Iron, Ferroptosis, and Head and Neck Cancer
Abstract
:1. Introduction
2. Ferroptosis
2.1. The Development of the Concept of Ferroptosis
2.2. Features and Components of Ferroptosis
2.3. The Mechanism and Regulation of Ferroptosis
3. The Function of Ferroptosis in Head and Neck Squamous Cell Carcinoma (HNSCC)
3.1. Ferroptosis and Tumor Cell Death
3.2. Ferroptosis and Tumor Metastasis
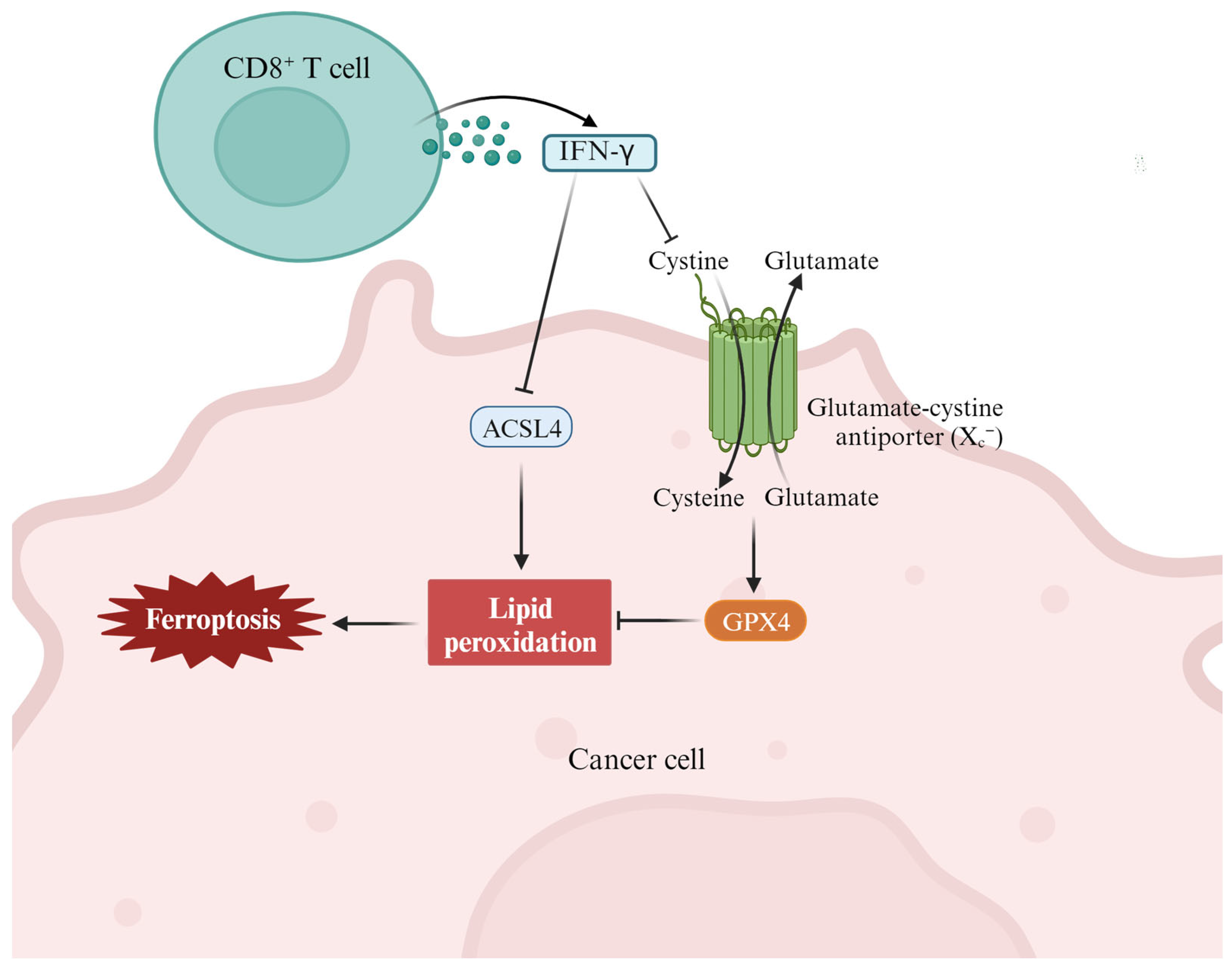
3.3. Ferroptosis and Antitumor Immunity
3.4. Ferroptosis and Drug Resistance
4. Targeting Ferroptosis in the Prevention and Intervention of HNSCC
4.1. Ferroptosis and Cancer Diagnosis and Prognosis
4.2. Ferroptosis and HNSCC Therapeutic Strategy
4.3. Ferroptosis Resistance in HNSCC
5. Challenges and Prospects for Targeting Ferroptosis in Cancer Therapy
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| AA | Arachidonoyl |
| ACD | Accidental cell death |
| ACSL4 | Acyl-CoA synthetase 4 |
| AdA | Adenoid/adrenoyl |
| CoA | Coenzyme A |
| DAMPs | Damage-associated molecular patterns |
| EMT | Epithelial mesenchymal transition |
| FRGs | Growth-related genes |
| GCL | Glutamate-cysteine ligase |
| GSH | Glutathione |
| GSS | Glutathione synthetase |
| GPX4 | Glutathione peroxidase 4 |
| HNSCC | Head and neck squamous cell carcinoma |
| ICB | Immune checkpoint blockade |
| IFN-γ | Interferon-γ |
| IFRMs | Immuno-ferroptosis-related mRNAs |
| LPCAT3 | Lysophosphatidyllecithin acyltransferase 3 |
| NADPH | Nicotinamide adenine dinucleotide phosphate oxidase |
| POR | P450 oxidoreductase |
| PUFA | Polyunsaturated fatty acid |
| ROC | Receiver operating characteristic |
| ROS | Reactive oxygen species |
| RCD | Regulated cell death |
| Tf | Transferrin |
| TFRC | Transferrin receptor |
| xCT | xc−-cystine/glutamate antiporter |
References
- Torti, S.V.; Torti, F.M. Iron: The cancer connection. Mol. Asp. Med. 2020, 75, 100860. [Google Scholar] [CrossRef] [PubMed]
- Lelievre, P.; Sancey, L.; Coll, J.L.; Deniaud, A.; Busser, B. Iron Dysregulation in Human Cancer: Altered Metabolism, Biomarkers for Diagnosis, Prognosis, Monitoring and Rationale for Therapy. Cancers 2020, 12, 3524. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Gao, Z.; Wu, H.; Sun, K.; Ning, L.; Lin, F.; Sun, B.; Wang, F. Reactive oxygen species mediated theranostics using a Fenton reaction activable lipo-polymersome. J. Mater. Chem. B 2019, 7, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kroemer, G. Ferroptosis. Curr. Biol. 2020, 30, R1292–R1297. [Google Scholar] [CrossRef]
- Crielaard, B.J.; Lammers, T.; Rivella, S. Targeting iron metabolism in drug discovery and delivery. Nat. Rev. Drug Discov. 2017, 16, 400–423. [Google Scholar] [CrossRef] [PubMed]
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, X.; Guan, L.; Qin, L.; Ding, J.; Zhou, L. The link between ferroptosis and airway inflammatory diseases: A novel target for treatment. Front. Mol. Biosci. 2022, 9, 985571. [Google Scholar] [CrossRef]
