The Combination of Glucocorticoids and Hyaluronic Acid Enhances Efficacy in IL-1β/IL-17-Treated Bovine Osteochondral Grafts Compared with Individual Application
Abstract
:1. Introduction
2. Results
2.1. Harvesting of Osteochondral Grafts
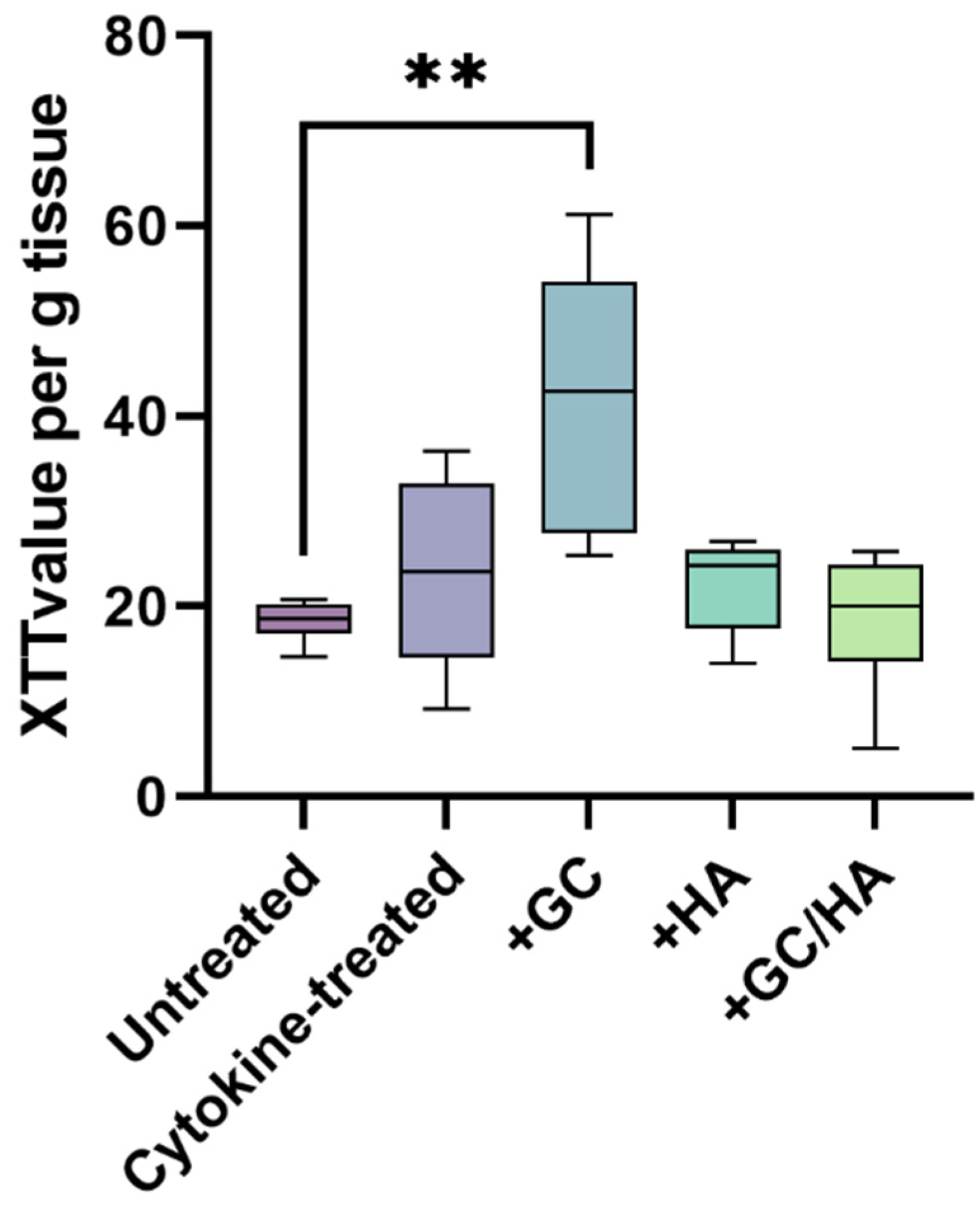
2.2. Metabolic Activity of Chondrocytes
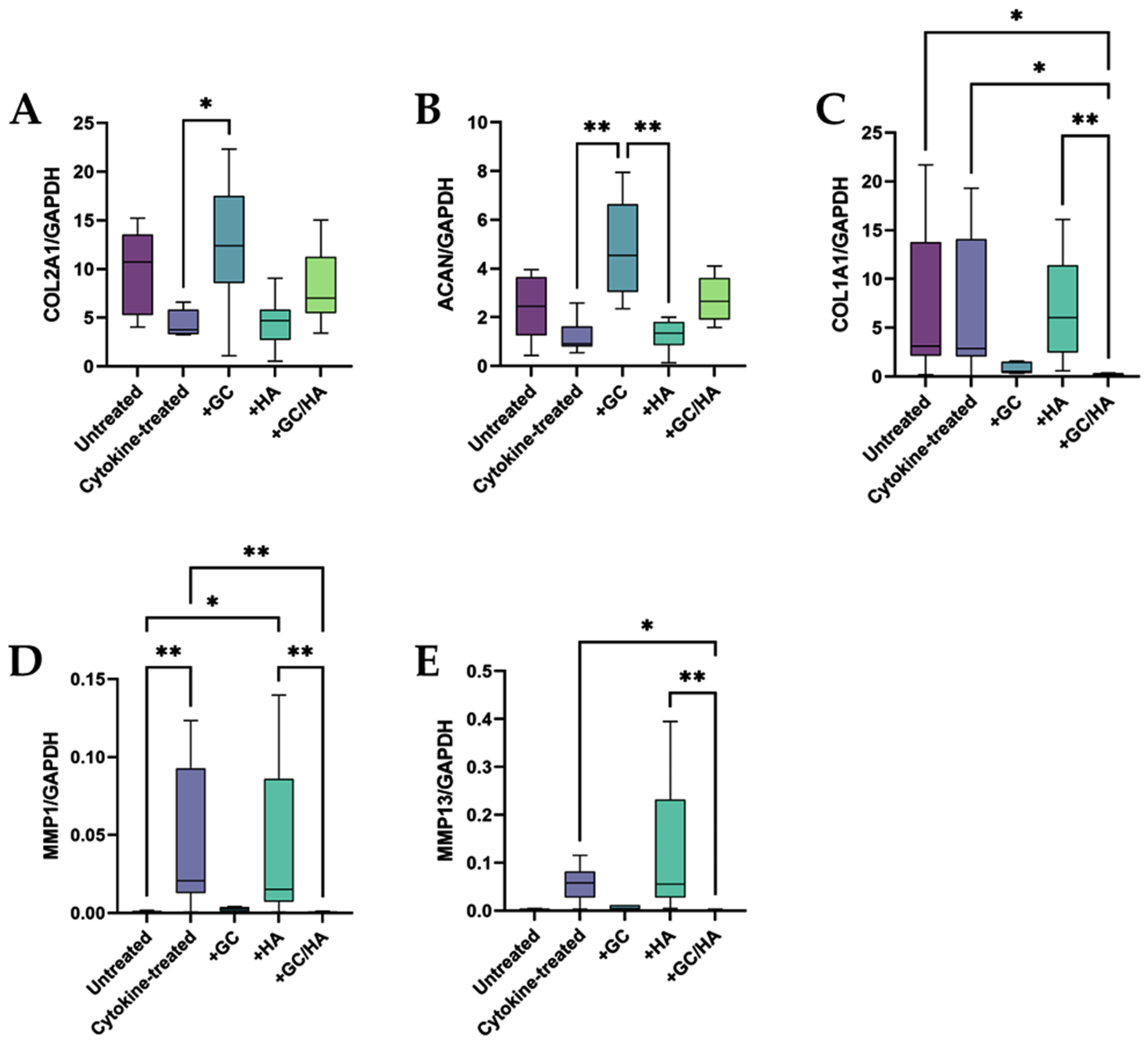
2.3. Expression of Anabolic and Catabolic Genes
2.4. Live/Dead Staining
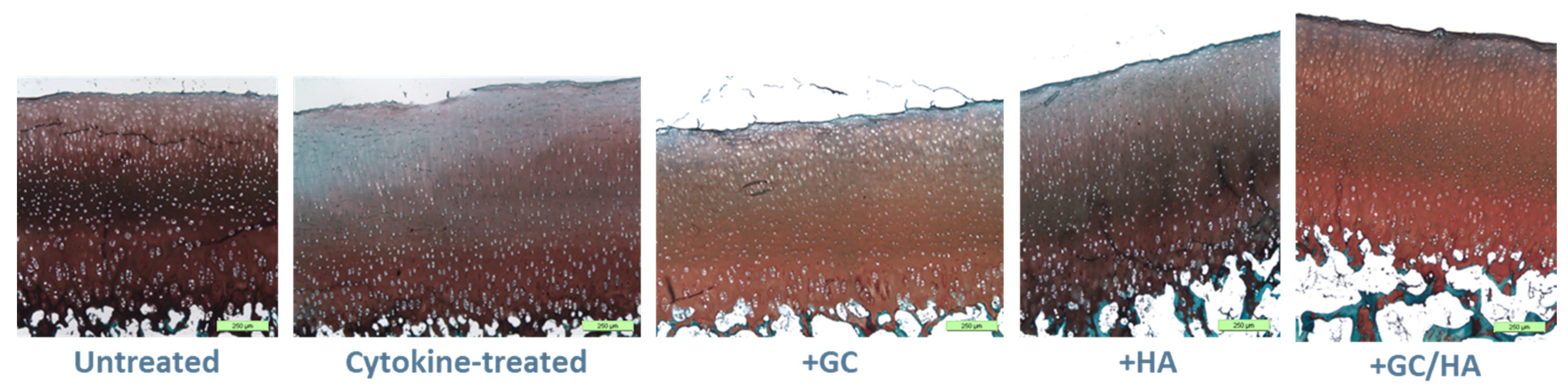
2.5. Histology
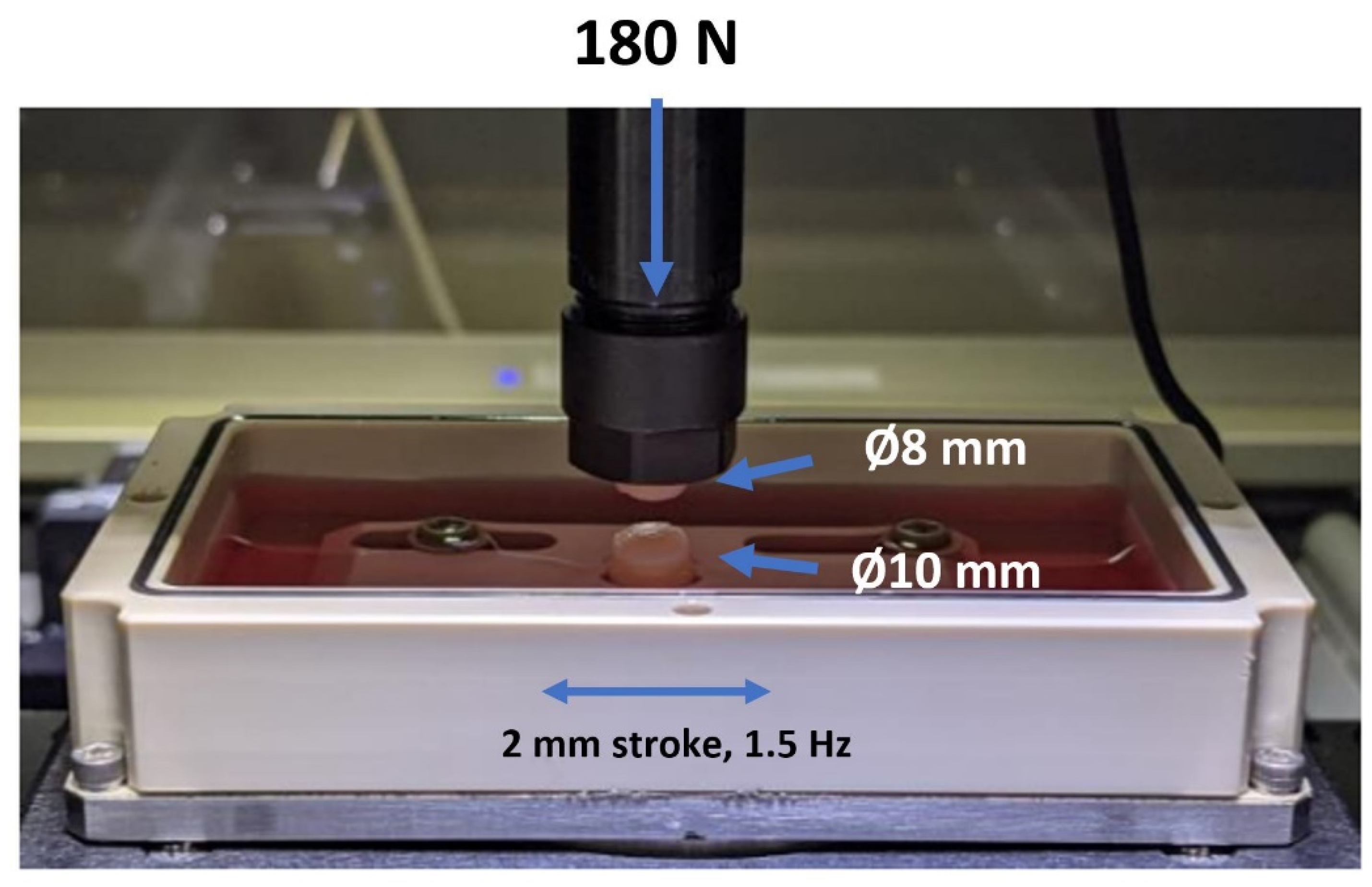
2.6. Coefficient of Friction (COF)
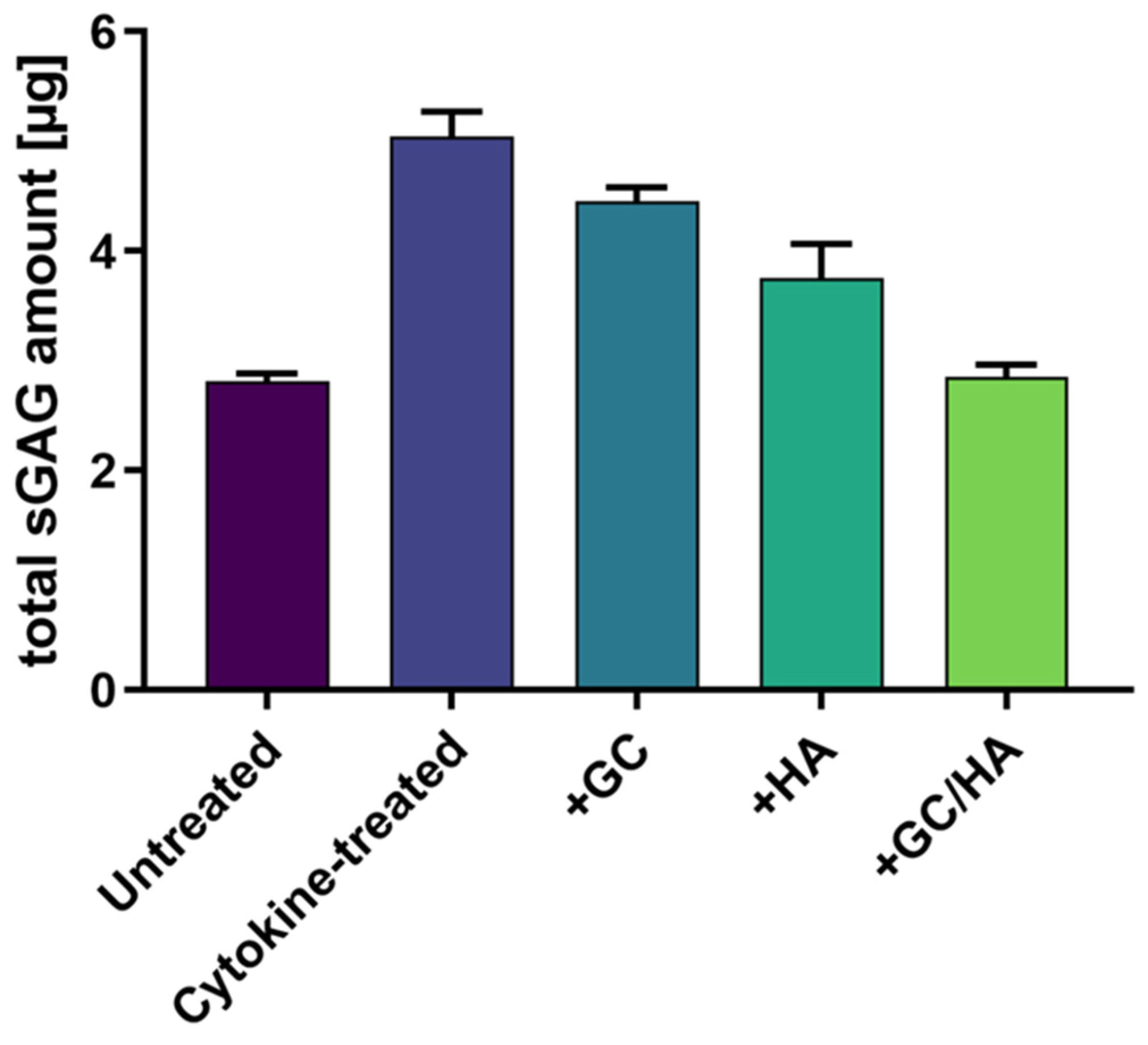
2.7. sGAG in the Supernatant
3. Discussion
4. Materials and Methods
4.1. Specimen Preparation and Study Design
4.2. Metabolic Activity
4.3. RNA Isolation
4.4. Gene Expression Analysis
4.5. Histology
4.6. Live/Dead Staining
4.7. Biotribological Test System
4.8. Sulfated Glycosaminoglycans (sGAGs)
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Goldring, S.R. Articular Cartilage and Subchondral Bone in the Pathogenesis of Osteoarthritis. Proc. Ann. N. Y. Acad. Sci. 2010, 1192, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Deng, Z.; Chen, K.; Jian, S.; Zhou, F.; Yang, Y.; Fu, Z.; Xie, H.; Xiong, J.; Zhu, W. Cartilage Tissue Engineering: From Pro-inflammatory and Anti-inflammatory Cytokines to Osteoarthritis Treatments (Review). Mol. Med. Rep. 2022, 25, 99. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Smith, J.R.; Allaway, D.; Harris, P.; Liddell, S.; Mobasheri, A. Carprofen Inhibits the Release of Matrix Metalloproteinases 1, 3, and 13 in the Secretome of an Explant Model of Articular Cartilage Stimulated with Interleukin 1β. Arthritis Res. Ther. 2013, 15, R223. [Google Scholar] [CrossRef]
