Photo- and Sono-Active Food Colorants Inactivating Bacteria
Abstract
1. Introduction
2. Results and Discussion
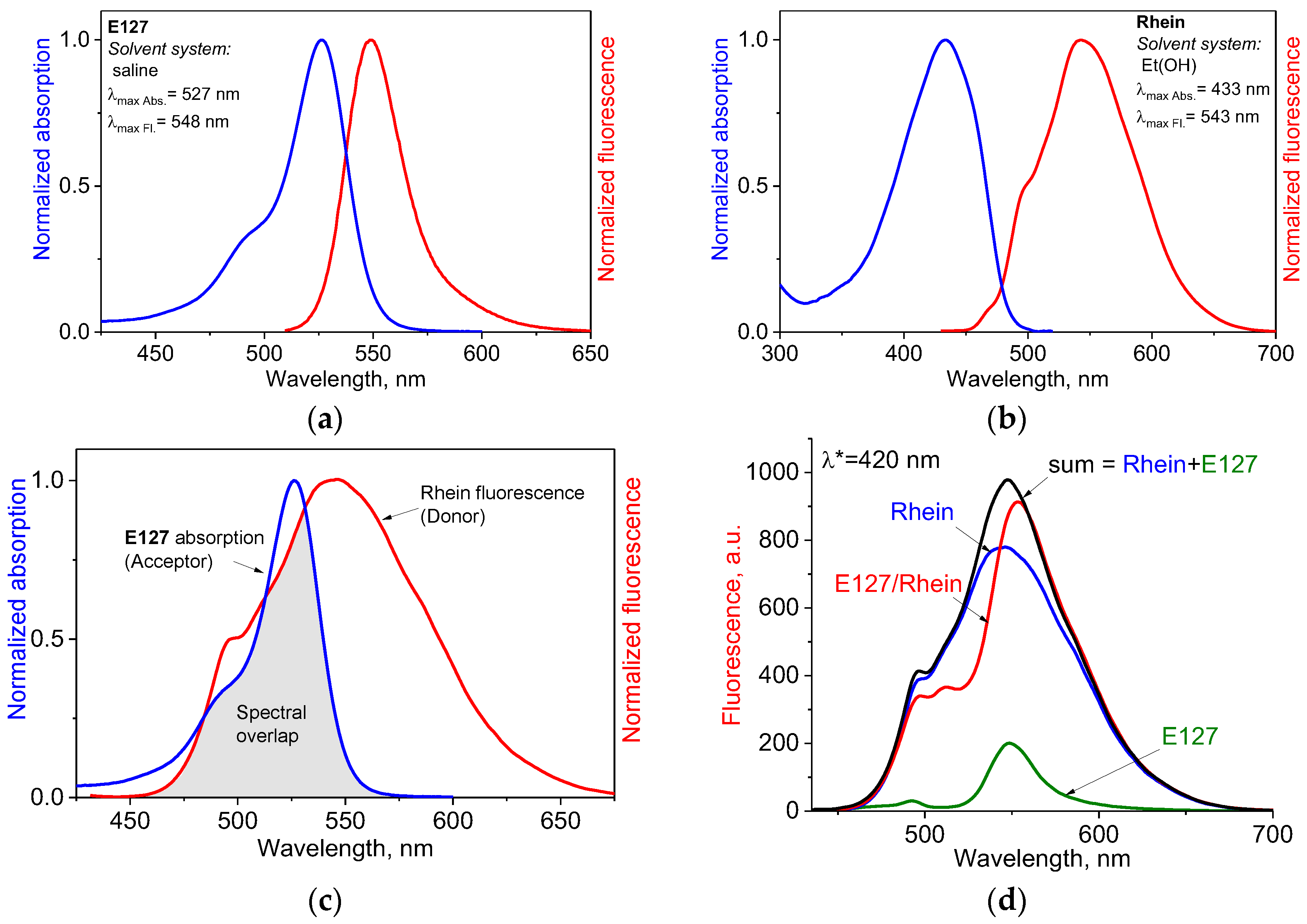
2.1. Spectral Characterization of the Colorants
2.2. Determination of MIC Values of Food Colorants against S. aureus and E. coli
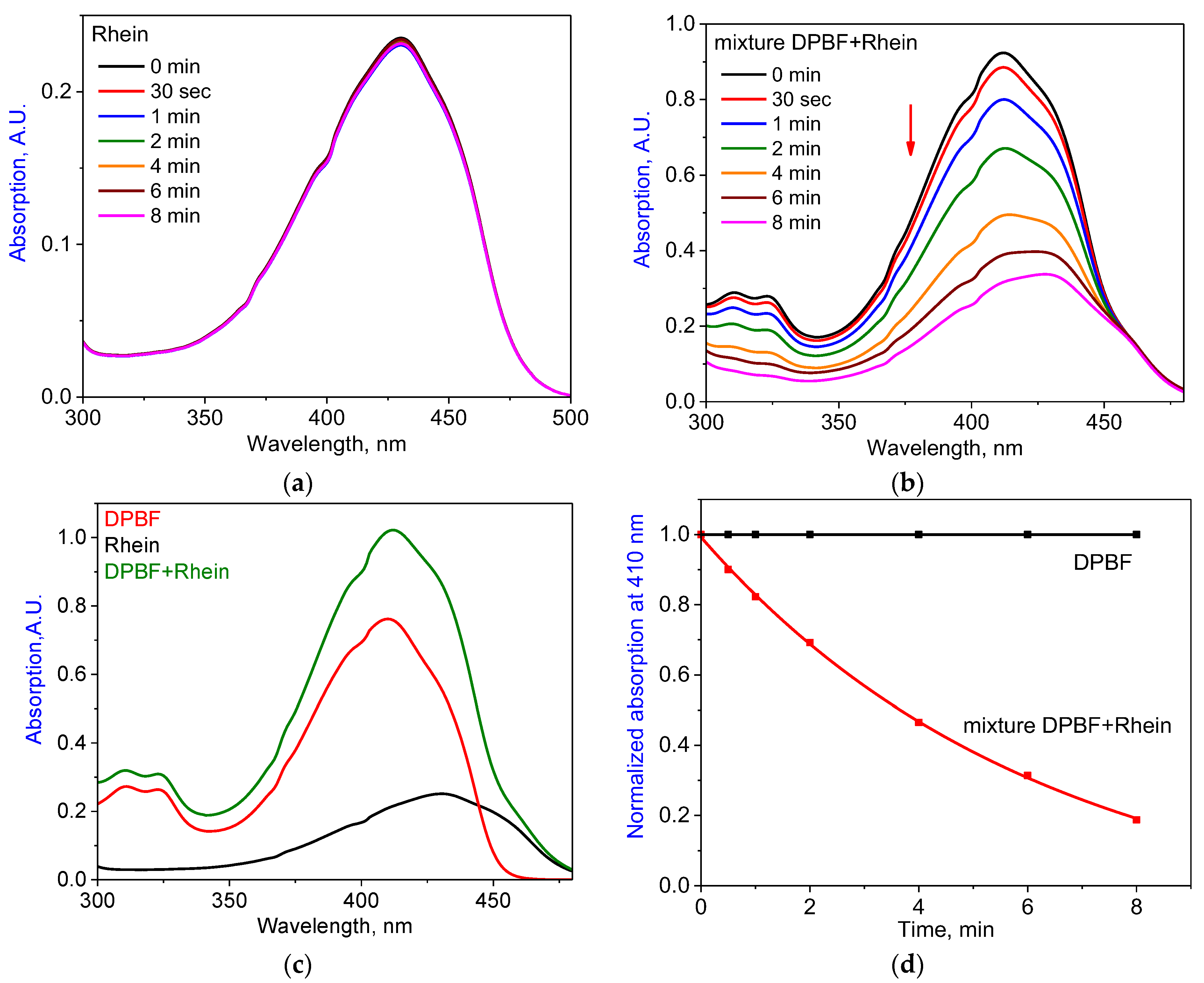
2.3. Photodynamic Activity of Food Colorants against S. aureus and E. coli
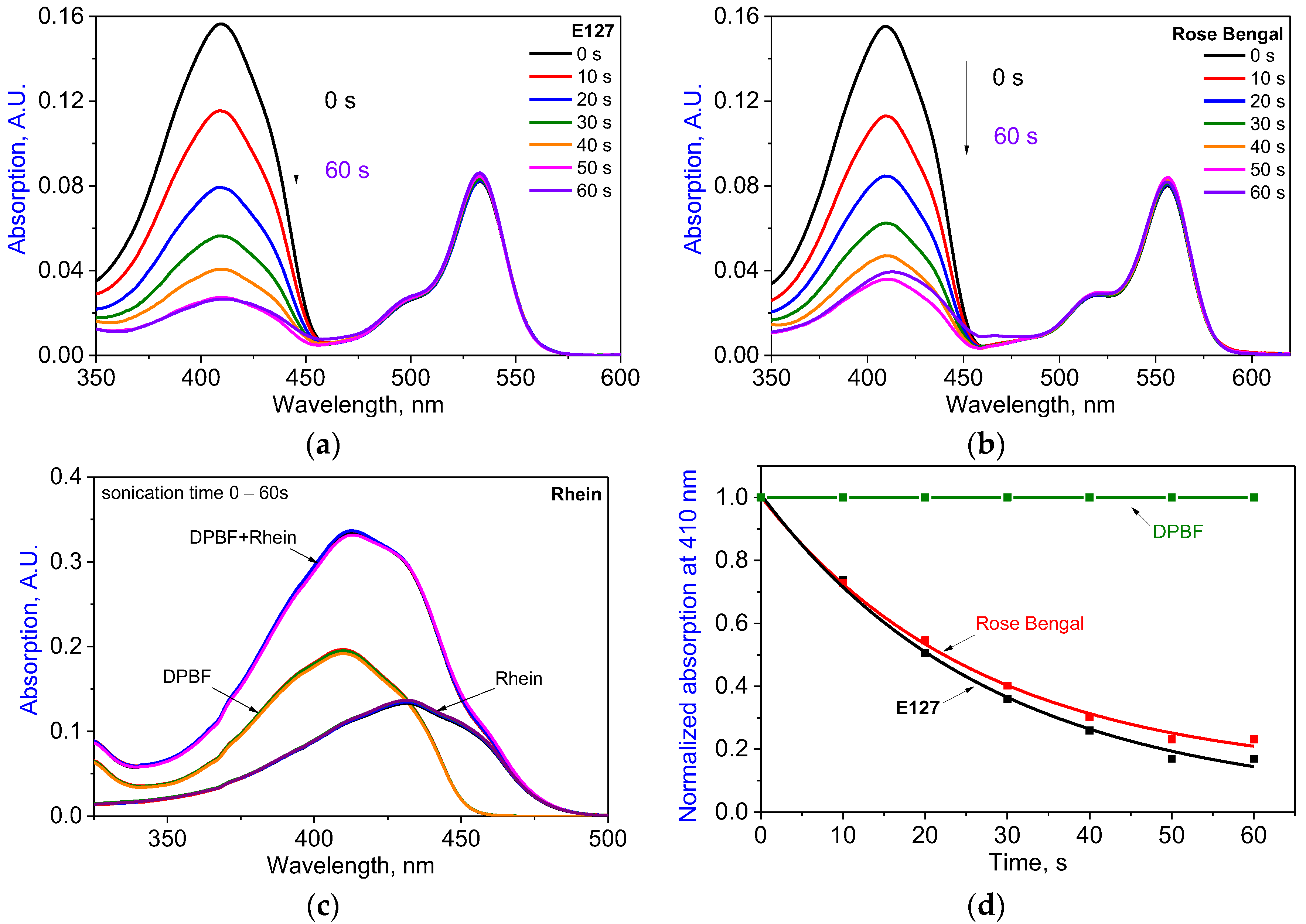
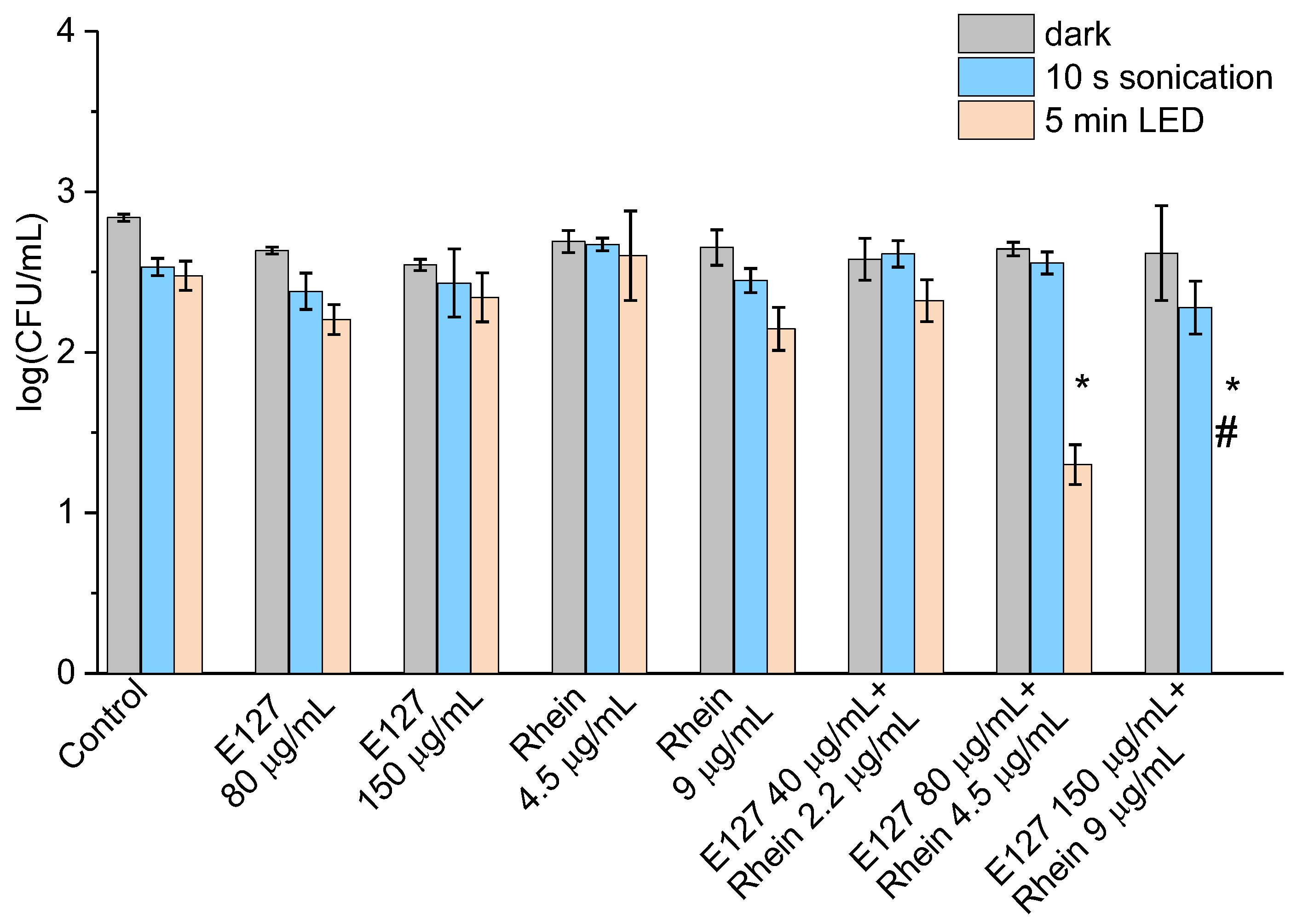
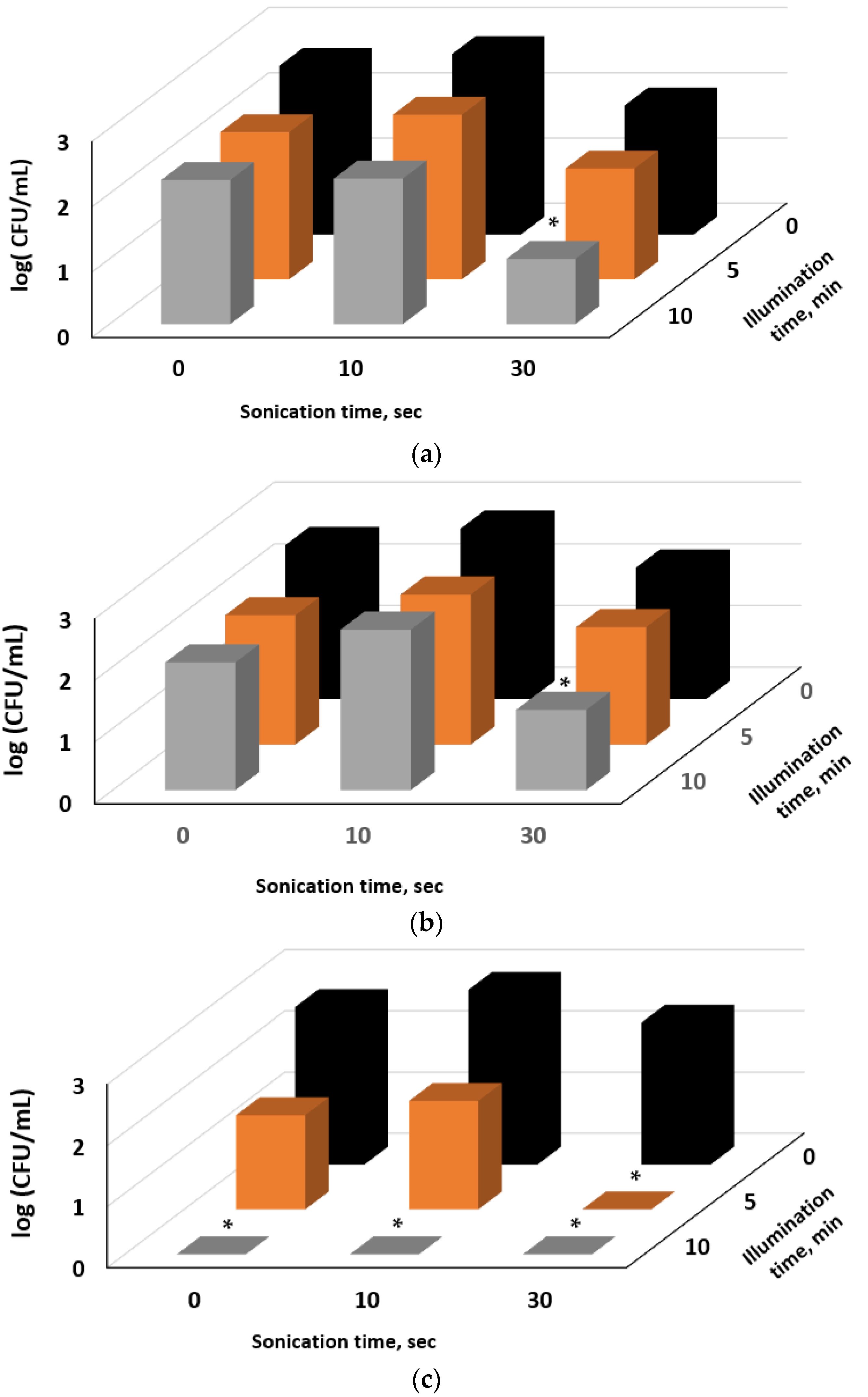
2.4. Inactivation of E. coli by Sono- and Photodynamic Treatment in Series Mediated by E127, Rhein, and E127/Rhein Combination
3. Materials and Methods
3.1. Bacterial Growth
3.2. Preparation of Colorant Stocks
3.3. Minimal Inhibitory Concentration (MIC) Assay
3.4. Absorption and Fluorescence Measurements
3.5. Singlet Oxygen Production Study
3.6. Photodynamic Cyto-Inactivation Study
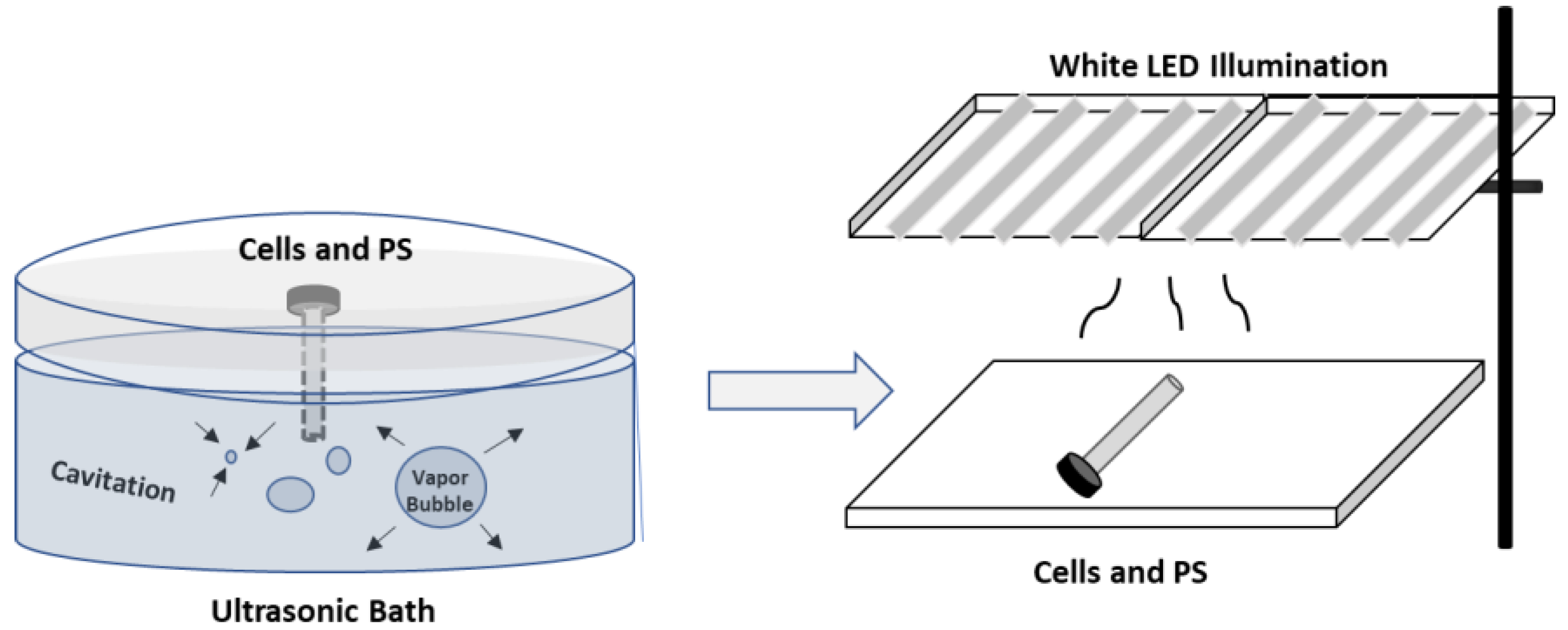
3.7. Ultrasonic Treatment of Cells
3.8. Inactivation of Bacteria by Ultrasonic and Photodynamic Treatment in Series
3.9. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Coultate, T.; Blackburn, R.S. Food colorants: Their past, present and future. Color. Technol. 2018, 134, 165–186. [Google Scholar] [CrossRef]
- Perez-Ibarbia, L.; Majdanski, T.; Schubert, S.; Windhab, N.; Schubert, U.S. Safety and regulatory review of dyes commonly used as excipients in pharmaceutical and nutraceutical applications. Eur. J. Pharm. Sci. 2016, 93, 264–273. [Google Scholar] [CrossRef]
- Hochma, E.; Yarmolinsky, L.; Khalfin, B.; Nisnevitch, M.; Ben-Shabat, S.; Nakonechny, F. Antimicrobial Effect of Phytochemicals from Edible Plants. Processes 2021, 9, 2089. [Google Scholar] [CrossRef]
- Cho, G.-L.; Ha, J.-W. Erythrosine B (Red Dye No. 3): A potential photosensitizer for the photodynamic inactivation of foodborne pathogens in tomato juice. J. Food Saf. 2020, 40, 1–9. [Google Scholar] [CrossRef]
