Cholinesterase Inhibitory and Anti-Inflammatory Activity of the Naphtho- and Thienobenzo-Triazole Photoproducts: Experimental and Computational Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of New Thienobenzo/Naphtho-Triazoles 1–13
2.2. Anti-Inflammatory Activity of 1,2,3-Triazole Derivatives 1–31
2.3. Cholinesterase Inhibition of 1,2,3-Triazole Derivatives 1–13
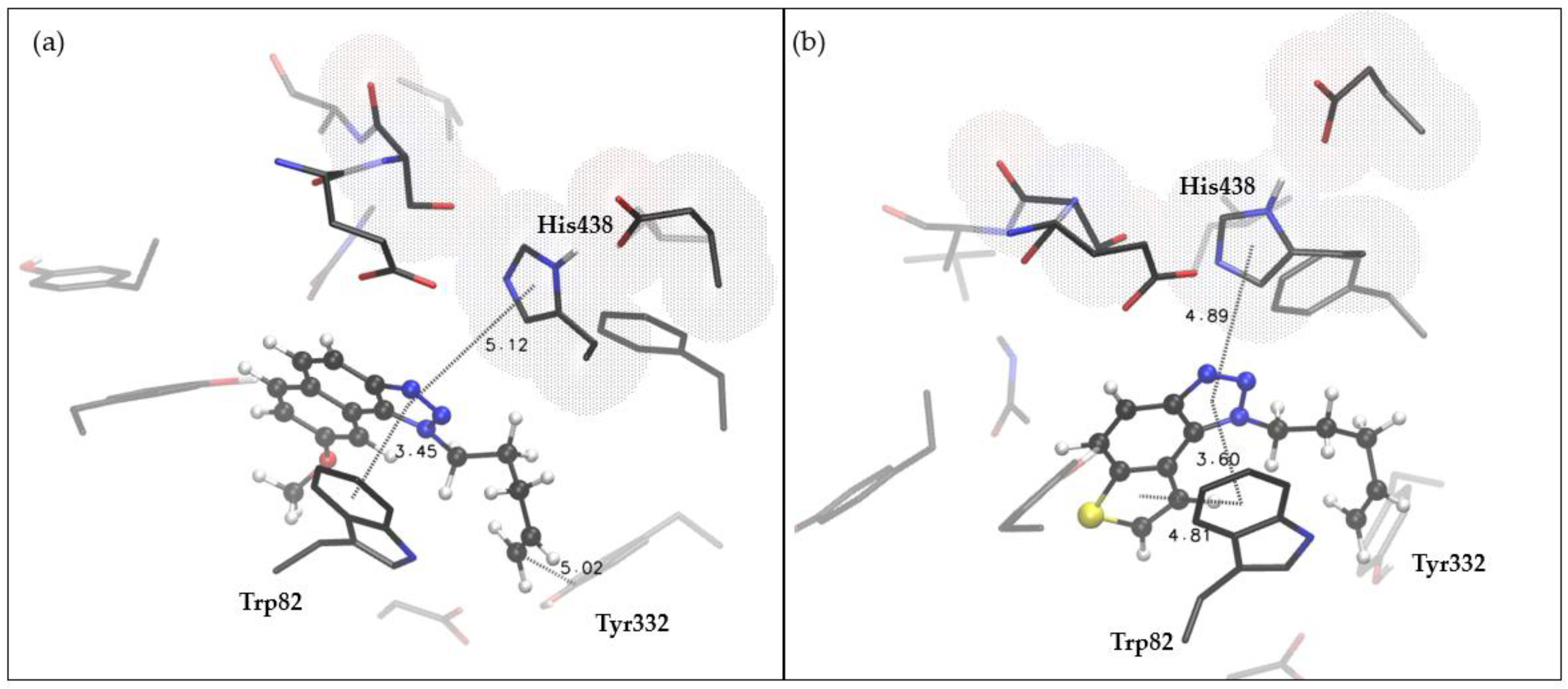
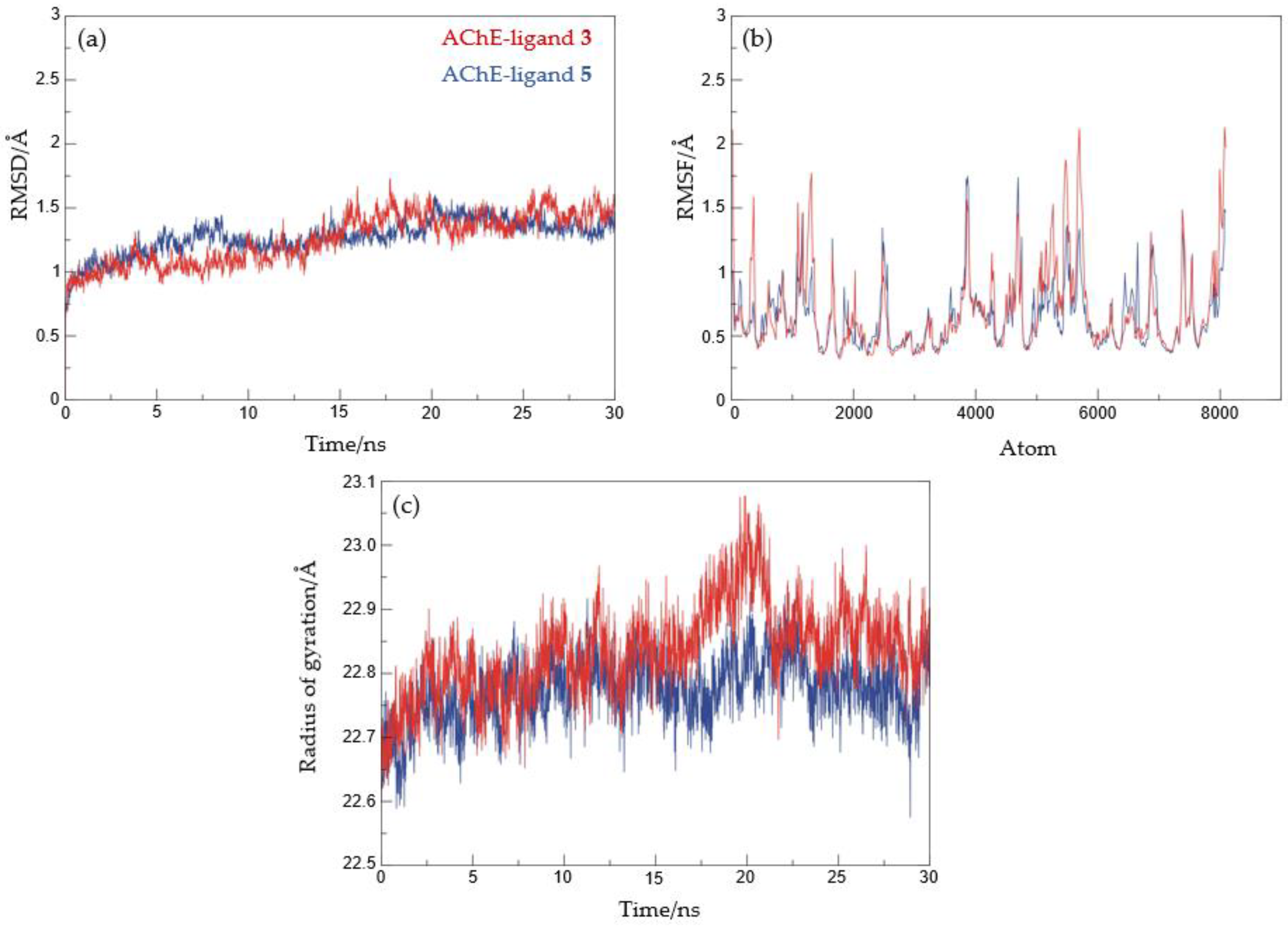
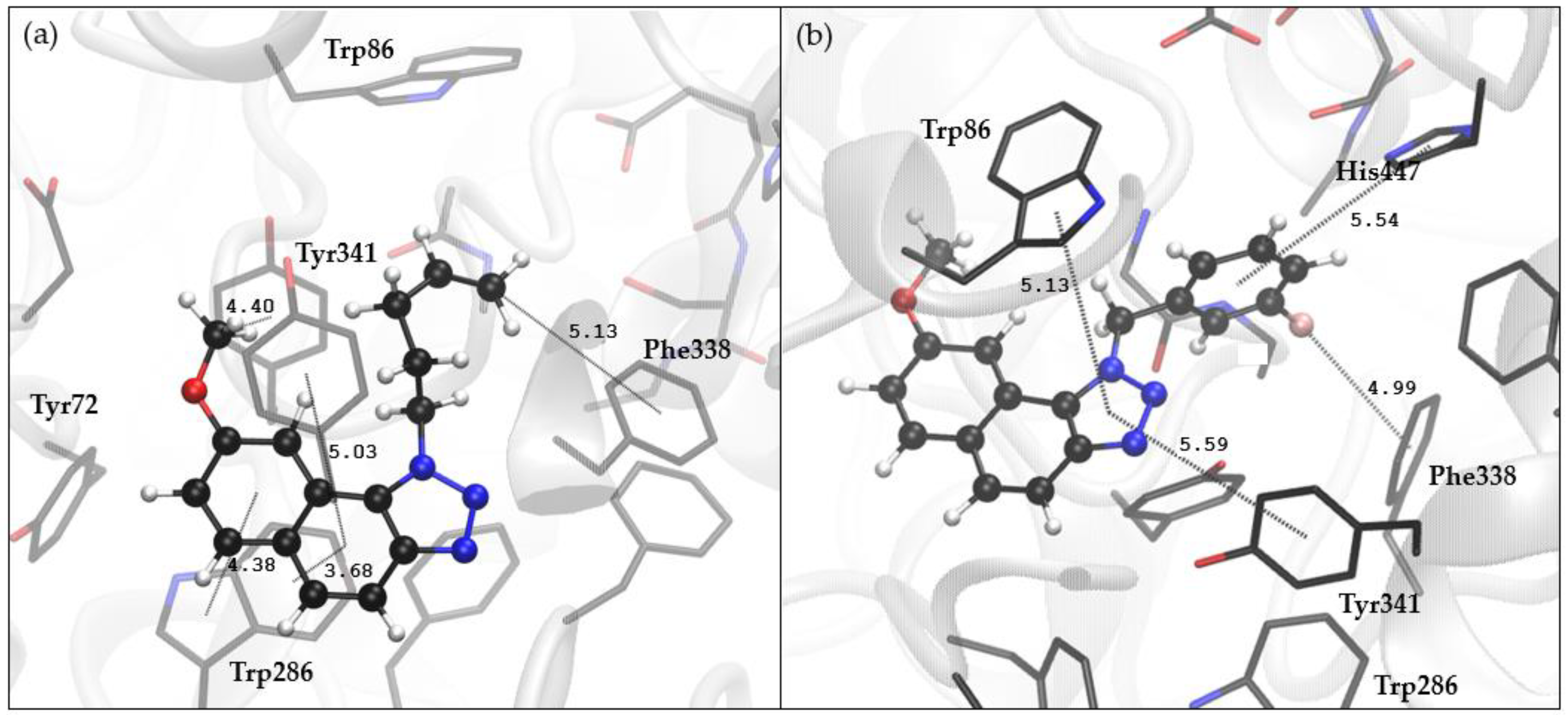
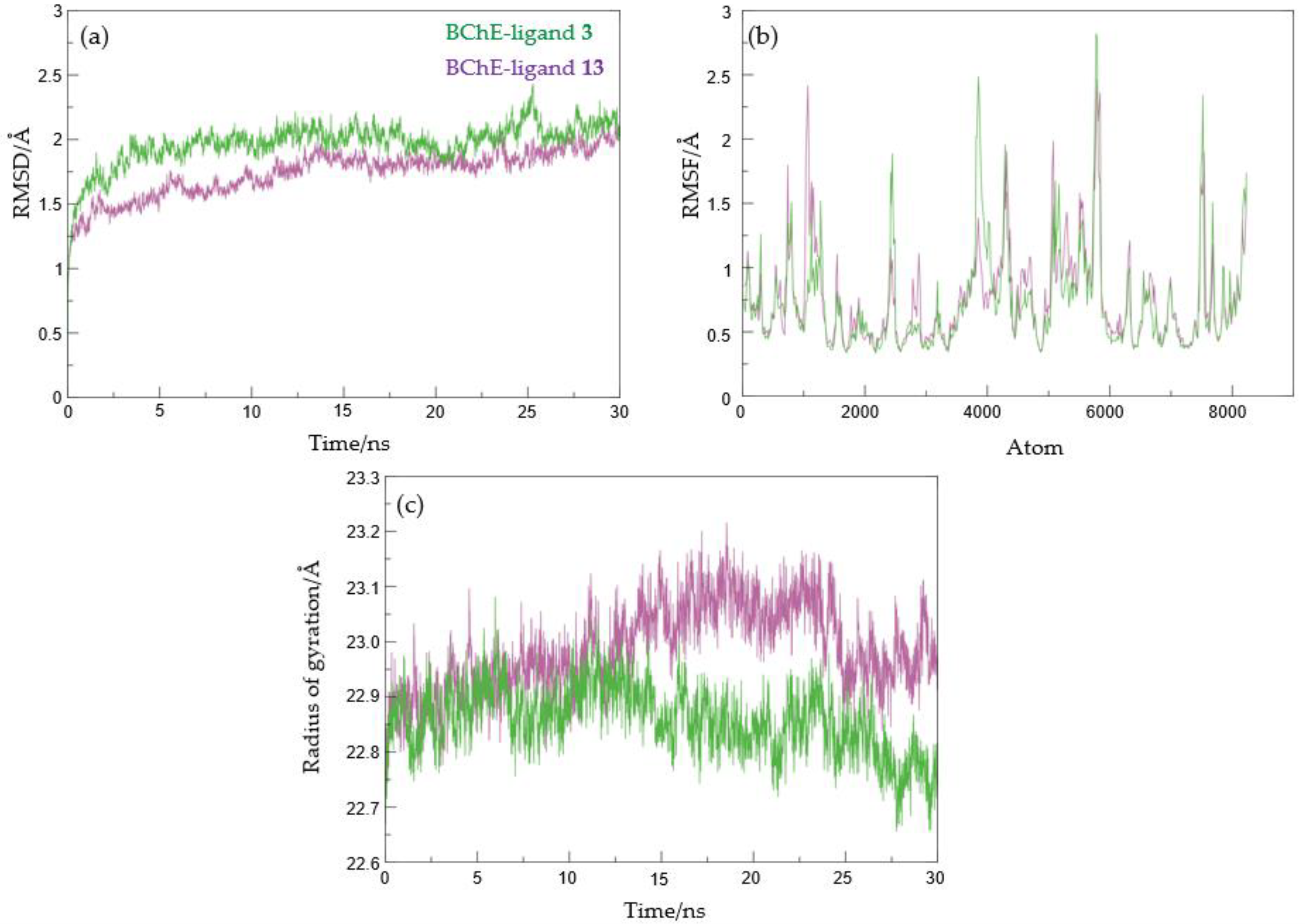
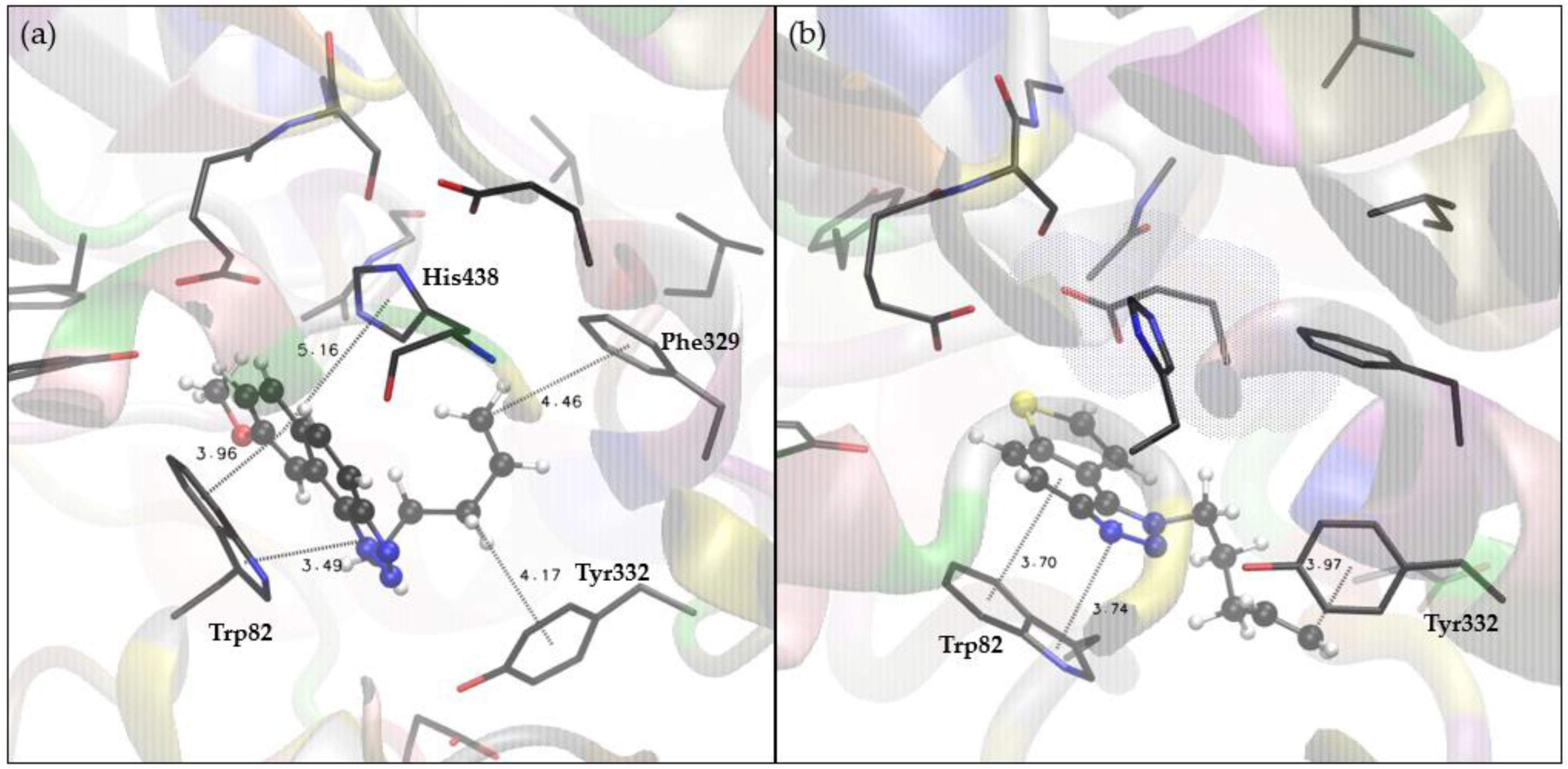
2.4. Docking and Molecular Dynamics Study of 1,2,3-Triazole Derivatives
2.5. Genotoxicity Testing on 1,2,3-Triazole Derivatives 1–31
3. Materials and Methods
3.1. General Remarks
3.2. Synthesis of Starting Thieno-Triazole Stilbenes 1a–13a
3.3. Synthesis of Cyclization Photoproducts 1–13
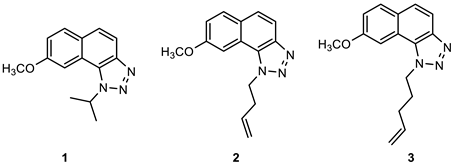
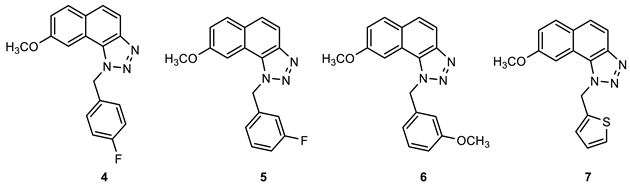
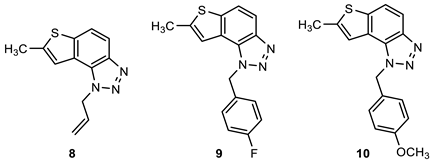
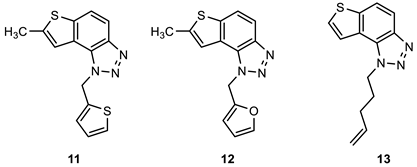
3.4. In Vitro Biological Activity
3.5. In Vitro ChE Activity Assay
3.6. Computational Details
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahu, J.K.; Ganguly, S.; Kaushik, A. Triazoles: A valuable insight recent developments and biological activities. Chin. J. Nat. Med. 2013, 11, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Choe, Y.S.; Choi, Y.J.; Lee, K.H.; Kim, B.T. Synthesis and Evaluation of 18F-Labeled Styryltriazole and Resveratrol Derivatives for β-Amyloid Plaque Imaging. J. Med. Chem. 2012, 55, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Guang, S.; Xu, H.; Ke, F.; Qin, X. Fluorene-based click polymers: Relationship between molecular structure and nonlinear optical properties. J. Appl. Polym. Sci. 2014, 131, 40878. [Google Scholar] [CrossRef]
