Signals of nerve impulses are transmitted to excitatory cells to induce the action of organs via the activation of Ca2+ entry through voltage-gated Ca2+ channels (VGCC), which are classified based on their activation threshold into high- and low-voltage activated channels, expressed specifically for each organ. Ca2+ entering into excitatory cells binds to Ca2+-binding proteins, which are classified into two groups of Ca2+ sensors: C2 domain and EF hand proteins. Within transient Ca2+ elevation, each Ca2+ sensor, having different affinity and binding speeds to Ca2+, determines the timing of protein–protein interactions to produce the specific action of each organ. This Special Issue aims to focus on investigations ranging from the control of Ca2+ entry at the plasma membrane of excitatory cells to detailed studies on the intracellular mediation of Ca2+-binding proteins that catalyze organ-specific action. The submitted papers are studies of Ca2+ channels and Ca2+ dynamics controlled by Ca2+ elevation in neurons, cardiomyocytes and osteosarcoma. Thus, recent findings of Ca2+ channels’ regulation and Ca2+ dynamics controlled by Ca2+ elevation in neurons are discussed before summarizing the papers in this Special Issue.
In the nervous system, VGCCs are locally activated in different populations of neurons and subcellular compartments [1]. A certain fraction of the total VGCC population expressed in the membrane is composed into signaling complexes, with their signaling capacity being dependent on kinetic properties and their interaction with intracellular binding partners. However, another recently proposed possibility for the regulation of the distribution and efficiency of Ca2+ transients is a change in the local density of VGCCs [1]. For example, the activity-dependent regulation of the postsynaptic spine apparatus of hippocampal neurons depends on the activation of the perisynaptic L-type VGCCs and ryanodine receptors (RyRs) in the endoplasmic reticulum (ER) membrane, to induce Ca2+ induced Ca2+ release (CICR), and Ca2+-sensing stromal interaction molecule 1 (STIM1), a component of ER, which leads to the inactivation of L-type VGCC within seconds to minutes, resulting in more effective Ca2+ entry from the N-methyl-d-aspartate receptors (NMDARs) in spines (Figure 1A) [2]. In the suprachiasmatic nucleus (SCN), neuron changes in the density of dendritic L-type VGCCs during the circadian rhythm induce an alternation in CICR, which has consequences for the activation of large conductance Ca2+-activated K+ channels (BK channels) and controls the neuronal excitability (Figure 1B) [3]. Fast interactions in the order of a few milliseconds are effective for the communication between Ca2+ nanodomains of somatic P/Q-type VGCCs activating CICR and trigger the opening of BK channels, which affects the excitability of the somatic compartment of cartwheel interneurons (Figure 1C) [4]. BK or KCa1.1 channels are members of the Ca2+-activated potassium (KCa) channel family [5]. Within the KCa channel family, BK channels are the only member activated by both voltage and an increase in intracellular Ca2+ concentration [Ca2+]i ≥ 10 µm [6]. In presynaptic terminals, pore-forming α1 subunits of CaV channels are also associated with BK channels via RIM-binding proteins/RIM [7], major active zone proteins. The BK channel N terminus is an endogenous ligand of the auxiliary CaVα2δ subunit, displacing CaVα2δ from the CaV channel complex at the plasma membrane [8]. Neurotransmitter release is precisely controlled by the time window of Ca2+ elevation in the synaptic vesicle release site.
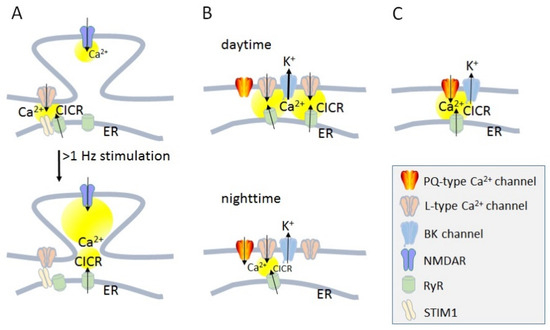
Figure 1.
