Quetiapine Moderates Doxorubicin-Induced Cognitive Deficits: Influence of Oxidative Stress, Neuroinflammation, and Cellular Apoptosis
Abstract
1. Introduction
2. Results
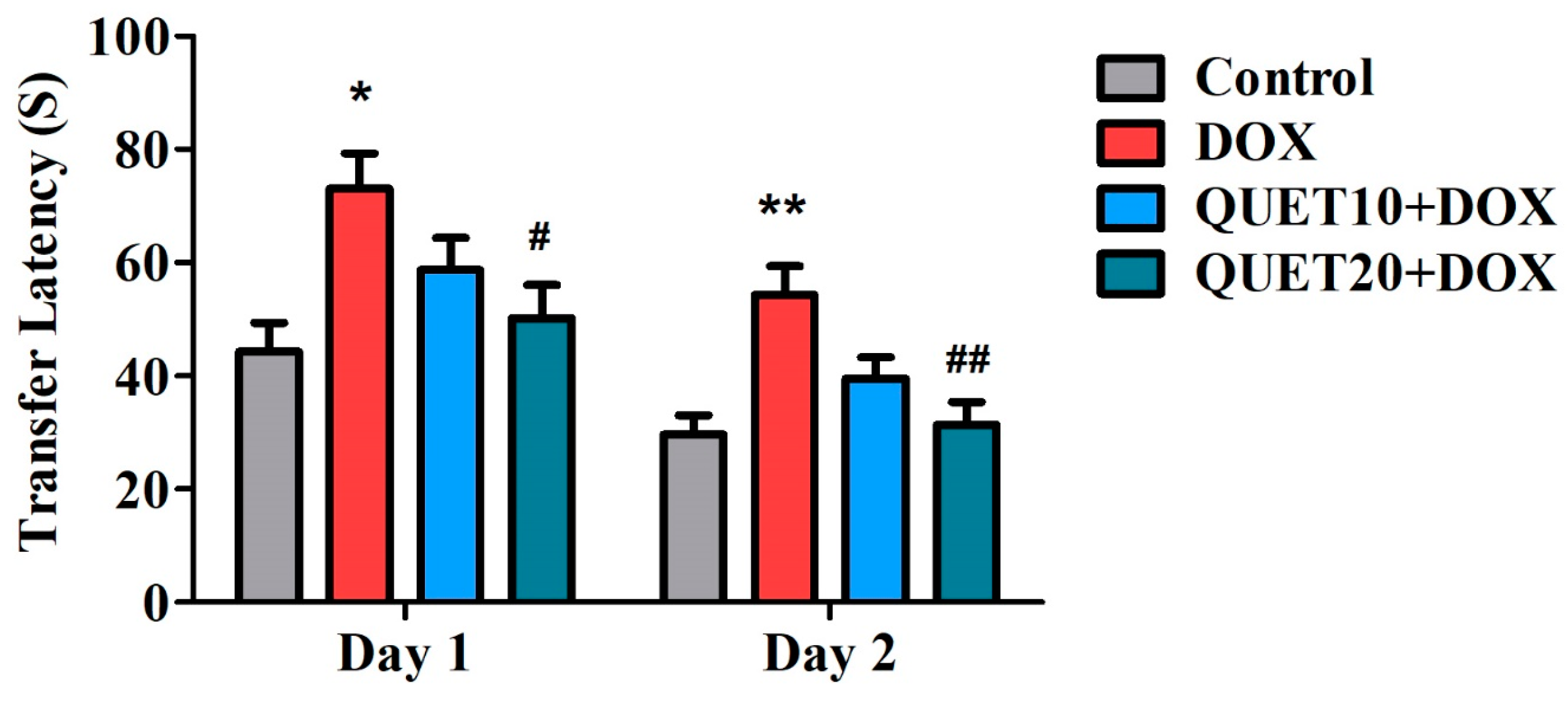
2.1. QUET-Attenuated Memory Deficits in DOX-Induced Rats in an Elevated Plus Maze (EPM) Test
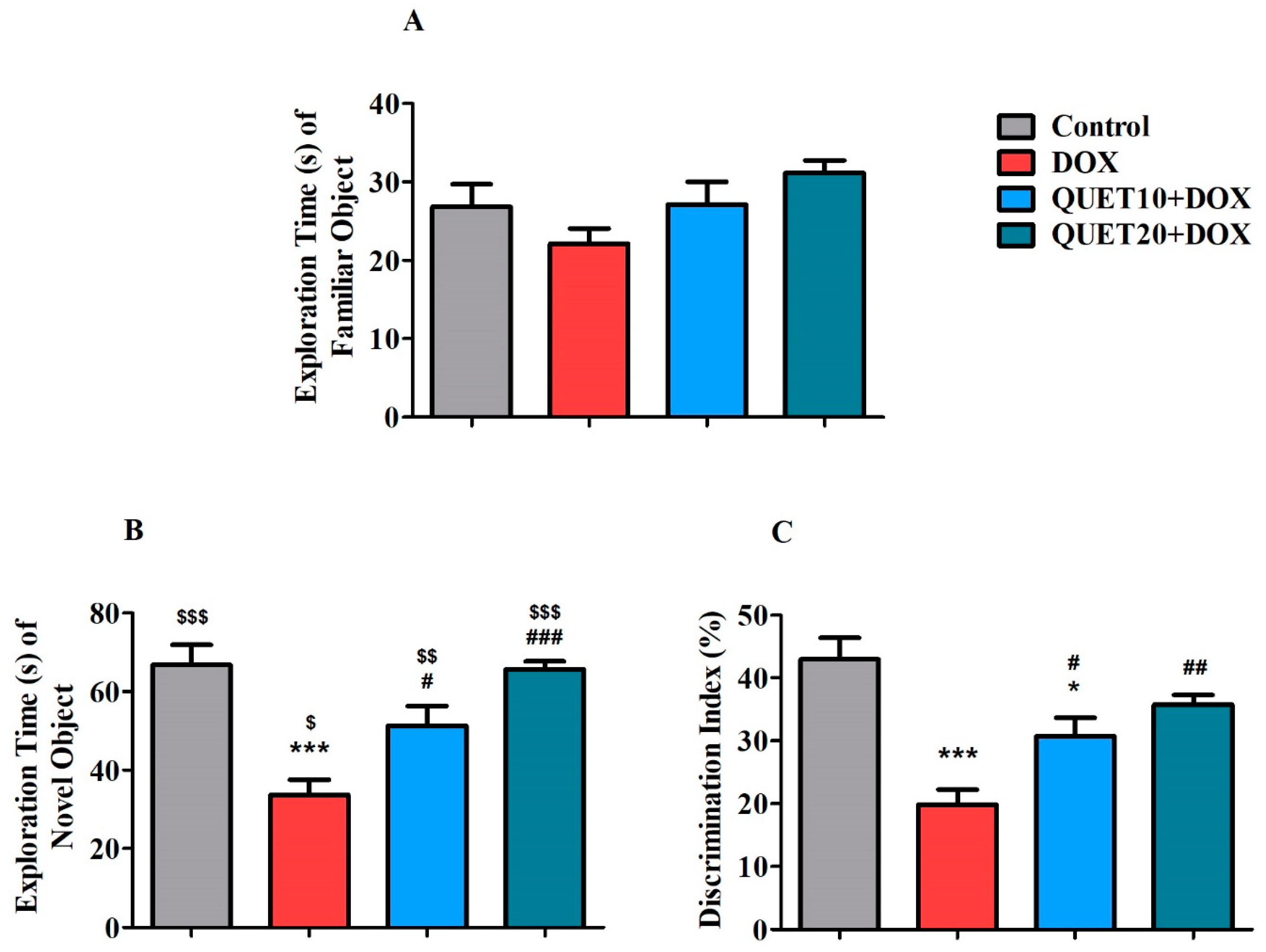
2.2. QUET-Improved Cognitive Functions in DOX-Induced Rats in Novel Object Recognition (NOR) Test
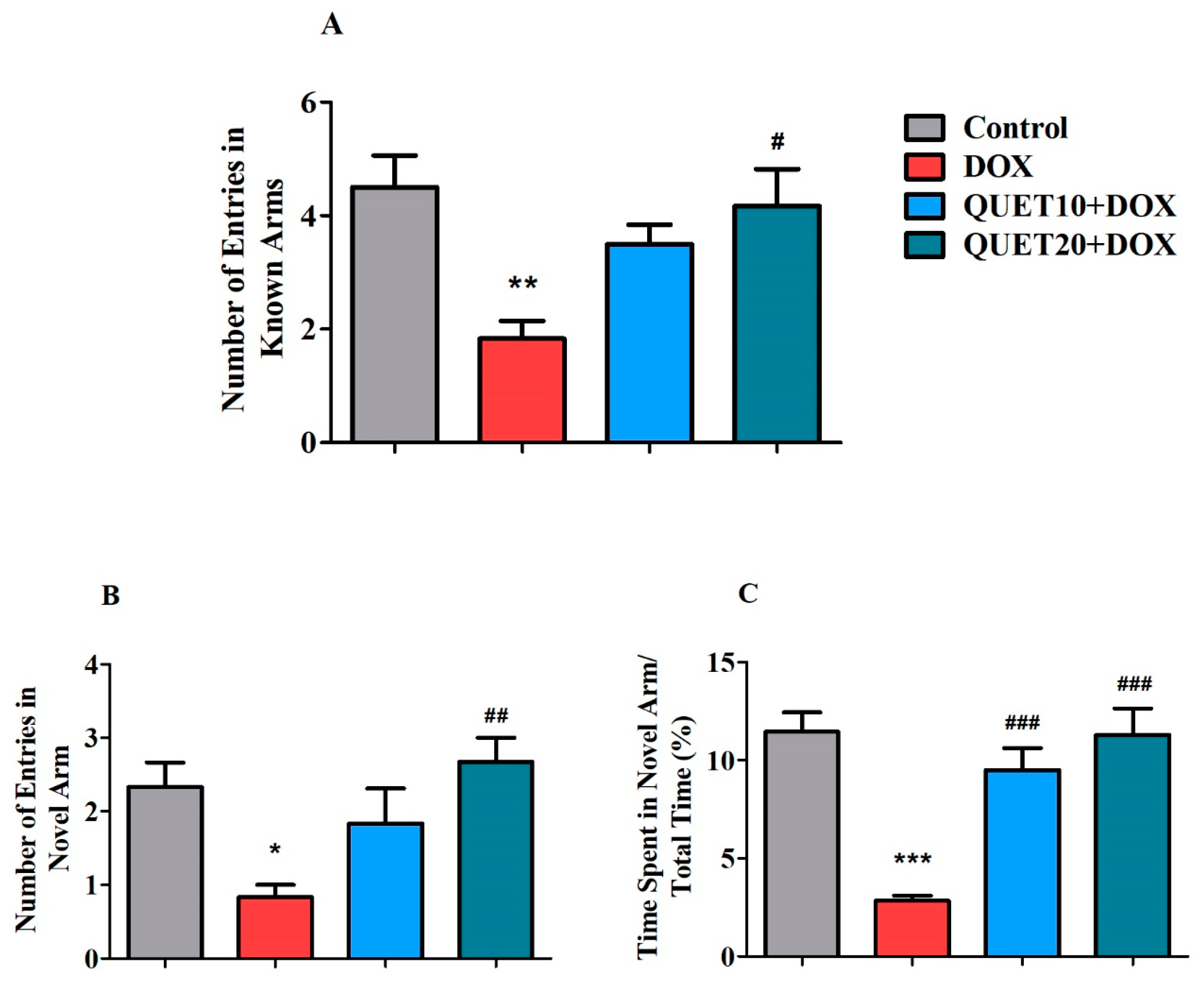
2.3. QUET-Enhanced Cognitive Functions in DOX-Induced Rats in Y-Maze Test
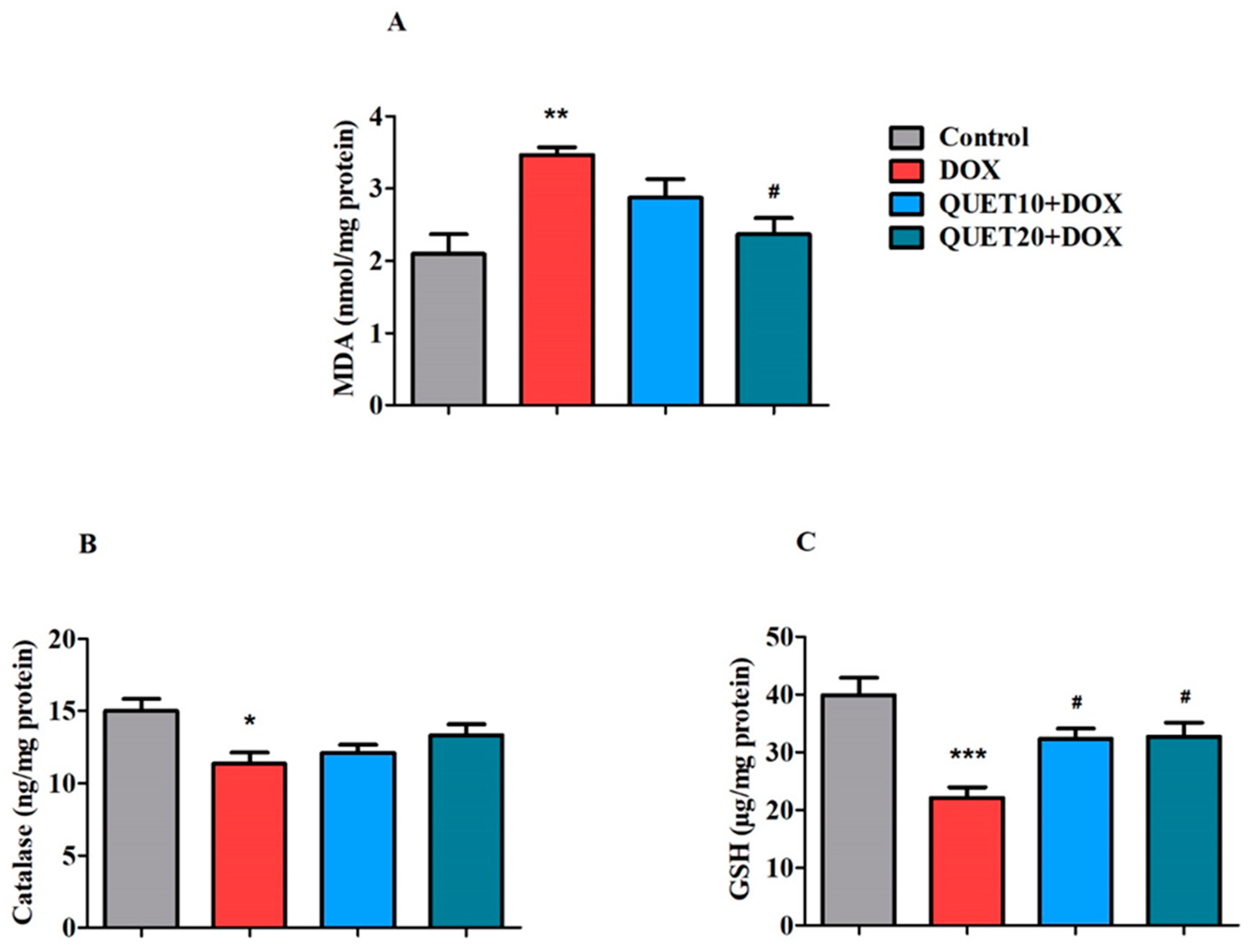
2.4. QUET-Ameliorated Oxidative Stress in Brain Tissues of DOX-Induced Rats
2.5. QUET-Reduced Neuro-Inflammatory Mediators in the Brain Tissues of DOX-Induced Rats
2.6. QUET-Reduced Apoptosis in the Brain Tissues of DOX-Induced Rats
3. Discussion
4. Materials and Methods
4.1. Animals
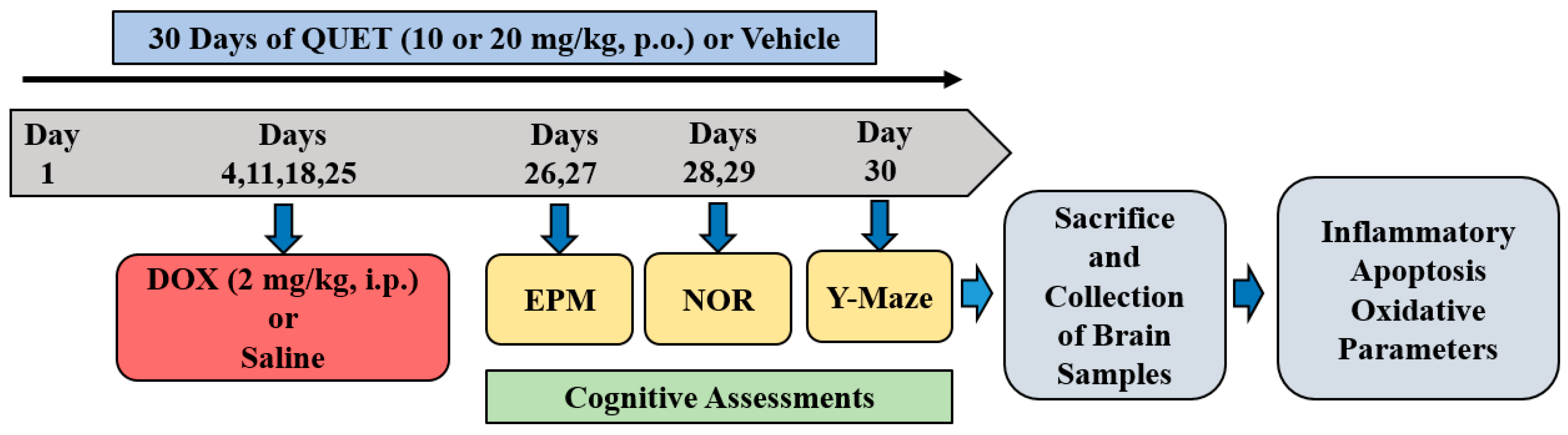
4.2. Treatment Groups and Schedule
4.3. Cognitive Assessments
4.3.1. Elevated Plus Maze (EPM)
4.3.2. Novel Object Recognition (NOR)
4.3.3. Y-Maze
4.4. Biochemical Analysis—Enzyme-Linked Immunosorbent (ELISA) Assay
4.4.1. Preparation of Brain Homogenate
4.4.2. Neuronal Oxidative Stress
4.4.3. Neuronal Inflammation
4.4.4. Neuronal Apoptosis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morean, D.F.; O’Dwyer, L.; Cherney, L.R. Therapies for cognitive deficits associated with chemotherapy for breast cancer: A systematic review of objective outcomes. Arch. Phys. Med. Rehabil. 2015, 96, 1880–1897. [Google Scholar] [CrossRef] [PubMed]
- Philpot, R.M.; Ficken, M.; Wecker, L. Doxorubicin and cyclophosphamide lead to long-lasting impairment of spatial memory in female, but not male mice. Behav. Brain Res. 2016, 307, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, S.; Assiri, H.; Alsalem, M.; Alharbi, M.A. Secondary psychosis following neoadjuvant AC-T chemotherapy for triple-negative breast cancer: Case report and literature review of psychosis postchemotherapy. Case Rep. Psychiatry 2022, 2022, 4939219. [Google Scholar] [CrossRef] [PubMed]
