Pharmacohistory of Cannabis Use—A New Possibility in Future Drug Development for Gastrointestinal Diseases
Abstract
1. Introduction
2. History of Cannabis Use
3. Ethnomedicinal Uses of Cannabis in Gastrointestinal Disorders
4. Parts of Cannabis Plant Utilised in Traditional Medicines
5. Chemical Profile of Cannabis
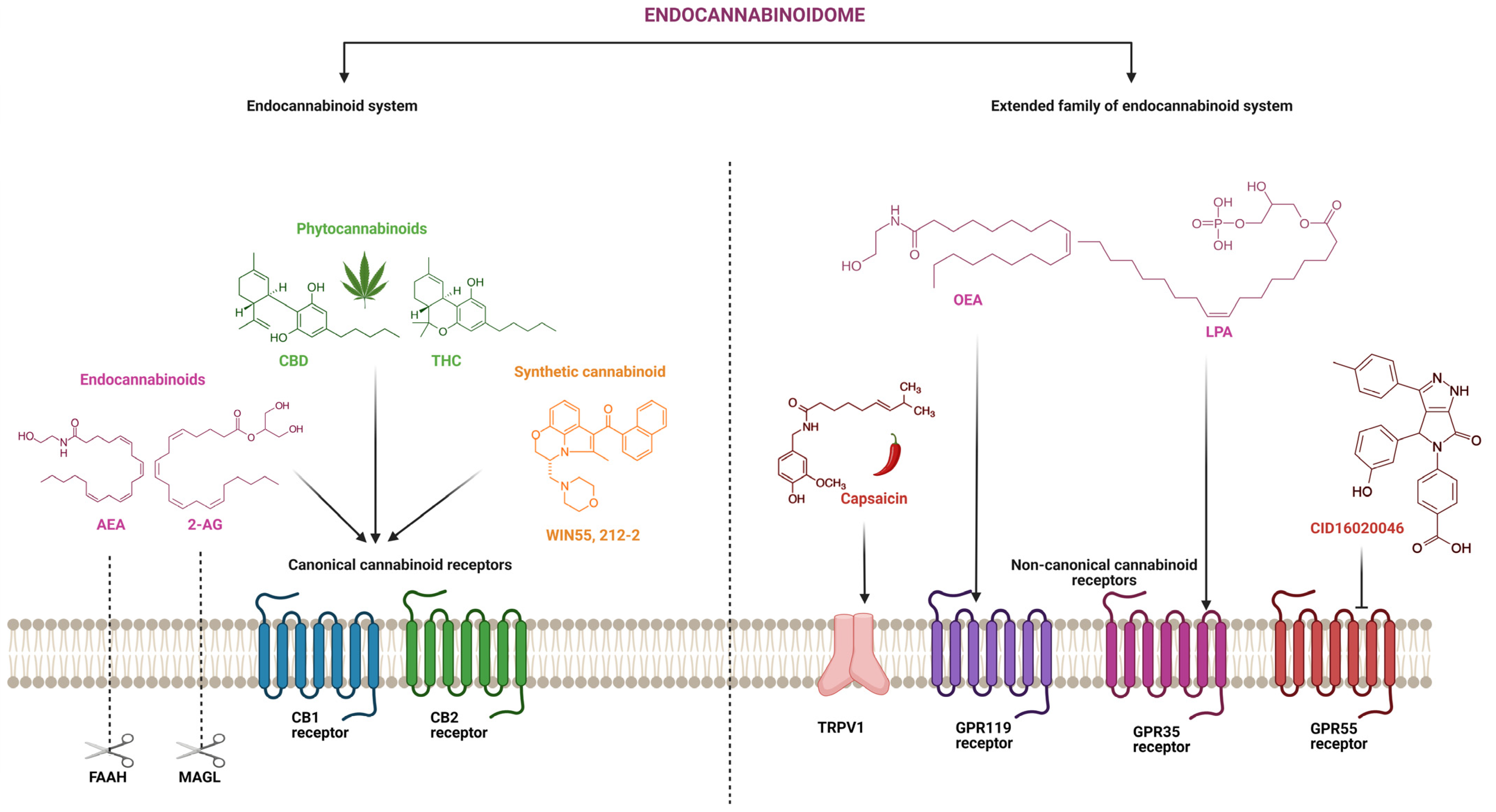
6. The Expanded Endocannabinoid System or the Endocannabinoidome
7. The Endocannabinoidome and Inflammatory Bowel Disease
8. Role of the Expanded Family of ECS in IBD
9. Clinically Available Cannabinoids-Based Drugs
10. Clinical Trials Investigating the Use of Cannabis and Cannabinoids in GI Disorders
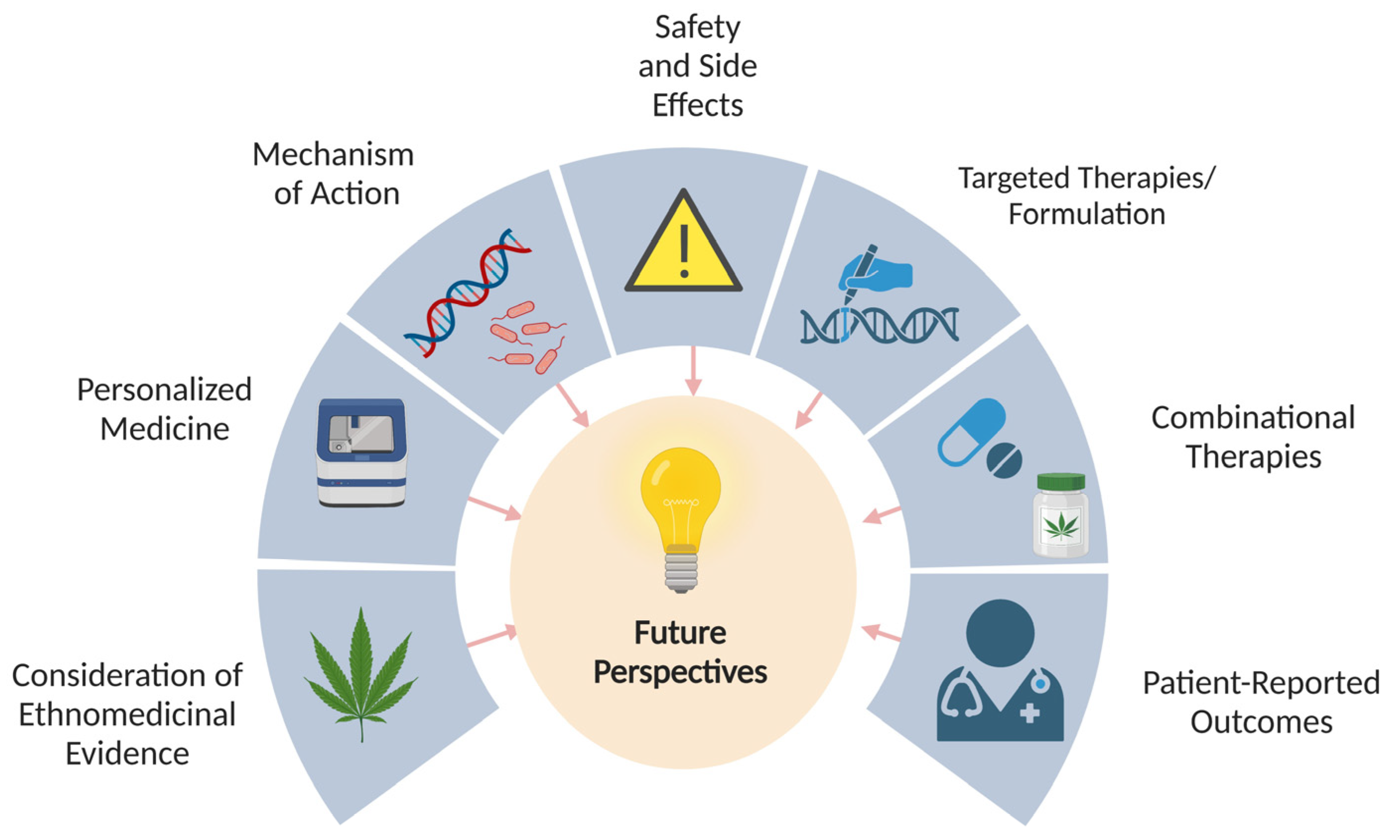
11. Conclusions and Future Perspectives
11.1. Consideration of Ethnomedicinal Evidence
11.2. Personalized Medicine
11.3. Further Understanding the Mechanisms of Action
11.4. Safety and Side-Effects
11.5. Targeted Therapies Exploring the Effects of Different Cannabinoids
11.6. Studying the Effects of Cannabinoids in Combination with Other Treatments
11.7. Patient-Reported Outcomes
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katz, L.; Baltz, R.H. Natural Product Discovery: Past, Present, and Future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Thomford, N.; Senthebane, D.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural Products for the Treatment of Type 2 Diabetes Mellitus: Pharmacology and Mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Song, L.; Yang, Z.; Qiu, M.; Wang, J.; Shi, S. Anthocyanins: Promising Natural Products with Diverse Pharmacological Activities. Molecules 2021, 26, 3807. [Google Scholar] [CrossRef]
- Veeresham, C. Natural Products Derived from Plants as a Source of Drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Balant, M.; Gras, A.; Gálvez, F.; Garnatje, T.; Vallès, J.; Vitales, D. CANNUSE, a Database of Traditional Cannabis Uses—An Opportunity for New Research. Database 2021, 2021, baab024. [Google Scholar] [CrossRef]
- Pisanti, S.; Bifulco, M. Medical Cannabis: A Plurimillennial History of an Evergreen. J. Cell. Physiol. 2019, 234, 8342–8351. [Google Scholar] [CrossRef]
- Wedman-St Louis, B. (Ed.) Cannabis as Medicine, 1st ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Oxford, UK, 2019; A CRC title, part of the Taylor & Francis imprint, a member of the Taylor & Francis Group, the academic division of T&F Informa plc; ISBN 978-0-429-05472-3. [Google Scholar]
- Russo, E. Cannabis in India: Ancient Lore and Modern Medicine. In Cannabinoids as Therapeutics; Mechoulam, R., Ed.; Milestones in Drug Therapy MDT; Birkhäuser: Basel, Switzerland, 2005; pp. 1–22. ISBN 978-3-7643-7055-8. [Google Scholar]
- Russo, E.B. Cannabis and Epilepsy: An Ancient Treatment Returns to the Fore. Epilepsy Behav. 2017, 70, 292–297. [Google Scholar] [CrossRef]
- Touw, M. The Religious and Medicinal Uses of Cannabis in China, India and Tibet. J. Psychoact. Drugs 1981, 13, 23–34. [Google Scholar] [CrossRef]
- Acharya, S.L.; Howard, J.; Panta, S.B.; Mahatma, S.S.; Copeland, J. Cannabis, Lord Shiva and Holy Men: Cannabis Use Among Sadhus in Nepal. J. Psychiatr. Assoc. Nepal 2015, 3, 9–14. [Google Scholar] [CrossRef]
- Godlaski, T.M. Shiva, Lord of Bhang. Subst. Use Misuse 2012, 47, 1067–1072. [Google Scholar] [CrossRef]
- Rubin, V. (Ed.) Cannabis and Culture; De Gruyter Mouton: Berlin, Germany, 1975; ISBN 978-90-279-7669-7. [Google Scholar]
- Tripp, D.A.; Nickel, J.C.; Katz, L.; Krsmanovic, A.; Ware, M.A.; Santor, D. A Survey of Cannabis (Marijuana) Use and Self-Reported Benefit in Men with Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Can. Urol. Assoc. J. 2014, 8, 901. [Google Scholar] [CrossRef]
- Jose, A.; Thomas, L.; Baburaj, G.; Munisamy, M.; Rao, M. Cannabinoids as an Alternative Option for Conventional Analgesics in Cancer Pain Management: A Pharmacogenomics Perspective. Indian J. Palliat. Care 2020, 26, 129. [Google Scholar] [CrossRef]
- Goyal, H.; Singla, U.; Gupta, U.; May, E. Role of Cannabis in Digestive Disorders. Eur. J. Gastroenterol. Hepatol. 2017, 29, 135–143. [Google Scholar] [CrossRef]
- Smith, L.A.; Azariah, F.; Lavender, V.T.; Stoner, N.S.; Bettiol, S. Cannabinoids for Nausea and Vomiting in Adults with Cancer Receiving Chemotherapy. Cochrane Database Syst. Rev. 2015, 2021, CD009464. [Google Scholar] [CrossRef]
- Crocq, M.-A. History of Cannabis and the Endocannabinoid System. Dialogues Clin. Neurosci. 2020, 22, 223–228. [Google Scholar] [CrossRef]
- Abel, E.L. Cannabis in the Ancient World. In Marihuana; Springer: Boston, MA, USA, 1980; pp. 3–35. ISBN 978-1-4899-2191-8. [Google Scholar]
