Protein Kinase C Is Involved in Vegetative Development, Stress Response and Pathogenicity in Verticillium dahliae
Abstract
:1. Introduction
2. Results
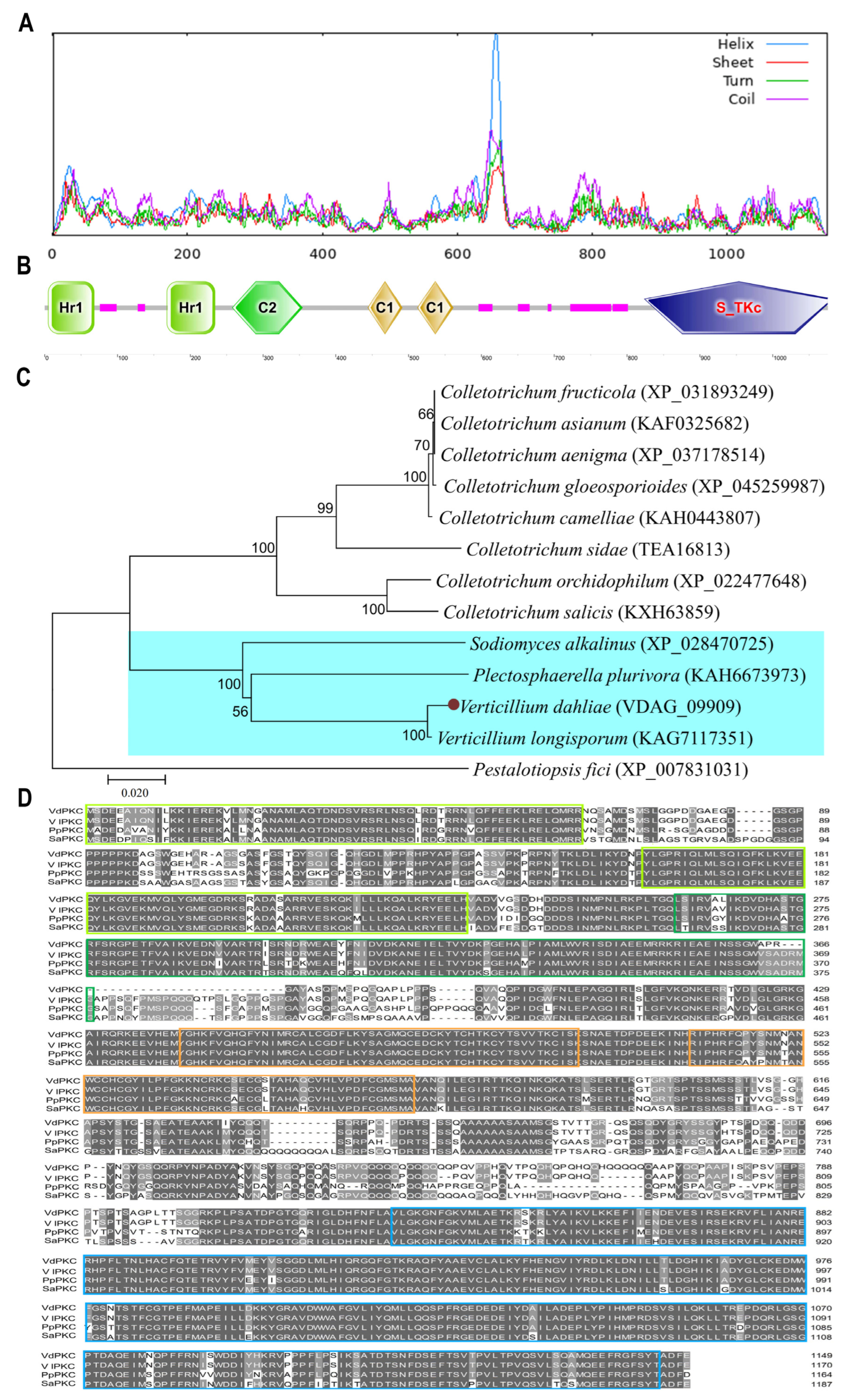
2.1. Sequence Analysis of VdPKC
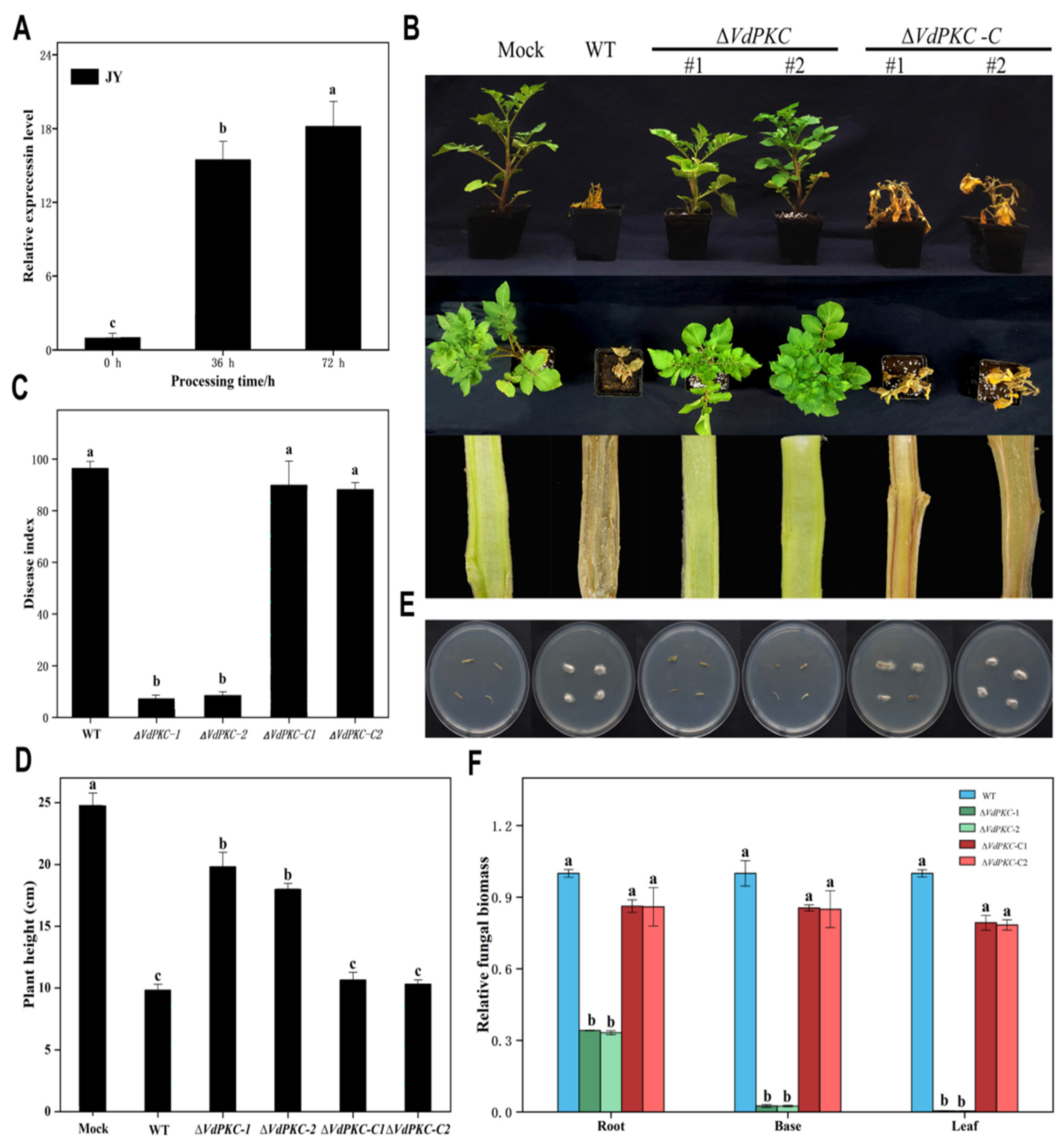
2.2. VdPKC Is Essential for Pathogenicity in Potato
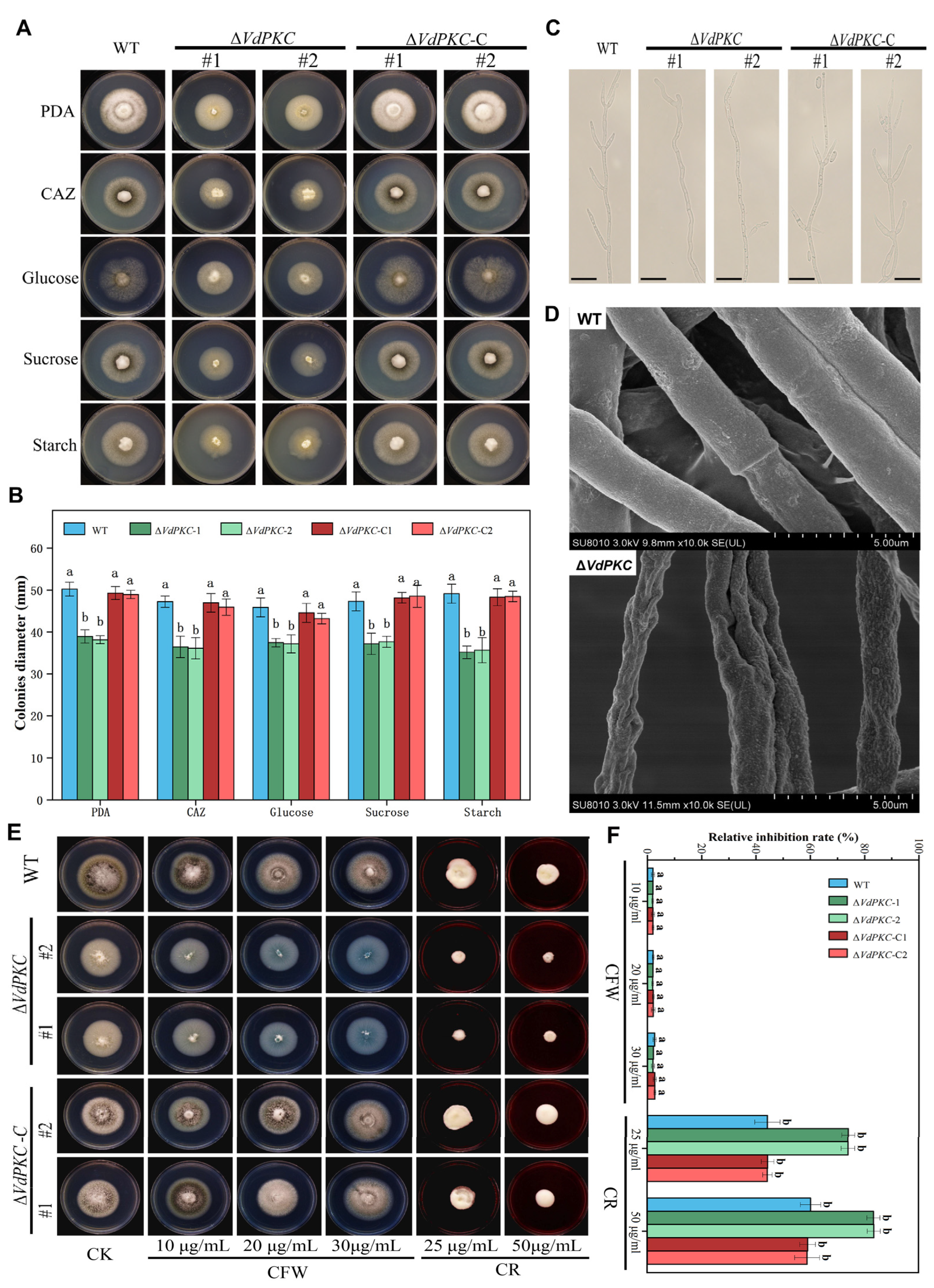
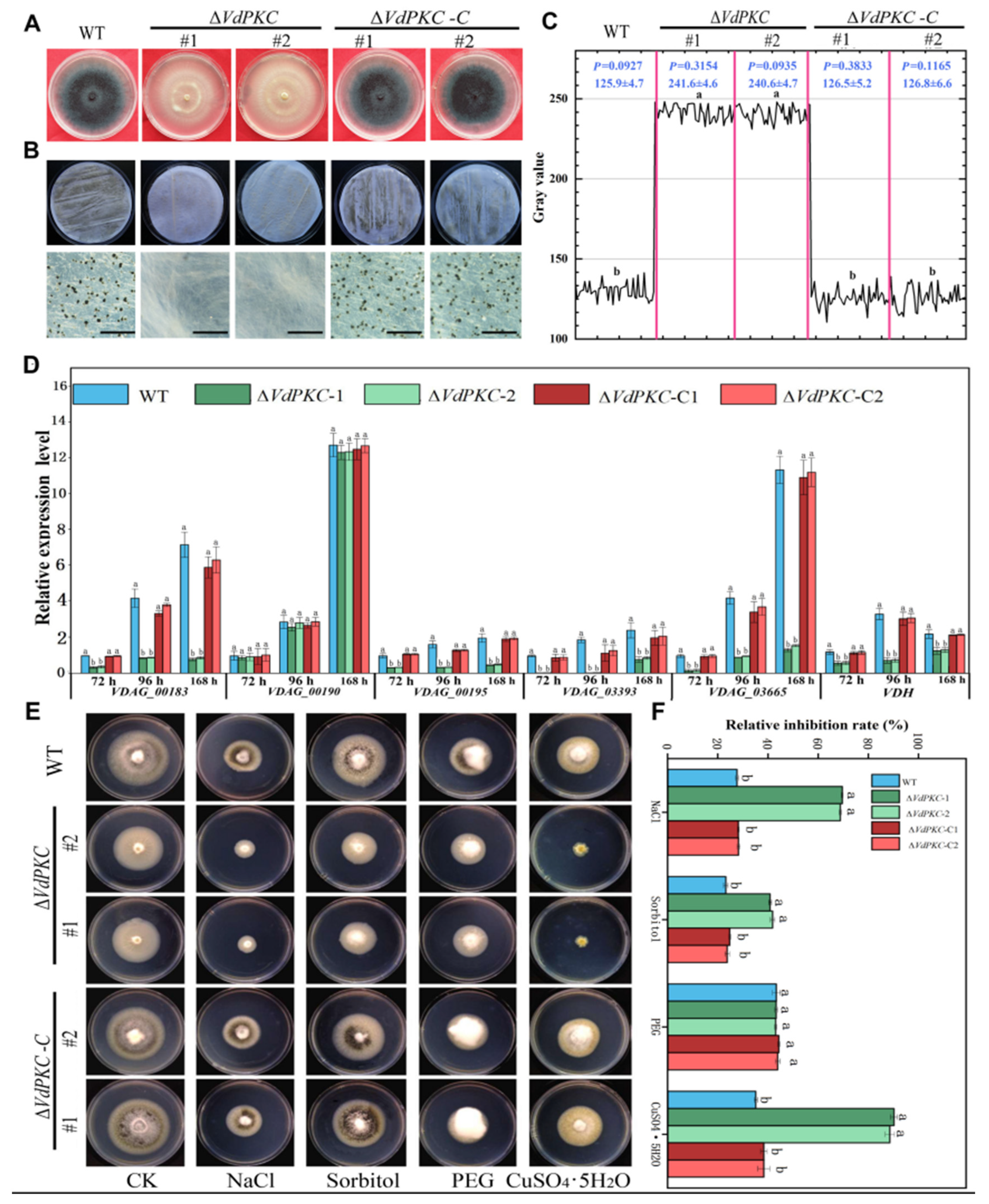
2.3. VdPKC Mediates the Vegetative Growth of V. dahliae
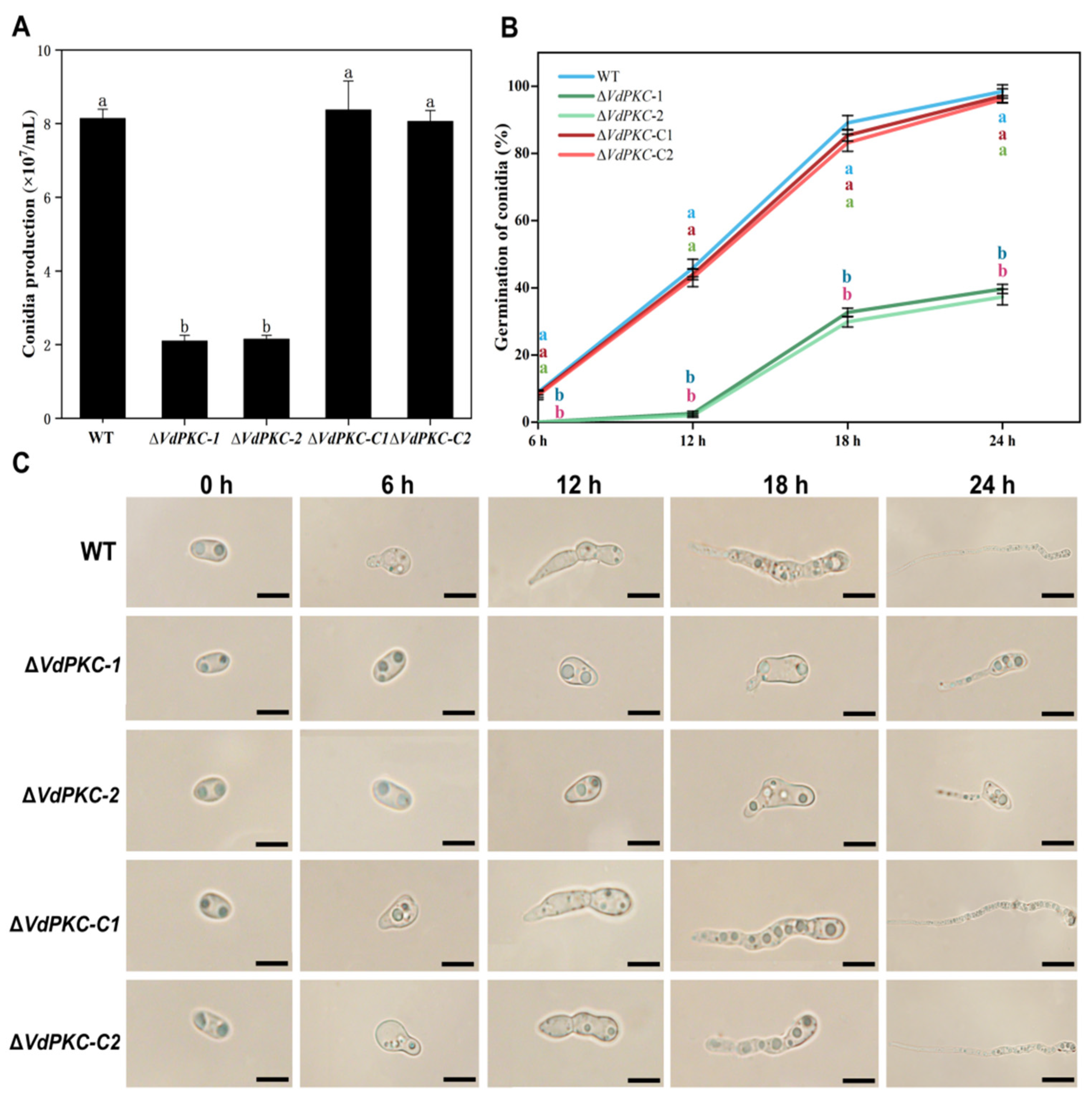
2.4. VdPKC Is Involved in the Formation and Germination of Conidia in V. dahliae
2.5. VdPKC Is Involved in the Formation of Microsclerotia and Melanin in V. dahliae
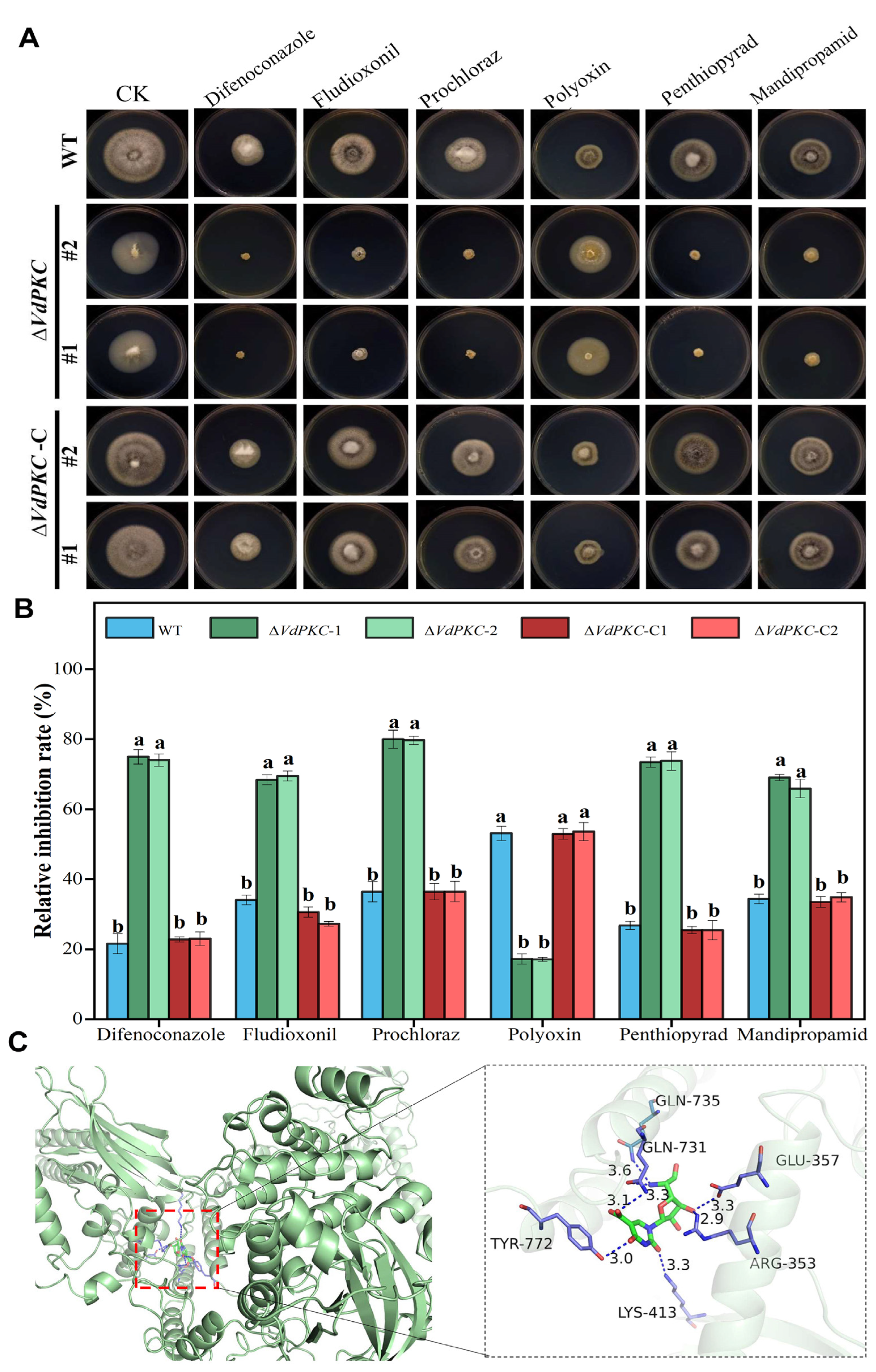
2.6. VdPKC Is Involved in the Resistance of V. dahliae to Different Fungicides
3. Discussion
4. Materials and Methods
4.1. Plants, Microbial Strains, and Culture Conditions
4.2. Gene Cloning and Sequence Analysis
4.3. Gene Knockout and Construction of Complementary Mutants
4.4. Pathogenicity and Penetration Assays
4.5. Vegetative Growth Assay
4.6. Conidial Yield and Determination of Germination Rate
4.7. Observation of Microsclerotia Formation
4.8. Analysis of Abiotic Stress Response
4.9. Molecular Docking
4.10. Data Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.Y.; Xie, P.D.; Li, Y.C.; Bi, Y.; Prusky, B.D. Updating insights into the regulatory mechanisms of calcineurin-activated transcription factor Crz1 in pathogenic fungi. J. Fungi 2022, 10, 1082. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A. Calcium and cell death. Subcell. Biochem. 2007, 45, 465–480. [Google Scholar]
- Zhu, Q.L.; Sun, L.; Lian, J.J.; Gao, X.L.; Zhao, L.; Ding, M.Y.; Li, J.; Liang, Y.C. The phospholipase C (FgPLC1) is involved in regulation of development, pathogenicity, and stress responses in Fusarium graminearum. Fungal Genet. Biol. 2016, 97, 1–9. [Google Scholar] [CrossRef]
- Tamuli, R.J.; Deka, R.; Borkovich, K.A. Calcineurin subunits A and B interact to regulate growth and asexual and sexual development in Neurospora crassa. PLoS ONE 2017, 11, e0151867. [Google Scholar] [CrossRef]
- Laxmi, V.; Tamuli, R. The calmodulin gene in Neurospora crassa is required for normal vegetative growth, ultraviolet survival, and sexual development. Arch. Microbiol. 2017, 199, 531–542. [Google Scholar] [CrossRef]
