRef]
- Li, Y.; Lin, J.; Wang, P.; Luo, Q.; Lin, H.; Zhang, Y.; Hou, Z.; Liu, J.; Liu, X. Tumor Microenvironment Responsive Shape-Reversal Self-Targeting Virus-Inspired Nanodrug for Imaging-Guided Near-Infrared-II Photothermal Chemotherapy. ACS Nano 2019, 13, 12912–12928. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; Gao, J.; Lu, X. Bacteria-Derived Nanoparticles: Multifunctional Containers for Diagnostic and Therapeutic Applications. Adv. Healthc. Mater. 2020, 9, 2000893. [Google Scholar] [CrossRef]
- Villageliu, D.N.; Samuelson, D.R. The Role of Bacterial Membrane Vesicles in Human Health and Disease. Front. Microbiol. 2022, 13, 828704. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Wang, S.; Weng, W.; Jing, Y.; Su, J. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: Advances and perspectives. Bioact. Mater. 2022, 14, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Wen, Z.J.; Li, Y.M.; Sun, W.R.; Hu, X.Q.; Zhu, J.Z.; Li, X.Y.; Wang, P.Y.; Pedraz, J.L.; Lee, J.-H.; et al. Bioengineered Bacterial Membrane Vesicles with Multifunctional Nanoparticles as a Versatile Platform for Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2023, 15, 3744–3759. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.-H.; Wang, M.-Z.; Chu, Y.; Zhang, L.; Niu, J.; Shao, H.-T.; Yuan, T.-J.; Jiang, Z.-H.; Gao, J.-Q.; Ning, X.-H. Engineering bacterial outer membrane vesicles as transdermal nanoplatforms for photo-TRAIL–programmed therapy against melanoma. Sci. Adv. 2020, 6, eaba2735. [Google Scholar] [CrossRef]
- Serna, N.; Sánchez-García, L.; Unzueta, U.; Díaz, R.; Vázquez, E.; Mangues, R.; Villaverde, A. Protein-Based Therapeutic Killing for Cancer Therapies. Trends Biotechnol. 2018, 36, 318–335. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Behnam Azad, B.; Nimmagadda, S. The Intricate Role of CXCR4 in Cancer. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 124, pp. 31–82. ISBN 978-0-12-411638-2. [Google Scholar]
