Differential Effects of Hypothermia and SZR72 on Cerebral Kynurenine and Kynurenic Acid in a Piglet Model of Hypoxic–Ischemic Encephalopathy
Abstract
:1. Introduction
2. Results
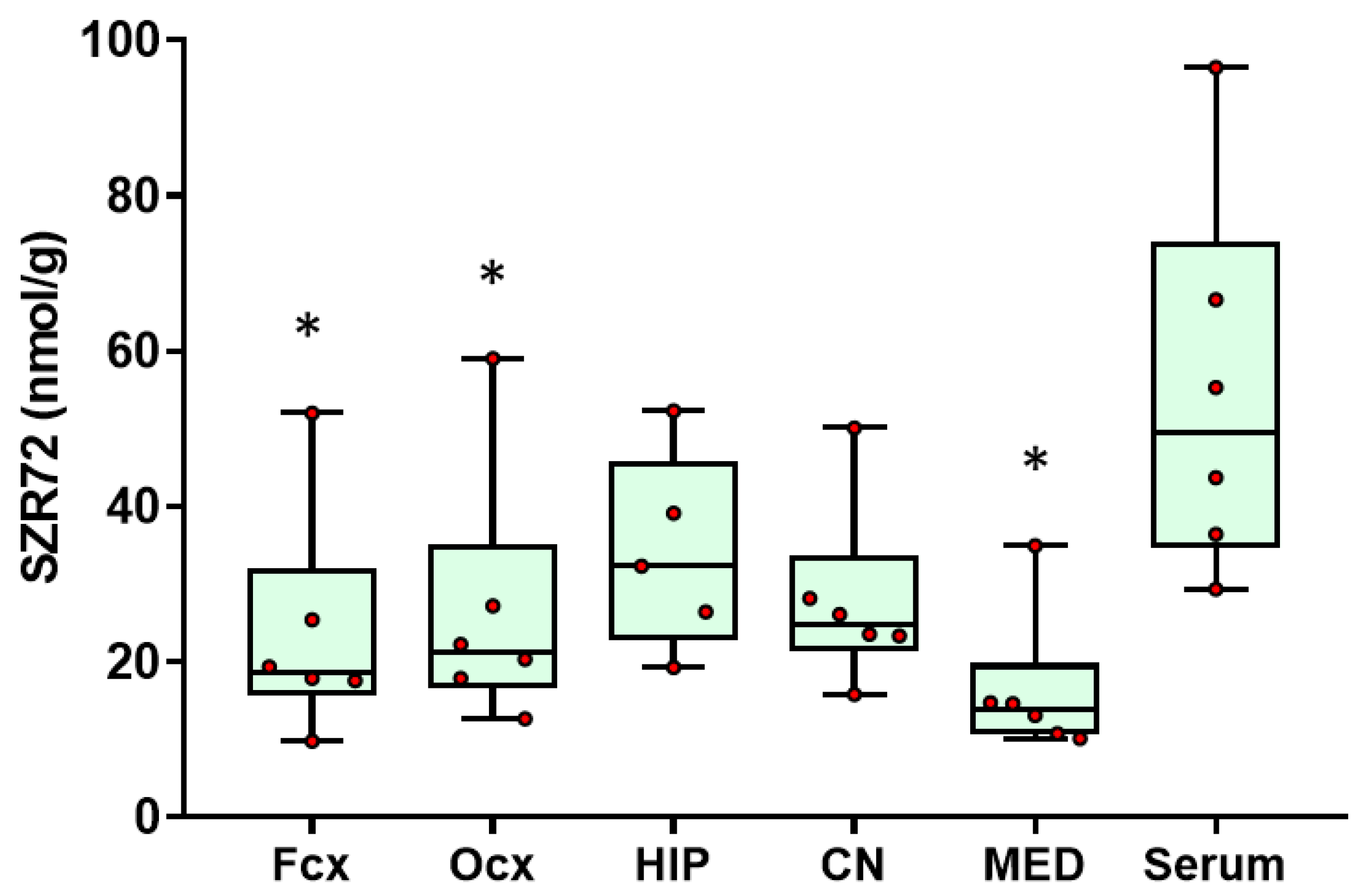
2.1. SZR72 Levels
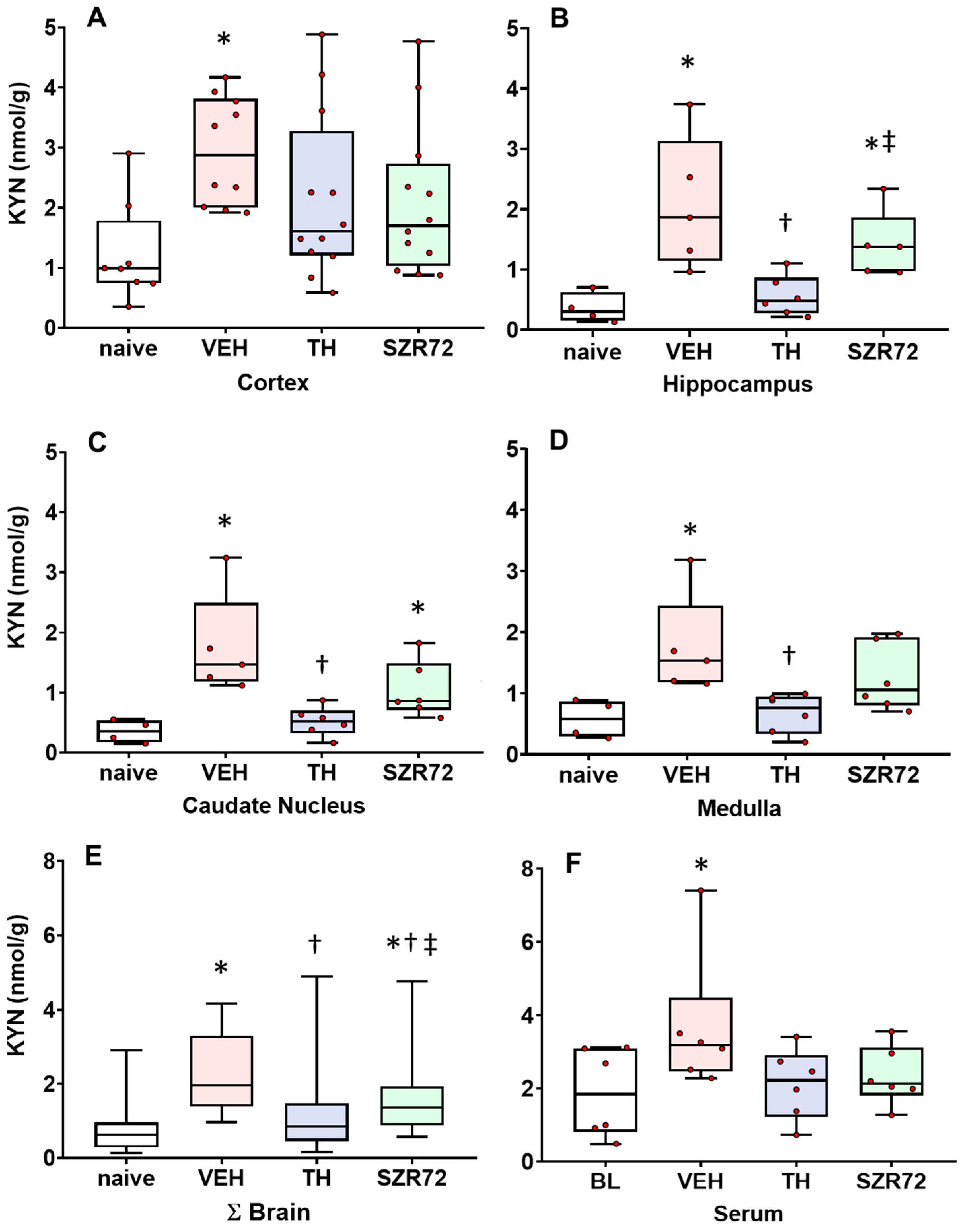
2.2. KYN Levels
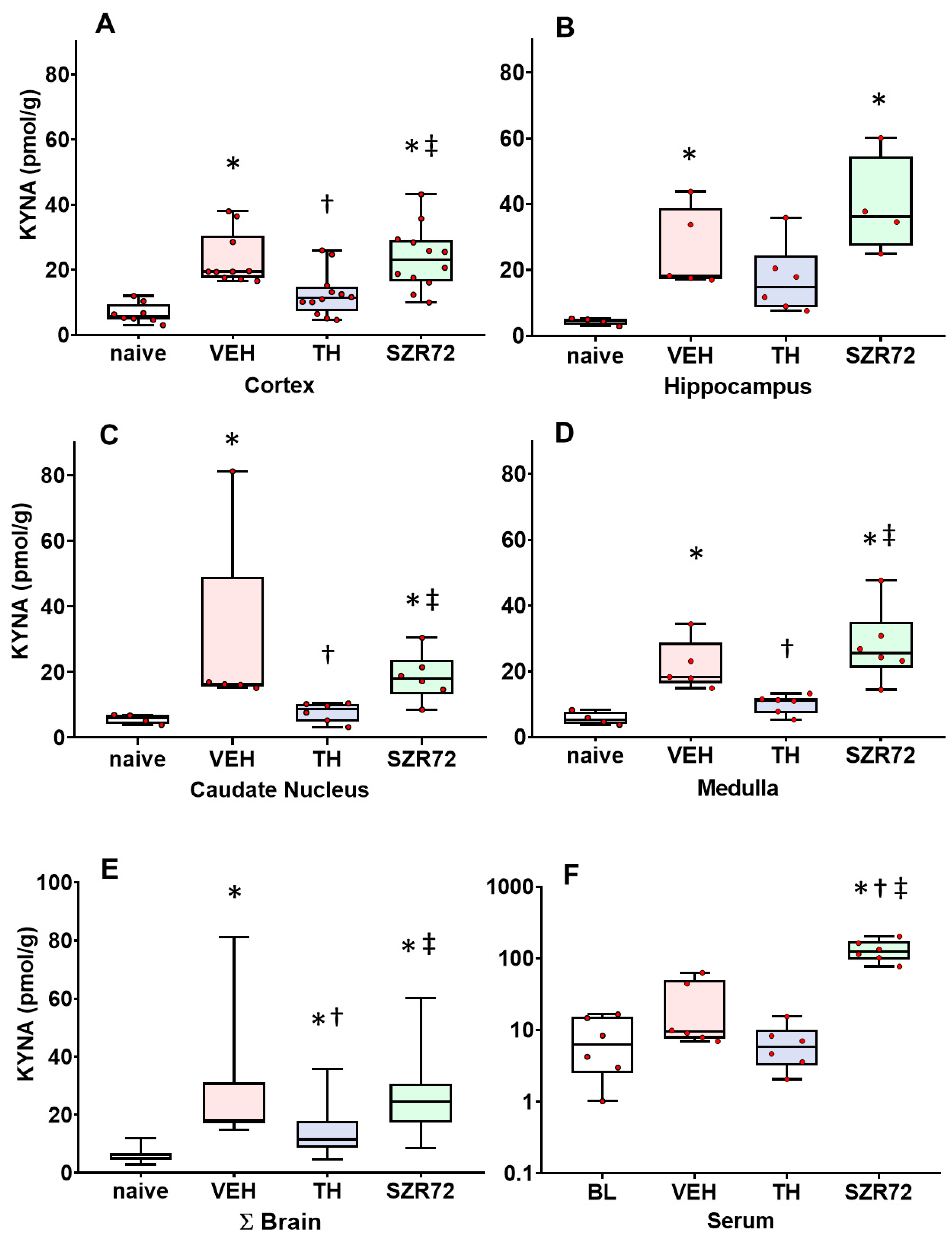
2.3. KYNA Levels
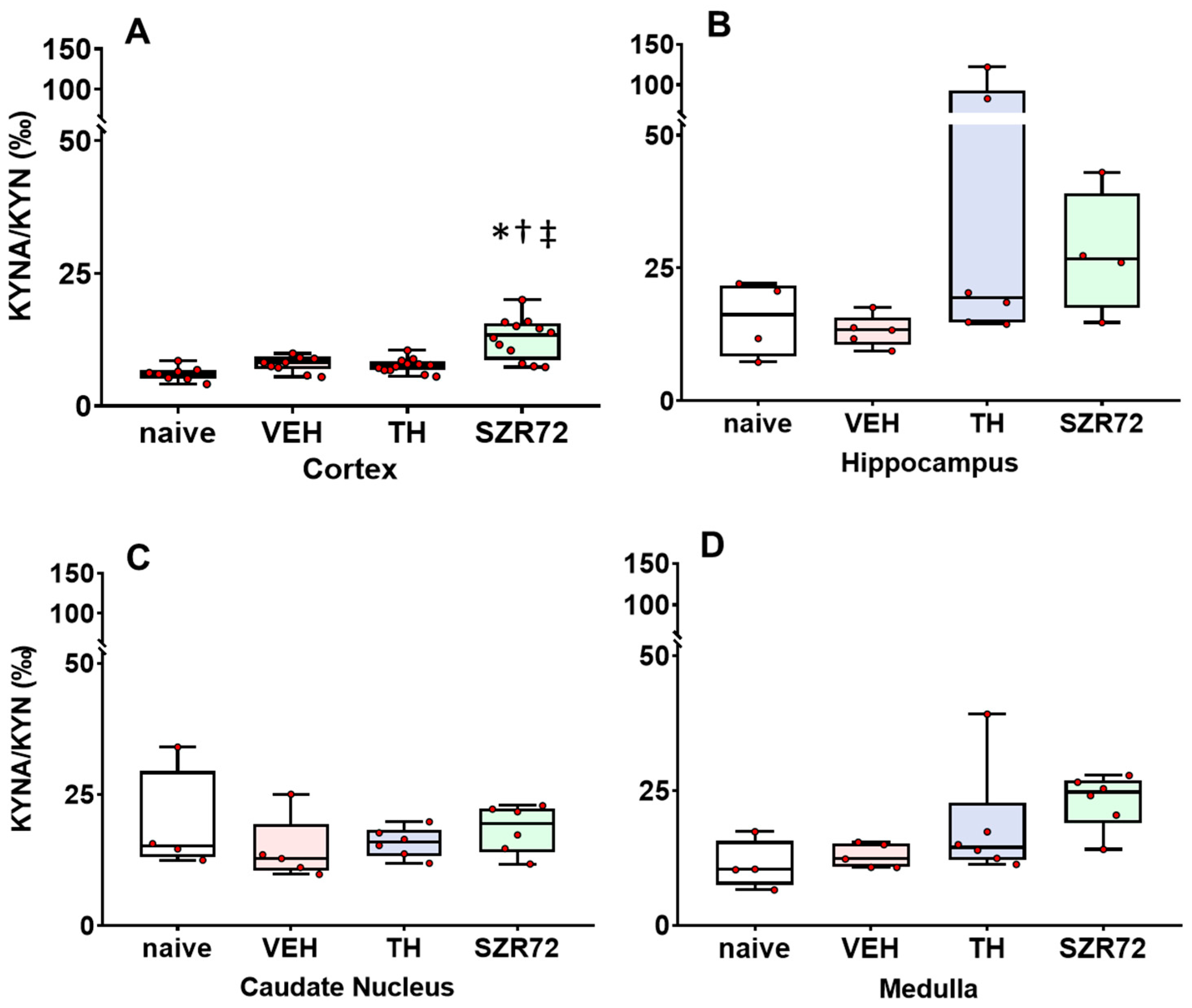
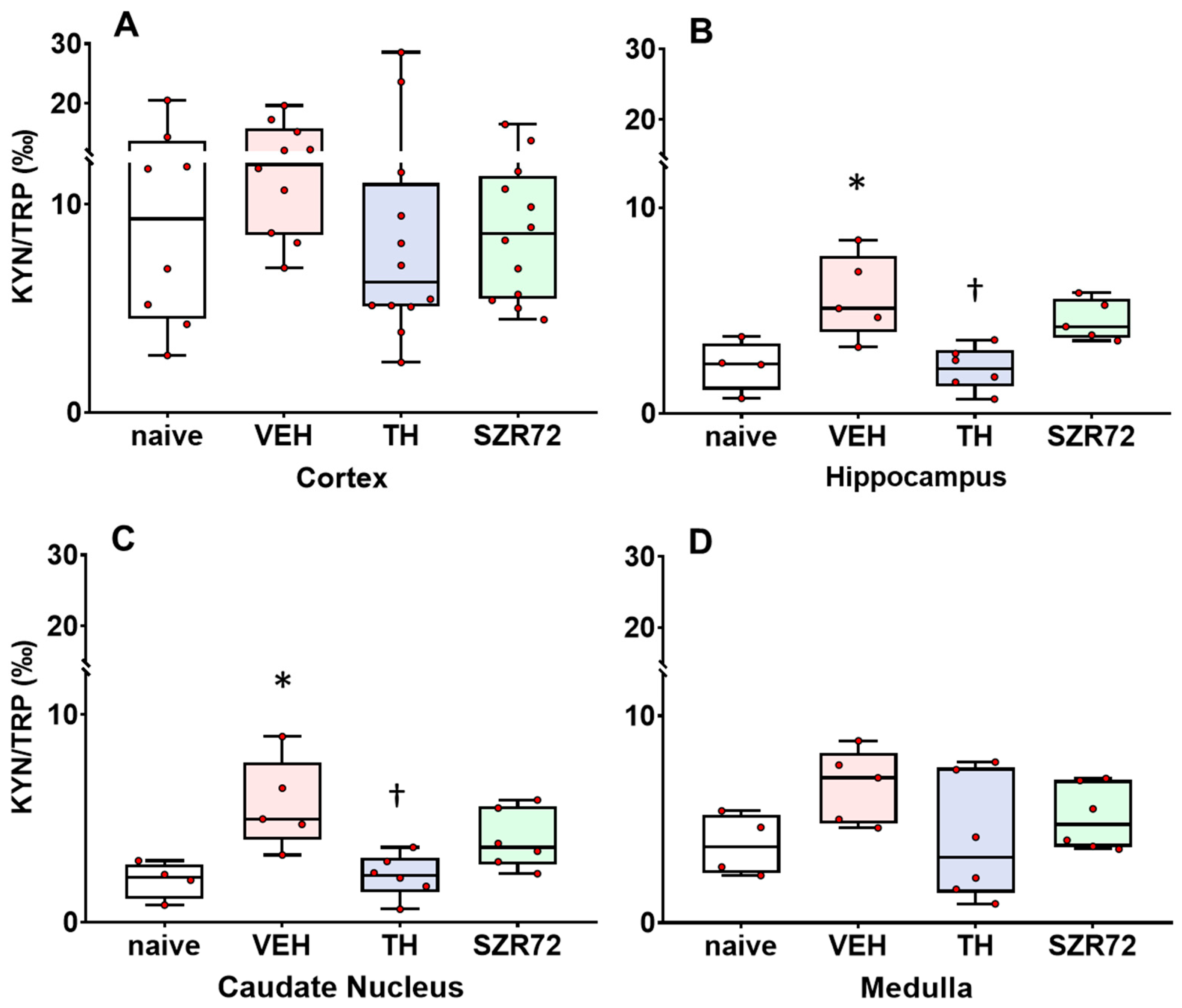
2.4. KYNA/KYN and KYN/TRP Ratios
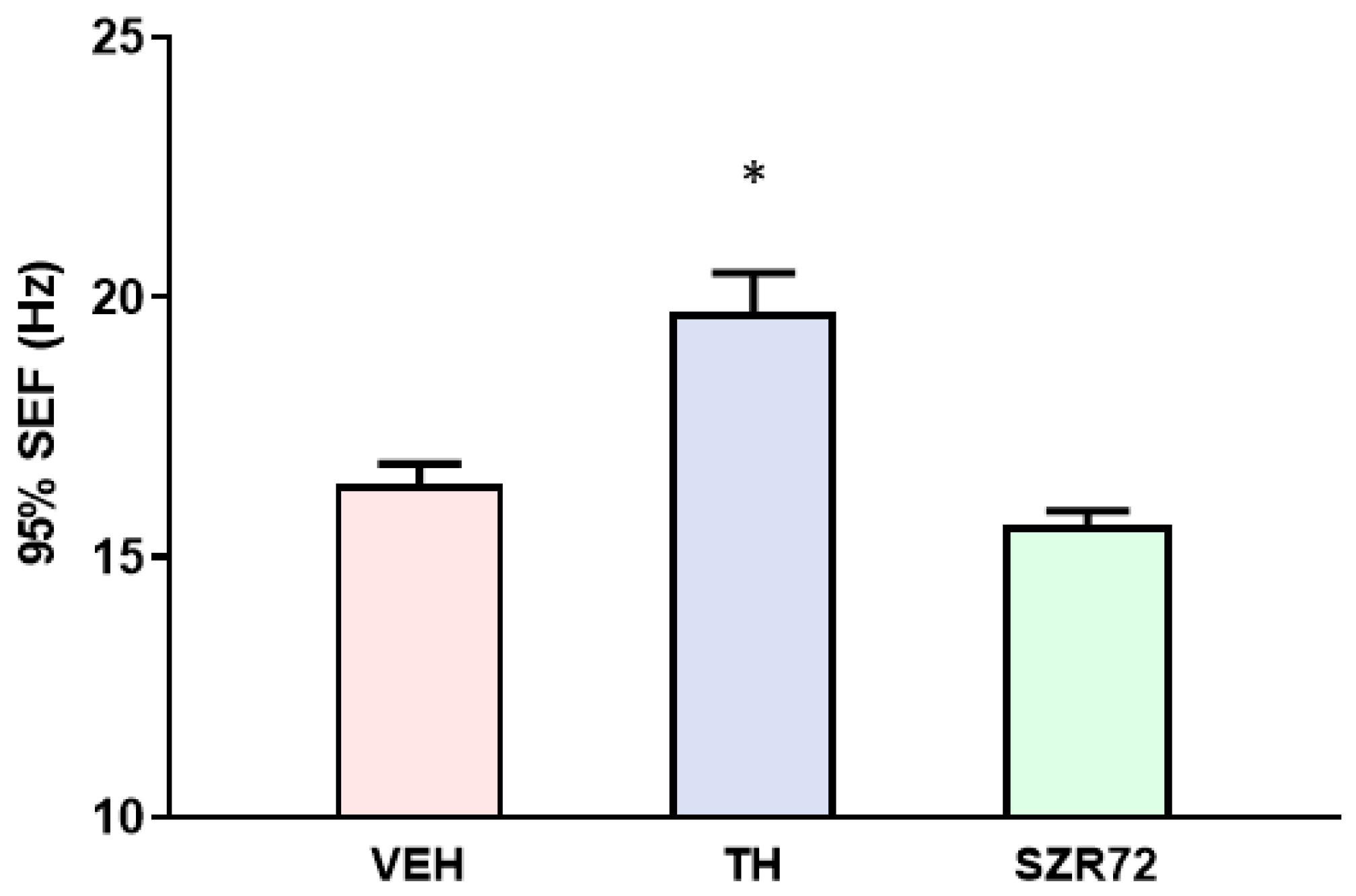
2.5. Spectral Edge Frequency (SEF)
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Materials
4.3. Sample Preparation of Brain Regions for Quantitative Analysis
4.4. Targeted Ultra-High-Performance Liquid Chromatography Coupled with High-Resolution Tandem Mass Spectrometry (UHPLC–MS/HRMS) Parameters for Quantitation of KYN, TRP, KYNA, and SZR72
4.5. Spectral Edge Frequency (SEF) Analysis
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jacobs, S.E.; Berg, M.; Hunt, R.; Tarnow-Mordi, W.O.; Inder, T.E.; Davis, P.G. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013, CD003311. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, S.; Nelson, K.B.; Mulkey, S.B.; Lechpammer, M.; Molloy, E.; Badawi, N. Neonatal encephalopathy: Focus on epidemiology and underexplored aspects of etiology. Semin. Fetal Neonatal Med. 2021, 26, 101265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhu, P.; Fujino, M.; Zhuang, J.; Guo, H.; Sheikh, I.; Zhao, L.; Li, X.K. Oxidative stress in hypoxic-ischemic encephalopathy: Molecular mechanisms and therapeutic strategies. Int. J. Mol. Sci. 2016, 17, 2078. [Google Scholar] [CrossRef]
- Lorek, A.; Takei, Y.; Cady, E.B.; Wyatt, J.S.; Penrice, J.; Edwards, A.D.; Peebles, D.; Wylezinska, M.; Owen-Reece, H.; Kirkbride, V.; et al. Delayed (“Secondary”) Cerebral Energy Failure after Acute Hypoxia-Ischemia in the Newborn Piglet: Continuous 48-Hour Studies by Phosphorus Magnetic Resonance Spectroscopy. Pediatr. Res. 1994, 36, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Rainaldi, M.A.; Perlman, J.M. Pathophysiology of Birth Asphyxia. Clin. Perinatol. 2016, 43, 409–422. [Google Scholar] [CrossRef]