- Stefan Christopher, P.; Zhang, N.N.; Sokabe, T.; Rivetta, A.; Slayman Clifford, L.; Montell, C.; Cunningham Kyle, W. Activation of an essential calcium signaling pathway in Saccharomyces cerevisiae by Kch1 and Kch2, putative low-affinity potassium transporters. Eukaryot. Cell 2013, 12, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Marcos, V.A.; Júlio, C.R.; Heloá, Q.; Rogelio, B.; Ieso, C. Acid stress response in Saccharomyces cerevisiae involves ionic homeostasis and calcium signaling pathway. BMC Proc. 2014, 8, 193. [Google Scholar]
- Chen, J.Y.; Xiao, H.L.; Gui, Y.J.; Zhang, D.D.; Li, L.; Bao, Y.M.; Dai, X.F. Characterization of the Verticillium dahliae exoproteome involves in pathogenicity from cotton-containing medium. Front. Microbiol. 2016, 7, 1709. [Google Scholar] [CrossRef]
- Shishodia, S.K.; Tiwari, S.; Hoda, S.; Vijayaraghavan, P.; Shankar, J. SEM and qRT-PCR revealed quercetin inhibits morphogenesis of Aspergillus flavus conidia via modulating calcineurin-Crz1 signalling pathway. Mycology 2020, 11, 118–125. [Google Scholar] [CrossRef]
- Zhang, Z.; Priddey, G.; Gurr, S.J. The barley powdery mildew protein kinase C gene, pkc1 and pkc-like gene, are differentially expressed during morphogenesis. Mol. Plant Pathol. 2001, 2, 327–337. [Google Scholar] [CrossRef]
- Silva, R.D.; Saraiva, L.; Coutinho, I.; Gonçalves, J.; Côrte-Real, M. Yeast as a powerful model system for the study of apoptosis regulation by protein kinase C isoforms. Curr. Pharm. Des. 2012, 18, 2492–2500. [Google Scholar] [CrossRef] [PubMed]
- Ansary, M.W.R.; Hoque, E.; West, H.M.; Rahman, M.M.; Akanda, A.M.; Wang, Y.; Islam, M.T. Medicinal plant extracts and protein kinase C inhibitor suppress zoosporogenesis and impair motility of Phytophthora capsici zoospores. Plant Prot. Sci. 2016, 54, 113–122. [Google Scholar] [CrossRef]
- Evans John, H.; Murray, D.; Leslie, C.C.; Falke Joseph, J. Specific translocation of protein kinase Calpha to the plasma membrane requires both Ca2+ and PIP2 recognition by its C2 domain. Mol. Biol. Cell 2006, 17, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Gallegos Lisa, L.; Newton Alexandra, C. Spatiotemporal dynamics of lipid signaling: Protein kinase C as a paradigm. IUBMB Life 2008, 60, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zou, G.; Wang, J.Z.; Wang, J.; Liu, R.; Jiang, Y.P.; Zhao, G.P.; Zhou, Z.H. Characterization of the Ca(2+) -responsive signaling pathway in regulating the expression and secretion of cellulases in Trichoderma reesei Rut-C30. Mol. Microbiol. 2016, 100, 560–575. [Google Scholar] [CrossRef]
