A Comparative Study of the Inhibitory Effect of Some Flavonoids and a Conjugate of Taxifolin with Glyoxylic Acid on the Oxidative Burst of Neutrophils
Abstract
1. Introduction
2. Results
2.1. Hydrogen Peroxide Scavenging Activity
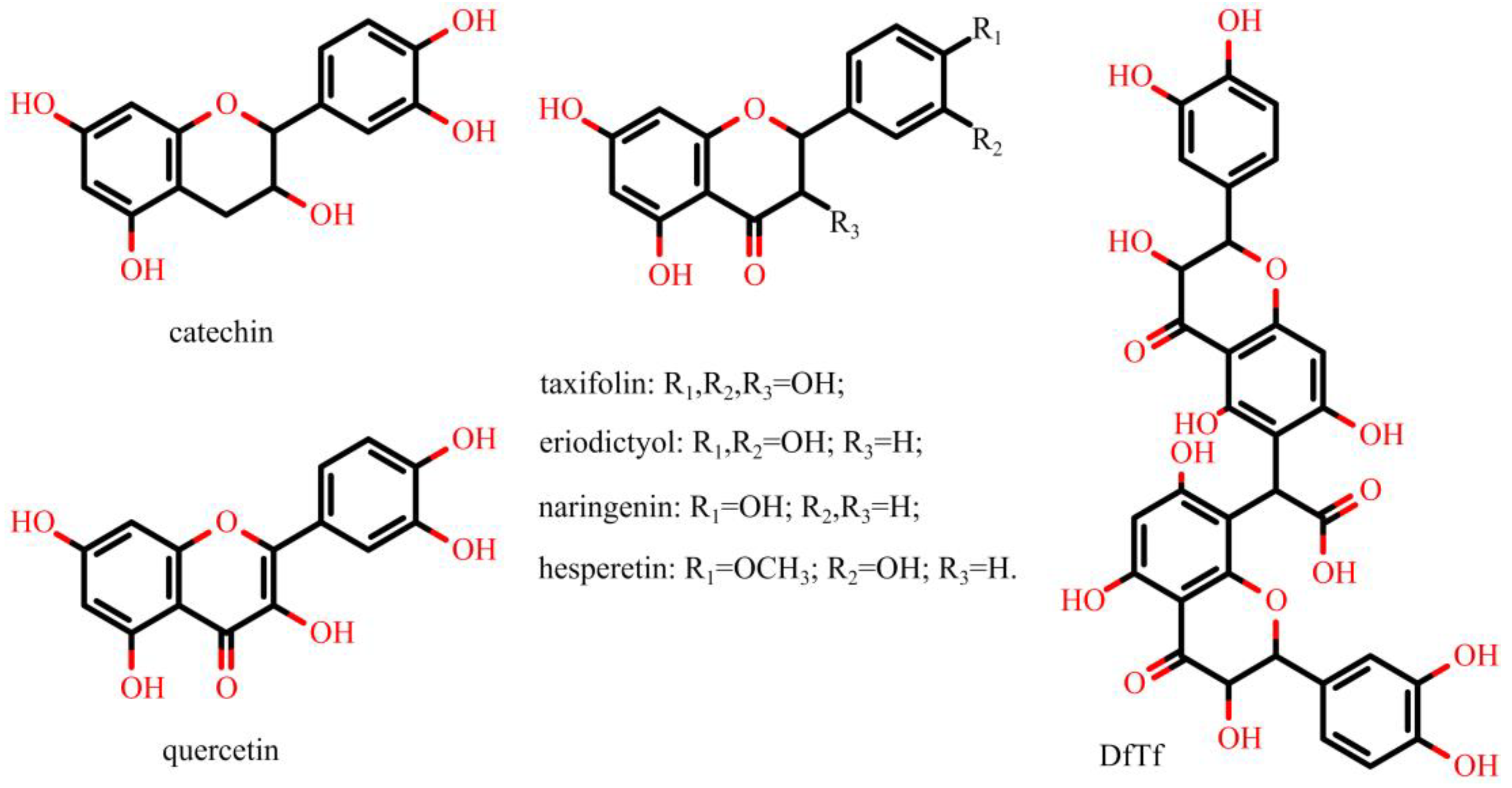
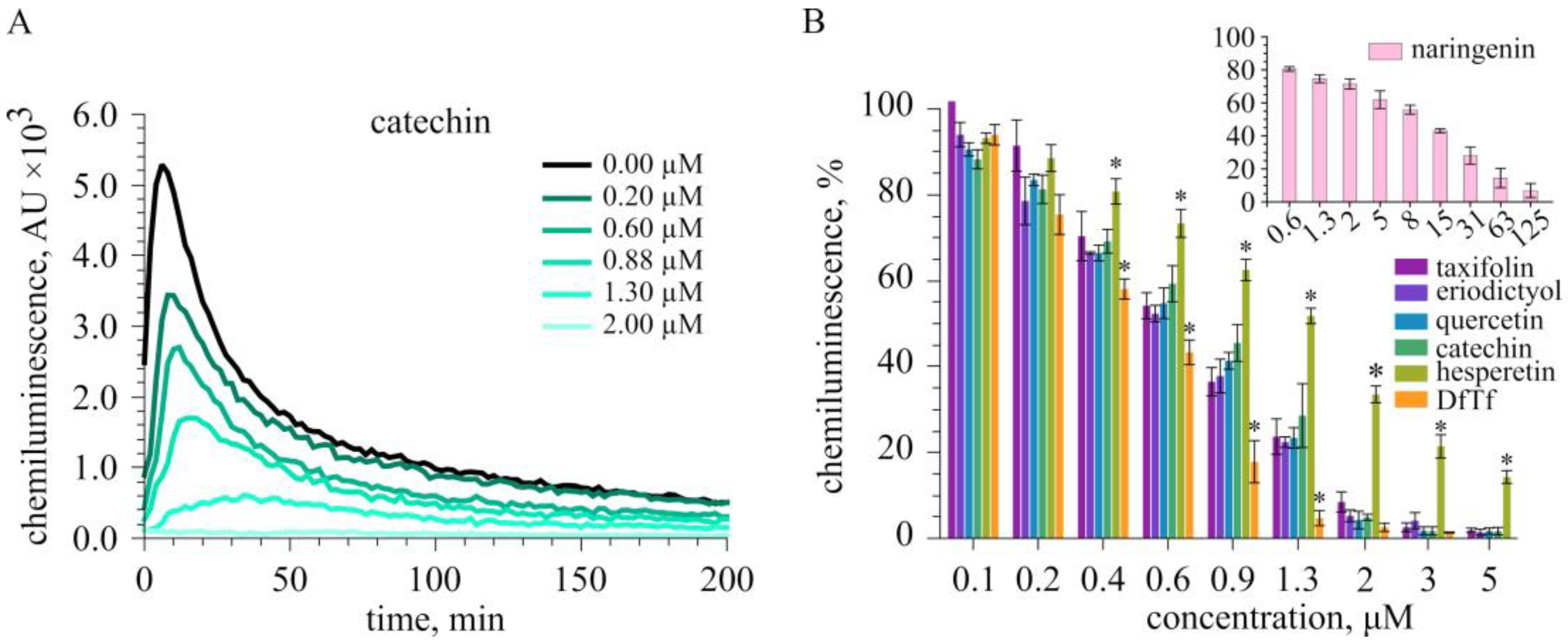
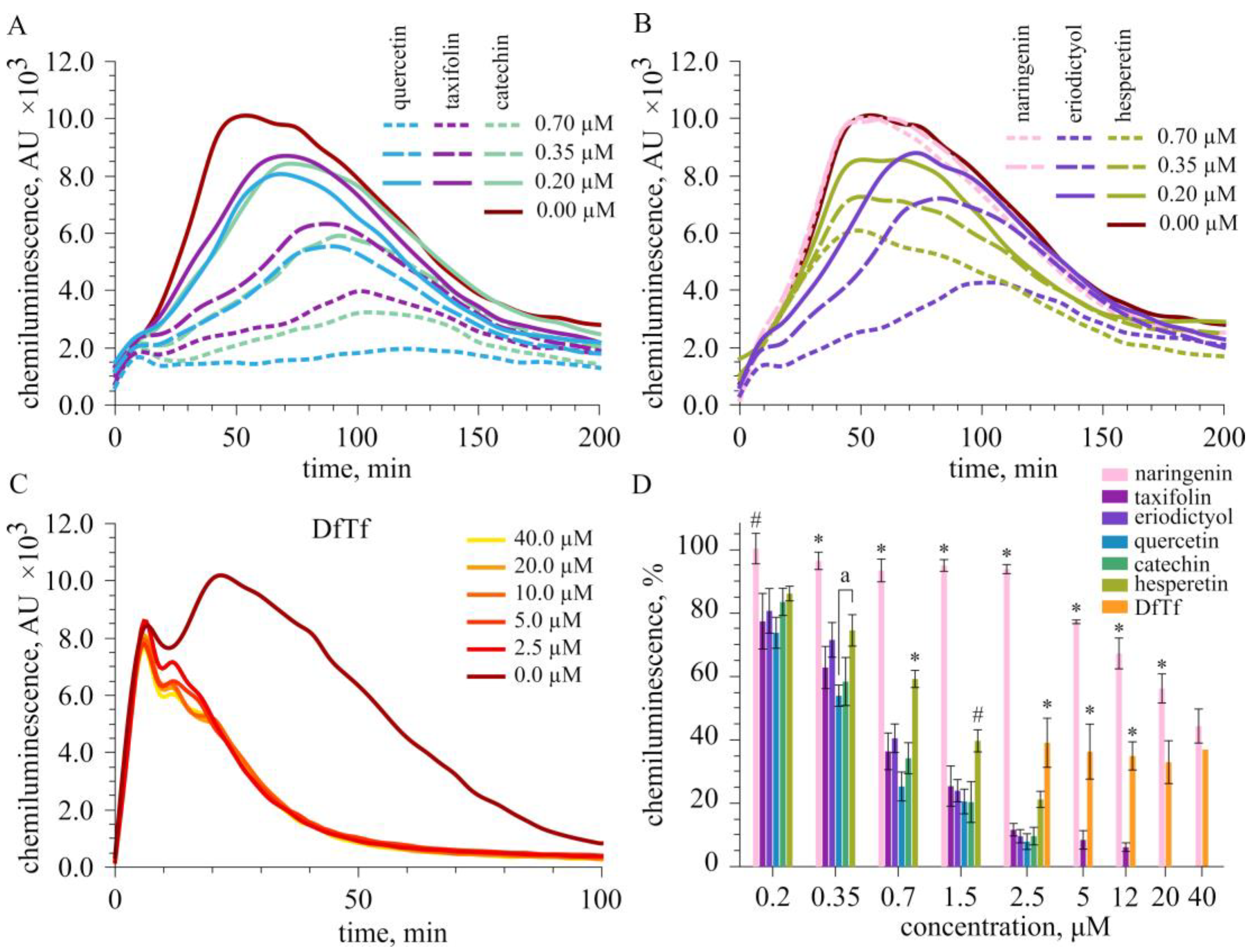
2.2. Effect of Polyphenols on the Total Production of ROS
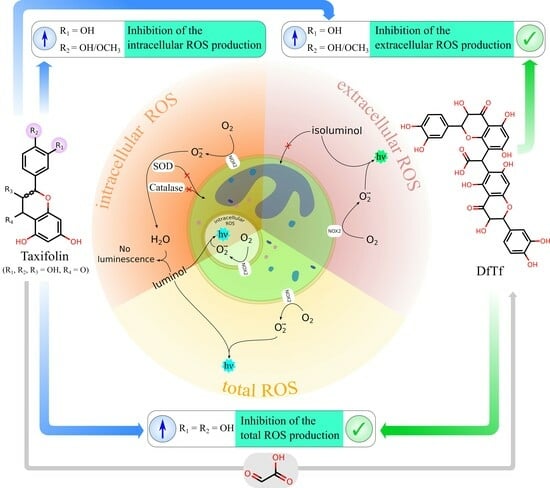
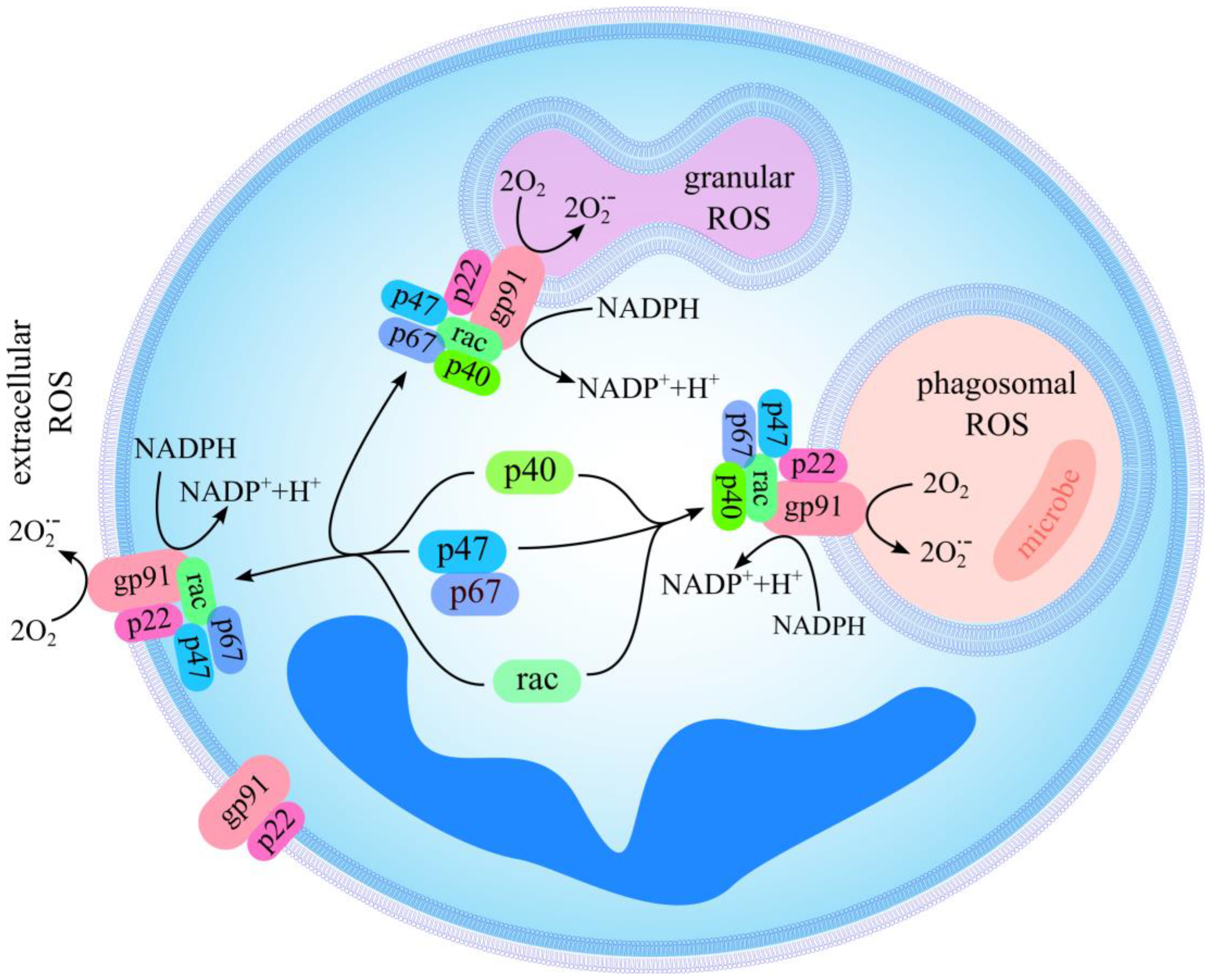
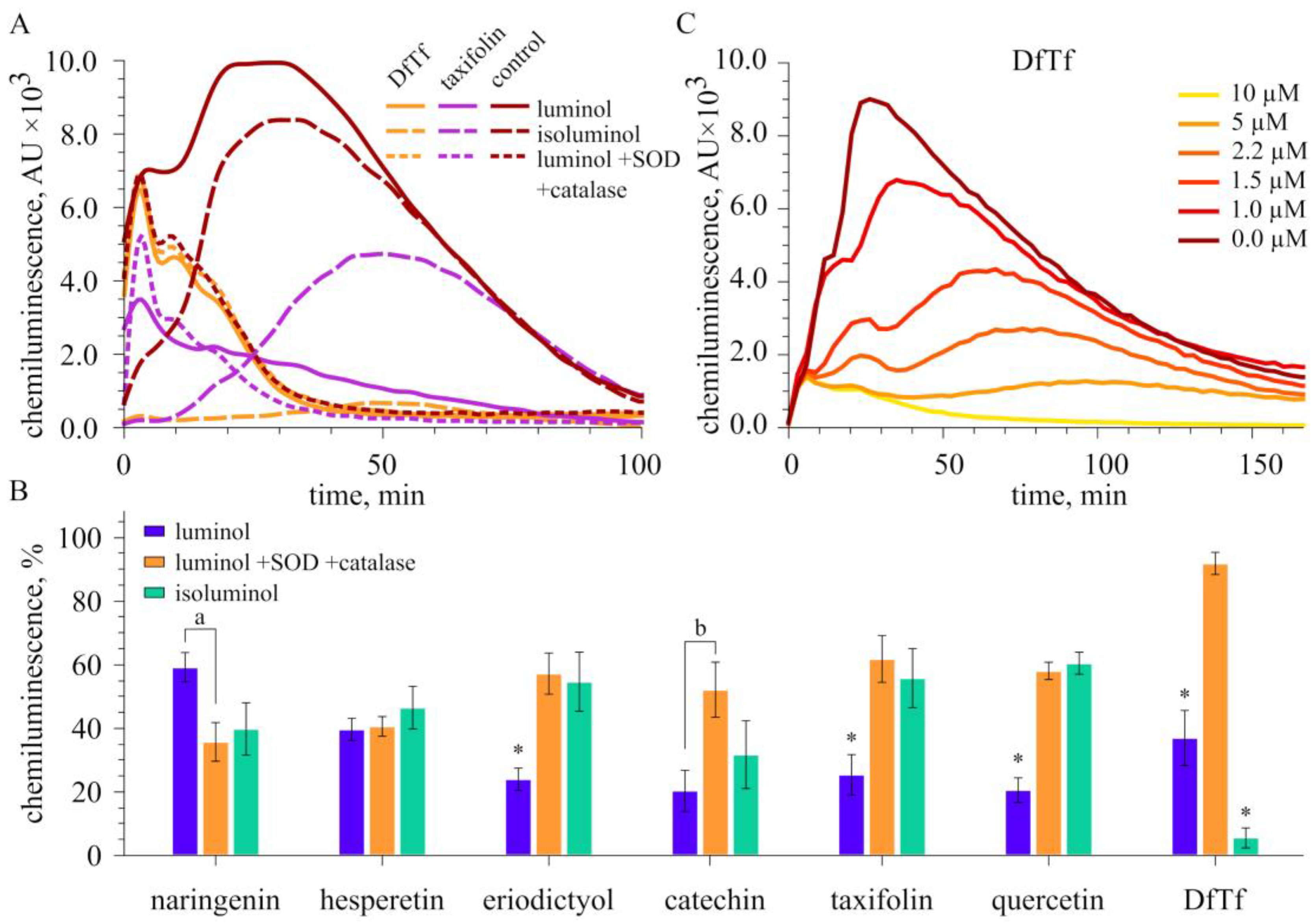
2.3. Effect of Polyphenols on Extracellular and Intracellular ROS Production
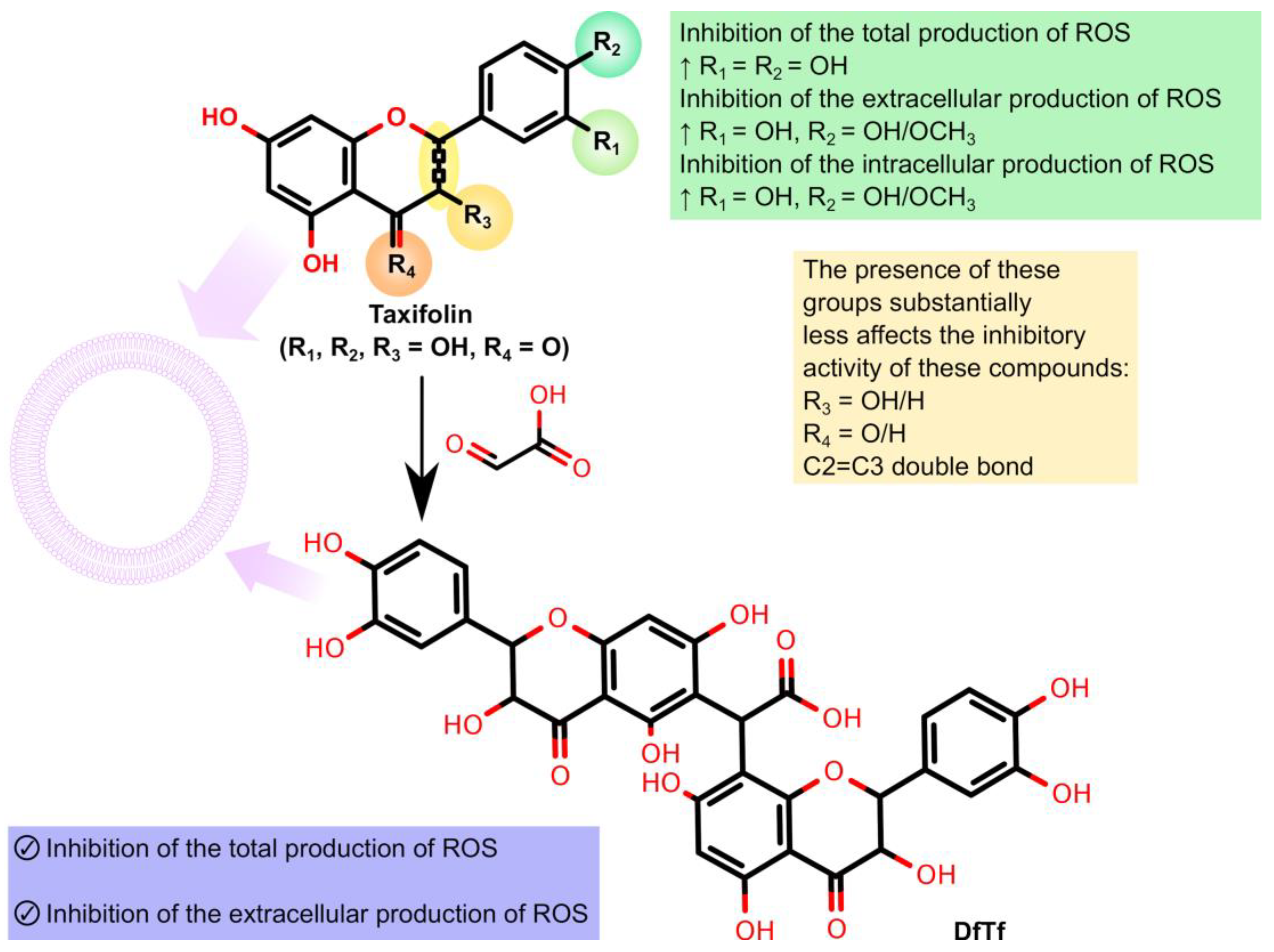
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis of DfTf
4.3. Hydrogen Peroxide-Scavenging Activity
4.4. Isolation of Neutrophils
4.5. Cell Viability
4.6. Production of ROS
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse Association between Habitual Polyphenol Intake and Incidence of Cardiovascular Events in the PREDIMED Study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 639–647. [Google Scholar] [CrossRef]
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual Intake of Anthocyanins and Flavanones and Risk of Cardiovascular Disease in Men. Am. J. Clin. Nutr. 2016, 104, 587–594. [Google Scholar] [CrossRef]
- Rienks, J.; Barbaresko, J.; Nöthlings, U. Association of Polyphenol Biomarkers with Cardiovascular Disease and Mortality Risk: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2017, 9, 415. [Google Scholar] [CrossRef]
- Lindsay, J. Risk Factors for Alzheimer’s Disease: A Prospective Analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002, 156, 445–453. [Google Scholar] [CrossRef]
- Devore, E.E.; Kang, J.H.; Breteler, M.M.B.; Grodstein, F. Dietary Intakes of Berries and Flavonoids in Relation to Cognitive Decline. Ann Neurol. 2012, 72, 135–143. [Google Scholar] [CrossRef]
- Letenneur, L.; Proust-Lima, C.; Le Gouge, A.; Dartigues, J.; Barberger-Gateau, P. Flavonoid Intake and Cognitive Decline over a 10-Year Period. Am. J. Epidemiol. 2007, 165, 1364–1371. [Google Scholar] [CrossRef]
- Commenges, D.; Scotet, V.; Renaud, S.; Jacqmin-Gadda, H.; Barberger-Gateau, P.; Dartigues, J.F. Intake of Flavonoids and Risk of Dementia. Eur. J. Epidemiol. 2000, 16, 357–363. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef]
- Goetz, M.E.; Judd, S.E.; Hartman, T.J.; McClellan, W.; Anderson, A.; Vaccarino, V. Flavanone Intake Is Inversely Associated with Risk of Incident Ischemic Stroke in the REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. J. Nutr. 2016, 146, 2233–2243. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Cai, Y.-Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure–Radical Scavenging Activity Relationships of Phenolic Compounds from Traditional Chinese Medicinal Plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as Antimicrobial Agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Hrubša, M.; Konečný, L.; Paclíková, M.; Parvin, M.S.; Skořepa, P.; Musil, F.; Karlíčková, J.; Javorská, L.; Matoušová, K.; Krčmová, L.K.; et al. The Antiplatelet Effect of 4-Methylcatechol in a Real Population Sample and Determination of the Mechanism of Action. Nutrients 2022, 14, 4798. [Google Scholar] [CrossRef] [PubMed]
- Konečný, L.; Hrubša, M.; Karlíčková, J.; Carazo, A.; Javorská, L.; Matoušová, K.; Krčmová, L.K.; Šmahelová, A.; Blaha, V.; Bláha, M.; et al. The Effect of 4-Methylcatechol on Platelets in Familial Hypercholesterolemic Patients Treated with Lipid Apheresis and/or Proprotein Convertase Subtilisin Kexin 9 Monoclonal Antibodies. Nutrients 2023, 15, 1842. [Google Scholar] [CrossRef] [PubMed]
- Ed Nignpense, B.; Chinkwo, K.A.; Blanchard, C.L.; Santhakumar, A.B. Polyphenols: Modulators of Platelet Function and Platelet Microparticle Generation? Int. J. Mol. Sci. 2019, 21, 146. [Google Scholar] [CrossRef]
- Lescano, C.H.; Freitas De Lima, F.; Mendes-Silvério, C.B.; Justo, A.F.O.; Da Silva Baldivia, D.; Vieira, C.P.; Sanjinez-Argandoña, E.J.; Cardoso, C.A.L.; Mónica, F.Z.; Pires De Oliveira, I. Effect of Polyphenols From Campomanesia Adamantium on Platelet Aggregation and Inhibition of Cyclooxygenases: Molecular Docking and in vitro Analysis. Front. Pharmacol. 2018, 9, 617. [Google Scholar] [CrossRef]
