Heat Stress Alleviation by Exogenous Calcium in the Orchid Dendrobium nobile Lindl: A Biochemical and Transcriptomic Analysis
Abstract
:1. Introduction
2. Results
2.1. Effect of CaCl2 on the Growth of D. nobile under Heat Stress
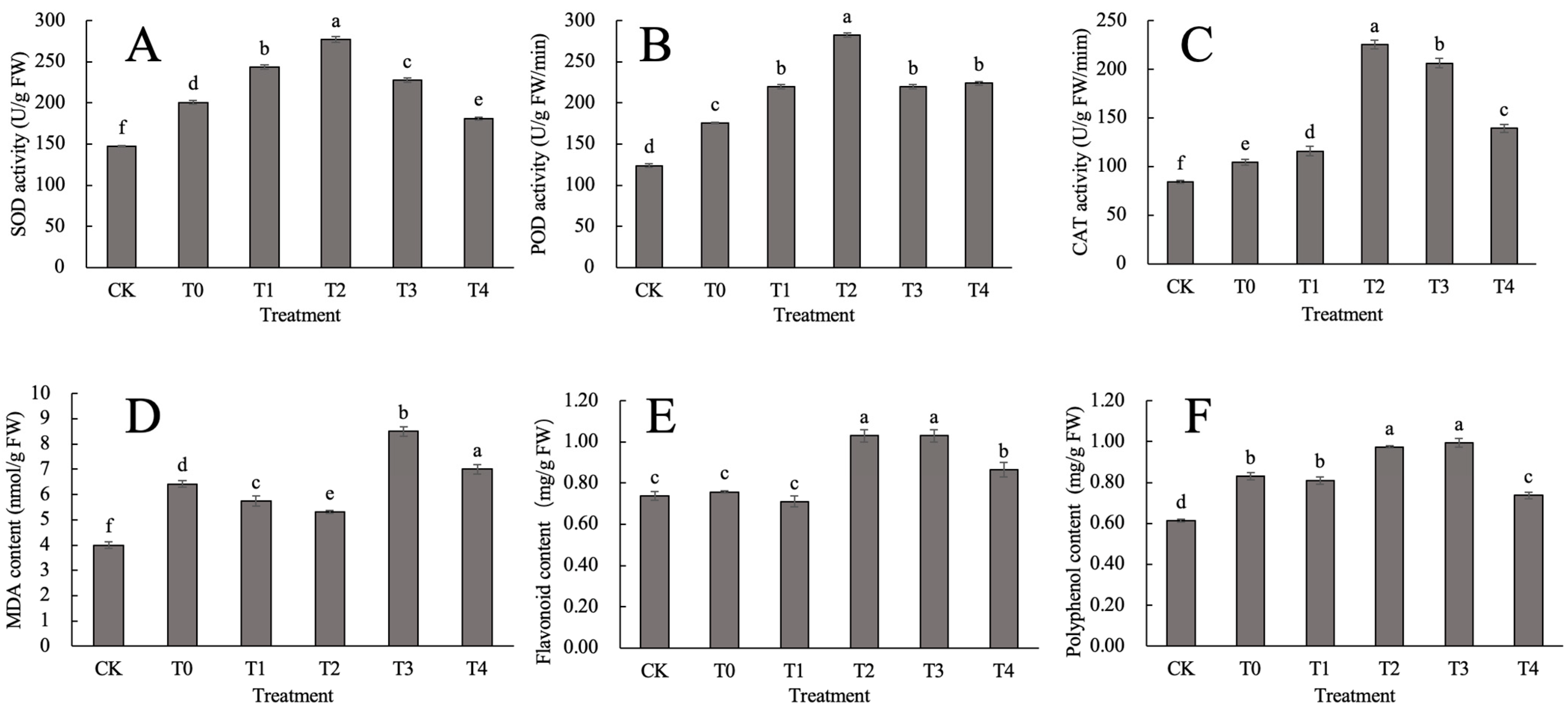
2.2. Effect of CaCl2 on the Antioxidant System of D. nobile under Heat Stress
2.3. Effect of CaCl2 on Photosynthetic Pigments in D. nobile under Heat Stress
2.4. Effect of CaCl2 on the Osmotic Regulation System of D. nobile under Heat Stress
2.5. Effect of CaCl2 on the Ascorbic Acid Glutathione Cycle in D. nobile under Heat Stress
2.6. Comprehensive Evaluation
2.6.1. Correlation Analysis
2.6.2. Principal Component Analysis
2.6.3. Comprehensive Analysis of Membership Functions
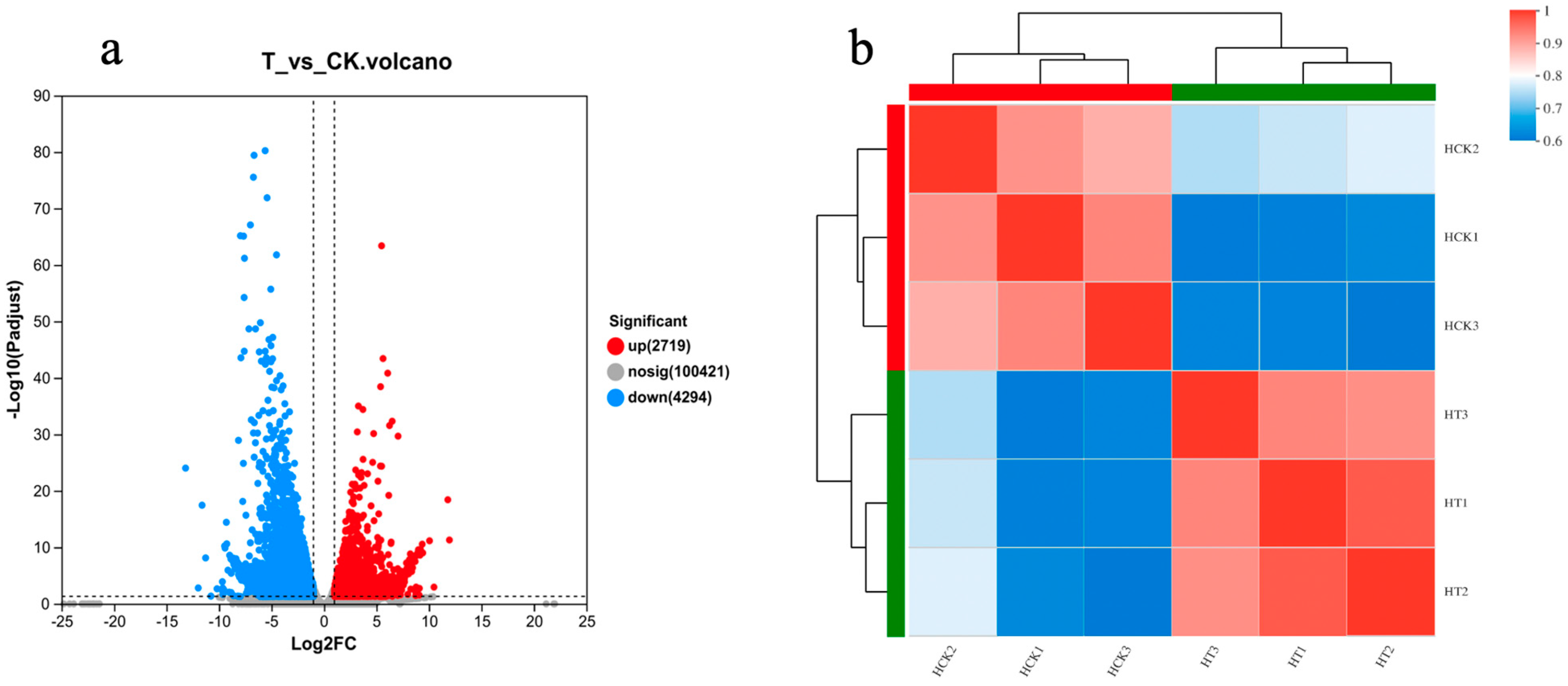
2.7. Transcriptome Sequencing Quality Analysis and Differential Gene Screening
2.8. GO Functional Enrichment of DGEs
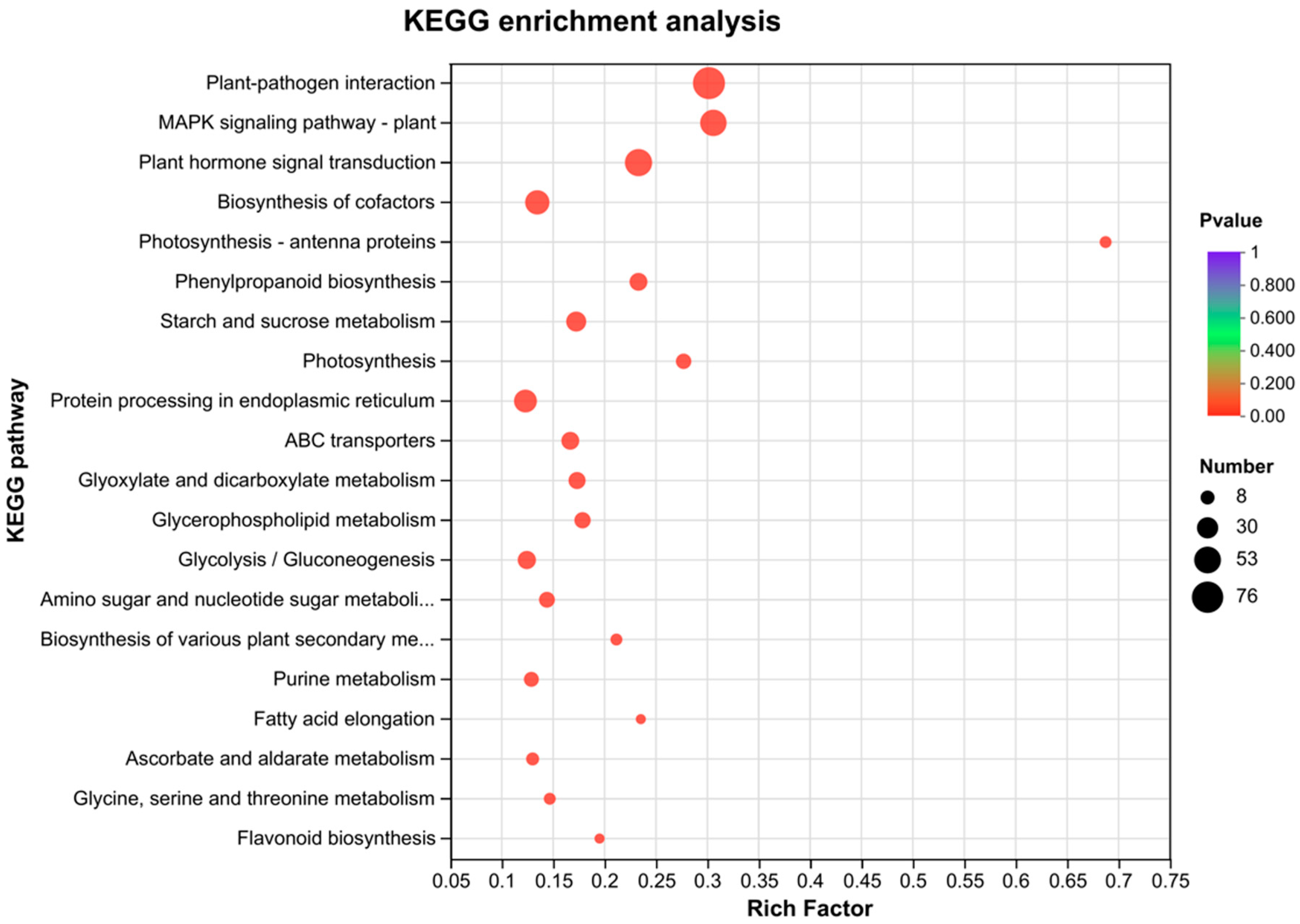
2.9. KEGG Analysis of DEGs
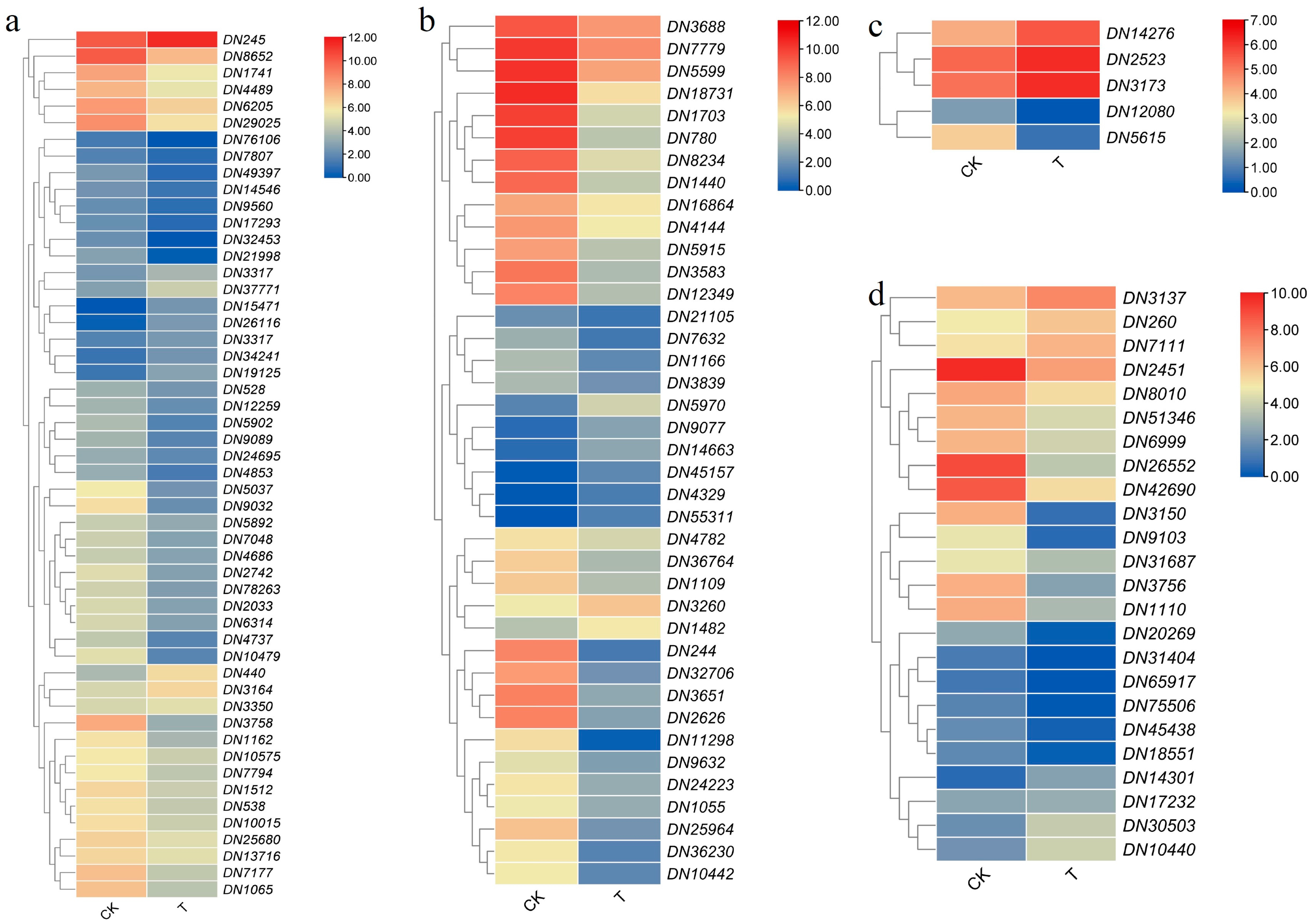
2.10. Key Gene Analysis
2.11. Transcription Factor Analysis
2.12. qRT-PCR Analysis of Differential Gene Expression Levels
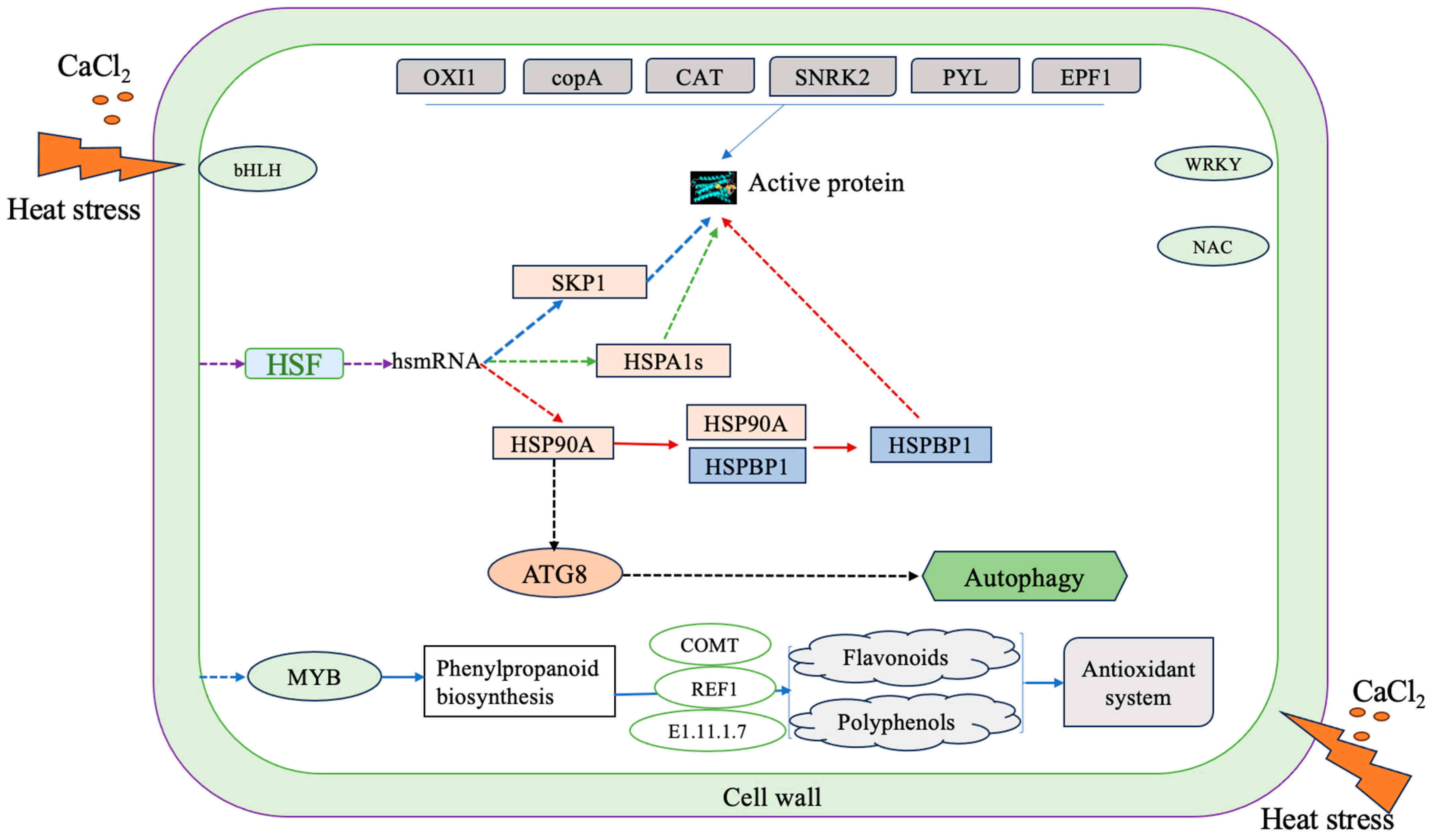
3. Discussion
3.1. Effects of Exogenous Calcium on Physiological and Biochemical Characteristics of D. nobile under Heat Stress
3.2. Effect of Exogenous Calcium on Gene Expression in D. nobile under Heat Stress
3.3. The Role of Transcription Factors in Enhancing the Tolerance of D. nobile to Heat Stress by Exogenous Calcium
4. Materials and Methods
4.1. Materials and Treatments
4.2. Detection of Growth Indicators
4.3. Determination of Physiological and Biochemical Indicators
4.4. Transcriptome Sequencing
4.4.1. Illumina Sequencing
4.4.2. Differential Gene Enrichment Analysis
4.4.3. qRT-PCR
4.5. Data Statistics and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Hu, Y.; Niu, Z.; Li, C.; Ou, J.; Xue, Q.; Liu, W.; Chen, J.; Ding, X. Diversity Evaluation of Dendrobium nobile Germplasm Resources Based on Phenotypic Traits. China Biotechnol. 2022, 42, 5–17. [Google Scholar] [CrossRef]