- Azzopardi, D.; Strohm, B.; Marlow, N.; Brocklehurst, P.; Deierl, A.; Eddama, O.; Goodwin, J.; Halliday, H.L.; Juszczak, E.; Kapellou, O.; et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N. Engl. J. Med. 2014, 371, 140–149. [Google Scholar] [CrossRef]
- Book, S.A.; Bustad, L.K. The Fetal and Neonatal Pig in Biomedical Research. Am. J. Anim. Sci. 1974, 38, 997–1002. [Google Scholar] [CrossRef]
- Dobbing, J.; Sands, J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979, 311, 79–83. [Google Scholar] [CrossRef]
- Koehler, R.C.; Yang, Z.J.; Lee, J.K.; Martin, L.J. Perinatal hypoxic-ischemic brain injury in large animal models: Relevance to human neonatal encephalopathy. J. Cereb. Blood Flow Metab. 2018, 38, 2092–2111. [Google Scholar] [CrossRef]
- Schwarcz, R.; Bruno, P.J.; Muchowski, J.P.; Wu, H.-Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- Colpo, G.D.; Venna, V.R.; McCullough, L.D.; Teixeira, A.L. Systematic review on the involvement of the kynurenine pathway in stroke: Pre-clinical and Clinical Evidence. Front. Neurol. 2019, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Fulop, F.; Szatmari, I.; Vamos, E.; Zadori, D.; Toldi, J.; Vecsei, L. Syntheses, Transformations and Pharmaceutical Applications of Kynurenic Acid Derivatives. Curr. Med. Chem. 2009, 16, 4828–4842. [Google Scholar] [CrossRef] [PubMed]
- Gellért, L.; Fuzik, J.; Göblös, A.; Sárközi, K.; Marosi, M.; Kis, Z.; Farkas, T.; Szatmári, I.; Fülöp, F.; Vécsei, L.; et al. Neuroprotection with a new kynurenic acid analog in the four-vessel occlusion model of ischemia. Eur. J. Pharmacol. 