- Balamurugan, S.; Nithyanandan, S.; Selvarasu, C.; Yeap, G.Y.; Kannan, P. Photophysical and photochemical investigations on triazole ring linked chalcone containing polymethacrylates. Polymer 2012, 53, 4104–4111. [Google Scholar] [CrossRef]
- Bhowmik, D.; Samphat Kumar, K.P.; Chandira, M.; Jayakar, B. Turmeric: A Herbal and Traditional Medicine. Arch. Appl. Sci. Res. 2009, 1, 86–108. [Google Scholar]
- Agrawa, D.K.; Mishra, P.K. Curcumin and its analogues: Potential anticancer agents. Med. Res. Rev. 2010, 30, 818–860. [Google Scholar]
- Caprioglio, D.; Torretta, S.; Ferrari, M.; Travelli, C.; Grolla, A.A.; Condorelli, F.; Genazzani, A.A.; Minassi, A. Triazole-curcuminoids: A new class of derivatives for ‘tuning’ curcumin bioactivities? Bioorg. Med. Chem. 2016, 24, 140–152. [Google Scholar] [CrossRef]
- Li, W.; Jia, Q.; Du, Z.; Wang, J. Direct access to triazole-olefins through catalytic cycloaddition of azides to unsaturated aldehydes. Chem. Commun. 2013, 49, 10187–10189. [Google Scholar] [CrossRef]
- Allison, M.C.; Howatson, A.G.; Torrance, C.J.; Lee, F.D.; Russell, R.I. Gastrointestinal damage associated with use of nonsteroidal anti-inflammatory drugs. N. Engl. J. Med. 1992, 327, 749–754. [Google Scholar] [CrossRef]
- Angajala, K.K.; Vianala, S.; Macha, R.; Raghavender, M.; Thupurani, M.K.; Pathi, P.J. Synthesis, anti-inflammatory, bactericidal activities and docking studies of novel 1,2,3-triazole derived from ibuprofen using click chemistry. Springerplus 2016, 5, 423. [Google Scholar] [CrossRef]
- Rajasekaran, A.; Rajagopal, K.A. Synthesis of some novel triazole derivatives as anti-nociceptive and anti-inflammatory agents. Acta Pharm. 2009, 59, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, A.; Murugesan, S.; Anandarajagopal, K. Antibacterial, antifungal and anticonvulsant evaluation of newly synthesized 1-1-[2-(1H-tetrazol-5-yl)ethyl]-1H-benzo[d][1,2,3]triazoles. Arch. Pharm. Res. 2006, 29, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Priya, V.; Bhasin, R.; Verma, K. An exploration of sundry nature of benzotriazole derivatives: A Review. Int. Res. J. Pharm. 2019, 10, 25–35. [Google Scholar] [CrossRef]
