1. Introduction
Zinc (Zn) is an essential trace element for most living organisms and forms an integral component of numerous enzyme systems. In humans, it ranks as the second most abundant transition metal after iron and is involved in a plethora of biological processes, including but not limited to structural, catalytic, and signaling functions [
1]. Zinc is predominantly intracellular (95%), with half of it being found in the cytoplasm, 40% in the nucleus, and 10% associated with the membranes [
2,
3]. The control of cellular zinc homeostasis is regulated by two prominent families of transporters: the Zrt- and Irt-like proteins (ZIP) and zinc transporters (ZnT). On one hand, ZIP transporters facilitate the influx of zinc ions from the extracellular milieu into the cell, while ZnT transporters mediate the efflux of zinc ions from the cytosol to the extracellular space. On the other hand, these transporters also play a crucial role in maintaining zinc ion homeostasis by facilitating the transport of zinc ions between the cytosol and specific organelles such as the Golgi apparatus, endoplasmic reticulum (ER), and lysosomes [
3]. The concentration of cytosolic labile zinc ions, also referred to as free zinc, is maintained at a minimal level, as it may prove harmful for several physiological reactions. As such, metallothioneins regulate this level to the picomolar range [
4]. These small cysteine-rich proteins, present in four major isoforms, are key proteins involved in zinc and copper homeostasis in mammals. It is estimated that at least 10% of the proteome is capable of binding zinc [
5]. In proteins, zinc can bind to nitrogen, oxygen, and sulfur atoms, enabling its coordination with several amino acids. Zinc-binding proteins play an essential role in cell growth, division, differentiation, cellular signalization, apoptosis, redox homeostasis, membrane structure, transcription, and DNA replication. Zinc finger proteins (ZFs) are a major class of zinc-binding proteins that have non-covalently bound zinc to cysteine and histidine amino acid residues, ensuring proper folding of the protein and facilitating its activity [
6,
7,
8]. Generally, zinc finger domains enable interactions with a variety of biomolecules, such as DNA, RNA, proteins, or lipids, depending on the zinc finger motif. Zinc finger proteins are abundant in eukaryotes, but they are also present in bacteria, archaea, and viruses.
The human immunodeficiency virus (HIV) is an enveloped, linear, positive-sense single-stranded RNA virus belonging to the Retroviridae (Group VI) family under the genus
Lentivirus. HIV is the etiological agent of acquired immunodeficiency syndrome (AIDS), and it targets and infects immune cells, ultimately leading to immunosuppression. With roughly 1.5 million deaths attributable to AIDS each year, HIV infection is a critical global health issue. HIV has two types, HIV-1 and HIV-2, which vary in their epidemiology and pathogenesis. While HIV-2 is largely limited to West Africa, HIV-1 has spread worldwide, owing to its high infectivity and virulence. The HIV-1 genome, which comprises roughly 9.7 kilobases of genomic RNA (gRNA), is reverse transcribed into DNA upon entering the host cell, followed by integration into the host genome to produce new infectious particles. The viral life cycle is intricate, subject to extensive transcriptional and translational regulation, and involves interactions with numerous host cell components [
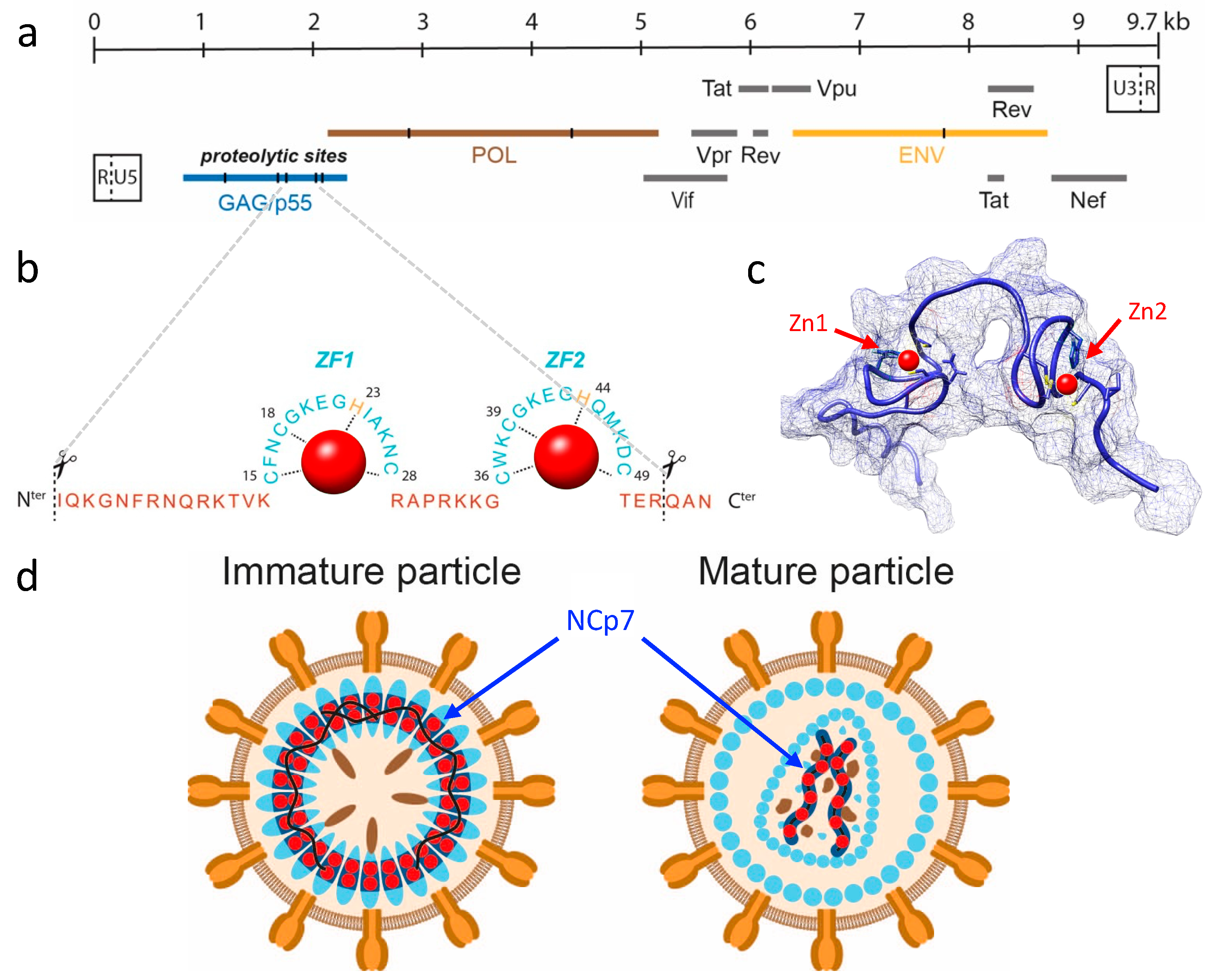
9]. As illustrated in
Figure 1a, the HIV-1 genome encodes 15 viral proteins and 2 peptides expressed from 9 open reading frames (ORFs), each with specific roles in the infection process. Among these proteins, nucleocapsid (NCp7), a small structural protein of 55 amino acids (7 kDa), is generated via sequential processing of the p55 Gag precursor polyprotein (Pr55
gag; see
Figure 1b,c). NCp7 is involved in multiple stages of HIV-1 replication, such as reverse transcription of gRNA, integration of DNA into the host genome, and recruitment of two gRNA molecules into viral particles (
Figure 1d) [
10,
11]. NCp7 contains two CCHC zinc-finger motifs, which are required for its nucleic acid binding activities. It is estimated that each HIV-1 viral particle contains approximately 5000 NCp7 molecules, equating to 10,000 zinc atoms [
12]. Given the inner diameter of a spherical HIV-1 virion (approximately 100 nm) and its volume of roughly 4.2 × 10
−18 L, a high intra-particle zinc concentration is inferred to be in the order of 4 millimolar, rendering this element one of the most abundant inorganic components in HIV-1 particles.