- Zhang, S.; Tu, H.; Zhu, J.; Liang, A.; Huo, P.; Shan, K.; He, J.; Zhao, M.; Chen, X.; Lei, X. Dendrobium nobile Lindl. polysaccharides improve follicular development in PCOS rats. Int. J. Biol. Macromol. 2020, 149, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xing, J.; Li, L.; Zhan, M.; Li, F.; Lin, L.; Wang, L.; Yang, W. Optimization of PEG 200 extraction process and in vitro antioxidant activity of polyphenols from Dendrobium nobile flowers. Sci. Technol. Food Ind. 2023, 44, 260–267. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Shi, Y.C.; Zhang, S.B. Metabolic and transcriptomic analyses elucidate a novel insight into the network for biosynthesis of carbohydrate and secondary metabolites in the stems of a medicinal orchid Dendrobium nobile. Plant Divers. 2023, 3, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Guo, M.; Li, F.; Shi, J.S. Dendrobium nobile Lindl. alkaloids (DNLA) inhibits d-galactose-induced hippocampal neuronal senescence through the SIRT1-FoxO1-autophagy axis. Pharmacol. Res.-Mod. Chin. Med. 2023, 8, 100288. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, H.; Liu, P.; Sun, Z.; Wei, X. Study on the hypoglycemic effect of water extract from Dendrobium nobile on streptozotocin induced hyperglycemia in mice. Acta Chin. Med. Pharmacol. 2013, 41, 15–16. [Google Scholar] [CrossRef]
- Liang, J.S.; Deng, Y.C.; Yu, G.C.; Gan, Y.K. Anti-fatigue Effects of Polysaccharides Derived from Dendrobium nobile Lindl. in Mice. Food Sci. 2012, 33, 282–288. [Google Scholar]
- Hong, S.; Kim, E.Y.; Lim, S.E.; Kim, J.H.; Sohn, Y.; Jung, H.S. Dendrobium nobile Lindley Administration Attenuates Atopic Dermatitis-like Lesions by Modulating Immune Cells. Int. J. Mol. Sci. 2022, 23, 4470. [Google Scholar] [CrossRef]
- Wang, J.H.; Luo, J.P.; Zha, X.Q.; Feng, B.J. Comparison of antitumor activities of different polysaccharide fractions from the stems of Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 79, 114–118. [Google Scholar] [CrossRef]
- Ye, G.; Zhang, J.; Xu, X.; Zeng, C.; Ye, Q.; Wang, Z. Comparative Analysis of Water-Soluble Polysaccharides from Dendrobium Second Love ‘Tokimeki’ and Dendrobium nobile in Structure, Antioxidant, and Anti-Tumor Activity In Vitro. Int. J. Mol. Sci. 2023, 24, 10361. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Si, J.P. Present status and sustainable development of Dendrobium officinale industry. Chin. J. Chin. Mater. Med. 2010, 35, 2033–2036. [Google Scholar] [CrossRef]