- Heinisch, J.J.; Rodicio, R. Protein kinase C in fungi-more than just cell wall integrity. FEMS Microbiol. Rev. 2018, 42, fux051. [Google Scholar] [CrossRef]
- Dickman, M.B.; Ha, Y.S.; Yang, Z.; Adams, B.; Huang, C. A protein kinase from Colletotrichum trifolii is induced by plant cutin and is required for appressorium formation. Mol. Plant. Microbe. Interact. 2003, 16, 411–421. [Google Scholar] [CrossRef]
- Franchi, L.; Fulci, V.; Macino, G. Protein kinase C modulates light responses in Neurospora by regulating the blue light photoreceptor WC-1. Mol. Microbiol. 2005, 56, 334–345. [Google Scholar] [CrossRef]
- Masayuki, I.; Hirotaka, U.; Yukako, K.; Akinori, O.; Hiroyuki, H. A protein kinase C-encoding gene, pkcA, is essential to the viability of the filamentous fungus Aspergillus nidulans. Biosci. Biotechnol. Biochem. 2007, 71, 2787–2799. [Google Scholar]
- Ronen, R.; Sharon, H.; Levdansky, E.; Romano, J.; Shadkchan, Y.; Osherov, N. The Aspergillus nidulans pkcA gene is involved in polarized growth, morphogenesis and maintenance of cell wall integrity. Curr. Genet. 2007, 51, 321–329. [Google Scholar] [CrossRef]
- Johnson, E.A.; Dung, J.K.S. Verticillium Wilt of potato-the pathogen, diseaseand management. Can. J. Plant Pathol. Rev. Can. Phytopathol. 2010, 32, 58–67. [Google Scholar] [CrossRef]
- Giovanni, B.; Antonio, D.M.; Lior, G.; Leah, T. Evaluation of thiophanate-methyl in controlling Verticillium wilt of potato and artichoke. Crop. Prot. 2019, 119, 1–8. [Google Scholar]
- Prieto, P.; Navarro-Raya, C.; Valverde-Corredor, A.; Amyotte, S.G.; Dobinson, K.F.; Mercado-Blanco, J. Colonization process of olive tissues by Verticillium dahliae and its in planta interaction with the biocontrol root endophyte Pseudomonas fluorescens PICF7. Microb. Biotechnol. 2009, 2, 499–511. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Zhou, T.T.; Guo, H.S. Hyphopodium-specific VdNoxB/VdPls1-dependent ROS-Ca2+ signaling is required for plant infection by Verticillium dahliae. PLoS Pathog. 2016, 12, e1005793. [Google Scholar] [CrossRef]
- Lizarazo, I.; Rodriguez, J.L.; Cristancho, O.; Olaya, F.; Duarte, M.; Prieto, F. Identification of symptoms related to potato Verticillium wilt from UAV-based multispectral imagery using an ensemble of gradient boosting machines. Smart Agric. Technol. 2023, 3, 100138. [Google Scholar] [CrossRef]