- Rajasekaran, A.; Rajamanickam, V.; Kumaresan, P.T.; Murugesan, S.; Sivakumar, V. Analgesic and anti-epileptic activity of N-Mannich bases of some substituted benztriazole. Int. J. Chem. Sci. 2004, 2, 445–449. [Google Scholar]
- Jamkhandi, C.M.; Disouza, J.I.; Magdum, S.B.; Swami, K.B.; Kumbhar, P.S. Synthesis, characterization, in vitro antiinflammatory activity and qsar evaluation of benzotriazolyl)-3-{5-(carboxymethyl) diazenyl]-2-hydroxyphenyl} prop-2-enoic acid derivatives. Eur. J. Pharm. Med. Res. 2016, 2, 302–306. [Google Scholar]
- Martelli, D.; McKinley, M.J.; McAllen, R.M. The cholinergic anti-inflammatory pathway: A critical review. Auton. Neurosci. 2014, 182, 65–69. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Wang, H.; Czura, C.J.; Friedman, S.G.; Tracey, K.J. The cholinergic anti-inflammatory pathway: A missing link in neuroimmunomodulation. Mol. Med. 2003, 9, 125–134. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Y.; Li, H. The regulation effect of a7nAChRs and M1AChRs on inflammation and immunity in sepsis. Mediat. Inflamm. 2021, 2021, 9059601. [Google Scholar] [CrossRef]
- Pohanka, M.; Hrabinova, M.; Kuca, K.; Simonato, J.-P. Assessment of Acetylcholinesterase Activity Using Indoxylacetate and Comparison with the Standard Ellman’s Method. Int. J. Mol. Sci. 2011, 12, 2631–2640. [Google Scholar] [CrossRef]
- Pohanka, M. Alpha7 Nicotinic Acetylcholine Receptor Is a Target in Pharmacology and Toxicology. Int. J. Mol. Sci. 2012, 13, 2219–2238. [Google Scholar] [CrossRef]
