Tiger Nut Milk’s Antiviral Properties against Enveloped and Non-Enveloped Viruses: Effect of Concentration and Adding Sugar
Abstract
1. Introduction
2. Results and Discussion
2.1. Antiviral Tests against phi 6 Bacteriophage
2.2. Antiviral Tests against Bacteriophage MS2
3. Materials and Methods
3.1. Materials
3.2. Antiviral Tests against phi 6 Bacteriophage
3.3. Antiviral Tests against MS2 Bacteriophage
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tong, T.; Hu, H.; Zhou, J.; Deng, S.; Zhang, X.; Tang, W.; Fang, L.; Xiao, S.; Liang, J. Glycyrrhizic-Acid-Based Carbon Dots with High Antiviral Activity by Multisite Inhibition Mechanisms. Small 2020, 16, e1906206. [Google Scholar] [CrossRef]
- Ashfaq, U.A.; Masoud, M.S.; Nawaz, Z.; Riazuddin, S. Glycyrrhizin as Antiviral Agent against Hepatitis C Virus. J. Transl. Med. 2011, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Glycyrrhizin, an Active Component of Liquorice Roots, and Replication of SARS-Associated Coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Vergoten, G. Glycyrrhizin: An Alternative Drug for the Treatment of COVID-19 Infection and the Associated Respiratory Syndrome? Pharmacol. Ther. 2020, 214, 107618. [Google Scholar] [PubMed]
- Rane, H.S.; Bernardo, S.M.; Howell, A.B.; Lee, S.A. Cranberry-Derived Proanthocyanidins Prevent Formation of Candida Albicans Biofilms in Artificial Urine through Biofilm- and Adherence-Specific Mechanisms. J. Antimicrob. Chemother. 2014, 69, 428–436. [Google Scholar] [CrossRef]