- Keefe, R.S.; Fenton, W.S. How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophr. Bull. 2007, 33, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Rabbani, S.I.; Shariq, A.; Amirthalingam, P.; Arfeen, M. Piracetam as a therapeutic agent for doxorubicin-induced cognitive deficits by enhancing cholinergic functions and reducing neuronal inflammation, apoptosis, and oxidative stress in rats. Pharmaceuticals 2022, 15, 1563. [Google Scholar] [CrossRef]
- Cardoso, C.V.; de Barros, M.P.; Bachi, A.L.L.; Bernardi, M.M.; Kirsten, T.B.; de Fátima Monteiro Martins, M.; Rocha, P.R.D.; da Silva Rodrigues, P.; Bondan, E.F. Chemobrain in rats: Behavioral, morphological, oxidative and inflammatory effects of doxorubicin administration. Behav. Brain Res. 2020, 378, 112233. [Google Scholar] [CrossRef]
- Liao, D.; Shangguan, D.; Wu, Y.; Chen, Y.; Liu, N.; Tang, J.; Yao, D.; Shi, Y. Curcumin protects against doxorubicin induced oxidative stress by regulating the Keap1-Nrf2-ARE and autophagy signaling pathways. Psychopharmacology 2023, 240, 1179–1190. [Google Scholar] [CrossRef]
- Alhowail, A.H.; Pinky, P.D.; Eggert, M.; Bloemer, J.; Woodie, L.N.; Buabeid, M.A.; Bhattacharya, S.; Jasper, S.L.; Bhattacharya, D.; Dhanasekaran, M.; et al. Doxorubicin induces dysregulation of AMPA receptor and impairs hippocampal synaptic plasticity leading to learning and memory deficits. Heliyon 2021, 7, e07456. [Google Scholar] [CrossRef]
- Lyu, W.; Ouyang, M.; Ma, X.; Han, T.; Pi, D.; Qiu, S. Kai-Xin-San attenuates doxorubicin-induced cognitive impairment by reducing inflammation, oxidative stress, and neural degeneration in 4T1 breast cancer mice. Evid. Based Complement. Alternat. Med. 2021, 2021, 5521739. [Google Scholar] [CrossRef]
- Wang, X.M.; Walitt, B.; Saligan, L.; Tiwari, A.F.; Cheung, C.W.; Zhang, Z.J. Chemobrain: A critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy. Cytokine 2015, 72, 86–96. [Google Scholar] [CrossRef]
