Xanthones Isolated from Cratoxylum cochinchinensis Reduced Oxidative Stress in Periodontal Ligament Stem Cells
Abstract
:1. Introduction
2. Results
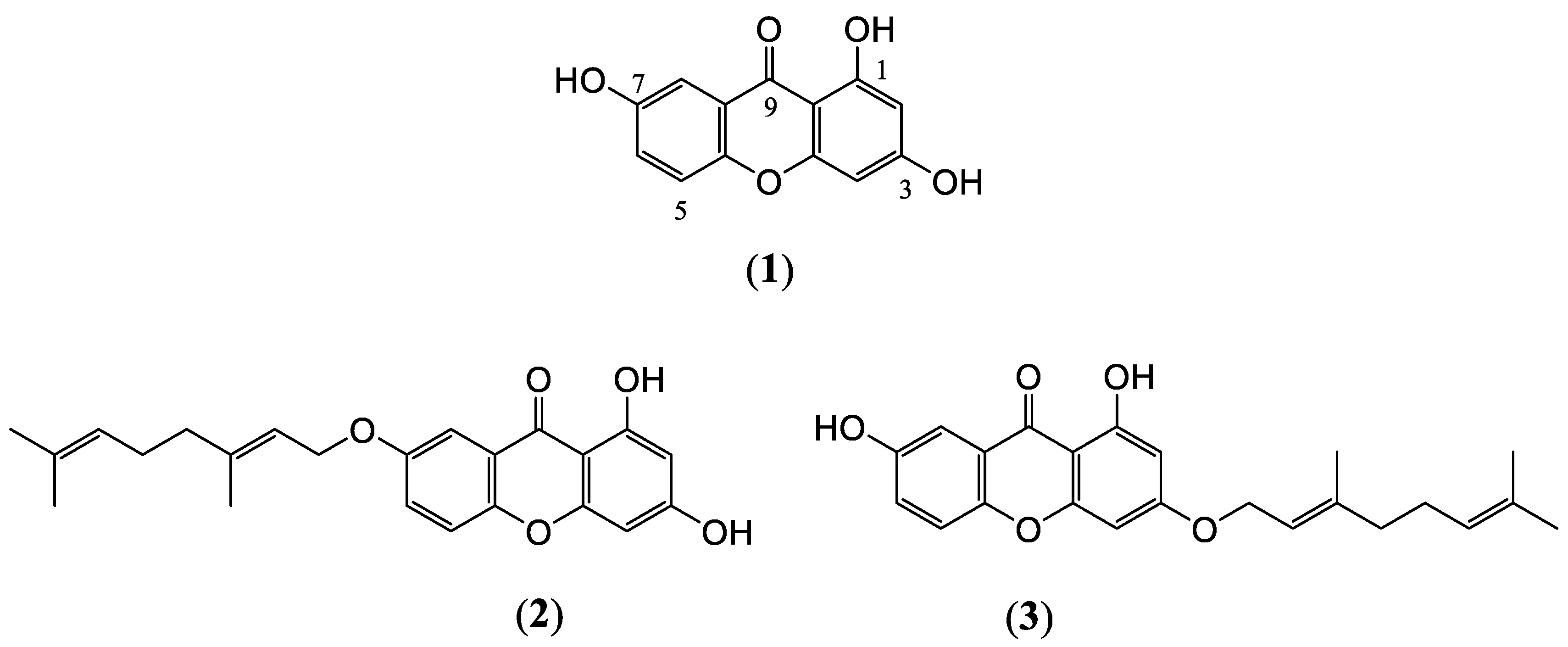
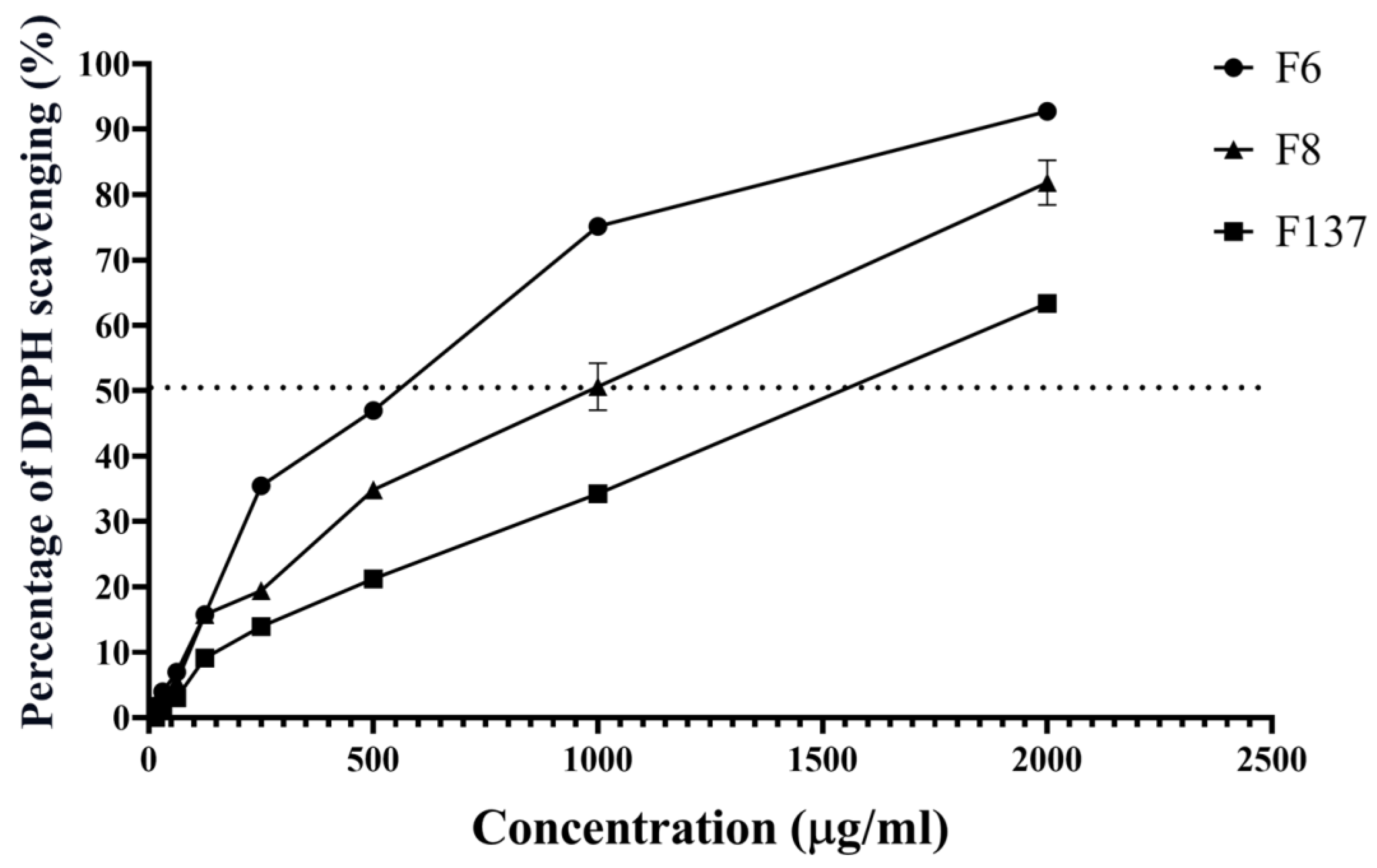
2.1. Antioxidant Activity of C. cochinchinensis Derivatives
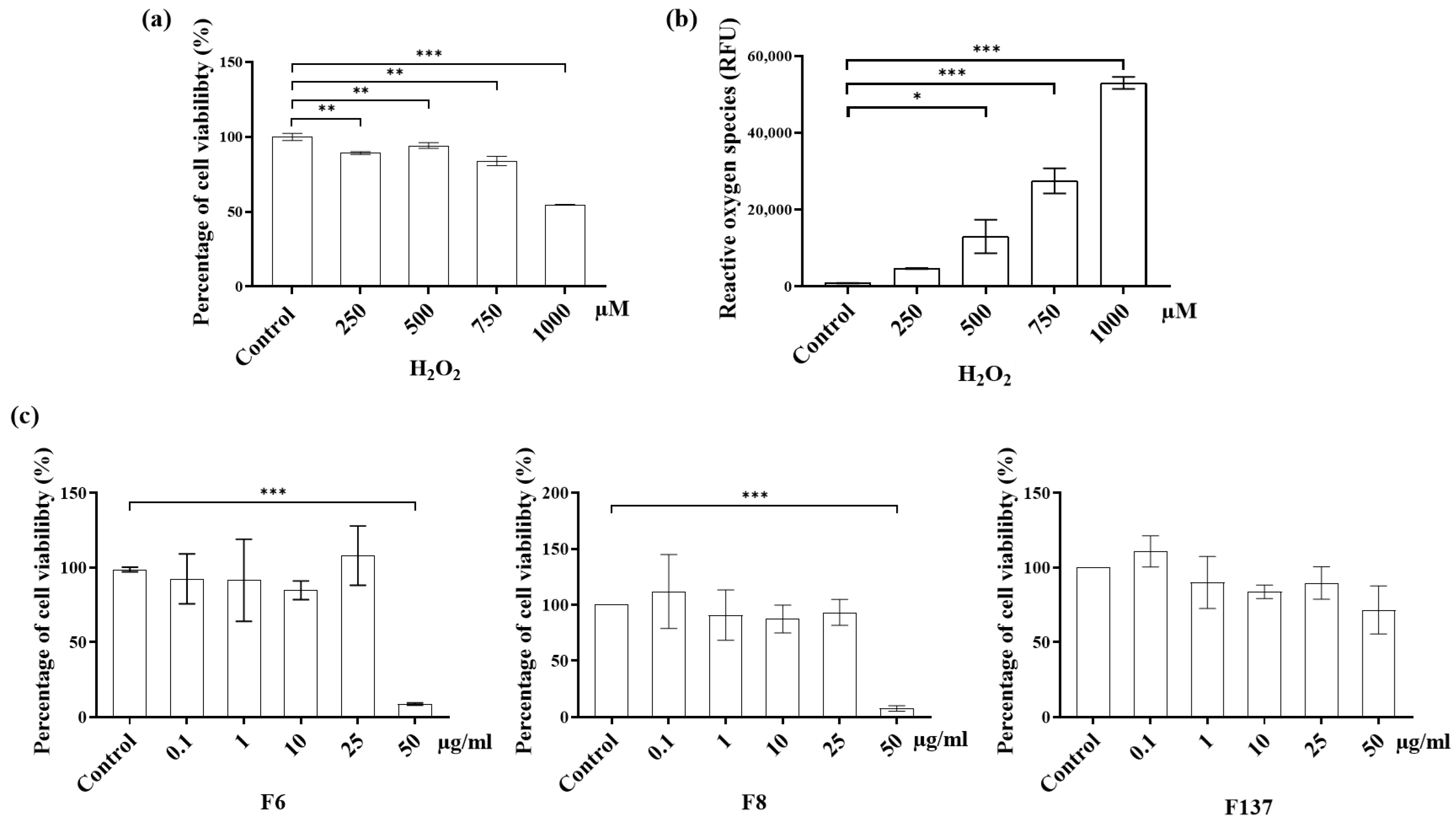
2.2. Cytotoxicity and ROS Production of C. cochinchinensis Derivatives in PDLSCs
2.3. Antioxidant Effects of C. cochinchinensis Derivatives
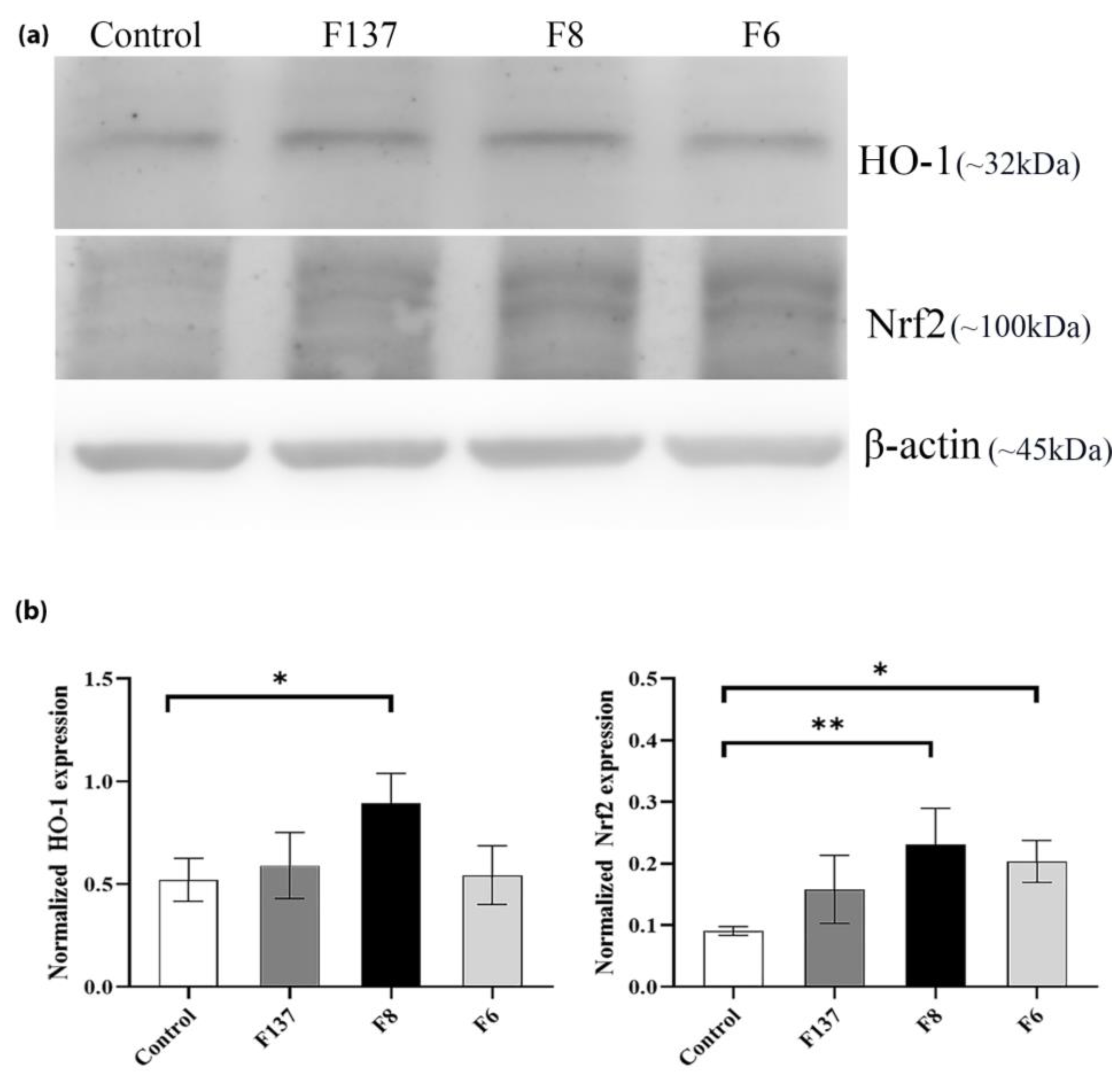
2.4. Effects of C. cochinchinensis Derivatives on HO-1 and Nrf2 Expression in PDLSCs
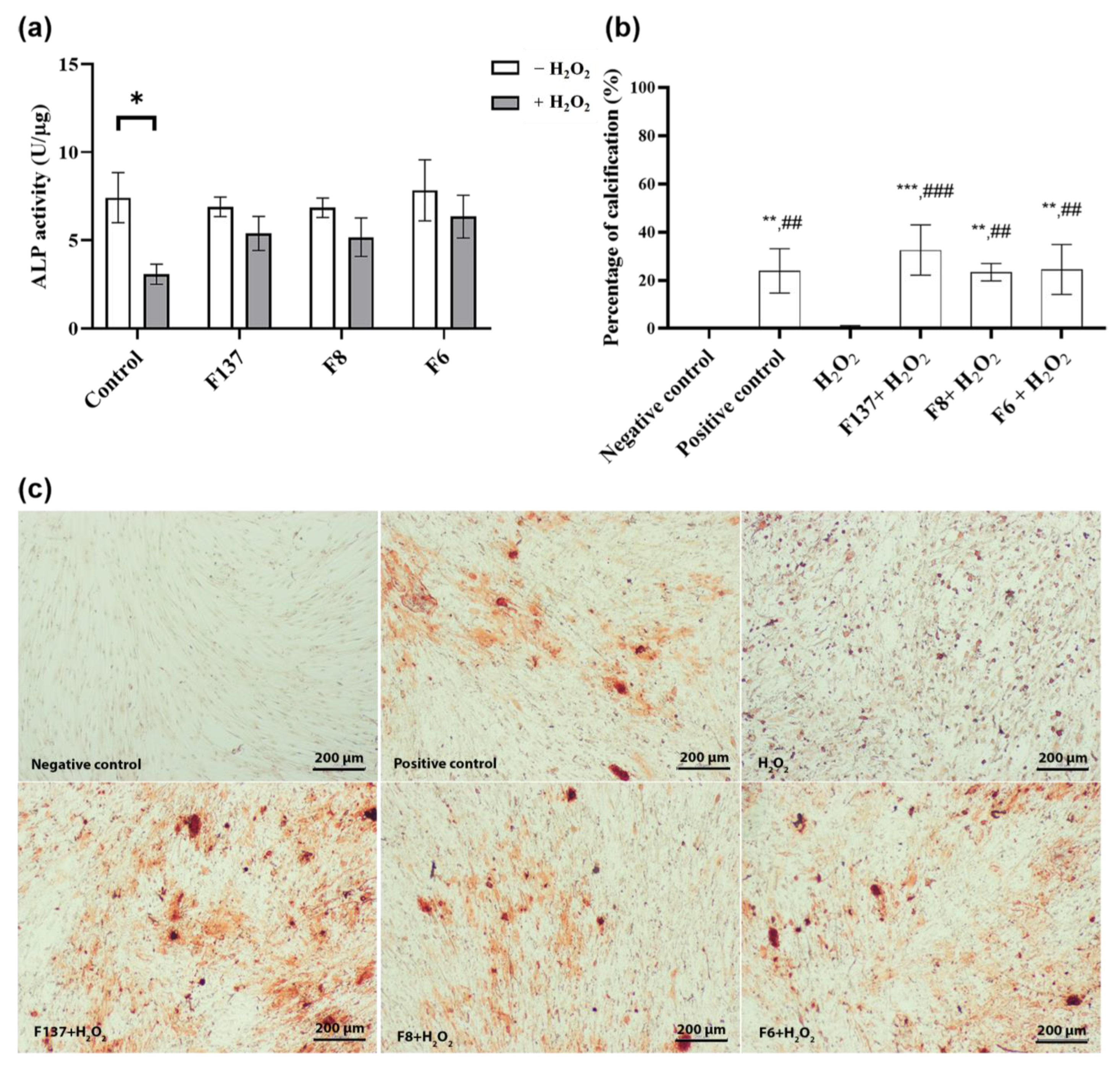
2.5. Effects of C. cochinchinensis Derivatives on PDLSCs Osteogenic Differentiation
3. Discussion
4. Materials and Methods
4.1. Extraction and Isolation of C. cochinchinensis Derivatives
4.2. Antioxidant Activity of C. cochinchinensis Derivatives
4.3. PDLSCs Isolation and Characterization
4.4. Cytotoxicity and ROS Production of C. cochinchinensis Derivatives in PDLSCs
4.5. Effects of Cratoxylum cochinchinensis Derivatives on Oxidative Stress
4.6. Western Blot Analysis of HO-1 and Nrf2 Expression in PDLSCs
4.7. Effects of C. cochinchinensis Derivatives on PDLSCs Osteogenic Differentiation