2011, 667, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Kovács, V.; Remzső, G.; Körmöczi, T.; Berkecz, R.; Tóth-Szűki, V.; Pénzes, A.; Vécsei, L.; Domoki, F. The kynurenic acid analog SZR72 enhances neuronal activity after asphyxia but is not neuroprotective in a translational model of neonatal hypoxic ischemic encephalopathy. Int. J. Mol. Sci. 2021, 22, 4822. [Google Scholar] [CrossRef]
- Ristagno, G.; Fries, M.; Brunelli, L.; Fumagalli, F.; Bagnati, R.; Russo, I.; Staszewsky, L.; Masson, S.; Li Volti, G.; Zappalà, A.; et al. Early kynurenine pathway activation following cardiac arrest in rats, pigs, and humans. Resuscitation 2013, 84, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Solberg, R.; Enot, D.; Deigner, H.P.; Koal, T.; Scholl-Bürgi, S.; Saugstad, O.D.; Keller, M. Metabolomic analyses of plasma reveals new insights into asphyxia and resuscitation in pigs. PLoS ONE 2010, 5, e9606. [Google Scholar] [CrossRef]
- Denihan, N.M.; Kirwan, J.A.; Walsh, B.H.; Dunn, W.B.; Broadhurst, D.I.; Boylan, G.B.; Murray, D.M. Untargeted metabolomic analysis and pathway discovery in perinatal asphyxia and hypoxic-ischaemic encephalopathy. J. Cereb. Blood Flow Metab. 2019, 39, 147–162. [Google Scholar] [CrossRef]
- Bari, F.; Nagy, K.; Guidetti, P.; Schwarcz, R.; Busija, D.W.; Domoki, F. Kynurenic acid attenuates NMDA-induced pial arteriolar dilation in newborn pigs. Brain Res. 2006, 1069, 39–46. [Google Scholar] [CrossRef]
- Azzopardi, D.; Brocklehurst, P.; Edwards, D.; Halliday, H.; Levene, M.; Thoresen, M.; Whitelaw, A. The TOBY study. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: A randomised controlled trial. BMC Pediatr. 2008, 8, 17. [Google Scholar] [CrossRef]
- Randall, G.C.B. Studies on the effect of acute asphyxia on the fetal pig in utero. Biol. Neonatorum 1979, 36, 63–69. [Google Scholar] [CrossRef]
