Baicalin Attenuates Panton–Valentine Leukocidin (PVL)-Induced Cytoskeleton Rearrangement via Regulating the RhoA/ROCK/LIMK and PI3K/AKT/GSK-3β Pathways in Bovine Mammary Epithelial Cells
Abstract
:1. Introduction
2. Results
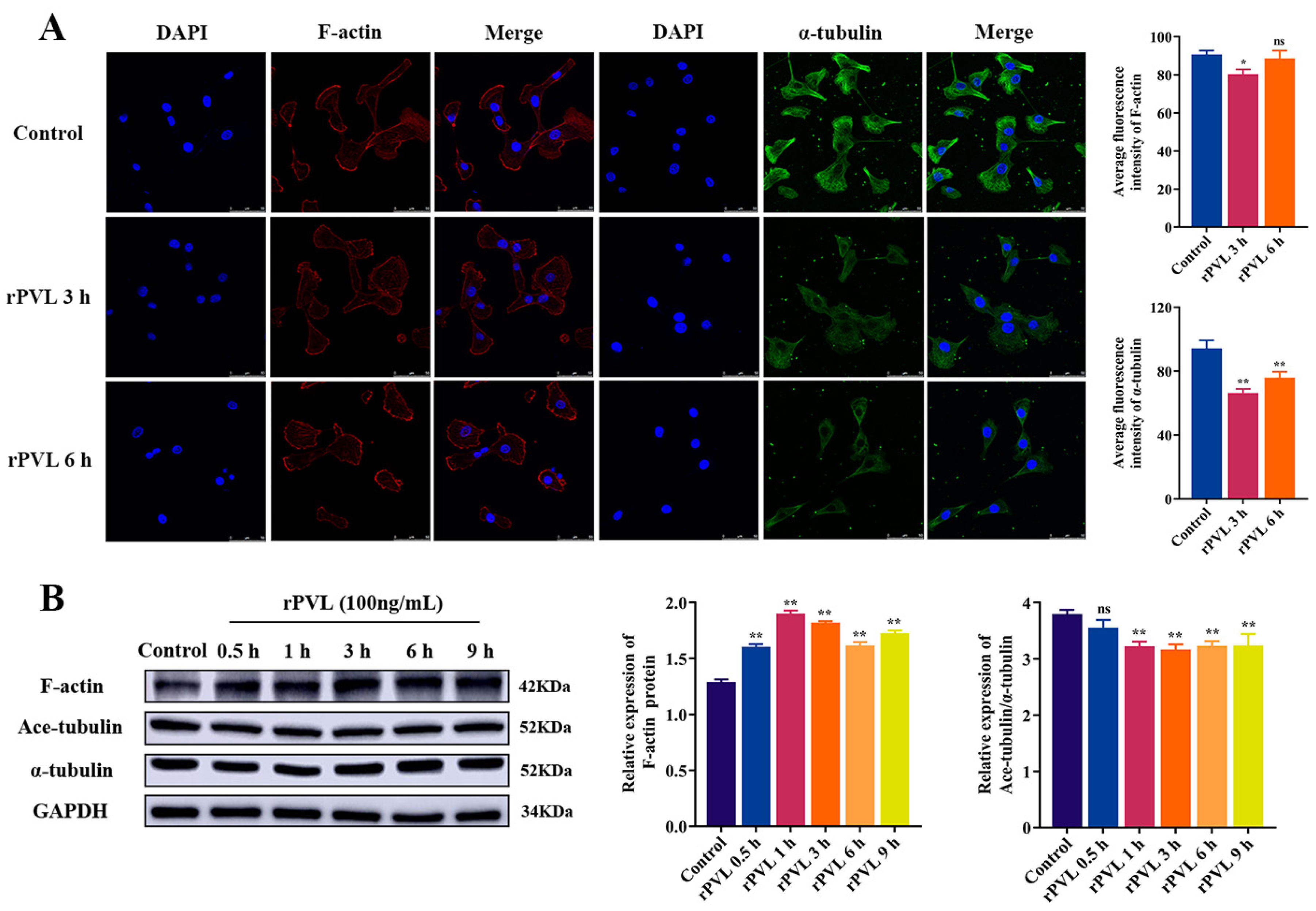
2.1. rPVL Caused the Rearrangement of the Microfilaments and Microtubules in BMECs
2.2. S. aureus Infection-Induced Microfilament and Microtubule Rearrangement Mainly through PVL
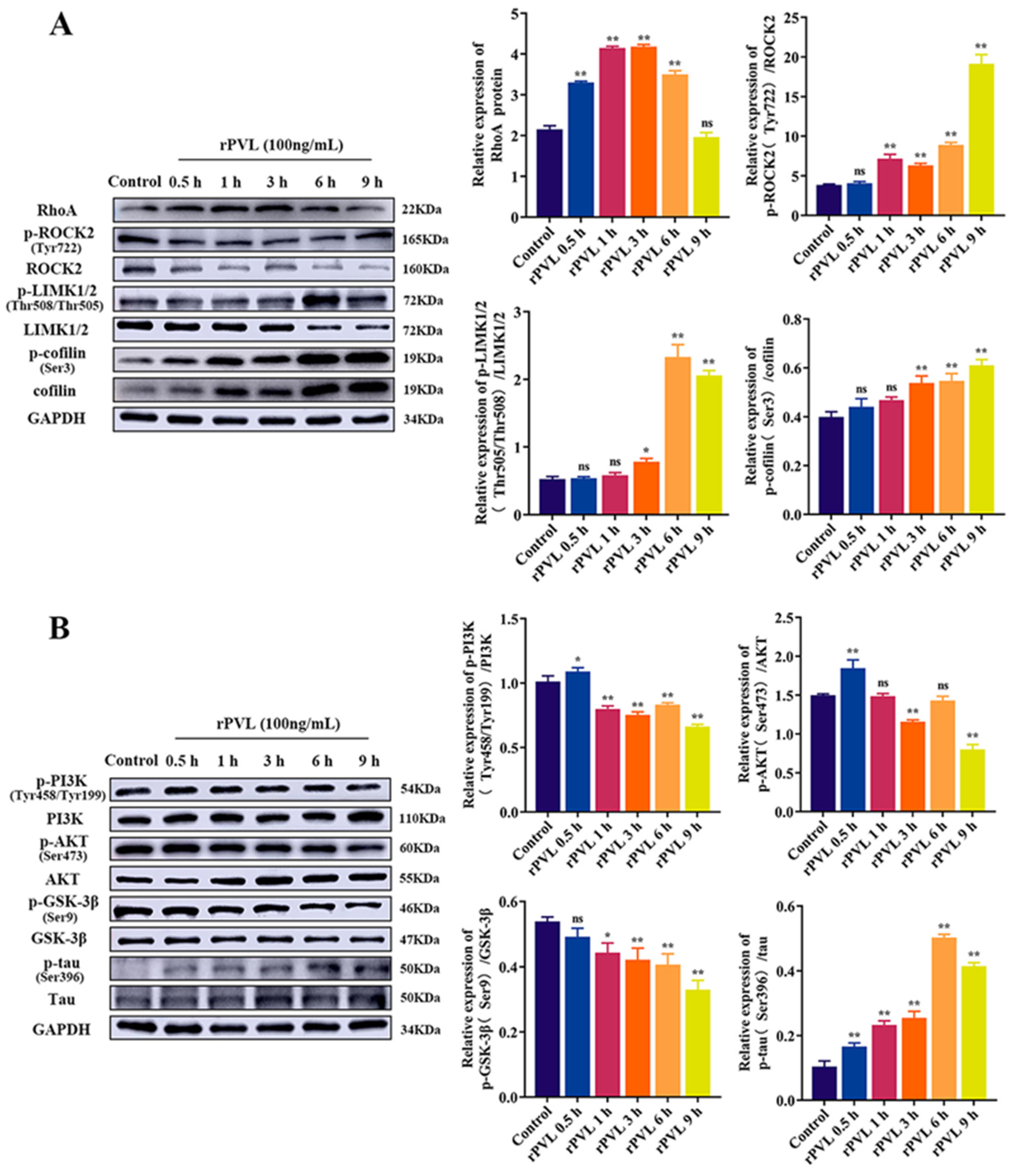
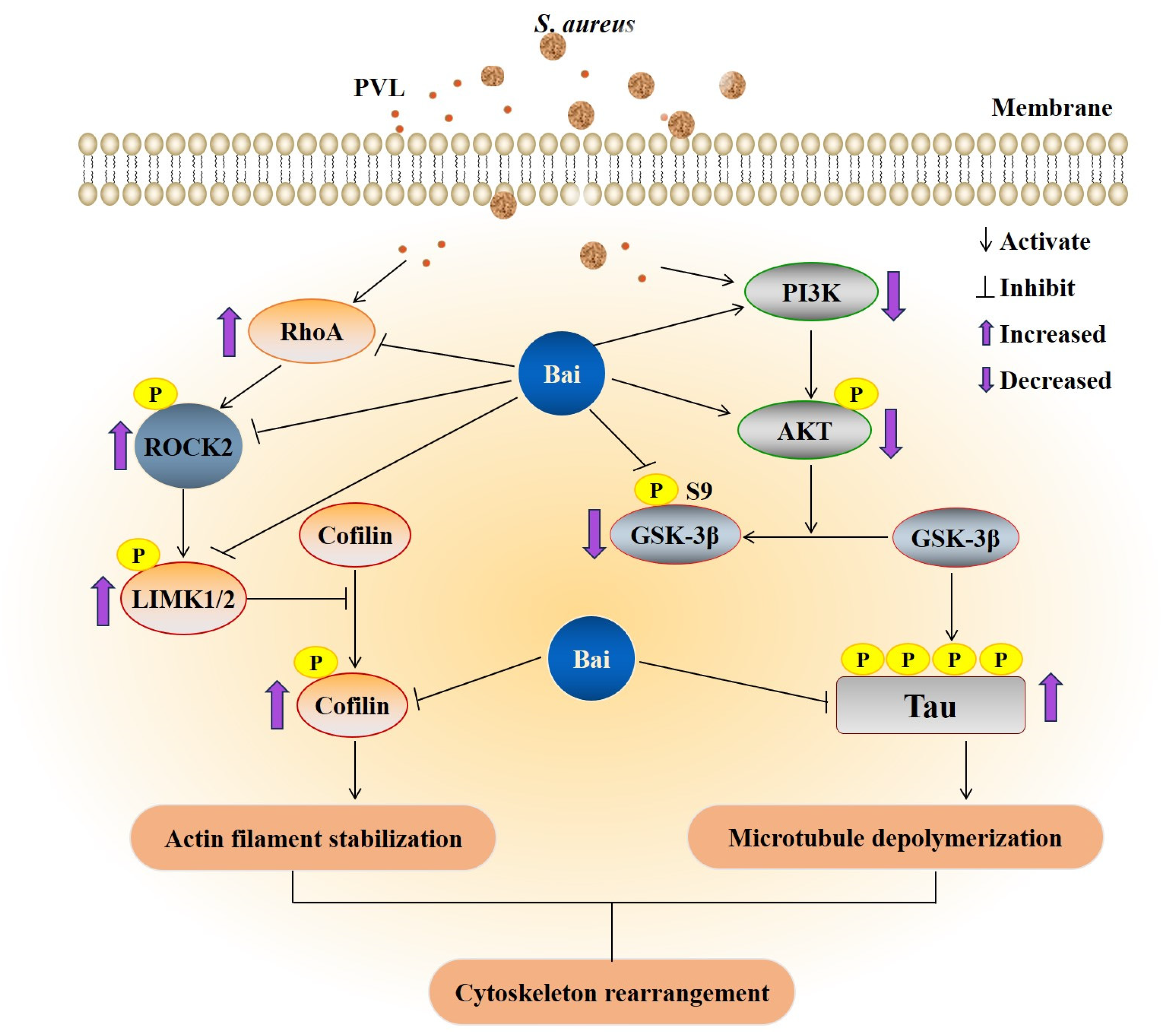
2.3. rPVL Activated the RhoA/ROCK/LIMK and Inhibited PI3K/AKT/GSK-3β Pathways in BMECs
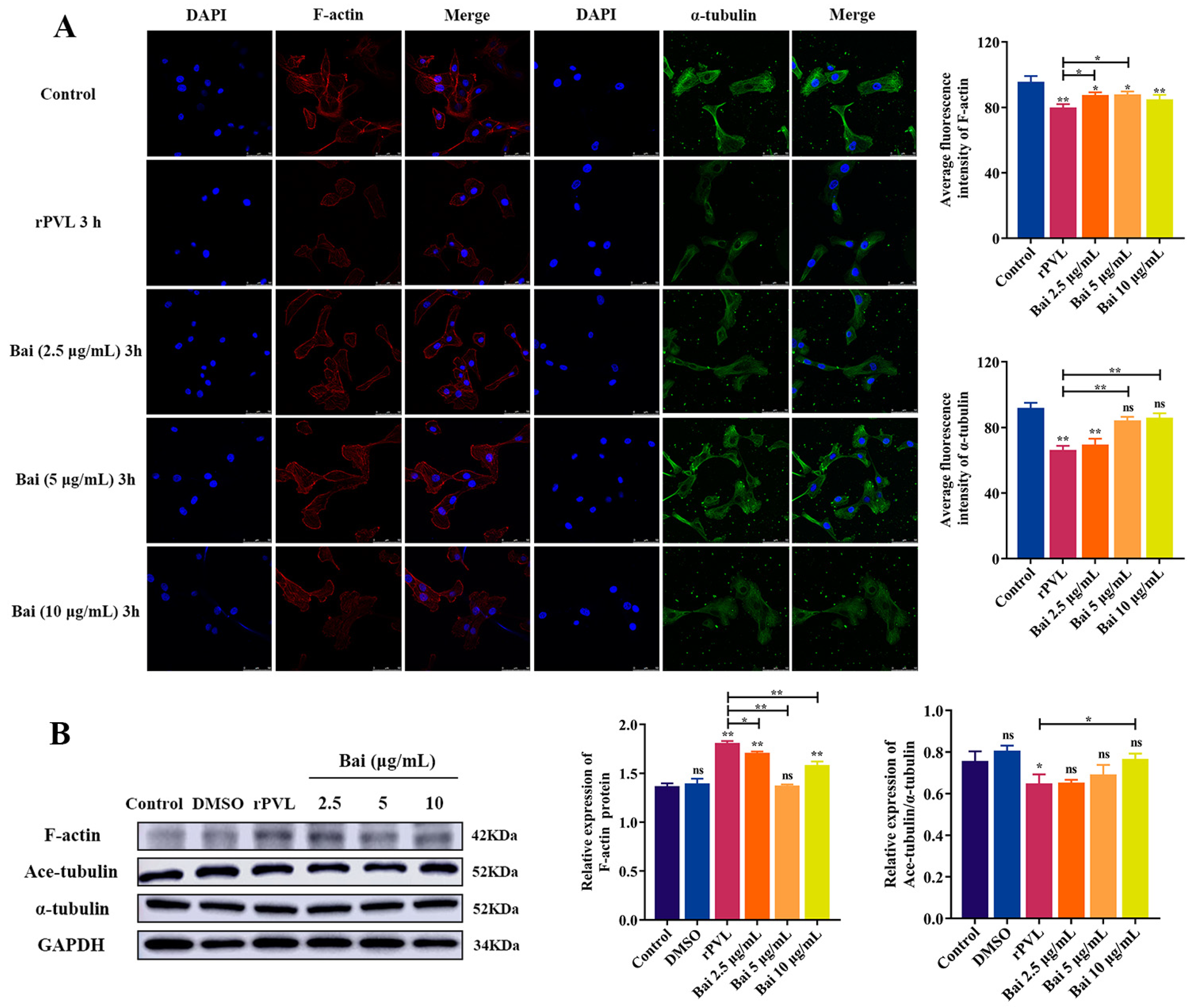
2.4. Baicalin Attenuated rPVL-Induced Rearrangement of Microfilaments and Microtubules in BMECs
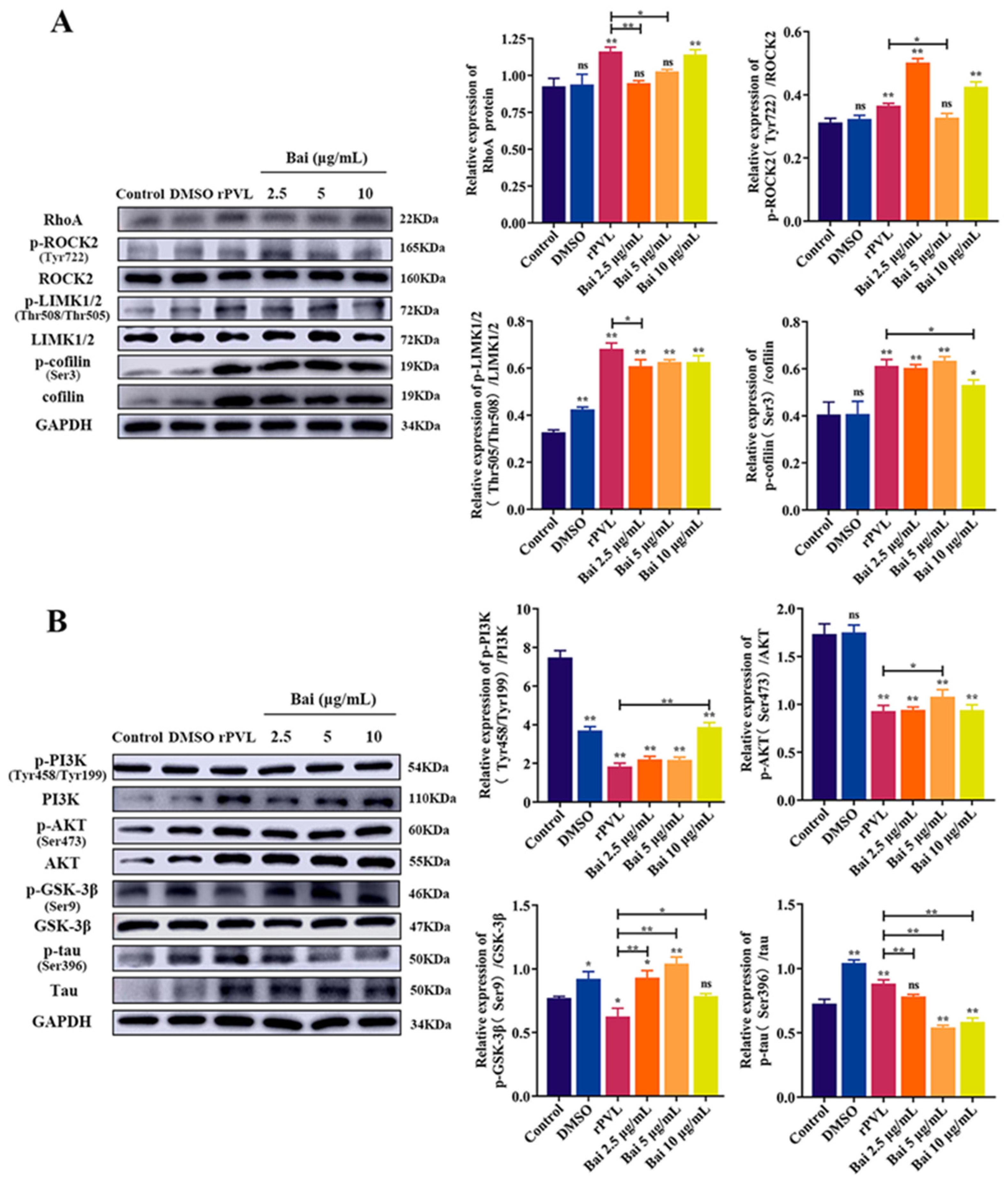
2.5. Baicalin Attenuated the rPVL-Induced Rearrangement of Microfilaments and Microtubules in BMECs via the RhoA/ROCK/LIMK and PI3K/AKT/GSK-3β Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Cell Culture
4.2. Antibodies and Other Reagents
4.3. rPVL Preparation
4.4. Lysostaphin Protection Assays and In Vitro Inhibitor Treatment
4.5. Immunofluorescence
4.6. Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rainard, P.; Foucras, G.; Fitzgerald, J.R.; Watts, J.L.; Koop, G.; Middleton, J.R. Knowledge gaps and research priorities in Staphylococcus aureus mastitis control. Transbound. Emerg. Dis. 2018, 65 (Suppl. 1), 149–165. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus aureus toxins. Curr. Opin. Microbiol. 2014, 17, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.; Borges, A.; Simões, M. Staphylococcus aureus toxins and their molecular activity in infectious diseases. Toxins 2018, 10, 252. [Google Scholar] [CrossRef]
- Seilie, E.S.; Bubeck Wardenburg, J. Staphylococcus aureus pore-forming toxins: The interface of pathogen and host complexity. Semin. Cell Dev. Biol. 2017, 72, 101–116. [Google Scholar] [CrossRef]