- Maida, V.; Shi, R.B.; Fazzari, F.G.T.; Zomparelli, L. Topical Cannabis-based Medicines—A Novel Adjuvant Treatment for Venous Leg Ulcers: An Open-label Trial. Exp. Dermatol. 2021, 30, 1258–1267. [Google Scholar] [CrossRef]
- Zheng, Z.; Qi, J.; Hu, L.; Ouyang, D.; Wang, H.; Sun, Q.; Lin, L.; You, L.; Tang, B. A Cannabidiol-Containing Alginate Based Hydrogel as Novel Multifunctional Wound Dressing for Promoting Wound Healing. Biomater. Adv. 2022, 134, 112560. [Google Scholar] [CrossRef]
- Hall, W. The Indian Hemp Drugs Commission 1893–1894. Addiction 2019, 114, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Kalant, O.J. Report of the Indian Hemp Drugs Commission, 1893–1894: A Critical Review. Int. J. Addict. 1972, 7, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.; Chaudhary, R.P.; Taylor, R.S. Ethnomedicinal Plants Used by the People of Manang District, Central Nepal. J. Ethnobiol. Ethnomed. 2006, 2, 41. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.R.; Joshi, K. Indigenous Knowledge and Uses of Medicinal Plants by Local Communities of the Kali Gandaki Watershed Area, Nepal. J. Ethnopharmacol. 2000, 73, 175–183. [Google Scholar] [CrossRef]
- Balami, N.P. Ethnomedicinal Uses of Plants among the Newar Community of Pharping Village of Kathmandu District, Nepal. Tribhuvan Univ. J. 1970, 24, 13–19. [Google Scholar] [CrossRef]
- Bhattarai, S.; Chaudhary, R.P.; Taylor, R.S. Ethno-Medicinal Plants Used by the People of Nawalparasi District, Central Nepal. Our Nat. 1970, 7, 82–99. [Google Scholar] [CrossRef]
- Malla, B.; Gauchan, D.P.; Chhetri, R.B. Medico-Ethnobotanical Investigations in Parbat District of Western Nepal. J. Med. Plants Res. 2014, 8, 95–108. [Google Scholar] [CrossRef]
- Pradhan, S.P.; Chaudhary, R.P.; Sidgel, S.; Pandey, B.P. Ethnobotanical Knowledge of Khandadevi and Gokulganga Rural Municipality of Ramechhap District of Nepal. Ethnobot. Res. Appl. 2020, 20, 1–32. [Google Scholar] [CrossRef]
- Kunwar, R.M.; Nepal, B.K.; Kshhetri, H.B.; Rai, S.K.; Bussmann, R.W. Ethnomedicine in Himalaya: A Case Study from Dolpa, Humla, Jumla and Mustang Districts of Nepal. J. Ethnobiol. Ethnomed. 2006, 2, 27. [Google Scholar] [CrossRef]
- Rokaya, M.B.; Münzbergová, Z.; Timsina, B. Ethnobotanical Study of Medicinal Plants from the Humla District of Western Nepal. J. Ethnopharmacol. 2010, 130, 485–504. [Google Scholar] [CrossRef]
- Singh, A.G.; Panthi, M.P.; Tewari, D.D. Ethno Medicinal Plants Used by the Tharu and Magar Communities of Rupandehi District, Western Nepal. Curr. Bot. 2011, 2, 30–33. [Google Scholar]
- Badola, H.K.; Pradhan, B.K. Ethnomedicinal Plant Use by Lepcha Tribe of Dzongu Valley, Bordering Khangchendzonga Biosphere Reserve, in North Sikkim, India. J. Ethnobiol. Ethnomed. 2008, 4, 22. [Google Scholar]
- Bonn-Miller, M.O.; ElSohly, M.A.; Loflin, M.J.E.; Chandra, S.; Vandrey, R. Cannabis and Cannabinoid Drug Development: Evaluating Botanical versus Single Molecule Approaches. Int. Rev. Psychiatry 2018, 30, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Samant, S.S.; Arya, S.C. Diversity and Indigenous Household Remedies of the Inhabitants Surrounding Mornaula Reserve Forest in West Himalaya. Indian J. Tradit. Knowl. 2009, 8, 606–610. [Google Scholar]
- Saha, J.; Sarkar, P.K.; Chattopadhay, S. A Survey of Ethnomedicinal Plants of Darjeeling Hills for Their Antimicrobial and Antioxidant Activities. Indian J. Nat. Prod. Resour. 2011, 2, 479–492. [Google Scholar]
- Lee, S.; Xiao, C.; Pei, S. Ethnobotanical Survey of Medicinal Plants at Periodic Markets of Honghe Prefecture in Yunnan Province, SW China. J. Ethnopharmacol. 2008, 117, 362–377. [Google Scholar] [CrossRef]
- Zhong, L.L.D.; Zheng, G.; Da Ge, L.; Lin, C.Y.; Huang, T.; Zhao, L.; Lu, C.; Lu, A.P.; Bian, Z.X. Chinese Herbal Medicine for Constipation: Zheng-Based Associations among Herbs, Formulae, Proprietary Medicines, and Herb–Drug Interactions. Chin. Med. 2016, 11, 28. [Google Scholar] [CrossRef]
- Abbasi, A.M.; Ajab Khan, M.; Ahmed, M.; Zafar, M. Herbal Medicines Used to Cure Various Ailments by the Inhabitants of Abbottabad District, North West Frontier Province, Pakistan. Indian J. Tradit. Knowl. 2010, 9, 175–183. [Google Scholar]
- Abouri, M.; Mousadik, A.E.; Msanda, F.; Boubaker, H.; Saadi, B.; Cherifi, K. An Ethnobotanical Survey of Medicinal Plants Used in the Tata Province, Morocco. Int. J. Med. Plant Res. 2012, 1, 99–123. [Google Scholar]
- Afolayan, A.; Mbaebie, B. Ethnobotanical Study of Medicinal Plants Used as Anti-Obesity Remedies in Nkonkobe Municipality of South Africa. Pharmacogn. J. 2010, 2, 368–373. [Google Scholar] [CrossRef]