- Liskova, S.; Cacanyiova, S.; Cebova, M.; Berenyiova, A.; Kluknavsky, M.; Micurova, A.; Valachova, K.; Soltes, L.; Bernatova, I. Taxifolin Reduces Blood Pressure via Improvement of Vascular Function and Mitigating the Vascular Inflammatory Response in Spontaneously Hypertensive Rats. Int. J. Mol. Sci. 2023, 24, 12616. [Google Scholar] [CrossRef]
- Bernatova, I.; Liskova, S. Mechanisms Modified by (−)-Epicatechin and Taxifolin Relevant for the Treatment of Hypertension and Viral Infection: Knowledge from Preclinical Studies. Antioxidants 2021, 10, 467. [Google Scholar] [CrossRef]
- Maaliki, D.; Shaito, A.A.; Pintus, G.; El-Yazbi, A.; Eid, A.H. Flavonoids in Hypertension: A Brief Review of the Underlying Mechanisms. Curr. Opin. Pharmacol. 2019, 45, 57–65. [Google Scholar] [CrossRef]
- Jasenovec, T.; Radosinska, D.; Kollarova, M.; Balis, P.; Zorad, S.; Vrbjar, N.; Bernatova, I.; Cacanyiova, S.; Tothova, L.; Radosinska, J. Effects of Taxifolin in Spontaneously Hypertensive Rats with a Focus on Erythrocyte Quality. Life 2022, 12, 2045. [Google Scholar] [CrossRef]
- Estrela, J.M.; Mena, S.; Obrador, E.; Benlloch, M.; Castellano, G.; Salvador, R.; Dellinger, R.W. Polyphenolic Phytochemicals in Cancer Prevention and Therapy: Bioavailability versus Bioefficacy. J. Med. Chem. 2017, 60, 9413–9436. [Google Scholar] [CrossRef]
- Lund, M.N. Reactions of Plant Polyphenols in Foods: Impact of Molecular Structure. Trends Food Sci. Technol. 2021, 112, 241–251. [Google Scholar] [CrossRef]
- Goszcz, K.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Bioactive Polyphenols and Cardiovascular Disease: Chemical Antagonists, Pharmacological Agents or Xenobiotics That Drive an Adaptive Response?: Bioactive Polyphenols and Cardiovascular Disease. Br. J. Pharmacol. 2017, 174, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Debelo, H.; Li, M.; Ferruzzi, M.G. Processing Influences on Food Polyphenol Profiles and Biological Activity. Curr. Opin. Food Sci. 2020, 32, 90–102. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Cheynier, V.; Moutounet, M. Study of the Reactions between (+)-Catechin and Furfural Derivatives in the Presence or Absence of Anthocyanins and Their Implication in Food Color Change. J. Agric. Food Chem. 2000, 48, 5946–5954. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Cheynier, V.; Moutounet, M. Role of Aldehydic Derivatives in the Condensation of Phenolic Compounds with Emphasis on the Sensorial Properties of Fruit-Derived Foods. J. Agric. Food Chem. 2002, 50, 5571–5585. [Google Scholar] [CrossRef]
- Lo, C.-Y.; Li, S.; Tan, D.; Pan, M.-H.; Sang, S.; Ho, C.-T. Trapping Reactions of Reactive Carbonyl Species with Tea Polyphenols in Simulated Physiological Conditions. Mol. Nutr. Food Res. 2006, 50, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Totlani, V.M.; Peterson, D.G. Epicatechin Carbonyl-Trapping Reactions in Aqueous Maillard Systems: Identification and Structural Elucidation. J. Agric. Food Chem. 2006, 54, 7311–7318. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xia, Q.; Lu, Y.; Zheng, T.; Sang, S.; Lv, L. Influence of Quercetin and Its Methylglyoxal Adducts on the Formation of α-Dicarbonyl Compounds in a Lysine/Glucose Model System. J. Agric. Food Chem. 2017, 65, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.M.; Hidalgo, F.J.; Zamora, R. Antagonism between Lipid-Derived Reactive Carbonyls and Phenolic Compounds in the Strecker Degradation of Amino Acids. Food Chem. 2016, 194, 1143–1148. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Delgado, R.M.; Zamora, R. Protective Effect of Phenolic Compounds on Carbonyl-Amine Reactions Produced by Lipid-Derived Reactive Carbonyls. Food Chem. 2017, 229, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Aguilar, I.; Hidalgo, F.J. Epoxyalkenal-Trapping Ability of Phenolic Compounds. Food Chem. 2017, 237, 444–452. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, H.; Huang, C.; Liu, P.; Fei, J.; Ou, J.; Ou, S.; Zheng, J. Identification and Cytotoxic Evaluation of the Novel Rutin–Methylglyoxal Adducts with Dione Structures in vivo and in Foods. Food Chem. 2022, 377, 132008. [Google Scholar] [CrossRef]
- Chen, M.; Liu, P.; Zhou, H.; Huang, C.; Zhai, W.; Xiao, Y.; Ou, J.; He, J.; El-Nezami, H.; Zheng, J. Formation and Metabolism of 6-(1-Acetol)-8-(1-Acetol)-Rutin in Foods and in vivo, and Their Cytotoxicity. Front. Nutr. 2022, 9, 973048. [Google Scholar] [CrossRef]