- Campos, B.; Pickering, A.C.; Rocha, L.S.; Aguilar, A.P.; Fabres-Klein, M.H.; De Oliveira Mendes, T.A.; Fitzgerald, J.R.; De Oliveira Barros Ribon, A. Diversity and pathogenesis of Staphylococcus aureus from bovine mastitis: Current understanding and future perspectives. BMC Vet. Res. 2022, 18, 115. [Google Scholar] [CrossRef] [PubMed]
- Vann, J.M.; Proctor, R.A. Cytotoxic effects of ingested Staphylococcus aureus on bovine endothelial cells: Role of S. aureus alpha-hemolysin. Microb. Pathog. 1988, 4, 443–453. [Google Scholar] [CrossRef]
- Cifrian, E.; Guidry, A.J.; Bramley, A.J.; Norcross, N.L.; Bastida-Corcuera, F.D.; Marquardt, W.W. Effect of staphylococcal beta toxin on the cytotoxicity, proliferation and adherence of Staphylococcus aureus to bovine mammary epithelial cells. Vet. Microbiol. 1996, 48, 187–198. [Google Scholar] [CrossRef]
- Deplanche, M.; Alekseeva, L.; Semenovskaya, K.; Fu, C.L.; Dessauge, F.; Finot, L.; Petzl, W.; Zerbe, H.; Le Loir, Y.; Rainard, P.; et al. Staphylococcus aureus Phenol-Soluble Modulins impair interleukin expression in bovine mammary epithelial cells. Infect. Immun. 2016, 84, 1682–1692. [Google Scholar] [CrossRef]
- Günther, J.; Petzl, W.; Bauer, I.; Ponsuksili, S.; Zerbe, H.; Schuberth, H.J.; Brunner, R.M.; Seyfert, H.M. Differentiating Staphylococcus aureus from Escherichia coli mastitis: S. aureus triggers unbalanced immune-dampening and host cell invasion immediately after udder infection. Sci. Rep. 2017, 7, 4811. [Google Scholar] [CrossRef]
- Dziewanowska, K.; Patti, J.M.; Deobald, C.F.; Bayles, K.W.; Trumble, W.R.; Bohach, G.A. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect. Immun. 1999, 67, 4673–4678. [Google Scholar] [CrossRef]
- Aepfelbacher, M.; Essler, M.; Huber, E.; Sugai, M.; Weber, P.C. Bacterial toxins block endothelial wound repair. Evidence that Rho GTPases control cytoskeletal rearrangements in migrating endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Pian, Y.; Chen, S.; Hao, H.; Zheng, Y.; Zhu, L.; Xu, B.; Liu, K.; Li, M.; Jiang, H.; et al. Phenol-soluble modulin α4 mediates Staphylococcus aureus-associated vascular leakage by stimulating heparin-binding protein release from neutrophils. Sci. Rep. 2016, 6, 29373. [Google Scholar] [CrossRef] [PubMed]
