Propolis as a Potential Therapeutic Agent to Counteract Age-Related Changes in Cartilage: An In Vivo Study
Abstract
:1. Introduction
2. Results
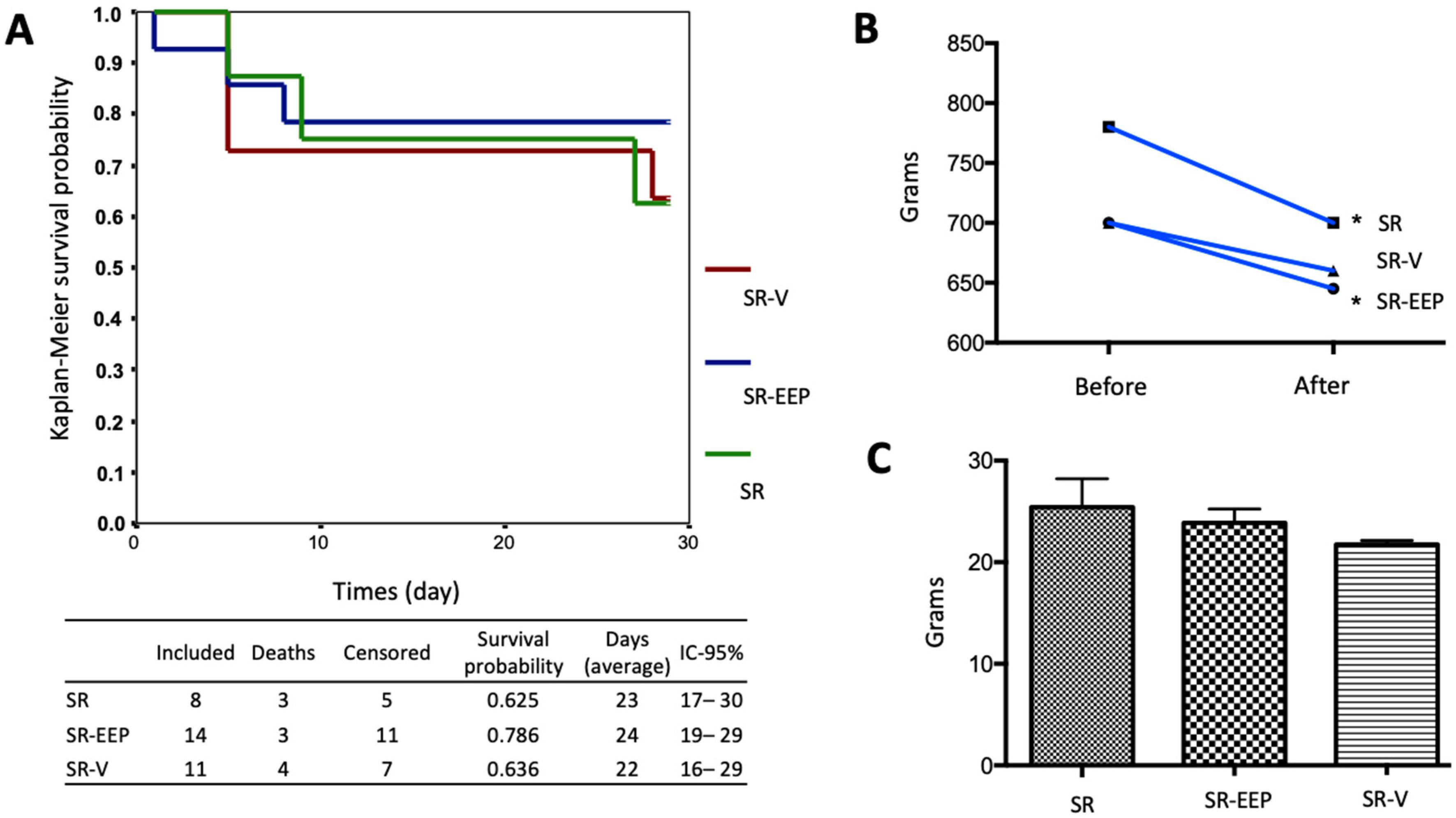
2.1. In Vivo Treatment with EEP Does Not Generate Significant Changes in the Survival of the Animals
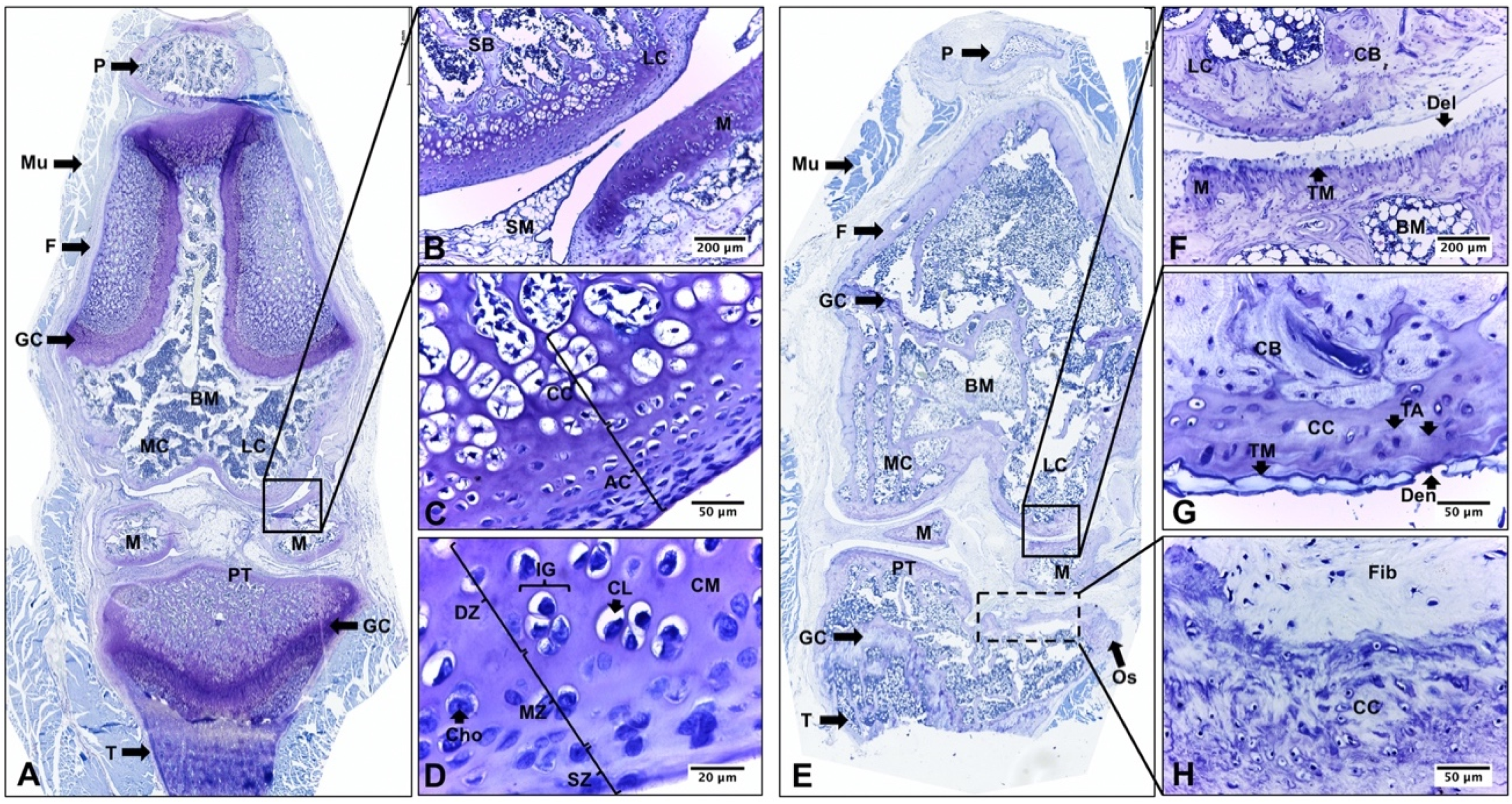
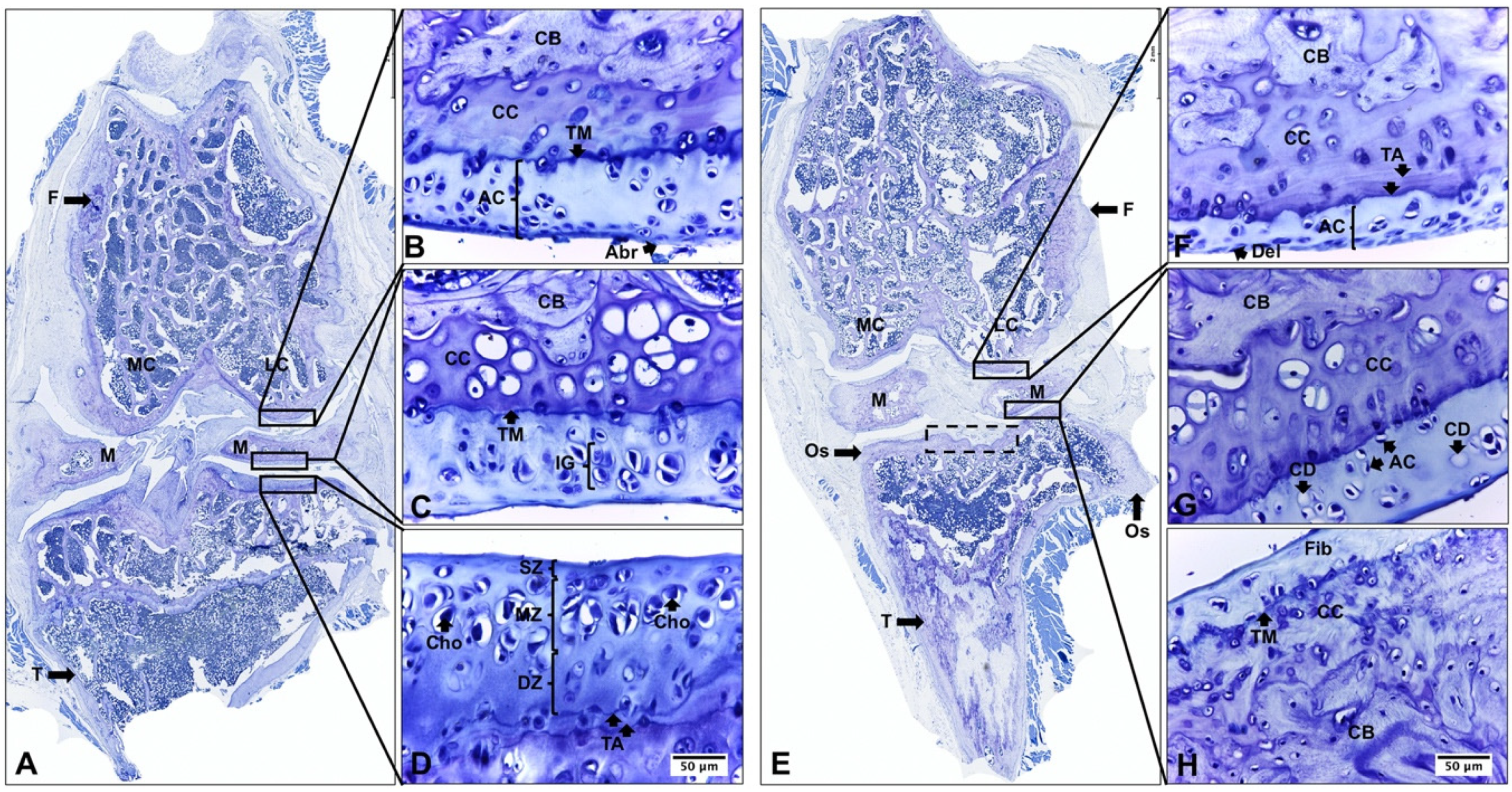
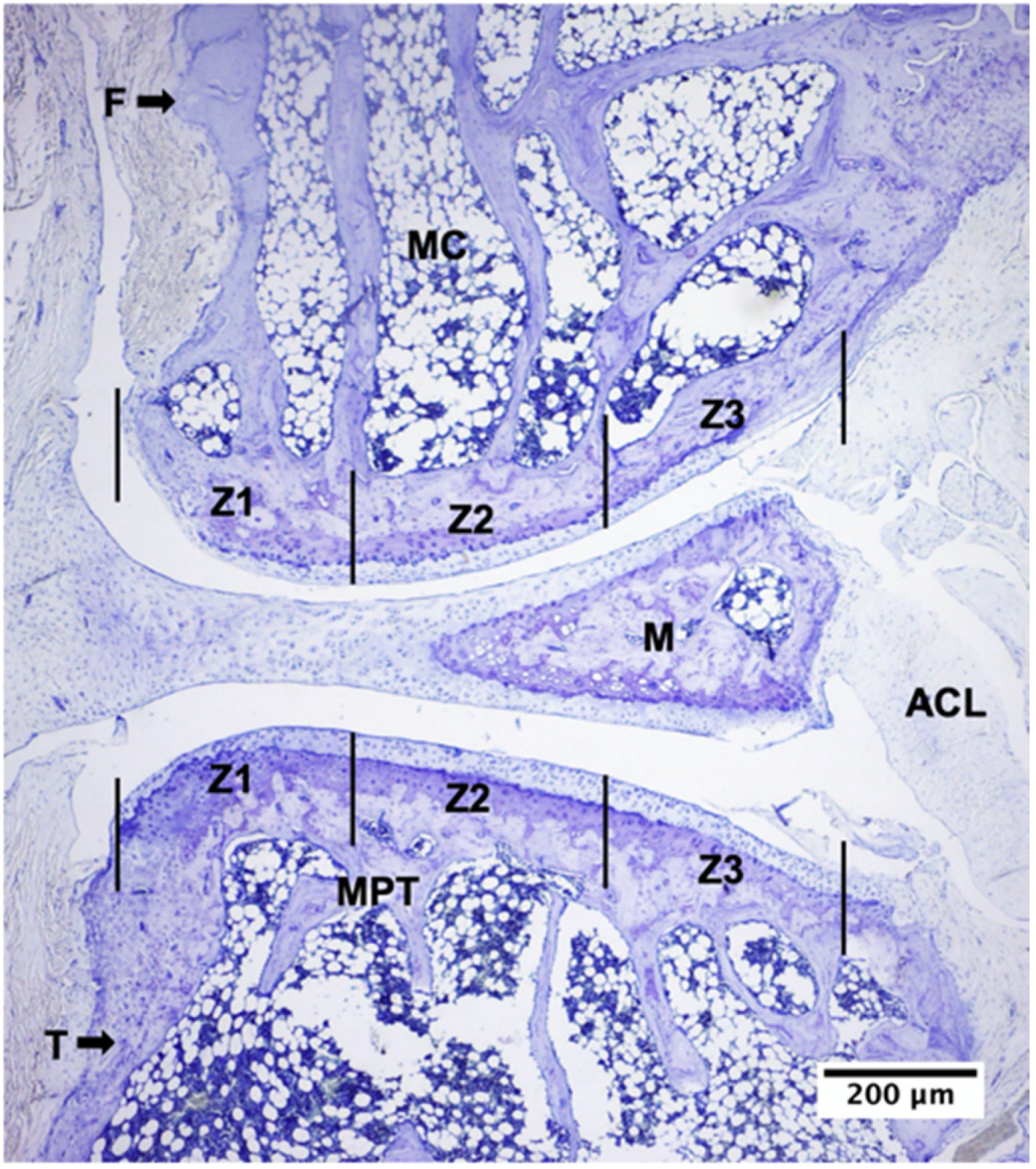
2.2. Descriptive Histological Analysis of Knee Articular Cartilage Suggests Key Differences between Young and Senescent Animals
2.3. Descriptive Histological Analysis of Knee Articular Cartilage Suggests Beneficial Effects of In Vivo EEP Treatment
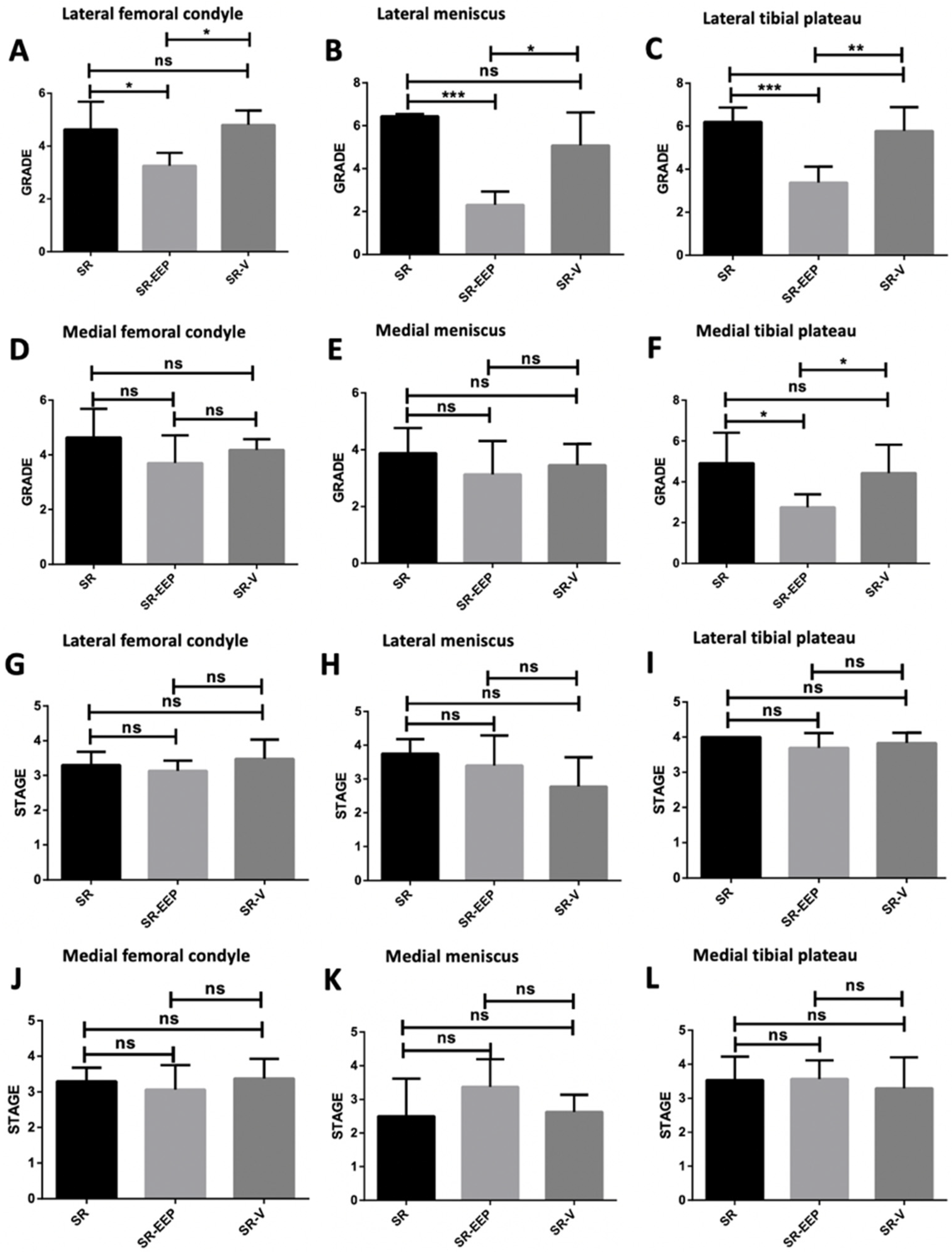
2.4. In Vivo EEP Treatment Reduces Osteoarthritis Severity in Knee Articular Cartilage
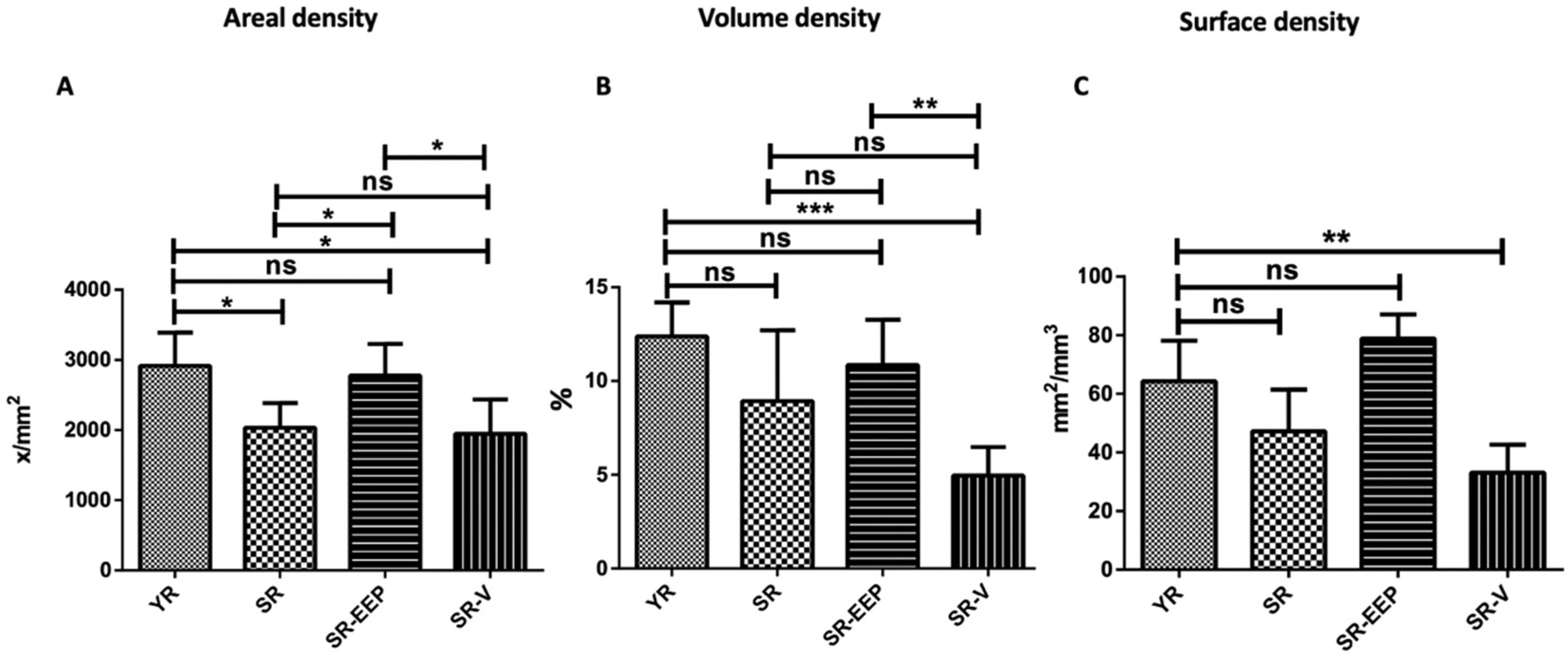
2.5. Stereological Analysis Revealed That Propolis Treatment Significantly Reduces Chondrocyte Depletion Caused by Aging
3. Discussion
4. Materials and Methods
4.1. Characterization of Ethanolic Extract of Propolis (EEP)
4.2. Animals
4.3. Histological Processing
4.4. Histological Analysis
4.5. Stereological Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mobasheri, A.; Matta, C.; Zákány, R.; Musumeci, G. Chondrosenescence: Definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas 2015, 80, 237–244. [Google Scholar] [CrossRef]
- Sen, R.; Hurley, J.A. Osteoarthritis; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Weng, T.; Xie, Y.; Yi, L.; Huang, J.; Luo, F.; Du, X.; Chen, L.; Liu, C.; Chen, D. Loss of Vhl in cartilage accelerated the progression of age-associated and surgically induced murine osteoarthritis. Osteoarthr. Cartil. 2014, 22, 1197–1205. [Google Scholar] [CrossRef]
- Mobasheri, A.; Kalamegam, G.; Musumeci, G.; Batt, M.E. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas 2014, 78, 188–198. [Google Scholar] [CrossRef]
- Loeser, R.F. Aging processes and the development of osteoarthritis. Curr. Opin. Rheumatol. 2013, 25, 108–113. [Google Scholar] [CrossRef]
- Allen, R.T.; Robertson, C.M.; Harwood, F.L.; Sasho, T.; Williams, S.K.; Pomerleau, A.C.; Amiel, D. Characterization of mature vs aged rabbit articular cartilage: Analysis of cell density, apoptosis-related gene expression and mechanisms controlling chondrocyte apoptosis. Osteoarthr. Cartil. 2004, 12, 917–923. [Google Scholar] [CrossRef]
- Barter, M.J.; Young, D.A. Epigenetic Mechanisms and Non-coding RNAs in Osteoarthritis. Curr. Rheumatol. Rep. 2013, 15, 353. [Google Scholar] [CrossRef]