- Jongberg, S.; Tørngren, M.A.; Gunvig, A.; Skibsted, L.H.; Lund, M.N. Effect of Green Tea or Rosemary Extract on Protein Oxidation in Bologna Type Sausages Prepared from Oxidatively Stressed Pork. Meat Sci. 2013, 93, 538–546. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. The Triple Defensive Barrier of Phenolic Compounds against the Lipid Oxidation-Induced Damage in Food Products. Trends Food Sci. Technol. 2016, 54, 165–174. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Carbonyl–Phenol Adducts: An Alternative Sink for Reactive and Potentially Toxic Lipid Oxidation Products. J. Agric. Food Chem. 2018, 66, 1320–1324. [Google Scholar] [CrossRef]
- Zhu, H.; Poojary, M.M.; Andersen, M.L.; Lund, M.N. Trapping of Carbonyl Compounds by Epicatechin: Reaction Kinetics and Identification of Epicatechin Adducts in Stored UHT Milk. J. Agric. Food Chem. 2020, 68, 7718–7726. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, J.; Andersen, M.L.; Peters, G.H.J.; Lund, M.N. Predicting the Reaction Rates between Flavonoids and Methylglyoxal by Combining Molecular Properties and Machine Learning. Food Biosci. 2023, 54, 102890. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Competition between (+)-Catechin and (−)-Epicatechin in Acetaldehyde-Induced Polymerization of Flavanols. J. Agric. Food Chem. 1999, 47, 2088–2095. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Studies on the Acetaldehyde-Induced Condensation of (−)-Epicatechin and Malvidin 3- O -Glucoside in a Model Solution System. J. Agric. Food Chem. 1999, 47, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Poojary, M.M.; Andersen, M.L.; Lund, M.N. Effect of PH on the Reaction between Naringenin and Methylglyoxal: A Kinetic Study. Food Chem. 2019, 298, 125086. [Google Scholar] [CrossRef]
- Wang, C.; Lu, Y.; Huang, Q.; Zheng, T.; Sang, S.; Lv, L. Levels and Formation of α-Dicarbonyl Compounds in Beverages and the Preventive Effects of Flavonoids. J. Food Sci. Technol. 2017, 54, 2030–2040. [Google Scholar] [CrossRef]
- Shubina, V.S.; Shatalin, Y.V. Antioxidant and Iron-Chelating Properties of Taxifolin and Its Condensation Product with Glyoxylic Acid. J. Food Sci. Technol. 2017, 54, 1467–1475. [Google Scholar] [CrossRef]
- Zhu, H.; Poojary, M.M.; Andersen, M.L.; Lund, M.N. The Effect of Molecular Structure of Polyphenols on the Kinetics of the Trapping Reactions with Methylglyoxal. Food Chem. 2020, 319, 126500. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, L.; Lv, L.; Sang, S. Trapping Methylglyoxal by Myricetin and Its Metabolites in Mice. J. Agric. Food Chem. 2020, 68, 9408–9414. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the Gastrointestinal Tract: Local and Systemic Effects. Mol. Asp. Med. 2018, 61, 41–49. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. A Novel Function of Red Wine Polyphenols in Humans: Prevention of Absorption of Cytotoxic Lipid Peroxidation Products. FASEB J. 2008, 22, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. The Stomach as a “Bioreactor”: When Red Meat Meets Red Wine. J. Agric. Food Chem. 2008, 56, 5002–5007. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J.; Gorelik, S.; Roman, S.; Kohen, R. Protection by Polyphenols of Postprandial Human Plasma and Low-Density Lipoprotein Modification: The Stomach as a Bioreactor. J. Agric. Food Chem. 2012, 60, 8790–8796. [Google Scholar] [CrossRef] [PubMed]
- Sirota, R.; Gorelik, S.; Harris, R.; Kohen, R.; Kanner, J. Coffee Polyphenols Protect Human Plasma from Postprandial Carbonyl Modifications. Mol. Nutr. Food Res. 2013, 57, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J.; Selhub, J.; Shpaizer, A.; Rabkin, B.; Shacham, I.; Tirosh, O. Redox Homeostasis in Stomach Medium by Foods: The Postprandial Oxidative Stress Index (POSI) for Balancing Nutrition and Human Health. Redox Biol. 2017, 12, 929–936. [Google Scholar] [CrossRef]
- Wang, P.; Chen, H.; Sang, S. Trapping Methylglyoxal by Genistein and Its Metabolites in Mice. Chem. Res. Toxicol. 2016, 29, 406–414. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, P.; Sang, S. Dietary Genistein Inhibits Methylglyoxal-Induced Advanced Glycation End Product Formation in Mice Fed a High-Fat Diet. J. Nutr. 2019, 149, 776–787. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, P.; Zhu, Y.; Lv, L.; Sang, S. Additive Capacity of [6]-Shogaol and Epicatechin To Trap Methylglyoxal. J. Agric. Food Chem. 2017, 65, 8356–8362. [Google Scholar] [CrossRef]