- Ziesemer, S.; Eiffler, I.; Schönberg, A.; Müller, C.; Hochgräfe, F.; Beule, A.G.; Hildebrandt, J.P. Staphylococcus aureus α-Toxin induces actin filament remodeling in human airway epithelial model cells. Am. J. Respir. Cell Mol. Biol. 2018, 58, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef]
- De Souza Santos, M.; Orth, K. Subversion of the cytoskeleton by intracellular bacteria: Lessons from Listeria, Salmonella and Vibrio. Cell. Microbiol. 2015, 17, 164–173. [Google Scholar] [CrossRef]
- Almeida, R.A.; Matthews, K.R.; Cifrian, E.; Guidry, A.J.; Oliver, S.P. Staphylococcus aureus invasion of bovine mammary epithelial cells. J. Dairy Sci. 1996, 79, 1021–1026. [Google Scholar] [CrossRef]
- Dogan, B.; Klaessig, S.; Rishniw, M.; Almeida, R.A.; Oliver, S.P.; Simpson, K.; Schukken, Y.H. Adherent and invasive Escherichia coli are associated with persistent bovine mastitis. Vet. Microbiol. 2006, 116, 270–282. [Google Scholar] [CrossRef]
- Döpfer, D.; Nederbragt, H.; Almeida, R.A.; Gaastra, W. Studies about the mechanism of internalization by mammary epithelial cells of Escherichia coli isolated from persistent bovine mastitis. Vet. Microbiol. 2001, 80, 285–296. [Google Scholar] [CrossRef]
- Fiorentini, C.; Falzano, L.; Travaglione, S.; Fabbri, A. Hijacking Rho GTPases by protein toxins and apoptosis: Molecular strategies of pathogenic bacteria. Cell Death Differ. 2003, 10, 147–152. [Google Scholar] [CrossRef]
- Barbieri, J.T.; Riese, M.J.; Aktories, K. Bacterial toxins that modify the actin cytoskeleton. Annu. Rev. Cell. Dev. Biol. 2002, 18, 315–344. [Google Scholar] [CrossRef]