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kinane, D.; Bouchard, P.; Group E of the European Workshop on Periodontology. Periodontal diseases and health: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Fine, N.; Hassanpour, S.; Borenstein, A.; Sima, C.; Oveisi, M.; Scholey, J.; Cherney, D.; Glogauer, M. Distinct Oral Neutrophil Subsets Define Health and Periodontal Disease States. J. Dent. Res. 2016, 95, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, S.; Wang, J.; Jin, F. Periodontitis promotes the proliferation and suppresses the differentiation potential of human periodontal ligament stem cells. Int. J. Mol. Med. 2015, 36, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Shih, Y.R.; Kuo, T.K.; Lee, O.K.; Wei, Y.H. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem. Cells 2008, 26, 960–968. [Google Scholar] [CrossRef]
- Waddington, R.J.; Moseley, R.; Embery, G. Reactive oxygen species: A potential role in the pathogenesis of periodontal diseases. Oral Dis. 2000, 6, 138–151. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, J.Y.; Park, Y.D.; Kang, K.L.; Lee, J.C.; Heo, J.S. Hesperetin alleviates the inhibitory effects of high glucose on the osteoblastic differentiation of periodontal ligament stem cells. PLoS ONE 2013, 8, e67504. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chow, J.M.; Lin, C.W.; Wu, C.Y.; Shen, S.C. Baicalein inhibition of oxidative-stress-induced apoptosis via modulation of ERKs activation and induction of HO-1 gene expression in rat glioma cells C6. Toxicol Appl Pharmacol 2006, 216, 263–273. [Google Scholar] [CrossRef]