- de Boer, F.Y.; Imhof, A.; Velikov, K.P. Encapsulation of colorants by natural polymers for food applications. Color. Technol. 2019, 135, 183–194. [Google Scholar] [CrossRef]
- Dhiman, R.; Aggarwal, N.K. Efficacy of Plant Antimicrobials as Preservative in Food. In Food Preservation and Waste Exploitation; Socaci, S.A., Farcas, A.C., Aussenac, T., Laguerre, J.-C., Eds.; Intechopen: London, UK, 2019; pp. 1–19. [Google Scholar]
- Hamblin, M.; Abrahamse, H. Oxygen-independent antimicrobial photoinactivation: Type III photochemical mechanism? Antibiotics 2020, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.; Ayala, E.T.P.; Pratavieira, S. Sonophotodynamic Inactivation: The power of light and ultrasound in the battle against microorganisms. J. Photochem. Photobiol. 2021, 7, 100039. [Google Scholar] [CrossRef]
- Caires, C.S.A.; Silva, C.M.; Lima, A.R.; Alves, L.M.; Lima, T.H.N.; Rodrigues, A.C.S.; Chang, M.R.; Oliveira, S.L.; Whitby, C.; Nascimento, V.A.; et al. Photodynamic Inactivation of Methicillin-Resistant Staphylococcus aureus by a Natural Food Colorant (E-141ii). Molecules 2020, 25, 4464. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, F.F.; Huang, Y.Y.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Kill Gram-negative Bacteria. Recent Pat Antiinfect Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef]
- Macdonald, I.J.; Douherty, T.J. Basic Principles of Photodynamic Therapy. J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Maisch, T. Anti-Microbial Photodynamic Therapy: Useful in the Future? Lasers Med. Sci. 2007, 22, 83–91. [Google Scholar] [CrossRef]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current States and Future Views in Photodynamic Therapy. J. Photochem. Photobiol. C Photochem. Reviews. 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Maisch, T.; Szeimies, R.M.; Jori, G.; Abels, C. Antibacterial Photodynamic Therapy in Dermatology. Photochem. Photobiol. Sci. 2004, 3, 907–917. [Google Scholar] [CrossRef]