- Kitagishi, Y.; Nakanishi, A.; Ogura, Y.; Matsuda, S. Dietary regulation of PI3K/AKT/GSK-3β pathway in Alzheimer’s disease. Alzheimers Res. Ther. 2014, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, R.; Gu, J.; Yin, X.; Jin, N.; Xie, S.; Wang, Y.; Chang, H.; Qian, W.; Shi, J.; et al. Cross talk between PI3K-AKT-GSK-3β and PP2A pathways determines tau hyperphosphorylation. Neurobiol. Aging 2015, 36, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Onishi, K.; Higuchi, M.; Asakura, T.; Masuyama, N.; Gotoh, Y. The PI3K-Akt pathway promotes microtubule stabilization in migrating fibroblasts. Genes Cells 2007, 12, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.R.; Pan, Y.J.; Liu, J.Y.; Chen, C.T.; Lin, T.L.; Wang, J.T. Klebsiella pneumoniae translocates across the intestinal epithelium via Rho GTPase- and phosphatidylinositol 3-kinase/Akt-dependent cell invasion. Infect. Immun. 2015, 83, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Mansell, A.; Khelef, N.; Cossart, P.; O’neill, L.A. Internalin B activates nuclear factor-kappa B via Ras, phosphoinositide 3-kinase, and Akt. J. Biol. Chem. 2001, 276, 43597–43603. [Google Scholar] [CrossRef]
- Zhao, W.D.; Liu, W.; Fang, W.G.; Kim, K.S.; Chen, Y.H. Vascular endothelial growth factor receptor 1 contributes to Escherichia coli K1 invasion of human brain microvascular endothelial cells through the phosphatidylinositol 3-kinase/Akt signaling pathway. Infect. Immun. 2010, 78, 4809–4816. [Google Scholar] [CrossRef]
- Kierbel, A.; Gassama-Diagne, A.; Mostov, K.; Engel, J.N. The phosphoinositol-3-kinase-protein kinase B/Akt pathway is critical for Pseudomonas aeruginosa strain PAK internalization. Mol. Biol. Cell 2005, 16, 2577–2585. [Google Scholar] [CrossRef]
- Lina, G.; Piémont, Y.; Godail-Gamot, F.; Bes, M.; Peter, M.O.; Gauduchon, V.; Vandenesch, F.; Etienne, J. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 1999, 29, 1128–1132. [Google Scholar] [CrossRef]
- Zaatout, N.; Hezil, D. A meta-analysis of the global prevalence of methicillin-resistant Staphylococcus aureus (MRSA) isolated from clinical and subclinical bovine mastitis. J. Appl. Microbiol. 2022, 132, 140–154. [Google Scholar] [CrossRef]