- Falgàs, A.; Garcia-León, A.; Núñez, Y.; Serna, N.; Sánchez-Garcia, L.; Unzueta, U.; Voltà-Durán, E.; Aragó, M.; Álamo, P.; Alba-Castellón, L.; et al. A diphtheria toxin-based nanoparticle achieves specific cytotoxic effect on CXCR4+ lymphoma cells without toxicity in immunocompromised and immunocompetent mice. Biomed. Pharmacother. 2022, 150, 112940. [Google Scholar] [CrossRef]
- Medina-Gutiérrez, E.; García-León, A.; Gallardo, A.; Álamo, P.; Alba-Castellón, L.; Unzueta, U.; Villaverde, A.; Vázquez, E.; Casanova, I.; Mangues, R. Potent Anticancer Activity of CXCR4-Targeted Nanostructured Toxins in Aggressive Endometrial Cancer Models. Cancers 2022, 15, 85. [Google Scholar] [CrossRef]
- Falgàs, A.; Pallarès, V.; Unzueta, U.; Céspedes, M.V.; Arroyo-Solera, I.; Moreno, M.J.; Sierra, J.; Gallardo, A.; Mangues, M.A.; Vázquez, E.; et al. A CXCR4-targeted nanocarrier achieves highly selective tumor uptake in diffuse large B-cell lymphoma mouse models. Haematologica 2020, 105, 741–753. [Google Scholar] [CrossRef]
- Serna, N.; Álamo, P.; Ramesh, P.; Vinokurova, D.; Sánchez-García, L.; Unzueta, U.; Gallardo, A.; Céspedes, M.V.; Vázquez, E.; Villaverde, A.; et al. Nanostructured toxins for the selective destruction of drug-resistant human CXCR4+ colorectal cancer stem cells. J. Control. Release 2020, 320, 96–104. [Google Scholar] [CrossRef]
- Díaz, R.; Pallarès, V.; Cano-Garrido, O.; Serna, N.; Sánchez-García, L.; Falgàs, A.; Pesarrodona, M.; Unzueta, U.; Sánchez-Chardi, A.; Sánchez, J.M.; et al. Selective CXCR4+ Cancer Cell Targeting and Potent Antineoplastic Effect by a Nanostructured Version of Recombinant Ricin. Small 2018, 14, 1800665. [Google Scholar] [CrossRef]
- Cohen-Inbar, O.; Zaaroor, M. Glioblastoma multiforme targeted therapy: The Chlorotoxin story. J. Clin. Neurosci. 2016, 33, 52–58. [Google Scholar] [CrossRef]