- Jiang, H.; Li, D. Polyphenols as Reactive Carbonyl Species Scavengers—The Solution to the Current Puzzle of Polyphenols’ Health Effects. Med. Hypotheses 2020, 142, 110144. [Google Scholar] [CrossRef]
- Shubina, V.S.; Kozina, V.I.; Shatalin, Y.V. Comparison of Antioxidant Properties of a Conjugate of Taxifolin with Glyoxylic Acid and Selected Flavonoids. Antioxidants 2021, 10, 1262. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, W.; Yang, F.; Xu, Y.; Feng, C.; Zhao, Y. The Regulatory Roles of Neutrophils in Adaptive Immunity. Cell Commun. Signal 2019, 17, 147. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in Chronic Inflammatory Diseases. Cell. Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef]
- Laforge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.-J.; Becker, C. Tissue Damage from Neutrophil-Induced Oxidative Stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Aviello, G.; Knaus, U. ROS in Gastrointestinal Inflammation: Rescue Or Sabotage? ROS and GI Inflammation. Br. J. Pharmacol. 2017, 174, 1704–1718. [Google Scholar] [CrossRef]
- Drury, B.; Hardisty, G.; Gray, R.D.; Ho, G. Neutrophil Extracellular Traps in Inflammatory Bowel Disease: Pathogenic Mechanisms and Clinical Translation. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 321–333. [Google Scholar] [CrossRef]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH Oxidases: An Overview from Structure to Innate Immunity-Associated Pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef]
- Dahlgren, C.; Karlsson, A.; Bylund, J. Intracellular Neutrophil Oxidants: From Laboratory Curiosity to Clinical Reality. J. Immunol. 2019, 202, 3127–3134. [Google Scholar] [CrossRef] [PubMed]
- Bylund, J.; Brown, K.L.; Movitz, C.; Dahlgren, C.; Karlsson, A. Intracellular Generation of Superoxide by the Phagocyte NADPH Oxidase: How, Where, and What For? Free Radic. Biol. Med. 2010, 49, 1834–1845. [Google Scholar] [CrossRef]
- Lundqvist, H.; Foilin, P.; Khalfan, L.; Dahlgren, C. Phorbol Myristate Acetate-Induced NADPH Oxidase Activity in Human Neutrophils: Only Half the Story Has Been Told. J. Leukoc. Biol. 1996, 59, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Björnsdottir, H.; Welin, A.; Dahlgren, C.; Karlsson, A.; Bylund, J. Quantification of Heterotypic Granule Fusion in Human Neutrophils by Imaging Flow Cytometry. Data Brief 2016, 6, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Matute, J.D.; Arias, A.A.; Wright, N.A.M.; Wrobel, I.; Waterhouse, C.C.M.; Li, X.J.; Marchal, C.C.; Stull, N.D.; Lewis, D.B.; Steele, M.; et al. A New Genetic Subgroup of Chronic Granulomatous Disease with Autosomal Recessive Mutations in P40phox and Selective Defects in Neutrophil NADPH Oxidase Activity. Blood 2009, 114, 3309–3315. [Google Scholar] [CrossRef]
- Jancinová, V.; Drábiková, K.; Nosál, R.; Racková, L.; Májeková, M.; Holománová, D. The Combined Luminol/Isoluminol Chemiluminescence Method for Differentiating between Extracellular and Intracellular Oxidant Production by Neutrophils. Redox Rep. 2006, 11, 110–116. [Google Scholar] [CrossRef]
- Ellson, C.D.; Riça, I.G.; Kim, J.S.; Huang, Y.M.; Lim, D.; Mitra, T.; Hsu, A.; Wei, E.X.; Barrett, C.D.; Otterbein, L.E.; et al. An Integrated Pharmacological, Structural, and Genetic Analysis of Extracellular Versus Intracellular ROS Production in Neutrophils. J. Mol. Biol. 2022, 434, 167533. [Google Scholar] [CrossRef]
- Quinn, M.T.; DeLeo, F.R. (Eds.) Neutrophil Methods and Protocols; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2014; Volume 1124, ISBN 978-1-62703-844-7. [Google Scholar]
- Saleh, L.; Plieth, C. Total Low-Molecular-Weight Antioxidants as a Summary Parameter, Quantified in Biological Samples by a Chemiluminescence Inhibition Assay. Nat. Protoc. 2010, 5, 1627–1634. [Google Scholar] [CrossRef]
- Schroeder, H.R.; Yeager, F.M. Chemiluminescence Yields and Detection Limits of Some Isoluminol Derivatives in Various Oxidation Systems. Anal. Chem. 1978, 50, 1114–1120. [Google Scholar] [CrossRef]
