Biomarkers for Immunotherapy in Driver-Gene-Negative Advanced NSCLC
Abstract
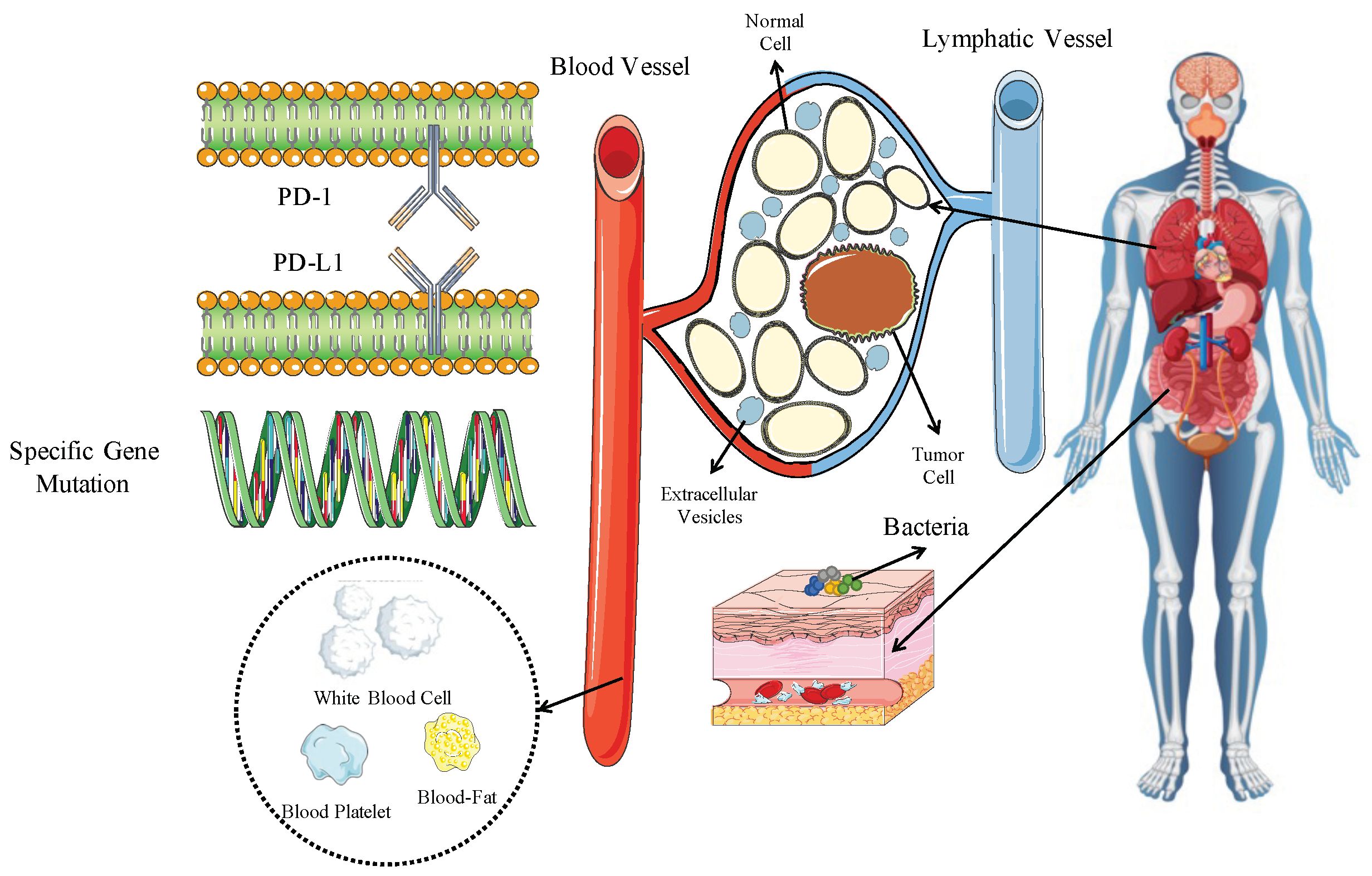
:1. Introduction
2. Tumor-Related
2.1. PD-L1
| Clinical Trial | Pathological Type | Treatment Arms | HR for mOS (95% CI) by PD-L1 (%) | HR for PFS (95% CI) by PD-L1 (%) |
|---|---|---|---|---|
| Squamous | ||||
| IMpower131 [29] | SQ | Atezolizumab + Nab-paclitaxel + carboplatin vs. Nab-paclitaxel + carboplatin | TC/IC 3 HR = 0.48, 95% CI 0.29–0.81 | TC/IC 3 HR = 0.41, 95% CI 0.25–0.68 |
| TC/IC 1/2 HR = 1.08, 95% CI 0.81–1.45 | TC/IC 1/2 HR = 0.70, 95% CI 0.54–0.91 | |||
| TC/IC 0 HR = 0.87, 95% CI 0.67–1.13 | TC/IC 0 HR = 0.82, 95% CI 0.65–1.04 | |||
| KEYNOTE-407 [30] | SQ | Pembrolizumab + chemotherapy vs. chemotherapy | ≥50% HR = 0.68, 95% CI 0.47–0.97 | ≥50% HR = 0.48, 95% CI 0.33–0.69 |
| 1–49% HR = 0.61, 95% CI 0.45–0.83 | 1–49% HR = 0.6, 95% CI 0.45–0.81 | |||
| <1% HR = 0.83, 95% CI 0.61–1.13 | <1% HR = 0.7, 95% CI 0.52–0.95 | |||
| RATIONALE-307 [31] | SQ | Tislelizumab + paclitaxel/nab-paclitaxel and carboplatin vs. paclitaxel and carboplatin | NA | ≥50% = 0.50, 95% CI 0.28–0.89 |
| 1–49% = 0.44, 95% CI 0.22–0.87 | ||||
| <1% = 0.64, 95% CI 0.37–1.10 | ||||
| ≥50% = 0.43, 95% CI 0.23–0.78 | ||||
| 1–49% = 0.31, 95% CI 0.15–0.66 | ||||
| <1% = 0.69, 95% CI 0.41–1.18 | ||||
| CameL-sq [16,17] | SQ | Camrelizumab + chemotherapy vs. chemotherapy | ≥50% HR = 0.48, 95% CI 0.21–1.12 | ≥50% HR = 0.30, 95% CI 0.17–0.55 |
| 1–49% HR 0.6, 95% CI 0.27–1.0 | 1–49% HR 0.6, 95% CI 0.20–0.51 | |||
| HR 0.7, 95% CI 0.41–0.94 | <1% HR 0.7, 95% CI 0.35–0.68 | |||
| ORIENT-12 [32] | SQ | Sintilimab + chemotherapy vs. chemotherapy | NA | ≥50% HR = 0.458, 95% CI 0.302–0.695 |
| 1–49% HR 0.620, 95% CI 0.408–0.941 | ||||
| <1% HR 0.548, 95% CI 0.368–0.815 | ||||
| Non-squamous | ||||
| IMpower130 [33] | NSQ | Atezolizumab + Nab-paclitaxel + carboplatin vs. Nab-paclitaxel + carboplatin | TC/IC 3 HR = 0.84, 95% CI 0.51–1.39 | NA |
| TC/IC 1/2 HR = 0.7, 95% CI 0.45–1.08 | ||||
| TC/IC 0 HR = 0.81, 95% CI 0.61–1.08 | ||||
| IMpower132 [18] | NSQ | Atezolizumab + Carboplatin/ cisplatin + pemetrexed vs. Carboplatin/cisplatin + pemetrexed | TC/IC 3 HR = 0.73, 95% CI 0.31–1.73 | NA |
| TC/IC 1/2 HR = 1.18, 95% CI 0.80–1.76 | ||||
| TC/IC 0 HR = 0.67, 95% CI 0.46–0.96 | ||||
| KEYNOTE-189 [19] | NSQ | Pembrolizumab + chemotherapy vs. chemotherapy | ≥50% HR = 0.59, 95% CI 0.40–0.86 | |
| 1–49% HR = 0.66, 95% CI 0.46–0.96 | ||||
| <1% HR = 0.51, 95% CI 0.36–0.71 | ||||
| RATIONALE-304 [34] | NSQ | Tislelizumab + chemotherapy vs. chemotherapy | NA | ≥50% HR = 0.336, 95% CI 0.185–0.611 |
| 1–49% HR = 1.095, 95% CI 0.526–2.277 | ||||
| <1% HR = 0.733, 95% CI 0.456–1.179 | ||||
| CameL [35] | NSQ | Camrelizumab + chemotherapy vs. chemotherapy | NA | ≥50% HR = 0.39, 95% CI 0.14–0.99 |
| 1–49% HR = 0.62, 95% CI 0.40–0.94 | ||||
| <1% HR = 0.76, 95% CI 0.45–1.26 | ||||
| ORIENT 11 [36] | NSQ | Sintilimab + chemotherapy vs. chemotherapy | ≥1% HR = 0.56, 95% CI 0.40–0.77 | NA |
| <1% HR = 0.75, 95% CI 0.48–1.19 | ||||
| NSCLC | ||||
| GEMSTONE-302 [20] | NSCLC | Sugemalimab + chemotherapy vs. chemotherapy | NA | HR = 0.41, 95% CI 0.27–0.62 |
| 1–49% HR = 0.53, 95% CI 0.35–0.79 | ||||
| <1% HR = 0.56, 95% CI 0.40–0.77 | ||||
| CHOICE-01 [37] | NSCLC | Toripalimab + chemotherapy vs. chemotherapy | All HR = 0.69, 95% CI 0.53–0.92 | HR = 0.49; 95% CI 0.39–0.61 |
| HR = 0.56, 95% CI 0.36–0.86 | TC HR = 0.45; 95%CI 0.27–0.78 | |||
| 1–49% HR = 0.72, 95% CI 0.48–1.07 | TC < 50% HR = 0.56; 95% CI 0.40–0.78 | |||
| <1% HR = 0.79, 95% CI 0.49–1.31 | TC < 1% HR = 0.47; 95%CI 0.32–0.71 | |||
| EMPOWER-Lung 3 [38] | NSCLC (EGFR/ALK/ROS1WT) | Cemiplimab + chemotherapy vs. chemotherapy | All HR = 0.65, 95% CI: 0.51–0.82 | NA |
| ≥50% HR = 0.56, 95% CI 0.36–0.86 | ||||
| 1–49% HR = 0.50, 95% CI 0.34–0.74 | ||||
| <1% HR = 0.94, 95% CI 0.62–1.42 |
| Clinical Trial | Pathological Type | Treatment Arms | HR for mOS (95% CI) by PD-L1 (%) | HR for PFS (or mPFS) (95% CI) by PD-L1 (%) |
|---|---|---|---|---|
| KEYNOTE-010 [21] | NSCLC | Pembrolizumab vs. Docetaxel | TPS : 16.9 vs. 8.2 mo, HR = 0.55 (0.44–0.69) | TPS : 5.3 vs. 4.2 mo, HR = 0.57 (0.46–0.71) |
| TPS 1–49%: HR = 0.79(0.65–0.94) | TPS : 4.0 vs. 4.1 mo, HR = 0.84 (0.73–0.96) | |||
| TPS : 11.8 vs. 8.4mo, HR = 0.70 (0.61–0.80) | ||||
| OAK [22] | NSCLC | Atezolizumab vs. Docetaxel | ITT: 13.8 vs. 9.6 mo, HR 0.73 (0.62–0.87) | ITT: 2.8 vs. 4.0 mo, HR 0.95 (0.82–1.10) |
| TC3 or IC3: 20.5 vs. 8.9 mo, HR 0.41(0·27–0·64) | TC3 or IC3: 4.2 vs. 3.3 mo, HR 0.63(0.43–0.91) | |||
| TC2/3 or IC2/3: 16.3 vs. 10.8 mo, HR 0.67 (0.49–0.90) | TC2/3 or IC2/3: 4.1 vs. 3.6 mo, HR 0.76(0.58–0.99) | |||
| TC1/2/3 or IC1/2/3: 15.7 vs. 10.3 mo, HR 0·74 (0·58–0.93) | TC1/2/3 or IC1/2/3: 2.8 vs. 4.1 mo, HR 0.91 (0·74–1.12) | |||
| TC0 and IC0: 12.6 vs. 8.9 mo, HR 0.75 (CI 0.59–0.96) | TC0 and IC0: 2.6–4.0 mo, HR 1.00 (0.80–1.25) | |||
| POPLAR [23] | NSCLC | Atezolizumab vs. Docetaxel | TC3 or IC3: 15.5 vs. 11.1 mo, HR 0·49 (0·22–1.07) | TC3 or IC3: 7.8 vs. 3.9 mo, HR 0.60 (0.31–1.16) |
| TC2/3 or IC2/3: 15.1 vs. 7.4 mo, HR 0.54 (0.33–0.89) | TC2/3 or IC2/3: 3.4 vs. 2.8 mo, HR 0.72 (0.47–1.10) | |||