Calcium dynamics with the activation of Ca2+ channels and Ca2+-induced Ca2+ release in neurons. (A) Ca2+-induced Ca2+ release (CICR) and activity-dependent regulation of postsynaptic voltage-gated Ca2+ channels (VGCC). (B) Circadian regulation of dendritic Ca2+ dynamics. (C) Dual Ca2+ nanodomain in Soma. Yellow circles indicate predicted Ca2+ elevation. Reproduced with permission from Ref. [1]; published by Elsevier, 2020.
Membrane–membrane contacts controlling Ca2+ signaling are a complex built between L-type VGCCs at the plasma membrane and RyRs in the ER, well known from muscle cells [9,10]. Store-operated Ca2+ channels (SOCCs), also known as calcium release-activated Ca2+ channels [11], consist of calcium-sensing stromal interaction molecules (STIMs) within the ER membrane and pore-forming Orai proteins in the plasma membrane [12]. STIM1 is a transmembrane protein originally described in immune cells [13] that plays a key role as a main activator of store-operated channels and membrane-associated calcium sensors in the ER [14]. STIM1 also binds directly to the C-terminus of the CaV1.2 α1 subunit, suppressing their depolarization-triggered opening (Figure 1A) and inducing their internalization [15,16]. Recently, adaptor proteins such as SH3 and cysteine-rich containing proteins (STAC proteins) have been found as molecules that form a molecular complex between VGCCs in the plasma membrane and RyRs in the ER membrane, and participate in the coordination of CICR [17,18].
Ca2+ transients are terminated calcium pumps which ensure low [Ca2+]i in the cytosol and are efficient in controlling sub-membrane calcium concentrations [19]. In excitable cells, the active transport of Ca2+ against the ion gradient is carried out primarily by the Na+/Ca2+ exchanger (NCX) and the plasma membrane Ca2+-ATPase (PMCA). A dynamic association of PMCAs and VGCCs mediates the fine-tuning of local Ca2+ domains and the regulation of [Ca2+]i via Ca2+-mediated communication between STIM proteins in the ER membrane and VGCCs in the plasma membrane [1].
The piriform cortex (PC) is a central component of olfactory information processing, underlying odor discrimination and contextualization [20]. The PC, situated in the ventrolateral forebrain, has a laminar structure consisting of layers of pyramidal and semilunar neurons (layers II and III) whose dendrites extend out to the lateral olfactory tract (LOT) near the tissue surface, forming synaptic connections in adjacent layers. The inner synaptic layer (Ib) consists of associational cortico-cortical synapses, while the outer layer (Ia) makes synaptic connections with the LOT, passing afferent olfactory information via fibers from the olfactory bulb [21]. In conjunction with its role in olfactory encoding, the PC is a site involved in associative memory formation, exhibiting a high degree of synaptic plasticity during early developmental periods, which moderately persists into adulthood [22,23,24]. Similar to the hippocampus, the high- and low-frequency stimulation of afferent and associational fibers within the PC produce patterned Ca2+ influx via NMDARs to induce the long-term potentiation (LTP) or long-term depression (LTD) of synaptic activity, respectively [22,23,24]. NMDARs and voltage-gated L-type calcium channels (LTCCs) initiate diverse Ca2+-dependent signaling cascades that underlie the molecular basis of associative memory across different developmental stages. In this Special Issue, Rajani et al. demonstrated age-dependent changes in synaptic plasticity [25]. They investigated the expression and contribution of NMDARs and LTCCs in LTD of the PC associational fiber pathway in three cohorts of Sprague Dawley rats: neonatal (1–2 weeks), young adult (2–3 months) and aged (20–25 months). Using a combination of slice electrophysiology, Western blotting, fluorescent immunohistochemistry and confocal imaging, a shift from an NMDAR to LTCC mediation in LTD in aged rats was observed, despite there being no difference in the amount of LTD expression. These changes in plasticity are related to age-dependent differential receptor expression in the PC. LTCC Cav1.2 expression relative to postsynaptic density protein 95 was increased in the associational pathway of the aged PC layer Ib. Enhanced LTCC contribution in synaptic depression in the PC may contribute to altered olfactory function and learning with aging. These results suggest age-dependent contributions of NMDAR and LTCC to LTD in the PC.