- Lv, H.; Yu, Z.; Zheng, Y.; Wang, L.; Qin, X.; Cheng, G.; Ci, X. Isovitexin Exerts Anti-Inflammatory and Anti-Oxidant Activities on Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting MAPK and NF-kappaB and Activating HO-1/Nrf2 Pathways. Int. J. Biol. Sci. 2016, 12, 72–86. [Google Scholar] [CrossRef]
- Smitinand, T. Thai Plant Names; Prachachon Publishing: Bangkok, Thailand, 2001. [Google Scholar]
- Kuvatanasuchati, J.; Laphookhieo, S.; Rodanant, P. Antimicrobial activity against periodontopathic bacteria and cytotoxic study of Cratoxylum formosum and Clausena lansium. J. Med. Plants Res. 2011, 5, 5988–5992. [Google Scholar]
- Rodanant, P.; Boonnak, N.; Surarit, R.; Kuvatanasuchati, J.; Lertsooksawat, W. Antibacterial, anti-inflammatory and anti-oxidatant activities of various isolated compounds from Cratoxylum species. Pak. J. Pharm. Sci. 2017, 30, 667–674. [Google Scholar]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer prevention: A review. J. Am. Diet. Assoc. 1996, 96, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.R.; Halim, L. Antioxidant and antiproliferative activities of common edible nut seeds. Lwt-Food Sci. Technol. 2009, 42, 1–8. [Google Scholar] [CrossRef]
- Suksamrarn, S.; Komutiban, O.; Ratananukul, P.; Chimnoi, N.; Lartpornmatulee, N.; Suksamrarn, A. Cytotoxic prenylated xanthones from the young fruit of Garcinia mangostana. Chem. Pharm. Bull. 2006, 54, 301–305. [Google Scholar] [CrossRef]