- Qin, C.; He, B.; Dai, W.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Wang, G.; Yin, L.; Zhang, Q. Inhibition of Metastatic Tumor Growth and Metastasis via Targeting Metastatic Breast Cancer by Chlorotoxin-Modified Liposomes. Mol. Pharm. 2014, 11, 3233–3241. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Gunn, J.W.; Kievit, F.M.; Sun, C.; Fang, C.; Lee, J.S.H.; Zhang, M. Inhibition of Tumor-Cell Invasion with Chlorotoxin-Bound Superparamagnetic Nanoparticles. Small 2008, 5, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Kievit, F.M.; Jeon, M.; Silber, J.R.; Ellenbogen, R.G.; Zhang, M. Nanoparticle-Mediated Target Delivery of TRAIL as Gene Therapy for Glioblastoma. Adv. Healthc. Mater. 2015, 4, 2719–2726. [Google Scholar] [CrossRef]
- Stephen, Z.R.; Chiarelli, P.A.; Revia, R.A.; Wang, K.; Kievit, F.; Dayringer, C.; Jeon, M.; Ellenbogen, R.; Zhang, M. Time-Resolved MRI Assessment of Convection-Enhanced Delivery by Targeted and Nontargeted Nanoparticles in a Human Glioblastoma Mouse Model. Cancer Res. 2019, 79, 4776–4786. [Google Scholar] [CrossRef]
- Chung, S.; Sugimoto, Y.; Huang, J.; Zhang, M. Iron Oxide Nanoparticles Decorated with Functional Peptides for a Targeted siRNA Delivery to Glioma Cells. ACS Appl. Mater. Interfaces 2023, 15, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, C. Therapeutic Uses of Bacterial Subunit Toxins. Toxins 2021, 13, 378. [Google Scholar] [CrossRef]
- Engedal, N.; Skotland, T.; Torgersen, M.L.; Sandvig, K. Shiga toxin and its use in targeted cancer therapy and imaging: Shiga toxin in cancer therapy and imaging. Microb. Biotechnol. 2011, 4, 32–46. [Google Scholar] [CrossRef]
- Deville-Foillard, S.; Billet, A.; Dubuisson, R.-M.; Johannes, L.; Durand, P.; Schmidt, F.; Volk, A. High-Relaxivity Molecular MRI Contrast Agent to Target Gb3-Expressing Cancer Cells. Bioconjug. Chem. 2022, 33, 180–193. [Google Scholar] [CrossRef]
- Kostova, V.; Dransart, E.; Azoulay, M.; Brulle, L.; Bai, S.-K.; Florent, J.-C.; Johannes, L.; Schmidt, F. Targeted Shiga toxin–drug conjugates prepared via Cu-free click chemistry. Bioorg. Med. Chem. 2015, 23, 7150–7157. [Google Scholar] [CrossRef]