- Su, X.; Howell, A.B.; D’Souza, D.H. Antiviral Effects of Cranberry Juice and Cranberry Proanthocyanidins on Foodborne Viral Surrogates--a Time Dependence Study in Vitro. Food Microbiol. 2010, 27, 985–991. [Google Scholar] [CrossRef]
- Lipson, S.M.; Sethi, L.; Cohen, P.; Gordon, R.E.; Tan, I.P.; Burdowski, A.; Stotzky, G. Antiviral Effects on Bacteriophages and Rotavirus by Cranberry Juice. Phytomedicine 2007, 14, 23–30. [Google Scholar] [CrossRef]
- Takayama, K.; Tuñón-Molina, A.; Cano-Vicent, A.; Muramoto, Y.; Noda, T.; Aparicio-Collado, J.L.; Sabater I Serra, R.; Martí, M.; Serrano-Aroca, Á. Non-Woven Infection Prevention Fabrics Coated with Biobased Cranberry Extracts Inactivate Enveloped Viruses Such as SARS-CoV-2 and Multidrug-Resistant Bacteria. Int. J. Mol. Sci. 2021, 22, 12719. [Google Scholar] [CrossRef]
- Da Costa Neto, J.J.G.; Gomes, T.L.M.; Justo, T.F.; Pereira, K.S.; Amaral, P.F.F.; Rocha Leão, M.H.M.; Fontes Sant’Ana, G.C. Microencapsulation of Tiger Nut Milk by Lyophilization: Morphological Characteristics, Shelf Life and Microbiological Stability. Food Chem. 2019, 284, 133–139. [Google Scholar] [CrossRef]
- Selma-Royo, M.; García-Mantrana, I.; Collado, M.C.; Perez-Martínez, G. Intake of Natural, Unprocessed Tiger Nuts (Cyperus Esculentus L.) Drink Significantly Favors Intestinal Beneficial Bacteria in a Short Period of Time. Nutrients 2022, 14, 1709. [Google Scholar] [CrossRef]
- Chukwuma, E.R.; Obioma, N.; Christopher, O.I. The Phytochemical Composition and Some Biochemical Effects of Nigerian Tigernut (Cyperus Esculentus L.) Tuber. Pak. J. Nutr. 2010, 9, 709–715. [Google Scholar] [CrossRef]