| TC1/2/3 or IC1/2/3: 15.5 vs. 9.2 mo, HR 0·59 (0·40–0·85) | TC1/2/3 or IC1/2/3: 2.8 vs. 3.0 mo, HR 0.85 (0.63–1.16) | |||
| TC0 and IC0: 9.7 vs. 9.7 mo, HR 1.04 (0.62–1.75) | TC0 and IC0: 1.7 vs. 4.1mo, HR 1.12 (1.4–4.2) | |||
| Checkmate-017 and Checkmate-057 [24] | NSCLC | Nivolumab vs. Docetaxel | PD-L1 : 13.4 vs. 8.5 mo, HR 0.61 (0.49–0.76) | PD-L1 : 3.7 vs. 3.6 mo, HR = 0.66 (0.53–0.84) |
| PD-L1 : 9.7 vs. 7.8 mo, HR 0.76 (0.61–0.96) | PD-L1 : 2.1 vs. 3.5 mo, HR 0.99 (0.78–1.26) | |||
| Checkmate-078 [25] | NSCLC | Nivolumab vs. Docetaxel | PD-L1 %: 12.0 vs. 7.9 mo, HR 0.71 (0.54–0.95) | PD-L1 tumor expression : 2.8 vs. 2.6 mo, HR 0.75 (0.56–0.99) |
| PD-L1 : 11.4 vs. 10.2 mo, HR 0.73 (0.53–1.02) | PD-L1 expression : 2.9 vs. 2.8 mo, HR 0.77 (0.56–1.07) | |||
| RATIONALE-303 [26] | NSCLC | Tislelizumab vs. docetaxel | PD-L1 expression TC: 19.3 vs. 11.5 mo, HR 0.53 (0.40–0.70) | PD-L1 expression TC: 0.37 (0.28–0.49) |
| PD-L1 expression TC: 15.2 vs. 12.3 mo, HR 0.77 (0.62–0.96) |
| Clinical Trial | Pathological Type | Treatment Arms | HR for mOS (95% CI) by PD-L1 (%) | HR for PFS (or mPFS) (95% CI) by PD-L1 (%) |
|---|---|---|---|---|
| TORG1630 [39] | NSCLC | Nivolumab vs. Nivolumab plus Docetaxel | PD-L1 ≥ 50% (N = 5): HR 1.03 (0.09–11.55) | NA |
| PD-L1 1–49% (N = 23): HR 0.32 (0.10–1.07) | ||||
| PD-L1 0% (N = 22): HR 0.41 (0.14–1.22) | ||||
| PROLUNG [40] | NSCLC | Pembrolizumab plus Docetaxel vs. Docetaxel | NA | PD-L1 (+) (N = 30): 16.8 vs. 3.9 mo, HR 0.16 (0.05–0.52) |
| PD-L1 (−) (N = 30): 6.3 vs. 4.4 mo, HR 0.41 (0.16–1.05) |
2.2. Tumor Mutation Burden (TMB)
2.2.1. Tissue TMB (tTMB)
2.2.2. Blood TMB (bTMB)
2.3. Specific Genetic Mutations
3. Tumor Microenvironment (TME)-Related Biomarkers
3.1. Biomarkers in Extracellular Vesicles (EVs)
3.2. Roles of T-Cell Receptors(TCR) in Prediction
4. Host-Related
4.1. Biomarkers Relating to Systemic Inflammation
4.2. Circulating Fatty Acid Profile
4.3. Microbiome
5. Discussion
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ASVs | Amplicon Sequence Variants |
| bTMB | Blood-Tumor Mutation Burden |
| CI | Confidence Interval |
| CRP | C-reactive Protein |
| CT | Chemotherapy |
| D | Durvalumab |
| EVs | Extracellular Vesicles |
| HR | Hazard Ratio |
| IC | Immune Cell |
| ICIs | Immune checkpoint inhibitors |
| ITT | Intention-to-treat |
| KEAP1 | Kelch-like ECH-associated Protein 1 |
| LC | Lymphocyte Count |
| LDH | Lactate Dehydrogenase |
| LIPI | Lung Immune Prognostic Index |
| mOS | Median Overall Survival |
| mPFS | Median Progression-Free Survival |
| NA | Not Available |
| NC | Neutrophil Count |
| NCCN | National Comprehensive Cancer Network |
| NE | Not Estimable |
| NLR | Neutrophil-to-lymphocyte Ratio |
| NPV | Negative Predictive Value |
| NRF2 | Nuclear Factor Erythroid 2-related Factor 2 |
| NSCLC | Non-Small Cell Lung Cancer |
| NSQ NSCLC | Non-Squamous Non-Small Cell Lung Cancer |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| PLR | Platelet-to-Lymphocyte Ratio |
| SCFAs | Short-Chain Fatty Acids |
| SIPS | Scottish Inflammatory Prognostic Score |
| SQ NSCLC | Squamous Non-Small Cell Lung Cancer |
| STK11 | Serine/Threonine Kinase 11 |
| T | Tremelimumab |
| TC | Tumor Cell |
| TCR | T-cell Receptors |
| TGF- | Transforming Growth Factor- |
| TIL | Tumor-iInfiltrating T Lymphocyte |
| TMAO | Trimethylamine N-Oxide |
| TMB | Tumor Mutation Burden |
| TME | Tumor Immune Microenvironment |
| TPS | Tumor Proportion Score |
| tTMB | Tissue-TMB |
| WCC | White Cell Count |
| WT | Wild Type |
References
- Hanna, N.H.; Schneider, B.J.; Temin, S.; Baker, S., Jr.; Brahmer, J.; Ellis, P.M.; Gaspar, L.E.; Haddad, R.Y.; Hesketh, P.J.; Jain, D.; et al. Therapy for stage IV non–small-cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J. Clin. Oncol. 2020, 38, 1608–1632. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer, version 7.2021; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2021. [Google Scholar]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 3.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 20, 497–530. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Garon, E.B.; Kim, D.W.; Cho, B.C.; Perez-Gracia, J.L.; Han, J.Y.; Arvis, C.D.; Majem, M.; Forster, M.D.; Monnet, I.; et al. Long-Term Outcomes and Retreatment Among Patients with Previously Treated, Programmed Death-Ligand 1-Positive, Advanced Non-Small-Cell Lung Cancer in the KEYNOTE-010 Study. J. Clin. Oncol. 2020, 38, 1580–1590. [Google Scholar] [CrossRef]
- Kerr, K.M.; Nicolson, M.C. Non-Small Cell Lung Cancer, PD-L1, and the Pathologist. Arch. Pathol. Lab. Med. 2016, 140, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Koomen, B.M.; Badrising, S.K.; van den Heuvel, M.M.; Willems, S.M. Comparability of PD-L1 immunohistochemistry assays for non-small-cell lung cancer: A systematic review. Histopathology 2020, 76, 793–802. [Google Scholar] [CrossRef]
- Marchetti, A.; Barberis, M.; Franco, R.; De Luca, G.; Pace, M.V.; Staibano, S.; Volante, M.; Buttitta, F.; Guerini-Rocco, E.; Righi, L.; et al. Multicenter Comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) Assays to Test PD-L1 Expression for NSCLC Patients to Be Treated with Immune Checkpoint Inhibitors. J. Thorac. Oncol. 2017, 12, 1654–1663. [Google Scholar] [CrossRef]