- Navarro-Palomares, E.; García-Hevia, L.; Padín-González, E.; Bañobre-López, M.; Villegas, J.C.; Valiente, R.; Fanarraga, M.L. Targeting Nanomaterials to Head and Neck Cancer Cells Using a Fragment of the Shiga Toxin as a Potent Natural Ligand. Cancers 2021, 13, 4920. [Google Scholar] [CrossRef]
- Navarro-Palomares, E.; García-Hevia, L.; Galán-Vidal, J.; Gandarillas, A.; García-Reija, F.; Sánchez-Iglesias, A.; Liz-Marzán, L.M.; Valiente, R.; Fanarraga, M.L. Shiga Toxin-B Targeted Gold Nanorods for Local Photothermal Treatment in Oral Cancer Clinical Samples. Int. J. Nanomed. 2022, 17, 5747–5760. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, A.; Shields, C.L.; Rishi, P.; Atalay, H.T.; Pellegrini, M.; McLaughlin, J.P.; Patrick, K.A.; Morton, S.J.; Remmer, M.H.; Parendo, A.; et al. Primary transpupillary thermotherapy for choroidal melanoma in 391 cases: Importance of risk factors in tumor control. Ophthalmology 2015, 122, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ferrel, G.L.; Guerra, M.C.; Hode, T.; Lunn, J.A.; Adalsteinsson, O.; Nordquist, R.E.; Liu, H.; Chen, W.R. Preliminary safety and efficacy results of laser immunotherapy for the treatment of metastatic breast cancer patients. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2011, 10, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef]
- Rawlins, J.; Din, J.N.; Talwar, S.; O’Kane, P. Coronary Intervention with the Excimer Laser: Review of the Technology and Outcome Data. Interv. Cardiol. Rev. 2016, 11, 27–32. [Google Scholar] [CrossRef]
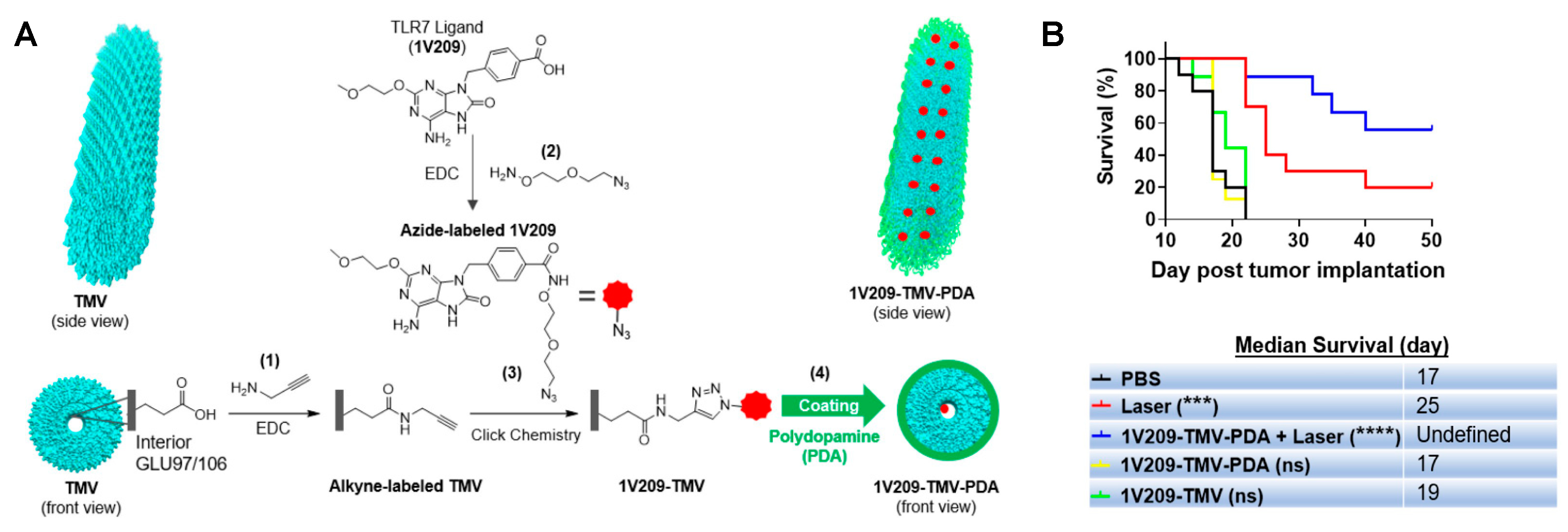
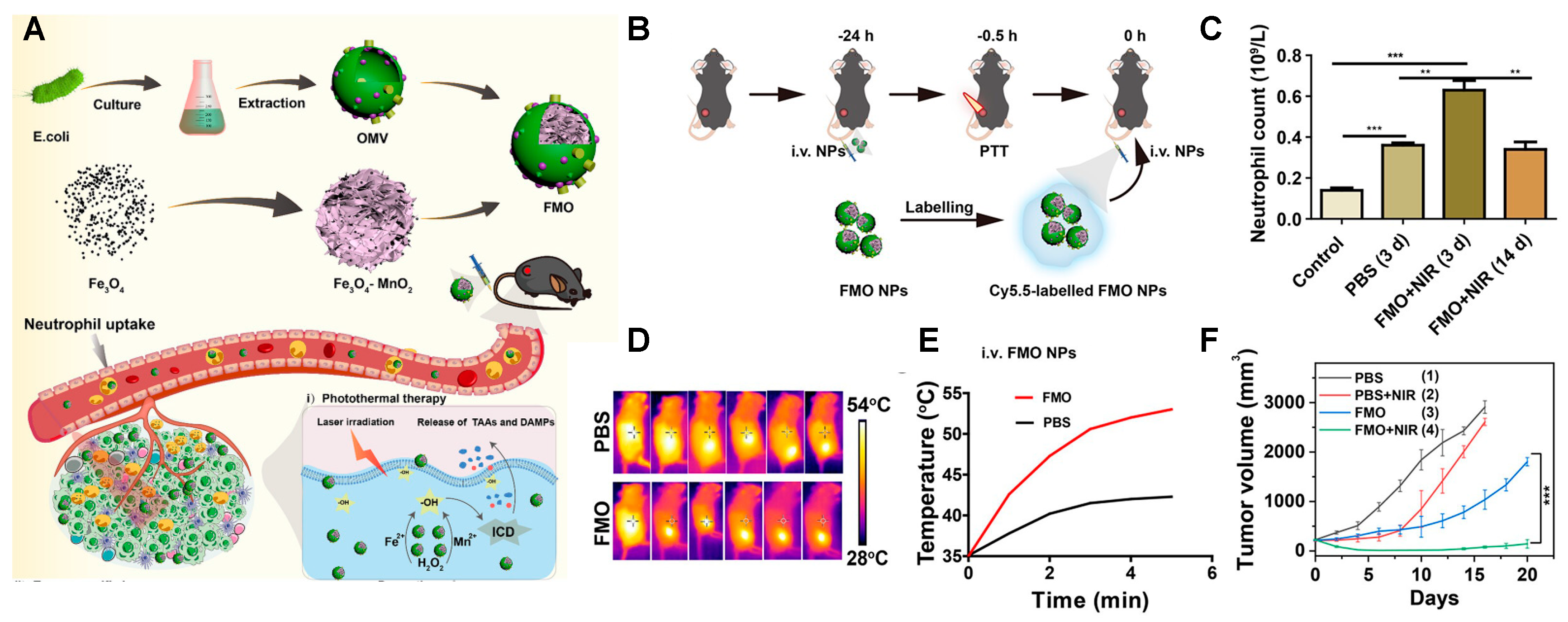
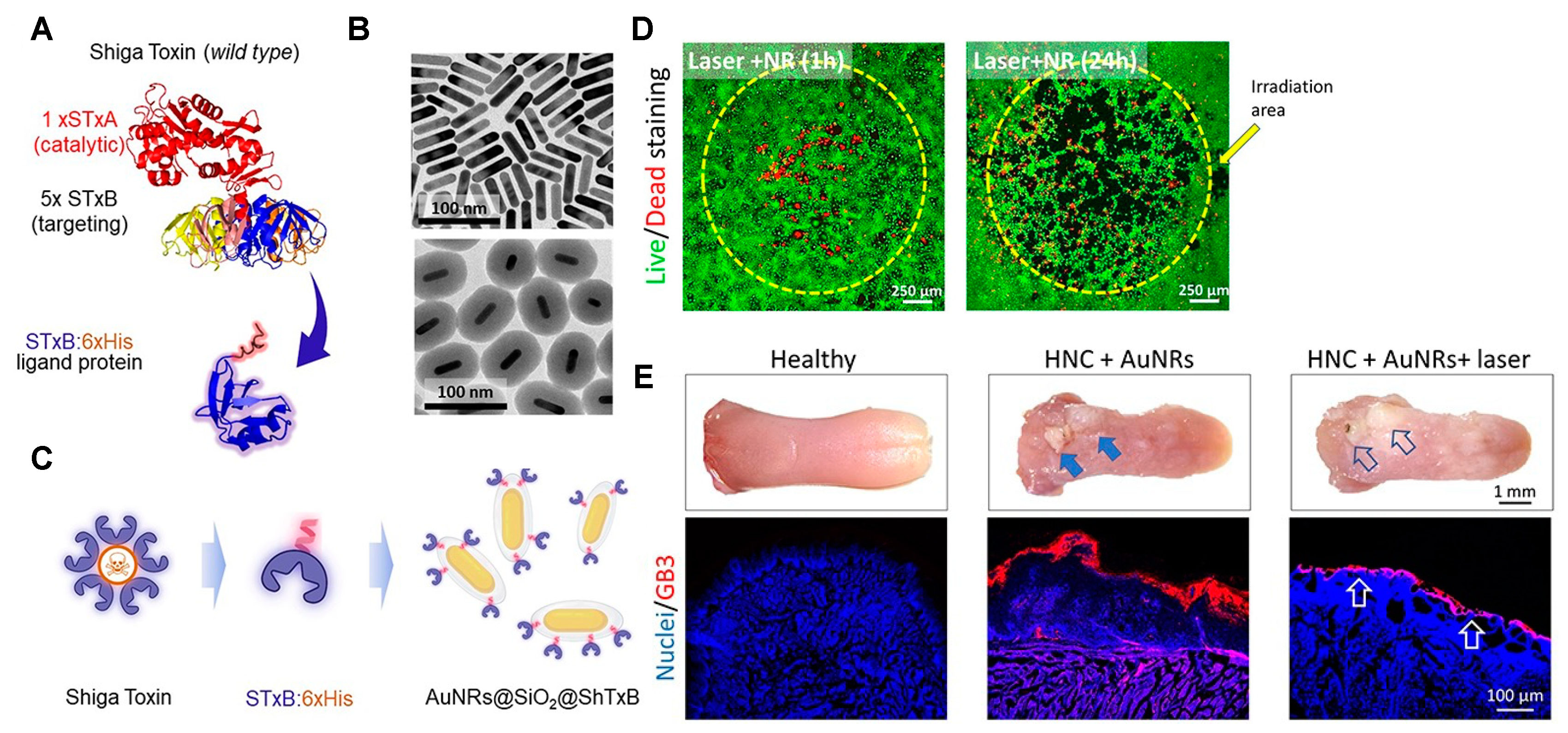
| Shell (Cell Membrane) | Core (NIR-Responsive Materials) | Therapy | Cancer | Study Models | Reference |
|---|---|---|---|---|---|
| Sheep Red blood cells (RBC) | Titanium dioxide/gold nanorods | PTT | Human breast cancer | In vitro: MCF7 cells | [43] |
| Mouse RBC | Gold nanocages | Murine breast cancer | In vitro: HepG2 cells; In vivo: BALB/c mouse bearing 4T1 | [44] | |
| Iron oxide nanoclusters | Human breast cancer | In vitro: MCF-7 cells; In vivo: Nude mice bearing MCF-7 | [45] | ||