- Di Veroli, G.Y.; Fornari, C.; Goldlust, I.; Mills, G.; Koh, S.B.; Bramhall, J.L.; Richards, F.M.; Jodrell, D.I. An Automated Fitting Procedure and Software for Dose-Response Curves with Multiphasic Features. Sci. Rep. 2015, 5, 14701. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Structure-Activity Relationships Analysis of Monomeric and Polymeric Polyphenols (Quercetin, Rutin and Catechin) Obtained by Various Polymerization Methods. Chem. Biodivers. 2019, 16, e1900426. [Google Scholar] [CrossRef]
- Hashemi Gahruie, H.; Niakousari, M. Antioxidant, Antimicrobial, Cell Viability and Enzymatic Inhibitory of Antioxidant Polymers as Biological Macromolecules. Int. J. Biol. Macromol. 2017, 104, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.E.; Kurisawa, M.; Kim, Y.-J.; Uyama, H.; Kobayashi, S. Amplification of Antioxidant Activity of Catechin by Polycondensation with Acetaldehyde. Biomacromolecules 2004, 5, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Uyama, H.; Kobayashi, S. Inhibition Effects of (+)-Catechin–Aldehyde Polycondensates on Proteinases Causing Proteolytic Degradation of Extracellular Matrix. Biochem. Biophys. Res. Commun. 2004, 320, 256–261. [Google Scholar] [CrossRef]
- Kurisawa, M.; Chung, J.E.; Uyama, H.; Kobayashi, S. Enzymatic Synthesis and Antioxidant Properties of Poly(Rutin). Biomacromolecules 2003, 4, 1394–1399. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A.; Piotrowska, M. Polymeric Forms of Plant Flavonoids Obtained by Enzymatic Reactions. Molecules 2022, 27, 3702. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A.; Piotrowska, M. Novel Polymeric Biomaterial Based on Naringenin. Materials 2021, 14, 2142. [Google Scholar] [CrossRef]
- Desentis-Mendoza, R.M.; Hernández-Sánchez, H.; Moreno, A.; Rojas Del C., E.; Chel-Guerrero, L.; Tamariz, J.; Jaramillo-Flores, M.E. Enzymatic Polymerization of Phenolic Compounds Using Laccase and Tyrosinase from Ustilago m Aydis. Biomacromolecules 2006, 7, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Kurisawa, M.; Chung, J.E.; Uyama, H.; Kobayashi, S. Oxidative Coupling of Epigallocatechin Gallate Amplifies Antioxidant Activity and Inhibits Xanthine Oxidase Activity. Chem. Commun. 2004, 3, 294–295. [Google Scholar] [CrossRef]
- Falleh, H.; Ksouri, R.; Boulaaba, M.; Guyot, S.; Abdelly, C.; Magné, C. Phenolic Nature, Occurrence and Polymerization Degree as Marker of Environmental Adaptation in the Edible Halophyte Mesembryanthemum Edule. S. Afr. J. Bot. 2012, 79, 117–124. [Google Scholar] [CrossRef]
- Jerez, M.; Touriño, S.; Sineiro, J.; Torres, J.L.; Núñez, M.J. Procyanidins from Pine Bark: Relationships between Structure, Composition and Antiradical Activity. Food Chem. 2007, 104, 518–527. [Google Scholar] [CrossRef]
- Spranger, I.; Sun, B.; Mateus, A.M.; de Freitas, V.; Ricardo-da-Silva, J.M. Chemical Characterization and Antioxidant Activities of Oligomeric and Polymeric Procyanidin Fractions from Grape Seeds. Food Chem. 2008, 108, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-C.; Tam, N.F.; Lin, Y.-M.; Ding, Z.-H.; Chai, W.-M.; Wei, S.-D. Relationships between Degree of Polymerization and Antioxidant Activities: A Study on Proanthocyanidins from the Leaves of a Medicinal Mangrove Plant Ceriops Tagal. PLoS ONE 2014, 9, e107606. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Tao, F.; Hou, Y.; Lu, Y.; Zheng, T.; Sang, S.; Lv, L. Dual Effects of Propyl Gallate and Its Methylglyoxal Adduct on Carbonyl Stress and Oxidative Stress. Food Chem. 2018, 265, 227–232. [Google Scholar] [CrossRef]
- Shubina, V.S.; Kozina, V.I.; Shatalin, Y.V. Effect of Complex Formation by Taxifolin and Naringenin with Cu(i) Ions on the Distribution of the Components of Complexes in the Octanol–Water System. Russ. J. Bioorg. Chem. 2017, 43, 463–470. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Day, A.J.; Morgan, M.R.A. Experimental Determination of Octanol−Water Partition Coefficients of Quercetin and Related Flavonoids. J. Agric. Food Chem. 2005, 53, 4355–4360. [Google Scholar] [CrossRef]
- Wangkarn, S.; Grudpan, K.; Khanongnuch, C.; Pattananandecha, T.; Apichai, S.; Saenjum, C. Development of HPLC Method for Catechins and Related Compounds Determination and Standardization in Miang (Traditional Lanna Fermented Tea Leaf in Northern Thailand). Molecules 2021, 26, 6052. [Google Scholar] [CrossRef] [PubMed]
- Perrissoud, D.; Testa, B. Inhibiting or Potentiating Effects of Flavonoids on Carbon Tetrachloride-Induced Toxicity in Isolated Rat Hepatocytes. Arzneimittelforschung 1986, 36, 1249–1253. [Google Scholar]