- Hui, W.; Young, D.A.; Rowan, A.D.; Xu, X.; Cawston, T.E.; Proctor, C.J. Oxidative changes and signalling pathways are pivotal in initiating age-related changes in articular cartilage. Rheumatology 2016, 75, 449–458. [Google Scholar] [CrossRef]
- Baugé, C.; Duval, E.; Ollitrault, D.; Girard, N.; Leclercq, S.; Galéra, P.; Boumédiene, K. Type II TGFβ receptor modulates chondrocyte phenotype. Age 2013, 35, 1105–1116. [Google Scholar] [CrossRef]
- Martin, J.A.; Buckwalter, J.A. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology 2002, 3, 257–264. [Google Scholar] [CrossRef]
- Li, Y.P.; Wei, X.C.; Zhou, J.M.; Wei, L. The age-related changes in cartilage and osteoarthritis. BioMed Res. Int. 2013, 2013, 916530. [Google Scholar] [CrossRef]
- Itoh, S.; Hattori, T.; Tomita, N.; Aoyama, E.; Yutani, Y.; Yamashiro, T.; Takigawa, M. CCN Family Member 2/Connective Tissue Growth Factor (CCN2/CTGF) Has Anti-Aging Effects That Protect Articular Cartilage from Age-Related Degenerative Changes. PLoS ONE 2013, 8, e71156. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F. Aging and osteoarthritis: The role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr. Cartil. 2009, 17, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F. Aging cartilage and osteoarthritis—What’s the link? Sci. Aging Knowl. Environ. 2004, 2004, pe31. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pei, M. Cell SENESCENCe: A challenge in cartilage engineering and regeneration. Tissue Eng. Part B Rev. 2012, 18, 270–287. [Google Scholar] [CrossRef]
- Legendre, F.; Ollitrault, D.; Hervieu, M.; Baugé, C.; Maneix, L.; Goux, D.; Chajra, H.; Mallein-Gerin, F.; Boumediene, K.; Galera, P.; et al. Enhanced hyaline cartilage matrix synthesis in collagen sponge scaffolds by using siRNA to stabilize chondrocytes phenotype cultured with bone morphogenetic protein-2 under hypoxia. Tissue Eng. Part C Methods 2013, 19, 550–567. [Google Scholar] [CrossRef]
- Wang, X.-H.; Zhu, L.; Hong, X.; Wang, Y.-T.; Wang, F.; Bao, J.-P.; Xie, X.-H.; Liu, L.; Wu, X.-T. Resveratrol attenuated TNF-α–induced MMP-3 expression in human nucleus pulposus cells by activating autophagy via AMPK/SIRT1 signaling pathway. Exp. Biol. Med. 2016, 241, 848–853. [Google Scholar] [CrossRef]
- Chen, K.; Yang, Y.-H.; Jiang, S.-D.; Jiang, L.-S. Decreased activity of osteocyte autophagy with aging may contribute to the bone loss in senile population. Histochem. 2014, 142, 285–295. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Emami, A.; Namdari, H.; Parvizpour, F.; Arabpour, Z. Challenges in osteoarthritis treatment. Tissue Cell 2023, 80, 101992. [Google Scholar] [CrossRef]