- Demeter, I.; Nagy, K.; Farkas, T.; Kis, Z.; Kocsis, K.; Knapp, L.; Gellert, L.; Fülöp, F.; Vecsei, L.; Toldi, J. Paradox effects of kynurenines on LTP induction in the Wistar rat. An in vivo study. Neurosci. Lett. 2013, 553, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Rózsa, É.; Robotka, H.; Vécsei, L.; Toldi, J. The Janus-face kynurenic acid. J. Neural Transm. 2008, 115, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Remzso, G.; Németh, J.; Varga, V.; Kovács, V.; TóthSzuki, V.; Kaila, K.; Voipio, J.; Domoki, F. Brain interstitial pH changes in the subacute phase of hypoxic-ischemic encephalopathy in newborn pigs. PLoS ONE 2020, 15, e233851. [Google Scholar] [CrossRef]
- Uetrecht, J.P.; Freeman, R.W.; Woosley, R.L. The implications of procainamide metabolism to its induction of lupus. Arthritis Rheum. 1981, 24, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Uwai, Y.; Honjo, H.; Iwamoto, K. Interaction and transport of kynurenic acid via human organic anion transporters hOAT1 and hOAT3. Pharmacol. Res. 2012, 65, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Huitrón, R.; Blanco-Ayala, T.; Ugalde-Muñiz, P.; Carrillo-Mora, P.; Pedraza-Chaverrí, J.; Silva-Adaya, D.; Maldonado, P.D.; Torres, I.; Pinzón, E.; Ortiz-Islas, E.; et al. On the antioxidant properties of kynurenic acid: Free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol. Teratol. 2011, 33, 538–547. [Google Scholar] [CrossRef]
- Nozaki, K.; Beal, M.F. Neuroprotective effects of L-kynurenine on hypoxia-ischemia and NMDA lesions in neonatal rats. J. Cereb. Blood Flow Metab. 1992, 12, 400–407. [Google Scholar] [CrossRef]
- Kratimenos, P.; Vij, A.; Vidva, R.; Koutroulis, I.; Delivoria-Papadopoulos, M.; Gallo, V.; Sathyanesan, A. Computational analysis of cortical neuronal excitotoxicity in a large animal model of neonatal brain injury. J. Neurodev. Disord. 2022, 14, 26. [Google Scholar] [CrossRef]
- Thornton, C.; Leaw, B.; Mallard, C.; Nair, S.; Jinnai, M.; Hagberg, H. Cell death in the developing brain after hypoxia-ischemia. Front. Cell Neurosci. 2017, 11, 00248. [Google Scholar] [CrossRef]