- Ribeiro, D.; Freitas, M.; Tomé, S.M.; Silva, A.M.S.; Porto, G.; Fernandes, E. Modulation of Human Neutrophils’ Oxidative Burst by Flavonoids. Eur. J. Med. Chem. 2013, 67, 280–292. [Google Scholar] [CrossRef]
- Ribeiro, D.; Fernandes, E.; Freitas, M. Flavonoids as Modulators of Neutrophils’ Oxidative Burst: Structure-Activity Relationship. In Polyphenols: Mechanisms of Action in Human Health and Disease; Elsevier: London, UK, 2018; pp. 261–276. ISBN 978-0-12-813006-3. [Google Scholar]
- Saroni Arwa, P.; Zeraik, M.L.; Farias Ximenes, V.; Da Fonseca, L.M.; Da Silva Bolzani, V.; Siqueira Silva, D.H. Redox-Active Biflavonoids from Garcinia Brasiliensis as Inhibitors of Neutrophil Oxidative Burst and Human Erythrocyte Membrane Damage. J. Ethnopharmacol. 2015, 174, 410–418. [Google Scholar] [CrossRef]
- Czerwińska, M.E.; Dudek, M.K.; Pawłowska, K.A.; Pruś, A.; Ziaja, M.; Granica, S. The Influence of Procyanidins Isolated from Small-Leaved Lime Flowers (Tilia Cordata Mill.) on Human Neutrophils. Fitoterapia 2018, 127, 115–122. [Google Scholar] [CrossRef]
- Ciz, M.; Denev, P.; Kratchanova, M.; Vasicek, O.; Ambrozova, G.; Lojek, A. Flavonoids Inhibit the Respiratory Burst of Neutrophils in Mammals. Oxid. Med. Cell. Longev. 2012, 2012, 181295. [Google Scholar] [CrossRef]
- Vuotto, M.L.; Miranda, R.; Ritieni, A.; Basile, A.; Ricciardi, L.; Di Prisco, R.; Nicolosi, G.; Mascolo, N. Improvement of (+)-Catechin Inhibitory Activity on Human PMN Respiratory Burst by (+)-3-O-Propionyl and (-)-3-O-Valeryl Substitution. J. Pharm. Pharmacol. 2003, 55, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Drábiková, K.; Perečko, T.; Nosál’, R.; Harmatha, J.; Šmidrkal, J.; Jančinová, V. Study of Possible Mechanisms Involved in the Inhibitory Effects of Coumarin Derivatives on Neutrophil Activity. Oxid. Med. Cell. Longev. 2013, 2013, 136570. [Google Scholar] [CrossRef] [PubMed]
- Jančinová, V.; Perečko, T.; Nosáľ, R.; Košťálová, D.; Bauerová, K.; Drábiková, K. Decreased Activity of Neutrophils in the Presence of Diferuloylmethane (Curcumin) Involves Protein Kinase C Inhibition. Eur. J. Pharmacol. 2009, 612, 161–166. [Google Scholar] [CrossRef]
- Genaro-Mattos, T.C.; Maurício, Â.Q.; Rettori, D.; Alonso, A.; Hermes-Lima, M. Antioxidant Activity of Caffeic Acid against Iron-Induced Free Radical Generation—A Chemical Approach. PLoS ONE 2015, 10, e0129963. [Google Scholar] [CrossRef]
- Jomova, K.; Hudecova, L.; Lauro, P.; Simunková, M.; Barbierikova, Z.; Malcek, M.; Alwasel, S.H.; Alhazza, I.M.; Rhodes, C.J.; Valko, M. The Effect of Luteolin on DNA Damage Mediated by a Copper Catalyzed Fenton Reaction. J. Inorg. Biochem. 2022, 226, 111635. [Google Scholar] [CrossRef]
- Jomova, K.; Lawson, M.; Drostinova, L.; Lauro, P.; Poprac, P.; Brezova, V.; Michalik, M.; Lukes, V.; Valko, M. Protective Role of Quercetin against Copper(II)-Induced Oxidative Stress: A Spectroscopic, Theoretical and DNA Damage Study. Food Chem. Toxicol. 2017, 110, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Cvik, M.; Lauro, P.; Valko, M.; Cizmar, E.; Alomar, S.Y.; Alwasel, S.H.; Oleksak, P.; Chrienova, Z.; Nepovimova, E.; et al. The Role of Redox Active Copper(II) on Antioxidant Properties of the Flavonoid Baicalein: DNA Protection under Cu(II)-Fenton Reaction and Cu(II)-Ascorbate System Conditions. J. Inorg. Biochem. 2023, 245, 112244. [Google Scholar] [CrossRef]
- Lomozová, Z.; Hrubša, M.; Conte, P.F.; Papastefanaki, E.; Moravcová, M.; Catapano, M.C.; Proietti Silvestri, I.; Karlíčková, J.; Kučera, R.; Macáková, K.; et al. The Effect of Flavonoids on the Reduction of Cupric Ions, the Copper-Driven Fenton Reaction and Copper-Triggered Haemolysis. Food Chem. 2022, 394, 133461. [Google Scholar] [CrossRef]
- Lomozová, Z.; Catapano, M.C.; Hrubša, M.; Karlíčková, J.; Macáková, K.; Kučera, R.; Mladěnka, P. Chelation of Iron and Copper by Quercetin B-Ring Methyl Metabolites, Isorhamnetin and Tamarixetin, and Their Effect on Metal-Based Fenton Chemistry. J. Agric. Food Chem. 2021, 69, 5926–5937. [Google Scholar] [CrossRef]
- Salama, S.A.; Kabel, A.M. Taxifolin Ameliorates Iron Overload-Induced Hepatocellular Injury: Modulating PI3K/AKT and P38 MAPK Signaling, Inflammatory Response, and Hepatocellular Regeneration. Chem.-Biol. Interact. 2020, 330, 109230. [Google Scholar] [CrossRef]
