Study on Spontaneous Reactivation and Aging of Acetylcholinesterase Inhibited by Paraoxon and Malaoxon in Ten Species
Abstract
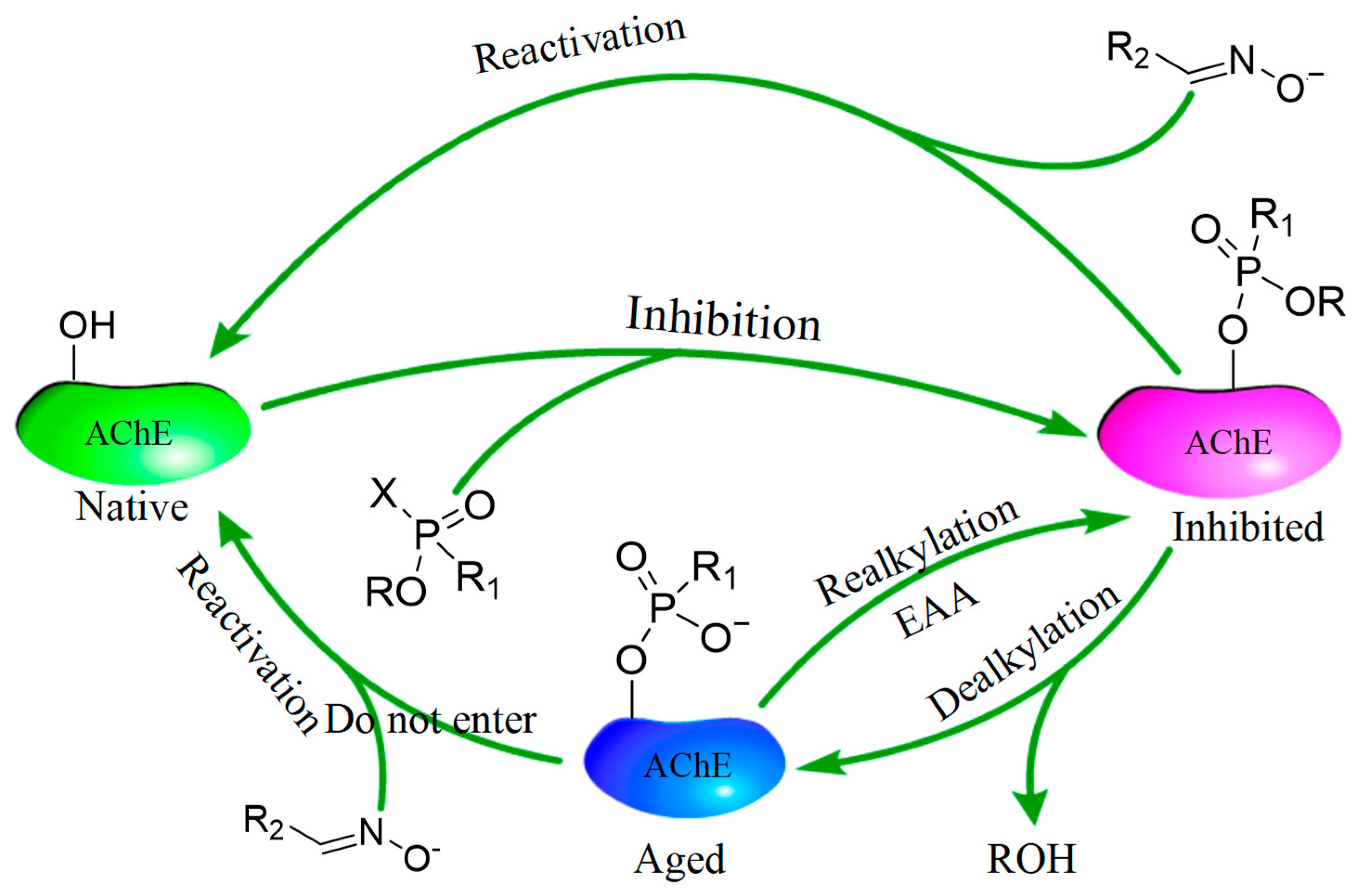
:1. Introduction
2. Results
2.1. Sensitivity of AChE to Paraoxon and Malaoxon
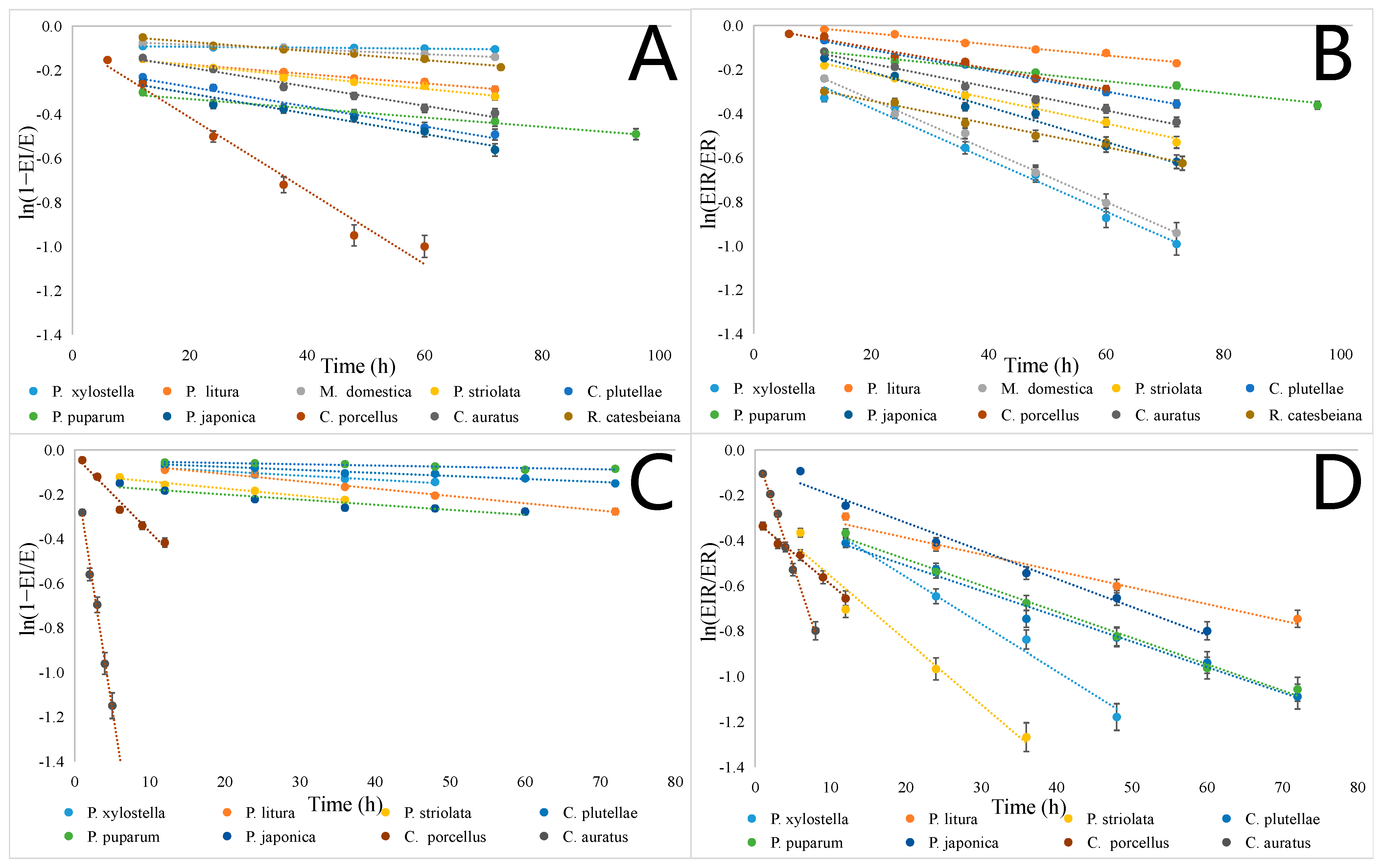
2.2. The Spontaneous Reactivation Rate Constants (Ks) of AChE Inhibited by Paraoxon and Malaoxon
2.3. The Aging Rate Constant (Ka) of AChE Inhibited by Paraoxon and Malaoxon
3. Discussion
4. Materials and Methods
4.1. Sources of Biological Materials
4.2. Chemicals
4.3. Preparation of AChE
4.4. Determination of AChE Activity
4.5. Determination of the Sensitivity of AChE Inhibited by Paraoxon and Malaoxon
4.6. Spontaneous Reactivation Rate Constants (Ks) of AChE Inhibited by Paraoxon and Malaoxon
4.7. Aging Rate Constants (Ka) of AChE Inhibited by Paraoxon and Malaoxon
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rabeler, C.; Gong, T.; Ireland, D.; Cochet-Escartin, O.; Collins, E.S. Acetylcholinesterase Activity Staining in Freshwater Planarians. Curr. Protoc. 2023, 3, e674. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Young, A.; Callam, C.S.; McElroy, C.A.; Ekici, Ö.D.; Yoder, R.J.; Hadad, C.M. Efforts toward treatments against aging of organophosphorus-inhibited acetylcholinesterase. Ann. N. Y. Acad. Sci. 2016, 1374, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.D.; Todd, S.W.; Lumsden, E.; Mullins, R.J.; Mamczarz, J.; Fawcett, W.P.; Gullapalli, R.P.; Randall, W.R.; Pereira, E.F.R.; Albuquerque, E.X. Developmental neurotoxicity of the organophosphorus insecticide chlorpyrifos: From clinical findings to preclinical models and potential mechanisms. J. Neurochem. 2017, 142, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xu, W.; Zhang, W.; Wu, H.; Guang, C.; Mu, W. Overview of a bioremediation tool: Organophosphorus hydrolase and its significant application in the food, environmental, and therapy fields. Appl. Microbiol. Biotechnol. 2021, 105, 8241–8253. [Google Scholar] [CrossRef] [PubMed]
- Sheikhi, S.; Dehghanzadeh, R.; Aslani, H. Advanced oxidation processes for chlorpyrifos removal from aqueous solution: A systematic review. J. Environ. Health Sci. Eng. 2021, 19, 1249–1262. [Google Scholar] [CrossRef]