- Kumar, K.; Pawar, A.P.; Pavankumar, K.; Sivakumar, T. Synthesis and Investigation of Anti-microbial and Anti-inflammatory Activity of Substituted Flavones. J. Appl. Pharm. Sci. 2012, 2, 143–150. [Google Scholar]
- Kumar Singh, A.; Lohani, M.; Parthsarthy, R. Synthesis, characterization and anti-inflammatory activity of some 1, 3,4 -oxadiazole derivatives. Iran. J. Pharm. Res. IJPR 2013, 12, 319–323. [Google Scholar]
- Tatakis, D.N.; Kumar, P.S. Etiology and pathogenesis of periodontal diseases. Dent. Clin. N. Am. 2005, 49, 491–516. [Google Scholar] [CrossRef]
- Maisuthisakul, P.; Pongsawatmanit, R.; Gordon, M.H. Antioxidant properties of Teaw (Cratoxylum formosum Dyer) extract in soybean oil and emulsions. J. Agric. Food Chem. 2006, 54, 2719–2725. [Google Scholar] [CrossRef]
- Maisuthisakul, P.; Pongsawatmanit, R.; Gordon, M.H. Characterization of the phytochemicals and antioxidant properties of extracts from Teaw (Cratoxylum formosum Dyer). Food Chem. 2007, 100, 1620–1629. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Reprint of “Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays”. J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants-A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef]
- Apak, R.; Capanoglu, E.; Shahidi, F. Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Negi, J.S.; Bisht, V.K.; Singh, P.; Rawat, M.S.M.; Joshi, G.P. Naturally Occurring Xanthones: Chemistry and Biology. J. Appl. Chem. 2013, 2013, 621459. [Google Scholar] [CrossRef]