- Du, J.; Zhang, A.; Li, J.; Liu, X.; Wu, S.; Wang, B.; Wang, Y.; Jia, H. Doxorubicin-induced cognitive impairment: The mechanistic insights. Front. Oncol. 2021, 11, 673340. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Keeney, J.T.R.; Miriyala, S.; Noel, T.; Powell, D.K.; Chaiswing, L.; Bondada, S.; St Clair, D.K.; Butterfield, D.A. The triangle of death of neurons: Oxidative damage, mitochondrial dysfunction, and loss of choline-containing biomolecules in brains of mice treated with doxorubicin. Advanced insights into mechanisms of chemotherapy induced cognitive impairment (“chemobrain”) involving TNF-α. Free Radic. Biol. Med. 2019, 134, 1–8. [Google Scholar] [CrossRef]
- Sardi, I.; la Marca, G.; Cardellicchio, S.; Giunti, L.; Malvagia, S.; Genitori, L.; Massimino, M.; de Martino, M.; Giovannini, M.G. Pharmacological modulation of blood-brain barrier increases permeability of doxorubicin into the rat brain. Am. J. Cancer Res. 2013, 3, 424–432. [Google Scholar]
- Licht, T.; Sasson, E.; Bell, B.; Grunewald, M.; Kumar, S.; Kreisel, T.; Ben-Zvi, A.; Keshet, E. Hippocampal neural stem cells facilitate access from circulation via apical cytoplasmic processes. eLife 2020, 9, e52134. [Google Scholar] [CrossRef] [PubMed]
- Jaehne, E.J.; Corrigan, F.; Toben, C.; Jawahar, M.C.; Baune, B.T. The effect of the antipsychotic drug quetiapine and its metabolite norquetiapine on acute inflammation, memory and anhedonia. Pharmacol. Biochem. Behav. 2015, 135, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Riedel, M.; Spellmann, I.; Strassnig, M.; Douhet, A.; Dehning, S.; Opgen-Rhein, M.; Valdevit, R.; Engel, R.R.; Kleindienst, N.; Müller, N.; et al. Effects of risperidone and quetiapine on cognition in patients with schizophrenia and predominantly negative symptoms. Eur. Arch. Psychiatry Clin. Neurosci. 2007, 257, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Riedel, M.; Schennach-Wolff, R.; Musil, R.; Dehning, S.; Cerovecki, A.; Opgen-Rhein, M.; Matz, J.; Seemüller, F.; Obermeier, M.; Engel, R.R.; et al. Neurocognition and its influencing factors in the treatment of schizophrenia-effects of aripiprazole, olanzapine, quetiapine and risperidone. Hum. Psychopharmacol. 2010, 25, 116–125. [Google Scholar] [CrossRef]