- Rimm, D.L.; Han, G.; Taube, J.M.; Yi, E.S.; Bridge, J.A.; Flieder, D.B.; Homer, R.; West, W.W.; Wu, H.; Roden, A.C.; et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol. 2017, 3, 1051–1058. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.J.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Jassem, J.; de Marinis, F.; Giaccone, G.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Oprean, C.; Kim, Y.C.; Andric, Z.; et al. Updated Overall Survival Analysis From IMpower110: Atezolizumab Versus Platinum-Based Chemotherapy in Treatment-Naive Programmed Death-Ligand 1-Selected NSCLC. J. Thorac. Oncol. 2021, 16, 1872–1882. [Google Scholar] [CrossRef]
- Sezer, A.; Kilickap, S.; Gümüş, M.; Bondarenko, I.; Özgüroğlu, M.; Gogishvili, M.; Turk, H.M.; Cicin, I.; Bentsion, D.; Gladkov, O.; et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50open-label, global, phase 3, randomised, controlled trial. Lancet 2021, 397, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.E.; Badin, F.; et al. First-line nivolumab in stage IV or recurrent non–small-cell lung cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Cho, B.C.; Reinmuth, N.; Lee, K.H.; Luft, A.; Ahn, M.J.; Van Den Heuvel, M.M.; Cobo, M.; Vicente, D.; Smolin, A.; et al. Durvalumab with or without tremelimumab vs. standard chemotherapy in first-line treatment of metastatic non–small cell lung cancer: The MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 2020, 6, 661–674. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, J.; Xu, X.; Cheng, Y.; Chen, G.; Pan, Y.; Fang, Y.; Wang, Q.; Huang, Y.; Yao, W.; et al. On-treatment blood TMB as predictors for camrelizumab plus chemotherapy in advanced lung squamous cell carcinoma: Biomarker analysis of a phase III trial. Mol. Cancer 2022, 21, 4. [Google Scholar] [CrossRef]
- Ren, S.; Chen, J.; Xu, X.; Jiang, T.; Cheng, Y.; Chen, G.; Pan, Y.; Fang, Y.; Wang, Q.; Huang, Y.; et al. Camrelizumab Plus Carboplatin and Paclitaxel as First-Line Treatment for Advanced Squamous NSCLC (CameL-Sq): A Phase 3 Trial. J. Thorac. Oncol. 2022, 17, 544–557. [Google Scholar] [CrossRef]
- Nishio, M.; Barlesi, F.; West, H.; Ball, S.; Bordoni, R.; Cobo, M.; Longeras, P.D.; Goldschmidt, J.; Novello, S.; Orlandi, F.; et al. Atezolizumab Plus Chemotherapy for First-Line Treatment of Nonsquamous NSCLC: Results From the Randomized Phase 3 IMpower132 Trial. J. Thorac. Oncol. 2021, 16, 653–664. [Google Scholar] [CrossRef]
- Garassino, M.C.; Gadgeel, S.; Speranza, G.; Felip, E.; Esteban, E.; Dómine, M.; Hochmair, M.J.; Powell, S.F.; Bischoff, H.G.; Peled, N.; et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 1992–1998. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Z.; Sun, Y.; Cao, L.; Ma, Z.; Wu, R.; Yu, Y.; Yao, W.; Chang, J.; Chen, J.; et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): Interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol. 2022, 23, 220–233. [Google Scholar] [CrossRef]
- Herbst, R.S.; Garon, E.B.; Kim, D.W.; Cho, B.C.; Gervais, R.; Perez-Gracia, J.L.; Han, J.Y.; Majem, M.; Forster, M.D.; Monnet, I.; et al. Five Year Survival Update From KEYNOTE-010: Pembrolizumab Versus Docetaxel for Previously Treated, Programmed Death-Ligand 1-Positive Advanced NSCLC. J. Thorac. Oncol. 2021, 16, 1718–1732. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Borghaei, H.; Gettinger, S.; Vokes, E.E.; Chow, L.Q.M.; Burgio, M.A.; de Castro Carpeno, J.; Pluzanski, A.; Arrieta, O.; Frontera, O.A.; Chiari, R.; et al. Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2021, 39, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Cheng, Y.; Mok, T.; Chang, J.; Zhang, L.; Feng, J.; Tu, H.Y.; Wu, L.; Zhang, Y.; et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non-small cell lung cancer: 2-year follow-up from a randomized, open-label, phase 3 study (CheckMate 078). Lung Cancer 2021, 152, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Huang, D.; Fan, Y.; Yu, X.; Liu, Y.; Shu, Y.; Ma, Z.; Wang, Z.; Cheng, Y.; Wang, J.; et al. Tislelizumab Versus Docetaxel in Patients With Previously Treated Advanced NSCLC (RATIONALE-303): A Phase 3, Open-Label, Randomized Controlled Trial. J. Thorac. Oncol. 2023, 18, 93–105. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Perez-Gracia, J.L.; Felip, E.; Kim, D.W.; Han, J.Y.; Molina, J.R.; Kim, J.H.; Dubos Arvis, C.; Ahn, M.J.; et al. Use of archival versus newly collected tumor samples for assessing PD-L1 expression and overall survival: An updated analysis of KEYNOTE-010 trial. Ann. Oncol. 2019, 30, 281–289. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Jotte, R.; Cappuzzo, F.; Vynnychenko, I.; Stroyakovskiy, D.; Rodríguez-Abreu, D.; Hussein, M.; Soo, R.; Conter, H.J.; Kozuki, T.; Huang, K.C.; et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): Results from a randomized phase III trial. J. Thorac. Oncol. 2020, 15, 1351–1360. [Google Scholar] [CrossRef]