- Irwin, R.M.; Feeney, E.; Secchieri, C.; Galesso, D.; Cohen, I.; Oliviero, F.; Ramonda, R.; Bonassar, L.J. Distinct Tribological Endotypes of Pathological Human Synovial Fluid Reveal Characteristic Biomarkers and Variation in Efficacy of Viscosupplementation at Reducing Local Strains in Articular C artilage. Osteoarthr. Cartil. 2020, 28, 492–501. [Google Scholar] [CrossRef]
- Vaishya, R.; Pariyo, G.B.; Agarwal, A.K.; Vijay, V. Non-Operative Management of Osteoarthritis of the Knee Joint. J. Clin. Orthop. Trauma 2016, 7, 170–176. [Google Scholar] [CrossRef]
- Fokter, S.K.; Kuhta, M.; Hojnik, M.; Ledinek, Ž.; Kostanjšek, R. Tissue Integration of Calcium Phosphate Compound after Subchondroplasty: 4-Year Follow-Up in a 76-Year-Old Female Patient. Bioengineering 2023, 10, 208. [Google Scholar] [CrossRef]
- Gerwin, N.; Hops, C.; Lucke, A. Intraarticular Drug Delivery in Osteoarthritis. Adv. Drug Deliv. Rev. 2006, 58, 226–242. [Google Scholar] [CrossRef]
- McAlindon, T.E.; LaValley, M.P.; Harvey, W.F.; Price, L.L.; Driban, J.B.; Zhang, M.; Ward, R.J. Effect of Intra-Articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients with Knee Osteoarthritis a Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2017, 317, 1967–1975. [Google Scholar] [CrossRef]
- Bowden, D.J.; Byrne, C.A.; Alkhayat, A.; Eustace, S.J.; Kavanagh, E.C. Injectable Viscoelastic Supplements: A Review for Radiologists. AJR Am. J. Roentgenol. 2017, 209, 883–888. [Google Scholar] [CrossRef]
- Bowden, D.J.; Eustace, S.J.; Kavanagh, E.C. The Value of Injectable Viscoelastic Supplements for Joints. Skelet. Radiol. 2023, 52, 933–940. [Google Scholar] [CrossRef]
- Katta, J.; Jin, Z.; Ingham, E.; Fisher, J. Effect of Nominal Stress on the Long Term Friction, Deformation and Wear of Native and Glycosaminoglycan Deficient Articular Cartilage. Osteoarthr. Cartil. 2009, 17, 662–668. [Google Scholar] [CrossRef]
- Pastrama, M.I.; Ortiz, A.C.; Zevenbergen, L.; Famaey, N.; Gsell, W.; Neu, C.P.; Himmelreich, U.; Jonkers, I. Combined Enzymatic Degradation of Proteoglycans and Collagen Significantly Alters Intratissue Strains in Articular Cartilage during Cyclic Compression. J. Mech. Behav. Biomed. Mater. 2019, 98, 383–394. [Google Scholar] [CrossRef]