- Wang, K.; Chen, X.Z.; Wang, Y.H.; Cheng, X.L.; Zhao, Y.; Zhou, L.Y.; Wang, K. Emerging roles of ferroptosis in cardiovascular diseases. Cell Death. Discov. 2022, 8, 394. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- De Bortoli, M.; Taverna, E.; Maffioli, E.; Casalini, P.; Crisafi, F.; Kumar, V.; Caccia, C.; Polli, D.; Tedeschi, G.; Bongarzone, I. Lipid accumulation in human breast cancer cells injured by iron depletors. J. Exp. Clin. Cancer. Res. 2018, 37, 75. [Google Scholar] [CrossRef] [PubMed]
- Hino, K.; Yanatori, I.; Hara, Y.; Nishina, S. Iron and liver cancer: An inseparable connection. FEBS J. 2022, 289, 7810–7829. [Google Scholar] [CrossRef]
- Wang, D.; Tang, L.; Zhang, Y.; Ge, G.; Jiang, X.; Mo, Y.; Wu, P.; Deng, X.; Li, L.; Zuo, S.; et al. Regulatory pathways and drugs associated with ferroptosis in tumors. Cell Death. Dis. 2022, 13, 544. [Google Scholar] [CrossRef] [PubMed]
- Rishi, G.; Huang, G.; Subramaniam, V.N. Cancer: The role of iron and ferroptosis. Int. J. Biochem. Cell Biol. 2021, 141, 106094. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.T.; Cheng, J.Y.; Lin, Q.S.; Ni, Z.H. Autophagy-dependent ferroptosis in kidney disease. Front. Med. 2023, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Ji, Q.; Wu, M.; Tu, Z.; Lei, K.; Luo, M.; Liu, J.; Lin, L.; Li, K.; Li, J.; et al. Ferroptosis in tumor immunity and therapy. J. Cell Mol. Med. 2022, 26, 5565–5579. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Chen, M.; Gao, Y.; Huang, D.; Cao, H.; Peng, Y.; Guo, N.; Wang, F.; Zhang, S. Ferroptosis and Tumor Drug Resistance: Current Status and Major Challenges. Front. Pharmacol. 2022, 13, 879317. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lin, D.; Yu, Q.; Li, Z.; Lenahan, C.; Dong, Y.; Wei, Q.; Shao, A. A Promising Future of Ferroptosis in Tumor Therapy. Front. Cell Dev. Biol. 2021, 9, 629150. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.J.; Peng, W.; Xia, Q.X.; Zhou, H.H.; Mao, X.Y. Ferroptosis-related gene signature predicts prognosis and immunotherapy in glioma. CNS Neurosci. Ther. 2021, 27, 973–986. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, N.; Adelian, S.; Shakerian, S.; Afshinpour, M.; Chaleshtori, S.R.; Rostami, N.; Rezaei-Tazangi, F.; Beiranvand, S.; Hamblin, M.R.; Aref, A.R. Crosstalk between ferroptosis and the epithelial-mesenchymal transition: Implications for inflammation and cancer therapy. Cytokine Growth Factor Rev. 2022, 64, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.; Hermann, E.; Lin, D.; Chowanadisai, W.; Hull, E.; Montgomery, M. HDAC inhibition induces EMT and alterations in cellular iron homeostasis to augment ferroptosis sensitivity in SW13 cells. Redox Biol. 2021, 47, 102149. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, Y.; Li, M.; Sun, Z.; Liu, T.; Zhao, M.; Li, Z. Single-Cell RNA-Seq Reveals the Promoting Role of Ferroptosis Tendency During Lung Adenocarcinoma EMT Progression. Front. Cell Dev. Biol. 2021, 9, 822315. [Google Scholar] [CrossRef]
- Edinger, A.L.; Thompson, C.B. Death by design: Apoptosis, necrosis and autophagy. Curr. Opin. Cell Biol. 2004, 16, 663–669. [Google Scholar] [CrossRef]
- Ray, L.B. How RAS mutations really work. Science 2020, 370, 927–928. [Google Scholar] [CrossRef]
- Prior, I.A.; Hood, F.E.; Hartley, J.L. The Frequency of Ras Mutations in Cancer. Cancer Res. 2020, 80, 2969–2974. [Google Scholar] [CrossRef] [PubMed]