- Wassink, G.; Barrett, R.D.; Davidson, J.O.; Bennet, L.; Galinsky, R.; Dragunow, M.; Gunn, A.J. Hypothermic neuroprotection is associated with recovery of spectral edge frequency after asphyxia in preterm fetal sheep. Stroke 2015, 46, 585–587. [Google Scholar] [CrossRef]
- Robotka, H.; Sas, K.; Ágoston, M.; Rózsa, É.; Szénási, G.; Gigler, G.; Vécsei, L.; Toldi, J. Neuroprotection achieved in the ischaemic rat cortex with l-kynurenine sulphate. Life Sci. 2008, 82, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Gellért, L.; Knapp, L.; Németh, K.; Herédi, J.; Varga, D.; Oláh, G.; Kocsis, K.; Menyhárt, Á.; Kis, Z.; Farkas, T.; et al. Post-ischemic treatment with L-kynurenine sulfate exacerbates neuronal damage after transient middle cerebral artery occlusion. Neuroscience 2013, 247, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Osredkar, D.; Thoresen, M.; Maes, E.; Flatebø, T.; Elstad, M.; Sabir, H. Hypothermia is not neuroprotective after infection-sensitized neonatal hypoxic-ischemic brain injury. Resuscitation 2014, 85, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Poles, M.Z.; Nászai, A.; Gulácsi, L.; Czakó, B.L.; Gál, K.G.; Glenz, R.J.; Dookhun, D.; Rutai, A.; Tallósy, S.P.; Szabó, A.; et al. Kynurenic Acid and Its Synthetic Derivatives Protect Against Sepsis-Associated Neutrophil Activation and Brain Mitochondrial Dysfunction in Rats. Front. Immunol. 2021, 12, 717157. [Google Scholar] [CrossRef]
- Szabo, M.; Lajkó, N.; Dulka, K.; Szatmári, I.; Fülöp, F.; Mihály, A.; Vécsei, L.; Gulya, K. Kynurenic Acid and Its Analog SZR104 Exhibit Strong Antiinflammatory Effects and Alter the Intracellular Distribution and Methylation Patterns of H3 Histones in Immunochallenged Microglia-Enriched Cultures of Newborn Rat Brains. Int. J. Mol. Sci. 2022, 23, 1079. [Google Scholar] [CrossRef]