- Nosáľ, R.; Drábiková, K.; Jančinová, V.; Mačičková, T.; Pečivová, J.; Perečko, T.; Harmatha, J. Pharmacological Intervention with Oxidative Burst in Human Neutrophils. Interdiscip. Toxicol. 2017, 10, 56–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nosáľ, R.; Drábiková, K.; Jančinová, V.; Perečko, T.; Ambrožová, G.; Číž, M.; Lojek, A.; Pekarová, M.; Šmidrkal, J.; Harmatha, J. On the Molecular Pharmacology of Resveratrol on Oxidative Burst Inhibition in Professional Phagocytes. Oxid. Med. Cell. Longev. 2014, 2014, 706269. [Google Scholar] [CrossRef] [PubMed]
- Takemura, O.S.; Banno, Y.; Nozawa, Y. Inhibition of N-Formylmethionyl-Leucylphenylalanine-Stimulated Tyrosine Phosphorylation and Phospholipase d Activation by Quercetin in Rabbit Neutrophils. Biochem. Pharmacol. 1997, 53, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-P.; Chang, L.-C.; Hsu, M.-F.; Lin, C.-N. The Blockade of Formyl Peptide-Induced Respiratory Burst by 2’,5’-Dihydroxy-2-Furfurylchalcone Involves Phospholipase D Signaling in Neutrophils. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2003, 368, 166–174. [Google Scholar] [CrossRef]
- Issuree, P.D.A.; Pushparaj, P.N.; Pervaiz, S.; Melendez, A.J. Resveratrol Attenuates C5a-induced Inflammatory Responses in vitro and in vivo by Inhibiting Phospholipase D and Sphingosine Kinase Activities. FASEB J. 2009, 23, 2412–2424. [Google Scholar] [CrossRef]
- Spalteholz, H.; Furtmüller, P.G.; Jakopitsch, C.; Obinger, C.; Schewe, T.; Sies, H.; Arnhold, J. Kinetic Evidence for Rapid Oxidation of (–)-Epicatechin by Human Myeloperoxidase. Biochem. Biophys. Res. Commun. 2008, 371, 810–813. [Google Scholar] [CrossRef]
- Steffen, Y.; Gruber, C.; Schewe, T.; Sies, H. Mono-O-Methylated Flavanols and Other Flavonoids as Inhibitors of Endothelial NADPH Oxidase. Arch. Biochem. Biophys. 2008, 469, 209–219. [Google Scholar] [CrossRef]
- Lu, N.; Sui, Y.; Tian, R.; Peng, Y.-Y. Inhibitive Effects of Quercetin on Myeloperoxidase-Dependent Hypochlorous Acid Formation and Vascular Endothelial Injury. J. Agric. Food Chem. 2018, 66, 4933–4940. [Google Scholar] [CrossRef]
- Tian, R.; Jin, Z.; Zhou, L.; Zeng, X.-P.; Lu, N. Quercetin Attenuated Myeloperoxidase-Dependent HOCl Generation and Endothelial Dysfunction in Diabetic Vasculature. J. Agric. Food Chem. 2021, 69, 404–413. [Google Scholar] [CrossRef]
- Nocella, C.; D’Amico, A.; Cammisotto, V.; Bartimoccia, S.; Castellani, V.; Loffredo, L.; Marini, L.; Ferrara, G.; Testa, M.; Motta, G.; et al. Structure, Activation, and Regulation of NOX2: At the Crossroad between the Innate Immunity and Oxidative Stress-Mediated Pathologies. Antioxidants 2023, 12, 429. [Google Scholar] [CrossRef]
- Qi, S.; Feng, Z.; Li, Q.; Qi, Z.; Zhang, Y. Myricitrin Modulates NADPH Oxidase-Dependent ROS Production to Inhibit Endotoxin-Mediated Inflammation by Blocking the JAK/STAT1 and NOX2/P47 phox Pathways. Oxid. Med. Cell. Longev. 2017, 2017, 9738745. [Google Scholar] [CrossRef]
- Stefanska, J.; Pawliczak, R. Apocynin: Molecular Aptitudes. Mediat. Inflamm. 2008, 2008, 106507. [Google Scholar] [CrossRef] [PubMed]
- Altenhöfer, S.; Radermacher, K.A.; Kleikers, P.W.M.; Wingler, K.; Schmidt, H.H.H.W. Evolution of NADPH Oxidase Inhibitors: Selectivity and Mechanisms for Target Engagement. Antioxid. Redox Signal. 2015, 23, 406–427. [Google Scholar] [CrossRef]
- Stolk, J.; Hiltermann, T.J.; Dijkman, J.H.; Verhoeven, A.J. Characteristics of the Inhibition of NADPH Oxidase Activation in Neutrophils by Apocynin, a Methoxy-Substituted Catechol. Am. J. Respir. Cell Mol. Biol. 1994, 11, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, V.F.; Kanegae, M.P.P.; Rissato, S.R.; Galhiane, M.S. The Oxidation of Apocynin Catalyzed by Myeloperoxidase: Proposal for NADPH Oxidase Inhibition. Arch. Biochem. Biophys. 2007, 457, 134–141. [Google Scholar] [CrossRef]
- Johnson, D.K.; Schillinger, K.J.; Kwait, D.M.; Hughes, C.V.; McNamara, E.J.; Ishmael, F.; O’Donnell, R.W.; Chang, M.-M.; Hogg, M.G.; Dordick, J.S.; et al. Inhibition of NADPH Oxidase Activation in Endothelial Cells by Ortho -Methoxy-Substituted Catechols. Endothelium 2002, 9, 191–203. [Google Scholar] [CrossRef]
- Santos, W.H.D.; Yoguim, M.I.; Daré, R.G.; Da Silva-Filho, L.C.; Lautenschlager, S.O.S.; Ximenes, V.F. Development of a Caffeic Acid–Phthalimide Hybrid Compound for NADPH Oxidase Inhibition. RSC Adv. 2021, 11, 17880–17890. [Google Scholar] [CrossRef] [PubMed]