Zinc is a mixture of five stable isotopes, which differ solely in their neutron number and exhibit varying relative abundances:
64Zn (48.268%),
66Zn (27.975%),
67Zn (4.102%),
68Zn (19.024%), and
70Zn (0.631%). As long employed by geologists, the isotopic ratios of a particular chemical element can display distinct behaviors during physical, chemical, or biochemical processes, leading to variations in the isotopic ratios [
14,
15,
16]. Over the past two decades, a rising number of reports have indicated isotopic fractionation in biological processes, particularly for calcium, iron, copper, and zinc [
17,
18,
19,
20,
21,
22]. Zinc isotope fractionation has been identified in various physiological [
23,
24] and pathological conditions [
24,
25,
26,
27], including the brains of Alzheimer’s and Creutzfeldt–Jakob disease-specific mouse models, relative to control mice. Moreover, in humans, the zinc isotopic composition of blood became increasingly enriched with heavy isotopes with aging, as reported in [
23]. While the exact reasons for the isotopic fractionation of zinc remain unknown, it is suggested that variations in the binding of zinc to various zinc finger proteins and metallothioneins, which exhibit different expressions based on the tissue or its pathophysiological state, may be a contributing factor. Ab initio calculations have demonstrated that heavy zinc tends to be enriched in complexes with histidine relative to cysteine [
28]. In biology, the molecular configuration, redox conditions, and kinetics are known to be the primary driving forces for isotopic variations [
15,
22]. Since zinc does not participate in redox reactions, the sole factors influencing its isotopic fluctuations encompass molecular binding as well as transportation across membranes, either through active mechanisms (ZIP or ZnT) or passive diffusion. Therefore, zinc is an element that exhibits a relatively low range of isotopic fractionation.
In addition to natural isotopic fractionation, the use of isotopes for labeling purposes has played a critical role in biochemical research, particularly in elucidating various metabolic pathways [
21,
29]. Isotope exchange and all other applications of isotopes for labeling purposes rely on the assumption that isotopic substitution of an atom in a molecule does not affect its chemical properties and that any effect on its kinetic properties is sufficiently small to be neglected in the analysis. Conversely, the analysis of isotopic effects assumes that measurable differences exist between the kinetic or equilibrium properties of molecules substituted with isotopes. The mass difference between the isotopes of a chemical element can indeed be sufficient to modify the physical and chemical properties of molecules made from different isotopes. In the case of zinc and other transition metals, the analysis of isotopic effects on biological or biochemical activity has never been reported to the best of our knowledge.
The replication and infectivity of HIV-1 virus critically depend on the activity of its zinc finger protein NCp7, which itself relies on the presence of zinc atoms [
11]. Even slight perturbations of the zinc finger modules of NCp7 can induce detectable changes in its viral activity since it is involved in multiple stages of viral replication [
30]. Therefore, this model would be highly relevant for investigating a potential isotopic effect of zinc on the function of a zinc finger protein by following the viral production and infectivity of the produced particles. Initially, our work aims at determining whether viral particles can recruit enough zinc to measure isotopic fractionation and compare them to cells and a culture medium. In addition, we have designed and developed an experimental protocol to enrich cells with specific zinc isotopes in order to measure the impact of isotopically enriched zinc on the production, morphology, and infectivity of HIV-1.
3. Discussion
The investigation of isotopic composition variations in biology has garnered increasing interest, as isotopic fractionation can arise from biological processes and be a biomarker of physiological changes or pathological conditions [
14,
15]. In addition, the use of stable isotopes addresses fundamental questions about metabolism, cell signaling, protein dynamics, and other biological processes [
17,
18,
19,
20,
21,
22]. Most importantly, the use of stable isotopes as tracers assumes that the isotopic nature does not influence the chemical reactivity of the element [
21,
29]. In the present study, we challenged this presumption within the exceptionally delicate framework of viral replication by investigating the correlation between zinc isotopes and the zinc-binding structural protein NCp7 of HIV-1.
The manipulation of zinc by viruses remains poorly elucidated to date, despite the fact that numerous viruses encode proteins that bind zinc for structural or enzymatic functions. A bioinformatics analysis of 226 distinct viral genomes revealed that the majority of viral strains (72%) expressed zinc-binding proteins, indicating the significance of this essential metal ion to viruses [
32]. Furthermore, 77% of the zinc-binding domains within viral proteomes belong to the zinc finger type, recognized as the most highly conserved fold. Among these viral proteins, those with a structural role within viral particles are only found in a limited number of viruses. This is the case for the nucleocapsid of retroviruses, such as NCp7 for HIV-1. This is also true for the nucleocapsid of some arenaviruses (Guanarito mammarenavirus, Junin mammarenavirus, Lassa virus, and Lymphocytic choriomeningitis virus) [
32]. A few other examples involve capsid proteins in some plant and insect viruses (Caulivirus, Begomovirus, and Baculovirus). In summary, although zinc plays an important role in viral replication, its involvement in the core of the virion particle remains relatively limited to a subset of viruses including retroviruses.