The suprachiasmatic nucleus (SCN) is the central clock that coordinates peripheral oscillators to control circadian rhythms in mammals [26]. Photic cues, conveying information from the retina to the SCN via the glutamatergic retinohypothalamic tract, produce biphasic phase shifts, with delays in the early night and advances in the late night, during the dark phase of the light-dark cycle [27]. The glutamate-induced phase shifts involve Ca2+ entry, intracellular Ca2+ signaling mechanisms, gene expression, and protein synthesis [28,29]. In this Special Issue, Cheng et al. [30], using a ratiometric Ca2+ and Na+ imaging technique, investigated glutamate-evoked intracellular Ca2+ signaling that mediates the photic entrainment of the central clock in the SCN. The application of glutamate (100 µM) or high (20 mM) K+ induced an increase in [Ca2+]i. The Ca2+ clearance of the glutamate-induced Ca2+ transient was slower than that of the high K+-induced Ca2+ transient, and followed by the Ca2+ rebound. The time course of the Ca2+ clearance and the Ca2+ rebound depended on the duration of glutamate exposure. The application of glutamate, but not high K+, increased [Na+]i. In addition, the Ca2+ rebound was abolished by ouabain, which inhibits Na+/K+-ATPase (NKA), monensin, which increases Na+ by activating NKA, Na+-free solution, or nimodipine, which blocks L-type channels. These results suggest that glutamate-induced Ca2+ rebound originates from Na+ loads through activated NKA. Ouabain or Na+-free solution also slow Ca2+ clearance, apparently by retarding the Na+/Ca2+-exchanger (NCX)-mediated Ca2+ extrusion. Thus, the time cause of the glutamate-evoked Ca2+ response is controlled by glutamate-induced Na+ loads and NKA and NCX activation. The glutamate-activated NKA promotes Na+ extrusion and mediates rebound Ca2+ suppression, which accelerates Ca2+ clearance. In the absence of external Na+, Ca2+ clearance is still slower for the Ca2+ response to glutamate than for high K+, suggesting the participation of additional Ca2+ handlers in the slower Ca2+ clearance under this condition.
The soma, dendrites, and axon of neurons may display the Ca2+-dependent release of transmitters and peptides. Such a release is named extrasynaptic because it occurs in the absence of synaptic structures. In this Special Issue, De-Miguel reviewed the cooperative actions of three Ca2+ sources on somatic exocytosis [31]. The somatic release of serotonin was investigated using the classical leech Retzius neuron, which allowed detailed studies on the fine steps from excitation to exocytosis. Trains of action potentials induced transmembrane Ca2+ entry through L-type VGCCs. For action potential frequencies above 5 Hz, the summation of Ca2+ transients on individual action potentials activated the second calcium source: RyRs produced CICR. The resulting Ca2+ tsunami activated mitochondrial ATP synthesis to fuel the transport of vesicles to the plasma membrane. Serotonin released from the vesicles maintained a large-scale exocytosis by activating the third Ca2+ source; serotonin autoreceptors coupled to phospholipase C promoted inositol 1,4,5-trisphosphate (IP3) production. Activated IP3 receptors in peripheral ER released Ca2+ that promotes vesicle fusion. The machinery for somatic exocytosis has a striking disadvantage. The essential Ca2+ releasing ER near the plasma membrane prevents vesicle transport, drastically reducing the thermodynamic efficiency of the ATP expenses and elevating the energy cost of release.