- He, J.; Luo, H.; Yan, B.; Yu, Y.; Wang, H.; Wei, Z.; Zhang, Y.; Xu, H.; Tempier, A.; Li, X.; et al. Beneficial effects of quetiapine in a transgenic mouse model of Alzheimer’s disease. Neurobiol. Aging 2009, 30, 1205–1216. [Google Scholar] [CrossRef]
- Luo, G.; Liu, M.; He, J.; Guo, H.; Xue, M.; Wang, X.; Li, X.M. Quetiapine attenuates recognition memory impairment and hippocampal oxidative stress in a transgenic mouse model of Alzheimer’s disease. Neuroreport 2014, 25, 647–650. [Google Scholar] [CrossRef]
- He, J.; Xu, H.; Yang, Y.; Rajakumar, D.; Li, X.; Li, X.M. The effects of chronic administration of quetiapine on the phencyclidine-induced reference memory impairment and decrease of Bcl-XL/Bax ratio in the posterior cingulate cortex in rats. Behav. Brain Res. 2006, 168, 236–242. [Google Scholar] [CrossRef]
- He, J.; Yang, Y.; Yu, Y.; Li, X.; Li, X.M. The effects of chronic administration of quetiapine on the methamphetamine-induced recognition memory impairment and dopaminergic terminal deficit in rats. Behav. Brain Res. 2006, 172, 39–45. [Google Scholar] [CrossRef]
- Wang, K.; Song, F.; Wang, H.; Wang, J.H.; Sun, Y. Quetiapine attenuates the neuroinflammation and executive function deficit in streptozotocin-induced diabetic mice. Mediat. inflamm. 2019, 2019, 1236082. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin-an agent with multiple mechanisms of anticancer activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef]
- Mani, V.; Arfeen, M.; Rabbani, S.I.; Shariq, A.; Amirthalingam, P. Levetiracetam ameliorates doxorubicin-induced chemobrain by enhancing cholinergic transmission and reducing neuroinflammation using an experimental rat model and molecular docking study. Molecules 2022, 27, 7364. [Google Scholar] [CrossRef]
- Mani, V.; Arfeen, M.; Dhaked, D.K.; Mohammed, H.A.; Amirthalingam, P.; Elsisi, H.A. Neuroprotective effect of methanolic Ajwa seed extract on lipopolysaccharide-induced memory dysfunction and neuroinflammation: In vivo, molecular docking and dynamics studies. Plants 2023, 12, 934. [Google Scholar] [CrossRef]