In the context of HIV-1 viral particles, it is noteworthy that additional zinc-binding proteins, namely Vif, Tat, and integrase, are also present within the virions, albeit in significantly lower quantities compared with NCp7 [
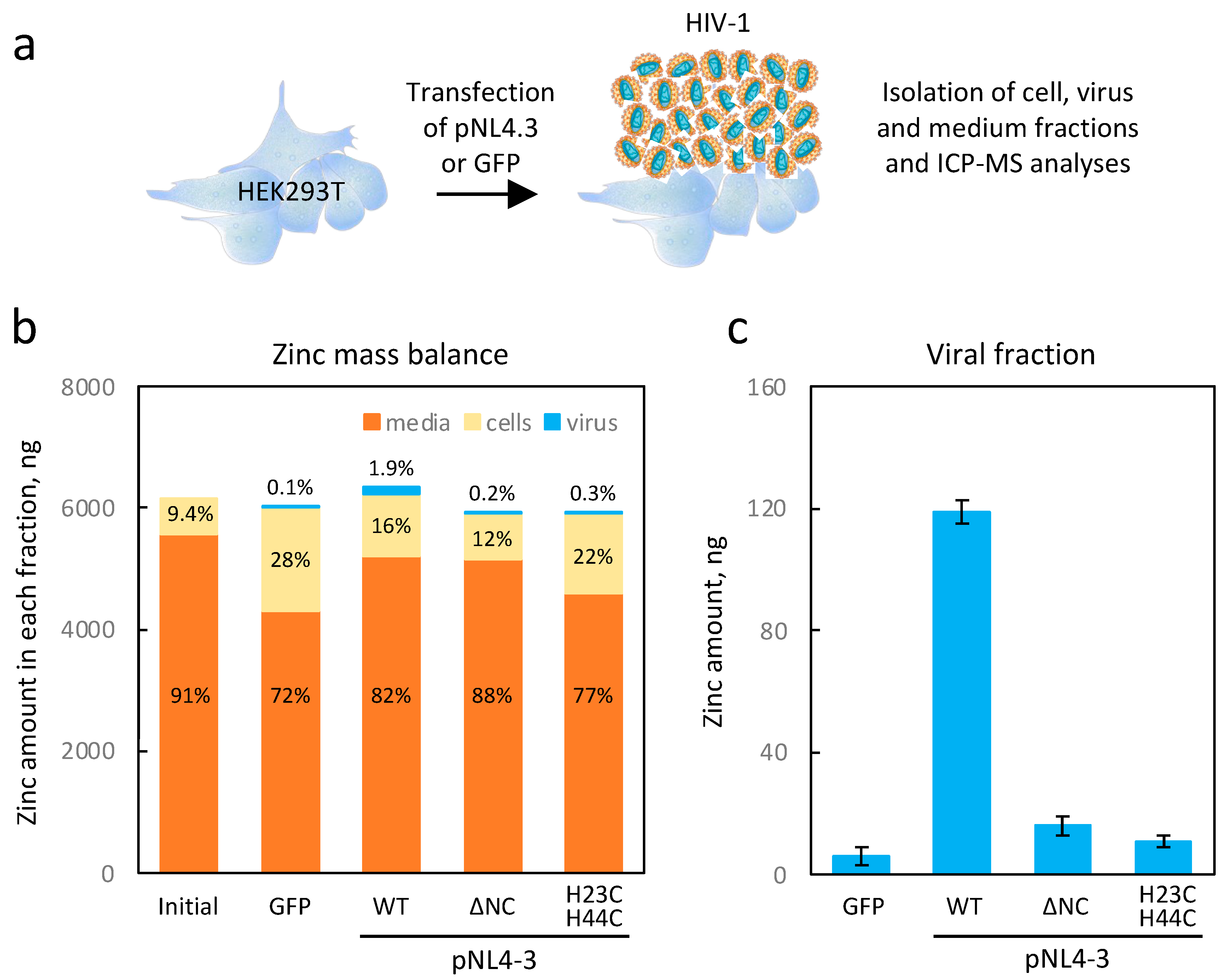
12]. In our study, we have demonstrated that high viral production results in the accumulation of zinc in HIV-1 particles (
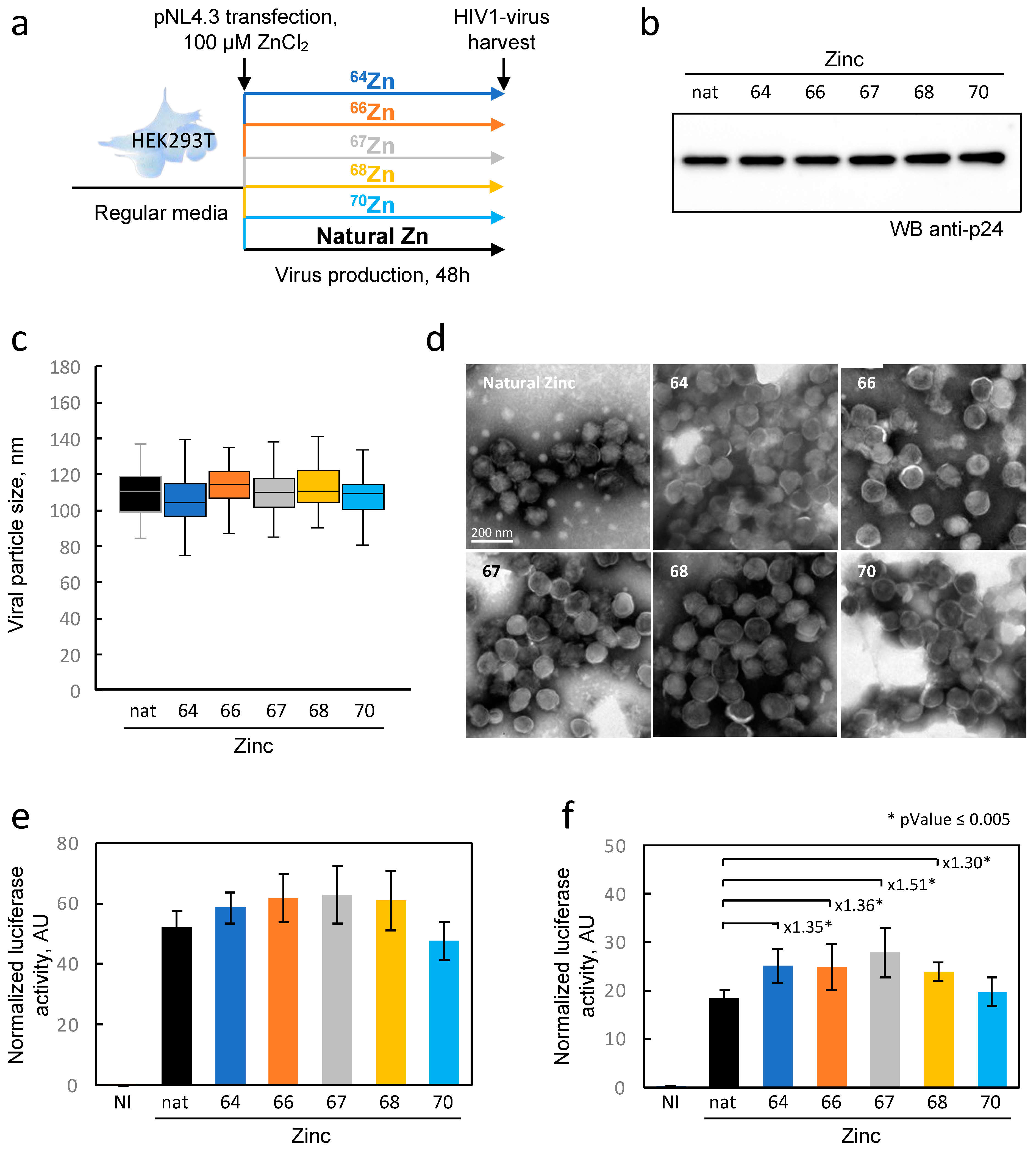
Figure 2). Indeed, we substantiated through ICP-MS analysis that the zinc concentration within the HIV viral particle amounted to approximately 16 mM (~40,000 atoms per particle), potentially rendering it one of the most concentrated inorganic elements in the HIV-1 virion. Our experiments on HEK293T cells transfected with the pNL4-3 plasmid showed that the virions captured up to 10% of the amount of cellular zinc within 48 h. More importantly, by using mutants of NCp7, we have provided evidence that this zinc uptake was merely due to the properties of NCp7 and confirmed that the contributions of Vif, Tat, and integrase proteins to the zinc capture within HIV-1 particles remained low. Such data were a prerequisite to establish a potential isotopic fractionation associated with the presence of NCp7 within the viral particle.
As mentioned earlier, a significant amount of cellular zinc is captured by HIV-1 virions. The question of whether this uptake of zinc by the produced viral particles can cause a zinc deficiency in the organism remains to be determined. However, it is worth noting that zinc deficiencies often stem from the inflammatory processes linked to viral infections, as evidenced notably in hospitalized patients with SARS-CoV-2 [
33,