- Hangody, L.; Szody, R.; Lukasik, P.; Zgadzaj, W.; Lénárt, E.; Dokoupilova, E.; Bichovsk, D.; Berta, A.; Vasarhelyi, G.; Ficzere, A.; et al. Intraarticular Injection of a Cross-Linked Sodium Hyaluronate Combined with Triamcinolone Hexacetonide (Cingal) to Provide Symptomatic Relief of Osteoarthritis of the Knee: A Randomized, Double-Blind, Placebo-Controlled Multicenter Clinical Trial. Cartilage 2018, 9, 276–283. [Google Scholar] [CrossRef]
- Ozturk, C.; Atamaz, F.; Hepguler, S.; Argin, M.; Arkun, R. The Safety and Efficacy of Intraarticular Hyaluronan with/without Corticosteroid in Knee Osteoarthritis: 1-Year, Single-Blind, Randomized Study. Rheumatol. Int. 2006, 26, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Moreland, L.W. Intra-Articular Hyaluronan (Hyaluronic Acid) and Hylans for the Treatment of Osteoarthritis: Mechanisms of Action. Arthritis Res. Ther. 2003, 5, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Niculescu-Morzsa, E.; Jeyakumar, V.; Kern, D.; Späth, S.S.; Nehrer, S. Chondroprotective Effect of High-Molecular-Weight Hyaluronic Acid on Osteoarthritic Chondrocytes in a Co-Cultivation Inflammation Model with M1 Macrophages. J. Inflamm. 2016, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Hackel, J.; Niazi, F.; Shaw, P.; Nicholls, M. Efficacy and Safety of Repeated Courses of Hyaluronic Acid Injections for Knee Osteoarthritis: A Systematic Review. Semin. Arthritis Rheum. 2018, 48, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cai, G.-Q.; Peng, J.-P.; Shen, C. Glucocorticoids Induce Apoptosis and Matrix Metalloproteinase-13 Expression in Chondrocytes through the NOX4/ROS/P38 MAPK Pathway. J. Steroid Biochem. Mol. Biol. 2018, 181, 52–62. [Google Scholar] [CrossRef]