- Assi, R.; Quintiens, J.; Monteagudo, S.; Lories, R.J. Innovation in Targeted Intra-articular Therapies for Osteoarthritis. Drugs 2023, 83, 649–663. [Google Scholar] [CrossRef]
- de Figueroa, P.L.; Lotz, M.K.; Blanco, F.J.; Caramés, B. Autophagy activation and protection from mitochondrial dysfunction in human chondrocytes. Arthritis Rheumatol. 2015, 67, 966–976. [Google Scholar] [CrossRef]
- Joven, J.; Micol, V.; Segura-Carretero, A.; Alonso-Villaverde, C.; Menéndez, J.A.; Aragonès, G.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Camps, J.; Cufí, S.; et al. Polyphenols and the Modulation of Gene Expression Pathways: Can We Eat Our Way Out of the Danger of Chronic Disease? Crit. Rev. Food Sci. Nutr. 2014, 54, 985–1001. [Google Scholar] [CrossRef]
- Schiano, C.; Vietri, M.T.; Grimaldi, V.; Picascia, A.; De Pascale, M.R.; Napoli, C. Epigenetic-related therapeutic challenges in cardiovascular disease. Trends Pharmacol. Sci. 2015, 36, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, A. Evidence on the Health Benefits of Supplemental Propolis. Nutrients 2019, 11, 2705. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Hamaguchi, M.; Bamba, R.; Nakajima, H.; Yoshimura, Y.; Kimura, T.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; et al. Brazilian green propolis improves gut microbiota dysbiosis and protects against sarcopenic obesity. J. Cachex-Sarcopenia Muscle 2022, 13, 3028–3047. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.A.D.; De Jong, D.; Berretta, A.A.; Galvão, E.B.d.S.; Ribeiro, J.C.; Cerqueira-Silva, T.; Amorim, T.C.; da Conceição, L.F.M.R.; Gomes, M.M.D.; Teixeira, M.B.; et al. Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct treatment for hospitalized COVID-19 patients: A randomized, controlled clinical trial. BioMedicine 2021, 138, 111526. [Google Scholar] [CrossRef]
- Al-Kahtani, S.N.; Alaqil, A.A.; Abbas, A.O. Modulation of Antioxidant Defense, Immune Response, and Growth Performance by Inclusion of Propolis and Bee Pollen into Broiler Diets. Animals 2022, 12, 1658. [Google Scholar] [CrossRef]
- Ma, X.; Guo, Z.; Li, Y.; Yang, K.; Li, X.; Liu, Y.; Shen, Z.; Zhao, L.; Zhang, Z. Phytochemical Constituents of Propolis Flavonoid, Immunological Enhancement, and Anti-porcine Parvovirus Activities Isolated From Propolis. Front. Veter-Sci. 2022, 9, 857183. [Google Scholar] [CrossRef]
- Cardile, V.; Panico, A.; Gentile, B.; Borrelli, F.; Russo, A. Effect of propolis on human cartilage and chondrocytes. Life Sci. 2003, 73, 1027–1035. [Google Scholar] [CrossRef]