- Wang, H.N.; Peng, Y.; Tan, Q.R.; Chen, Y.C.; Zhang, R.G.; Qiao, Y.T.; Wang, H.H.; Liu, L.; Kuang, F.; Wang, B.R.; et al. Quetiapine ameliorates anxiety-like behavior and cognitive impairments in stressed rats: Implications for the treatment of posttraumatic stress disorder. Physiol. Res. 2010, 59, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Mathiasen, J.R.; DiCamillo, A. Novel object recognition in the rat: A facile assay for cognitive function. Curr. Protoc. Pharmacol. 2010, 49, 5–59. [Google Scholar] [CrossRef]
- Lueptow, L.M. Novel object recognition test for the investigation of learning and memory in mice. J. Vis. Exp. 2017, 126, 55718. [Google Scholar] [CrossRef]
- Mani, V.; Arfeen, M.; Sajid, S.; Almogbel, Y. Aqueous Ajwa dates seeds extract improves memory impairment in type-2 diabetes mellitus rats by reducing blood glucose levels and enhancing brain cholinergic transmission. Saudi J. Biol. Sci. 2022, 29, 2738–2748. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol. Biol. 2019, 1916, 105–111. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, L.; Jiang, W.; Xu, H.; Xiao, L.; Bi, X.; Wang, J.; Zhu, S.; Zhang, R.; et al. Quetiapine enhances oligodendrocyte regeneration and myelin repair after cuprizone-induced demyelination. Schizophr. Res. 2012, 138, 8–17. [Google Scholar] [CrossRef]
- Tempier, A.; He, J.; Zhu, S.; Zhang, R.; Kong, L.; Tan, Q.; Luo, H.; Kong, J.; Li, X.M. Quetiapine modulates conditioned anxiety and alternation behavior in Alzheimer’s transgenic mice. Curr. Alzheimer Res. 2013, 10, 199–206. [Google Scholar] [CrossRef]
- Amin, S.N.; Gamal, S.M.; Esmail, R.S.; Aziz, T.M.; Rashed, L.A. Cognitive effects of acute restraint stress in male albino rats and the impact of pretreatment with quetiapine versus ghrelin. J. Integr. Neurosci. 2014, 13, 669–692. [Google Scholar] [CrossRef]