- Rosas-Ballina, M.; Tracey, K.J. Cholinergic control of inflammation. J. Intern. Med. 2009, 265, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Mesulam, M.M.; Guillozet, A.; Shaw, P.; Levey, A.; Duysen, E.G.; Lockridge, O. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience 2002, 110, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Darvesh, S.; Walsh, R.; Kumar, R.; Caines, A.; Roberts, S.; Magee, D.; Rockwood, K.; Martin, E. Inhibition of Human Cholinesterases by Drugs Used to Treat Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2003, 17, 117–126. [Google Scholar] [CrossRef] [PubMed]
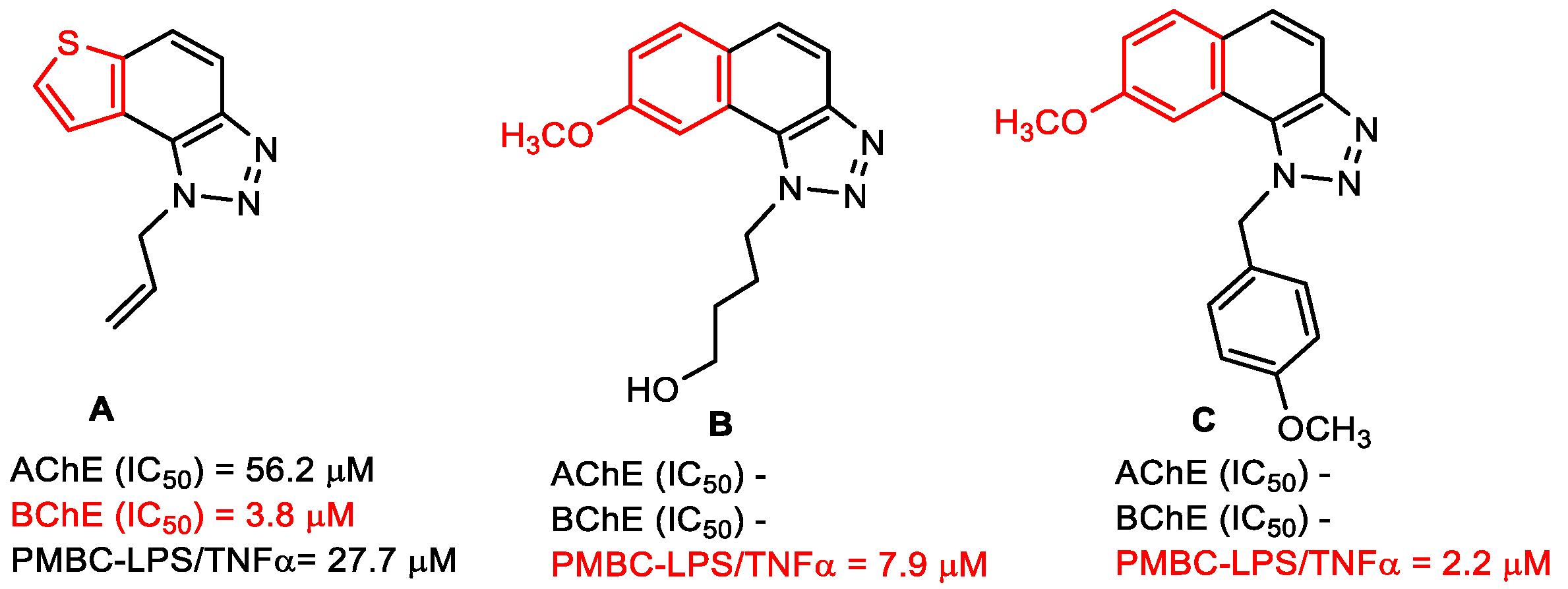
- Mlakić, M.; Faraho, I.; Odak, I.; Talić, S.; Vukovinski, A.; Raspudić, A.; Bosnar, M.; Zadravec, R.; Ratković, A.; Lasić, K.; et al. Synthesis, photochemistry and computational study of novel 1,2,3-triazole heterostilbenes: Expressed biological activity of their electrocyclization photoproducts. Bioorg. Chem. 2022, 121, 105701. [Google Scholar] [CrossRef] [PubMed]
- Mlakić, M.; Odak, I.; Faraho, I.; Talić, S.; Bosnar, M.; Lasić, K.; Barić, D.; Škorić, I. New naphtho/thienobenzo-triazoles with interconnected anti-inflammatory and cholinesterase inhibitory activity. Eur. J. Med. Chem. 2022, 241, 114616. [Google Scholar] [CrossRef]
- Opsomer, T.; Valkeneers, K.; Ratković, A.; Dehaen, W. 1-(4-Nitrophenyl)-1H-1,2,3-triazole-4-carbaldehyde: Scalable synthesis and its use in the preparation of 1-alkyl-4-formyl-1,2,3-triazoles. Organics 2021, 2, 404–414. [Google Scholar] [CrossRef]
- Mlakić, M.; Selec, I.; Ćaleta, I.; Odak, I.; Barić, D.; Ratković, A.; Molčanov, K.; Škorić, I. New thienobenzo/naphtha-triazoles as butyrylcholinesterase inhibitors: Design, synthesis and computational study. Int. J. Mol. Sci. 2023, 24, 5879. [Google Scholar] [CrossRef]
- Pohanka, M. Inhibitors of Acetylcholinesterase and Butyrylcholinesterase Meet Immunity. Int. J. Mol. Sci. 2014, 15, 9809–9825. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Safety SCCS. Opinion on Hair Dye 1,2,4-Trihydroxybenzene (1,2,4-THB) COLIPA n° A33, Submission VI. Available online: https://health.ec.europa.eu/system/files/2021-08/sccs_o_222_0.pdf/ (accessed on 21 June 2019).