- El-Ghazaly, M.A.; El-Naby, D.H.A.; Khayyal, M.T. The influence of irradiation on the potential chondroprotective effect of aqueous extract of propolis in rats. Int. J. Radiat. Biol. 2011, 87, 254–262. [Google Scholar] [CrossRef]
- Araújo, C.; Oliveira, R.D.; Pinto-Ribeiro, F.; Almeida-Aguiar, C. An Insight on the Biomedical Potential of Portuguese Propolis from Gerês. Foods 2022, 11, 3431. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.; Saavedra, N.; Saavedra, K.; Alvear, M.; Cuevas, A.; Maria-Engler, S.S.; Abdalla, D.S.P.; Salazar, L.A. Propolis reduces the expression of autophagy-related proteins in chondrocytes under interleukin-1β stimulus. Int. J. Mol. Sci. 2019, 20, 3768. [Google Scholar] [CrossRef] [PubMed]
- Baki, M.E.; Özcan, M.; Kerimoğlu, G. Oral propolis treatment decelerates experimentally induced osteoarthritis in rats. J. Exp. Clin. Med. 2017, 34, 191–194. [Google Scholar]
- Meimandi-Parizi, A.; Oryan, A.; Sayahi, E.; Bigham-Sadegh, A. Propolis extract a new reinforcement material in improving bone healing: An in vivo study. Int. J. Surg. 2018, 56, 94–101. [Google Scholar] [CrossRef]
- Darmadi, D. The Effect of Propolis on Increasing the Number of Osteoblasts and Chondrocytes, and Decreasing the Number of Osteoclasts in Wistar Rats (Rattusnovergicus)with Femoral Bone Fracture. J. Dent. Med. Sci. 2016, 15, 90–95. [Google Scholar]
- Kim, D.H.; Auh, J.-H.; Oh, J.; Hong, S.; Choi, S.; Shin, E.J.; Woo, S.O.; Lim, T.-G.; Byun, S. Propolis suppresses UV-induced photoaging in human skin through directly targeting phosphoinositide 3-kinase. Nutrients 2020, 12, 3790. [Google Scholar] [CrossRef]
- Zullkiflee, N.; Taha, H.; Usman, A. Propolis: Its Role and Efficacy in Human Health and Diseases. Molecules 2022, 27, 6120. [Google Scholar] [CrossRef] [PubMed]
- Zulhendri, F.; Ravalia, M.; Kripal, K.; Chandrasekaran, K.; Fearnley, J.; Perera, C.O. Propolis in Metabolic Syndrome and Its Associated Chronic Diseases: A Narrative Review. Antioxidants 2021, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Gurău, F.; Baldoni, S.; Prattichizzo, F.; Espinosa, E.; Amenta, F.; Procopio, A.D.; Albertini, M.C.; Bonafè, M.; Olivieri, F. Anti-senescence compounds: A potential nutraceutical approach to healthy aging. Ageing Res. Rev. 2018, 46, 14–31. [Google Scholar] [CrossRef]
- Grimmel, M.; Backhaus, C.; Proikas-Cezanne, T. WIPI-Mediated Autophagy and Longevity. Cells 2015, 4, 202–217. [Google Scholar] [CrossRef]
- Ji, M.-L.; Jiang, H.; Li, Z.; Geng, R.; Hu, J.Z.; Lin, Y.C.; Lu, J. Sirt6 attenuates chondrocyte senescence and osteoarthritis progression. Nat. Commun. 2022, 13, 7658. [Google Scholar] [CrossRef]