- Shokoohinia, Y.; Hosseinzadeh, L.; Moieni-Arya, M.; Mostafaie, A.; Mohammadi-Motlagh, H.R. Osthole attenuates doxorubicin-induced apoptosis in PC12 cells through inhibition of mitochondrial dysfunction and ROS production. Biomed. Res. Int. 2014, 2014, 156848. [Google Scholar] [CrossRef]
- Mounier, N.M.; Wahdan, S.A.; Gad, A.M.; Azab, S.S. Role of inflammatory, oxidative, and ER stress signaling in the neuroprotective effect of atorvastatin against doxorubicin-induced cognitive impairment in rats. Naunyn. Schmiedebergs Arch. Pharmacol. 2021, 394, 1537–1551. [Google Scholar] [CrossRef]
- Patel, M. Targeting oxidative stress in central nervous system disorders. Trends Pharmacol. Sci. 2016, 37, 768–778. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Okudan, N.; Belviranlı, M.; Sezer, T. Potential protective effect of coenzyme Q10 on doxorubicin-induced neurotoxicity and behavioral disturbances in rats. Neurochem. Res. 2022, 47, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, A.A.; Le Maitre, C.L.; Haywood-Small, S.L.; Cross, N.A.; Jordan-Mahy, N. Glutathione is key to the synergistic enhancement of doxorubicin and etoposide by polyphenols in leukaemia cell lines. Cell Death Dis. 2015, 6, e2028. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.K.; Mason, R.P. Is metabolic activation of topoisomerase II poisons important in the mechanism of cytotoxicity? J. Drug Metab. Toxicol. 2015, 6, 186. [Google Scholar] [CrossRef]
- Ignácio, Z.M.; Réus, G.Z.; Abelaira, H.M.; de Moura, A.B.; de Souza, T.G.; Matos, D.; Goldim, M.P.; Mathias, K.; Garbossa, L.; Petronilho, F.; et al. Acute and chronic treatment with quetiapine induces antidepressant-like behavior and exerts antioxidant effects in the rat brain. Metab. Brain Dis. 2017, 32, 1195–1208. [Google Scholar] [CrossRef]
- Han, J.H.; Tian, H.Z.; Lian, Y.Y.; Yu, Y.; Lu, C.B.; Li, X.M.; Zhang, R.L.; Xu, H. Quetiapine mitigates the ethanol-induced oxidative stress in brain tissue, but not in the liver, of the rat. Neuropsychiatr. Dis. Treat. 2015, 11, 1473–1482. [Google Scholar] [CrossRef]