- Wang, G.; Wu, W.; Zhu, J.-J.; Pang, D. The promise of low-intensity ultrasound: A review on sonosensitizers and sonocatalysts by ultrasonic activation for bacterial killing. Ultrason. Sonochemistry 2021, 79, 105781. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, P.; Li, J.; Yang, Y.; Zeng, S.; Qiu, J.; Lin, S. Curcumin-Mediated Sono-Photodynamic Treatment Inactivates Listeria monocytogenes via ROS-Induced Physical Disruption and Oxidative Damage. Foods 2022, 11, 808. [Google Scholar] [CrossRef]
- Bhavya, M.L.; Hebbar, H.U. Sono-photodynamic inactivation of Escherichia coli and Staphylococcus aureus in orange juice. Ultrasonic. Sonochem. 2019, 57, 108–115. [Google Scholar] [CrossRef]
- Chequer, F.M.; Venâncio, V.P.; Bianchi, M.L.; Antunes, L.M. Genotoxic and mutagenic effects of erythrosine B, a xanthene food dye, on HepG2 cells. Food Chem Toxicol. 2012, 50, 3447–3451. [Google Scholar] [CrossRef]
- Patel, M.; Paredes, R.; Hassan, M.; Boyd, T. Oral Care Compos. Use Oral Light. Device. Patent WO 2011/084744 A1, 14 July 2011. [Google Scholar]
- Melgoza, D.; Hernandez-Ramirez, A.; Peralta-Hernandez, J.M. Comparative efficiencies of the decolourisation of Methylene Blue using Fenton’s and photo-Fenton’s reactions. Photochem. Photobiol. Sci. 2009, 8, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Waring, D.R. Heterocyclic Dyes and Pigments. In Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Ed.; Pergamon Press: New York, NY, USA, 1984; pp. 317–346. [Google Scholar]
- Chavan, R.B. Environmentally friendly dyes. In Handbook of Textile and Industrial Dyeing; Clark, M., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2011; Volume 1, pp. 515–561. [Google Scholar]
- Corradini, M.G. Synthetic Food Colors. In Encyclopedia of Food Chemistry; Varelis, P., Melton, L., Shahidi, F., Eds.; Elsevier Science & Technology: Amsterdam, The Netherlands, 2018; pp. 291–296. [Google Scholar]