- Eyer, F.; Meischner, V.; Kiderlen, D.; Thiermann, H.; Worek, F.; Haberkorn, M.; Felgenhauer, N.; Zilker, T.; Eyer, P. Human parathion poisoning: A toxicokinetic analysis. Toxicol. Rev. 2003, 22, 143–163. [Google Scholar] [CrossRef]
- Fukuto, T.R. Mechanism of action of organophosphorus and carbamate insecticides. Env. Health Perspect 1990, 87, 245–254. [Google Scholar] [CrossRef]
- Casida, J.E.; Quistad, G.B. Golden Age of Insecticide Research: Past, Present, or Future? Annu. Rev. Èntomol. 1998, 43, 1–16. [Google Scholar] [CrossRef]
- Mew, E.J.; Padmanathan, P.; Konradsen, F.; Eddleston, M.; Chang, S.-S.; Phillips, M.R.; Gunnell, D. The global burden of fatal self-poisoning with pesticides 2006-15: Systematic review. J. Affect. Disord. 2017, 219, 93–104. [Google Scholar] [CrossRef]
- Yang, L.-L.; Yang, X.; Li, G.-B.; Fan, K.-G.; Yin, P.-F.; Chen, X.-G. An integrated molecular docking and rescoring method for predicting the sensitivity spectrum of various serine hydrolases to organophosphorus pesticides. J. Sci. Food Agric. 2016, 96, 2184–2192. [Google Scholar] [CrossRef]
- Silman, I.; Sussman, J.L. Acetylcholinesterase: ‘classical’ and ‘non-classical’ functions and pharmacology. Curr. Opin. Pharmacol. 2005, 5, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Tsao, T.C.-Y.; Juang, Y.-C.; Lan, R.-S.; Shieh, W.-B.; Lee, C.-H. Respiratory Failure of Acute Organophosphate and Carbamate Poisoning. Chest 1990, 98, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, T.H.; Apland, J.P.; Braga, M.F.M.; Marini, A.M. Acute and long-term consequences of exposure to organophosphate nerve agents in humans. Epilepsia 2018, 59, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Blumenberg, A.; Benabbas, R.; Desouza, I.S.; Conigliaro, A.; Paladino, L.; Warman, E.; Sinert, R.; Wiener, S.W. Utility of 2-Pyridine Aldoxime Methyl Chloride (2-PAM) for Acute Organophosphate Poisoning: A Systematic Review and Meta-Analysis. J. Med. Toxicol. 2018, 14, 91–98. [Google Scholar] [CrossRef]
- Tang, X.W.; Zhu, L.D.; Chen, J.M.; Hu, J.T.; Zhang, Q.Z.; Wang, W. Reaction pathway for reactivation and aging of paraoxon-inhibited-acetylcholinesterase: A QM/MM study. Comput. Theor. Chem. 2014, 1035, 44–50. [Google Scholar] [CrossRef]