- Sánchez-Zapata, E.; Fernández-López, J.; Angel Pérez-Alvarez, J. Tiger Nut (Cyperus Esculentus) Commercialization: Health Aspects, Composition, Properties, and Food Applications. Compr. Rev. Food Sci. Food Saf. 2012, 11, 366–377. [Google Scholar] [CrossRef]
- Adejuyitan, J.A.; Otunola, E.T.; Akande, E.A.; Bolarinwa, I.F.; Oladokun, F.M. Some Physicochemical Properties of Flour Obtained from Fermentation of Tigernut (Cyperus Esculentus) Sourced from a Market in Ogbomoso, Nigeria. Afr. J. Food Sci. 2009, 3, 51–55. [Google Scholar]
- Falcó, I.; Randazzo, W.; Rodríguez-Díaz, J.; Gozalbo-Rovira, R.; Luque, D.; Aznar, R.; Sánchez, G. Antiviral Activity of Aged Green Tea Extract in Model Food Systems and under Gastric Conditions. Int. J. Food Microbiol. 2019, 292, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, L.C.; Edo, G.I.; Özgör, E. The Phytochemical, Proximate, Pharmacological, GC-MS Analysis of Cyperus Esculentus (Tiger Nut): A Fully Validated Approach in Health, Food and Nutrition. Food Biosci. 2022, 46, 101551. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á. Antiviral Characterization of Advanced Materials: Use of Bacteriophage Phi 6 as Surrogate of Enveloped Viruses Such as SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 5335. [Google Scholar] [CrossRef]
- Tejera, E.; Pérez-Castillo, Y.; Toscano, G.; Noboa, A.L.; Ochoa-Herrera, V.; Giampieri, F.; Álvarez-Suarez, J.M. Computational Modeling Predicts Potential Effects of the Herbal Infusion “Horchata” against COVID-19. Food Chem. 2022, 366, 130589. [Google Scholar] [CrossRef]