- Suntiparpluacha, M.; Tammachote, N.; Tammachote, R. Triamcinolone Acetonide Reduces Viability, Induces Oxidative Stress, and Alters Gene Expressions of Human Chondrocytes. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4985–4992. [Google Scholar]
- Bauer, C.; Moser, L.B.; Jeyakumar, V.; Niculescu-Morzsa, E.; Kern, D.; Nehrer, S. Increased Chondroprotective Effect of Combining Hyaluronic Acid with a Glucocorticoid Compared to Separate Administration on Cytokine-Treated Osteoarthritic Chondrocytes in a 2D Culture. Biomedicines 2022, 10, 1733. [Google Scholar] [CrossRef] [PubMed]
- Meehan, R.T.; Regan, E.A.; Hoffman, E.D.; Wolf, M.L.; Gill, M.T.; Crooks, J.L.; Parmar, P.J.; Scheuring, R.A.; Hill, J.C.; Pacheco, K.A.; et al. Synovial Fluid Cytokines, Chemokines and MMP Levels in Osteoarthritis Patients with Knee Pain Display a Profile Similar to Many Rheumatoid Arthritis Patients. J. Clin. Med. 2021, 10, 5027. [Google Scholar] [CrossRef] [PubMed]
- Trevino, R.L.; Stoia, J.; Laurent, M.P.; Pacione, C.A.; Chubinskaya, S.; Wimmer, M.A. Establishing a Live Cartilage-on-Cartilage Interface for Tribological Testing. Biotribology 2017, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.; Chen, T.; Hutchinson, I.D.; Choi, D.; Voigt, C.; Warren, R.F.; Maher, S.A. Dynamic Contact Mechanics on the Tibial Plateau of the Human Knee during Activities of Daily Living. J. Biomech. 2014, 47, 2006–2012. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Gitelis, S.L.; Bodker, A.; Laurent, M.P.; Kirk, S.S.; Filardo, G.; Meyer, M.A.; Hakimiyan, A.A.; Rappoport, L.; Wimmer, M.A.; Cole, B.J.; et al. The Effect of Surgical Insertion and Pro-inflammatory Cytokines on Osteochondral Allograft Survival and Metabolism. Cartilage 2018, 9, 284–292. [Google Scholar] [CrossRef]
- Lotz, M. Cytokines in Cartilage Injury and Repair. Clin. Orthop. Relat. Res. 2001, 391, S108–S115. [Google Scholar] [CrossRef]