- Baran, H.; Kepplinger, B.; Herrera-Marschitz, M.; Stolze, K.; Lubec, G.; Nohl, H. Increased kynurenic acid in the brain after neonatal asphyxia. Life Sci. 2001, 69, 1249–1256. [Google Scholar] [CrossRef]
- Luchowska, E.; Luchowski, P.; Sarnowska, A.; Wielosz, M.; Turski, W.A.; Urbańska, E.M. Endogenous level of kynurenic acid and activities of kynurenine aminotransferases following transient global ischemia in the gerbil hippocampus. Pol. J. Pharmacol. 2003, 55, 443–447. [Google Scholar]
- Mangas, A.; Yajeya, J.; González, N.; Ruiz, I.; Duleu, S.; Geffard, M.; Coveñas, R. Overexpression of kynurenic acid in stroke: An endogenous neuroprotector? Ann. Anat. 2017, 211, 33–38. [Google Scholar] [CrossRef]
- Bajnok, A.; Berta, L.; Orbán, C.; Veres, G.; Zádori, D.; Barta, H.; Méder, Ü.; Vécsei, L.; Tulassay, T.; Szabó, M.; et al. Distinct cytokine patterns may regulate the severity of neonatal asphyxia-an observational study. J. Neuroinflamm. 2017, 14, 244. [Google Scholar] [CrossRef]
- Guidetti, P.; Hoffman, G.E.; Melendez-Ferro, M.; Albuquerque, E.X.; Schwarcz, R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia 2007, 55, 78–92. [Google Scholar] [CrossRef]
- Cuartero, M.I.; Ballesteros, I.; De La Parra, J.; Harkin, A.L.; Abautret-Daly, A.; Sherwin, E.; Fernández-Salguero, P.; Corbí, Á.L.; Lizasoain, I.; Moro, M.A. L-kynurenine/aryl hydrocarbon receptor pathway mediates brain damage after experimental stroke. Circulation 2014, 130, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Gál, E.M.; Sherman, A.D. Synthesis and metabolism of L-kynurenine in rat brain. J. Neurochem. 1978, 30, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Nakamura, T.; Funakoshi, H. Identification and characterization of novel variants of the tryptophan 2,3-dioxygenase gene: Differential regulation in the mouse nervous system during development. Neurosci. Res. 2009, 64, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, C.C.; Chou, C.M.; Wang, C.Y.; Hung, C.C.; Chen, J.Y.; Chang, H.W.; Chen, Y.C.; Yeh, G.C.; Lee, Y.H. Knockdown of the aryl hydrocarbon receptor attenuates excitotoxicity and enhances NMDA-induced BDNF expression in cortical neurons. J. Neurochem. 2009, 111, 777–789. [Google Scholar] [CrossRef]