- Chang, J.; Wang, L.; Ma, D.; Qu, X.; Guo, H.; Xu, X.; Mason, P.M.; Bourne, N.; Moriarty, R.; Gu, B.; et al. Novel Imino Sugar Derivatives Demonstrate Potent Antiviral Activity against Flaviviruses. Antimicrob. Agents Chemother. 2009, 53, 1501–1508. [Google Scholar] [CrossRef]
- Howe, J.D.; Smith, N.; Lee, M.J.R.; Ardes-Guisot, N.; Vauzeilles, B.; Désiré, J.; Baron, A.; Blériot, Y.; Sollogoub, M.; Alonzi, D.S.; et al. Novel Imino Sugar α-Glucosidase Inhibitors as Antiviral Compounds. Bioorganic Med. Chem. 2013, 21, 4831–4838. [Google Scholar] [CrossRef]
- Kifli, N.; Htar, T.T.; De Clercq, E.; Balzarini, J.; Simons, C. Novel Bicyclic Sugar Modified Nucleosides: Synthesis, Conformational Analysis and Antiviral Evaluation. Bioorganic Med. Chem. 2004, 12, 3247–3257. [Google Scholar] [CrossRef]
- Sanmartín-Santos, I.; Gandía-Llop, S.; Salesa, B.; Martí, M.; Lillelund Aachmann, F.; Serrano-Aroca, Á. Enhancement of Antimicrobial Activity of Alginate Films with a Low Amount of Carbon Nanofibers (0.1% w/w). Appl. Sci. 2021, 11, 2311. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, X.; Zhang, T.; Zhao, C.; Guan, S.; Pu, Y.; Gao, F. Tiger Nut (Cyperus Esculentus L.): Nutrition, Processing, Function and Applications. Foods 2022, 11, 601. [Google Scholar] [CrossRef]
- Johari, J.; Kianmehr, A.; Mustafa, M.R.; Abubakar, S.; Zandi, K. Antiviral Activity of Baicalein and Quercetin against the Japanese Encephalitis Virus. Int. J. Mol. Sci. 2012, 13, 16785–16795. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Ramos, F.A.; Takaishi, Y.; Shirotori, M.; Kawaguchi, Y.; Tsuchiya, K.; Shibata, H.; Higuti, T.; Tadokoro, T.; Takeuchi, M. Antibacterial and Antioxidant Activities of Quercetin Oxidation Products from Yellow Onion (Allium Cepa) Skin. J. Agric. Food Chem. 2006, 54, 3551–3557. [Google Scholar] [CrossRef]
- Sharma, K.; Ko, E.Y.; Assefa, A.D.; Ha, S.; Nile, S.H.; Lee, E.T.; Park, S.W. Temperature-Dependent Studies on the Total Phenolics, Flavonoids, Antioxidant Activities, and Sugar Content in Six Onion Varieties. J. Food Drug Anal. 2015, 23, 243–252. [Google Scholar] [CrossRef]
- Tuñón-Molina, A.; Cano-Vicent, A.; Serrano-Aroca, Á. Antimicrobial Lipstick: Bio-Based Composition against Viruses, Bacteria, and Fungi. ACS Appl. Mater. Interfaces 2022, 14, 56658–56665. [Google Scholar] [CrossRef] [PubMed]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of Bacteriophages by Double Agar Overlay Plaque Assay. In Bacteriophages; Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 501, pp. 69–76. [Google Scholar] [CrossRef]
- Martí, M.; Tuñón-Molina, A.; Aachmann, F.L.; Muramoto, Y.; Noda, T.; Takayama, K.; Serrano-Aroca, Á. Protective Face Mask Filter Capable of Inactivating SARS-CoV-2, and Methicillin-Resistant Staphylococcus Aureus and Staphylococcus Epidermidis. Polymers 2021, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Tuñón-Molina, A.; Martí, M.; Muramoto, Y.; Noda, T.; Takayama, K.; Serrano-Aroca, Á. Antimicrobial Face Shield: Next Generation of Facial Protective Equipment against SARS-CoV-2 and Multidrug-Resistant Bacteria. Int. J. Mol. Sci. 2021, 22, 9518. [Google Scholar] [CrossRef] [PubMed]