- Tripathi, R.; O’Brien, R. Effect of organophosphates in vivo upon acetylcholinesterase isozymes from housefly head and thorax. Pestic. Biochem. Physiol. 1973, 2, 418–424. [Google Scholar] [CrossRef]
- Worek, F.; Aurbek, N.; Wille, T.; Eyer, P.; Thiermann, H. Kinetic analysis of interactions of paraoxon and oximes with human, Rhesus monkey, swine, rabbit, rat and guinea pig acetylcholinesterase. Toxicol. Lett. 2011, 200, 19–23. [Google Scholar] [CrossRef]
- Carr, R.L.; Chambers, J.E. Kinetic Analysis of thein VitroInhibition, Aging, and Reactivation of Brain Acetylcholinesterase from Rat and Channel Catfish by Paraoxon and Chlorpyrifos-oxon. Toxicol. Appl. Pharmacol. 1996, 139, 365–373. [Google Scholar] [CrossRef]
- Johnson, J.A.; Wallace, K.B. Species-related differences in the inhibition of brain acetylcholinesterase by paraoxon and malaoxon. Toxicol. Appl. Pharmacol. 1987, 88, 234–241. [Google Scholar] [CrossRef]
- Wu, G.; Miyata, T. Susceptibilities to methamidophos and enzymatic characteristics in 18 species of pest insects and their natural enemies in crucifer vegetable crops. Pestic. Biochem. Physiol. 2005, 82, 79–93. [Google Scholar] [CrossRef]
- De Castro, A.A.; Assis, L.C.; Soares, F.V.; Kuca, K.; Polisel, D.A.; da Cunha, E.F.F.; Ramalho, T.C. Trends in the Recent Patent Literature on Cholinesterase Reactivators (2016–2019). Biomolecules 2020, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Gerlits, O.; Fajer, M.; Cheng, X.L.; Blumenthal, K.D.; Radić, Z.; Kovalevsky, A. Structural and dynamic effects of paraoxon binding to human acetylcholinesterase by X-ray crystallography and inelastic neutron scattering. Structure 2022, 30, 1538–1549.e3. [Google Scholar] [CrossRef] [PubMed]
- Sette, K.N.; Alugubelly, N.; Glenn, L.B.; Guo-Ross, S.X.; Parkes, M.K.; Wilson, J.R.; Seay, C.N.; Carr, R.L. The mechanistic basis for the toxicity difference between juvenile rats and mice following exposure to the agricultural insecticide chlorpyrifos. Toxicology 2022, 480, 153317. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Castellanos, L.; Sanchez-Hernandez, J.C. Chemical reactivation and aging kinetics of organophosphorus-inhibited cholinesterases from two earthworm species. Environ. Toxicol. Chem. 2007, 26, 1992–2000. [Google Scholar] [CrossRef]
