Development and Characterization of Electrospun Composites Built on Polycaprolactone and Cerium-Containing Phases
Abstract
:1. Introduction
2. Results and Discussion
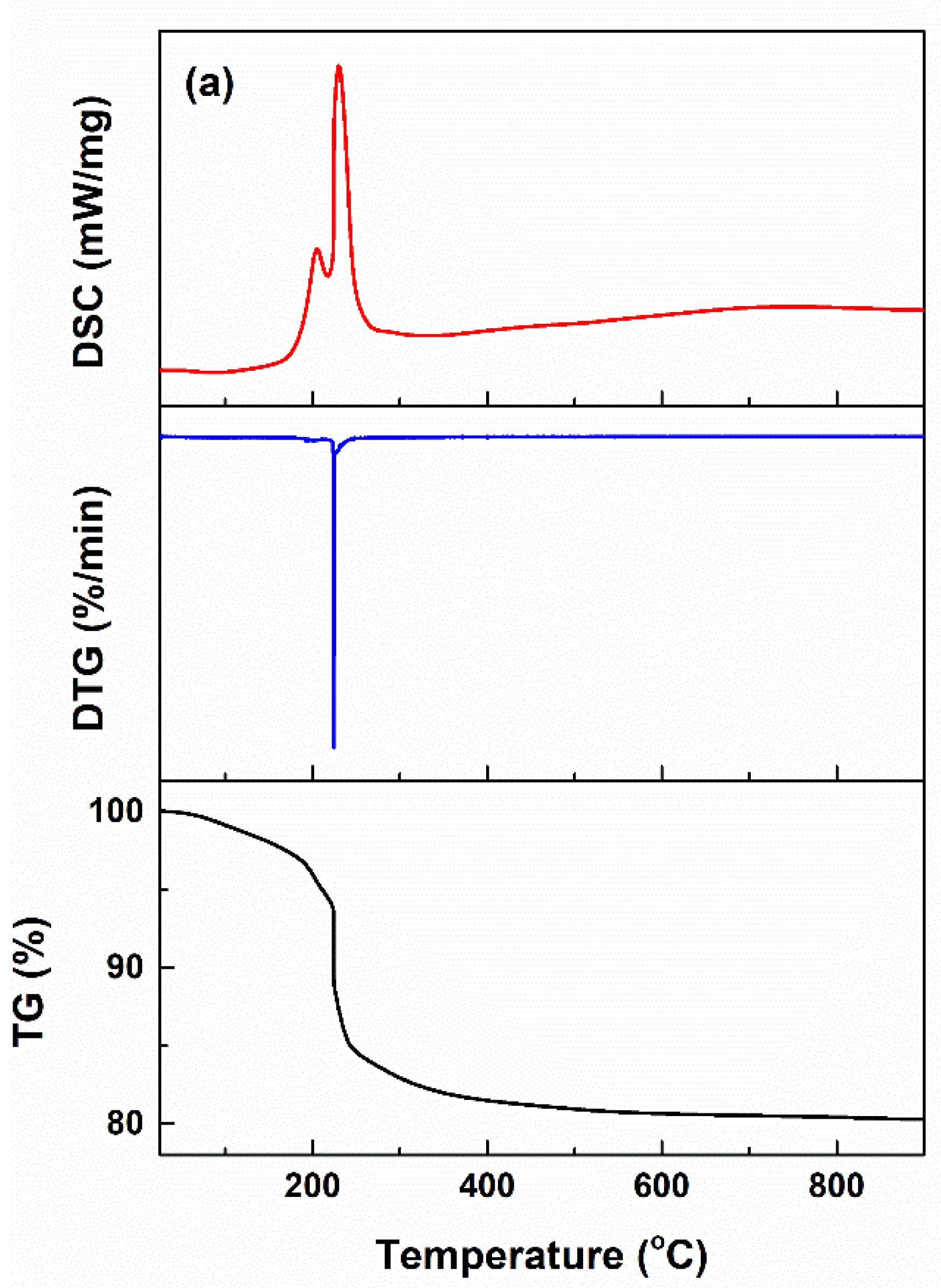