- Pitsillides, A.A.; Beier, F. Cartilage Biology in Osteoarthritis—Lessons from Developmental Biology. Nat. Rev. Rheumatol. 2011, 7, 654–663. [Google Scholar] [CrossRef]
- Sherman, S.L.; Khazai, R.S.; James, C.H.; Stoker, A.M.; Flood, D.L.; Cook, J.L. In Vitro Toxicity of Local Anesthetics and Corticosteroids on Chondrocyte and Synoviocyte Viability and Metabolism. Cartilage 2015, 6, 233–240. [Google Scholar] [CrossRef]
- Euppayo, T.; Siengdee, P.; Buddhachat, K.; Pradit, W.; Chomdej, S.; Ongchai, S.; Nganvongpanit, K. In Vitro Effects of Triamcinolone Acetonide and in Combination with Hyaluronan on Canine Normal and Spontaneous Osteoarthritis Articular Cartilage. In Vitro Cell. Dev. Biol. 2016, 52, 723–735. [Google Scholar] [CrossRef]
- Tschon, M.; Salamanna, F.; Martini, L.; Giavaresi, G.; Lorenzini, L.; Calzà, L.; Fini, M. Boosting the Intra-Articular Efficacy of Low Dose Corticosteroid through a Biopolymeric Matrix: An In Vivo Model of Osteoarthritis. Cells 2020, 9, 1571. [Google Scholar] [CrossRef]
- Eskelinen, A.S.A.; Tanska, P.; Florea, C.; Orozco, G.A.; Julkunen, P.; Grodzinsky, A.J.; Korhonen, R.K. Mechanobiological Model for Simulation of Injured Cartilage Degradation via Pro-Inflammatory Cytokines and Mechanical Stimulus. PLoS Comput. Biol. 2020, 16, e1007998. [Google Scholar] [CrossRef]
- Bauer, C.; Göçerler, H.; Niculescu-Morzsa, E.; Jeyakumar, V.; Stotter, C.; Klestil, T.; Franek, F.; Nehrer, S. Biotribological Tests of Osteochondral Grafts after Treatment with Pro-Inflammatory Cytokines. Cartilage 2021, 13, 496S–508S. [Google Scholar] [CrossRef]
- Charnley, J. The Lubrication of Animal Joints in Relation to Surgical Reconstruction by Arthroplasty. Ann. Rheum. Dis. 1960, 19, 10–19. [Google Scholar] [CrossRef]
- Farnham, M.S.; Price, C. Translational Cartilage Tribology: How Close Are We to Physiologically Relevant Benchtop Testing? Tribol. Lubr. Technol. 2020, 1–34. Available online: https://par.nsf.gov/servlets/purl/10213551 (accessed on 16 August 2023).