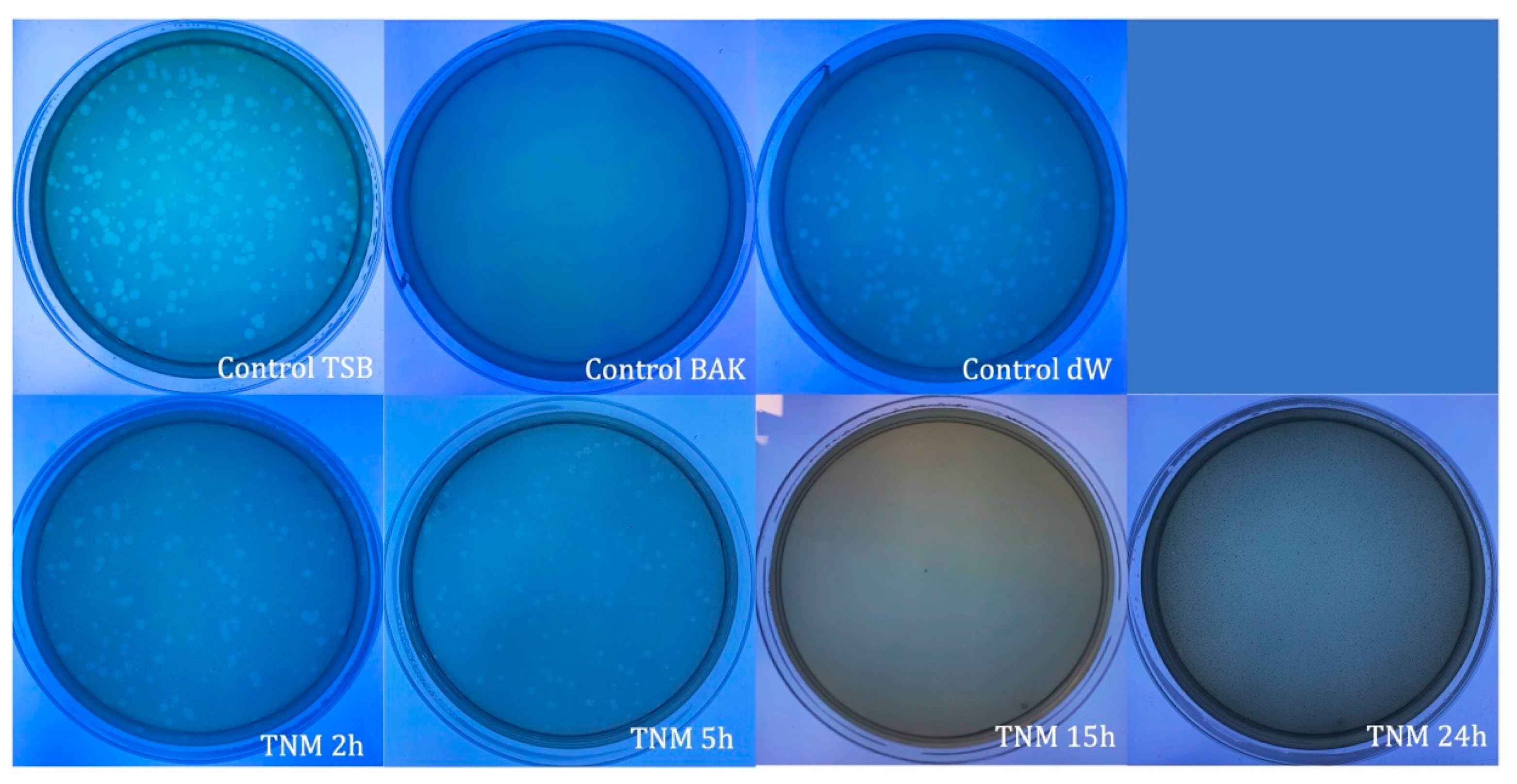
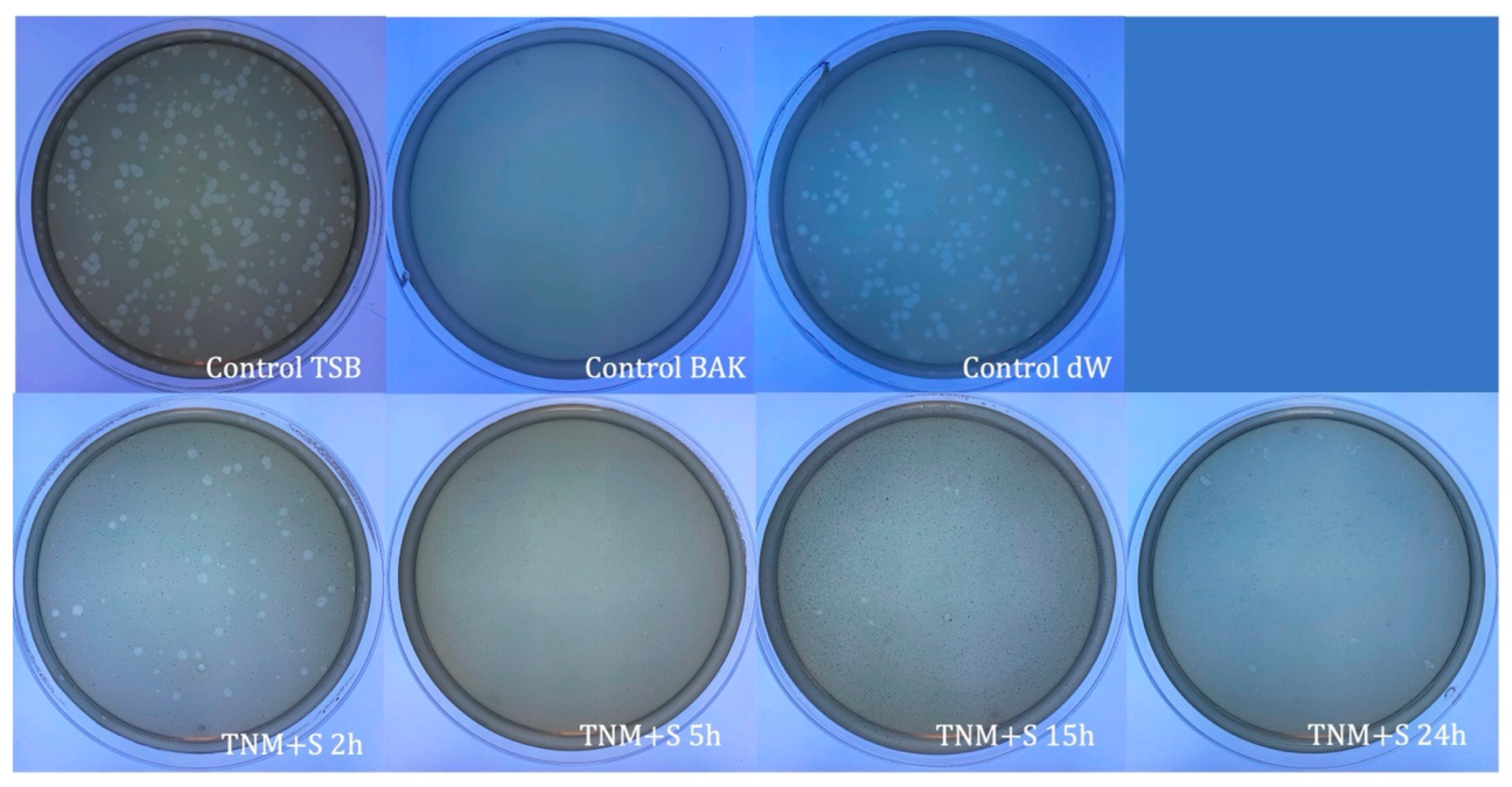
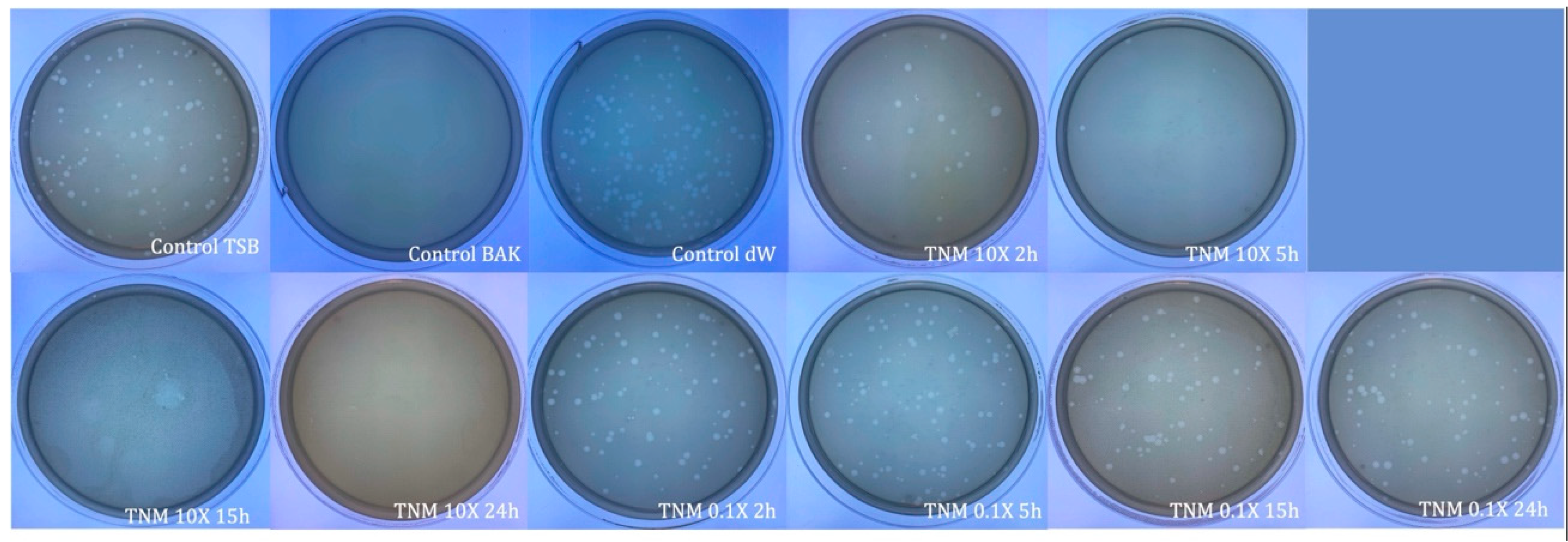
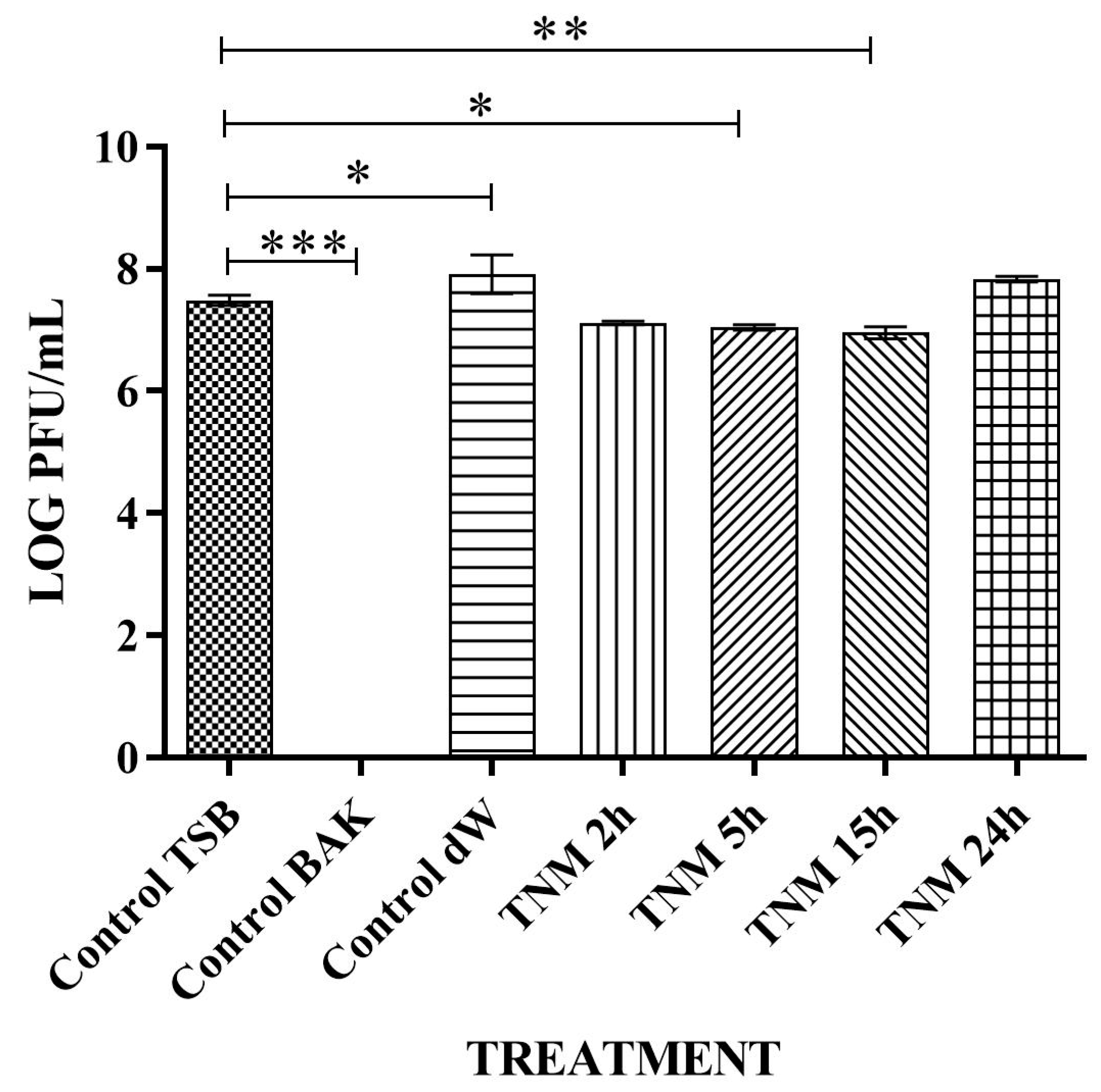
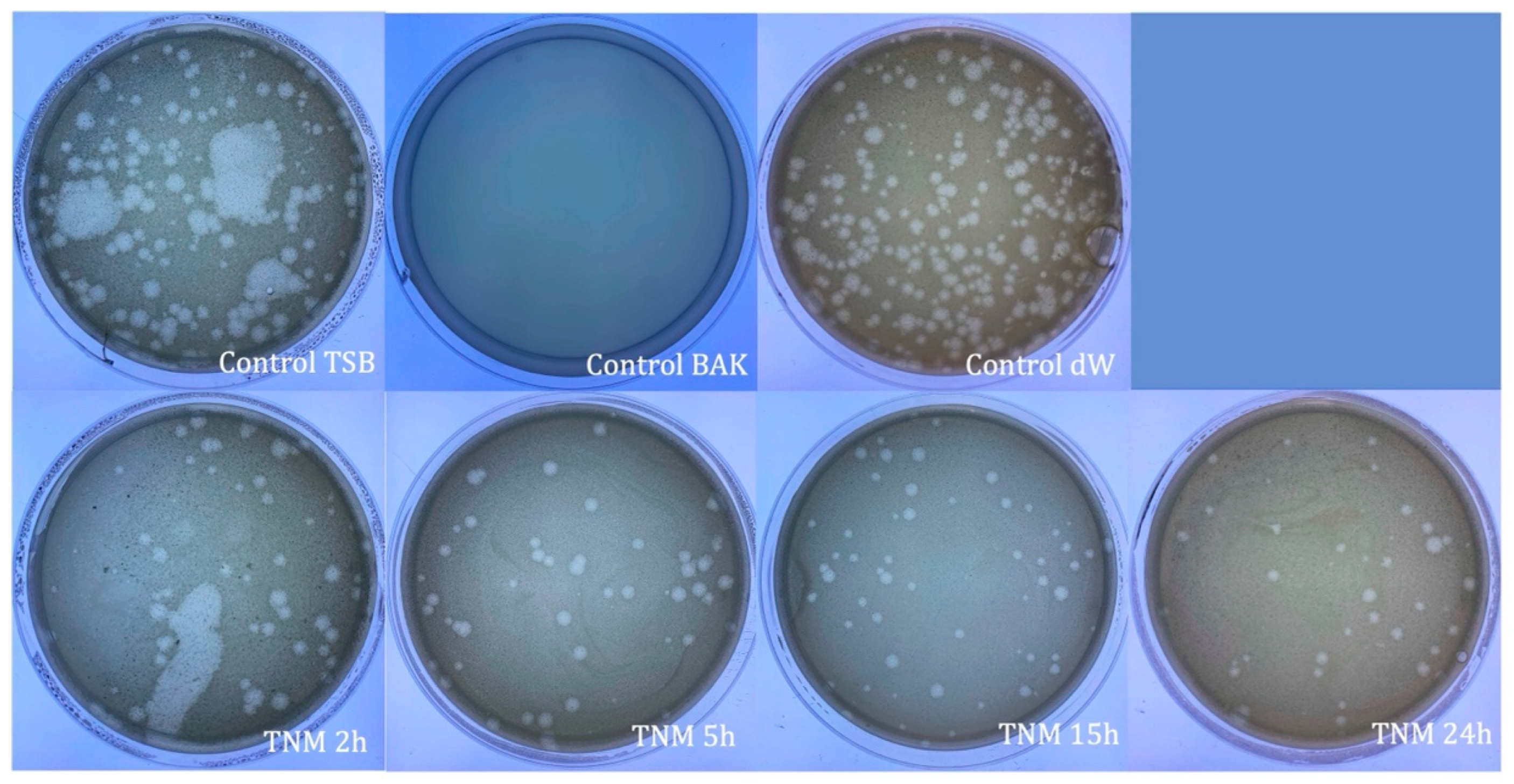
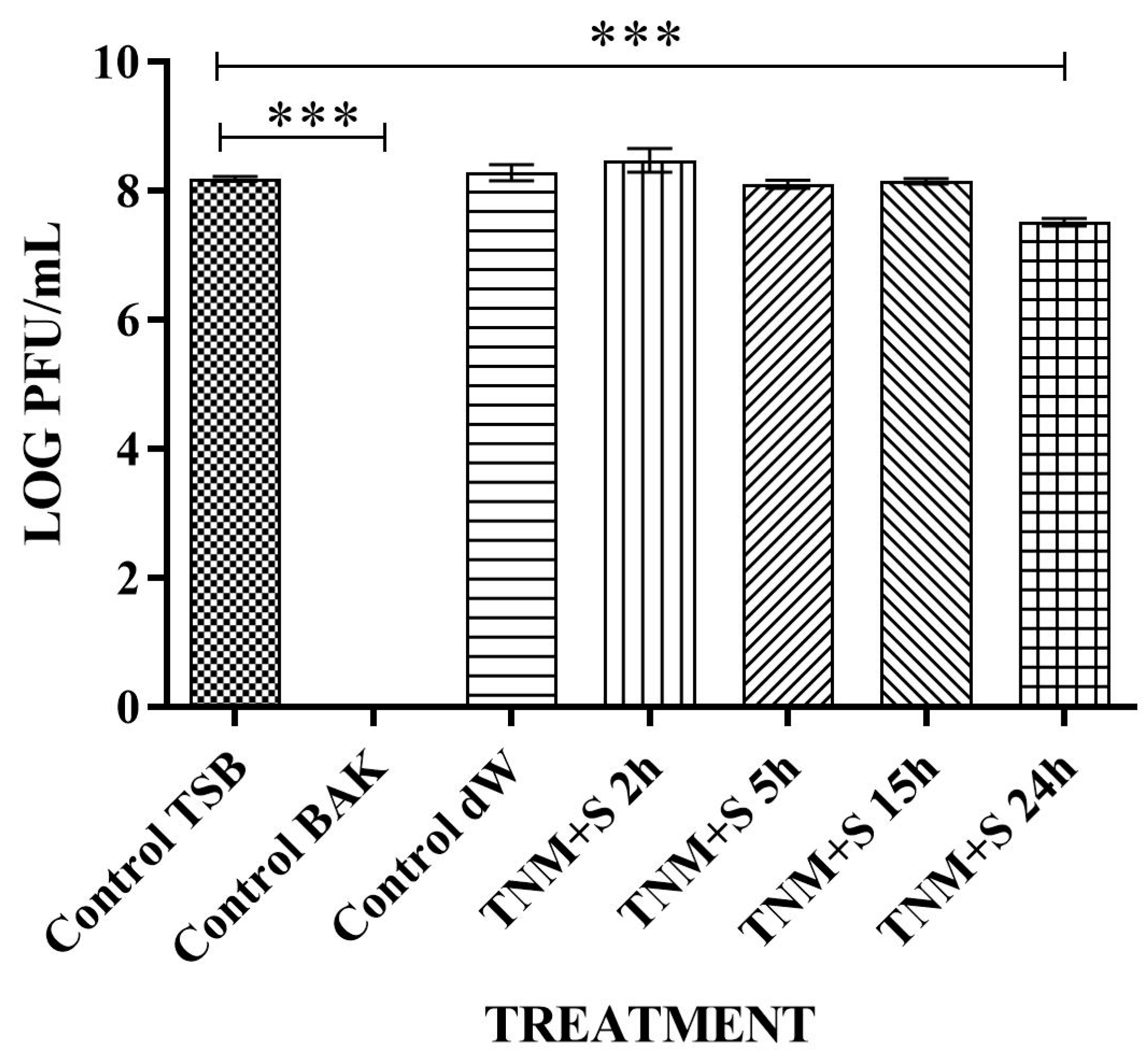
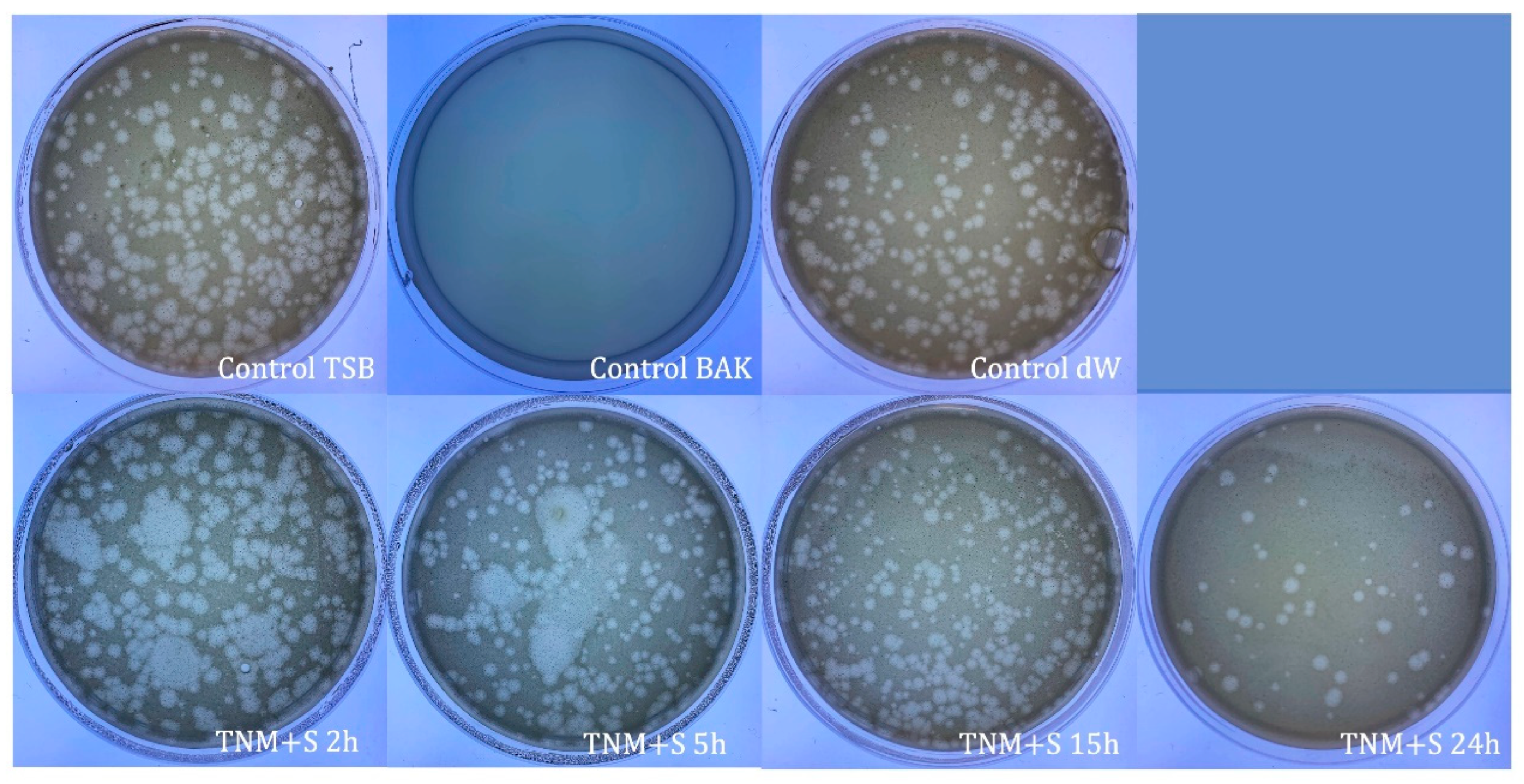
| SAMPLE | VIRUS CONCENTRATION (PFU/mL) | Log10 REDUCTION | VIRAL INACTIVATION (%) |
|---|---|---|---|
| control TSB | 4.96 × 106 ± 4.53 × 105 | - | - |
| control BAK | 0.00 ± 0.00 | ~7 | 100 |
| control dW | 3.11 × 106 ± 3.72 × 105 | ~0 | ~0 |
| TNM 2h | 3.01 × 106 ± 6.52 × 105 | ~0 | ~0 |
| TNM 5h | 3.21 × 106 ± 1.97 × 105 | ~0 | ~0 |
| TNM 15h | 0.00 ± 0.00 | ~7 | 100 |
| TNM 24h | 0.00 ± 0.00 | ~7 | 10 |
| SAMPLE | VIRUS CONCENTRATION (PFU/mL) | Log10 REDUCTION | VIRAL INACTIVATION (%) |
|---|---|---|---|
| control TSB | 2.37 × 106 ± 5.33 × 105 | - | - |
| control BAK | 0.00 ± 0.00 | ~6 | 100 |
| control dW | 3.10 × 106 ± 8.77 × 105 | ~0 | ~0 |
| TNM+S 2h | 1.57 × 106 ± 1.22 × 105 | ~0 | ~0 |
| TNM+S 5h | 0.00 ± 0.00 | ~6 | 100 |
| TNM+S 15h | 0.00 ± 0.00 | ~6 | 100 |
| TNM+S 24h | 0.00 ± 0.00 | ~6 | 100 |
| SAMPLE | VIRUS CONCENTRATION (PFU/mL) | Log10 REDUCTION | VIRAL INACTIVATION (%) |
|---|---|---|---|
| control TSB | 2.04 × 106 ± 2.83 × 105 | - | - |
| control BAK | 0.00 ± 0.00 | ~6 | 100 |
| control dW | 3.43 × 106 ± 1.84 × 105 | ~0 | ~0 |
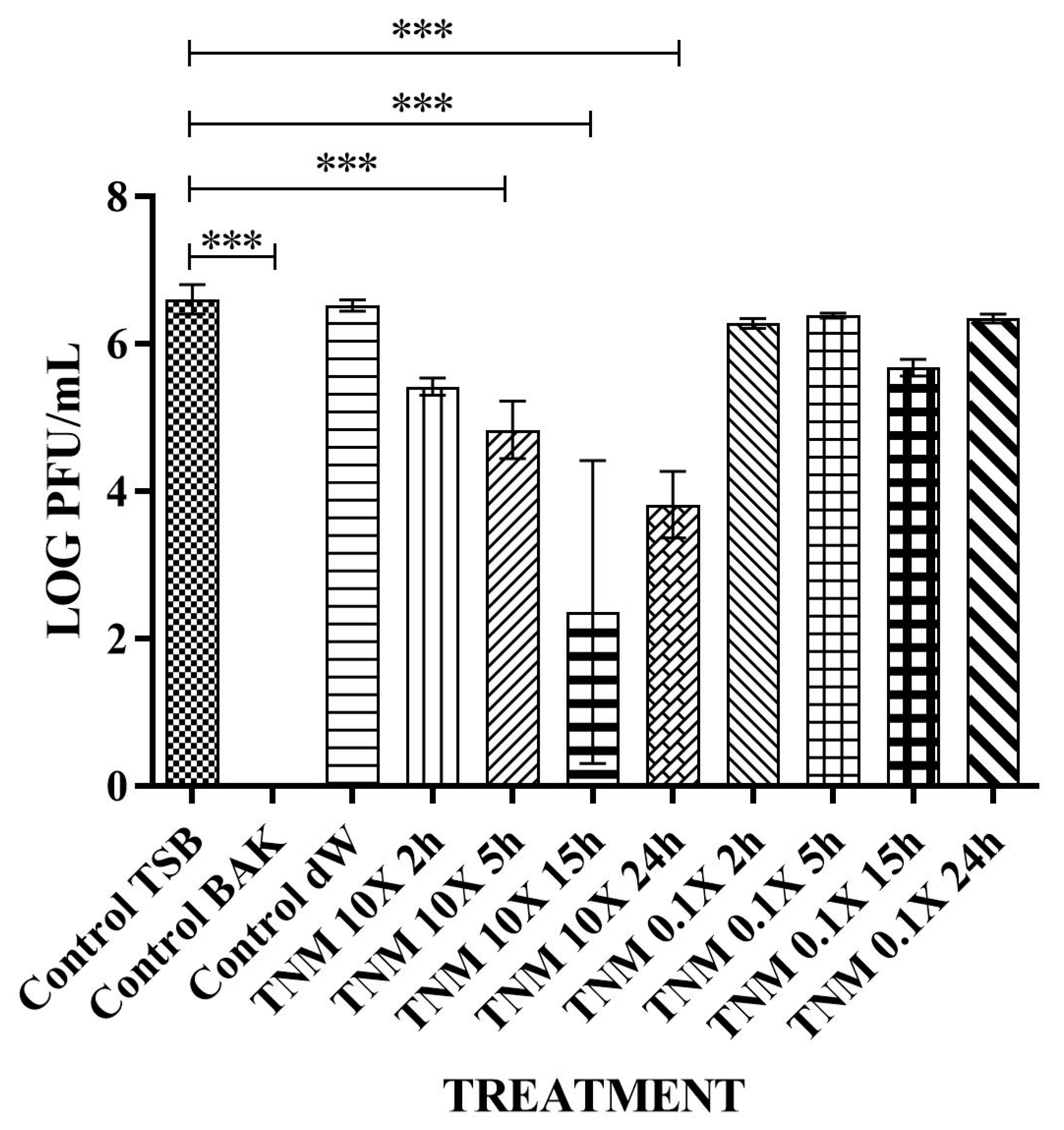
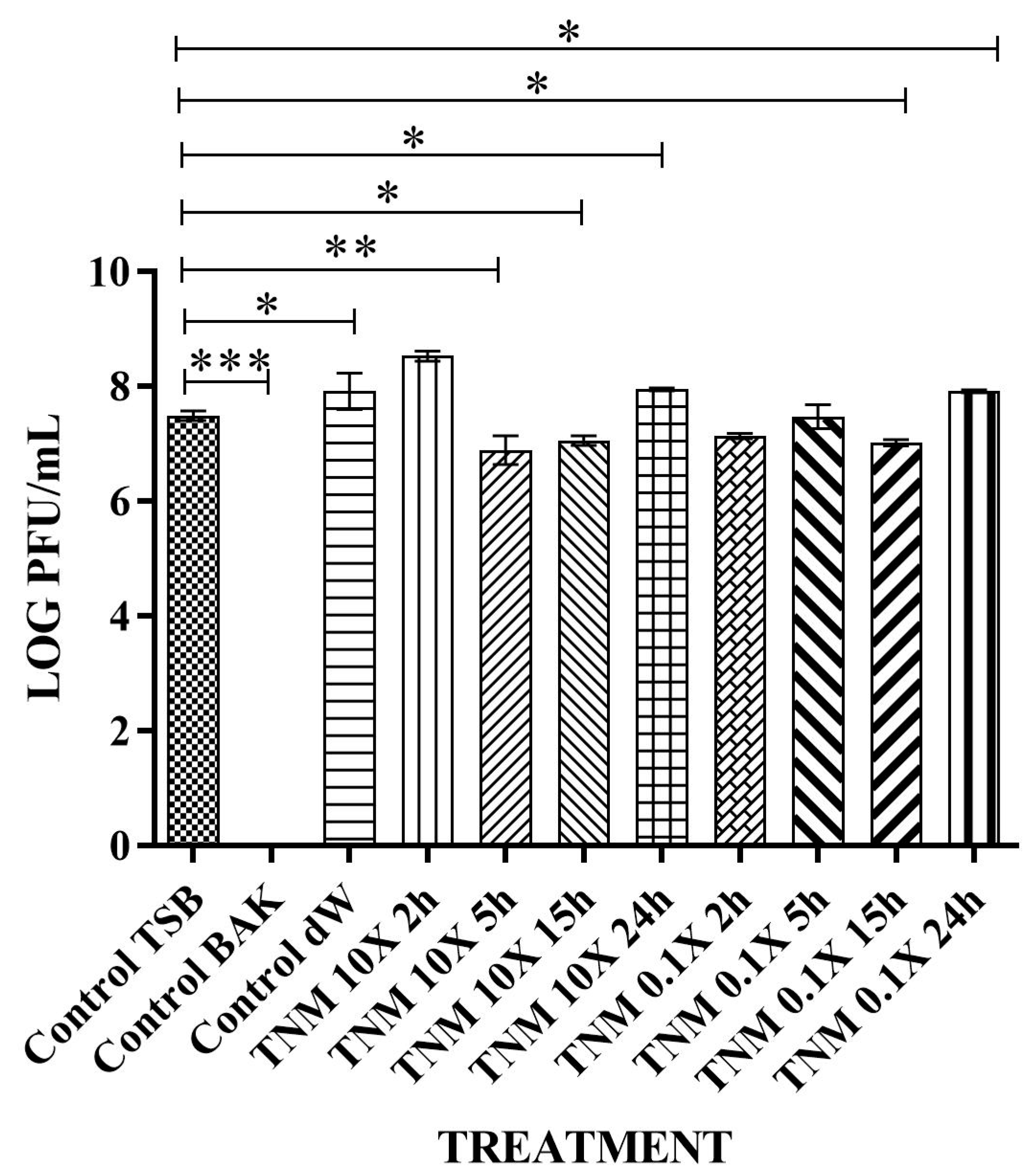
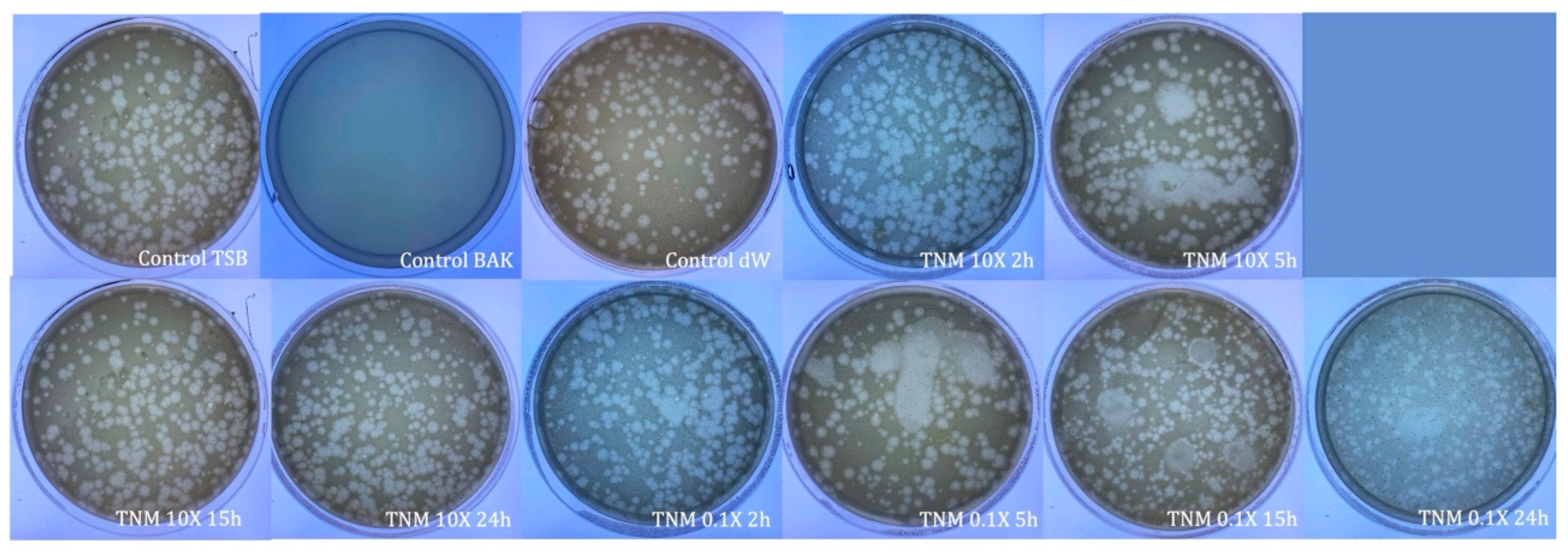
| TNM 10X 2h | 2.69 × 105 ± 7.26 × 104 | ~1 | ~87 |