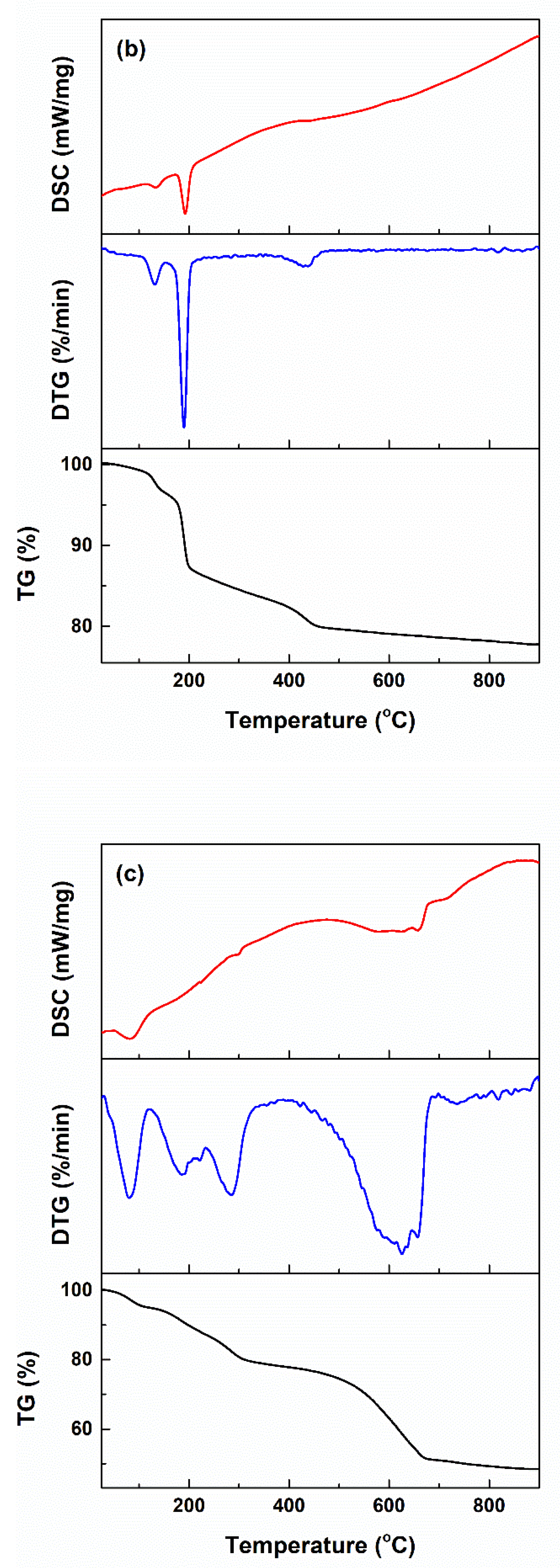
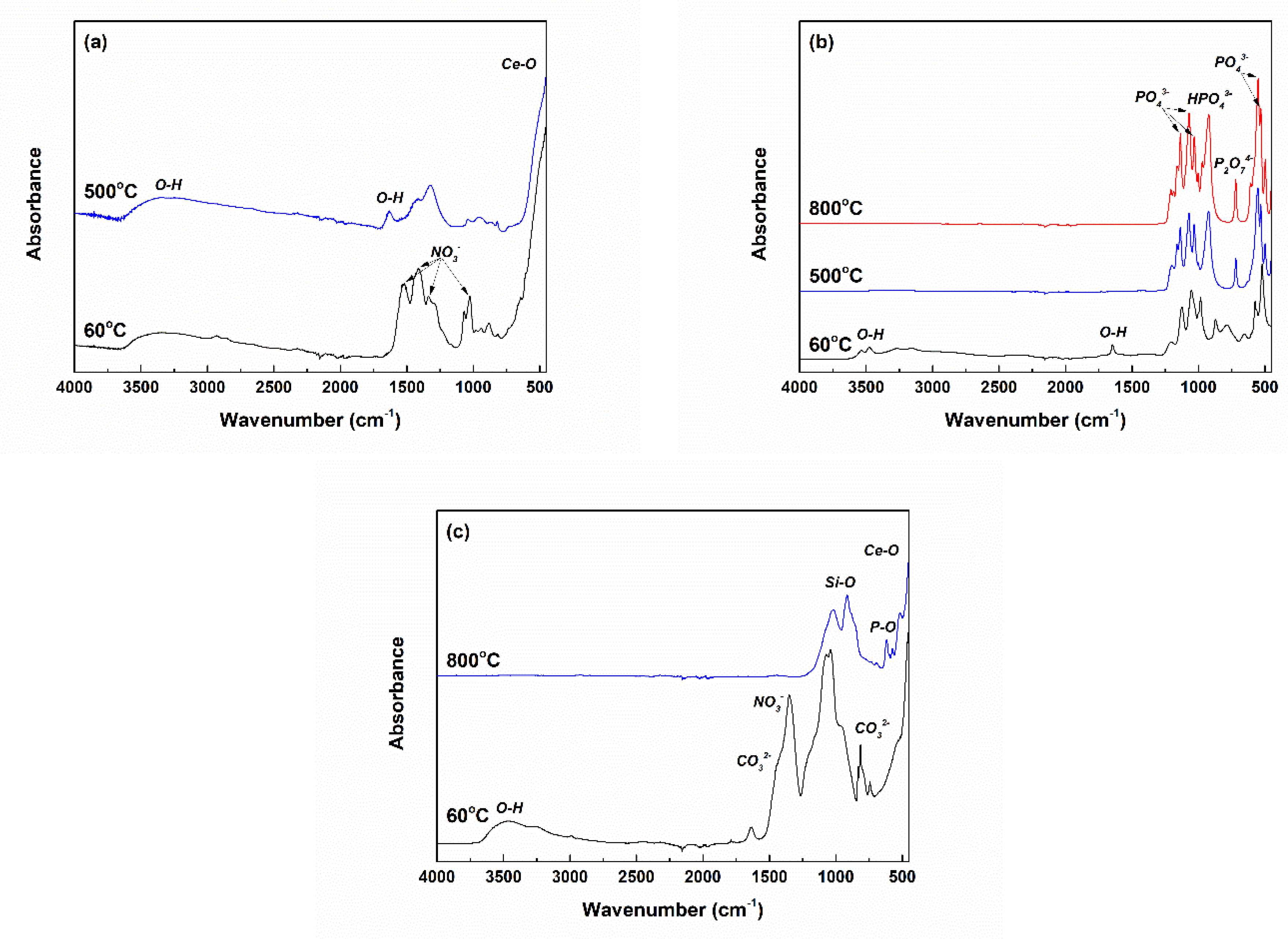