34]. Often, viral infections not only increase the requirement for micronutrients but also result in their depletion, thereby leading to deficiencies that can be compensated by the supplementation of micronutrients [
35].
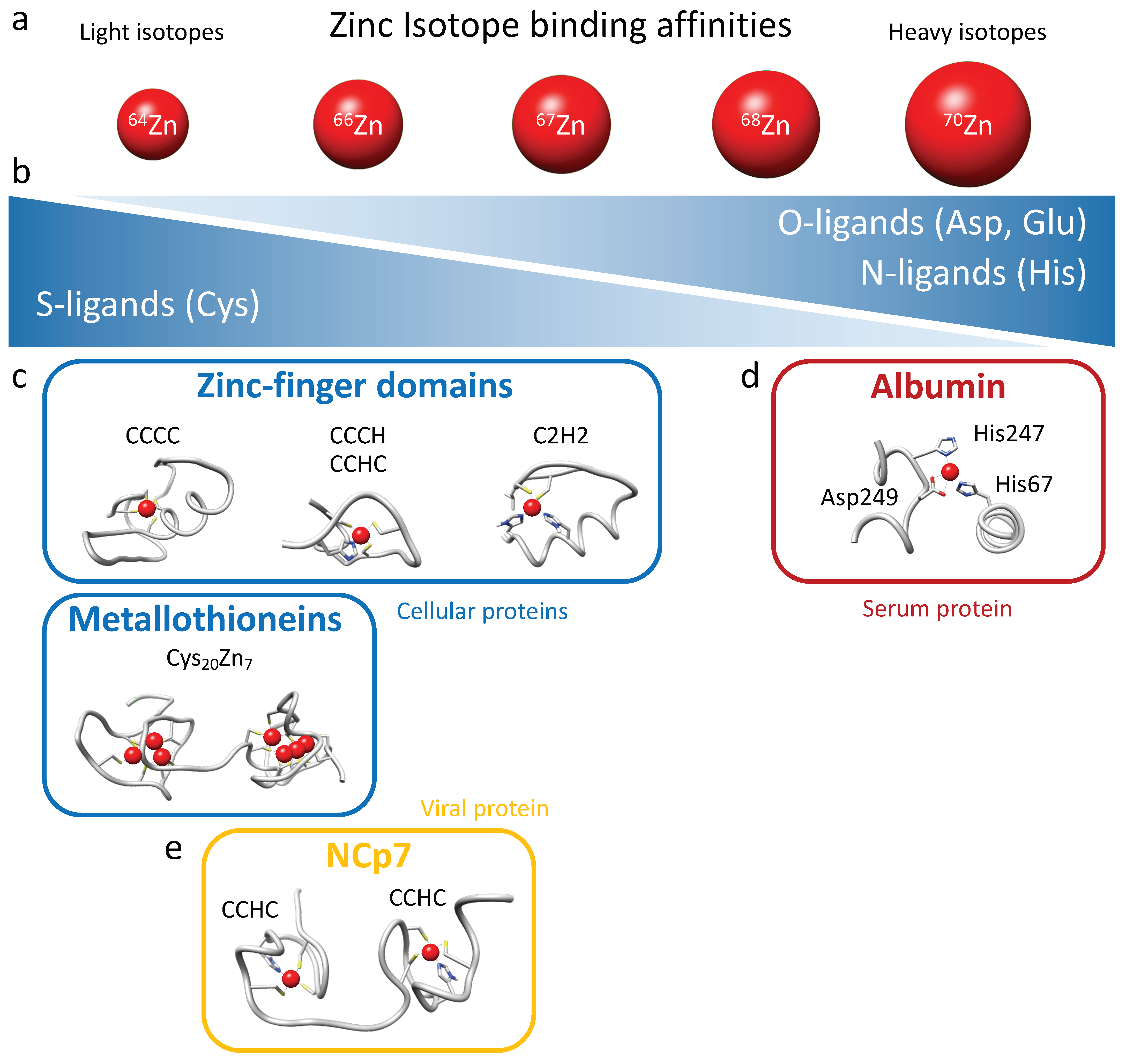
The isotopic fractionation of zinc isotopes in cells is influenced by coordination chemistry [
28]. Heavier isotopes have higher bond energies, and thus they tend to be enriched in the strongest ligand bonds. Therefore, cysteine (S-ligands) preferentially binds lighter zinc isotopes, while histidine (N-ligands) and aspartate (O-ligands), which generally exhibit stronger binding, preferentially interact with heavier zinc isotopes (
Figure 6a,b). Isotopic fractionation is increasingly being investigated in biology research due to the advancement of analytical techniques that can now determine the isotopic fractionation of elements present in extremely low concentrations in samples (to the order of nanograms per gram of sample) [
21,
22,
24]. The presence of zinc in numerous chemical compounds makes the analysis of its isotopic fractionation challenging and laborious. Moreover, in general, zinc exhibits relatively low isotopic fractionation since it is redox-inactive. For these reasons, isotopic fractionation of zinc has never been measured in viruses, not even during viral infection. In the present study, we demonstrated for the first time a significant and reproducible fractionation of zinc between different types of cells, whether they were adherent cells (HEK293T) or CD4 lymphocytes in suspension (Jurkat, CEM-T4, and SupT1), and their respective culture media. All cells tested here were indeed enriched with lighter isotopes of zinc. Zinc isotopic fractionation in cellular zinc uptake can be attributed to a wide range of factors due to the significant number of proteins binding this element. Binding ligands for zinc in proteins include histidine, glutamate, aspartate, and cysteine residues (