- Novello, S.; Kowalski, D.M.; Luft, A.; Gümüş, M.; Vicente, D.; Mazières, J.; Rodríguez-Cid, J.; Tafreshi, A.; Cheng, Y.; Lee, K.H.; et al. Pembrolizumab plus chemotherapy in squamous non–small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J. Clin. Oncol. 2023, 41, 1999. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, S.; Yu, X.; Hu, Y.; Sun, Y.; Wang, Z.; Zhao, J.; Yu, Y.; Hu, C.; Yang, K.; et al. Tislelizumab plus chemotherapy vs. chemotherapy alone as first-line treatment for advanced squamous non–small-cell lung cancer: A phase 3 randomized clinical trial. JAMA Oncol. 2021, 7, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, L.; Fan, Y.; Wang, Z.; Liu, L.; Chen, G.; Zhang, L.; Huang, D.; Cang, S.; Yang, Z.; et al. Sintilimab plus platinum and gemcitabine as first-line treatment for advanced or metastatic squamous NSCLC: Results from a randomized, double-blind, phase 3 trial (ORIENT-12). J. Thorac. Oncol. 2021, 16, 1501–1511. [Google Scholar] [CrossRef]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Yu, Y.; Yu, X.; Hu, Y.; Ai, X.; Ma, Z.; Li, X.; Zhuang, W.; Liu, Y.; et al. Tislelizumab plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous NSCLC (RATIONALE 304): A randomized phase 3 trial. J. Thorac. Oncol. 2021, 16, 1512–1522. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, G.; Huang, Y.; Zhou, J.; Lin, L.; Feng, J.; Wang, Z.; Shu, Y.; Shi, J.; Hu, Y.; et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): A randomised, open-label, multicentre, phase 3 trial. Lancet Respir. Med. 2021, 9, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.; Fang, J.; Yu, Q.; Han, B.; Cang, S.; Chen, G.; Mei, X.; Yang, Z.; Stefaniak, V.; et al. Final overall survival data of sintilimab plus pemetrexed and platinum as First-Line treatment for locally advanced or metastatic nonsquamous NSCLC in the Phase 3 ORIENT-11 study. Lung Cancer 2022, 171, 56–60. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, L.; Li, B.; Cheng, Y.; Li, X.; Wang, X.; Han, L.; Wu, X.; Fan, Y.; Yu, Y.; et al. Toripalimab Plus Chemotherapy for Patients With Treatment-Naive Advanced Non–Small-Cell Lung Cancer: A Multicenter Randomized Phase III Trial (CHOICE-01). J. Clin. Oncol. 2023, 41, 651. [Google Scholar] [CrossRef] [PubMed]
- Makharadze, T.; Gogishvili, M.; Melkadze, T.; Baramidze, A.; Giorgadze, D.; Penkov, K.; Laktionov, K.; Nemsadze, G.; Nechaeva, M.; Rozhkova, I.; et al. Cemiplimab plus chemotherapy versus chemotherapy alone in advanced NSCLC: 2-year follow-up from the phase 3 EMPOWER-Lung 3 part 2 trial. J. Thorac. Oncol. 2023, 18, 755–768. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Shimokawa, T.; Takiguchi, Y.; Misumi, T.; Nakamura, Y.; Kawashima, Y.; Furuya, N.; Shiraishi, Y.; Harada, T.; Tanaka, H.; et al. A Randomized Comparison of Nivolumab versus Nivolumab + Docetaxel for Previously Treated Advanced or Recurrent ICI-Naïve Non-Small Cell Lung Cancer: TORG1630. Clin. Cancer Res. 2022, 28, 4402–4409. [Google Scholar] [CrossRef]
- Arrieta, O.; Barrón, F.; Ramírez-Tirado, L.A.; Zatarain-Barrón, Z.L.; Cardona, A.F.; Díaz-García, D.; Yamamoto Ramos, M.; Mota-Vega, B.; Carmona, A.; Peralta Álvarez, M.P.; et al. Efficacy and Safety of Pembrolizumab Plus Docetaxel vs. Docetaxel Alone in Patients With Previously Treated Advanced Non-Small Cell Lung Cancer: The PROLUNG Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 856–864. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kim, S.; Keam, B.; Kim, T.M.; Kim, D.W.; Heo, D.S. Soluble PD-L1 is a predictive and prognostic biomarker in advanced cancer patients who receive immune checkpoint blockade treatment. Sci. Rep. 2021, 11, 19712. [Google Scholar] [CrossRef]
- Moran, J.A.; Adams, D.L.; Edelman, M.J.; Lopez, P.; He, J.; Qiao, Y.; Xu, T.; Liao, Z.; Gardner, K.P.; Tang, C.M.; et al. Monitoring PD-L1 Expression on Circulating Tumor-Associated Cells in Recurrent Metastatic Non-Small-Cell Lung Carcinoma Predicts Response to Immunotherapy With Radiation Therapy. JCO Precis. Oncol. 2022, 6, e2200457. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bai, H.; Wang, C.; Seery, S.; Wang, Z.; Duan, J.; Li, S.; Xue, P.; Wang, G.; Sun, Y.; et al. Efficacy and Safety of First-Line Immunotherapy Combinations for Advanced NSCLC: A Systematic Review and Network Meta-Analysis. J. Thorac. Oncol. 2021, 16, 1099–1117. [Google Scholar] [CrossRef] [PubMed]
- Fancello, L.; Gandini, S.; Pelicci, P.G.; Mazzarella, L. Tumor mutational burden quantification from targeted gene panels: Major advancements and challenges. J. Immunother. Cancer 2019, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Galvano, A.; Gristina, V.; Malapelle, U.; Pisapia, P.; Pepe, F.; Barraco, N.; Castiglia, M.; Perez, A.; Rolfo, C.; Troncone, G.; et al. The prognostic impact of tumor mutational burden (TMB) in the first-line management of advanced non-oncogene addicted non-small-cell lung cancer (NSCLC): A systematic review and meta-analysis of randomized controlled trials. ESMO Open 2021, 6, 100124. [Google Scholar] [CrossRef]
- Yu, Y.; Zeng, D.; Ou, Q.; Liu, S.; Li, A.; Chen, Y.; Lin, D.; Gao, Q.; Zhou, H.; Liao, W.; et al. Association of survival and immune-related biomarkers with immunotherapy in patients with non–small cell lung cancer: A meta-analysis and individual patient–level analysis. JAMA Netw. Open 2019, 2, e196879. [Google Scholar] [CrossRef]