- Ellman, G.L.; Courtnex, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDock-823 Tools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.; Burshteyn, F.; Cassidy, M.; Gary, E.; Love, J.; Height, J.; Franklin, M. Crystal Structure of Recombinant Human Acetylcholinesterase in Complex with Donepezil; PDB: Oakland, CA, USA, 2012. [Google Scholar] [CrossRef]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal Structure of Human Butyrylcholinesterase; PDB: Oakland, CA, USA, 2003. [Google Scholar] [CrossRef]
- Case, D.A.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; Ghoreishi, D.; Gilson, M.K.; et al. AMBER 2016; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
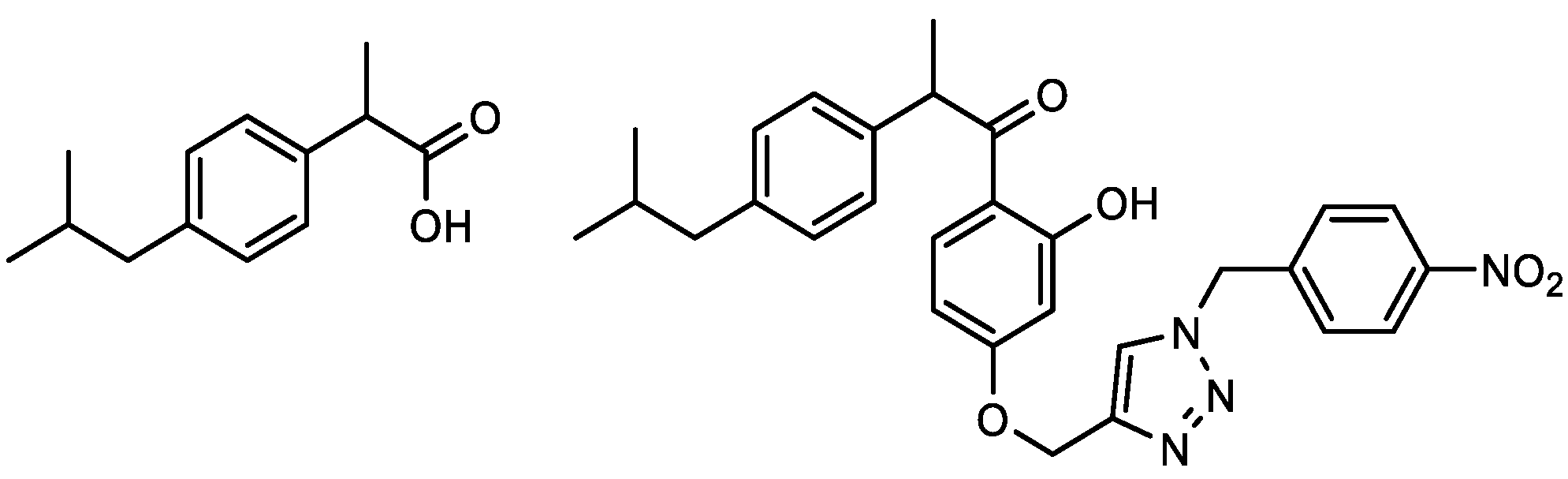
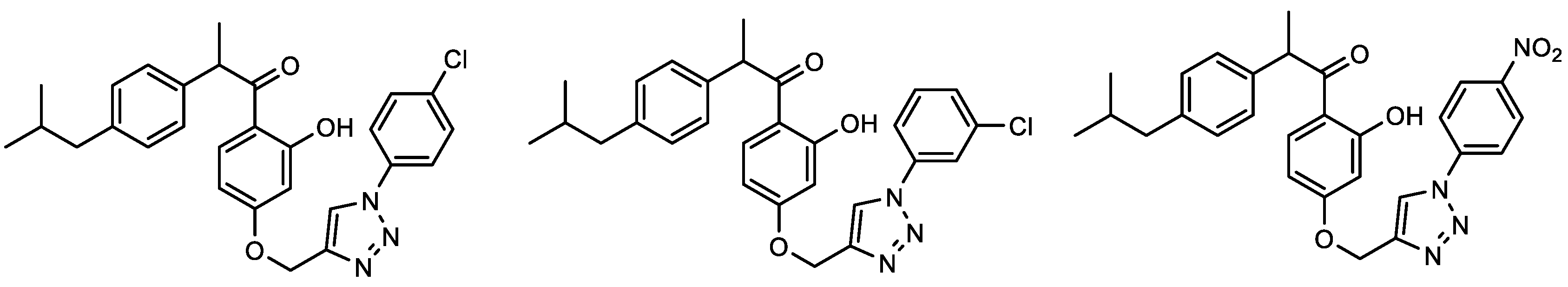
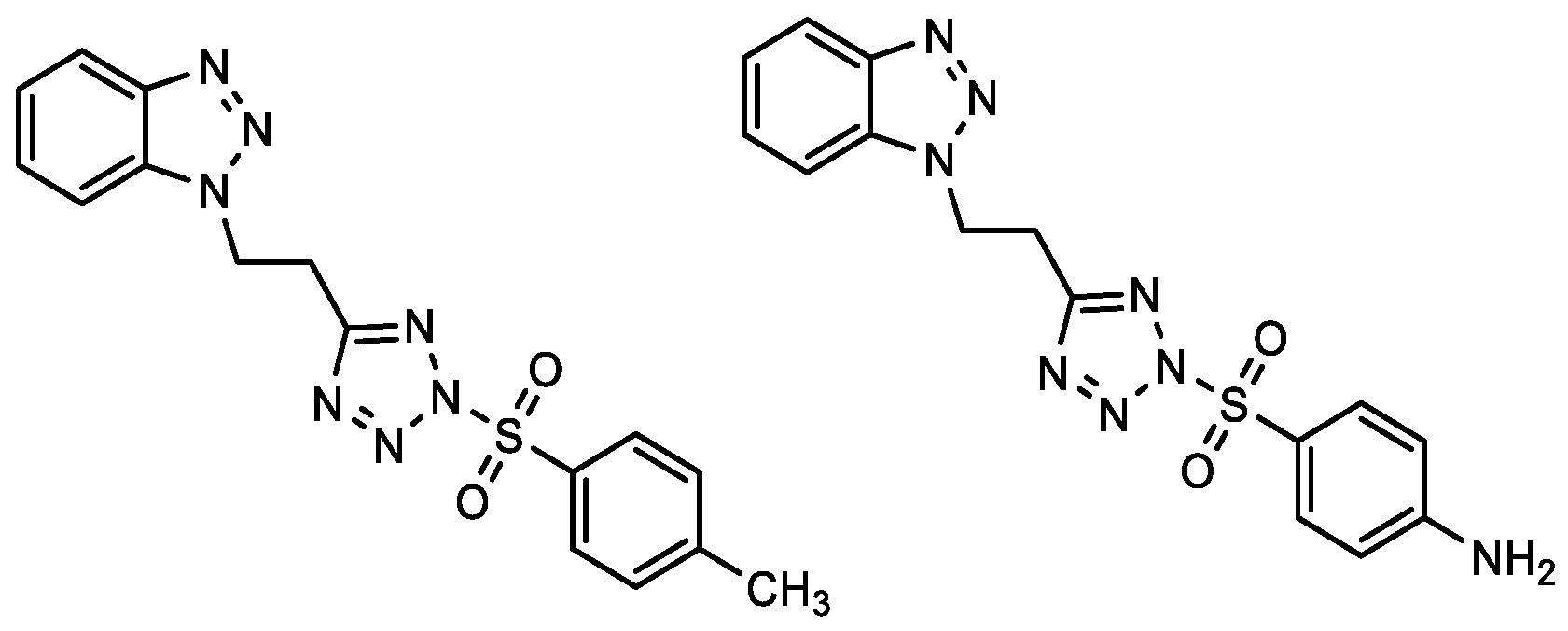
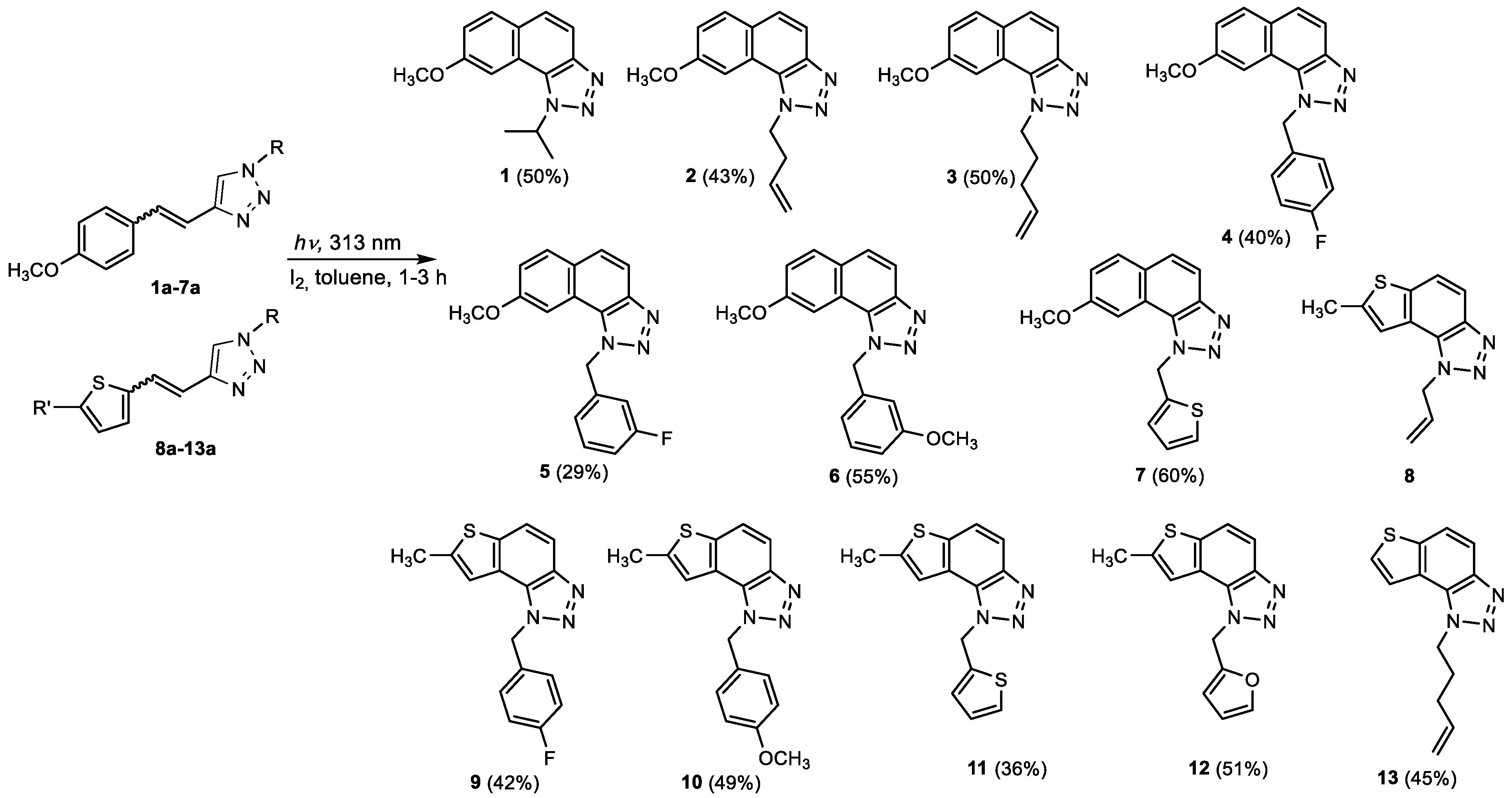
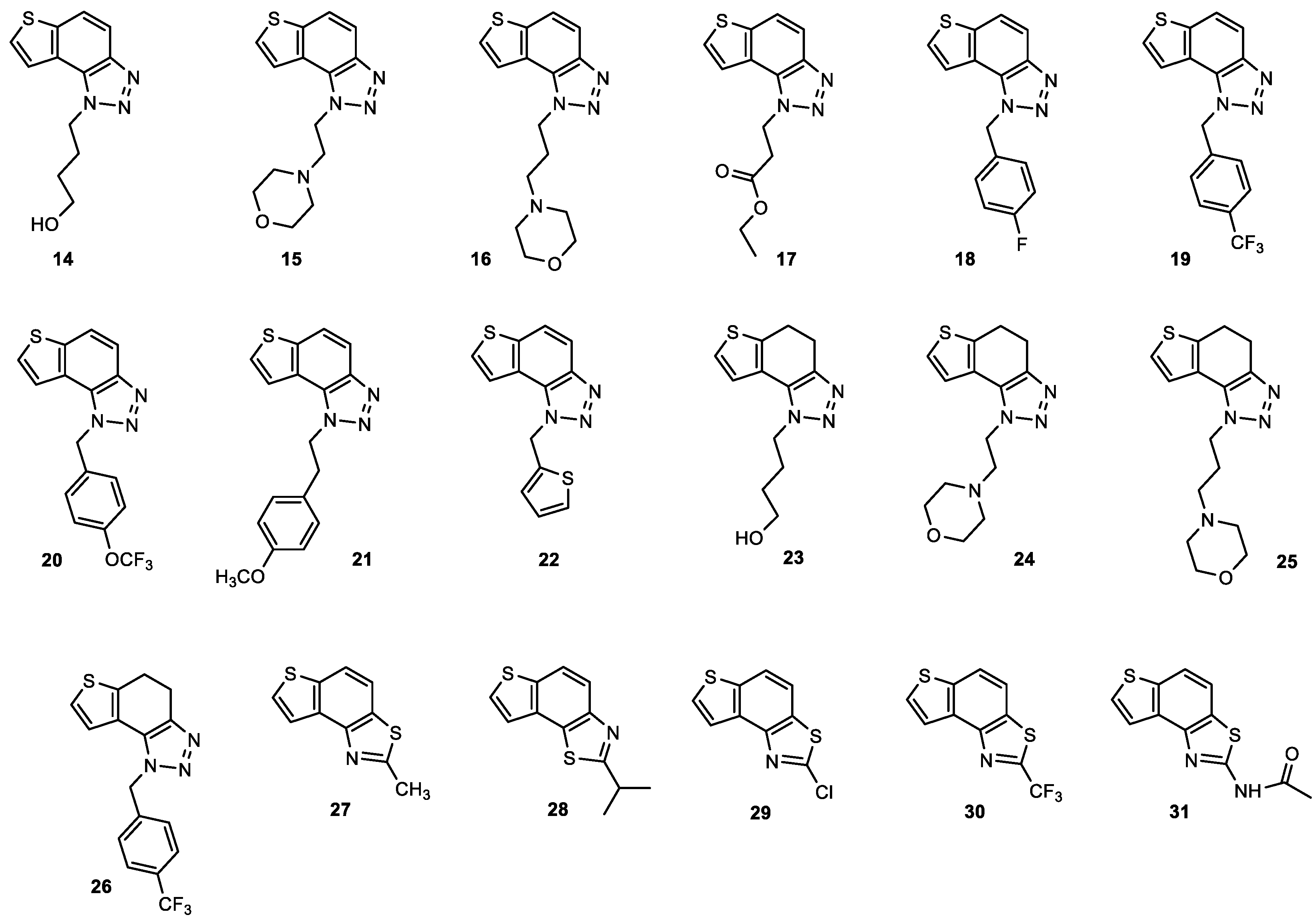
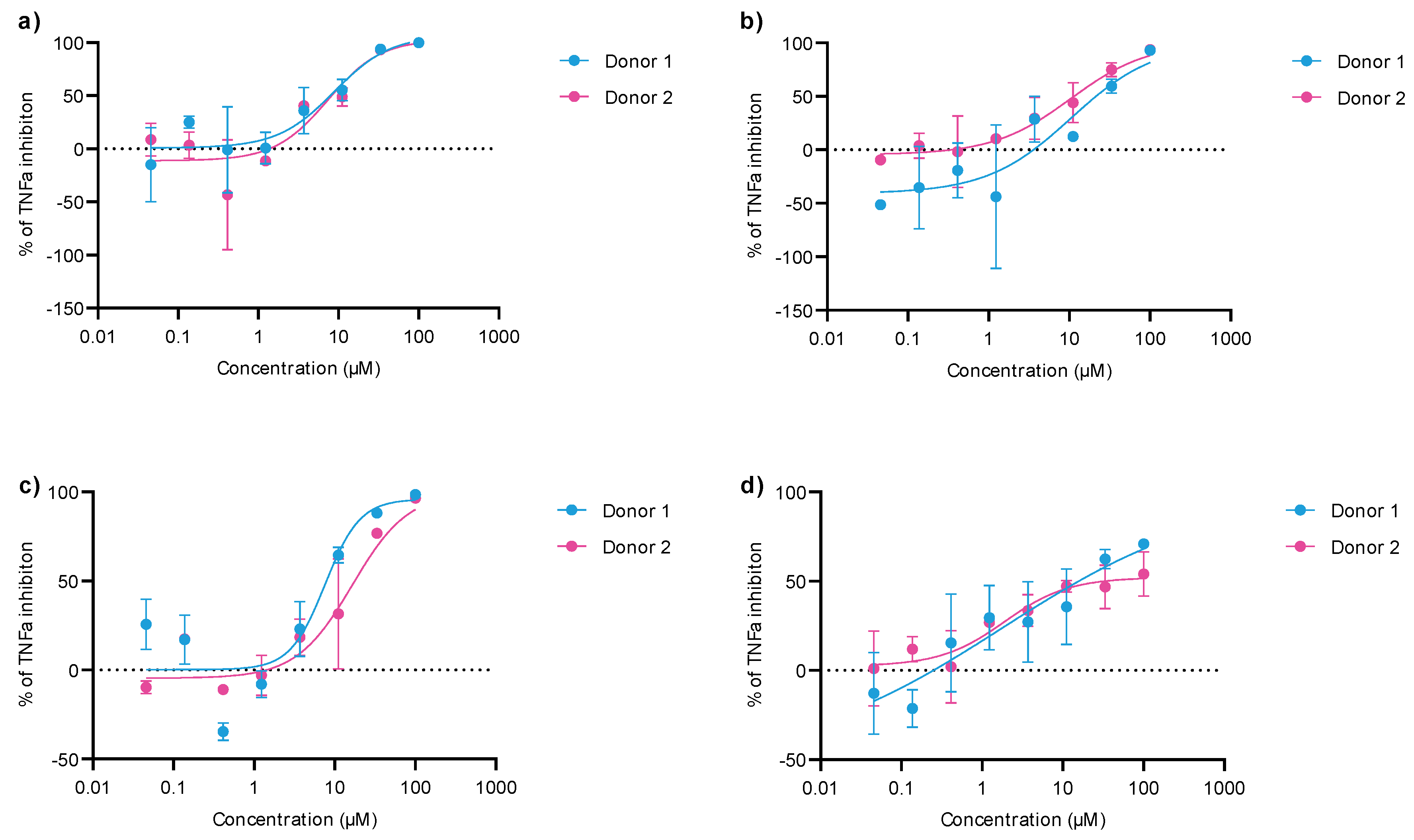
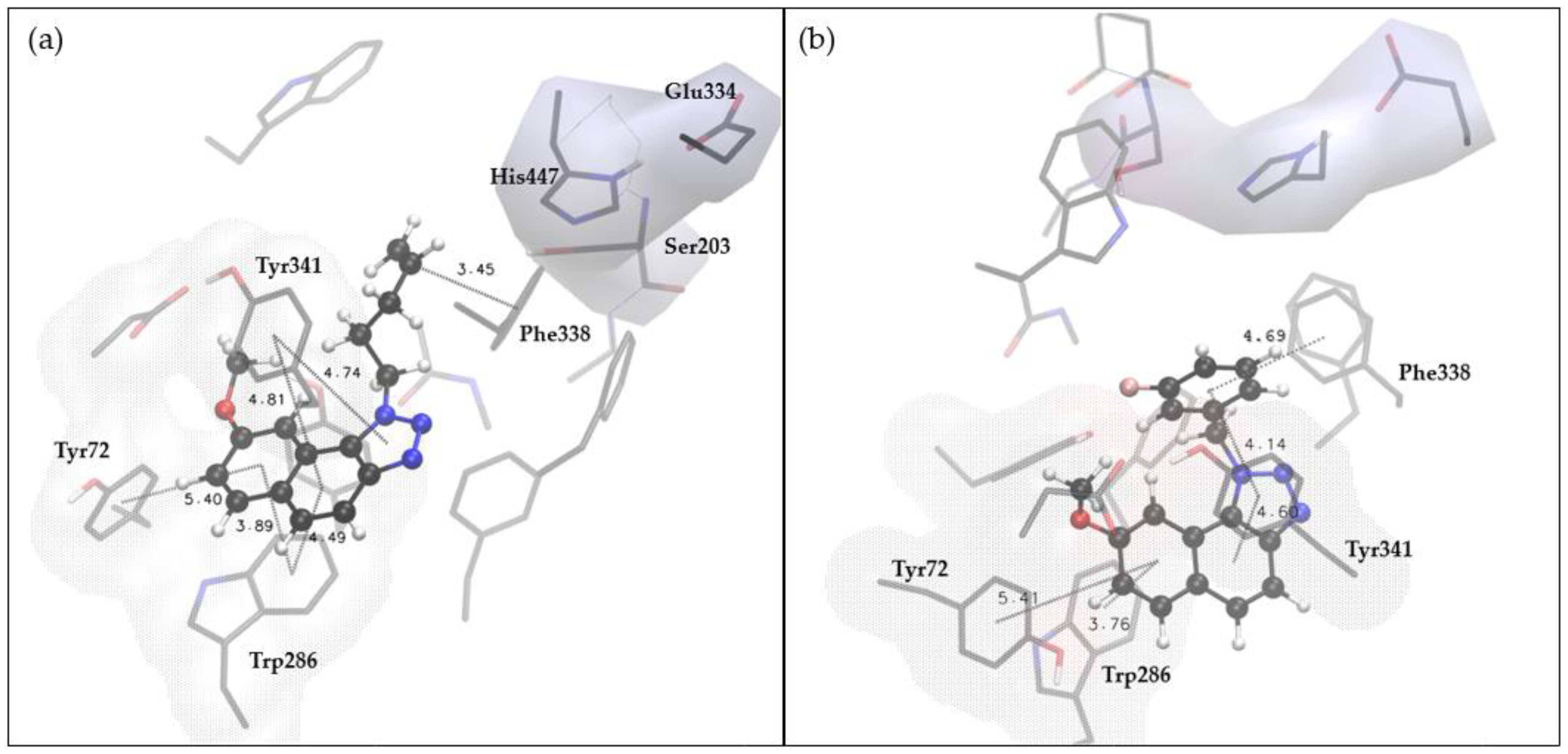
| Compound | IC50/μM (av.) PMBC-LPS/TNF-α | Compound | IC50/μM (av.) PMBC-LPS/TNF-α |
|---|---|---|---|
| 1 | 8.1 | 17 | >100 |
| 2 | 10.4 | 18 | 27.7 |
| 3 | 12.0 | 19 | 10.2 ** |
| 4 | 19.1 | 20 | 8.9 ** |
| 5 | >100 | 21 | 17.0 |
| 6 | 1.9 | 22 | 16.1 |
| 7 | >100 | 23 | >100 |
| 8 | >100 | 24 | >100 |