Figure 6b). Histidine-rich loops typically form the primary zinc-binding sites for ZIPs and ZnTs, while cysteine-rich ligands are involved in zinc binding on metallothionein and zinc finger proteins [
36]. Metallothioneins bind up to 7 Zn
2+ coordinated tetrahedrally using a set of 20 cysteines clustered into two distinct domains separated by a linker: an N-terminal β domain that uses 9 cysteines to bind 3 metals, and a C-terminal α domain that binds 4 metals with 11 cysteines (
Figure 6c) [
37,
38]. Cellular zinc finger domains exhibit significant variability in their composition of cysteine and histidine residues, albeit with a majority of cysteines participating in Zn
2+ coordination (CCCC, CCHC, CCCH, and C2H2 motifs) [
6,
7,
8]. Therefore, considering the abundance of metallothioneins, the composition of zinc finger motifs, and the quasi-absence of intracellular free zinc, it follows that intra-cellular zinc predominantly binds to cysteine residues in proteins and thus favors fractionation toward light isotopes in cells (
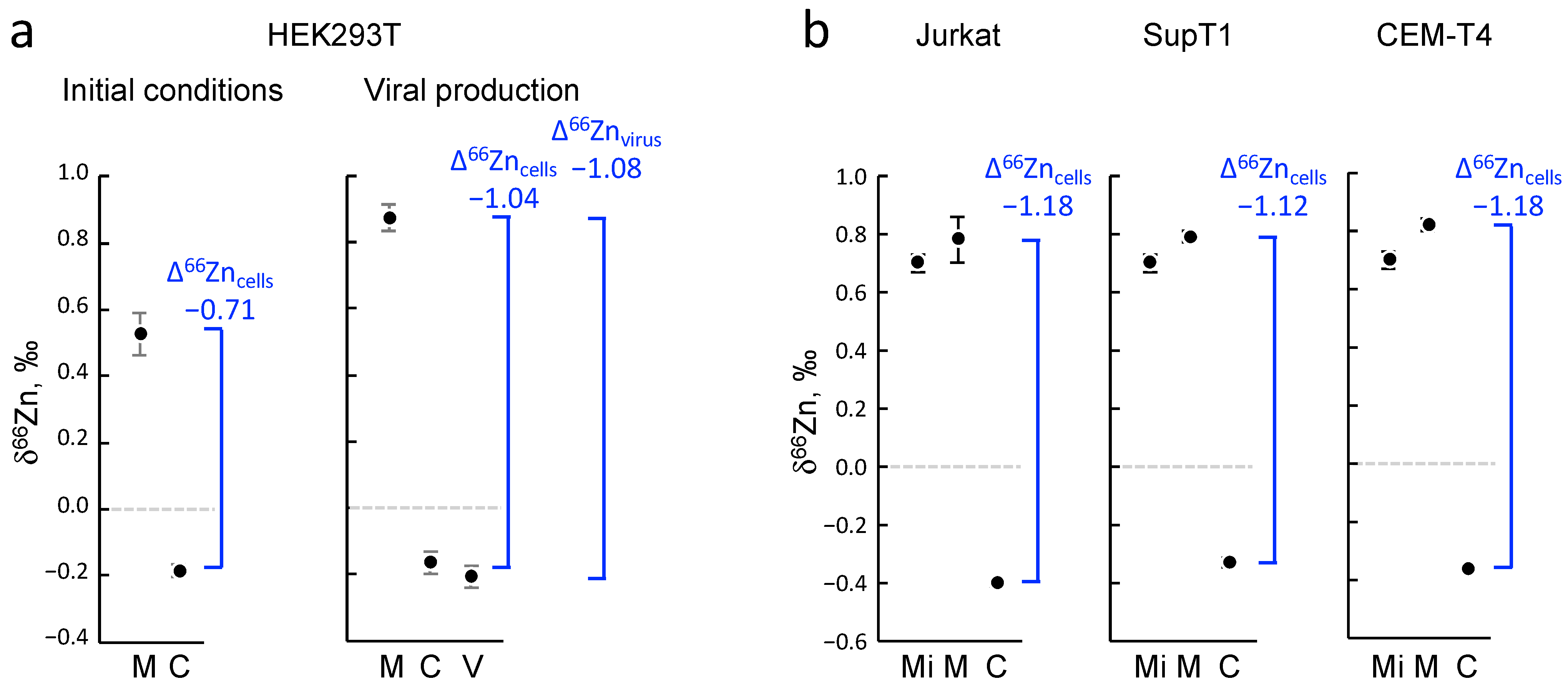
Figure 6). The variability in zinc finger composition and the predominance of metallothioneins play a critical role in tissue- or cell-specific isotopic fractionation. This phenomenon probably accounts for the enrichment of lighter isotopes in total intracellular zinc compared with the surrounding media, assuming that serum proteins predominantly bind zinc via histidine and aspartate residues.