- Rebenda, D.; Vrbka, M.; Čípek, P.; Toropitsyn, E.; Nečas, D.; Pravda, M.; Hartl, M. On the Dependence of Rheology of Hyaluronic Acid Solutions and Frictional Behavior of Articular Cartilage. Materials 2020, 13, 2659. [Google Scholar] [CrossRef]
- Chen, C.; Xie, J.; Rajappa, R.; Deng, L.; Fredberg, J.; Yang, L. Interleukin-1β and Tumor Necrosis Factor-α Increase Stiffness and Impair Contractile Function of Articular Chondrocytes. Acta Biochim. Biophys. Sin. 2015, 47, 121–129. [Google Scholar] [CrossRef]
- Altman, R.; Bedi, A.; Manjoo, A.; Niazi, F.; Shaw, P.; Mease, P. Anti-Inflammatory Effects of Intra-Articular Hyaluronic Acid: A Systematic Review. Cartilage 2019, 10, 43–52. [Google Scholar] [CrossRef]
- Masuko, K.; Murata, M.; Yudoh, K.; Kato, T.; Nakamura, H. Anti-Inflammatory Effects of Hyaluronan in Arthritis Therapy: Not Just for Viscosity. Int. J. Gen. Med. 2009, 2, 77–81. [Google Scholar] [CrossRef]
- Pemmari, A.; Leppänen, T.; Hämäläinen, M.; Moilanen, T.; Vuolteenaho, K.; Moilanen, E. Widespread Regulation of Gene Expression by Glucocorticoids in Chondrocytes from Patients with Osteoarthritis as Determined by RNA-Seq. Arthritis Res. Ther. 2020, 22, 271. [Google Scholar] [CrossRef]
- Lu, Y.C.S.; Evans, C.H.; Grodzinsky, A.J. Effects of Short-Term Glucocorticoid Treatment on Changes in Cartilage Matrix Degradation and Chondrocyte Gene Expression Induced by Mechanical Injury and Inflammatory Cytokines. Arthritis Res. Ther. 2011, 13, R142. [Google Scholar] [CrossRef]
- Wann, A.K.T.; Mistry, J.; Blain, E.J.; Michael-Titus, A.T.; Knight, M.M. Eicosapentaenoic Acid and Docosahexaenoic Acid Reduce Interleukin-1β-Mediated Cartilage Degradation. Arthritis Res. Ther. 2010, 12, R207. [Google Scholar] [CrossRef]