- Frederick, Z.A.; Cummings, T.F.; Johnson, D.A. The effect of alfalfa residue incorporation on soil bacterial communities and the quantity of Verticillium dahliae microsclerotia in potato fields in the columbia basin of washington state, USA. Am. J. Potato Res. 2018, 95, 15–25. [Google Scholar] [CrossRef]
- Fan, R.; Gong, X.; Gao, L.Q.; Shang, W.J.; Hu, X.P.; Xu, X.M. Temporal dynamics of the survival of Verticillium dahliae microsclerotia with or without melanin in soils amended with biocontrol agents. Eur. J. Plant Pathol. 2020, 157, 521–531. [Google Scholar] [CrossRef]
- Geng, Q.; Li, H.; Wang, D.; Sheng, R.C.; Zhu, H.; Klosterman, S.J.; Subbarao, K.V.; Chen, J.Y.; Chen, F.M.; Zhang, D.D. The Verticillium dahliae Spt-Ada-Gcn5 acetyltransferase complex subunit Ada1 is essential for conidia and microsclerotia production and contributes to virulence. Front. Microbiol. 2022, 13, 852571. [Google Scholar] [CrossRef]
- Sun, L.F.; Qin, J.; Rong, W.; Ni, H.; Guo, H.S.; Zhang, J. Cellophane surface-induced gene, VdCSIN1, regulates hyphopodium formation and pathogenesis via cAMP-mediated signalling in Verticillium dahliae. Mol. Plant Pathol. 2019, 20, 323–333. [Google Scholar] [CrossRef]
- Tian, L.Y.; Wang, Y.L.; Yu, J.; Xiong, D.G.; Zhao, H.J.; Tian, C.M. The mitogen-activated protein kinase kinase VdPbs2 of Verticillium dahliae regulates microsclerotia formation, stress response, and plant infection. Front. Microbiol. 2016, 7, 1532. [Google Scholar] [CrossRef]
- Yu, J.; Li, T.Y.; Tian, L.Y.; Tang, C.; Klosterman, S.J.; Tian, C.M.; Wang, Y.L. Two Verticillium dahliae MAPKKKs, VdSsk2 and VdSte11, have distinct roles in pathogenicity, microsclerotial formation, and stress adaptation. mSphere 2019, 4, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Tian, L.Y.; Xiong, D.G.; Klosterman, S.J.; Xiao, S.X.; Tian, C.M. The mitogen-activated protein kinase gene, VdHog1, regulates osmotic stress response, microsclerotia formation and virulence in Verticillium dahliae. Fungal Genet. Biol. 2016, 88, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Klosterman, S.J.; Wang, C.h.; Subbarao, K.V.; Xu, X.M.; Shang, W.J.; Hu, X.P. Vayg1 is required for microsclerotium formation and melanin production in Verticillium dahliae. Fungal Genet. Biol. 2017, 98, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.L.; Hu, X.P.; Fang, Y.L.; Anchieta, A.; Goldman, P.H.; Hernandez, G.; Klosterman, S.J. Transcription factor VdCmr1 is required for pigment production, protection from UV irradiation, and regulates expression of melanin biosynthetic genes in Verticillium dahliae. Microbiology 2018, 164, 685–696. [Google Scholar] [CrossRef]