- Anderson, A.S.; Loeser, R.F. Why is osteoarthritis an age-related disease? Best Pract. Res. Clin. Rheumatol. 2010, 24, 15–26. [Google Scholar] [CrossRef]
- Lotz, M.; Loeser, R.F. Effects of aging on articular cartilage homeostasis. Bone 2012, 51, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Altun, M.; Bergman, E.; Edström, E.; Johnson, H.; Ulfhake, B. Behavioral impairments of the aging rat. Physiol. Behav. 2007, 92, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.; Sousa-Victor, P. Regulation of inflammation as an anti-aging intervention. FEBS J. 2020, 287, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.; Rodrigues, I.; Napoleão, L.; Lira, L.; Marques, D.; Veríssimo, M.; Andrade, J.P.; Dourado, M. Non-steroidal anti-inflammatory drugs (NSAIDs), pain and aging: Adjusting prescription to patient features. BioMedicine 2022, 150, 112958. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef]
- Wauquier, F.; Boutin-Wittrant, L.; Viret, A.; Guilhaudis, L.; Oulyadi, H.; Bourafai-Aziez, A.; Charpentier, G.; Rousselot, G.; Cassin, E.; Descamps, S.; et al. Metabolic and anti-inflammatory protective properties of human enriched serum following artichoke leaf extract absorption: Results from an innovative ex vivo clinical trial. Nutrients 2021, 13, 2653. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, X.; Chen, K.; Lang, H.; Zhang, Y.; Hou, P.; Ran, L.; Zhou, M.; Zheng, J.; Yi, L.; et al. Resveratrol prevents sarcopenic obesity by reversing mitochondrial dysfunction and oxidative stress via the PKA/LKB1/AMPK pathway. Aging 2019, 11, 2217–2240. [Google Scholar] [CrossRef]
- Biswas, P.; Dellanoce, C.; Vezzoli, A.; Mrakic-Sposta, S.; Malnati, M.; Beretta, A.; Accinni, R. Antioxidant activity with increased endogenous levels of vitamin C, E and a following dietary supplementation with a combination of glutathione and resveratrol precursors. Nutrients 2020, 12, 3224. [Google Scholar] [CrossRef]
- Wang, N.; Luo, Z.; Jin, M.; Sheng, W.; Wang, H.-T.; Long, X.; Wu, Y.; Hu, P.; Xu, H.; Zhang, X. Exploration of age-related mitochondrial dysfunction and the anti-aging effects of resveratrol in zebrafish retina. Aging 2019, 11, 3117–3137. [Google Scholar] [CrossRef]
- Stavropoulou, M.-I.; Stathopoulou, K.; Cheilari, A.; Benaki, D.; Gardikis, K.; Chinou, I.; Aligiannis, N. NMR metabolic profiling of Greek propolis samples: Comparative evaluation of their phytochemical compositions and investigation of their anti-ageing and antioxidant properties. J. Pharm. Biomed. Anal. 2021, 194, 113814. [Google Scholar] [CrossRef]