Ca2+ is a critical second messenger in almost all cell types [32]. Likewise, Ca2+ plays a central role in the nervous system, where it is closely linked to the regulation of numerous neuronal functions such as neurotransmission, neuronal excitability, or gene expression [33]. On the other hand, an excessive increase in Ca2+ levels can also activate a series of harmful mechanisms for the cell, such as alterations in mitochondrial functioning and the generation of free radicals, which can eventually cause cell death through apoptosis [34]. Pesticides of different chemical classes exert their toxic effects on the nervous system by acting on the different regulatory mechanisms of Ca2+ homeostasis. Pesticides have been shown to alter Ca2+ homeostasis, mainly by increasing its intracellular concentration above physiological levels. Pesticide-induced Ca2+ overload occurs through two main mechanisms: the entry of Ca2+ from the extracellular medium through the different types of Ca2+ channels present in the plasma membrane, or its release into the cytoplasm from intracellular stocks, mainly from the ER. It has also been observed that intracellular increases in Ca2+ concentrations are maintained over time, because pesticides inhibit the enzymes involved in reducing its levels. Thus, the alteration of Ca2+ levels can lead to the activation of various signaling pathways that generate oxidative stress, neuroinflammation and, finally, neuronal death. In this Special Issue, Costas-Ferreira and Faro summarize the main mechanisms of pesticides’ actions on neuronal Ca2+ homeostasis (Figure 2) [35].
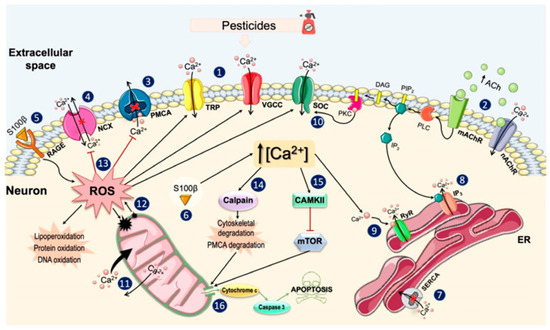
Figure 2.
Main mechanisms of action of pesticides on neuronal Ca2+ homeostasis. Reproduced from Ref. [35]. Published in this Special Issue by MDPI, 2021. Exposure to pesticides induces a series of changes in the plasma membrane that include: (1) the opening of the VGCCs (especially the L- and T-types) and some TRP channels, which allows the Ca2+ influx and enhances the membrane depolarization; (2) the activation of nicotinic acetylcholine receptors (nAChR) and muscarinic acetylcholine receptor (mAChR) through increasing the availability of acetylcholine and/or by binding directly to these receptors; (3) the inhibition of plasma membrane Ca2+-ATPase, the main Ca2+ extrusion mechanism; (4) alterations in the Na+/Ca2+ exchanger, completely inhibiting its activity or activating its reverse mode. Pesticides also increase the S100β levels, which (5) bind to the receptor for advanced glycation end products in the extracellular side and favor the production of reactive oxygen species (ROS) and (6) increase the Ca2+ levels in the intracellular medium. In the cytosol, pesticides induce the depletion of the endoplasmic reticulum (ER) Ca2+ reserves by (7) inhibiting the sarcoplasmic (endoplasmic) reticulum Ca2+-ATPase, responsible for sequestering Ca2+; (8) stimulating the inositol 1,4,5-trisphosphate (IP3)-induced Ca2+ release; and (9) via Ca2+ release through RyRs stimulated by cytosolic Ca2+. When the Ca2+ content of the ER begins to decline, the protein kinase C (PKC) stimulates the influx of Ca2+ through the store-operated channels (SOC) (10) and the mitochondria assume the role of the Ca2+ reservoir, rapidly accumulating large amounts of Ca2+ and slowly releasing it (11). The overload of Ca2+ in the mitochondria increases ROS levels and its release to the cytosol (12), where they enhance the [Ca2+]i by stimulating Ca2+ channels and inhibiting their expulsion mechanisms, damaging lipids, cell proteins, and DNA (13). Increases in ROS and Ca2+ levels activate calpains and Ca2+/calmodulin-dependent protein kinase II (CAMKII). Calpains induce the degradation of elements of the cellular cytoarchitecture (14), while CAMKII inhibits mammalian or mechanistic targets of rapamycin (mTOR) (15). These two pathways can ultimately cause the release of pro-apoptotic factors from the mitochondria, finally leading to cell death (16).