- Miano, R.S.; Picardal, J.P.; Alonso, C.A.G.; Reuyan, D. Ethnobotanical Inventory and Assessment of Medically-Important Plant Roots in Cebu Island, Philippines. Asian J. Biodivers. 2011, 2, 81–102. [Google Scholar] [CrossRef]
- Lazzarotto Rebelatto, E.R.; Rauber, G.S.; Caon, T. An Update of Nano-Based Drug Delivery Systems for Cannabinoids: Biopharmaceutical Aspects & Therapeutic Applications. Int. J. Pharm. 2023, 635, 122727. [Google Scholar] [CrossRef] [PubMed]
- Martínez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodríguez, S.A.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation Process: From Encapsulation to Drug Delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Neslihan Gursoy, R.; Benita, S. Self-Emulsifying Drug Delivery Systems (SEDDS) for Improved Oral Delivery of Lipophilic Drugs. Biomed. Pharmacother. 2004, 58, 173–182. [Google Scholar] [CrossRef]
- Nakano, Y.; Tajima, M.; Sugiyama, E.; Sato, V.H.; Sato, H. Development of a Novel Nano-emulsion Formulation to Improve Intestinal Absorption of Cannabidiol. Med. Cannabis Cannabinoids 2019, 2, 35–42. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Singh Chauhan, B.P.; Sharma, V. Challenges of Cannabinoid Delivery: How Can Nanomedicine Help? Nanomedicine 2020, 15, 2023–2028. [Google Scholar] [CrossRef]
- Zgair, A.; Wong, J.C.; Lee, J.B.; Mistry, J.; Sivak, O.; Wasan, K.M.; Hennig, I.M.; Barrett, D.A.; Constantinescu, C.S.; Fischer, P.M.; et al. Dietary Fats and Pharmaceutical Lipid Excipients Increase Systemic Exposure to Orally Administered Cannabis and Cannabis-Based Medicines. Am. J. Transl. Res. 2016, 8, 3448–3459. [Google Scholar]
- Patti, F.; Messina, S.; Solaro, C.; Amato, M.P.; Bergamaschi, R.; Bonavita, S.; Bossio, R.B.; Morra, V.B.; Costantino, G.F.; Cavalla, P.; et al. Efficacy and Safety of Cannabinoid Oromucosal Spray for Multiple Sclerosis Spasticity. J. Neurol. Neurosurg. Psychiatry 2016, 87, 944–951. [Google Scholar] [CrossRef]
- Atsmon, J.; Cherniakov, I.; Izgelov, D.; Hoffman, A.; Domb, A.J.; Deutsch, L.; Deutsch, F.; Heffetz, D.; Sacks, H. PTL401, a New Formulation Based on Pro-Nano Dispersion Technology, Improves Oral Cannabinoids Bioavailability in Healthy Volunteers. J. Pharm. Sci. 2018, 107, 1423–1429. [Google Scholar] [CrossRef]
- Huestis, M.A. Human Cannabinoid Pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef]
- Baron, E.P. Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science. Headache J. Head Face Pain 2018, 58, 1139–1186. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential Cannabis Synergy and Phytocannabinoid-Terpenoid Entourage Effects: Phytocannabinoid-Terpenoid Entourage Effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Atakan, Z. Cannabis, a Complex Plant: Different Compounds and Different Effects on Individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Reggio, P.H.; Jagerovic, N. An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol. Front. Pharmacol. 2017, 8, 422. [Google Scholar] [CrossRef] [PubMed]
- Biala, G. Cannabinoid dependence in animal models. Postep. Hig. Med. Dosw. Online 2008, 62, 258–262. [Google Scholar]
- Compton, D.R.; Dewey, W.L.; Martin, B.R. Cannabis Dependence and Tolerance Production. Adv. Alcohol Subst. Abuse 1990, 9, 129–147. [Google Scholar] [CrossRef]
- Gonzalez, S.; Cebeira, M.; Fernandez-Ruiz, J. Cannabinoid Tolerance and Dependence: A Review of Studies in Laboratory Animals. Pharmacol. Biochem. Behav. 2005, 81, 300–318. [Google Scholar] [CrossRef]
- Bhamra, S.K.; Desai, A.; Imani-Berendjestanki, P.; Horgan, M. The Emerging Role of Cannabidiol (CBD) Products; a Survey Exploring the Public’s Use and Perceptions of CBD. Phytother. Res. 2021, 35, 5734–5740. [Google Scholar] [CrossRef]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. In Medicinal Plants; Joshee, N., Dhekney, S.A., Parajuli, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 333–359. ISBN 978-3-030-31268-8. [Google Scholar]
- Downer, E.J. Anti-Inflammatory Potential of Terpenes Present in Cannabis sativa L. ACS Chem. Neurosci. 2020, 11, 659–662. [Google Scholar] [CrossRef]
- Weston-Green, K.; Clunas, H.; Jimenez Naranjo, C. A Review of the Potential Use of Pinene and Linalool as Terpene-Based Medicines for Brain Health: Discovering Novel Therapeutics in the Flavours and Fragrances of Cannabis. Front. Psychiatry 2021, 12, 583211. [Google Scholar] [CrossRef] [PubMed]
- Save, M.; Hellaye, M.L.; De Villedon, V.; Adoumaz, I.; Pillet, M.; Atanase, L.; Lahcini, M.; Deniau, E.; Khoukh, A.; Pellerin, V.; et al. Biosourced Polymeric Emulsifiers for Miniemulsion Copolymerization of Myrcene and Styrene: Toward Biobased Waterborne Latex as Pickering Emulsion Stabilizer. Biomacromolecules 2022, 23, 2536–2551. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Mackie, K. Endocannabinoid Receptor Pharmacology. Curr. Top. Behav. Neurosci. 2009, 1, 37–63. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Kaur, R.; Ambwani, S.R.; Singh, S. Endocannabinoid System: A Multi-Facet Therapeutic Target. Curr. Clin. Pharmacol. 2016, 11, 110–117. [Google Scholar] [CrossRef]