- Murugan, A.K.; Grieco, M.; Tsuchida, N. RAS mutations in human cancers: Roles in precision medicine. Semin. Cancer Biol. 2019, 59, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Millstein, J.; Arai, H.; Battaglin, F.; Kawanishi, N.; Jayachandran, P.; Soni, S.; Wu, Z.; Mancao, C.; Cremolini, C.; et al. The role of genetic variants involved with ferroptosis regulator genes in predicting outcomes in patients (pts) with RAS-mutant metastatic colorectal cancer (mCRC): Data from MAVERICC and TRIBE trials. J. Clin. Oncol. 2022, 40, 197. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death. Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Loveless, R.; Bloomquist, R.; Teng, Y. Pyroptosis at the forefront of anticancer immunity. J. Exp. Clin. Cancer Res. 2021, 40, 264. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Hadian, K.; Stockwell, B.R. SnapShot: Ferroptosis. Cell 2020, 181, 1188–1188.e1. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.F.; Zou, T.; Tuo, Q.Z.; Xu, S.; Li, H.; Belaidi, A.A.; Lei, P. Ferroptosis: Mechanisms and links with diseases. Signal Transduct. Target. Ther. 2021, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Stockwell, B.R. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? PLoS Biol. 2018, 16, e2006203. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Zhang, H.; Chen, Z.; Sun, X.; Zhu, S.; Nan, K.; Chen, W.; Miao, C. The Role of Ferroptosis in Acute Respiratory Distress Syndrome. Front. Med. 2021, 8, 651552. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Y.; Dixon, S.J. Mechanisms of ferroptosis. Cell Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; You, J.H.; Kim, M.S.; Roh, J.L. Epigenetic reprogramming of epithelial-mesenchymal transition promotes ferroptosis of head and neck cancer. Redox Biol. 2020, 37, 101697. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Lin, C.Y.; Chen, C.Y.; Hsueh, C.W.; Chang, Y.W.; Wang, C.C.; Chu, P.Y.; Tai, S.K.; Yang, M.H. Ferroptosis Signature Shapes the Immune Profiles to Enhance the Response to Immune Checkpoint Inhibitors in Head and Neck Cancer. Adv. Sci. 2023, 10, e2204514. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Yao, C.; Ma, Y.; Liu, Y. Epithelial Membrane Protein 1 Promotes Sensitivity to RSL3-Induced Ferroptosis and Intensifies Gefitinib Resistance in Head and Neck Cancer. Oxid. Med. Cell. Longev. 2022, 2022, 4750671. [Google Scholar] [CrossRef]
- Li, M.; Jin, S.; Zhang, Z.; Ma, H.; Yang, X. Interleukin-6 facilitates tumor progression by inducing ferroptosis resistance in head and neck squamous cell carcinoma. Cancer Lett. 2022, 527, 28–40. [Google Scholar] [CrossRef]
- Jehl, A.; Conrad, O.; Burgy, M.; Foppolo, S.; Vauchelles, R.; Ronzani, C.; Etienne-Selloum, N.; Chenard, M.P.; Danic, A.; Dourlhes, T.; et al. Blocking EREG/GPX4 Sensitizes Head and Neck Cancer to Cetuximab through Ferroptosis Induction. Cells 2023, 12, 733. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; You, J.H.; Shin, D.; Roh, J.L. Inhibition of Glutaredoxin 5 predisposes Cisplatin-resistant Head and Neck Cancer Cells to Ferroptosis. Theranostics 2020, 10, 7775–7786. [Google Scholar] [CrossRef]