- Ren, Q.; Yao, Y.; Ao, Q.; Huang, F.; Xie, H. Study on the meteorological insurance index of high temperature and drought of Dendrobium nobile in Chishui City. J. Green Sci. Technol. 2023, 25, 36–39. [Google Scholar] [CrossRef]
- Yu, Q.; Sun, W.; Han, Y.; Hao, J.; Qin, X.; Liu, C. Exogenous spermidine improves the sucrose metabolism of lettuce to resist high-temperature stress. Plant Growth Regul. 2022, 96, 497–509. [Google Scholar] [CrossRef]
- Sarkar, S.; Aminul Islam, A.K.M.; Barma, N.C.D.; Ahmed, J.U. Tolerance mechanisms for breeding wheat against heat stress: A review. S. Afr. J. Bot. 2021, 138, 262–277. [Google Scholar] [CrossRef]
- Liu, Y.; Xi, M.; Li, Y.; Cheng, Z.; Kong, F. Improvement in salt tolerance of iris pseudacorus l. in constructed wetland by exogenous application of salicylic acid and calcium chloride. J. Environ. Manag. 2021, 300, 113703. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bheri, M.; Pandey, G.K. Delineating Calcium Signaling Machinery in Plants: Tapping the Potential through Functional Genomics. Curr. Genom. 2021, 22, 404. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, Y.; Ren, X. Calcium regulates antioxidative isozyme activity for enhancing rice adaption to acid rain stress. Plant Sci. 2021, 306, 110876. [Google Scholar] [CrossRef]
- Tan, W.; Meng, Q.W.; Brestic, M.; Olsovska, K.; Yang, X. Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J. Plant Physiol. 2011, 168, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, Y.; Zhang, Y.; Liu, J.; Gul, Z.; Guo, X.-R.; Abozeid, A.; Tang, Z.-H. Effects of Exogenous Calcium on Adaptive Growth, Photosynthesis, Ion Homeostasis and Phenolics of Gleditsia sinensis Lam. Plants under Salt Stress. Agriculture 2021, 11, 978. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, P.; Chen, C.; Song, G.; Tang, H. Effects of Exogenous Ca2+ on the Growth, Physiological Characters of Sophora viciifolia Seedlings in Karst Mountain Area under the Drought Stress. J. Nucl. Agric. Sci. 2017, 31, 2039–2046. [Google Scholar]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Minoru, K.; Michihiro, A.; Susumu, G.; Masahiro, H.; Mika, H.; Masumi, I.; Toshiaki, K.; Shuichi, K.; Shujiro, O.; Toshiaki, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, 480–484. [Google Scholar]