- Nannelli, A.; Rossignolo, F.; Tolando, R.; Rossato, P.; Longo, V.; Gervasi, P.G. Effect of β-naphthoflavone on AhR-regulated genes (CYP1A1, 1A2, 1B1, 2S1, Nrf2, and GST) and antioxidant enzymes in various brain regions of pig. Toxicology 2009, 265, 69–79. [Google Scholar] [CrossRef]
- Hilmas, C.; Pereira, E.F.R.; Alkondon, M.; Rassoulpour, A.; Schwarcz, R. The Brain Metabolite Kynurenic Acid Inhibits α7 Nicotinic Receptor Activity and Increases Non-α7 Nicotinic Receptor Expression: Physiopathological Implications. J. Neurosci. 2001, 21, 7463–7473. [Google Scholar] [CrossRef]
- Stone, T.W. Does kynurenic acid act on nicotinic receptors? An assessment of the evidence. J. Neurochem. 2020, 152, 627–649. [Google Scholar] [CrossRef]
- Alkondon, M.; Pereira, E.F.R.; Todd, S.W.; Randall, W.R.; Lane, M.V.; Albuquerque, E.X. Functional G-protein-coupled receptor 35 is expressed by neurons in the CA1 field of the hippocampus. Biochem. Pharmacol. 2015, 93, 506–518. [Google Scholar] [CrossRef]
- Stone, T.W.; Clanchy, F.I.L.; Huang, Y.S.; Chiang, N.Y.; Darlington, L.G.; Williams, R.O. An integrated cytokine and kynurenine network as the basis of neuroimmune communication. Front. Neurosci. 2022, 16, 1002004. [Google Scholar] [CrossRef]
- Lai, Y.; Liu, C.W.; Chi, L.; Ru, H.; Lu, K. High-Resolution Metabolomics of 50 Neurotransmitters and Tryptophan Metabolites in Feces, Serum, and Brain Tissues Using UHPLC-ESI-Q Exactive Mass Spectrometry. ACS Omega 2021, 6, 8094–8103. [Google Scholar] [CrossRef] [PubMed]
- Poojary, M.M.; Tiwari, B.K.; Lund, M.N. Selective and sensitive UHPLC-ESI-Orbitrap MS method to quantify protein oxidation markers. Talanta 2021, 234, 122700. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