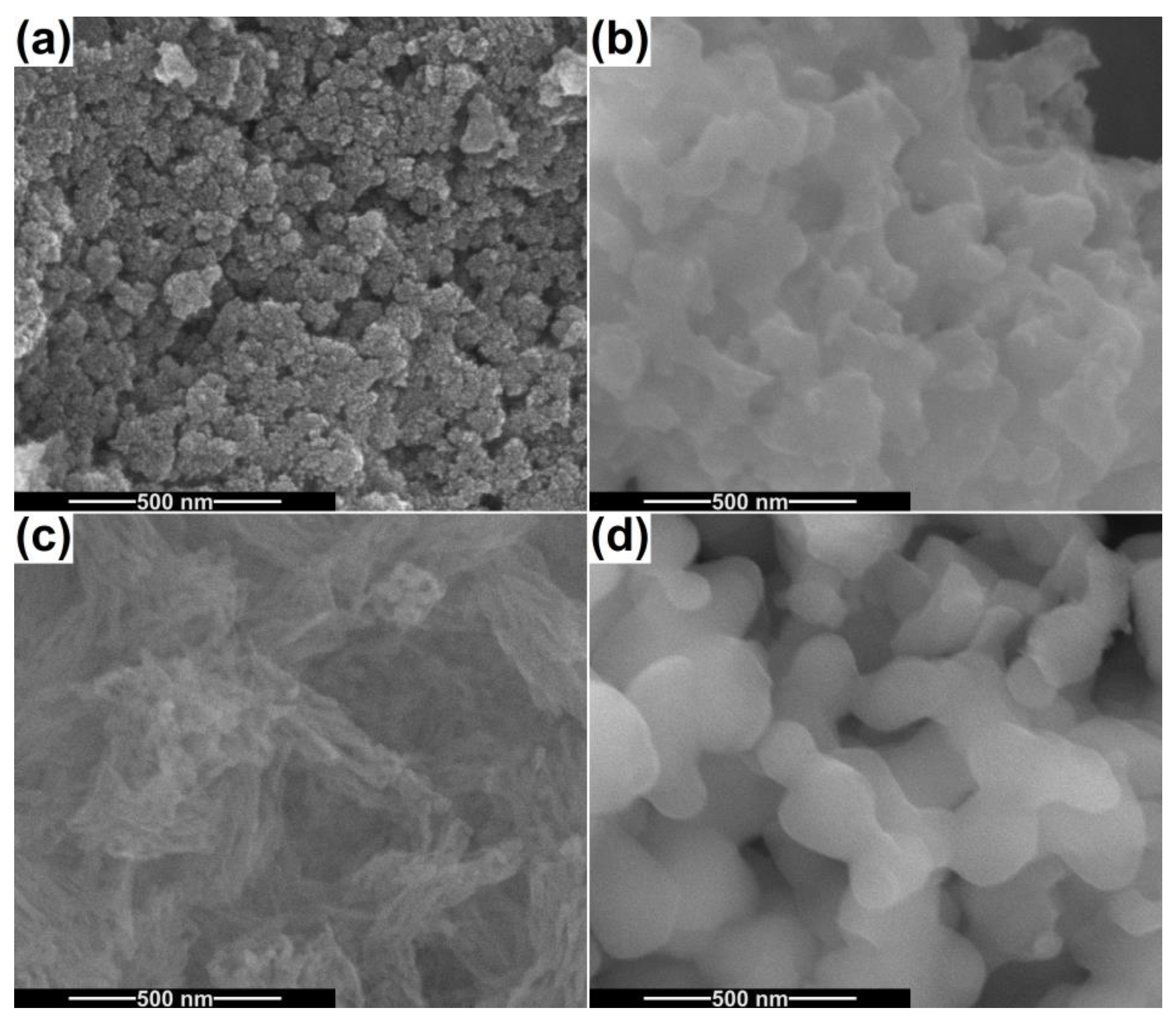
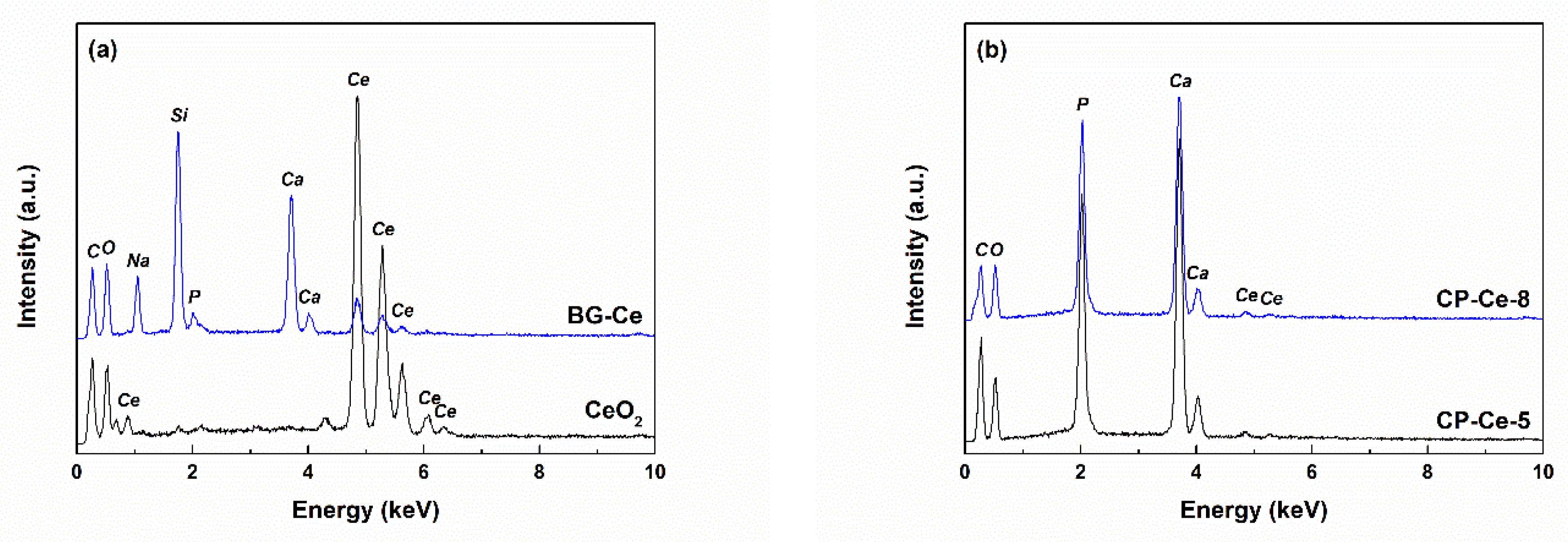