- Liu, S.; Yan, S.; Zhu, J.; Lu, R.; Kang, C.; Tang, K.; Zeng, J.; Ding, M.; Guo, Z.; Lai, X.; et al. Combination RSL3 Treatment Sensitizes Ferroptosis- and EGFR-Inhibition-Resistant HNSCCs to Cetuximab. Int. J. Mol. Sci. 2022, 23, 9014. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.W.; Wen, Y.H.; Ma, R.Q.; Chen, L.; Zeng, X.L.; Wen, W.P.; Sun, W. Ferroptosis Driver SOCS1 and Suppressor FTH1 Independently Correlate With M1 and M2 Macrophage Infiltration in Head and Neck Squamous Cell Carcinoma. Front. Cell Dev. Biol. 2021, 9, 727762. [Google Scholar] [CrossRef]
- Roh, J.L.; Kim, E.H.; Jang, H.; Shin, D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017, 11, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Zhang, Z.; Chen, L.; Zhou, Y.; Zou, P.; Feng, C.; Wang, L.; Liang, G. Dihydroartemisinin (DHA) induces ferroptosis and causes cell cycle arrest in head and neck carcinoma cells. Cancer Lett. 2016, 381, 165–175. [Google Scholar] [CrossRef]
- Li, J.; Xiao, W.; Wei, W.; Wu, M.; Xiong, K.; Lyu, J.; Li, Y. HSPA5, as a ferroptosis regulator, may serve as a potential therapeutic for head and neck squamous cell carcinoma. Mol. Immunol. 2023, 158, 79–90. [Google Scholar] [CrossRef]
- Shin, D.; Kim, E.H.; Lee, J.; Roh, J.L. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic. Biol. Med. 2018, 129, 454–462. [Google Scholar] [CrossRef]
- You, J.H.; Lee, J.; Roh, J.L. Mitochondrial pyruvate carrier 1 regulates ferroptosis in drug-tolerant persister head and neck cancer cells via epithelial-mesenchymal transition. Cancer Lett. 2021, 507, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Lee, J.; You, J.H.; Kim, D.; Roh, J.L. Dihydrolipoamide dehydrogenase regulates cystine deprivation-induced ferroptosis in head and neck cancer. Redox Biol. 2020, 30, 101418. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Tang, L.; Li, Q.; Chen, L.; Pan, Y.; Yin, Z.; He, J.; Tian, J. Single-cell transcriptomics uncover the key ferroptosis regulators contribute to cancer progression in head and neck squamous cell carcinoma. Front. Mol. Biosci. 2022, 9, 962742. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Z.; Pan, K.; Li, J.; Chen, Q. The function and mechanism of ferroptosis in cancer. Apoptosis 2020, 25, 786–798. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhu, S.; Song, X.; Sun, X.; Fan, Y.; Liu, J.; Zhong, M.; Yuan, H.; Zhang, L.; Billiar, T.R.; et al. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 2017, 20, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, J.; Yuan, S.; Zhuang, X.; Qiao, T. Activation of the P62-Keap1-NRF2 Pathway Protects against Ferroptosis in Radiation-Induced Lung Injury. Oxid. Med. Cell. Longev. 2022, 2022, 8973509. [Google Scholar] [CrossRef] [PubMed]
- Cheff, D.M.; Huang, C.; Scholzen, K.C.; Gencheva, R.; Ronzetti, M.H.; Cheng, Q.; Hall, M.D.; Arner, E.S.J. The ferroptosis inducing compounds RSL3 and ML162 are not direct inhibitors of GPX4 but of TXNRD1. Redox Biol. 2023, 62, 102703. [Google Scholar] [CrossRef] [PubMed]
- Read, M.L.; Modasia, B.; Fletcher, A.; Thompson, R.J.; Brookes, K.; Rae, P.C.; Nieto, H.R.; Poole, V.L.; Roberts, S.; Campbell, M.J.; et al. PTTG and PBF Functionally Interact with p53 and Predict Overall Survival in Head and Neck Cancer. Cancer Res. 2018, 78, 5863–5876. [Google Scholar] [CrossRef] [PubMed]