- Wallace, K.B.; Herzberg, U. Reactivation and aging of phosphorylated brain acetylcholinesterase from fish and rodents. Toxicol. Appl. Pharmacol. 1988, 92, 307–314. [Google Scholar] [CrossRef]
- Alfonso, M.; Durán, R.; Fajardo, D.; Justo, L.; Faro, L.R. Mechanisms of action of paraoxon, an organophosphorus pesticide, on in vivo dopamine release in conscious and freely moving rats. Neurochem. Int. 2019, 124, 130–140. [Google Scholar] [CrossRef]
- Karina, M.A.; Ignacio, A.P.L.; Eustacio, R.F.M.A.; Nieves, T.T.; Luis, A.O.C. Growth, photosynthesis and removal responses of the cyanobacteria Chroococcus sp. to malathion and malaoxon. J. Environ. Sci. Health. Part B 2018, 53, 771–776. [Google Scholar]
- Aker, W.G.; Hu, X.; Wang, P.; Hwang, H. Comparing the relative toxicity of malathion and malaoxon in blue catfish Ictalurus furcatus. Environ. Toxicol. 2008, 23, 548–554. [Google Scholar] [CrossRef]
- Cohen, S.D.; Williams, R.A.; Killinger, J.M.; Freudenthal, R.I. Comparative sensitivity of bovine and rodent acetylcholinesterase to in vitro inhibition by organophosphate insecticides. Toxicol. Appl. Pharmacol. 1985, 81, 452–459. [Google Scholar] [CrossRef]
- Millard, C.B.; Koellner, G.; Ordentlich, A.; Shafferman, A.; Silman, I.; Sussman, J.L. Reaction Products of Acetylcholinesterase and VX Reveal a Mobile Histidine in the Catalytic Triad. J. Am. Chem. Soc. 1999, 121, 9883–9884. [Google Scholar] [CrossRef]
- Haddi, K.; Berger, M.; Bielza, P.; Rapisarda, C.; Williamson, M.S.; Moores, G.; Bass, C. Mutation in the ace-1 gene of the tomato leaf miner (Tuta absoluta) associated with organophosphates resistance. J. Appl. Entomol. 2017, 141, 612–619. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Zhan, C.-G. Reaction Pathway and Free-Energy Barrier for Reactivation of Dimethylphosphoryl-Inhibited Human Acetylcholinesterase. J. Phys. Chem. B 2009, 113, 16226–16236. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, X.; Du, L.; Zhang, Q.; Wang, W. QM/MM study on the spontaneous reactivation mechanism of (±)methamidophos-inhibited-acetylcholinesterase. Comput. Theor. Chem. 2012, 980, 108–114. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Miyata, T.; Wu, Z.J.; Wu, G.; Xie, L.H. Hydrolysis of acetylthiocholine iodide and reactivation of phoxim-inhibited acetylcholinesterase by pralidoxime chloride, obidoxime chloride and trimedoxime. Arch. Toxicol. 2007, 81, 785–792. [Google Scholar] [CrossRef]