- Wang, W.; Lin, X.; Jiang, T.; Peng, Z.; Xu, J.; Yi, L.; Li, F.; Fanning, S.; Baloch, Z. Prevalence and characterization of Staphylococcus aureus cultured from raw milk taken from dairy cows with mastitis in Beijing, China. Front. Microbiol. 2018, 9, 1123. [Google Scholar] [CrossRef]
- Sadat, A.; Shata, R.R.; Farag, A.M.M.; Ramadan, H.; Alkhedaide, A.; Soliman, M.M.; Elbadawy, M.; Abugomaa, A.; Awad, A. Prevalence and characterization of PVL-positive Staphylococcus aureus isolated from raw cow’s milk. Toxins 2022, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Ma, W.; Zhang, X.; Wang, D.; Zhou, X. Matrine and baicalin inhibit apoptosis induced by Panton-Valentine leukocidin of Staphylococcus aureus in bovine mammary epithelial cells. J. Dairy. Sci. 2020, 103, 2731–2742. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Liu, Y.; Zhang, C. Pharmacokinetics and bioavailability enhancement of baicalin: A review. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yu, S.; Deng, R.; Liu, H.; Ding, Y.; Sun, Y.; Chen, W.; Wang, A.; Wei, Z.; Lu, Y. TRPV4 promotes metastasis in melanoma by regulating cell motility through cytoskeletal rearrangement. Int. J. Mol. Sci. 2022, 23, 15155. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, P.; Ma, J.; Zhu, L.; Xu, A.; Zhang, J. Damage and phenotype change in PC12 cells induced by lipopolysaccharide can be inhibited by antioxidants through reduced cytoskeleton protein synthesis. Inflammation 2019, 42, 2246–2256. [Google Scholar] [CrossRef]
- Aktories, K.; Schwan, C.; Papatheodorou, P.; Lang, A.E. Bidirectional attack on the actin cytoskeleton. Bacterial protein toxins causing polymerization or depolymerization of actin. Toxicon 2012, 60, 572–581. [Google Scholar] [CrossRef]
- Aktories, K.; Barbieri, J.T. Bacterial cytotoxins: Targeting eukaryotic switches. Nat. Rev. Microbiol. 2005, 3, 397–410. [Google Scholar] [CrossRef]
- Aktories, K.; Lang, A.E.; Schwan, C.; Mannherz, H.G. Actin as target for modification by bacterial protein toxins. FEBS J. 2011, 278, 4526–4543. [Google Scholar] [CrossRef]