- He, Z.; Chen, L.; Catalan-Dibene, J.; Bongers, G.; Faith, J.J.; Suebsuwong, C.; DeVita, R.J.; Shen, Z.; Fox, J.G.; Lafaille, J.J.; et al. Food colorants metabolized by commensal bacteria promote colitis in mice with dysregulated expression of interleukin-23. Cell Metab. 2021, 33, 1358–1371. [Google Scholar] [CrossRef]
- Folliero, V.; Dell’Annunziata, F.; Roscetto, E.; Amato, A.; Gasparro, R.; Zannella, C.; Casolaro, V.; Filippis, A.D.; Catania, M.R.; Franci, G.; et al. Rhein: A novel antibacterial compound against Streptococcus mutans infection. Microbiol. Res. 2022, 261, 127062. [Google Scholar] [CrossRef] [PubMed]
- Henamayee, S.; Banik, K.; Sailo, B.L.; Shabnam, B.; Harsha, C.; Srilakshmi, S.; VGM, N.; Baek, S.H.; Ahn, K.S.; Kunnumakkara, A.B. Therapeutic Emergence of Rhein as a Potential Anticancer Drug: A Review of Its Molecular Targets and Anticancer Properties. Molecules 2020, 25, 2278. [Google Scholar] [CrossRef]
- Azelmat, J.; Larente, J.F.; Grenier, D. The anthraquinone Rhein exhibits synergistic antibacterial activity in association with metronidazole or natural compounds and attenuates virulence gene expression in Porphyromonas gingivalis. Arch. Oral Biol. 2015, 60, 342–346. [Google Scholar] [CrossRef]
- Annunziata, F.D.; Folliero, V.; Palma, F.; Crudele, V.; Finamore, E.; Sanna, G.; Manzin, A.; Filippis, A.D.; Galdiero, M. Anthraquinone Rhein Exhibits Antibacterial Activity against Staphylococcus aureus. Appl. Sci. 2022, 12, 8691. [Google Scholar]
- Joung, D.K.; Joung, H.; Yang, D.W.; Kwon, D.Y.; Choi, J.G.; Woo, S.; Shin, D.Y.; Kweon, O.H.; Kweon, K.T.; Shin, D.W. Synergistic effect of Rhein in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. Exp. Ther. Med. 2012, 3, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Reboredo, F.H.; Lidon, F.C. Food Colour Additives: A Synoptical Overview on Their Chemical Properties, Applications in Food Products, and Health Side Effects. Foods 2022, 11, 379. [Google Scholar] [CrossRef]