- Li, J.J.; Zhou, L.; Yin, C.M.; Zhang, D.D.; Klosterman, S.J.; Wang, B.L.; Song, J.; Wang, D.; Hu, X.P.; Subbarao, K.V.; et al. The Verticillium dahliae Sho1-MAPK pathway regulates melanin biosynthesis and is required for cotton infection. Environ. Microbiol. 2019, 21, 4852–4874. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.Y.; Li, Y.Q.; Zeng, J.G.; Wang, G.L.; Deng, C.Y.; Guo, W.Z. Host-induced gene silencing of a regulator of G protein signalling gene (VdRGS1) confers resistance to Verticillium wilt in cotton. Plant Biotechnol. J. 2018, 16, 1629–1643. [Google Scholar] [CrossRef]
- Xiong, D.G.; Wang, Y.L.; Tang, C.; Fang, Y.L.; Zou, J.Y.; Tian, C.M. VdCrz1 is involved in microsclerotia formation and required for full virulence in Verticillium dahliae. Fungal Genet. Biol. 2015, 82, 201–212. [Google Scholar] [CrossRef]
- Tian, L.L.; Xu, J.; Zhou, L.; Guo, W.Z. VdMsb regulates virulence and microsclerotia production in the fungal plant pathogen Verticillium dahliae. Gene 2014, 550, 238–244. [Google Scholar] [CrossRef]
- Tzima, A.K.; Paplomatas, E.J.; Tsitsigiannis, D.I.; Kang, K. The G protein β subunit controls virulence and multiple growth- and development-related traits in Verticillium dahliae. Fungal Genet. Biol. 2012, 49, 271–283. [Google Scholar] [CrossRef]
- Wang, D.H.; Wen, S.L.; Zhao, Z.B.; Long, Y.H.; Fan, R. Hypothetical protein VDAG_07742 is required for Verticillium dahliae pathogenicity in potato. Int. J. Mol. Sci. 2023, 24, 3630. [Google Scholar] [CrossRef]
- Emil, R.; Ivana, P.; Svetlana, M.M.; Miloš, S.; Biljana, T.; Milica, M. Efficacy of seaweed concentrate from ecklonia maxima (osbeck) and conventional fungicides in the control of Verticillium wilt of pepper. Pestic. Fitomedicina 2010, 25, 319–324. [Google Scholar]
- Zheng, J.Y.; Tang, C.; Deng, C.L.; Wang, Y.L. Involvement of a response regulator VdSsk1 in stress response, melanin biosynthesis and full virulence in Verticillium dahliae. Front. Microbiol. 2019, 10, 606. [Google Scholar] [CrossRef]