- Doyle, A.J.; Stewart, A.A.; Constable, P.D.; Eurell, J.A.C.; Freeman, D.E.; Griffon, D.J. Effects of Sodium Hyaluronate and Methylprednisolone Acetate on Proteoglycan Synthesis in Equine Articular Cartilage Explants. Am. J. Vet. Res. 2005, 66, 48–53. [Google Scholar] [CrossRef]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Szapary, H.J.; Flaman, L.; Frank, E.; Chubinskaya, S.; Dwivedi, G.; Grodzinsky, A.J. Effects of Dexamethasone and Dynamic Loading on Cartilage of Human Osteochondral Explants Challenged with Inflammatory Cytokines. J. Biomech. 2023, 149, 111480. [Google Scholar] [CrossRef]
- Bauer, C.; Niculescu-Morzsa, E.; Nehrer, S. A Protocol for Gene Expression Analysis of Chondrocytes from Bovine Osteochondral Plugs Used for Biotribological Applications. MethodsX 2017, 4, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Steklov, N.; Song, L.; Bae, W.C.; D’Lima, D.D. Comparative Biomechanical Analysis of Human and Caprine Knee Articular Cartilage. Knee 2014, 21, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, I.; Garcia, S.; Barbier-Chassefière, V.; Caruelle, J.P.; Martelly, I.; Papy-García, D. Improved and Simple Micro Assay for Sulfated Glycosaminoglycans Quantification in Biological Extracts and Its Use in Skin and Muscle Tissue Studies. Glycobiology 2003, 13, 647–653. [Google Scholar] [CrossRef] [PubMed]



| No. of Condition | Medium Composition |
|---|---|
| 1 | Growth Medium |
| 2 | Growth Medium + Cytokines |
| 3 | Growth Medium + Cytokines + 10% GC [4.5 mg/mL] |
| 4 | Growth Medium + Cytokines + 10% HA [22 mg/mL] |
| 5 | Growth Medium + Cytokines + 10% GC/HA [4.5 mg/mL and 22 mg/mL] |
| Primer | Abreviation | Sequence (3′-5′) |
|---|---|---|
| Glyceraldehyde-3-phophate dehydrogenase | GAPDH | |
| Forward | ATGTTCCAGTATGATTCCACCC | |
| Probe | AGCTTCCCGTTCTCTGCCTTGAC | |
| Reverse | ATACTCAGCACCAGCATCAC | |
| Aggrecan core protein I | ACAN | |
| Forward | ACCTACGATGTCTACTGCTACG | |