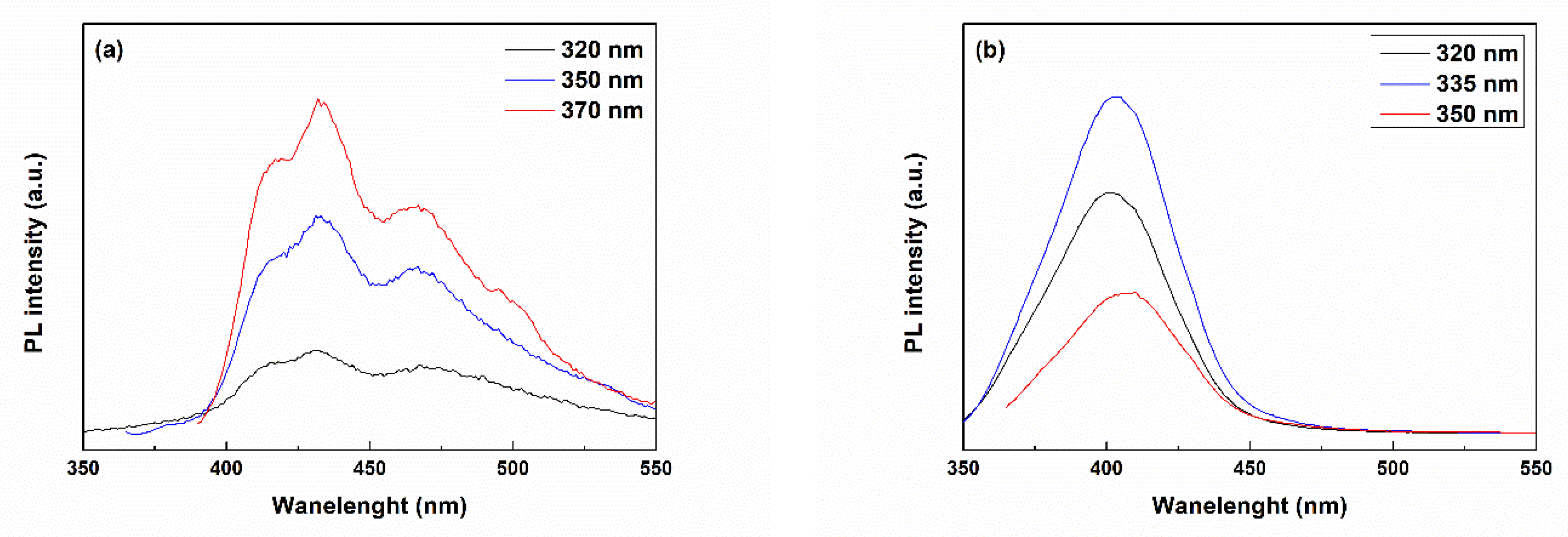