- Jhelum, H.; Čerina, D.; Harbort, C.; Lindner, A.; Hanitsch, L.G.; Leistner, R.; Schröder, J.-T.; Von Bernuth, H.; Stegemann, M.S.; Schürmann, M. Patients with recurrent PVL-Staphylococcus aureus infections show enhanced sensitivity to PVL-mediated formation of atypical NETs. bioRxiv 2021, 2021.2011.2026.470012. [Google Scholar]
- Lee, S.H.; Dominguez, R. Regulation of actin cytoskeleton dynamics in cells. Mol. Cells 2010, 29, 311–325. [Google Scholar] [CrossRef]
- Aktories, K.; Schmidt, G.; Just, I. Rho GTPases as targets of bacterial protein toxins. Biol. Chem. 2000, 381, 421–426. [Google Scholar] [CrossRef]
- Guo, Z.; Ma, Y.; Jia, Z.; Wang, L.; Lu, X.; Chen, Y.; Wang, Y.; Hao, H.; Yu, S.; Wang, Z. Crosstalk between integrin/FAK and Crk/Vps25 governs invasion of bovine mammary epithelial cells by S. agalactiae. iScience 2023, 26, 107884. [Google Scholar] [CrossRef]
- Oviedo-Boyso, J.; Cortés-Vieyra, R.; Huante-Mendoza, A.; Yu, H.B.; Valdez-Alarcón, J.J.; Bravo-Patiño, A.; Cajero-Juárez, M.; Finlay, B.B.; Baizabal-Aguirre, V.M. The phosphoinositide-3-kinase-Akt signaling pathway is important for Staphylococcus aureus internalization by endothelial cells. Infect. Immun. 2011, 79, 4569–4577. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Shi, Y.; Dai, Y.; Wang, F.; Chen, X.; Li, X. Baicalin clears inflammation by enhancing macrophage efferocytosis via inhibition of RhoA/ROCK signaling pathway and regulating macrophage polarization. Int. Immunopharmacol. 2022, 105, 108532. [Google Scholar] [CrossRef]
- Liu, P.; Xiao, Z.; Yan, H.; Lu, X.; Zhang, X.; Luo, L.; Long, C.; Zhu, Y. Baicalin suppresses Th1 and Th17 responses and promotes Treg response to ameliorate sepsis-associated pancreatic injury via the RhoA-ROCK pathway. Int. Immunopharmacol. 2020, 86, 106685. [Google Scholar] [CrossRef]