- Pietrowska, A.; Hołowacz, I.; Ulatowska-Jarża, A.; Guźniczak, M.; Matczuk, A.K.; Wieliczko, A.; Wolf-Baca, M.; Buzalewicz, I. The Enhancement of Antimicrobial Photodynamic Therapy of Escherichia Coli by a Functionalized Combination of Photosensitizers: In Vitro Examination of Single Cells by Quantitative Phase Imaging. Int. J. Mol. Sci. 2022, 23, 6137. [Google Scholar] [CrossRef]
- Fitzgerald, D.J.; Stratford, M.; Gasson, M.J.; Ueckert, J.; Bos, A.; Narbad, A. Mode of antimicrobial action of vanillin against Escherichia coli, Lactobacillus plantarum and Listeria innocua. J. Appl. Microbiol. 2004, 97, 104–113. [Google Scholar] [CrossRef]
- Wood, S.; Metcalf, D.; Devine, D.; Robinson, C. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J. Antimicrob. Chemother. 2006, 57, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Park, H.-W.; Lee, J.-H.; Seo, H.-W.; Lee, S.-Y. The photodynamic therapy on Streptococcus mutans biofilms using erythrosine and dental halogen curing unit. Int. J. Oral Sci. 2012, 4, 196–201. [Google Scholar] [CrossRef][Green Version]
- Pellosi, D.S.; Estevão, B.M.; Freitas, C.F.; Tsubone, T.M.; Caetano, W.; Hioka, N. Photophysical properties of erythrosin ester derivatives in ionic and non-ionic micelles. Dye. Pigment. 2013, 99, 705–712. [Google Scholar] [CrossRef]
- Entradas, T.; Waldron, S.; Volk, M. The detection sensitivity of commonly used singlet oxygen probes in aqueous environments. J. Photochem. Photobiol. B 2020, 204, 1011–1344. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Zhao, H.; Ji, X.; Cao, W.; Zhang, Z.; Meng, P. Photophysical properties, singlet oxygen generation efficiency and cytotoxic effects of aloe emodin as a blue light photosensitizer for photodynamic therapy in dermatological treatment. Photochem. Photobiol. Sci. 2017, 16, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, B.B.; Ganguly, P. Photophysical studies of some dyes in aqueous solution of triton X-100. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 62, 808–813. [Google Scholar] [CrossRef]