- Membreno, P.V.; Luttrell, J.B.; Mamidala, M.P.; Schwartz, D.L.; Hayes, D.N.; Gleysteen, J.P.; Gillespie, M.B. Outcomes of primary radiotherapy with or without chemotherapy for advanced oral cavity squamous cell carcinoma: Systematic review. Head Neck 2021, 43, 3165–3176. [Google Scholar] [CrossRef]
- Teng, Y.; Gao, L.; Loveless, R.; Rodrigo, J.P.; Strojan, P.; Willems, S.M.; Nathan, C.A.; Mäkitie, A.A.; Saba, N.F.; Ferlito, A. The Hidden Link of Exosomes to Head and Neck Cancer. Cancers 2021, 13, 5802. [Google Scholar] [CrossRef]
- Saba, N.F.; Steuer, C.E.; Ekpenyong, A.; McCook-Veal, A.; Magliocca, K.; Patel, M.; Schmitt, N.C.; Stokes, W.; Bates, J.E.; Rudra, S.; et al. Pembrolizumab and cabozantinib in recurrent metastatic head and neck squamous cell carcinoma: A phase 2 trial. Nat. Med. 2023, 29, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Wang, W.; Wang, W.; Kryczek, I.; Li, X.; Bian, Y.; Sell, A.; Wei, S.; Grove, S.; Johnson, J.K.; et al. CD8(+) T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 2022, 40, 365–378.e366. [Google Scholar] [CrossRef] [PubMed]
- Orcutt, K.P.; Parsons, A.D.; Sibenaller, Z.A.; Scarbrough, P.M.; Zhu, Y.; Sobhakumari, A.; Wilke, W.W.; Kalen, A.L.; Goswami, P.; Miller, F.J., Jr.; et al. Erlotinib-mediated inhibition of EGFR signaling induces metabolic oxidative stress through NOX4. Cancer Res. 2011, 71, 3932–3940. [Google Scholar] [CrossRef] [PubMed]
- Anthonymuthu, T.S.; Tyurina, Y.Y.; Sun, W.Y.; Mikulska-Ruminska, K.; Shrivastava, I.H.; Tyurin, V.A.; Cinemre, F.B.; Dar, H.H.; VanDemark, A.P.; Holman, T.R.; et al. Resolving the paradox of ferroptotic cell death: Ferrostatin-1 binds to 15LOX/PEBP1 complex, suppresses generation of peroxidized ETE-PE, and protects against ferroptosis. Redox Biol. 2021, 38, 101744. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E.; Tyurina, Y.Y.; Zhao, J.; St Croix, C.M.; Dar, H.H.; Mao, G.; Tyurin, V.A.; Anthonymuthu, T.S.; Kapralov, A.A.; Amoscato, A.A.; et al. PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals. Cell 2017, 171, 628–641.e626. [Google Scholar] [CrossRef] [PubMed]
- Kapralov, A.A.; Yang, Q.; Dar, H.H.; Tyurina, Y.Y.; Anthonymuthu, T.S.; Kim, R.; St Croix, C.M.; Mikulska-Ruminska, K.; Liu, B.; Shrivastava, I.H.; et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat. Chem. Biol. 2020, 16, 278–290. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, H.; Zhao, C.; Liu, D.; Luo, J.; Ying, Y.; Zhong, Y. DDIT4 promotes malignancy of head and neck squamous cell carcinoma. Mol. Carcinog. 2023, 62, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Chan, C.; Lin, W. Nanoparticle-Mediated Immunogenic Cell Death Enables and Potentiates Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2019, 58, 670–680. [Google Scholar] [CrossRef]
- Zhang, F.; Li, F.; Lu, G.H.; Nie, W.; Zhang, L.; Lv, Y.; Bao, W.; Gao, X.; Wei, W.; Pu, K.; et al. Engineering Magnetosomes for Ferroptosis/Immunomodulation Synergism in Cancer. ACS Nano. 2019, 13, 5662–5673. [Google Scholar] [CrossRef]