| 9 | >100 | 25 | >100 |
| 10 | 19.2 | 26 | 9.4 ** |
| 11 | 38.7 | 27 | 12.2 |
| 12 | 40.6 | 28 | >100 |
| 13 | 21.3 | 29 | >100 |
| 14 | 38.8 | 30 | >100 |
| 15 | n/a * | 31 | >100 |
| 16 | >100 | dexamethasone | 0.007 |
| Compound | AChE | BChE | ||
|---|---|---|---|---|
| IC50/μM | % Inhibition * | IC50/μM | % Inhibition * | |
| 1 | 329.0 | 58.1 ± 1.6 (500) | 261.1 | 65.3 ± 0.7 (500) |
| 2 | 91.8 | 77.9 ± 3.6 (500) | 109.6 | 80.2 ± 0.7 (500) |
| 3 | 51.3 | 64.8 ± 5.3 (250) | 53.5 | 76.6 ± 1.8 (250) |
| 4 | - | 35.1 ± 2.0 (500) | - | 25.0 ± 0.5 (500) |
| 5 | 55.5 | 80.8 ± 1.8 (250) | - | 43.8 ± 1.8 (250) |
| 6 | 176.7 | 78.6 ± 3.8 (250) | - | 47.8 ± 0.5 (250) |
| 7 | 109.5 | 81.4 ± 0.8 (500) | 294.2 | 60.9 ± 0.4 (500) |
| 8 | - | 43.5 ± 2.1 (350) | 206.5 | 62.6 ± 0.2 (350) |
| 9 | 83.0 | 78.8 ± 1.9 (350) | 69.8 | 76.1 ± 0.8 (350) |
| 10 | 66.6 | 83.6 ± 3.7 (350) | 99.7 | 65.2 ± 0.3 (350) |
| 11 | 217.7 | 71.3 ± 0.3 (350) | 122.7 | 72.3 ± 5.6 (350) |
| 12 | - | 41.8 ± 1.0 (350) | - | 46.1 ± 4.(350) |
| 13 | - | 46.4 ± 2.5 (250) | 40.7 | 86.5 ± 5.0 (250) |
| Galantamine | 0.15 | 90.0 ± 1.5 (60) | 7.9 | 90.1 ± 3.4 (4.5) |
| Structure | ICH M7 Class | Derek Prediction | Sarah Prediction | Experimental Data | Overall In Silico |
|---|---|---|---|---|---|