- Worek, F.; Diepold, C.; Eyer, P. Dimethylphosphoryl-inhibited human cholinesterases: Inhibition, reactivation, and aging kinetics. Arch. Toxicol. 1999, 73, 7–14. [Google Scholar] [CrossRef]
| Species | Paraoxon | ||
| Toxicity Regression Equation | I50 (mol·L−1) | I90 (mol·L−1) | |
| Plutella xylostella (moth) | y = 1.02x + 3.57 | (5.88 ± 0.434) × 10−6 A | (1.297 ± 0.446) × 10−4 |
| Prodenia litura (cotton leafworm) | y = 1.25x + 4.61 | (4.46 ± 0.516) × 10−7 B | (5.234 ± 0.592) × 10−6 |
| Musca domestica (housefly) | y = 0.87x + 3.94 | (3.17 ± 0.378) × 10−6 C | (1.127 ± 0.064) × 10−4 |
| Phyllotreta striolata (flea beetle) | y = 1.10x + 4.83 | (3.27 ± 0.220) × 10−7 D | (4.811 ± 0.285) × 10−6 |
| Cotesia plutellae (wasp) | y = 2.49x + 6.19 | (7.21 ± 0.064) × 10−8 E | (2.334 ± 0.004) × 10−7 |
| Pteromalus puparum (parasitic wasp) | y = 2.20x + 5.96 | (7.77 ± 0.128) × 10−8 F | (2.973 ± 0.047) × 10−7 |
| Propylea japonica (ladybird) | y = 2.11x + 5.79 | (9.85 ± 0.086) × 10−8 G | (4.467 ± 0.077) × 10−7 |
| Cavia porcellus (guinea pig) | y = 3.02x + 6.17 | (8.40 ± 0.300) × 10−8 H | (2.308 ± 0.054) × 10−7 |
| Carassius auratus (goldfish) | y = 2.45x + 1.18 | (7.50 ± 0.201) × 10−6 I | (2.698 ± 0.112) × 10−5 |
| Rana catesbeiana (frog) | y = 2.31x – 1.71 | (1.74 ± 0.006) × 10−4 J | (6.130 ± 0.144) × 10−4 |
| Species | Malaoxon | ||
| Toxicity Regression Equation | I50 (mol·L−1) | I90 (mol·L−1) | |
| Plutella xylostella | y = 1.80x + 1.83 | (1.06 ± 0.022) × 10−5 A * | (4.34 ± 0.146) × 10−5 |
| Prodenia litura | y = 1.50x + 3.68 | (1.40 ± 0.026) × 10−6 B * | (9.38 ± 0.671) × 10−6 |
| Musca domestica | y = 1.53x + 3.22 | (2.74 ± 0.036) × 10−6 C * | (1.92 ± 0.060) × 10−5 |
| Phyllotreta striolata | y = 0.84x + 4.18 | (1.73 ± 0.016) × 10−6 B * | (2.76 ± 0.443) × 10−5 |
| Cotesia plutellae | y = 1.85x + 4.36 | (5.21 ± 0.406) × 10−7 D * | (4.30 ± 0.656) × 10−6 |
| Pteromalus puparum | y = 2.35x + 4.26 | (3.88 ± 0.055) × 10−7 E * | (1.39 ± 0.038) × 10−6 |
| Propylea japonica | y = 1.84x + 3.72 | (9.42 ± 0.321) × 10−7 F * | (3.49 ± 0.077) × 10−6 |
| Cavia porcellus | y = 1.63x + 2.33 | (7.74 ± 0.300) × 10−6 G * | (4.72 ± 0.278) × 10−5 |
| Carassius auratus | y = 1.23x + 2.88 | (1.02 ± 0.040) × 10−5 A * | (1.10 ± 0.056) × 10−4 |