- Lu, Q.; Wang, Y.; Yang, H. Effect of exogenous calcium on physiological characteristics of salt tolerance in Tartary buckwheat. Biologia 2021, 76, 3621–3630. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Li, Q.; Chen, X.; Li, X. Comparative transcriptome analysis to elucidate the enhanced thermotolerance of tea plants (Camellia sinensis) treated with exogenous calcium. Planta 2019, 249, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Qi, J.; Deng, X.; Han, X. Effects of Exogenous Calcium on the Metabolic System of Pinus sylvestris var. mongolica under Drought Stress. J. Shenyang Agric. Univ. 2018, 49, 559–565. [Google Scholar]
- Hu, W.; Tian, S.B.; Di, Q.; Duan, S.H.; Dai, K. Effects of exogenous calcium on mesophyll cell ultrastructure, gas exchange, and photosystem II in tobacco (Nicotiana tabacum Linn.) under drought stress. Photosynthetica 2018, 56, 1204–1211. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, J.; Xing, Y.; Li, B.; Zhang, Q. The Influence of Different Exogenous Substances on Physiology and Chlorophyll Fluorescence Parameters of Trollius chinensis Seedling under High Temperature Stress. Mol. Plant Breed. 2022, 1–22. Available online: http://kns.cnki.net/kcms/detail/46.1068.s.20220106.1706.006.html (accessed on 10 January 2022).
- Xie, Y.; Liu, J.; Liao, X.; Li, P. The effect of calcium stress on the physiological and biochemical characteristics of honeysuckle. Jiangsu Agric. Sci. 2017, 45, 144–146. [Google Scholar] [CrossRef]
- Xia, H.; Gao, F.; Hu, L.; Lu, X.; Liang, L. Effects of Melatonin Applicationon Antioxidant Capacityin Kiwifruit Seedings under High Temperature Stress. Acta Bot. Boreali-Occident. Sin. 2019, 39, 1425–1433. [Google Scholar]
- Du, J.H.; Fan, D.M.; Feng, X.D. Effect of CaCl2 on the physiological characteristics of eggplant seedlings under high temperature stress. Mol. Plant Breed. 2021, 19, 5503–5511. [Google Scholar] [CrossRef]
- Sangwan, V.; Orvar, B.L.; Beyerly, J.; Hirt, H.; Dhindsa, R.S. Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J. 2010, 31, 629–638. [Google Scholar] [CrossRef]