| Iron oxide nanoclusters + Cypate | Human colon cancer | In vitro: HCT-116 cells; In vivo: Balb/c nude mice bearing HCT-116 | [46] | ||
| BSA + indocyanine green (ICG) + gambogic acid | Chemo-PTT | Human breast and cervical cancer | In vitro: HeLa and MCF7 cells; In vivo: Nude mice bearing HeLa | [47] | |
| Mouse RBC + anti-EpCam | Gold nanocages + paclitaxel | Murine breast cancer | In vitro: 4T1 cancer cells | [48] | |
| Mouse or human RBC + Folic acid | Graphene oxide + ICG + doxorubicin (DOX) | Human cervical cancer | In vitro: HeLa cells; In vivo: BALB/c mice bearing HeLa | [49] | |
| Mouse or human RBC + Hyaluronic acid | Prussian blue + gamabufotalin | Human breast cancer | In vitro: MDA-MB-231 cells; In vivo: BALB/c mice bearing MDA-MB-231 | [50] | |
| Mouse Platelets (PLT) | Bismuth-silica nanorods | Radio-PTT | Murine breast cancer | In vitro: 4T1 cancer cells; In vivo: BALB/c mice bearing 4T1 | [51] |
| Polypyrrole + DOX | Chemo-PTT | Human hepatocellular carcinoma | In vitro: Huh7 cells; In vivo: BALB/c nude mice bearing Huh7 | [52] | |
| PLGA + IR780 + DOX | Murine breast cancer | In vitro: 4T1 cancer cells; In vivo: BALB/c mice bearing 4T1 | [53] | ||
| Iron oxide + sulfasalazine | Chemo-PTT + immunotherapy | Murine breast cancer | In vitro: 4T1 cancer cells; In vivo: BALB/c mice bearing 4T1 | [54] | |
| Mouse Macrophage (Ma) | Iron oxide nanoparticles | PTT | Human breast cancer | In vitro: MCF7 cells; In vivo: BALB/c nude mice bearing MCF-7 | [55] |
| Gold–silica nanoshells | Murine breast cancer | In vitro: 4T1 cancer cells; In vivo: BALB/c mice bearing 4T1 | [56] | ||
| Rat alveolar Ma | Murine glioma | In vitro: C6 glioma cells; In vivo: Sprague-Dawley rat glioma | [57] | ||
| Human umbilical cord mesenchymal stem cell (UCMS) | polydopamine (PDA) nanoparticles + SN38 | Chemo-PTT | Human osteosarcoma | In vitro: MG63 cancer cells; In vivo: BALB/c mice bearing MG63 | [58] |
| PDA—iron oxide nanoparticles + Plk1 siRNA | Gene-PTT | Human prostate cancer | In vitro: DU145 cancer cells; In vivo: Nude mice bearing DU145 | [59] |