- Zhang, Z.; Nong, L.; Chen, M.; Gu, X.; Zhao, W.; Liu, M.; Cheng, W. Baicalein suppresses vasculogenic mimicry through inhibiting RhoA/ROCK expression in lung cancer A549 cell line. Acta Biochim. Biophys. Sin. 2020, 52, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Ma, X.; Zhao, X.; Zhang, S. Baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des. Devel. Ther. 2018, 12, 3961–3972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Lu, P.; Guo, Q.Y.; Zhang, Z.H.; Meng, X.Y. Baicalein induces apoptosis in esophageal squamous cell carcinoma cells through modulation of the PI3K/Akt pathway. Oncol. Lett. 2013, 5, 722–728. [Google Scholar] [CrossRef]
- Yu, M.; Qi, B.; Xiaoxiang, W.; Xu, J.; Liu, X. Baicalein increases cisplatin sensitivity of A549 lung adenocarcinoma cells via PI3K/Akt/NF-κB pathway. Biomed. Pharmacother. 2017, 90, 677–685. [Google Scholar] [CrossRef]
- Suarez, G.; Sierra, J.C.; Erova, T.E.; Sha, J.; Horneman, A.J.; Chopra, A.K. A type VI secretion system effector protein, VgrG1, from Aeromonas hydrophila that induces host cell toxicity by ADP ribosylation of actin. J. Bacteriol. 2010, 192, 155–168. [Google Scholar] [CrossRef]
- Petit, P.; Bréard, J.; Montalescot, V.; El Hadj, N.B.; Levade, T.; Popoff, M.; Geny, B. Lethal toxin from Clostridium sordellii induces apoptotic cell death by disruption of mitochondrial homeostasis in HL-60 cells. Cell. Microbiol. 2003, 5, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.Y.; Yuan, F.W.; Liang, T.; Liang, X.C.; Luo, Y.R.; Jiang, M.; Qing, S.Z.; Zhang, W.M. Baicalin inhibits Escherichia coli isolates in bovine mastitic milk and reduces antimicrobial resistance. J. Dairy. Sci. 2018, 101, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhang, N.; Li, D.; Liang, D.; Liu, Z.; Li, F.; Fu, Y.; Cao, Y.; Deng, X.; Yang, Z. Baicalin plays an anti-inflammatory role through reducing nuclear factor-κB and p38 phosphorylation in S. aureus-induced mastitis. Int. Immunopharmacol. 2013, 16, 125–130. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wei, Z.; Zhou, E.; Chen, L.; Kou, J.; Wang, J.; Yang, Z. Baicalein attenuates inflammatory responses by suppressing TLR4 mediated NF-κB and MAPK signaling pathways in LPS-induced mastitis in mice. Int. Immunopharmacol. 2015, 28, 470–476. [Google Scholar] [CrossRef]