| 5 | Class 5 |  |  | Carc: UnspecifiedAmes: Unspecified | Negative |
| 6 | Class 5 |  |  | Carc: UnspecifiedAmes: Unspecified | Negative |
| 7 | Class 5 |  |  | Carc: UnspecifiedAmes: Unspecified | Negative |
| 9 | Class 5 |  |  | Carc: UnspecifiedAmes: Unspecified | Negative |
| 10 | Class 5 |  |  | Carc: UnspecifiedAmes: Unspecified | Negative |
| 11 | Class 5 |  |  | Carc: UnspecifiedAmes: Unspecified | Negative |
| 15 | Class 5 |  |  | Carc: UnspecifiedAmes: Unspecified | Negative |
| 18 | Class 5 |  |  | Carc: UnspecifiedAmes: Unspecified | Negative |
| 21 | Class 5 |  |  | Carc: UnspecifiedAmes: Unspecified | Negative |
| 22 | Class 5 |  |  | Carc: UnspecifiedAmes: Unspecified | Negative |
| 24 | Class 5 |  |  | Carc: UnspecifiedAmes: Unspecified | Negative |
| 27 | Class 5 |  |  | Carc: UnspecifiedAmes: Unspecified | Negative |
| 29 | Class 5 |  |  | Carc: UnspecifiedAmes: Unspecified | Negative |
| 30 | Class 5 |  |  | Carc: UnspecifiedAmes: Unspecified | Negative |
| 31 | Class 3 |  |  | Carc: UnspecifiedAmes: Unspecified | Positive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlakić, M.; Faraho, I.; Odak, I.; Kovačević, B.; Raspudić, A.; Šagud, I.; Bosnar, M.; Škorić, I.; Barić, D. Cholinesterase Inhibitory and Anti-Inflammatory Activity of the Naphtho- and Thienobenzo-Triazole Photoproducts: Experimental and Computational Study. Int. J. Mol. Sci. 2023, 24, 14676. https://doi.org/10.3390/ijms241914676