- Stamer, W.D.; Golightly, S.F.; Hosohata, Y.; Ryan, E.P.; Porter, A.C.; Varga, E.; Noecker, R.J.; Felder, C.C.; Yamamura, H.I. Cannabinoid CB1 Receptor Expression, Activation and Detection of Endogenous Ligand in Trabecular Meshwork and Ciliary Process Tissues. Eur. J. Pharmacol. 2001, 431, 277–286. [Google Scholar] [CrossRef]
- Croxford, J.L.; Yamamura, T. Cannabinoids and the Immune System: Potential for the Treatment of Inflammatory Diseases? J. Neuroimmunol. 2005, 166, 3–18. [Google Scholar] [CrossRef]
- Atwood, B.K.; Mackie, K. CB2: A Cannabinoid Receptor with an Identity Crisis. Br. J. Pharmacol. 2010, 160, 467–479. [Google Scholar] [CrossRef]
- Hauser, A.S.; Chavali, S.; Masuho, I.; Jahn, L.J.; Martemyanov, K.A.; Gloriam, D.E.; Babu, M.M. Pharmacogenomics of GPCR Drug Targets. Cell 2018, 172, 41–54.e19. [Google Scholar] [CrossRef]
- Sriram, K.; Insel, P.A. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol. Pharmacol. 2018, 93, 251–258. [Google Scholar] [CrossRef]
- Veilleux, A.; Di Marzo, V.; Silvestri, C. The Expanded Endocannabinoid System/Endocannabinoidome as a Potential Target for Treating Diabetes Mellitus. Curr. Diab. Rep. 2019, 19, 117. [Google Scholar] [CrossRef] [PubMed]
- M’Koma, A.E. Inflammatory Bowel Disease: An Expanding Global Health Problem. Clin. Med. Insights Gastroenterol. 2013, 6, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet Lond. Engl. 2018, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Fousekis, F.S.; Katsanos, A.H.; Kourtis, G.; Saridi, M.; Albani, E.; Katsanos, K.H.; Christodoulou, D.K. Inflammatory Bowel Disease and Patients with Mental Disorders: What Do We Know? J. Clin. Med. Res. 2021, 13, 466–473. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Y.; He, L.; Sun, J.; Wang, X.; Li, H. Inflammatory Bowel Disease and Risk of Dementia: An Updated Meta-Analysis. Front. Aging Neurosci. 2022, 14, 962681. [Google Scholar] [CrossRef]
- Al-Bawardy, B.; Shivashankar, R.; Proctor, D.D. Novel and Emerging Therapies for Inflammatory Bowel Disease. Front. Pharmacol. 2021, 12, 651415. [Google Scholar] [CrossRef]
- Lin, S.C.; Cheifetz, A.S. The Use of Complementary and Alternative Medicine in Patients with Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2018, 14, 415–425. [Google Scholar]
- Greuter, T.; Rieder, F.; Kucharzik, T.; Peyrin-Biroulet, L.; Schoepfer, A.M.; Rubin, D.T.; Vavricka, S.R. Emerging Treatment Options for Extraintestinal Manifestations in IBD. Gut 2021, 70, 796–802. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- Gravina, A.G.; Pellegrino, R.; Zingone, F. Editorial: Challenges in Inflammatory Bowel Disease: Current, Future and Unmet Needs. Front. Med. 2022, 9, 979535. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Naganuma, M.; Sugimoto, S.; Nanki, K.; Mizuno, S.; Mutaguchi, M.; Nakazato, Y.; Inoue, N.; Ogata, H.; Iwao, Y.; et al. The Risk Factor of Clinical Relapse in Ulcerative Colitis Patients with Low Dose 5-Aminosalicylic Acid as Maintenance Therapy: A Report from the IBD Registry. PLoS ONE 2017, 12, e0187737. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.L.; Kane, S.V. Treatment Nonadherence in Inflammatory Bowel Disease: Identification, Scope, and Management Strategies. Inflamm. Bowel Dis. 2015, 21, 2979–2984. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lal, S.; Prasad, N.; Ryan, M.; Tangri, S.; Silverberg, M.S.; Gordon, A.; Steinhart, H. Cannabis Use amongst Patients with Inflammatory Bowel Disease. Eur. J. Gastroenterol. Hepatol. 2011, 23, 891–896. [Google Scholar] [CrossRef]
- Pi, S.; Rosenfeld, G.; Enns, R.; Bressler, B.; Wong, A.; Enns, C.; MacDonnell, C.; Leung, Y. Patterns and Motivations of Cannabis Use amongst Patients with Inflammatory Bowel Disease. GastroHep 2019, 1, 100–107. [Google Scholar] [CrossRef]
- Ahmed, W.; Katz, S. Therapeutic Use of Cannabis in Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2016, 12, 668–679. [Google Scholar]
- Leinwand, K.L.; Gerich, M.E.; Hoffenberg, E.J.; Collins, C.B. Manipulation of the Endocannabinoid System in Colitis: A Comprehensive Review. Inflamm. Bowel Dis. 2017, 23, 192–199. [Google Scholar] [CrossRef]
- Szabady, R.L.; Louissaint, C.; Lubben, A.; Xie, B.; Reeksting, S.; Tuohy, C.; Demma, Z.; Foley, S.E.; Faherty, C.S.; Llanos-Chea, A.; et al. Intestinal P-Glycoprotein Exports Endocannabinoids to Prevent Inflammation and Maintain Homeostasis. J. Clin. Investig. 2018, 128, 4044–4056. [Google Scholar] [CrossRef]