- Jiang, Q.Q.; Mao, R.Y.; Li, Y.C.; Bi, Y.; Liu, Y.X.; Zhang, M.; Li, R.; Yang, Y.Y.; Dov, B.P. AaCaM is required for infection structure differentiation and secondary metabolites in pear fungal pathogen Alternaria alternata. Mol. Microbiol. 2022, 133, 2631–2641. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.A.; Sossin, W.S. The role of C2 domains in PKC signaling. Adv. Exp. Med. Biol. 2012, 740, 663–683. [Google Scholar] [PubMed]
- Madrid, M.; Jiménez, R.; Sánchez-Mir, L.; Soto, T.; Franco, A.; Vicente-Soler, J.; Gacto, M.; Pérez, P.; Cansado, J. Multiple layers of regulation influence cell integrity control by the PKC ortholog Pck2 in fission yeast. J. Cell Sci. 2015, 128, 266–280. [Google Scholar]
- Gregorio, B.; Teresa, S.; Marisa, M.; Andrés, N.; Jeronima, V.; Mariano, G.; José, C. Activation of the cell integrity pathway is channelled through diverse signalling elements in fission yeast. Cell. Signal. 2008, 20, 748–757. [Google Scholar]
- Arellano, M.; Valdivieso, M.H.; Calonge, T.M.; Coll, P.M.; Duran, A.; Perez, P. Schizosaccharomyces pombe protein kinase C homologues, pck1p and pck2p, are targets of rho1p and rho2p and differentially regulate cell integrity. J. Cell Sci. 1999, 112, 3569–3578. [Google Scholar] [CrossRef]
- Laura, S.M.; Teresa, S.; Alejandro, F.; Marisa, M.; Raúl, A.V.; Jero, V.; Mariano, G.; Pilar, P.; José, C. Rho1 GTPase and PKC ortholog Pck1 are upstream activators of the cell integrity MAPK pathway in fission yeast. PLoS ONE 2017, 9, e88020. [Google Scholar]
- Madrid, M.; Vázquez-Marín, B.; Soto, T.; Franco, A.; Gómez-Gil, E.; Vicente-Soler, J.; Gacto, M.; Pérez, P.; Cansado, J. Differential functional regulation of protein kinase C (PKC) orthologs in fission yeast. J. Biol. Chem. 2017, 292, 11374–11387. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, B.; Hua, C.L.; Meng, P.; Wang, S.; Chen, Z.R.; Du, Y.J.; Gao, F.; Huang, J.F. VdPKS1 is required for melanin formation and virulence in a cotton wilt pathogen Verticillium dahliae. Sci. China. Life Sci. 2017, 60, 868–879. [Google Scholar] [CrossRef]
- Wang, H.F.; Tang, C.; Deng, C.L.; Li, W.W.; Klosterman, S.J.; Wang, Y.L. Septins regulate virulence in Verticillium dahliae and differentially contribute to microsclerotial formation and stress responses. Phytopathol. Res. 2022, 4, 40. [Google Scholar] [CrossRef]