- Keeney, J.T.R.; Ren, X.; Warrier, G.; Noel, T.; Powell, D.K.; Brelsfoard, J.M.; Sultana, R.; Saatman, K.E.; Clair, D.K.S.; Butterfield, D.A. Doxorubicin-induced elevated oxidative stress and neurochemical alterations in brain and cognitive decline: Protection by MESNA and insights into mechanisms of chemotherapy-induced cognitive impairment (“chemobrain”). Oncotarget 2018, 9, 30324–30339. [Google Scholar] [CrossRef]
- Tangpong, J.; Cole, M.P.; Sultana, R.; Joshi, G.; Estus, S.; Vore, M.; St Clair, W.; Ratanachaiyavong, S.; St Clair, D.K.; Butterfield, D.A. Adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiol. Dis. 2006, 23, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Shaker, F.H.; El-Derany, M.O.; Wahdan, S.A.; El-Demerdash, E.; El-Mesallamy, H.O. Berberine ameliorates doxorubicin-induced cognitive impairment (chemobrain) in rats. Life Sci. 2021, 269, 119078. [Google Scholar] [CrossRef] [PubMed]
- El-Agamy, S.E.; Abdel-Aziz, A.K.; Wahdan, S.; Esmat, A.; Azab, S.S. Astaxanthin ameliorates doxorubicin-induced cognitive impairment (chemobrain) in experimental rat model: Impact on oxidative, inflammatory, and apoptotic machineries. Mol. Neurobiol. 2018, 55, 5727–5740. [Google Scholar] [CrossRef] [PubMed]
- Westphal, D.; Dewson, G.; Czabotar, P.E.; Kluck, R.M. Molecular biology of Bax and Bak activation and action. Biochim. Biophys. Acta 2011, 1813, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Means, J.C.; Gerdes, B.C.; Kaja, S.; Sumien, N.; Payne, A.J.; Stark, D.A.; Borden, P.K.; Price, J.L.; Koulen, P. Caspase-3-dependent proteolytic cleavage of tau causes neurofibrillary tangles and results in cognitive impairment during normal aging. Neurochem Res. 2016, 41, 2278–2288. [Google Scholar] [CrossRef]
- Alhowail, A.H.; Bloemer, J.; Majrashi, M.; Pinky, P.D.; Bhattacharya, S.; Yongli, Z.; Bhattacharya, D.; Eggert, M.; Woodie, L.; Buabeid, M.A.; et al. Doxorubicin-induced neurotoxicity is associated with acute alterations in synaptic plasticity, apoptosis, and lipid peroxidation. Toxicol. Mech. Methods. 2019, 29, 457–466. [Google Scholar] [CrossRef]