- Mok, T.; Lopes, G.; Cho, B.; Kowalski, D.; Kasahara, K.; Wu, Y.L.; de Castro Jr, G.; Turna, H.; Cristescu, R.; Aurora-Garg, D.; et al. Associations of tissue tumor mutational burden and mutational status with clinical outcomes in KEYNOTE-042: Pembrolizumab versus chemotherapy for advanced PD-L1-positive NSCLC. Ann. Oncol. 2023, 34, 377–388. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Griesinger, F.; Kowanetz, M.; Zou, W.; Shames, D.; Cummings, C.; Rizvi, N.; Spira, A.; Frampton, G.; Leveque, V.; Flynn, S.; et al. Tumor mutation burden (TMB) is associated with improved efficacy of atezolizumab in 1L and 2L+ NSCLC patients. In Proceedings of the Oncology Research and Treatment; Karger: Basel, Switzerland, 2017; Volume 40, pp. 220–+. [Google Scholar]
- Gandara, D.R.; Paul, S.M.; Kowanetz, M.; Schleifman, E.; Zou, W.; Li, Y.; Rittmeyer, A.; Fehrenbacher, L.; Otto, G.; Malboeuf, C.; et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med. 2018, 24, 1441–1448. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Ciuleanu, T.E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N. Engl. J. Med. 2018, 378, 2093–2104. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Nathanson, T.; Rizvi, H.; Creelan, B.C.; Sanchez-Vega, F.; Ahuja, A.; Ni, A.; Novik, J.B.; Mangarin, L.M.; Abu-Akeel, M.; et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell 2018, 33, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Di Capua, D.; Bracken-Clarke, D.; Ronan, K.; Baird, A.M.; Finn, S. The liquid biopsy for lung cancer: State of the art, limitations and future developments. Cancers 2021, 13, 3923. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fang, L.; Zhu, Y.; Bao, Z.; Wang, Q.; Liu, R.; Sun, W.; Du, H.; Lin, J.; Yu, B.; et al. Blood tumor mutation burden can predict the clinical response to immune checkpoint inhibitors in advanced non-small cell lung cancer patients. Cancer Immunol. Immunother. 2021, 70, 3513–3524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chang, L.; Yang, Y.; Fang, W.; Guan, Y.; Wu, A.; Hong, S.; Zhou, H.; Chen, G.; Chen, X.; et al. The correlations of tumor mutational burden among single-region tissue, multi-region tissues and blood in non-small cell lung cancer. J. Immunother. Cancer 2019, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.t.; Seeruttun, S.R.; Wu, X.y.; Wang, Z.X. Maximum somatic allele frequency in combination with blood-based tumor mutational burden to predict the efficacy of atezolizumab in advanced non-small cell lung cancer: A pooled analysis of the randomized POPLAR and OAK studies. Front. Oncol. 2019, 9, 1432. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Velcheti, V.; Mekhail, T.; Leal, T.A.; Dowell, J.E.; Tsai, M.L.; Dakhil, C.S.; Stella, P.; Shen, V.; Hu, S.; et al. Primary efficacy results from B-F1RST, a prospective phase II trial evaluating blood-based tumour mutational burden (bTMB) as a predictive biomarker for atezolizumab (atezo) in 1L non-small cell lung cancer (NSCLC). Ann. Oncol. 2018, 29, viii744. [Google Scholar] [CrossRef]
- Aggarwal, C.; Ben-Shachar, R.; Gao, Y.; Hyun, S.W.; Rivers, Z.; Epstein, C.; Kaneva, K.; Sangli, C.; Nimeiri, H.; Patel, J. Assessment of Tumor Mutational Burden and Outcomes in Patients With Diverse Advanced Cancers Treated With Immunotherapy. JAMA Netw. Open 2023, 6, e2311181. [Google Scholar] [CrossRef]
- Peters, S.; Dziadziuszko, R.; Morabito, A.; Felip, E.; Gadgeel, S.M.; Cheema, P.; Cobo, M.; Andric, Z.; Barrios, C.H.; Yamaguchi, M.; et al. Atezolizumab versus chemotherapy in advanced or metastatic NSCLC with high blood-based tumor mutational burden: Primary analysis of BFAST cohort C randomized phase 3 trial. Nat. Med. 2022, 28, 1831–1839. [Google Scholar] [CrossRef]
- Garassino, M.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; Speranza, G.; Reck, M.; Hui, R.; Boyer, M.; Cristescu, R.; et al. OA04.06 evaluation of TMB in KEYNOTE-189: Pembrolizumab plus chemotherapy vs. placebo plus chemotherapy for nonsquamous NSCLC. J. Thorac. Oncol. 2019, 14, S216–S217. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Langer, C.; Novello, S.; Halmos, B.; Cheng, Y.; Gadgeel, S.; Hui, R.; Sugawara, S.; Borghaei, H.; Cristescu, R.; et al. Pembrolizumab (pembro) plus platinum-based chemotherapy (chemo) for metastatic NSCLC: Tissue TMB (tTMB) and outcomes in KEYNOTE-021, 189, and 407. Ann. Oncol. 2019, 30, v917–v918. [Google Scholar] [CrossRef]
- Kim, E.S.; Velcheti, V.; Mekhail, T.; Yun, C.; Shagan, S.M.; Hu, S.; Chae, Y.K.; Leal, T.A.; Dowell, J.E.; Tsai, M.L.; et al. Blood-based tumor mutational burden as a biomarker for atezolizumab in non-small cell lung cancer: The phase 2 B-F1RST trial. Nat. Med. 2022, 28, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Qian, J.; Xu, M.D.; Gu, K.; Qian, F.F.; Hu, M.J.; Lu, J.; Gan, L.; Zhang, X.Y.; Cao, S.H.; et al. A non-linear association between blood tumor mutation burden and prognosis in NSCLC patients receiving atezolizumab. Oncoimmunology 2020, 9, 1731072. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Duan, J.; Wang, G.; Zhao, J.; Xu, J.; Han, J.; Zhao, Z.; Zhao, J.; Zhu, B.; Zhuo, M.; et al. Allele Frequency-Adjusted Blood-Based Tumor Mutational Burden as a Predictor of Overall Survival for Patients With NSCLC Treated With PD-(L)1 Inhibitors. J. Thorac. Oncol. 2020, 15, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhu, Y.; Zhuo, M.; Chen, X.; Xie, Y.; Duan, J.; Bai, H.; Hao, S.; Yu, Z.; Yi, Y.; et al. Maximum Somatic Allele Frequency-Adjusted Blood-Based Tumor Mutational Burden Predicts the Efficacy of Immune Checkpoint Inhibitors in Advanced Non-Small Cell Lung Cancer. Cancers 2022, 14, 5649. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Wang, L.; Cai, Y.; Cao, W.; Sun, W.; Zou, X.; Li, B.; Zhang, Z.; Cai, S.; et al. bITH, a blood-based metric of intratumor heterogeneity, is associated with clinical response to immune checkpoint blockade in non-small cell lung cancer. EBioMedicine 2023, 91, 104564. [Google Scholar] [CrossRef]