- Wang, W.; Green, M.; Choi, J.E.; Gijon, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Green, M.D.; Wang, W.; Yu, J.; Choi, J.E.; Jiang, L.; Liao, P.; Zhou, J.; Zhang, Q.; Dow, A.; et al. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov. 2019, 9, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Hu, B.X.; Li, Z.L.; Du, T.; Shan, J.L.; Ye, Z.P.; Peng, X.D.; Li, X.; Huang, Y.; Zhu, X.Y.; et al. PKCbetaII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat. Cell. Biol. 2022, 24, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.; Horbath, A.; Li, Z.; Gan, B. PKCβII-ACSL4 pathway mediating ferroptosis execution and anti-tumor immunity. Cancer Commun. 2022, 42, 583–586. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Y.; Pan, X.; Wen, W. Relationships of Ferroptosis and Pyroptosis-Related Genes with Clinical Prognosis and Tumor Immune Microenvironment in Head and Neck Squamous Cell Carcinoma. Oxid. Med. Cell. Longev. 2022, 2022, 3713929. [Google Scholar] [CrossRef]
- Ran, J.; Liu, T.; Song, C.; Wei, Z.; Tang, C.; Cao, Z.; Zou, H.; Zhang, X.; Cai, Y.; Han, W. Rhythm Mild-Temperature Photothermal Therapy Enhancing Immunogenic Cell Death Response in Oral Squamous Cell Carcinoma. Adv. Healthc. Mater. 2023, 12, e2202360. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Lian, J.X.; Lan, Z.; Zou, K.L.; Wang, W.M.; Yu, G.T. Ferroptosis promotes anti-tumor immune response by inducing immunogenic exposure in HNSCC. Oral Dis. 2023, 29, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Z.; Tao, H.; Fan, Z.W.; Song, S.J.; Bai, J. Prognostic and Immunological Role of Key Genes of Ferroptosis in Pan-Cancer. Front. Cell Dev. Biol. 2021, 9, 748925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol. Cancer 2022, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Friedmann Angeli, J.P.; Krysko, D.V.; Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 2019, 19, 405–414. [Google Scholar] [CrossRef]
- Song, X.; Wang, X.; Liu, Z.; Yu, Z. Role of GPX4-Mediated Ferroptosis in the Sensitivity of Triple Negative Breast Cancer Cells to Gefitinib. Front. Oncol. 2020, 10, 597434. [Google Scholar] [CrossRef]
- Huang, W.; Chen, K.; Lu, Y.; Zhang, D.; Cheng, Y.; Li, L.; Huang, W.; He, G.; Liao, H.; Cai, L.; et al. ABCC5 facilitates the acquired resistance of sorafenib through the inhibition of SLC7A11-induced ferroptosis in hepatocellular carcinoma. Neoplasia 2021, 23, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Shin, D.; Lee, J.; Jung, A.R.; Roh, J.L. CISD2 inhibition overcomes resistance to sulfasalazine-induced ferroptotic cell death in head and neck cancer. Cancer Lett. 2018, 432, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, C.; Zhang, Y.J.; Wu, Z.H. Ferroptosis-Related Long Non-Coding RNA signature predicts the prognosis of Head and neck squamous cell carcinoma. Int. J. Biol. Sci. 2021, 17, 702–711. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Liao, S.; Xiao, L.; Cai, L.; You, M.; He, L.; Huang, W. Prognostic Value of a Ferroptosis-Related Gene Signature in Patients With Head and Neck Squamous Cell Carcinoma. Front. Cell. Dev. Biol. 2021, 9, 739011. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, Z.; Liu, Y.; Wang, X.; Li, J.; Ye, Q. Immunization Combined with Ferroptosis Related Genes to Construct a New Prognostic Model for Head and Neck Squamous Cell Carcinoma. Cancers 2022, 14, 4099. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wang, H.; Tian, W.; Li, X.; Zhu, Z.; Huang, R.; Luo, H. Targeting ferroptosis-based cancer therapy using nanomaterials: Strategies and applications. Theranostics 2021, 11, 9937–9952. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, T.; Jiang, C. Nanodrug delivery systems for ferroptosis-based cancer therapy. J. Control. Release. 2022, 344, 289–301. [Google Scholar] [CrossRef]
- Guan, Q.; Guo, R.; Huang, S.; Zhang, F.; Liu, J.; Wang, Z.; Yang, X.; Shuai, X.; Cao, Z. Mesoporous polydopamine carrying sorafenib and SPIO nanoparticles for MRI-guided ferroptosis cancer therapy. J. Control. Release. 2020, 320, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhang, X.; Yang, P.; Zhao, J.; Zhang, W.; Feng, N.; Yang, W.; Tang, J. Defect self-assembly of metal-organic framework triggers ferroptosis to overcome resistance. Bioact. Mater. 2023, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, S.; Wang, Q.; Ye, W.; Liu, S.; Wang, X.; Zhang, Z.; Cao, L.; Jiang, X. Tailored theranostic nanoparticles cause efficient ferroptosis in head and neck squamous cell carcinoma through a reactive oxygen species “butterfly effect”. Chem. Eng. J. 2021, 423, 130083. [Google Scholar] [CrossRef]
- Belvin, B.R.; Lewis, J.P. Ferroportin depletes iron needed for cell cycle progression in head and neck squamous cell carcinoma. Front. Oncol. 2022, 12, 1025434. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zhang, Z.; Pan, X.; Zhang, J.; Wang, X.; Wang, M.; Li, H.; Yan, M.; Chen, W. Caveolin-1 promotes cancer progression via inhibiting ferroptosis in head and neck squamous cell carcinoma. J. Oral Pathol. Med. 2022, 51, 52–62. [Google Scholar] [CrossRef]
- Brown, C.W.; Chhoy, P.; Mukhopadhyay, D.; Karner, E.R.; Mercurio, A.M. Targeting prominin2 transcription to overcome ferroptosis resistance in cancer. EMBO Mol. Med. 2021, 13, e13792. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.; Barbosa, D.J.; Benfeito, S.; Silva, V.; Chavarria, D.; Borges, F.; Remião, F.; Silva, R. Molecular mechanisms of ferroptosis and their involvement in brain diseases. Pharmacol. Ther. 2023, 244, 108373. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zhang, X.; Yang, M.; Dong, X. Recent Progress in Ferroptosis Inducers for Cancer Therapy. Adv. Mater. 2019, 31, e1904197. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R. A powerful cell-protection system prevents cell death by ferroptosis. Nature 2019, 575, 597–598. [Google Scholar] [CrossRef]
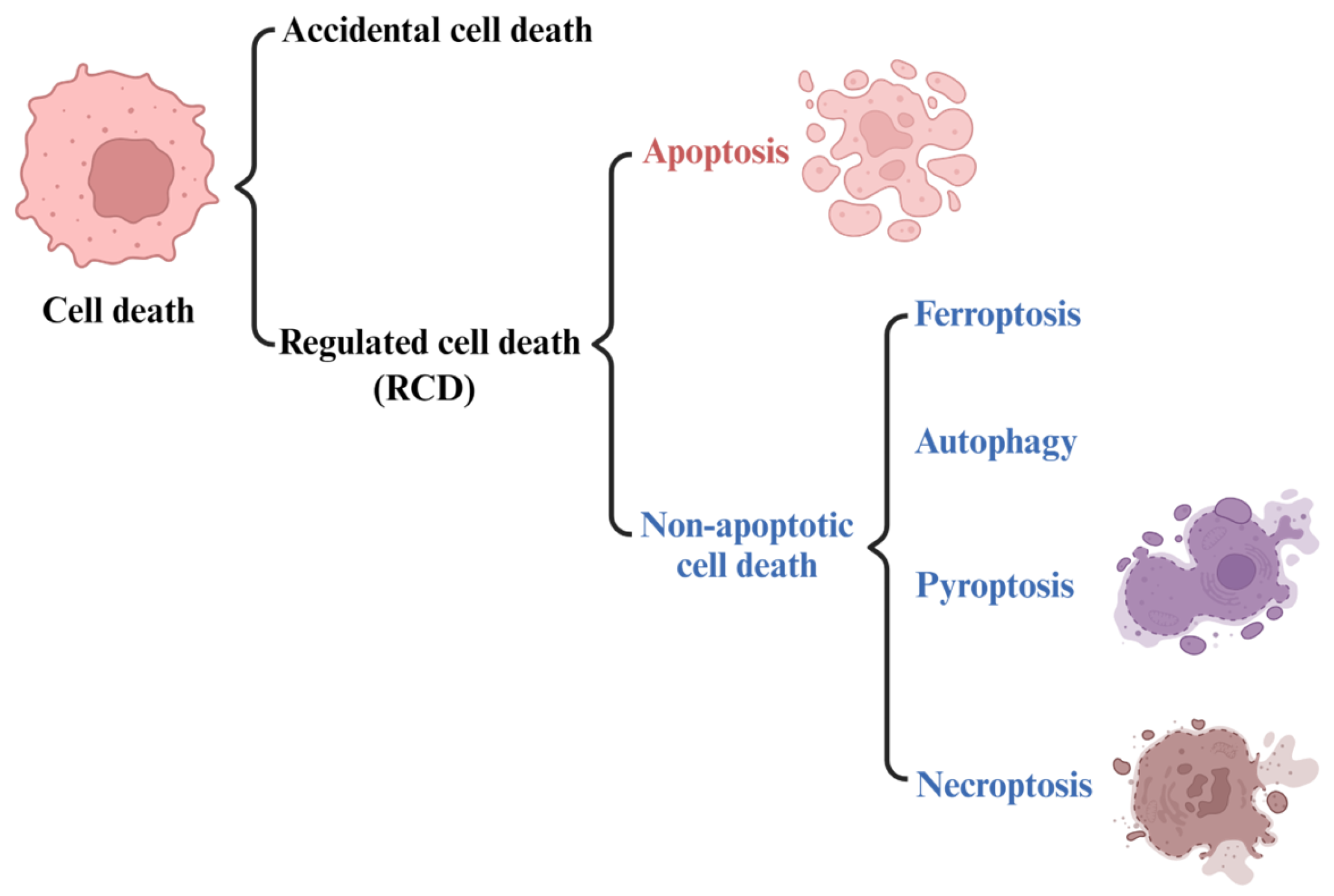
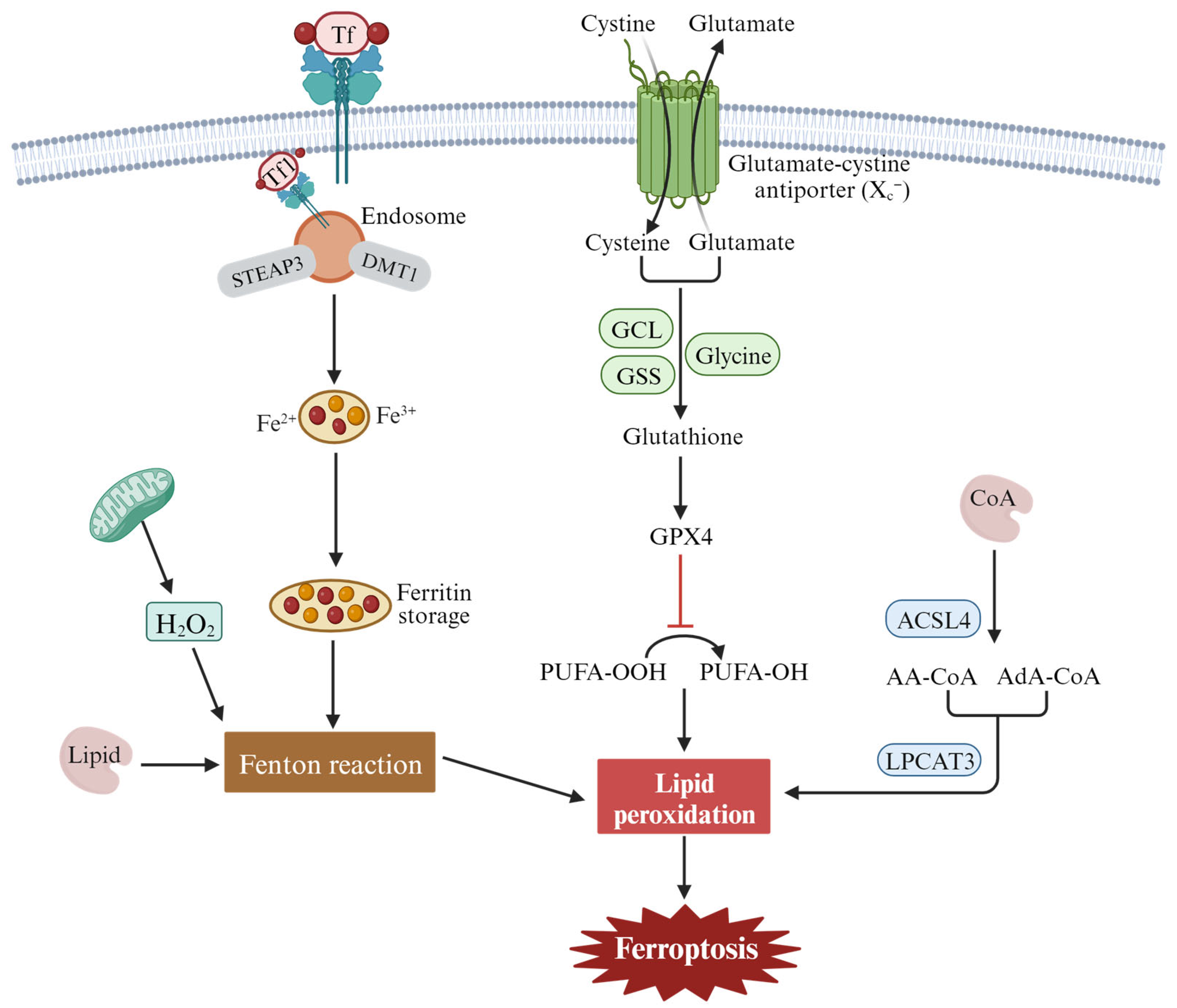
| Ferroptosis | Features | Morphology: reduced mitochondrial volume, reduced or disappeared mitochondrial ridge, broken mitochondrial outer membrane, increased double membrane density |
| Biochemical characteristics: Iron accumulation and lipid peroxidation | ||
| Immune characteristics: DAMPs | ||
| Key signature proteins: GPX4, p53, TFR1, SLC7A11, ACSL4, FSP1, Nrf2, Ferritin | ||