Mlakić M, Faraho I, Odak I, Kovačević B, Raspudić A, Šagud I, Bosnar M, Škorić I, Barić D. Cholinesterase Inhibitory and Anti-Inflammatory Activity of the Naphtho- and Thienobenzo-Triazole Photoproducts: Experimental and Computational Study. International Journal of Molecular Sciences. 2023; 24(19):14676. https://doi.org/10.3390/ijms241914676
Chicago/Turabian StyleMlakić, Milena, Ivan Faraho, Ilijana Odak, Borislav Kovačević, Anamarija Raspudić, Ivana Šagud, Martina Bosnar, Irena Škorić, and Danijela Barić. 2023. "Cholinesterase Inhibitory and Anti-Inflammatory Activity of the Naphtho- and Thienobenzo-Triazole Photoproducts: Experimental and Computational Study" International Journal of Molecular Sciences 24, no. 19: 14676. https://doi.org/10.3390/ijms241914676
APA StyleMlakić, M., Faraho, I., Odak, I., Kovačević, B., Raspudić, A., Šagud, I., Bosnar, M., Škorić, I., & Barić, D. (2023). Cholinesterase Inhibitory and Anti-Inflammatory Activity of the Naphtho- and Thienobenzo-Triazole Photoproducts: Experimental and Computational Study. International Journal of Molecular Sciences, 24(19), 14676. https://doi.org/10.3390/ijms241914676