- Zeb, A. Phenolic Antioxidants in Foods: Chemistry, Biochemistry and Analysis; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar]
- Martínez, A.; Hernández-Marin, E.; Galano, A. Xanthones as antioxidants: A theoretical study on the thermodynamics and kinetics of the single electron transfer mechanism. Food Funct. 2012, 3, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Salman, Z.; Yuqing, J.; Bin, L.; Cai-yun, P.; Iqbal, C.M.; Atta-ur, R.; Wei, W. Antioxidant Nature Adds Further Therapeutic Value: An Updated Review on Natural Xanthones and Their Glycosides. Digit. Chin. Med. 2019, 2, 166–192. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Abdul-Muneer, P.M. Nrf2 as a Potential Therapeutic Target for Traumatic Brain Injury. J. Integr. Neurosci. 2023, 22, 81. [Google Scholar] [CrossRef] [PubMed]
- Ghareghomi, S.; Habibi-Rezaei, M.; Arese, M.; Saso, L.; Moosavi-Movahedi, A.A. Nrf2 Modulation in Breast Cancer. Biomedicines 2022, 10, 2668. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Marzioni, D.; Mazzucchelli, R. Cellular Modulators of the NRF2/KEAP1 Signaling Pathway in Prostate Cancer. Front. Biosci. 2023, 28, 143. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Montanari, E.; Marzioni, D.; Goteri, G. Role of NRF2 in Ovarian Cancer. Antioxidants 2022, 11, 663. [Google Scholar] [CrossRef]