| Targeted Compound | Retention Time (min) | Precursor Ion (m/z) | Main Fragment Ion (m/z) | Collision Energy (eV) |
|---|---|---|---|---|
| KYN | 2.75 | 209.09207 | 192.06552 | 16 |
| TRP | 2.86 | 205.09715 | 188.07061 | 10 |
| KYNA | 3.41 | 190.04987 | 162.05496 | 20 |
| KYNA-D5 (IS *-1) | 3.41 | 195.08125 | 167.05615 | 20 |
| SZR72 | 2.98 | 260.13935 | 162.05496 | 38 |
| SZR73 (IS *-2) | 3.07 | 274.15500 | 162.05496 | 38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domoki, F.; Tóth-Szűki, V.; Kovács, V.; Remzső, G.; Körmöczi, T.; Vécsei, L.; Berkecz, R. Differential Effects of Hypothermia and SZR72 on Cerebral Kynurenine and Kynurenic Acid in a Piglet Model of Hypoxic–Ischemic Encephalopathy. Int. J. Mol. Sci. 2023, 24, 14522. https://doi.org/10.3390/ijms241914522
Domoki F, Tóth-Szűki V, Kovács V, Remzső G, Körmöczi T, Vécsei L, Berkecz R. Differential Effects of Hypothermia and SZR72 on Cerebral Kynurenine and Kynurenic Acid in a Piglet Model of Hypoxic–Ischemic Encephalopathy. International Journal of Molecular Sciences. 2023; 24(19):14522. https://doi.org/10.3390/ijms241914522
Chicago/Turabian StyleDomoki, Ferenc, Valéria Tóth-Szűki, Viktória Kovács, Gábor Remzső, Tímea Körmöczi, László Vécsei, and Róbert Berkecz. 2023. "Differential Effects of Hypothermia and SZR72 on Cerebral Kynurenine and Kynurenic Acid in a Piglet Model of Hypoxic–Ischemic Encephalopathy" International Journal of Molecular Sciences 24, no. 19: 14522. https://doi.org/10.3390/ijms241914522
APA StyleDomoki, F., Tóth-Szűki, V., Kovács, V., Remzső, G., Körmöczi, T., Vécsei, L., & Berkecz, R. (2023). Differential Effects of Hypothermia and SZR72 on Cerebral Kynurenine and Kynurenic Acid in a Piglet Model of Hypoxic–Ischemic Encephalopathy. International Journal of Molecular Sciences, 24(19), 14522. https://doi.org/10.3390/ijms241914522