- Fatahinia, M.; Khosravi, A.; Shokri, H. Propolis efficacy on TNF-α, IFN-γ and IL2 cytokines production in old mice with and without systemic candidiasis. J. Mycol. Med. 2012, 22, 237–242. [Google Scholar] [CrossRef]
- Gao, W.; Wu, J.; Wei, J.; Pu, L.; Guo, C.; Yang, J.; Yang, M.; Luo, H. Brazilian green propolis improves immune function in aged mice. J. Clin. Biochem. Nutr. 2014, 55, 7–10. [Google Scholar] [CrossRef]
- Zhu, A.; Wu, Z.; Zhong, X.; Ni, J.; Li, Y.; Meng, J.; Du, C.; Zhao, X.; Nakanishi, H.; Wu, S. Brazilian Green Propolis Prevents Cognitive Decline into Mild Cognitive Impairment in Elderly People Living at High Altitude. J. Alzheimer’s Dis. 2018, 63, 551–560. [Google Scholar] [CrossRef]
- Vasileva, B.; Staneva, D.; Grozdanova, T.; Petkov, H.; Trusheva, B.; Alipieva, K.; Popova, M.; Miloshev, G.; Bankova, V.; Georgieva, M. Natural Deep Eutectic Extracts of Propolis, Sideritis scardica, and Plantago major Reveal Potential Antiageing Activity during Yeast Chronological Lifespan. Oxidative Med. Cell. Longev. 2022, 2022, 8368717. [Google Scholar] [CrossRef]
- Lisbona, C.; Díaz-Castro, J.; Alférez, M.J.M.; Guisado, I.M.; Guisado, R.; López-Aliaga, I. Positive influence of a natural product as propolis on antioxidant status and lipid peroxidation in senescent rats. J. Physiol. Biochem. 2013, 69, 919–925. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxidative Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef] [PubMed]
- Piredda, M.; Facchinetti, G.; Biagioli, V.; Giannarelli, D.; Armento, G.; Tonini, G.; De Marinis, M.G. Propolis in the prevention of oral mucositis in breast cancer patients receiving adjuvant chemotherapy: A pilot randomised controlled trial. Eur. J. Cancer Care 2017, 26, e12757. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, A.; Saavedra, N.; Rudnicki, M.; Abdalla, D.S.P.; Salazar, L.A. ERK1/2 and HIF1α Are Involved in Antiangiogenic Effect of Polyphenols-Enriched Fraction from Chilean Propolis. Evid.-Based Complement. Altern. Med. 2015, 2015, 187575. [Google Scholar] [CrossRef] [PubMed]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer’s Disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef]
- Hossain, S.; Yousaf, M.; Liu, Y.; Chang, D.; Zhou, X. An Overview of the Evidence and Mechanism of Drug–Herb Interactions Between Propolis and Pharmaceutical Drugs. Front. Pharmacol. 2022, 13, 876183. [Google Scholar] [CrossRef]
- Waluyo, Y.; Artika, S.R.; Wahyuni, I.N.; Gunawan, A.M.A.K.; Zainal, A.T.F. Efficacy of Prolotherapy for Osteoarthritis: A Systematic Review. J. Rehabil. Med. 2023, 55, jrm00372. [Google Scholar] [CrossRef]