| Detection methods | Morphological observations (transmission electron microscopy), determination of cell activity, iron levels, ROS and lipid peroxidation, glutathione levels, metabolomics or lipidomics, changes in expression levels of signature proteins. |
| Trigger | Targets | Regulation Mechanism | Ref. |
|---|---|---|---|
| EMT | GSH | EMT contributes to promoting ferroptosis | [36] |
| Ferroptosis stress | NF-κB | Ferroptotic stress induces the inflammation signature and PD-L1 expression | [37] |
| EMP1 | Hippo-TAZ pathway, Rac1, and NOX1 | EMP1 overexpression enhances RSL3-induced ferroptosis | [38] |
| IL-6 | xCT | IL-6 transcriptionally upregulates xCT expression via the JAK2/STAT3 pathway | [39] |
| EREG | Lipid peroxidation, iron accumulation, and GPX4 | EREG deficiency induces ferroptosis and enhances the sensitivity of HNSCC cells to cetuximab | [40] |
| Sulfasalazine and GLRX5 | ROS and GSH | GLRX5 promotes ferroptosis by increasing the amount of intracellular free irons and lipid peroxidation | [41] |
| RSL3 and cetuximab | KRAS and FTH1 | FTH1 reduces the susceptibility of HNSCC to ferroptosis inducers and prevents ferroptosis. | [42] |
| SOCS1 and FTH1 | M1 and M2 macrophages | SOCS1 and FTH1 are independent prognostic factors that correlate with M1 and M2 macrophage infiltration in HNSCC | [43] |
| Artesunate | Nrf2-ARE pathway | Artesunate decreases cellular GSH level and increases lipid ROS level | [44] |
| Dihydroartemisinin (DHA) | ROS, GPX4, and Ras | DHA increases ROS levels and decreases GPX4 and Ras levels | [45] |
| HA15 | HSPA5 | HA15 reduces GPX4 and FTH1 expression and increases ACSL4 expression | [46] |
| RSL3 or ML-162 | Nrf2-ARE pathway | RSL3 or ML-162 upregulates p62 and Nrf2 expression, downregulates Keap1 expression, and activates the PERK-ATF4-SESN2 pathway | [47] |
| Mitochondrial pyruvate carrier 1 (MCP1) | GPX4 and xCT | MPC1 increases the susceptibility to ferroptosis by regulating interstitial traits and glutaminolysis | [48] |
| Dihydrolipoamide dehydrogenase (DLD) | Cystine | DLD induces ferroptosis via cystine deprivation | [49] |
| ACSL1, SLC39A14, TFRC, and PRNP | Unclear | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, Y.; Gao, L.; Mäkitie, A.A.; Florek, E.; Czarnywojtek, A.; Saba, N.F.; Ferlito, A. Iron, Ferroptosis, and Head and Neck Cancer. Int. J. Mol. Sci. 2023, 24, 15127. https://doi.org/10.3390/ijms242015127
Teng Y, Gao L, Mäkitie AA, Florek E, Czarnywojtek A, Saba NF, Ferlito A. Iron, Ferroptosis, and Head and Neck Cancer. International Journal of Molecular Sciences. 2023; 24(20):15127. https://doi.org/10.3390/ijms242015127
Chicago/Turabian StyleTeng, Yong, Lixia Gao, Antti A. Mäkitie, Ewa Florek, Agata Czarnywojtek, Nabil F. Saba, and Alfio Ferlito. 2023. "Iron, Ferroptosis, and Head and Neck Cancer" International Journal of Molecular Sciences 24, no. 20: 15127. https://doi.org/10.3390/ijms242015127
APA StyleTeng, Y., Gao, L., Mäkitie, A. A., Florek, E., Czarnywojtek, A., Saba, N. F., & Ferlito, A. (2023). Iron, Ferroptosis, and Head and Neck Cancer. International Journal of Molecular Sciences, 24(20), 15127. https://doi.org/10.3390/ijms242015127