- Nie, W.; Wang, Z.J.; Zhang, K.; Li, B.; Cai, Y.R.; Wen, F.C.; Zhang, D.; Bai, Y.Z.; Zhang, X.Y.; Wang, S.Y.; et al. ctDNA-adjusted bTMB as a predictive biomarker for patients with NSCLC treated with PD-(L)1 inhibitors. BMC Med. 2022, 20, 170. [Google Scholar] [CrossRef]
- Lu, S.; Sun, M.; Liu, Y.; Hu, Y.; Xie, Y.; Wang, Z.; Wang, D.; Yang, Z.; Liang, L.; Huo, Y.; et al. Abstract LB512: RATIONALE-304: The association of tumor mutational burden (TMB) with clinical outcomes of tislelizumab (TIS) + chemotherapy (chemo) versus chemo alone as first-line treatment for advanced non-squamous non-small cell lung cancer (nsq-NSCLC). Cancer Res. 2022, 82, LB512. [Google Scholar] [CrossRef]
- Arbour, K.; Shen, R.; Plodkowski, A.; Rizvi, H.; Ni, A.; Long, N.; Halpenny, D.; Sanchez-Vega, F.; Rudin, C.; Riely, G.; et al. MA19. 09 concurrent mutations in STK11 and KEAP1 is associated with resistance to PD-(L) 1 blockade in patients with NSCLC despite high TMB. J. Thorac. Oncol. 2018, 13, S424. [Google Scholar] [CrossRef]
- Song, M.Y.; Lee, D.Y.; Chun, K.S.; Kim, E.H. The role of NRF2/KEAP1 signaling pathway in cancer metabolism. Int. J. Mol. Sci. 2021, 22, 4376. [Google Scholar] [CrossRef]
- Perri, F.; Della Vittoria Scarpati, G.; Pontone, M.; Marciano, M.L.; Ottaiano, A.; Cascella, M.; Sabbatino, F.; Guida, A.; Santorsola, M.; Maiolino, P.; et al. Cancer Cell Metabolism Reprogramming and Its Potential Implications on Therapy in Squamous Cell Carcinoma of the Head and Neck: A Review. Cancers 2022, 14, 3560. [Google Scholar] [CrossRef]
- Papillon-Cavanagh, S.; Doshi, P.; Dobrin, R.; Szustakowski, J.; Walsh, A.M. STK11 and KEAP1 mutations as prognostic biomarkers in an observational real-world lung adenocarcinoma cohort. ESMO Open 2020, 5, e000706. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Gan, L.; Wang, X.; Gu, K.; Qian, F.F.; Hu, M.J.; Zhang, D.; Chen, S.Q.; Lu, J.; Cao, S.H.; et al. Atezolizumab prolongs overall survival over docetaxel in advanced non-small-cell lung cancer patients harboring STK11 or KEAP1 mutation. Oncoimmunology 2021, 10, 1865670. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, Q.; Wang, Q.; Wang, H.; Yue, W. EPHA5 mutation was associated with adverse outcome of atezolizumab treatment in late-stage non-small cell lung cancers. BMC Pulm. Med. 2022, 22, 356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, S.; Zhang, C.; Li, M.; Zheng, Y.; Hu, X.; Huang, M.; Cheng, X. Investigation of PALB2 Mutation and Correlation With Immunotherapy Biomarker in Chinese Non-Small Cell Lung Cancer Patients. Front. Oncol. 2021, 11, 742833. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, X.; Zhang, C.; Bu, S.; Zhao, C.; Wang, H. A 5-Genomic Mutation Signature Can Predict the Survival for Patients With NSCLC Receiving Atezolizumab. Front. Immunol. 2021, 12, 606027. [Google Scholar] [CrossRef]
- Zhang, K.; Hong, X.; Song, Z.; Xu, Y.; Li, C.; Wang, G.; Zhang, Y.; Zhao, X.; Zhao, Z.; Zhao, J.; et al. Identification of Deleterious NOTCH Mutation as Novel Predictor to Efficacious Immunotherapy in NSCLC. Clin. Cancer Res. 2020, 26, 3649–3661. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, J.; Wang, Z.; Fang, J.; Yu, Q.; Han, B.; Cang, S.; Chen, G.; Mei, X.; Yang, Z.; et al. Updated Overall Survival Data and Predictive Biomarkers of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC in the Phase 3 ORIENT-11 Study. J. Thorac. Oncol. 2021, 16, 2109–2120. [Google Scholar] [CrossRef]
- Van Niel, G.; d’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Yang, E.; Wang, X.; Gong, Z.; Yu, M.; Wu, H.; Zhang, D. Exosome-mediated metabolic reprogramming: The emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct. Target. Ther. 2020, 5, 242. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, S.; Li, J.; Zhang, Y.; Ling, X.; Zhang, L.; Zhou, Y.; Zhong, H. The predictive value of plasma exosomal lncRNAs/mRNAs in NSCLC patients receiving immunotherapy. Adv. Med. Sci. 2023, 68, 86–93. [Google Scholar] [CrossRef]
- Pantano, F.; Zalfa, F.; Iuliani, M.; Simonetti, S.; Manca, P.; Napolitano, A.; Tiberi, S.; Russano, M.; Citarella, F.; Foderaro, S.; et al. Large-scale profiling of extracellular vesicles identified miR-625-5p as a novel biomarker of immunotherapy response in advanced non-small-cell lung cancer patients. Cancers 2022, 14, 2435. [Google Scholar] [CrossRef] [PubMed]
- Keegan, A.; Ricciuti, B.; Garden, P.; Cohen, L.; Nishihara, R.; Adeni, A.; Paweletz, C.; Supplee, J.; Jänne, P.A.; Severgnini, M.; et al. Plasma IL-6 changes correlate to PD-1 inhibitor responses in NSCLC. J. Immunother. Cancer 2020, 8, e000678. [Google Scholar] [CrossRef] [PubMed]
- Ghahremanifard, P.; Chanda, A.; Bonni, S.; Bose, P. TGF-β Mediated Immune Evasion in Cancer—Spotlight on Cancer-Associated Fibroblasts. Cancers 2020, 12, 3650. [Google Scholar] [CrossRef]