- Gaigalas, A.K.; Wang, L. Measurement of the Fluorescence Quantum Yield Using a Spectrometer With an Integrating Sphere Detector. J. Res. Natl. Inst. Stand. Technol. 2008, 113, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Berney, C.; Danuser, G. FRET or no FRET: A quantitative comparison. Biophys. J. 2003, 84, 3992–4010. [Google Scholar] [CrossRef]
- Williams, R.M. Introduction to Electron Transfer, Adapted from: Fullerenes as Electron Accepting Components in Supramolecular and Covalently Linked Electron Transfer Systems. Ph.D. Thesis, University of Amsterdam, Amsterdam, The Netherlands, 1996. [Google Scholar]
- Doose, S.; Neuweiler, H.; Sauer, M. Fluorescence quenching by photoinduced electron transfer: A reporter for conformational dynamics of macromolecules. Chemphyschem A Eur. J. Chem. Phys. Phys. Chem. 2009, 10, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.J.; Jones, W.E., Jr. Studies of photoinduced electron transfer and energy migration in a conjugated polymer system for fluorescence “turn-on” chemosensor applications. J. Phys. Chem. B 2006, 110, 7777–7782. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zhu, B.; Chen, J.; Duan, X. Fluorescent sensing of fluoride in cellular system. Theranostics 2015, 5, 173–187. [Google Scholar] [CrossRef]
- Panchenko, P.A.; Fedorov, Y.V.; Fedorova, O.A.; Jonusauskas, G. FRET versus PET: Ratiometric chemosensors assembled from naphthalimide dyes and crown ethers. Phys. Chem. Chem. Phys. PCCP 2015, 17, 22749–22757. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Kim, K.Y. Rhein inhibits the growth of Propionibacterium acnes by blocking NADH dehydrogenase-2 activity. J. Med. Microbiol. 2020, 69, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, X.; Ma, G. Antimicrobial activities and mechanisms of extract and components of herbs in East Asia. RSC Adv. 2022, 12, 29197–29213. [Google Scholar] [CrossRef]