VGCCs are divided into high-voltage-activated L-type (Cav1.1, Cav1.2, Cav1.3, and Cav1.4), P/Q-type (Cav2.1), N-type (Cav2.2), and R-type (Cav2.3) channels because they are activated by relatively large depolarizations, as well as low-voltage-activated T-type (Cav3.1, Cav3.2, Cav3.3) channels, because they are activated by relatively small depolarizations. Among VGCCs, Cav1.2 is the dominant type in the cardiac working muscle, while Cav1.3 and Cav3.2 are dominant in pacemaker (nodal) cells. Ca2+ influx through Cav1.2 channels is regulated by the negative feedback mechanism, known as Ca2+-dependent inactivation (CDI), as well as the positive feedback mechanism, known as Ca2+-dependent facilitation (CDF) [36]. Calmodulin (CaM) is thought to play an important role in both CDI and CDF. Furthermore, CaM may have roles in channel trafficking and clustering/multimerization. In this Special Issue, Kameyama et al. reviewed the details of cardiac Cav1.2 channels’ regulation by CaM [37]. The direct interactions with CaM, a Ca2+-binding protein, causes Ca2+-dependent facilitation (CDF) and the inactivation (CDI) of the Cav1.2 channels. Ca2+-free CaM (apoCaM) also contributes to the regulation of Cav1.2 channels. A role of apoCaM in the channel ‘rundown’ phenomena and the related repriming of channels, and CDF, as well as the role of Ca2+/CaM in CDI, has been identified. Furthermore, CaM indirectly affects channel activity by activating CaM-dependent enzymes, such as CaM-dependent protein kinase II and calcineurin (a CaM-dependent protein phosphatase). In addition, Cav1.2 channels’ trafficking are regulated through the intrinsic properties of isoforms of the α1 subunit of the channel, the associated auxiliary subunits, and regulatory proteins such as CaM. However, Kameyama concluded that the role of CaM in the trafficking of Cav1.2 channels remains controversial and further studies are required.
The heart possesses huge amounts of mitochondria which serve to supply ATP via oxidative phosphorylation, meeting the cardiac energy demand. The mitochondrial Ca2+—one of the key factors of mitochondrial energetics—is strictly maintained within an appropriate range [38]. The mitochondrial Ca2+ in cardiomyocytes is balanced by an influx via the Ca2+ uniport activity and by an efflux via the Na+-Ca2+ exchange or the H+-Ca2+ exchange activities in which the former plays the major part [39,40]. The mitochondrial Na+-Ca2+ exchanger, NCLX, was reported to supply Ca2+ to the sarcoplasmic reticulum (SR)/ER, thereby modulating various cellular functions such as the rhythmicity of cardiomyocytes and cellular Ca2+ signaling upon antigen receptor stimulation and chemotaxis in B lymphocytes; however, there is little information on the spatial relationships of NCLX with SR Ca2+ handling proteins, and their physiological impact. In this Special Issue, Takeuchi and Matsuoka examined the tissue, focusing on the interaction of NCLX with an SR Ca2+ pump (SERCA) in cardiomyocytes [41]. A bimolecular fluorescence complementation assay using HEK293 cells revealed that the exogenously expressed NCLX was localized in close proximity to four exogenously expressed SERCA isoforms. Immunofluorescence analyses of isolated ventricular myocytes showed that the NCLX was localized to the edges of the mitochondria, forming a striped pattern. The co-localization coefficients in the super-resolution images were higher for NCLX–SERCA2 than for the NCLX–RyRs and NCLX–Na+/K+ ATPase α-1 subunit, confirming the close localization of endogenous NCLX and SERCA2 in cardiomyocytes. The mathematical model implemented with the spatial and functional coupling of NCLX and SERCA reproduced the NCLX inhibition-mediated modulations of SR Ca2+ reuptake in HL-1 cardiomyocytes well. These results indicated that NCLX and SERCA are spatially and functionally coupled in cardiomyocytes. The functional coupling plays pivotal roles in the SR Ca2+ dynamics and in the generation of automaticity in cardiomyocytes.