- Marquéz, L.; Suárez, J.; Iglesias, M.; Bermudez-Silva, F.J.; Rodríguez de Fonseca, F.; Andreu, M. Ulcerative Colitis Induces Changes on the Expression of the Endocannabinoid System in the Human Colonic Tissue. PLoS ONE 2009, 4, e6893. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Arbabi, E.; Atyabi, F.; Dinarvand, R. Application of Carbon Nanotubes as the Carriers of the Cannabinoid, 2-Arachidonoylglycerol: Towards a Novel Treatment Strategy in Colitis. Life Sci. 2017, 179, 66–72. [Google Scholar] [CrossRef]
- Alhouayek, M.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D.; Muccioli, G.G. Increasing Endogenous 2-arachidonoylglycerol Levels Counteracts Colitis and Related Systemic Inflammation. FASEB J. 2011, 25, 2711–2721. [Google Scholar] [CrossRef] [PubMed]
- Massa, F.; Marsicano, G.; Hermann, H.; Cannich, A.; Monory, K.; Cravatt, B.F.; Ferri, G.-L.; Sibaev, A.; Storr, M.; Lutz, B. The Endogenous Cannabinoid System Protects against Colonic Inflammation. J. Clin. Investig. 2004, 113, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Alhouayek, M.; Muccioli, G.G. COX-2-Derived Endocannabinoid Metabolites as Novel Inflammatory Mediators. Trends Pharmacol. Sci. 2014, 35, 284–292. [Google Scholar] [CrossRef]
- Grim, T.W.; Ghosh, S.; Hsu, K.-L.; Cravatt, B.F.; Kinsey, S.G.; Lichtman, A.H. Combined Inhibition of FAAH and COX Produces Enhanced Anti-Allodynic Effects in Mouse Neuropathic and Inflammatory Pain Models. Pharmacol. Biochem. Behav. 2014, 124, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Sasso, O.; Migliore, M.; Habrant, D.; Armirotti, A.; Albani, C.; Summa, M.; Moreno-Sanz, G.; Scarpelli, R.; Piomelli, D. Multitarget Fatty Acid Amide Hydrolase/Cyclooxygenase Blockade Suppresses Intestinal Inflammation and Protects against Nonsteroidal Anti-inflammatory Drug-dependent Gastrointestinal Damage. FASEB J. 2015, 29, 2616–2627. [Google Scholar] [CrossRef]
- Lima, P.A.; Berg, B.B.; Castor, M.G.M.E. Involvement of the Cannabinoid System in Chronic Inflammatory Intestinal Diseases: Opportunities for New Therapies. Intest. Res. 2022, 20, 392–417. [Google Scholar] [CrossRef]
- Lian, J.; Casari, I.; Falasca, M. Modulatory Role of the Endocannabinoidome in the Pathophysiology of the Gastrointestinal Tract. Pharmacol. Res. 2022, 175, 106025. [Google Scholar] [CrossRef]
- Ambrose, T.; Simmons, A. Cannabis, Cannabinoids, and the Endocannabinoid System—Is There Therapeutic Potential for Inflammatory Bowel Disease? J. Crohns Colitis 2019, 13, 525–535. [Google Scholar] [CrossRef]
- Bento, A.F.; Marcon, R.; Dutra, R.C.; Claudino, R.F.; Cola, M.; Pereira Leite, D.F.; Calixto, J.B. β-Caryophyllene Inhibits Dextran Sulfate Sodium-Induced Colitis in Mice through CB2 Receptor Activation and PPARγ Pathway. Am. J. Pathol. 2011, 178, 1153–1166. [Google Scholar] [CrossRef]
- De Filippis, D.; Esposito, G.; Cirillo, C.; Cipriano, M.; De Winter, B.Y.; Scuderi, C.; Sarnelli, G.; Cuomo, R.; Steardo, L.; De Man, J.G.; et al. Cannabidiol Reduces Intestinal Inflammation through the Control of Neuroimmune Axis. PLoS ONE 2011, 6, e28159. [Google Scholar] [CrossRef]
- Jamontt, J.; Molleman, A.; Pertwee, R.; Parsons, M. The Effects of Δ 9 -Tetrahydrocannabinol and Cannabidiol Alone and in Combination on Damage, Inflammation and in Vitro Motility Disturbances in Rat Colitis: Δ 9 -Tetrahydrocannabinol and Cannabidiol in Rat Colitis. Br. J. Pharmacol. 2010, 160, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Schicho, R.; Storr, M. Topical and Systemic Cannabidiol Improves Trinitrobenzene Sulfonic Acid Colitis in Mice. Pharmacology 2012, 89, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Pagano, E.; Capasso, R.; Piscitelli, F.; Romano, B.; Parisi, O.A.; Finizio, S.; Lauritano, A.; Marzo, V.D.; Izzo, A.A.; Borrelli, F. An Orally Active Cannabis Extract with High Content in Cannabidiol Attenuates Chemically-Induced Intestinal Inflammation and Hypermotility in the Mouse. Front. Pharmacol. 2016, 7, 341. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Fasolino, I.; Romano, B.; Capasso, R.; Maiello, F.; Coppola, D.; Orlando, P.; Battista, G.; Pagano, E.; Marzo, V.D.; et al. Beneficial Effect of the Non-Psychotropic Plant Cannabinoid Cannabigerol on Experimental Inflammatory Bowel Disease. Biochem. Pharmacol. 2013, 85, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Romano, B.; Borrelli, F.; Fasolino, I.; Capasso, R.; Piscitelli, F.; Cascio, M.; Pertwee, R.; Coppola, D.; Vassallo, L.; Orlando, P.; et al. The Cannabinoid TRPA1 Agonist Cannabichromene Inhibits Nitric Oxide Production in Macrophages and Ameliorates Murine Colitis: Cannabichromene, Macrophages and Colitis. Br. J. Pharmacol. 2013, 169, 213–229. [Google Scholar] [CrossRef]