- Liu, W.Y.; Yao, Z.; Chang-Zhen, W.; Qin, Y.; Luan, T.; Wang, L.-L.; Liu, S.-G.; Zhou, X.-Z.; Zhang, W.-J. Construction and growth characteristics analysis of PantonValentine Leukocidin deletion mutant of Staphylococcus aureus. Chin. J. Prev. Vet. Med. 2020, 42, 1104–1108. (In Chinese) [Google Scholar]
- Ma, W.; Zhou, X. Expression of Panton-Valenine Leukocidin from Staphylococcus aureus and its effects on bovine mammary epithelial cells. Acta Vet. Et Zootech. Sin. 2019, 50, 2105–2112. (In Chinese) [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Hai, Z.; Hou, L.; Liu, Y.; Zhang, D.; Zhou, X. Baicalin Attenuates Panton–Valentine Leukocidin (PVL)-Induced Cytoskeleton Rearrangement via Regulating the RhoA/ROCK/LIMK and PI3K/AKT/GSK-3β Pathways in Bovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 14520. https://doi.org/10.3390/ijms241914520
Yang J, Hai Z, Hou L, Liu Y, Zhang D, Zhou X. Baicalin Attenuates Panton–Valentine Leukocidin (PVL)-Induced Cytoskeleton Rearrangement via Regulating the RhoA/ROCK/LIMK and PI3K/AKT/GSK-3β Pathways in Bovine Mammary Epithelial Cells. International Journal of Molecular Sciences. 2023; 24(19):14520. https://doi.org/10.3390/ijms241914520
Chicago/Turabian StyleYang, Jiangliu, Zhenzhen Hai, Ling Hou, Yang Liu, Dongtao Zhang, and Xuezhang Zhou. 2023. "Baicalin Attenuates Panton–Valentine Leukocidin (PVL)-Induced Cytoskeleton Rearrangement via Regulating the RhoA/ROCK/LIMK and PI3K/AKT/GSK-3β Pathways in Bovine Mammary Epithelial Cells" International Journal of Molecular Sciences 24, no. 19: 14520. https://doi.org/10.3390/ijms241914520
APA StyleYang, J., Hai, Z., Hou, L., Liu, Y., Zhang, D., & Zhou, X. (2023). Baicalin Attenuates Panton–Valentine Leukocidin (PVL)-Induced Cytoskeleton Rearrangement via Regulating the RhoA/ROCK/LIMK and PI3K/AKT/GSK-3β Pathways in Bovine Mammary Epithelial Cells. International Journal of Molecular Sciences, 24(19), 14520. https://doi.org/10.3390/ijms241914520