| TNM 10X 5h | 9.13 × 104 ± 8.72 × 104 | ~2 | ~96 |
| TNM 10X 15h | 2.67 × 103 ± 3.06 × 103 | ~3 | ~100 |
| TNM 10X 24h | 8.67 × 103 ± 6.11 × 103 | ~3 | ~100 |
| TNM 0.1X 2h | 1.92 × 106 ± 2.88 × 105 | ~0 | ~0 |
| TNM 0.1X 5h | 2.43 × 106 ± 1.67 × 105 | ~0 | ~0 |
| TNM 0.1X 15h | 4.87 × 105 ± 1.30 × 105 | ~0.5 | ~76 |
| TNM 0.1X 24h | 2.21 × 106 ± 3.03 × 105 | ~0 | ~0 |
| SAMPLE | VIRUS CONCENTRATION (PFU/mL) | Log10 REDUCTION | VIRAL INACTIVATION (%) |
|---|---|---|---|
| control TSB | 4.40 × 107 ± 5.66 × 106 | - | - |
| control BAK | 0.00 ± 0.00 | ~8 | 100 |
| control dW | 2.17 × 107 ± 6.23 × 106 | ~0 | ~0 |
| TNM 2h | 1.31 × 107 ± 9.45 × 105 | ~0 | ~0 |
| TNM 5h | 1.11 × 107 ± 1.21 × 106 | ~0 | ~0 |
| TNM 15h | 9.27 × 106 ± 2.21 × 106 | ~0 | ~0 |
| TNM 24h | 6.80 × 107 ± 6.93 × 106 | ~0 | ~0 |
| SAMPLE | VIRUS CONCENTRATION (PFU/mL) | Log10 REDUCTION | VIRAL INACTIVATION (%) |
|---|---|---|---|
| control TSB | 1.54 × 108 ± 1.41 × 107 | - | - |
| control BAK | 0.00 ± 0.00 | ~8 | 100 |
| control dW | 2.17 × 108 ± 6.23 × 107 | ~0 | ~0 |
| TNM+S 2h | 7.47 × 108 ± 6.11 × 107 | ~0 | ~0 |
| TNM+S 5h | 1.14 × 108 ± 4.06 × 107 | ~0 | ~0 |
| TNM+S 15h | 1.17 × 108 ± 1.92 × 107 | ~0 | ~0 |
| TNM+S 24h | 3.23 × 107 ± 4.41 × 106 | ~1 | ~79 |
| SAMPLE | VIRUS CONCENTRATION (PFU/mL) | Log10 REDUCTION | VIRAL INACTIVATION (%) |
|---|---|---|---|
| control TSB | 2.64 × 107 ± 1.13 × 106 | - | - |
| control BAK | 0.00 ± 0.00 | ~7 | 100 |
| control dW | 5.64 × 107 ± 2.34 × 106 | ~0 | ~0 |
| TNM 10X 2h | 1.44 × 107 ± 2.31 × 106 | ~0 | ~0 |