- Wallace, J.L.; Flannigan, K.L.; McKnight, W.; Wang, L.; Ferraz, J.G.P.; Tuitt, D. Pro-Resolution, Protective and Anti-Nociceptive Effects of a Cannabis Extract in the Rat Gastrointestinal Tract. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2013, 64, 167–175. [Google Scholar]
- Becker, W.; Alrafas, H.R.; Wilson, K.; Miranda, K.; Culpepper, C.; Chatzistamou, I.; Cai, G.; Nagarkatti, M.; Nagarkatti, P.S. Activation of Cannabinoid Receptor 2 Prevents Colitis-Associated Colon Cancer through Myeloid Cell De-Activation Upstream of IL-22 Production. iScience 2020, 23, 101504. [Google Scholar] [CrossRef]
- Storr, M.A.; Keenan, C.M.; Zhang, H.; Patel, K.D.; Makriyannis, A.; Sharkey, K.A. Activation of the Cannabinoid 2 Receptor (CB2) Protects against Experimental Colitis. Inflamm. Bowel Dis. 2009, 15, 1678–1685. [Google Scholar] [CrossRef]
- Dunford, J.; Lee, A.T.; Morgan, M.M. Tetrahydrocannabinol (THC) Exacerbates Inflammatory Bowel Disease in Adolescent and Adult Female Rats. J. Pain 2021, 22, 1040–1047. [Google Scholar] [CrossRef]
- MacKenzie, A.E.; Lappin, J.E.; Taylor, D.L.; Nicklin, S.A.; Milligan, G. GPR35 as a Novel Therapeutic Target. Front. Endocrinol. 2011, 2, 68. [Google Scholar] [CrossRef]
- Farooq, S.M.; Hou, Y.; Li, H.; O’Meara, M.; Wang, Y.; Li, C.; Wang, J.-M. Disruption of GPR35 Exacerbates Dextran Sulfate Sodium-Induced Colitis in Mice. Dig. Dis. Sci. 2018, 63, 2910–2922. [Google Scholar] [CrossRef]
- Kaya, B.; Doñas, C.; Wuggenig, P.; Diaz, O.E.; Morales, R.A.; Melhem, H.; Hernández, P.P.; Kaymak, T.; Das, S.; Hruz, P.; et al. Lysophosphatidic Acid-Mediated GPR35 Signaling in CX3CR1+ Macrophages Regulates Intestinal Homeostasis. Cell Rep. 2020, 32, 107979. [Google Scholar] [CrossRef]
- Okumura, S.; Baba, H.; Kumada, T.; Nanmoku, K.; Nakajima, H.; Nakane, Y.; Hioki, K.; Ikenaka, K. Cloning of a G-Protein-Coupled Receptor That Shows an Activity to Transform NIH3T3 Cells and Is Expressed in Gastric Cancer Cells. Cancer Sci. 2004, 95, 131–135. [Google Scholar] [CrossRef]
- Wang, W.; Han, T.; Tong, W.; Zhao, J.; Qiu, X. Overexpression of GPR35 Confers Drug Resistance in NSCLC Cells by β-Arrestin/Akt Signaling. OncoTargets Ther. 2018, 11, 6249–6257. [Google Scholar] [CrossRef]
- Sawzdargo, M.; Nguyen, T.; Lee, D.K.; Lynch, K.R.; Cheng, R.; Heng, H.H.Q.; George, S.R.; O’Dowd, B.F. Identification and Cloning of Three Novel Human G Protein-Coupled Receptor Genes GPR52, ΨGPR53 and GPR55: GPR55 Is Extensively Expressed in Human Brain. Mol. Brain Res. 1999, 64, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, J.; Lehmann, C. GPR55—A Putative “Type 3” Cannabinoid Receptor in Inflammation. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.-O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The Orphan Receptor GPR55 Is a Novel Cannabinoid Receptor: GPR55, a Novel Cannabinoid Receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Schicho, R.; Storr, M. A Potential Role for GPR55 in Gastrointestinal Functions. Curr. Opin. Pharmacol. 2012, 12, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Fichna, J.; Schicho, R.; Saur, D.; Bashashati, M.; Mackie, K.; Li, Y.; Zimmer, A.; Göke, B.; Sharkey, K.A.; et al. A Role for O-1602 and G Protein-Coupled Receptor GPR55 in the Control of Colonic Motility in Mice. Neuropharmacology 2013, 71, 255–263. [Google Scholar] [CrossRef]
- Lin, X.-H.; Yuece, B.; Li, Y.-Y.; Feng, Y.-J.; Feng, J.-Y.; Yu, L.-Y.; Li, K.; Li, Y.-N.; Storr, M. A Novel CB Receptor GPR55 and Its Ligands Are Involved in Regulation of Gut Movement in Rodents: GPR55 and Its Ligands Regulate Gut Movement. Neurogastroenterol. Motil. 2011, 23, 862-e342. [Google Scholar] [CrossRef]
- Staton, P.C.; Hatcher, J.P.; Walker, D.J.; Morrison, A.D.; Shapland, E.M.; Hughes, J.P.; Chong, E.; Mander, P.K.; Green, P.J.; Billinton, A.; et al. The Putative Cannabinoid Receptor GPR55 Plays a Role in Mechanical Hyperalgesia Associated with Inflammatory and Neuropathic Pain. Pain 2008, 139, 225–236. [Google Scholar] [CrossRef]
- Stančić, A.; Jandl, K.; Hasenöhrl, C.; Reichmann, F.; Marsche, G.; Schuligoi, R.; Heinemann, A.; Storr, M.; Schicho, R. The GPR55 Antagonist CID16020046 Protects against Intestinal Inflammation. Neurogastroenterol. Motil. 2015, 27, 1432–1445. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Sobolewska-Włodarczyk, A.; Cygankiewicz, A.I.; Jacenik, D.; Krajewska, W.M.; Stec-Michalska, K.; Piechota-Polańczyk, A.; Wiśniewska-Jarosińska, M.; Fichna, J. G Protein-Coupled Receptor 55 (GPR55) Expresses Differently in Patients with Crohn’s Disease and Ulcerative Colitis. Scand. J. Gastroenterol. 2017, 52, 711–715. [Google Scholar] [CrossRef]