In the serum of mammals, approximately 75–80% of Zn
2+ is bound to albumin, accounting for as much as 98% of the exchangeable fraction of Zn
2+ in blood [
39,
40,
41]. The principal binding site, also called the “multi-metal binding site”, is a tetrahedral coordination of Zn
2+ by three amino acid residues (His67, His247, and Asp249;
Figure 6d), as inferred from X-ray crystallography of human (HSA) and equine serum albumins [
40]. Serum albumin effectively acts as an extracellular “zinc buffer” that controls the concentrations of “free” Zn
2+ ions that are available to other serum proteins or for cellular uptake through membrane-bound zinc transporters [
42]. The presence of albumin as the primary zinc binder in serum, through histidine and aspartate residues, provides an explanation for the fractionation toward the heavier zinc isotopes observed in the culture medium (
Figure 6d).
Our investigation sought to ascertain whether the absorption of zinc by HIV-1 viral particles exhibits any discernible isotopic fractionation with producing cells. While a distinct fractionation was observed between the cells and growth media, attributable to evident zinc-ligand differentiations, no significant disparity was noted between the HIV-1 particles and producer cells. This outcome was likely influenced by the minimal distinction in binding activity between the CCHC motifs of NCp7 and those of intracellular zinc-fingers proteins harboring CCCC, CCHC, CCCH, and C2H2 motifs and metallothioneins (
Figure 6c,e). Nevertheless, a clear distinction was observed between the HIV-1 particles and culture media.
In the absence of treatment, HIV infection progresses through three successive stages: the primary infection (a few weeks), a lengthy latency phase (10–12 years) without symptoms, and then the AIDS phase [
9,
43,
44]. Viral replication is most intense in the initial and final stages of the infection. Investigating the isotopic fractionation of zinc at these three stages would determine whether viremia alone is sufficient to alter the isotopic ratios in the serum through recruiting light isotopes in the HIV-1 viral particles. Additionally, the death of the CD4 cells during the AIDS phase triggers the release of intracellular zinc, and this event could also contribute to changes in the serum isotopic ratios. This would represent a novel instance of fractionation associated with a pathological condition. These hypotheses await further in vivo investigations.
To the best of our knowledge, there have been no prior reports, particularly in the context of zinc and other transition metals, regarding the investigation of isotopic influences on biological or biochemical functions. Here, we investigated a possible isotopic effect of zinc on HIV-1 viral fitness. We examined whether the loading of HIV-1 virus-producing cells with different zinc isotopes resulted in alterations in the quantity, morphology, and infectivity of the produced particles. It can be postulated that changes in atomic size or nuclear spin could have subtle but detectable effects in a highly sensitive system. HIV-1 viral replication involves the protein NCp7 at numerous key stages, and point mutations in zinc fingers can have deleterious effects on the quantity, morphology, and infectivity of the produced particles [
10,
11,
30].
All zinc isotopes have zero nuclear spin (even atomic numbers), except for the zinc isotope 67 (67Zn). Isotopes with nonzero nuclear spin possess an intrinsic magnetic moment that creates a local magnetic field around the nucleus, which can influence interactions with other atoms or molecules during the formation of chemical bonds. These magnetic interactions can have subtle yet significant effects on the chemical properties of the bond. Therefore, particular attention must be directed toward the 67Zn isotope among all others.
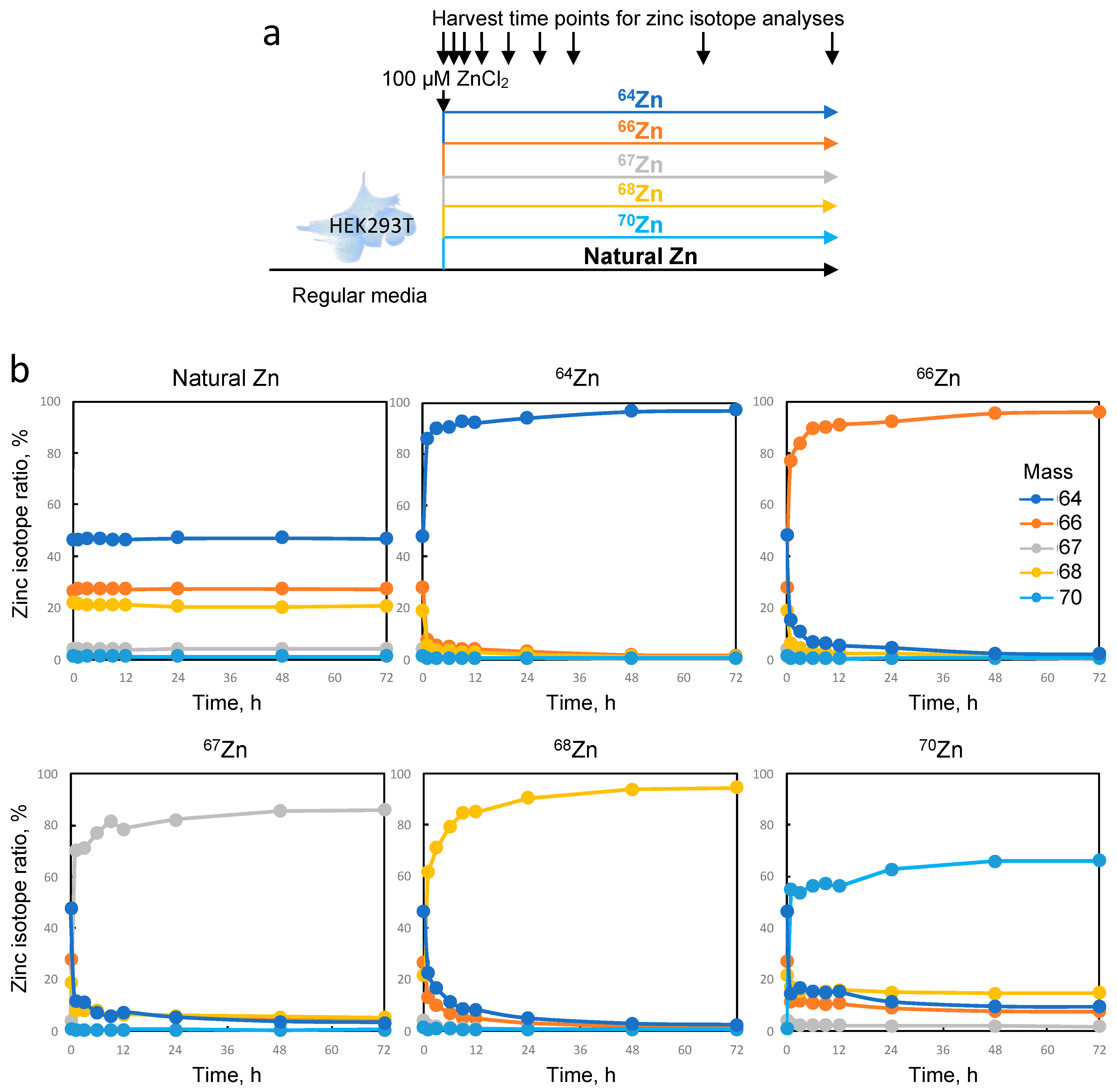
In our experimental protocol, we produced viral particles in HEK293T cells loaded with each of the enriched zinc isotopes. Although the isotopic purity was not absolute, this allowed us to load the cells with 97%, 96%, 86%, 94%, and 66% of isotopes 64Zn, 66Zn, 67Zn, 68Zn, and 70Zn, respectively, and to compare them to cells loaded with naturally distributed zinc. Although no significant alteration in particle morphology or quantity was observed, subtle variations in HIV-1 infectivity were detected between the enriched isotopes and natural isotopes. In the field of virology, these changes may not be considered drastic, but they could indicate a minimal influence of the isotope composition on NCp7 activity. Further experiments using recombinant purified proteins saturated with various zinc isotopes could provide further insights into the potential impact of isotopes on biological activity. To conclude, our data clearly provides evidence that the use of zinc isotopes as tracers for viral replication or any other research on cellular metabolism appears to be reasonable since these effects are minimal. Approaches in elemental imaging using these zinc tracers could potentially enable the visualization of the fate of zinc initially contained within the viral particle inside infected cells.