- Cotter, E.J.; Frank, R.M.; Mandelbaum, B. Management of osteoarthritis—Biological approaches: Current concepts. J. ISAKOS Jt. Disord. Orthop. Sports Med. 2020, 5, 27–31. [Google Scholar] [CrossRef]
- Roseti, L.; Desando, G.; Cavallo, C.; Petretta, M.; Grigolo, B. Articular cartilage regeneration in osteoarthritis. Cells 2019, 8, 1305. [Google Scholar] [CrossRef]
- Householder, N.A.; Raghuram, A.; Agyare, K.; Thipaphay, S.; Zumwalt, M. A Review of Recent Innovations in Cartilage Regeneration Strategies for the Treatment of Primary Osteoarthritis of the Knee: Intra-articular Injections. Orthop. J. Sports Med. 2023, 11, 23259671231155950. [Google Scholar] [CrossRef]
- Jain, K.; Ravikumar, P. Recent advances in treatments of cartilage regeneration for knee osteoarthritis. J. Drug Deliv. Sci. Technol. 2020, 60, 102014. [Google Scholar] [CrossRef]
- Albus, U. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2012; Volume 46. [Google Scholar] [CrossRef]
- Cuevas, A.; Saavedra, N.; Cavalcante, M.F.; Salazar, L.A.; Abdalla, D.S. Identification of microRNAs involved in the modulation of pro-angiogenic factors in atherosclerosis by a polyphenol-rich extract from propolis. Arch. Biochem. Biophys. 2014, 557, 28–35. [Google Scholar] [CrossRef]
- Cruz-Orive, L.M.; Snelling, E.P.; Seymour, R.S.; Green, J.E.F.; Meyer, L.C.R.; Fuller, A.; Haw, A.; Mitchell, D.; Farrell, A.P.; Costello, M.-A.; et al. Recent stereological methods for cell biology: A brief survey. Am. J. Physiol. Cell. Mol. Physiol. 1990, 258, L148–L156. [Google Scholar] [CrossRef]
- Mandarim-De-Lacerda, C.A.; Del-Sol, M. Tips for Studies with Quantitative Morphology (Morphometry and Stereology). Int. J. Morphol. 2017, 35, 1482–1494. [Google Scholar] [CrossRef]
- Gerwin, N.; Bendele, A.; Glasson, S.; Carlson, C. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 2010, 18, S24–S34. [Google Scholar] [CrossRef] [PubMed]
- Pritzker, K.P.H.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.-P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Pritzker, K.; Aigner, T. Terminology of osteoarthritis cartilage and bone histopathology—A proposal for a consensus. Osteoarthr. Cartil. 2010, 18, S7–S9. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.; Cook, J.; Gerwin, N.; Glasson, S.; Laverty, S.; Little, C.; McIlwraith, W.; Kraus, V. Histopathology atlas of animal model systems—Overview of guiding principles. Osteoarthr. Cartil. 2010, 18, S2–S6. [Google Scholar] [CrossRef] [PubMed]
| Structure | YR | SR |
|---|---|---|