- Paupiere, M.J.; Muller, F.; Li, H.J.; Rieu, L.; Tikunov, Y.M.; Visser, R.G.F.; Bovy, A.G. Untargeted metabolomic analysis of tomato pollen development and heat stress response. Plant Reprod. 2017, 30, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.L.; Xu, H.; Yuan, P.; He, K.J.; Wang, R.C.; Bu, F.W. Advance in relationship between heat shock protein 90 s and thermo-tolerance in plants. Shengwu Jishu Tongbao (Biotechnol. Bull.) 2020, 36, 173–179. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Yu, J.Q.; Chen, Z. Role and regulation of autophagy in heat stress responses of tomato plants. Front. Plant 2014, 5, 174. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.F.; Guo, M.; Wang, H.; Lu, J.P.; Liu, J.H.; Zhang, C.; Gong, Z.H.; Lu, M.H. Autophagy, a conversed mechanism for protein degradation, responds to heat, and other abiotic stresses in Capsicum annuum L. Front. Plant Sci. 2016, 7, 131. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, G.Y.; Shinozaki, K. The Plant Mitogen-activated Protein(MAP) Kinase. Acta Bot. Sin. 2000, 42, 661–667. [Google Scholar]
- Huang, H.J.; Fu, S.F.; Tai, Y.H.; Chou, W.C.; Huang, D.D. Expression of Oryza sativa MAP kinase gene is developmentally regulated and stress-responsive. Physiol. Plant 2002, 114, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Yelena, K.; Chiu, W.; Tena, G. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl. Acad. Sci. USA 2000, 97, 2940–2945. [Google Scholar]
- Han, L.H.; Liu, C.; Zhang, W.W.; Li, F.; Deng, F.H.; Ruan, Z.H. Gene family identification and bioinformatics analysis of heat shock transcription factors (Hsf) in Dendrobium officinale. J. South. Agric. 2019, 50, 677–684. [Google Scholar]
- Jaimes, F.; Montes, R.A.C. The plant MBF1 protein family: A bridge between stress and transcription. J. Exp. Bot. 2020, 71, 1782–1791. [Google Scholar] [CrossRef]
- Xu, X.P. Physical and functional interactions between pathogen-induced Arabidopsis WRKY18,WRKY40, andWRKY60 transcription factors. Plant Cell 2006, 18, 1310–1326. [Google Scholar] [CrossRef]