4. Materials and Methods
4.1. Materials
The Jurkat, SupT1, CEM-T4, and HEK293T cells lines used in this study were obtained from ATCC. The TZM-bl cells were obtained from the NIH AIDS Reagent program (Manassas, VA, USA). The cell culture media and supplements, NuPAGE 4–12% bis–Tris polyacrylamide gels, MOPS, and MES SDS running buffer were purchased from Life Technologies (ThermoFisher Scientific, Waltham, MA, USA). Fetal calf serum (FCS), sucrose, NaCl, Tris, DMSO, EDTA, Triton X100, glycerol, and DTT were purchased from Merck (Darmstadt, Germany). The microplate readers (FLUOSTAR OPTIMA and LUMISTAR OPTIMA) were from BMG Labtech (Champigny-sur-Marne, France). The plasmid pNL4.3 and anti-p24 antibodies were obtained from the NIH AIDS Reagent program. The mutant pNL4.3 plasmids were given by D. Muriaux and described in [
30]. The different isotopically enriched forms of zinc were purchased in metallic form from Isoflex (
64Zn,
66Zn, and
68Zn) and Eurisotop (
67Zn and
70Zn). The metal form of natural zinc was purchased from Merck (#31653).
In order to prevent sample contamination during collection, all plastic and Teflon equipment (Falcon tubes, tips, and ultra-centrifugation tubes) were pre-cleaned in a cleanroom facility with hydrochloric acid for a minimum of 48 h, followed by rinsing and drying. Chemical processing was carried out in a clean room equipped with a reverse filtration system comparable to a biosafety level 3 containment laboratory, specifically designed to protect samples from external contaminants and pollution. All the reagents used were purified by sub-boiling distillation, and appropriate dilutions were made with 18.2 MΩ cm-grade MilliQ water.
4.2. Cell Culture
The adherent cells (HEK293T and TZM-bl) were grown and maintained in 75 cm2 plates in Dulbecco’s Modified Eagle Medium (D-MEM). The cells in suspension (Jurkat, SupT1, and CEM-T4 cells) were cultured in Roswell Park Memorial Institute medium (RPMI). Media were supplemented with 10% fetal calf serum, 100 μg/mL streptomycin, 100 U/mL penicillin, and 2 mM L-glutamine. Only for the RPMI medium were 1 mM pyruvate and 10 mM HEPES added. The cells were cultivated at 37 °C in a humidified atmosphere containing 5% CO2.
4.3. HIV-1 Production in HEK293T for ICP-MS Analysis
HIV-1 viral particles were generated through transient calcium phosphate transfection of HEK293T cells with pNL4.3 plasmid DNA (wild type or mutants) in a biosafety level 3 containment laboratory. The HEK293T cells were seeded at a density of 9 × 106 cells per plate 15 cm in diameter the day before transfection. Each experimental condition required 8 plates to produce a sufficient quantity of virus for zinc isotopic fractionation measurement. A single plate was sufficient for a simple measurement of the total zinc quantity. Twenty-three micrograms of plasmid were transfected with calcium phosphate precipitation. The medium was then replaced with fresh medium 6 h later. Two days after transfection, both the medium and cells were collected. The supernatant was collected and centrifuged at 4000× g for 10 min to eliminate cellular debris and then ultracentrifuged at 110,000× g using a Beckman SW32 rotor for 1 h and 30 min on a TNE sucrose cushion (10 mM Tris, 100 mM NaCl, and 1 mM EDTA) with 20% sucrose to pellet the virus. An aliquot of the supernatant after ultracentrifugation was collected, and the virus pellet was resuspended in ultrapure water. In parallel, the cells were detached by scrapping in 1× PBS and washed by centrifugation at 500× g for 10 min. The cellular pellet was resuspended in ultrapure water. The supernatant, virus, and cells were either aliquoted for biochemical analysis or mixed in equal volumes with 13 N nitric acid in a Teflon beaker (Savillex®) for ICP-MS measurements. Nitric acid was used to inactivate the samples and remove them from the biosafety level 3 containment laboratory. To quantify the production of HIV-1 in HEK293T cells, we performed an anti-p24 ELISA (HIV-1 Gag p24 DuoSet ELISA, Biotechne) following the manufacturer instructions.