- Hou, Y.; Zhao, Y.; Wu, W.; Wu, X.; Xu, J. Antibacterial activities of rhubarb extract and the Bioactive compounds against Salmonella. Int. J. Nutr. Sci. Food Tech. 2015, 1, 1–9. [Google Scholar]
- Gong, R.; Lee, D.Y.; Lee, J.W.; Choi, D.J.; Kim, G.-S.; Lee, S.H.; Lee, Y.-S. Potentiating activity of Rhein in targeting of resistance genes in methicillin-resistant Staphylococcus aureus. Asian Pac. J. Trop. Med. 2019, 12, 14–18. [Google Scholar]
- Yuan, Y.; Zheng, J.; Wang, M.; Li, Y.; Ruan, J.; Zhang, H. Metabolic Activation of Rhein: Insights into the Potential Toxicity Induced by Rhein-Containing Herbs. J. Agric. Food Chem. 2016, 64, 5742–5750. [Google Scholar] [CrossRef]
- Nakonechny, F.; Nisnevitch, M.M.; Nitzan, Y.; Nisnevitch, M. Sonodynamic excitation of Rose Bengal for eradication of Gram-positive and Gram-negative bacteria. BioMed Res. Int. 2013, 2013, 684930. [Google Scholar] [CrossRef]
- Nakonechny, F.; Barel, M.; David, A.; Koretz, S.; Litvak, B.; Ragozin, E.; Etinger, A.; Livne, O.; Pinhasi, Y.; Gellerman, G.; et al. Dark Antibacterial Activity of Rose Bengal. Int. J. Mol. Sci. 2019, 20, 3196. [Google Scholar] [CrossRef] [PubMed]
- Vanerio, N.; Stijnen, M.; de Mol, B.A.J.M.; Kock, L.M. Biomedical Applications of Photo- and Sono-Activated Rose Bengal: A Review. Photobiomodulation Photomed. Laser Surg. 2019, 37, 383–394. [Google Scholar] [CrossRef]
- “General Standard for Food Additives”, Codex Stan 192-1995, Food and Agriculture Organization of the United Nations, Updated up to the 44th Session of the Codex Alimentarius Commission. 2021. Available online: https://www.fao.org/gsfaonline/additives/index.html (accessed on 14 July 2023).