| Shell (Cell Membrane) | Core (NIR-Responsive Materials) | Therapy | Cancer Target | Study Models | Reference |
|---|---|---|---|---|---|
| Human hepatocellular carcinoma (HepG2 cells) | Iron oxide @ silicon dioxide | PTT | Human hepatocellular carcinoma Human melanoma | In vitro: HepG2 cells In vitro: A375 cells | [68] |
| Human lung cancer (A549 cells) | PLGA @ ICG + perfluorocarbons | Human lung cancer | In vitro: A549 cells In vivo: Nude mice bearing A549 | [69] | |
| Human breast cancer (MCF7 cells)/ Mouse RBC | Melanin | Human breast cancer | In vitro: MCF-7 cells In vivo: Nude mice bearing MCF7 | [70] | |
| Human hepatocellular carcinoma (HepG2 cells) | Liposome@ICG + DOXO | Chemo-PTT | Human hepatocellular carcinoma | In vitro: HepG2 cells In vivo: BALB/c nude mice bearing HepG2 | [71] |
| Mouse melanoma (B16-F10 cells) | Hollow copper sulfide @ ICG + DOXO | Mouse melanoma | In vitro: B16-F10 cells In vivo: C57BL/6 mice bearing B16-F10 | [72] | |
| Mouse hepatocellular carcinoma (H22 cells)/ Mouse macrophage (RAW 264.7 cells) + anti-VEGFR | Hollow copper sulfide @ Sorafenib | Human and murine hepatocellular carcinoma | In vitro: HepG2 cells In vivo: BALB/c mice bearing H22 | [73] | |
| Mouse breast cancer (4T1 cells) | PLGA @ ICG + decitabine | Chemo-PTT + immunotherapy | Murine breast cancer | In vitro: 4T1 cells In vivo: BALB/c mice bearing 4T1 | [74] |
| Mouse melanoma (B16-F10 cells)/ bacterial outer membrane | Hollow polydopamine | PTT + immunotherapy | Human lung carcinoma; Human breast cancer; Mouse melanoma | In vitro: A549, MCF7 cells In vivo: C57BL/6 mice bearing B16-F10 | [75] |
| Shell (Extracellular Vesicles) | Core (NIR-Responsive Materials) | Therapy | Cancer | Study Models | Reference | |
|---|---|---|---|---|---|---|
| Healthy cell-derived EVs | Mouse adipose stem cells | Iron oxide (USPIO) | MRI | [79] | ||
| Human umbilical cord mesenchymal stem cell | Gold nanostars -TAT peptide | PTT | Human prostate cancer | In vitro: PC-3 cells; In vivo: Nude mice bearing PC-3 | [80] | |