| TNM 10X 5h | 8.47 × 106 ± 3.88 × 106 | ~0 | ~0 |
| TNM 10X 15h | 1.15 × 107 ± 2.19 × 106 | ~0 | ~0 |
| TNM 10X 24h | 8.85 × 107 ± 4.69 × 106 | ~0 | ~0 |
| TNM 0.1X 2h | 1.36 × 107 ± 1.51 × 106 | ~0 | ~0 |
| TNM 0.1X 5h | 3.18 × 107 ± 1.39 × 107 | ~0 | ~0 |
| TNM 0.1X 15h | 1.05 × 107 ± 1.22 × 106 | ~0 | ~0 |
| TNM 0.1X 24h | 8.13 × 107 ± 4.69 × 106 | ~0 | ~0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuñón-Molina, A.; Cano-Vicent, A.; Serrano-Aroca, Á. Tiger Nut Milk’s Antiviral Properties against Enveloped and Non-Enveloped Viruses: Effect of Concentration and Adding Sugar. Int. J. Mol. Sci. 2023, 24, 12018. https://doi.org/10.3390/ijms241512018
Tuñón-Molina A, Cano-Vicent A, Serrano-Aroca Á. Tiger Nut Milk’s Antiviral Properties against Enveloped and Non-Enveloped Viruses: Effect of Concentration and Adding Sugar. International Journal of Molecular Sciences. 2023; 24(15):12018. https://doi.org/10.3390/ijms241512018
Chicago/Turabian StyleTuñón-Molina, Alberto, Alba Cano-Vicent, and Ángel Serrano-Aroca. 2023. "Tiger Nut Milk’s Antiviral Properties against Enveloped and Non-Enveloped Viruses: Effect of Concentration and Adding Sugar" International Journal of Molecular Sciences 24, no. 15: 12018. https://doi.org/10.3390/ijms241512018
APA StyleTuñón-Molina, A., Cano-Vicent, A., & Serrano-Aroca, Á. (2023). Tiger Nut Milk’s Antiviral Properties against Enveloped and Non-Enveloped Viruses: Effect of Concentration and Adding Sugar. International Journal of Molecular Sciences, 24(15), 12018. https://doi.org/10.3390/ijms241512018