- Shiba, Y.; Kinoshita, T.; Chuman, H.; Taketani, Y.; Takeda, E.; Kato, Y.; Naito, M.; Kawabata, K.; Ishisaka, A.; Terao, J.; et al. Flavonoids as Substrates and Inhibitors of Myeloperoxidase: Molecular Actions of Aglycone and Metabolites. Chem. Res. Toxicol. 2008, 21, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Gau, J.; Furtmüller, P.G.; Obinger, C.; Prévost, M.; Van Antwerpen, P.; Arnhold, J.; Flemmig, J. Flavonoids as Promoters of the (Pseudo-)Halogenating Activity of Lactoperoxidase and Myeloperoxidase. Free Radic. Biol. Med. 2016, 97, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Ding, Y.; Peng, Y.-Y.; Lu, N. Inhibition of Myeloperoxidase- and Neutrophil-Mediated Hypochlorous Acid Formation in vitro and Endothelial Cell Injury by (−)-Epigallocatechin Gallate. J. Agric. Food Chem. 2017, 65, 3198–3203. [Google Scholar] [CrossRef]
- Yeoh, B.S.; Aguilera Olvera, R.; Singh, V.; Xiao, X.; Kennett, M.J.; Joe, B.; Lambert, J.D.; Vijay-Kumar, M. Epigallocatechin-3-Gallate Inhibition of Myeloperoxidase and Its Counter-Regulation by Dietary Iron and Lipocalin 2 in Murine Model of Gut Inflammation. Am. J. Pathol. 2016, 186, 912–926. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Lewandowska, U. Polyphenols and the Potential Mechanisms of Their Therapeutic Benefits against Inflammatory Bowel Diseases. J. Funct. Foods 2022, 95, 105181. [Google Scholar] [CrossRef]
- Rufino, A.T.; Freitas, M.; Proença, C.; Ferreira De Oliveira, J.M.P.; Fernandes, E.; Ribeiro, D. Rheumatoid Arthritis Molecular Targets and Their Importance to Flavonoid-based Therapy. Med. Res. Rev. 2023, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Dias, R.; Pereira, C.B.; Pérez-Gregorio, R.; Mateus, N.; Freitas, V. Recent Advances on Dietary Polyphenol’s Potential Roles in Celiac Disease. Trends Food Sci. Technol. 2021, 107, 213–225. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Beloborodova, N.; Bairamov, I.; Olenin, A.; Shubina, V.; Teplova, V.; Fedotcheva, N. Effect of Phenolic Acids of Microbial Origin on Production of Reactive Oxygen Species in Mitochondria and Neutrophils. J. Biomed. Sci. 2012, 19, 89. [Google Scholar] [CrossRef]

| Polyphenol | IC50, μM 1 | |
|---|---|---|
| Isoluminol-HRP-H2O2 | Luminol-HRP-H2O2 [53] | |
| DfTf | 0.44 ± 0.04 * | 0.48 ± 0.04 * |
| eriodictyol | 0.57 ± 0.04 | 0.73 ± 0.05 |
| quercetin | 0.60 ± 0.02 | 0.75 ± 0.05 |
| taxifolin | 0.64 ± 0.02 | 0.76 ± 0.05 |
| catechin | 0.66 ± 0.05 | 0.93 ± 0.09 |
| hesperetin | 1.19 ± 0.05 * | 2.17 ± 0.15 * |
| naringenin | 18.68 ± 2.67 * | 34.2 ± 4.32 * |
| Polyphenol | IC50, μM 1 |
|---|---|
| eriodictyol | 0.59 ± 0.06 |
| quercetin | 0.39 ± 0.03 # |
| taxifolin | 0.51 ± 0.08 |
| catechin | 0.49 ± 0.07 |
| hesperetin | 0.94 ± 0.03 * |
| naringenin | 26.6 ± 4.54 * |
| Polyphenol | IC50, μM 1 |
|---|---|
| DfTf | 1.70 ± 0.21 |
| eriodictyol | 1.74 ± 0.25 |
| quercetin | 1.43 ± 0.10 |
| taxifolin | 1.77 ± 0.20 |
| catechin | 0.84 ± 0.27 |
| hesperetin | 1.23 ± 0.14 |
| naringenin | 14.3 ± 2.17 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shubina, V.S.; Kozina, V.I.; Shatalin, Y.V. A Comparative Study of the Inhibitory Effect of Some Flavonoids and a Conjugate of Taxifolin with Glyoxylic Acid on the Oxidative Burst of Neutrophils. Int. J. Mol. Sci. 2023, 24, 15068. https://doi.org/10.3390/ijms242015068
Shubina VS, Kozina VI, Shatalin YV. A Comparative Study of the Inhibitory Effect of Some Flavonoids and a Conjugate of Taxifolin with Glyoxylic Acid on the Oxidative Burst of Neutrophils. International Journal of Molecular Sciences. 2023; 24(20):15068. https://doi.org/10.3390/ijms242015068
Chicago/Turabian StyleShubina, Victoria S., Victoria I. Kozina, and Yuri V. Shatalin. 2023. "A Comparative Study of the Inhibitory Effect of Some Flavonoids and a Conjugate of Taxifolin with Glyoxylic Acid on the Oxidative Burst of Neutrophils" International Journal of Molecular Sciences 24, no. 20: 15068. https://doi.org/10.3390/ijms242015068
APA StyleShubina, V. S., Kozina, V. I., & Shatalin, Y. V. (2023). A Comparative Study of the Inhibitory Effect of Some Flavonoids and a Conjugate of Taxifolin with Glyoxylic Acid on the Oxidative Burst of Neutrophils. International Journal of Molecular Sciences, 24(20), 15068. https://doi.org/10.3390/ijms242015068