- Wen, Y.; Zhou, J.L.; Feng, H.J.; Sun, W.Q.; Zhang, Y.L.; Zhao, L.H.; Cheng, Y.; Feng, Z.L.; Zhu, H.Q.; Wei, F. VdGAL4 modulates microsclerotium formation, conidial morphology, and germination to promote virulence in Verticillium dahliae. Microbiol. Spectr. 2022, 11, e03515-22. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.L.; Su, X.F.; Guo, H.M.; Qi, J.C.; Cheng, H.M. VdThit, a thiamine transport protein, is required for pathogenicity of the vascular pathogen Verticillium dahliae. Mol. Plant. Microbe. Interact. 2016, 29, 545–559. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, W.Y.; Abdul, H.H.; Guo, W. VdNop12, containing two tandem RRM domains, is a crucial factor for pathogenicity and cold adaption in Verticillium dahliae. Environ. Microbiol. 2020, 22, 5387–5401. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zhao, P.C.; Ye, Z.Q.; Sun, L.F.; Hu, X.P.; Zhang, J. Chitin synthase genes are differentially required for growth, stress response, and virulence in Verticillium dahliae. J. Fungi 2022, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Pu, M.L.; Chen, B.; Wang, G.D.; Li, C.L.; Zhang, X.X.; Yu, Y.J.; Wang, Z.; Kong, Z.S. Verticillium dahliae Asp1 regulates the transition from vegetative growth to asexual reproduction by modulating microtubule dynamic organization. Environ. Microbiol. 2022, 25, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Liu, K.Y.; Zhang, X.; Song, W.W.; Zhao, Q.; Dong, Y.H.; Guo, M.; Zheng, X.B.; Zhang, Z.G. A two-component histidine kinase, MoSLN1, is required for cell wall integrity and pathogenicity of the rice blast fungus, Magnaporthe oryzae. Curr. Genet. 2010, 56, 517–528. [Google Scholar] [CrossRef]
- Sugahara, A.; Yoshimi, A.; Shoji, F.; Fujioka, T.; Kawai, K.; Umeyama, H.; Komatsu, K.; Enomoto, M.; Kuwahara, S.; Hagiwara, D.; et al. Novel antifungal compound Z-705 specifically inhibits Protein Kinase C of filamentous fungi. Appl. Environ. Microbiol. 2019, 85, e02923-18. [Google Scholar] [CrossRef]
- Wang, J.Y.; Cai, Y.; Gou, J.Y.; Mao, Y.B.; Xu, Y.H.; Jiang, W.H.; Chen, X.Y. VdNEP, an elicitor from Verticillium dahliae, induces cotton plant wilting. Appl. Environ. Microbiol. 2004, 70, 4989–4995. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Li, J.; Wang, Y.; Yuan, J.; Zhan, J.; Wang, P.; Lin, Y.; Li, F.; Ge, X. Verticillium dahliae secreted protein Vd424Y is required for full virulence, targets the nucleus of plant cells, and induces cell death. Mol. Plant Pathol. 2021, 22, 1109–1120. [Google Scholar] [CrossRef]