- Li, S.J.; Fu, Q.T.; Chen, L.G.; Huang, W.D.; Yu, D.Q. Arabidopsis thaliana WRKY25,WRKY26,and WRKY33 coordinate induction of plant thermotolerance. Planta 2011, 233, 1237–1252. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Mizoi, J.; Tanaka, H.; Maruyama, K.; Qin, F.; Osakabe, Y.; Morimoto, K.; Ohori, T.; Kusakabe, K.; Nagata, M.; et al. Arabidopsis DPB3-1, a DREB2A interactor, specifically enhances heat stress-induced gene expression by forming a heat stress-specific transcriptional complex with NF-Ysubunits. Plant Cell 2014, 26, 4954–4973. [Google Scholar] [CrossRef]
- Liao, C.; Zheng, Y.; Guo, Y. MYB30 transcription factor regulates oxidative and heat stress responses through ANNEXIN-mediated cytosolic calcium signaling in Arabidopsis. New Phytol. 2017, 216, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Liang, L.X.; Li, L.B.; Wang, T. Genome-wide analysis of R2R3-MYB transcription factors in Phalaenopsis equestris. Linye Kexue Yanjiu (For. Res.) 2018, 31, 104–113. [Google Scholar] [CrossRef]
- Liu, T.; Shi, J.; Li, M.; Ye, X.; Qi, H. Trehalose triggers hydrogen peroxide and nitric oxide to participate in melon seedlings oxidative stress tolerance under cold stress. Environ. Exp. Bot. 2021, 184, 104379. [Google Scholar] [CrossRef]
- Fan, Y.J.; Yang, K.G.; Miao, R.S.; Wang, G.; Chun, Z.; Wu, S.D.; Pu, S.R.; Luo, A.X. Transcriptome analysis reveals the effects of red and blue light on the physiological and medicinal components of Dendrobium denneanum. Ind. Crops Prod. 2022, 180, 114798. [Google Scholar] [CrossRef]
- Fan, Y.; Li, X.; Wang, G.; Ma, J.; Liu, Y.; Xu, E.; Luo, A. Transcriptome analysis reveals the role of polysaccharide biosynthesis in the detoxification of Dendrobium nobile under zinc stress. Int. J. Biol. Macromol. 2023, 252, 126406. [Google Scholar] [CrossRef] [PubMed]
- Vuleta, A.; Jovanović, S.M.; Tucić, B. Adaptive flexibility of enzymatic antioxidants SOD, APX and CAT to high light stress: The clonal perennial monocot Iris pumila as a study case. Plant Physiol. Biochem. 2016, 100, 166–173. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, J.; Wang, G.; Li, X.; Liu, Y.; Xu, E.; Luo, A. Ultrasonic extraction, structural modification and gastric mucosal cells protective activity of a polysaccharide from Dendrobium denneanum. Arab. J. Chem. 2023, 16, 105033. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, E.; Ma, J.; Li, X.; Liu, Y.; Xu, L.; Luo, A. Isolation, Structural Characteristics Analysis of a Vigna unguiculata Polysaccharide VUP80-3 and Its Protective Effect on GES-1 Cells In Vitro. Molecules 2023, 28, 5566. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Shen, Y.X.; Zhang, C.J.; Luo, A.X.; Fan, Y.J. Contents of polyphenols and flavonoids in Dendrobium candidum and their correlation with antioxidant activity. Chin. J. Appl. Environ. Biol. 2014, 20, 438–442. [Google Scholar]
- Fan, Y.; Xu, E.; Wang, G.; He, D.; Ma, J.; Liu, Y.; Li, X.; Luo, A. Transcriptional and Physiological Analysis Reveal New Insights into the Regulation of Fertilization (N, P, K) on the Growth and Synthesis of Medicinal Components of Dendrobium denneanum. Int. J. Mol. Sci. 2023, 24, 1522. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]





| Treatment | CK | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|---|
| Leaf length (mm) | 2.64 ± 0.19a | 1.25 ± 0.15c | 1.44 ± 0.13c | 2.2 ± 0.11b | 1.33 ± 0.25c | 0.3 ± 0.12d |
| Leaf width (mm) | 1.55 ± 0.05a | 0.59 ± 0.1c | 0.68 ± 0.09c | 1.2 ± 0.07b | 0.38 ± 0.06d | 0.27 ± 0.06e |
| Plant height (mm) | 4.47 ± 0.21a | 3.7 ± 0.27c | 3.87 ± 0.16c | 4.18 ± 0.23b | 2.65 ± 0.25d | 2.48 ± 0.26d |
| Root Fresh Weight (g) | 11.71 ± 0.1a | 6.95 ± 0.09c | 7.1 ± 0.13c | 8.75 ± 0.07b | 6.47 ± 0.09d | 6.0 ± 0.14e |
| Stem Fresh Weight (g) | 64.06 ± 0.09a | 43.04 ± 0.09d | 46.21 ± 0.12c | 48.31 ± 0.06b | 40.39 ± 0.09e | 28.46 ± 0.1f |
| Leaf Fresh Weight (g) | 26.97 ± 0.1a | 11.01 ± 0.11d | 24.55 ± 0.07c | 24.98 ± 0.14b | 10.47 ± 0.07e | 9.69 ± 0.07f |
| Root Dry Weight (g) | 3.02 ± 0.08a | 1.89 ± 0.1c | 1.99 ± 0.12c | 2.28 ± 0.08b | 1.7 ± 0.06d | 1.44 ± 0.1e |
| Stem Dry Weight (g) | 9.27 ± 0.11a | 6.05 ± 0.11c | 6.07 ± 0.14c | 6.96 ± 0.07b | 4.63 ± 0.11d | 4.57 ± 0.07d |
| Leaf Dry Weight (g) | 4.88 ± 0.11a | 3.54 ± 0.08d | 3.94 ± 0.08c | 4.42 ± 0.08c | 3.07 ± 0.12e | 3.05 ± 0.12e |
| Yellow Leaf Rate (%) | 1.40 ± 1.63f | 58.44 ± 0.65b | 38.9 ± 1.04d | 27.78 ± 1.03e | 40.99 ± 0.73c | 74.2 ± 0.6a |
| Defoliation Rate (%) | 2.36 ± 1.66e | 22.1 ± 0.91b | 15.56 ± 1.19c | 10.65 ± 0.9d | 33.48 ± 1.09a | 16.71 ± 0.78c |
| Relative water content (%) | 92.95 ± 1.75a | 61.68 ± 1.65e | 79.16 ± 1.71c | 86.75 ± 1.51b | 74.25 ± 1.77d | 43.57 ± 1.32f |
| Treatment | Chla (mg/g) | Chlb (mg/g) | Chl a+b (mg/g) | Car (mg/g) |