The dysregulation of store-operated Ca2+ entry (SOCE) promotes cancer progression by changing Ca2+ levels in the cytosol or the ER. Stromal interaction molecule 1 (STIM1), which initiates SOCE by sensing severe ER Ca2+ store depletion, is upregulated in several types of cancer and responsible for cancer cell migration, invasion, and metastasis. In this Special Issue, Lin et al. [42] investigated the impact of STIM1-mediated SOCE on the turnover of focal adhesion (FA) and cancer cell migration. They overexpressed the wild-type and constitutively active or dominant negative variants of STIM1 in an osteosarcoma cell line, expecting that STIM1-mediated Ca2+ elevation may increase cell migration. However, constitutively active STIM1 dramatically increased the Ca2+ influx, calpain activity, and turnover of FA proteins, such as the focal adhesion kinase (FAK), paxillin, and vinculin, which impede the cell migration ability. In contrast, dominant negative STIM1 decreased the turnover of FA proteins as its wild-type variant compared to the cells without STIM1 overexpression while promoting cell migration. These results suggest that cancer cells need an appropriate amount of Ca2+ to control the assembly and disassembly of focal adhesions by regulating calpain activity. On the other hand, overloaded Ca2+ results in excessive calpain activity, which is not beneficial for cancer metastasis.
Papers in this Special Issue entitled “Calcium Channels and Calcium-Binding Proteins” demonstrated broad effects of Ca2+ dynamics controlled by various mechanisms for cell and organ-specific action.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
Abbreviations
| CDF | Ca2+-dependent facilitation |
| CDI | Ca2+-dependent inactivation |
| CICR | Ca2+ induced Ca2+ release |
| ER | Endoplasmic reticulum |
| FA | Focal adhesion |
| KCa | Ca2+-activated potassium |
| LOT | Lateral olfactory tract |
| LTCCs | L-type calcium channels |
| LTP | Long-term potentiation |
| LTD | Long-term depression |
| NCLX | Mitochondrial Na+-Ca2+ exchanger |
| NCX | Na+/Ca2+ exchanger |
| NKA | Na+/K+-ATPase |
| NMDARs | N-methyl-D-aspartate receptors |
| PC | Piriform cortex |
| PMCA | Plasma membrane Ca2+-ATPase |
| RIM | Rab3-interacting molecules |
| ROS | Reactive oxygen species |
| RyRs | Ryanodine receptors |
| SCN | Suprachiasmatic nucleus |
| SERCA | SR Ca2+ pump |
| SOCCs | Store-operated Ca2+ channels |
| SR | Sarcoplasmic reticulum |
| STIMs | Stromal interaction molecules |
| VGCC | Voltage-gated Ca2+ channels |
References
- Heine, M.; Heck, J.; Ciuraszkiewicz, A.; Bikbaev, A. Dynamic compartmentalization of calcium channel signalling in neurons. Neuropharmacology 2020, 169, 107556. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, P.J.; Wild, A.R.; Dell’Acqua, M.L.; Sather, W.A. STIM1 Ca2+ Sensor Control of L-type Ca2+-Channel-Dependent Dendritic Spine Structural Plasticity and Nuclear Signaling. Cell Rep. 2017, 19, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Whitt, J.P.; McNally, B.A.; Meredith, A.L. Differential contribution of Ca2+ sources to day and night BK current activation in the circadian clock. J. Gen. Physiol. 2017, 150, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Trussell, L.O. Double-Nanodomain Coupling of Calcium Channels, Ryanodine Receptors, and BK Channels Controls the Generation of Burst Firing. Neuron 2017, 96, 856–870.e854. [Google Scholar] [CrossRef]
- Latorre, R.; Castillo, K.; Carrasquel-Ursulaez, W.; Sepulveda, R.V.; Gonzalez-Nilo, F.; Gonzalez, C.; Alvarez, O. Molecular Determinants of BK Channel Functional Diversity and Functioning. Physiol. Rev. 2017, 97, 39–87. [Google Scholar] [CrossRef]