- Ono, T.; Yamashita, T.; Kano, R.; Inoue, M.; Okada, S.; Kano, K.; Koizumi, S.; Iwabuchi, K.; Hirabayashi, Y.; Matsuo, I.; et al. GPR55 Contributes to Neutrophil Recruitment and Mechanical Pain Induction after Spinal Cord Compression in Mice. Brain. Behav. Immun. 2023, 110, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Balenga, N.A.B.; Aflaki, E.; Kargl, J.; Platzer, W.; Schröder, R.; Blättermann, S.; Kostenis, E.; Brown, A.J.; Heinemann, A.; Waldhoer, M. GPR55 Regulates Cannabinoid 2 Receptor-Mediated Responses in Human Neutrophils. Cell Res. 2011, 21, 1452–1469. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Cairns, E.A.; Szczesniak, A.M.; Toguri, J.T.; Caldwell, M.D.; Kelly, M.E.M. The Cannabinoids Delta(8)THC, CBD, and HU-308 Act via Distinct Receptors to Reduce Corneal Pain and Inflammation. Cannabis Cannabinoid Res. 2018, 3, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Cairns, E.A.; Szczesniak, A.-M.; Kulkarni, P.M.; Straiker, A.J.; Thakur, G.A.; Kelly, M.E.M. Allosteric Cannabinoid Receptor 1 (CB1) Ligands Reduce Ocular Pain and Inflammation. Molecules 2020, 25, 417. [Google Scholar] [CrossRef]
- Quon, T.; Lin, L.-C.; Ganguly, A.; Tobin, A.B.; Milligan, G. Therapeutic Opportunities and Challenges in Targeting the Orphan G Protein-Coupled Receptor GPR35. ACS Pharmacol. Transl. Sci. 2020, 3, 801–812. [Google Scholar] [CrossRef]
- Yansen, Z.; Lingang, Z.; Dali, L.; Mingyao, L. Inflammatory Bowel Disease Susceptible Gene GPR35 Promotes Bowel Inflammation in Mice. Yi Chuan Hered. 2021, 43, 169–181. [Google Scholar] [CrossRef]
- Wong, B.S.; Camilleri, M.; Busciglio, I.; Carlson, P.; Szarka, L.A.; Burton, D.; Zinsmeister, A.R. Pharmacogenetic Trial of a Cannabinoid Agonist Shows Reduced Fasting Colonic Motility in Patients with Nonconstipated Irritable Bowel Syndrome. Gastroenterology 2011, 141, 1638–1647.e7. [Google Scholar] [CrossRef]
- Lahat, A.; Lang, A.; Ben-Horin, S. Impact of Cannabis Treatment on the Quality of Life, Weight and Clinical Disease Activity in Inflammatory Bowel Disease Patients: A Pilot Prospective Study. Digestion 2012, 85, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Naftali, T.; Bar-Lev Schleider, L.; Dotan, I.; Lansky, E.P.; Sklerovsky Benjaminov, F.; Konikoff, F.M. Cannabis Induces a Clinical Response in Patients with Crohn’s Disease: A Prospective Placebo-Controlled Study. Clin. Gastroenterol. Hepatol. 2013, 11, 1276–1280.e1. [Google Scholar] [CrossRef]
- Naftali, T.; Mechulam, R.; Marii, A.; Gabay, G.; Stein, A.; Bronshtain, M.; Laish, I.; Benjaminov, F.; Konikoff, F.M. Low-Dose Cannabidiol Is Safe but Not Effective in the Treatment for Crohn’s Disease, a Randomized Controlled Trial. Dig. Dis. Sci. 2017, 62, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Irving, P.M.; Iqbal, T.; Nwokolo, C.; Subramanian, S.; Bloom, S.; Prasad, N.; Hart, A.; Murray, C.; Lindsay, J.O.; Taylor, A.; et al. A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Pilot Study of Cannabidiol-Rich Botanical Extract in the Symptomatic Treatment of Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Naftali, T.; Bar-Lev Schleider, L.; Almog, S.; Meiri, D.; Konikoff, F.M. Oral CBD-Rich Cannabis Induces Clinical but Not Endoscopic Response in Patients with Crohn’s Disease, a Randomised Controlled Trial. J. Crohns Colitis 2021, 15, 1799–1806. [Google Scholar] [CrossRef]
- Naftali, T.; Bar-Lev Schleider, L.; Scklerovsky Benjaminov, F.; Konikoff, F.M.; Matalon, S.T.; Ringel, Y. Cannabis Is Associated with Clinical but Not Endoscopic Remission in Ulcerative Colitis: A Randomized Controlled Trial. PLoS ONE 2021, 16, e0246871. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.F.; Rissanen, A.M.; Scheen, A.J.; Ziegler, O.; Rössner, S. Effects of the Cannabinoid-1 Receptor Blocker Rimonabant on Weight Reduction and Cardiovascular Risk Factors in Overweight Patients: 1-Year Experience from the RIO-Europe Study. Lancet 2005, 365, 1389–1397. [Google Scholar] [CrossRef]
- Bird, S.M.; Bailey, R.A.; Grieve, A.P.; Senn, S. Statistical Issues in First-in-Human Studies on BIA 10-2474: Neglected Comparison of Protocol against Practice. Pharm. Stat. 2017, 16, 100–106. [Google Scholar] [CrossRef]
- Kaur, R.; Sidhu, P.; Singh, S. What Failed BIA 10–2474 Phase I Clinical Trial? Global Speculations and Recommendations for Future Phase I Trials. J. Pharmacol. Pharmacother. 2016, 7, 120–126. [Google Scholar] [CrossRef]


| GI Condition | Parts Used | Preparation/Formulation | Country | Reference |
|---|---|---|---|---|