- de Miguel-Perez, D.; Russo, A.; Gunasekaran, M.; Buemi, F.; Hester, L.; Fan, X.; Carter-Cooper, B.A.; Lapidus, R.G.; Peleg, A.; Arroyo-Hernández, M.; et al. Baseline extracellular vesicle TGF-β is a predictive biomarker for response to immune checkpoint inhibitors and survival in non–small cell lung cancer. Cancer 2023, 129, 521–530. [Google Scholar] [CrossRef]
- Riaz, N.; Havel, J.J.; Makarov, V.; Desrichard, A.; Urba, W.J.; Sims, J.S.; Hodi, F.S.; Martín-Algarra, S.; Mandal, R.; Sharfman, W.H.; et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell 2017, 171, 934–949. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Duan, J.; Bai, H.; Wang, Y.; Wan, R.; Wang, X.; Chen, S.; Tian, Y.; Wang, D.; Fei, K.; et al. TCR repertoire diversity of peripheral PD-1+ CD8+ T cells predicts clinical outcomes after immunotherapy in patients with non–small cell lung cancer. Cancer Immunol. Res. 2020, 8, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.P.L. Study of Prognostic Markers in Advanced Cancer; The University of Edinburgh: Edinburgh, UK, 2019. [Google Scholar]
- Stares, M.; Ding, T.E.; Stratton, C.; Thomson, F.; Baxter, M.; Cagney, H.; Cumming, K.; Swan, A.; Ross, F.; Barrie, C.; et al. Biomarkers of systemic inflammation predict survival with first-line immune checkpoint inhibitors in non-small-cell lung cancer. ESMO Open 2022, 7, 100445. [Google Scholar] [CrossRef]
- Olgun, P.; Diker, Ö. Sixth-week immune-nutritional-inflammatory biomarkers: Can they predict clinical outcomes in patients with advanced non-small-cell lung cancer treated with immune checkpoint inhibitors? Res. Sq. 2023. [Google Scholar] [CrossRef]
- Wang, M.; Zhai, X.; Li, J.; Guan, J.; Xu, S.; Li, Y.; Zhu, H. The role of cytokines in predicting the response and adverse events related to immune checkpoint inhibitors. Front. Immunol. 2021, 12, 2894. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef]
- Liu, C.; Yang, L.; Xu, H.; Zheng, S.; Wang, Z.; Wang, S.; Yang, Y.; Zhang, S.; Feng, X.; Sun, N.; et al. Systematic analysis of IL-6 as a predictive biomarker and desensitizer of immunotherapy responses in patients with non-small cell lung cancer. BMC Med. 2022, 20, 187. [Google Scholar]
- Chan, L.C. IL-6/JAK1 Drives PD-L1 Phosphorylation and Glycosylation to Promote Cancer Immune Evasion. Ph.D. Dissertation, University of Texas, Houston, TX, USA, 2019. [Google Scholar]
- Hummelink, K.; van der Noort, V.; Muller, M.; Schouten, R.D.; Lalezari, F.; Peters, D.; Theelen, W.S.; Koelzer, V.H.; Mertz, K.D.; Zippelius, A.; et al. PD-1T TILs as a predictive biomarker for clinical benefit to PD-1 blockade in patients with advanced NSCLC. Clin. Cancer Res. 2022, 28, 4893–4906. [Google Scholar] [CrossRef]
- Li, X.; Wenes, M.; Romero, P.; Huang, S.C.C.; Fendt, S.M.; Ho, P.C. Navigating metabolic pathways to enhance antitumour immunity and immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Carrer, A.; Trefely, S.; Zhao, S.; Campbell, S.L.; Norgard, R.J.; Schultz, K.C.; Sidoli, S.; Parris, J.L.; Affronti, H.C.; Sivanand, S.; et al. Acetyl-CoA metabolism supports multistep pancreatic tumorigenesis. Cancer Discov. 2019, 9, 416–435. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Thompson, C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 2019, 20, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.; Corsetto, P.A.; Proto, C.; Russo, G.L.; Ganzinelli, M.; Rulli, E.; Legramandi, L.; Morelli, D.; Ferrara, R.; Prelaj, A.; et al. Circulating fatty acid profile as a biomarker for immunotherapy in advanced non-small cell lung cancer. Clin. Lung Cancer 2022, 23, e489–e499. [Google Scholar] [CrossRef]
- Shi, R.; Tang, Y.Q.; Miao, H. Metabolism in tumor microenvironment: Implications for cancer immunotherapy. MedComm 2020, 1, 47–68. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, J.; Liu, D.; Lai, G.; Wen, X. Prognosis of non-small-cell lung cancer patients with lipid metabolism pathway alternations to immunotherapy. Front. Genet. 2021, 12, 646362. [Google Scholar] [CrossRef]
- Hosgood, H.D.; Cai, Q.; Hua, X.; Long, J.; Shi, J.; Wan, Y.; Yang, Y.; Abnet, C.; Bassig, B.A.; Hu, W.; et al. Variation in oral microbiome is associated with future risk of lung cancer among never-smokers. Thorax 2021, 76, 256–263. [Google Scholar] [CrossRef]
- Lurienne, L.; Cervesi, J.; Duhalde, L.; de Gunzburg, J.; Andremont, A.; Zalcman, G.; Buffet, R.; Bandinelli, P.A. NSCLC immunotherapy efficacy and antibiotic use: A systematic review and meta-analysis. J. Thorac. Oncol. 2020, 15, 1147–1159. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Fidelle, M.; Iebba, V.; Alla, L.; Pasolli, E.; Segata, N.; Desnoyer, A.; Pietrantonio, F.; Ferrere, G.; et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur. Urol. 2020, 78, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Wu, C.J.; Hung, Y.W.; Lee, C.J.; Chi, C.T.; Lee, I.C.; Yu-Lun, K.; Chou, S.H.; Luo, J.C.; Hou, M.C.; et al. Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor–treated unresectable hepatocellular carcinoma. J. Immunother. Cancer 2022, 10, e004779. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Wang, D.; Long, J.; Yang, X.; Lin, J.; Song, Y.; Xie, F.; Xun, Z.; Wang, Y.; Wang, Y.; et al. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J. Immunother. Cancer 2021, 9, e003334. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Cheng, S.; Kou, Y.; Wang, Z.; Jin, R.; Hu, H.; Zhang, X.; Gong, J.f.; Li, J.; Lu, M.; et al. The gut microbiome is associated with clinical response to anti–PD-1/PD-L1 immunotherapy in gastrointestinal cancer. Cancer Immunol. Res. 2020, 8, 1251–1261. [Google Scholar] [CrossRef]