4.4. Analyses of Zinc Concentrations through ICP-MS
All the samples coming out of the biosafety level 3 laboratory in concentrated nitric acid media were treated for elemental and isotopic analysis in a metal-free clean-room laboratory under positive air pressure to prevent contamination of the surrounding area. The culture medium, cells, and the viral fraction were digested in HNO3 15 N in Savillex® beakers at 120 °C for 1 day. After evaporation to dryness, they were digested once again in a mixture of HNO3 15 N and H2O2 (30%) at 120 °C for 1 day to ensure complete decomposition of the organic matter. The mixture could be very reactive, leading to the subsequent creation of nitric and carbon-based fumes that needed to be regularly evacuated to avoid any overpressure, particularly in the early hours of the digestion. After complete dissolution, the samples were dried down and taken up in HNO3 0.5 N media, from which an aliquot corresponding to 2% of the sample was collected for elemental concentration measurements. The remaining samples were reserved for chemical purification of the zinc prior to isotopic composition measurement.
The concentration of zinc was determined with inductively coupled plasma mass spectrometry (ICP-MS) with an Agilent 7500 Series ICP-MS or a Thermo Scientific iCAP Q ICP-MS at LGL-TPE. The concentrations were determined using calibration curves based on Zn elemental solutions. The instrumental drift over the course of the analytical session and matrix effects were monitored and corrected using these standard solutions combined with the measurement of indium as an internal standard.
4.5. Analyses of Zinc Isotopic Compositions through MC-ICP-MS
Zinc was isolated and purified by ion exchange chromatography using a Bio-Rad column filled with 0.5 mL of AG 1-X8 (200–400 mesh) anionic resin according to a procedure modified from [
45] and described more in detail in [
46]. Briefly, the samples were loaded onto the column in HBr 1.5 N media. After elimination of the sample matrix with 2 mL of HBr 1.5 N, zinc was eluted with 5 mL of HNO
3 0.5 N. The procedure was repeated twice to ensure the highest purity for better isotope ratio measurement.
The zinc isotopic compositions were measured with multi-collection inductively coupled plasma mass spectrometry (MC-ICP-MS) using Nu Plasma MC-ICP-MS (Nu Instrument, Nu Plasma LR) and Thermo Scientific Neptune Plus MC-ICP-MS. As described in [
47], instrumental mass discrimination and temporal drift were corrected with exponential law using the addition of copper as an internal standard, combined with a sample-standard bracketing technique. The isotopic compositions are reported in conventional delta notation (expressed in ‰) relative to the international isotopic standard solutions JMC 3–0749L Johnson Matthey Royston, UK (JMC Lyon):
The ∆
66Zn values represent the differences between two different δ
66Zn as follows:
All zinc isotopic data in the current study follow the theorized trend for mass-dependent isotopic fractionation, and therefore only δ66Zn is discussed. Wherever possible, the zinc isotopic compositions were analyzed multiple times (three replicate analyses for most samples), and the error values for all isotopic analyses have been reported as two times the standard deviation (2σ), in line with the convention.
4.6. HIV-1 Production in HEK293T in the Presence of Enriched Zinc
For each enriched isotope spike and natural zinc, metal zinc was transformed in ZnCl
2 with ultrapure hydrochroric acid. The concentration and isotopic composition of the zinc was measured with single-quadripole ICPMS (iCAP-Q), and the volumes were adjusted to reach a 100 mM concentration. The isotopic composition is given in
Table S1. The day prior to transfection, the HEK293T cells were seeded at 6.5 × 10
6 cells per plate 10 cm in diameter. The following day, the culture medium was changed, and the cells were transfected 2 h later with 10 μg of pNL4.3 plasmid using calcium phosphate precipitation. After 6 h, the medium was replaced with a medium containing either 100 μM of isotopically enriched or naturally abundant zinc. The medium was changed once more after 6 h with the same media, and the cells were incubated for 48 h at 37 °C with 5% CO
2.
4.7. Quantification of Infectious HIV-1 Produced by HEK293T Cells
The viral particles produced by HEK293T cells were analyzed for their capacity to infect TZM-bl cells, a cell line that carries the luciferase gene under the control of the HIV-1 promoter (LTR). The TZM-bl cells were seeded in 96 well plates. The following day, the virus was serially diluted (1/3) in triplicate and added to the cells, which were then incubated for 48 h at 37 °C with 5% CO2. The cell density in each well was determined using Cell-Titer Fluor (Promega). Then, the cells were lysed, and the luciferase activity was determined using the Luciferase assay system (Promega) following the manufacturer’s protocol. The luminescence was measured using a Tecan luminometer. The luciferase activity was normalized to the cell density for each well.
4.8. Transmission Electron Microscopy (TEM) Analysis
The viruses were concentrated from the supernatant on a TNE sucrose cushion as described above and purified over an optiprep gradient (5–20%). The fractions containing the viral particles were concentrated on a TNE sucrose cushion, and the pellet was then resuspended in 20 µL of a fixation solution containing 4% PFA. The suspensions were adsorbed on 200 mesh nickel grids coated with formvar-C for 10 min at RT. Then, grids with the suspension were colored with phosphotungstique acid 2% (Electron Microscopy Sciences, Hatfield, PA, USA) for 2 min and observed on a transmission electron microscope (Jeol 1400 JEM, Tokyo, Japan) equipped with a Gatan camera (Orius 1000) and Digital Micro-graph software (version 1.7).
4.9. Protein Extraction and Western Blot
Cellular protein extracts aliquoted for biochemical analysis were treated with lysis buffer (25 mM Tris-HCl, pH 7.8, 2 mM DTT, 2 mM EDTA, 1% Triton X-100, and 10% glycerol). Then, the protein concentrations were measured using a DC protein assay kit (Biorad, South Granville, Australia) in microplate assays. Equal protein amounts (30 µg) were separated in 4–12% Bis-Tris NuPAGE Novex Midi Gels and transferred onto nitrocellulose membranes using an iBlot Dry blotting system (ThermoFisher Scientific). The membranes were probed with anti-p24 primary antibodies and HRP-conjugated anti-mouse secondary antibodies. The chemiluminescence signal was detected using an ECL Select detection kit (GEHealthcare, Chicago, IL, USA) in a Chemidoc Imager (Biorad).