- Berkefeld, H.; Sailer, C.A.; Bildl, W.; Rohde, V.; Thumfart, J.O.; Eble, S.; Klugbauer, N.; Reisinger, E.; Bischofberger, J.; Oliver, D.; et al. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science 2006, 314, 615–620. [Google Scholar] [CrossRef]
- Sclip, A.; Acuna, C.; Luo, F.; Südhof, T.C. RIM-binding proteins recruit BK-channels to presynaptic release sites adjacent to voltage-gated Ca(2+)-channels. EMBO J. 2018, 37, e98637. [Google Scholar] [CrossRef]
- Zhang, F.X.; Gadotti, V.M.; Souza, I.A.; Chen, L.; Zamponi, G.W. BK Potassium Channels Suppress Cavα2δ Subunit Function to Reduce Inflammatory and Neuropathic Pain. Cell Rep. 2018, 22, 1956–1964. [Google Scholar] [CrossRef]
- Franzini-Armstrong, C. The relationship between form and function throughout the history of excitation–contraction coupling. J. Gen. Physiol. 2018, 150, 189–210. [Google Scholar] [CrossRef]
- Nakai, J.; Dirksen, R.T.; Nguyen, H.T.; Pessah, I.N.; Beam, K.G.; Allen, P.D. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature 1996, 380, 72–75. [Google Scholar] [CrossRef]
- Hoth, M.; Penner, R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 1992, 355, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Prakriya, M.; Lewis, R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef] [PubMed]
- Oritani, K.; Kincade, P.W. Identification of stromal cell products that interact with pre-B cells. J. Cell Biol. 1996, 134, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.; Kim, M.L.; Heo, W.D.; Jones, J.T.; Myers, J.W.; Ferrell, J.E., Jr.; Meyer, T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005, 15, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Shcheglovitov, A.; Dolmetsch, R. The CRAC Channel Activator STIM1 Binds and Inhibits L-Type Voltage-Gated Calcium Channels. Science 2010, 330, 101–105. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, X.; Mancarella, S.; Hendron, E.; Eguchi, S.; Soboloff, J.; Tang, X.D.; Gill, D.L. The Calcium Store Sensor, STIM1, Reciprocally Controls Orai and CaV1.2 Channels. Science 2010, 330, 105–109. [Google Scholar] [CrossRef]
- Campiglio, M.; Costé de Bagneaux, P.; Ortner, N.J.; Tuluc, P.; Van Petegem, F.; Flucher, B.E. STAC proteins associate to the IQ domain of Ca<sub>V</sub>1.2 and inhibit calcium-dependent inactivation. Proc. Natl. Acad. Sci. USA 2018, 115, 1376–1381. [Google Scholar] [CrossRef]
- Polster, A.; Dittmer, P.J.; Perni, S.; Bichraoui, H.; Sather, W.A.; Beam, K.G. Stac Proteins Suppress Ca2+-Dependent Inactivation of Neuronal l-type Ca2+ Channels. J. Neurosci. 2018, 38, 9215–9227. [Google Scholar] [CrossRef]
- Brini, M.; Carafoli, E. Calcium Pumps in Health and Disease. Physiol. Rev. 2009, 89, 1341–1378. [Google Scholar] [CrossRef]
- Blazing, R.M.; Franks, K.M. Odor coding in piriform cortex: Mechanistic insights into distributed coding. Curr. Opin. Neurobiol. 2020, 64, 96–102. [Google Scholar] [CrossRef]
- Martin-Lopez, E.; Ishiguro, K.; Greer, C.A. The Laminar Organization of Piriform Cortex Follows a Selective Developmental and Migratory Program Established by Cell Lineage. Cereb. Cortex 2019, 29, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lebel, D.; Grossman, Y.; Barkai, E. Olfactory Learning Modifies Predisposition for Long-term Potentiation and Long-term Depression Induction in the Rat Piriform (Olfactory) Cortex. Cereb. Cortex 2001, 11, 485–489. [Google Scholar] [CrossRef]