- Newsome, R.C.; Gharaibeh, R.Z.; Pierce, C.M.; da Silva, W.V.; Paul, S.; Hogue, S.R.; Yu, Q.; Antonia, S.; Conejo-Garcia, J.R.; Robinson, L.A.; et al. Interaction of bacterial genera associated with therapeutic response to immune checkpoint PD-1 blockade in a United States cohort. Genome Med. 2022, 14, 35. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Botticelli, A.; Vernocchi, P.; Marini, F.; Quagliariello, A.; Cerbelli, B.; Reddel, S.; Del Chierico, F.; Di Pietro, F.; Giusti, R.; Tomassini, A.; et al. Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment. J. Transl. Med. 2020, 18, 49. [Google Scholar] [CrossRef]
- Russo, G.L.; Prelaj, A.; Dolezal, J.; Beninato, T.; Agnelli, L.; Triulzi, T.; Fabbri, A.; Lorenzini, D.; Ferrara, R.; Brambilla, M.; et al. PEOPLE (NTC03447678), a phase II trial to test pembrolizumab as first-line treatment in patients with advanced NSCLC with PD-L1< 50%: A multiomics analysis. J. Immunother. Cancer 2023, 11, e006833. [Google Scholar]
- Chen, L.; Zhou, X.; Wang, Y.; Wang, D.; Ke, Y.; Zeng, X. Propionate and butyrate produced by gut microbiota after probiotic supplementation attenuate lung metastasis of melanoma cells in mice. Mol. Nutr. Food Res. 2021, 65, 2100096. [Google Scholar] [CrossRef]
- Mirji, G.; Worth, A.; Bhat, S.A.; El Sayed, M.; Kannan, T.; Goldman, A.R.; Tang, H.Y.; Liu, Q.; Auslander, N.; Dang, C.V.; et al. The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Sci. Immunol. 2022, 7, eabn0704. [Google Scholar] [CrossRef]
- Oster, P.; Vaillant, L.; Riva, E.; McMillan, B.; Begka, C.; Truntzer, C.; Richard, C.; Leblond, M.M.; Messaoudene, M.; Machremi, E.; et al. Helicobacter pylori infection has a detrimental impact on the efficacy of cancer immunotherapies. Gut 2022, 71, 457–466. [Google Scholar] [CrossRef]
- Tsay, J.C.J.; Wu, B.G.; Sulaiman, I.; Gershner, K.; Schluger, R.; Li, Y.; Yie, T.A.; Meyn, P.; Olsen, E.; Perez, L.; et al. Lower airway dysbiosis affects lung cancer progression. Cancer Discov. 2021, 11, 293–307. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Subbiah, V.; Solit, D.; Chan, T.; Kurzrock, R. The FDA approval of pembrolizumab for adult and pediatric patients with tumor mutational burden (TMB)≥10: A decision centered on empowering patients and their physicians. Ann. Oncol. 2020, 31, 1115–1118. [Google Scholar] [CrossRef]
- Wu, F.; Jiang, T.; Chen, G.; Huang, Y.; Zhou, J.; Lin, L.; Feng, J.; Wang, Z.; Shu, Y.; Shi, J.; et al. Multiplexed imaging of tumor immune microenvironmental markers in locally advanced or metastatic non-small-cell lung cancer characterizes the features of response to PD-1 blockade plus chemotherapy. Cancer Commun. 2022, 42, 1331–1346. [Google Scholar] [CrossRef]
| Clinical Trial | Pathological Type | Treatment Arms | HR for mOS (95% CI) by PD-L1 (%) | HR for PFS (or mPFS) (95% CI) by PD-L1 (%) |
|---|---|---|---|---|
| CheckMate-026 [14] | NSCLC (PD-L1 ≥ 1%) | Nivolumab vs. chemotherapy | ≥5% HR = 1.02, 95% CI 0.80–1.30 | ≥5% HR = 1.15, 95% CI 0.91–1.45 |
| MYSTIC [15] | NSCLC | Durvalumab vs. chemotherapy | TC ≥ 25% HR = 0.76; 97.54% CI 0.56–1.02 | |
| KEYNOTE-024 [10] | NSCLC (PD-L1 TPS ≥ 50% and EGFR/ALK WT) | Pembrolizumab vs. chemotherapy | ≥50% HR = 0.62, 95% CI 0.48–0.81 | |
| KEYNOTE-042 [11] | NSCLC (PD-L1 TPS ≥ 1% and EGFR/ALK WT) | Pembrolizumab vs. chemotherapy | ≥50% HR = 0.69, 95% CI 0.56–0.85 | |
| 1–49% HR = 0.92, 95% CI 0.77–1.11 | ||||
| ≥1% HR = 0.81, 95% CI 0.71–0.93 | ||||
| IMpower110 [12] | NSCLC | Atezolizumab vs. chemotherapy | TC/IC 3 HR = 0.59, 95% CI 0.4–0.89 | |
| TC/IC 2/3 HR = 0.72, 95% CI 0.52–0.99 | ||||
| TC/IC 1/2/3 HR = 0.83, 95% CI 0.65–1.07 | ||||
| TC 1/2 HR = 1.04, 95% CI 0.76–1.44 | ||||
| EMPOWER-Lung 1 [13] | NSCLC (PD-L1 TPS ≥ 50% and EGFR/ALK/ROS1 WT) | Cemiplimab vs. chemotherapy | ≥50% HR = 0.57, 95% CI 0.42–0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Chau, Y.-F.; Bai, H.; Wu, X.; Duan, J. Biomarkers for Immunotherapy in Driver-Gene-Negative Advanced NSCLC. Int. J. Mol. Sci. 2023, 24, 14521. https://doi.org/10.3390/ijms241914521
Huang Y, Chau Y-F, Bai H, Wu X, Duan J. Biomarkers for Immunotherapy in Driver-Gene-Negative Advanced NSCLC. International Journal of Molecular Sciences. 2023; 24(19):14521. https://doi.org/10.3390/ijms241914521
Chicago/Turabian StyleHuang, Yiyi, Yi-Fung Chau, Hua Bai, Xinyu Wu, and Jianchun Duan. 2023. "Biomarkers for Immunotherapy in Driver-Gene-Negative Advanced NSCLC" International Journal of Molecular Sciences 24, no. 19: 14521. https://doi.org/10.3390/ijms241914521
APA StyleHuang, Y., Chau, Y.-F., Bai, H., Wu, X., & Duan, J. (2023). Biomarkers for Immunotherapy in Driver-Gene-Negative Advanced NSCLC. International Journal of Molecular Sciences, 24(19), 14521. https://doi.org/10.3390/ijms241914521