| SZ | The surface is smooth and continuous. It presents small and flat or round chondrocytes, which are aligned parallel to the collagen fibers and the surface. | Irregular and discontinuity surface. Depending on the severity of OA and laterality of the joint structure, abrasion or focal delamination, erosion or denudation with a greater stage of involvement is observed. |
| MZ | It consists of undifferentiated cells and spherical chondrocytes that are grouped together and surrounded by a proteoglycan matrix (chondrons). | Presence of reactive (clustered with loss of chondron orientation) or dead chondrocytes. Depletion of matrix staining and focal rarefaction. |
| DZ | It presents round and larger chondrocytes, organized in isogenic groups. Deep hypertrophic chondrocytes are observed. | It presents similar characteristics to MZ. The borderline between MZ and DZ are not very evident due to the lesser thickness of the cartilage and loss of articular cartilage. |
| Tidemark | Absent | Present. Progress and duplication of the tidemark is observed. |
| CC | Chondrocytes are hypertrophic/apoptotic. Calcified cartilage matrix with traces of bony trabeculae and vascular infiltration. | Lower cellularity, with presence of dead and/or apoptotic chondrocytes. Calcified cartilage matrix. |
| SB | Subchondral bone of trabecular type. The bone marrow is dominated by blood elements. | Sclerotic bone. Thicker articular bone plate (bone/marrow ratio). Presence of reparative fibrocartilage. Thin trabeculae and wider medullary spaces, with predominance of yellow bone marrow. Activation of connective tissue at lateral interfaces. |
| Osteophytes | Absent | Present. They are mainly observed in TP. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias, C.; Vásquez, B.; Salazar, L.A. Propolis as a Potential Therapeutic Agent to Counteract Age-Related Changes in Cartilage: An In Vivo Study. Int. J. Mol. Sci. 2023, 24, 14272. https://doi.org/10.3390/ijms241814272
Arias C, Vásquez B, Salazar LA. Propolis as a Potential Therapeutic Agent to Counteract Age-Related Changes in Cartilage: An In Vivo Study. International Journal of Molecular Sciences. 2023; 24(18):14272. https://doi.org/10.3390/ijms241814272
Chicago/Turabian StyleArias, Consuelo, Bélgica Vásquez, and Luis A. Salazar. 2023. "Propolis as a Potential Therapeutic Agent to Counteract Age-Related Changes in Cartilage: An In Vivo Study" International Journal of Molecular Sciences 24, no. 18: 14272. https://doi.org/10.3390/ijms241814272
APA StyleArias, C., Vásquez, B., & Salazar, L. A. (2023). Propolis as a Potential Therapeutic Agent to Counteract Age-Related Changes in Cartilage: An In Vivo Study. International Journal of Molecular Sciences, 24(18), 14272. https://doi.org/10.3390/ijms241814272