| yCD::UPRT fusion gene human mesenchymal stem cells | iron oxide (Venofer) | Hyperthermia | Human prostate and breast cancer | In vitro: PC-3 cells and HeLa cells | [81] | |
| Mouse macrophage (RAW 264.7 cells) | Ag2S quantum dots @ DOX | Chemo-PTT | Mouse breast cancer | In vitro: 4T1 cells; In vivo: Balb/c mice bearing 4T1 | [82] | |
| Cancer cell-derived EVs | Mouse breast cancer (4T1 cells) | Mesoporous silica @ ICG + DOX | [83] | |||
| Mouse colon cancer (CD47-overexpressed CT26 cells) | Liposomes @ ICG + R837 | Immuno-PTT | Mouse colorectal carcinoma | In vitro: CT26 cells; In vivo: BALB/C mice bearing CT26 | [84] | |
| NIR-Responsive Material | Cancer Type | Clinical Trial | Recruitment Status |
|---|---|---|---|
| Indocyanine green (ICG) | Choroidal melanomas | NCT01253759 | Completed |
| ICG + Ranibizumab | NCT00680225 | Completed | |
| Silica–gold nanoshells (AuroShell®) | Head and neck | NCT00848042 | Completed |
| Lung tumors | NCT01679470 | Terminated | |
| Prostate | NCT02680535 | Completed | |
| NCT04240639 | Active, not recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, J.; Villasante, A. Recent Advances in Biomimetic Nanocarrier-Based Photothermal Therapy for Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 15484. https://doi.org/10.3390/ijms242015484
Gallo J, Villasante A. Recent Advances in Biomimetic Nanocarrier-Based Photothermal Therapy for Cancer Treatment. International Journal of Molecular Sciences. 2023; 24(20):15484. https://doi.org/10.3390/ijms242015484
Chicago/Turabian StyleGallo, Juan, and Aranzazu Villasante. 2023. "Recent Advances in Biomimetic Nanocarrier-Based Photothermal Therapy for Cancer Treatment" International Journal of Molecular Sciences 24, no. 20: 15484. https://doi.org/10.3390/ijms242015484
APA StyleGallo, J., & Villasante, A. (2023). Recent Advances in Biomimetic Nanocarrier-Based Photothermal Therapy for Cancer Treatment. International Journal of Molecular Sciences, 24(20), 15484. https://doi.org/10.3390/ijms242015484