| Rana catesbeiana | y = 2.24x – 0.24 | (3.95 ± 0.097) × 10−5 H * | (1.54 ± 0.062) × 10−4 |
| Species | Paraoxon | Malaoxon | ||
|---|---|---|---|---|
| Test Time (h) | Test Concentration (mol·L−1) | Test Time (h) | Test Concentration (mol·L−1) | |
| P.X. | 72 | (1.297 ± 0.446) × 10−4 | 48 | (4.34 ± 0.146) × 10−5 |
| P.L. | 72 | (5.234 ± 0.592) × 10−6 | 72 | (9.38 ± 0.671) × 10−6 |
| M.D. | 72 | (1.127 ± 0.064) × 10−4 | - | (1.92 ± 0.060) × 10−5 |
| P.S. | 72 | (4.811 ± 0.285) × 10−6 | 36 | (2.76 ± 0.443) × 10−5 |
| C.L. | 72 | (2.334 ± 0.004) × 10−7 | 72 | (4.30 ± 0.656) × 10−6 |
| P.P. | 96 | (2.973 ± 0.047) × 10−7 | 72 | (1.39 ± 0.038) × 10−6 |
| P.J. | 72 | (4.467 ± 0.077) × 10−7 | 60 | (3.49 ± 0.077) × 10−6 |
| C.P. | 60 | (2.308 ± 0.054) × 10−7 | 12 | (4.72 ± 0.278) × 10−5 |
| C.A. | 72 | (2.698 ± 0.112) × 10−5 | 8 | (1.10 ± 0.056) × 10−4 |
| R.C. | 73 | (6.130 ± 0.144) × 10−4 | - | (1.54 ± 0.062) × 10−4 |
| Species | Paraoxon | Malaoxon | ||
|---|---|---|---|---|
| Reactivation (Figure 2A) | Aging (Figure 2B) | Reactivation (Figure 2C) | Aging (Figure 2D) | |
| P. xylostella | y = −0.0002x − 0.0896 R2 = 0.9516 | y = −0.0118x − 0.1391 R2 = 0.9821 | y = −0.0018x − 0.0595 R2 = 0.9605 | y = −0.0208x − 0.1452 R2 = 0.9852 |
| P. litura | y = −0.0021x − 0.1324 R2 = 0.9758 | y = −0.0025x + 0.0155 R2 = 0.9865 | y = −0.0033x − 0.042 R2 = 0.984 | y = −0.0073x − 0.2411 R2 = 0.9709 |
| M. domestica | y = −0.0011x − 0.0634 R2 = 0.9932 | y = −0.0117x − 0.1019 R2 = 0.9955 | - | - |
| P. striolata | y = −0.0027x − 0.1249 R2 = 0.9743 | y = −0.0052x − 0.1055 R2 = 0.9853 | y = −0.0028x − 0.1092 R2 = 0.9775 | y = −0.0284x − 0.274 R2 = 0.9656 |
| C. plutellae | y = −0.0045x − 0.186 R2 = 0.975 | y = −0.0047x − 0.0154 R2 = 0.9946 | y = −0.0014x − 0.0488 R2 = 0.9743 | y = −0.0112x − 0.2867 R2 = 0.9859 |
| P. puparum | y = −0.0021x − 0.2893 R2 = 0.9768 | y = −0.0028x − 0.0866 R2 = 0.9874 | y = −0.0006x − 0.0468 R2 = 0.9012 | y = −0.0116x − 0.2516 R2 = 0.9939 |
| P. japonica | y = −0.0046x − 0.2135 R2 = 0.9607 | y = −0.0079x − 0.0518 R2 = 0.9835 | y = −0.0023x − 0.1542 R2 = 0.9042 | y = −0.0131x − 0.0735 R2 = 0.9824 |
| C. porcellus | y = −0.0171x − 0.0601 R2 = 0.9589 | y = −0.0047x − 0.0064 R2 = 0.9831 | y = −0.0348x − 0.0262 R2 = 0.9774 | y = −0.0281x − 0.3133 R2 = 0.9891 |