- Brouard, S.; Otterbein, L.E.; Anrather, J.; Tobiasch, E.; Bach, F.H.; Choi, A.M.; Soares, M.P. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J. Exp. Med. 2000, 192, 1015–1026. [Google Scholar] [CrossRef]
- Choi, A.M.; Alam, J. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell Mol. Biol. 1996, 15, 9–19. [Google Scholar] [CrossRef]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D.; Tonegawa, S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc. Natl. Acad. Sci. USA 1997, 94, 10919–10924. [Google Scholar] [CrossRef] [PubMed]
- Yachie, A.; Niida, Y.; Wada, T.; Igarashi, N.; Kaneda, H.; Toma, T.; Ohta, K.; Kasahara, Y.; Koizumi, S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Investig. 1999, 103, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Harder, B.; Jiang, T.; Wu, T.; Tao, S.; Rojo de la Vega, M.; Tian, W.; Chapman, E.; Zhang, D.D. Molecular mechanisms of Nrf2 regulation and how these influence chemical modulation for disease intervention. Biochem. Soc. Trans. 2015, 43, 680–686. [Google Scholar] [CrossRef]
- Sima, C.; Aboodi, G.M.; Lakschevitz, F.S.; Sun, C.; Goldberg, M.B.; Glogauer, M. Nuclear Factor Erythroid 2-Related Factor 2 Down-Regulation in Oral Neutrophils Is Associated with Periodontal Oxidative Damage and Severe Chronic Periodontitis. Am. J. Pathol. 2016, 186, 1417–1426. [Google Scholar] [CrossRef]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar] [CrossRef]