|---|---|---|---|---|
| CK | 0.7 ± 0.01a | 0.34 ± 0.01a | 1.04 ± 0.01a | 2.07 ± 0.01a |
| T0 | 0.53 ± 0.01d | 0.27 ± 0.01cd | 0.69 ± 0.02f | 1.74 ± 0.04d |
| T1 | 0.58 ± 0.01c | 0.28 ± 0.01c | 0.85 ± 0.02c | 1.83 ± 0.02c |
| T2 | 0.61 ± 0.01b | 0.3 ± 0.01b | 0.92 ± 0.01b | 1.95 ± 0.02b |
| T3 | 0.54 ± 0.01d | 0.27 ± 0.01d | 0.8 ± 0.02d | 1.72 ± 0.02d |
| T4 | 0.44 ± 0.01e | 0.25 ± 0.01e | 0.76 ± 0.02e | 1.57 ± 0.02e |
| Treatment | Score of Comprehensive Index | Membership Function Value | D Value | Sorting | ||||
|---|---|---|---|---|---|---|---|---|
| Y1 | Y2 | Y3 | U1 | U2 | U3 | |||
| T0 | −2.113 | −1.807 | −1.070 | 0.179 | 0.131 | 0.134 | 0.163 | 5 |
| T1 | 0.183 | −2.473 | 0.230 | 0.427 | 0.000 | 0.557 | 0.333 | 3 |
| T2 | 5.507 | 0.257 | 0.720 | 1.000 | 0.540 | 0.718 | 0.861 | 1 |
| T3 | 0.200 | 2.587 | −1.480 | 0.429 | 1.000 | 0.000 | 0.530 | 2 |
| T4 | −3.780 | 1.430 | 1.593 | 0.000 | 0.770 | 1.000 | 0.275 | 4 |
| Equal weighting | 0.667 | 0.244 | 0.087 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Ma, J.; Liu, Y.; Tan, X.; Li, X.; Xu, E.; Xu, L.; Luo, A. Heat Stress Alleviation by Exogenous Calcium in the Orchid Dendrobium nobile Lindl: A Biochemical and Transcriptomic Analysis. Int. J. Mol. Sci. 2023, 24, 14692. https://doi.org/10.3390/ijms241914692
Fan Y, Ma J, Liu Y, Tan X, Li X, Xu E, Xu L, Luo A. Heat Stress Alleviation by Exogenous Calcium in the Orchid Dendrobium nobile Lindl: A Biochemical and Transcriptomic Analysis. International Journal of Molecular Sciences. 2023; 24(19):14692. https://doi.org/10.3390/ijms241914692
Chicago/Turabian StyleFan, Yijun, Jie Ma, Yuanyuan Liu, Xueyan Tan, Xuebing Li, Erya Xu, Linlong Xu, and Aoxue Luo. 2023. "Heat Stress Alleviation by Exogenous Calcium in the Orchid Dendrobium nobile Lindl: A Biochemical and Transcriptomic Analysis" International Journal of Molecular Sciences 24, no. 19: 14692. https://doi.org/10.3390/ijms241914692
APA StyleFan, Y., Ma, J., Liu, Y., Tan, X., Li, X., Xu, E., Xu, L., & Luo, A. (2023). Heat Stress Alleviation by Exogenous Calcium in the Orchid Dendrobium nobile Lindl: A Biochemical and Transcriptomic Analysis. International Journal of Molecular Sciences, 24(19), 14692. https://doi.org/10.3390/ijms241914692