| Abdominal cramps and pain | In, Se | A pinch of seed and/or flower powder taken orally with hot water | Nepal | [27] |
| Abdominal cramps and pain | L, Se, | Crushed dried flowers and seeds taken orally alone or with foods/water | Nepal | [28] |
| Abdominal pain Antidiarrheal/Dysentery | Se, whole | Paste made from raw seeds/oral | Nepal | [28,29] |
| Antidiarrheal | Se | Fried seed in cow ghee taken orally | Nepal | [30] |
| Antidiarrheal, dysentery | In, Se | Dried leaves/flowers with milk | Nepal | [31] |
| Antidiarrheal | L, whole plant | Leaves juice/seeds | Nepal | [32] |
| Gastritis | Se | Seed oil/Seed chutney | Nepal | [33] |
| Appetite stimulant | In, L, Se | Mixed juice/orally | India, Nepal | [34,35] |
| Severe Stomach pain and wound healing | Resin | Small quantity of Resin/charas/orally | India | [36,37,38] |
| Indigestion | Rt | About 1 cm root chewed after dinner for 5 days | India | [39] |
| Constipation | Se | Administered orally in the form of decoction | China | [40,41] |
| Stomach and liver inflammation | L | Fresh leaf extract with sugar taken orally once in the morning | Pakistan | [42] |
| Stomach Pain | In, L, St | Dried powder is smoked | Morocco | [43] |
| Weight Loss | L | Fresh leaves are crushed, soaked in water, and extract is mixed with vinegar | South Africa | [44] |
| Stomach Pain | Rt | Boiled roots taken orally | Philippines | [45] |
| Study/NCT Trial Number | Participants/Enrolment | Intervention | Results |
|---|---|---|---|
| NCT01253408 | 75 (IBS) | 2.5 mg or 5 mg dronabinol and placebo | Reduced colonic motility [135] |
| [136] | 13 (IBD) | Cannabis oil containing CBD and THC | Improvement in clinical activity index, body mass index (BMI), and quality of life |
| NCT01040910 | 21 (Crohn’s) | Cannabis cigarettes containing 115 mg of THC | Improvement in clinical activity index and improved quality of life [137] |
| NCT01037322 | 20 (Crohn’s) | Low-dose CBD (10 mg) or placebo orally twice a day | CBD found to be safe, but no beneficial effects compared to placebo [138] |
| NCT01562314 | 60 (UC) | One to five 50 mg CBD capsules taken twice a day | CBD-rich botanical extract found to be beneficial for symptomatic treatment of UC [139] |
| [140] | 56 (Crohn’s) | CBD-enriched cannabis oil or placebo | Significant improvement in clinical parameters and quality of life but no change in inflammatory and endoscopic parameters between groups [140] |
| NCT01040910 | 32 (UC) | Cannabis cigarettes (0.5 g of dried flowers with 80 mg of THC) or placebo | Clinical remission and improved quality of life but no association with anti-inflammatory markers [141] |
| NCT03467620 | 36 (Crohn’s) | 25 mg of CBD capsules/day for 12 weeks | Study withdrawn due to insufficient funds |
| NCT04056442 | 28 (Crohn’s) | Synthetic CBD up to 300 mg/day to measure the safety, tolerability, and efficacy in steroid-dependent Crohn’s disease | Ongoing/patients recruiting |
| NCT05578313 | 1000 (UC, Crohn’s, pouchitis) | Clinically prescribed medical cannabis or healthy patients | Ongoing/patients recruiting |
| NCT03886532 | Aimed to treat IBD, neuropathic pain | IBD patients taking medical cannabis or CBD legally | Study withdrawn due to insufficient funds |
| NCT03422861 | 80 (IBD) | Nabilone 1 mg BID orally or placebo | Not yet recruiting |
| NCT04055662 | 100 (effective IBD bowel surgery) | Study the post-operative analgesia requirement in recreational cannabis users vs. non-cannabis users and IBD patients | Results not published yet |
| NCT01826188 | 50 (CD) | THC 5mg/mL and CBD 50mg/mL in olive oil taken BID | Patients recruited |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa, D.; Warne, L.N.; Falasca, M. Pharmacohistory of Cannabis Use—A New Possibility in Future Drug Development for Gastrointestinal Diseases. Int. J. Mol. Sci. 2023, 24, 14677. https://doi.org/10.3390/ijms241914677
Thapa D, Warne LN, Falasca M. Pharmacohistory of Cannabis Use—A New Possibility in Future Drug Development for Gastrointestinal Diseases. International Journal of Molecular Sciences. 2023; 24(19):14677. https://doi.org/10.3390/ijms241914677
Chicago/Turabian StyleThapa, Dinesh, Leon N. Warne, and Marco Falasca. 2023. "Pharmacohistory of Cannabis Use—A New Possibility in Future Drug Development for Gastrointestinal Diseases" International Journal of Molecular Sciences 24, no. 19: 14677. https://doi.org/10.3390/ijms241914677
APA StyleThapa, D., Warne, L. N., & Falasca, M. (2023). Pharmacohistory of Cannabis Use—A New Possibility in Future Drug Development for Gastrointestinal Diseases. International Journal of Molecular Sciences, 24(19), 14677. https://doi.org/10.3390/ijms241914677