| C. auratus | y = −0.0043x − 0.1013 R2 = 0.9776 | y = −0.0053x − 0.0658 R2 = 0.9862 | y = −0.2173x − 0.0816 R2 = 0.9845 | y = −0.1061x + 0.012 R2 = 0.9826 |
| R. catesbeiana | y = −0.0021x − 0.0308 R2 = 0.9832 | y = −0.0053x − 0.2359 R2 = 0.9857 | - | - |
| Species | Paraoxon (Ks, %/h) | Malaoxon (Ks, %/h) |
|---|---|---|
| Plutella xylostella | 0.02 ± 0.01 A | 0.18 ± 0.01 A * |
| Prodenia litura | 0.20 ± 0.01 B | 0.32 ± 0.02 B * |
| Musca domestica | 0.11 ± 0.01 C | - |
| Phyllotreta striolata | 0.27 ± 0.02 D | 0.27 ± 0.05 BD |
| Cotesia plutellae | 0.45 ± 0.02 E | 0.14 ± 0.03 A * |
| Pteromalus puparum | 0.21 ± 0.02 B | 0.05 ± 0.04 C * |
| Propylea japonica | 0.45 ± 0.01 E | 0.23 ± 0.03 D * |
| Cavia porcellus | 1.71 ± 0.03 F | 3.50 ± 0.10 E * |
| Carassius auratus | 0.44 ± 0.02 E | 21.71 ± 0.81 F * |
| Rana catesbeiana | 0.22 ± 0.01 B | - |
| Species | Paraoxon (Ka, %/h) | Malaoxon (Ka, %/h) |
|---|---|---|
| Plutella xylostella | 1.18 ± 0.06 A | 2.02 ± 0.07 A * |
| Prodenia litura | 0.25 ± 0.02 B | 0.73 ± 0.01 B * |
| Musca domestica | 1.17 ± 0.03 A | - |
| Phyllotreta striolata | 0.52 ± 0.04 C | 2.84 ± 0.29 C * |
| Cotesia plutellae | 0.47 ± 0.03 C | 1.12 ± 0.07 D * |
| Pteromalus puparum | 0.28 ± 0.02 D | 1.15 ± 0.03 D * |
| Propylea japonica | 0.78 ± 0.07 E | 1.30 ± 0.06 E * |
| Cavia porcellus | 0.47 ± 0.02 C | 2.81 ± 0.11 C * |
| Carassius auratus | 0.53 ± 0.04 C | 10.59 ± 1.06 F * |
| Rana catesbeiana | 0.48 ± 0.05 C | - |
| Species | Processing Method |
|---|---|
| Plutella xylostella (moth) | After eclosion, complete P. xylostella adults were raised for over 24 h using 10% honey water, before being frozen in liquid nitrogen and homogenized with a homogenizer. Approximately 4–5 adults were required per milliliter of homogenate. |
| Prodenia litura (cotton leafworm) | Their heads and chests were homogenized, after the freezing of complete adults in liquid nitrogen. Approximately two adult heads and chests were required for each milliliter of uniform slurry. |
| Musca domestica (housefly) | Raised with honey water until after M. domestica eclosion, complete adults were placed in liquid nitrogen, and their heads were homogenized. Approximately two M. domestica were required per milliliter of homogenate. |