- Fang, Y.L.; Klosterman, S.J.; Tian, C.M.; Wang, Y.L. Insights into VdCmr1-mediated protection against high temperature stress and UV irradiation in Verticillium dahliae. Environ. Microbiol. 2019, 21, 2977–2996. [Google Scholar] [CrossRef] [PubMed]
- Moranova, Z.; Virtudazo, E.; Hricova, K.; Ohkusu, M.; Kawamoto, S.; Husickova, V.; Raclavsky, V. The CRZ1/SP1-like gene links survival under limited aeration, cell integrity and biofilm formation in the pathogenic yeast Cryptococcus neoformans. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech Repub. 2014, 158, 21. [Google Scholar] [CrossRef] [PubMed]
- Akira, Y.; Ken, M.; Moriyuki, K.; Keietsu, A. Cell Wall Integrity and Its Industrial Applications in Filamentous Fungi. J. Fungi 2022, 8, 435. [Google Scholar]
- Zhang, Y.; Gao, Y.H.; Liang, Y.B.; Dong, Y.J.; Yang, X.F.; Yuan, J.J.; Qiu, D.W. The Verticillium dahliae snodprot1-like protein VdCP1 contributes to virulence and triggers the plant immune system. Front. Plant Sci. 2017, 8, 1880. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Anna, K.; Katherine, F.D. A hydrophobin gene, VDH1, is involved in microsclerotial development and spore viability in the plant pathogen Verticillium dahliae. Fungal Genet. Biol. FG 2006, 43, 283–294. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zhao, Z.; Long, Y.; Fan, R. Protein Kinase C Is Involved in Vegetative Development, Stress Response and Pathogenicity in Verticillium dahliae. Int. J. Mol. Sci. 2023, 24, 14266. https://doi.org/10.3390/ijms241814266
Wang D, Zhao Z, Long Y, Fan R. Protein Kinase C Is Involved in Vegetative Development, Stress Response and Pathogenicity in Verticillium dahliae. International Journal of Molecular Sciences. 2023; 24(18):14266. https://doi.org/10.3390/ijms241814266
Chicago/Turabian StyleWang, Dahui, Zhibo Zhao, Youhua Long, and Rong Fan. 2023. "Protein Kinase C Is Involved in Vegetative Development, Stress Response and Pathogenicity in Verticillium dahliae" International Journal of Molecular Sciences 24, no. 18: 14266. https://doi.org/10.3390/ijms241814266
APA StyleWang, D., Zhao, Z., Long, Y., & Fan, R. (2023). Protein Kinase C Is Involved in Vegetative Development, Stress Response and Pathogenicity in Verticillium dahliae. International Journal of Molecular Sciences, 24(18), 14266. https://doi.org/10.3390/ijms241814266