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Parker, C.A. Photoluminescence of Solutions with Applications to Photochemistry and Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 1968; p. 544. [Google Scholar]
- Rurack, K. Fluorescence Quantum Yields: Methods of Determination and Standards. In Standardization and Quality Assurance in Fluorescence Measurements I; Resch-Genger, U., Ed.; Springer Series on Fluorescence; Springer: Berlin/Heidelberg, Germany, 2008; pp. 101–145. [Google Scholar]
- Magde, D.; Wong, R.; Seybold, P.G. Fluorescence quantum yields and their relation to lifetimes of rhodamine 6G and fluorescein in nine solvents: Improved absolute standards for quantum yields. Photochem. Photobiol. 2002, 75, 327–334. [Google Scholar] [CrossRef]
- Würth, C.; Grabolle, M.; Pauli, J.; Spieles, M.; Resch-Genger, U. Relative and absolute determination of fluorescence quantum yields of transparent samples. Nat. Protoc. 2013, 8, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.E.; Elgeti, M.; Hildebrand, P.W.; Szczepek, M.; Hofmann, K.P.; Scheerer, P. Chapter Twenty-Six—Structure-Based Biophysical Analysis of the Interaction of Rhodopsin with G Protein and Arrestin. In Methods in Enzymology; Shukla, A.K., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 556, pp. 563–608. [Google Scholar]




| Food Colorant | Food Colorant Structure | λmax, nm in Visual Region * and Color |
|---|---|---|
| E129 Allura Red AC |  | 496 Red |
| E122 Azorubine S. | 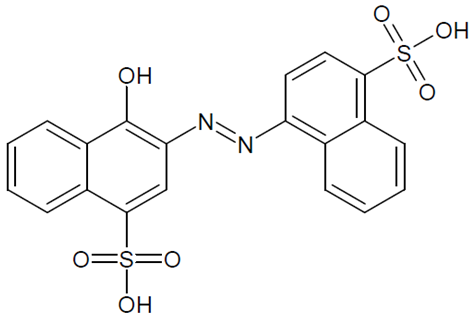 | 515 Red |
| E124 Ponceau 4R |  | 507 Red |
| E150a Caramel Caramel alkane Caramel olefins Caramel alkynes Vanillin | (C24H36O18)n (C36H50O25)n (C24H36O13)n  | ND ** Reddish brown |
| E133 Brilliant blue FCF | 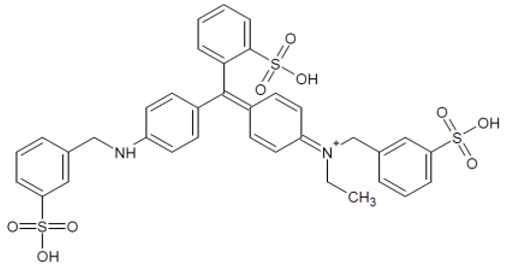 | 409 and 629 Blue |
| E127 Erythrosine B | 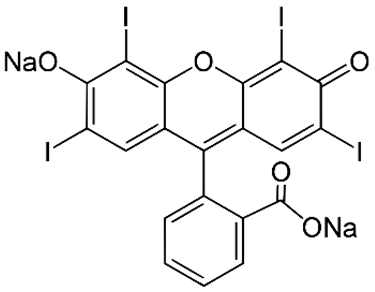 | 527 Red |
| Rhein | 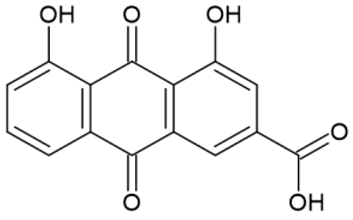 | 433 Yellow |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hochma, E.; Hovor, I.; Nakonechny, F.; Nisnevitch, M. Photo- and Sono-Active Food Colorants Inactivating Bacteria. Int. J. Mol. Sci. 2023, 24, 15126. https://doi.org/10.3390/ijms242015126
Hochma E, Hovor I, Nakonechny F, Nisnevitch M. Photo- and Sono-Active Food Colorants Inactivating Bacteria. International Journal of Molecular Sciences. 2023; 24(20):15126. https://doi.org/10.3390/ijms242015126
Chicago/Turabian StyleHochma, Efrat, Iryna Hovor, Faina Nakonechny, and Marina Nisnevitch. 2023. "Photo- and Sono-Active Food Colorants Inactivating Bacteria" International Journal of Molecular Sciences 24, no. 20: 15126. https://doi.org/10.3390/ijms242015126
APA StyleHochma, E., Hovor, I., Nakonechny, F., & Nisnevitch, M. (2023). Photo- and Sono-Active Food Colorants Inactivating Bacteria. International Journal of Molecular Sciences, 24(20), 15126. https://doi.org/10.3390/ijms242015126