- Morrison, G.L.; Fontaine, C.J.; Harley, C.W.; Yuan, Q. A role for the anterior piriform cortex in early odor preference learning: Evidence for multiple olfactory learning structures in the rat pup. J. Neurophysiol. 2013, 110, 141–152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poo, C.; Isaacson, J.S. An Early Critical Period for Long-Term Plasticity and Structural Modification of Sensory Synapses in Olfactory Cortex. J. Neurosci. 2007, 27, 7553–7558. [Google Scholar] [CrossRef] [PubMed]
- Rajani, V.; Maziar, A.; Man, K.N.M.; Hell, J.W.; Yuan, Q. Age-Dependent Contributions of NMDA Receptors and L-Type Calcium Channels to Long-Term Depression in the Piriform Cortex. Int. J. Mol. Sci. 2021, 22, 13551. [Google Scholar] [CrossRef] [PubMed]
- Dibner, C.; Schibler, U.; Albrecht, U. The Mammalian Circadian Timing System: Organization and Coordination of Central and Peripheral Clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef]
- Golombek, D.A.; Rosenstein, R.E. Physiology of Circadian Entrainment. Physiol. Rev. 2010, 90, 1063–1102. [Google Scholar] [CrossRef]
- Gillette, M.U.; Mitchell, J.W. Signaling in the suprachiasmatic nucleus: Selectively responsive and integrative. Cell Tissue Res. 2002, 309, 99–107. [Google Scholar] [CrossRef]
- Meijer, J.H.; Schwartz, W.J. In search of the pathways for light-induced pacemaker resetting in the suprachiasmatic nucleus. J. Biol. Rhythm. 2003, 18, 235–249. [Google Scholar] [CrossRef]
- Cheng, P.-C.; Cheng, R.-C.; Huang, R.-C. Glutamate-Evoked Ca2+ Responses in the Rat Suprachiasmatic Nucleus: Involvement of Na+/K+-ATPase and Na+/Ca2+-Exchanger. Int. J. Mol. Sci. 2023, 24, 6444. [Google Scholar] [CrossRef]
- De-Miguel, F.F. The Thermodynamically Expensive Contribution of Three Calcium Sources to Somatic Release of Serotonin. Int. J. Mol. Sci. 2022, 23, 1495. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Greenberg, M.E. Calcium signaling in neurons: Molecular mechanisms and cellular consequences. Science 1995, 268, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Toescu, E.C. Apoptosis and cell death in neuronal cells: Where does Ca2+ fit in? Cell Calcium 1998, 24, 387–403. [Google Scholar] [CrossRef]
- Costas-Ferreira, C.; Faro, L.R.F. Systematic Review of Calcium Channels and Intracellular Calcium Signaling: Relevance to Pesticide Neurotoxicity. Int. J. Mol. Sci. 2021, 22, 13376. [Google Scholar] [CrossRef]
- Hofmann, F.; Flockerzi, V.; Kahl, S.; Wegener, J.W. L-Type CaV1.2 Calcium Channels: From In Vitro Findings to In Vivo Function. Physiol. Rev. 2014, 94, 303–326. [Google Scholar] [CrossRef]
- Kameyama, M.; Minobe, E.; Shao, D.; Xu, J.; Gao, Q.; Hao, L. Regulation of Cardiac Cav1.2 Channels by Calmodulin. Int. J. Mol. Sci. 2023, 24, 6409. [Google Scholar] [CrossRef]
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 2017, 14, 238–250. [Google Scholar] [CrossRef]
- Carafoli, E.; Tiozzo, R.; Lugli, G.; Crovetti, F.; Kratzing, C. The release of calcium from heart mitochondria by sodium. J. Mol. Cell Cardiol. 1974, 6, 361–371. [Google Scholar] [CrossRef]
- Nicholls, D.G.; Crompton, M. Mitochondrial calcium transport. FEBS Lett. 1980, 111, 261–268. [Google Scholar] [CrossRef]
- Takeuchi, A.; Matsuoka, S. Spatial and Functional Crosstalk between the Mitochondrial Na+-Ca2+ Exchanger NCLX and the Sarcoplasmic Reticulum Ca2+ Pump SERCA in Cardiomyocytes. Int. J. Mol. Sci. 2022, 23, 7948. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Lin, Y.H.; Nguyen Thi, M.; Hsiao, S.C.; Chiu, W.T. STIM1 Controls the Focal Adhesion Dynamics and Cell Migration by Regulating SOCE in Osteosarcoma. Int. J. Mol. Sci. 2021, 23, 162. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).