| Phyllotreta striolata (flea beetle) | Complete adults were quickly homogenized, after treatment with liquid nitrogen. Approximately 20 adults were required per milliliter of homogenized liquid. |
| Cotesia plutellae (wasp) | Complete adults after eclosion were treated with liquid nitrogen, and then quickly homogenized. Approximately five adults were required per milliliter of homogenized liquid. |
| Pteromalus puparum (parasitic wasp) | After eclosion, P. puparums, parasitized within the puparium, were treated with liquid nitrogen, and quickly homogenized. Approximately 6–8 mL of homogenate can be prepared from the adult P. puparums produced by one bee pupa. |
| Propylea japonica (ladybird) | The head and chest were homogenized after the treatment of complete adults with liquid nitrogen. Approximately five adults were required for each milliliter of homogenate. |
| Cavia porcellus (guinea pig) | The brain of C. porcellus was homogenized at a ratio of 1:20 (w/v). |
| Carassius auratus (goldfish) | The brain of C. auratus was homogenized at a ratio of 1:20 (w/v). |
| Rana catesbeiana (frog) | The brain of R. catesbeiana was homogenized at a ratio of 1:20 (w/v). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Ni, Z.; Li, G.; Wu, G.; Huang, B. Study on Spontaneous Reactivation and Aging of Acetylcholinesterase Inhibited by Paraoxon and Malaoxon in Ten Species. Int. J. Mol. Sci. 2023, 24, 14213. https://doi.org/10.3390/ijms241814213
Gao M, Ni Z, Li G, Wu G, Huang B. Study on Spontaneous Reactivation and Aging of Acetylcholinesterase Inhibited by Paraoxon and Malaoxon in Ten Species. International Journal of Molecular Sciences. 2023; 24(18):14213. https://doi.org/10.3390/ijms241814213
Chicago/Turabian StyleGao, Mingwei, Zhongwen Ni, Guo Li, Gang Wu, and Binbin Huang. 2023. "Study on Spontaneous Reactivation and Aging of Acetylcholinesterase Inhibited by Paraoxon and Malaoxon in Ten Species" International Journal of Molecular Sciences 24, no. 18: 14213. https://doi.org/10.3390/ijms241814213
APA StyleGao, M., Ni, Z., Li, G., Wu, G., & Huang, B. (2023). Study on Spontaneous Reactivation and Aging of Acetylcholinesterase Inhibited by Paraoxon and Malaoxon in Ten Species. International Journal of Molecular Sciences, 24(18), 14213. https://doi.org/10.3390/ijms241814213