- Thanasrisuebwong, P.; Kiattavorncharoen, S.; Surarit, R.; Phruksaniyom, C.; Ruangsawasdi, N. Red and Yellow Injectable Platelet-Rich Fibrin Demonstrated Differential Effects on Periodontal Ligament Stem Cell Proliferation, Migration, and Osteogenic Differentiation. Int. J. Mol. Sci. 2020, 21, 5153. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruangsawasdi, N.; Boonnak, N.; Pruksaniyom, C.; Rodanant, P. Xanthones Isolated from Cratoxylum cochinchinensis Reduced Oxidative Stress in Periodontal Ligament Stem Cells. Int. J. Mol. Sci. 2023, 24, 14675. https://doi.org/10.3390/ijms241914675
Ruangsawasdi N, Boonnak N, Pruksaniyom C, Rodanant P. Xanthones Isolated from Cratoxylum cochinchinensis Reduced Oxidative Stress in Periodontal Ligament Stem Cells. International Journal of Molecular Sciences. 2023; 24(19):14675. https://doi.org/10.3390/ijms241914675
Chicago/Turabian StyleRuangsawasdi, Nisarat, Nawong Boonnak, Chareerut Pruksaniyom, and Pirasut Rodanant. 2023. "Xanthones Isolated from Cratoxylum cochinchinensis Reduced Oxidative Stress in Periodontal Ligament Stem Cells" International Journal of Molecular Sciences 24, no. 19: 14675. https://doi.org/10.3390/ijms241914675
APA StyleRuangsawasdi, N., Boonnak, N., Pruksaniyom, C., & Rodanant, P. (2023). Xanthones Isolated from Cratoxylum cochinchinensis Reduced Oxidative Stress in Periodontal Ligament Stem Cells. International Journal of Molecular Sciences, 24(19), 14675. https://doi.org/10.3390/ijms241914675