- Zhao, X.; Bausano, B.; Pike, B.R.; Newcomb-Fernandez, J.K.; Wang, K.K.; Shohami, E.; Ringger, N.C.; DeFord, S.M.; Anderson, D.K.; Hayes, R.L. TNF-alpha stimulates caspase-3 activation and apoptotic cell death in primary septo-hippocampal cultures. J. Neurosci. Res. 2001, 64, 121–131. [Google Scholar] [CrossRef]
- Lei, Y.; Hou, F.; Wu, X.; Yi, Y.; Xu, F.; Gong, Q.; Gao, J. Brucine-induced neurotoxicity by targeting caspase 3: Involvement of PPARγ/NF-κB/apoptosis signaling pathway. Neurotox. Res. 2022, 40, 2117–2131. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, M.B.; Tönge, M.; Emmez, H.; Kaymaz, F.; Kaymaz, M. Neuroprotective effects of quetiapine on neuronal apoptosis following experimental transient focal cerebral ischemia in rats. J. Korean Neurosurg. Soc. 2013, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.C.; Kulkarni, S.K. Evaluation of learning and memory mechanisms employing elevated plus-maze in rats and mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 1992, 16, 117–125. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mani, V.; Alshammeri, B.S. Quetiapine Moderates Doxorubicin-Induced Cognitive Deficits: Influence of Oxidative Stress, Neuroinflammation, and Cellular Apoptosis. Int. J. Mol. Sci. 2023, 24, 11525. https://doi.org/10.3390/ijms241411525
Mani V, Alshammeri BS. Quetiapine Moderates Doxorubicin-Induced Cognitive Deficits: Influence of Oxidative Stress, Neuroinflammation, and Cellular Apoptosis. International Journal of Molecular Sciences. 2023; 24(14):11525. https://doi.org/10.3390/ijms241411525
Chicago/Turabian StyleMani, Vasudevan, and Bander Shehail Alshammeri. 2023. "Quetiapine Moderates Doxorubicin-Induced Cognitive Deficits: Influence of Oxidative Stress, Neuroinflammation, and Cellular Apoptosis" International Journal of Molecular Sciences 24, no. 14: 11525. https://doi.org/10.3390/ijms241411525
APA StyleMani, V., & Alshammeri, B. S. (2023). Quetiapine Moderates Doxorubicin-Induced Cognitive Deficits: Influence of Oxidative Stress, Neuroinflammation, and Cellular Apoptosis. International Journal of Molecular Sciences, 24(14), 11525. https://doi.org/10.3390/ijms241411525