| Probe | AGAAGGTGAACTGCTCCAGGCG | |
| Reverse | AGAGTGGCGTTTTGGGATTC | |
| Collagen type II, alpha I | COL2A1 | |
| Forward | GTGCAACTGGTCCTCTGG | |
| Probe | CCTTGTTCGCCTTTGAAGCCAGC | |
| Reverse | ACCTCTTTTCCCTTCTTCACC | |
| Matrix metalloproteinase 1 | MMP1 | |
| Forward | TTCAACCAGGTGCAGGTATC | |
| Probe | AAATTCATGCGCTGCCACCCG | |
| Reverse | AGCCCCAATGTCA | |
| Matrix metalloproteinase 13 | MMP13 | |
| Forward | CTAAACATCCCAAAACGCCAG | |
| Probe | CCCTTGATGCCATAACCAGTCTCCG | |
| Reverse | ACAGCTCTGCTTCAACCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, C.; Moser, L.B.; Kern, D.; Jeyakumar, V.; Nehrer, S. The Combination of Glucocorticoids and Hyaluronic Acid Enhances Efficacy in IL-1β/IL-17-Treated Bovine Osteochondral Grafts Compared with Individual Application. Int. J. Mol. Sci. 2023, 24, 14338. https://doi.org/10.3390/ijms241814338
Bauer C, Moser LB, Kern D, Jeyakumar V, Nehrer S. The Combination of Glucocorticoids and Hyaluronic Acid Enhances Efficacy in IL-1β/IL-17-Treated Bovine Osteochondral Grafts Compared with Individual Application. International Journal of Molecular Sciences. 2023; 24(18):14338. https://doi.org/10.3390/ijms241814338
Chicago/Turabian StyleBauer, Christoph, Lukas B. Moser, Daniela Kern, Vivek Jeyakumar, and Stefan Nehrer. 2023. "The Combination of Glucocorticoids and Hyaluronic Acid Enhances Efficacy in IL-1β/IL-17-Treated Bovine Osteochondral Grafts Compared with Individual Application" International Journal of Molecular Sciences 24, no. 18: 14338. https://doi.org/10.3390/ijms241814338
APA StyleBauer, C., Moser, L. B., Kern, D., Jeyakumar, V., & Nehrer, S. (2023). The Combination of Glucocorticoids and Hyaluronic Acid Enhances Efficacy in IL-1β/IL-17-Treated Bovine Osteochondral Grafts Compared with Individual Application. International Journal of Molecular Sciences, 24(18), 14338. https://doi.org/10.3390/ijms241814338