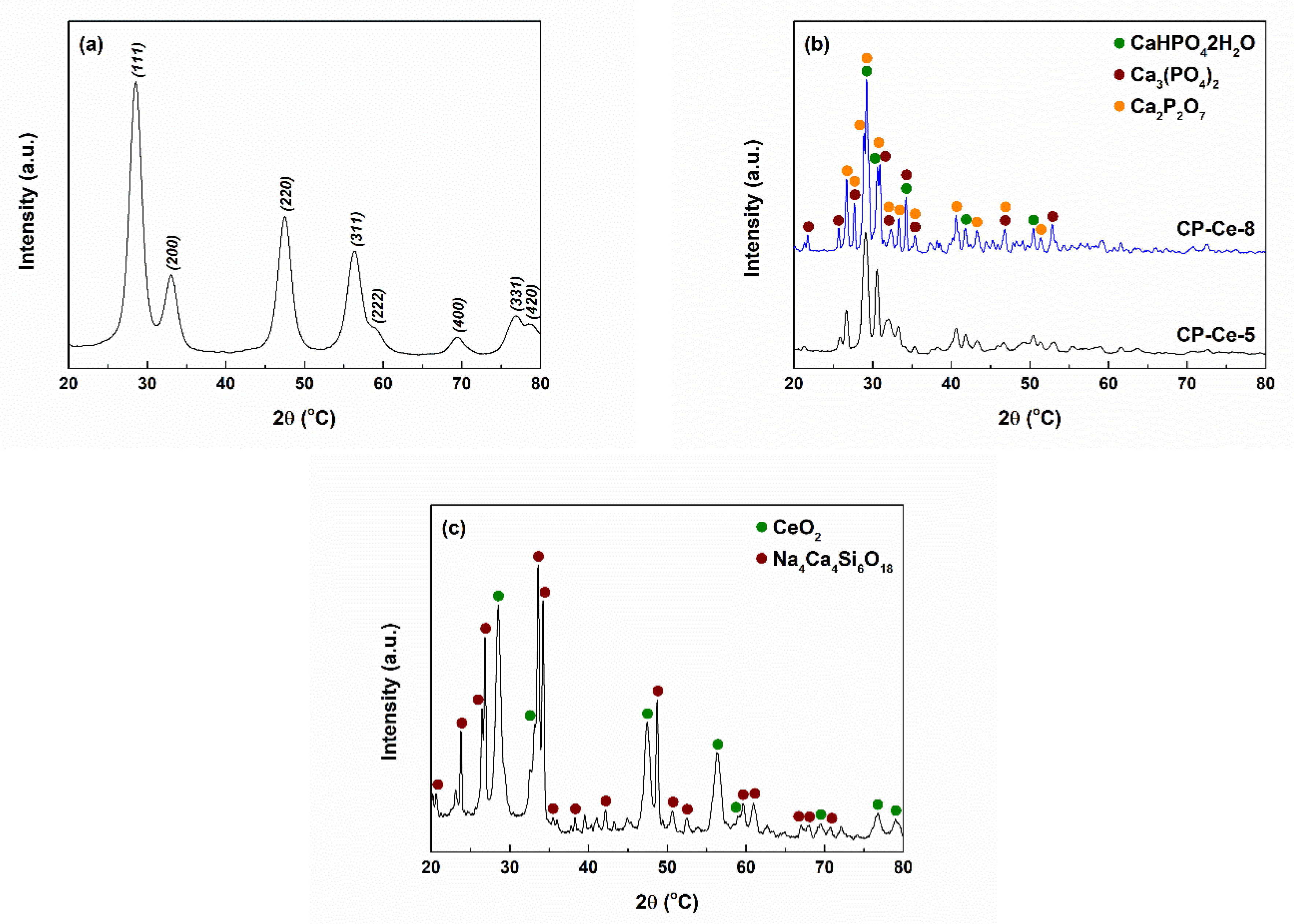
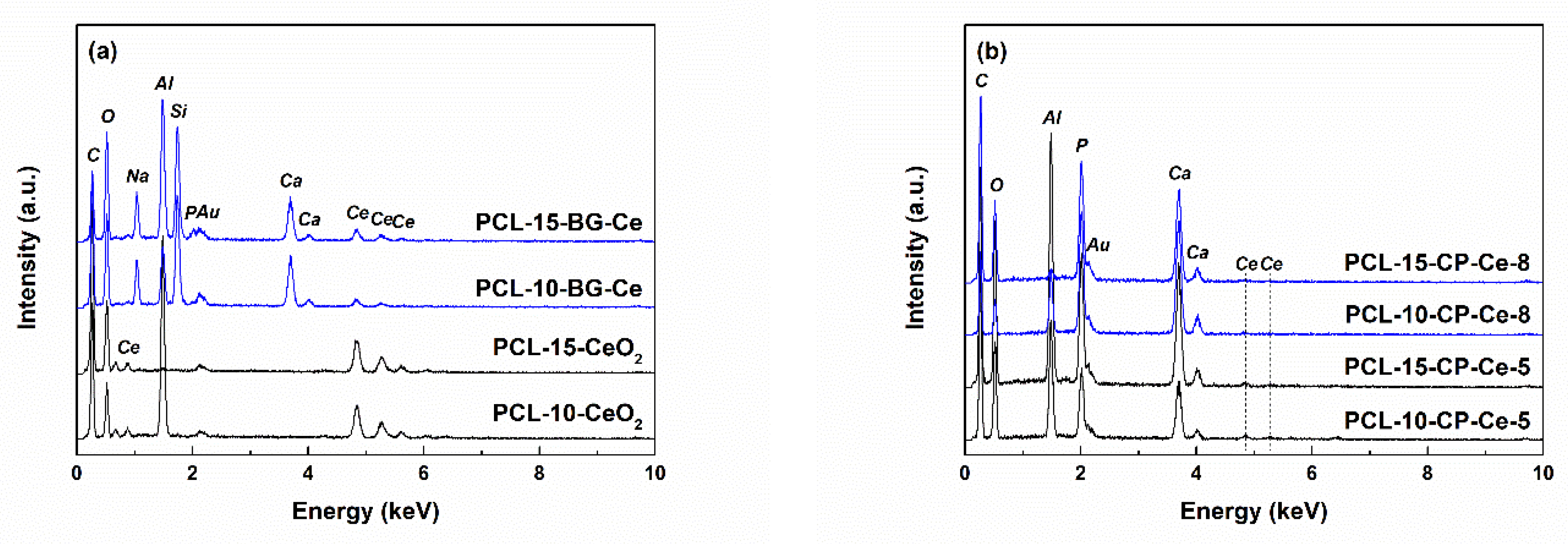
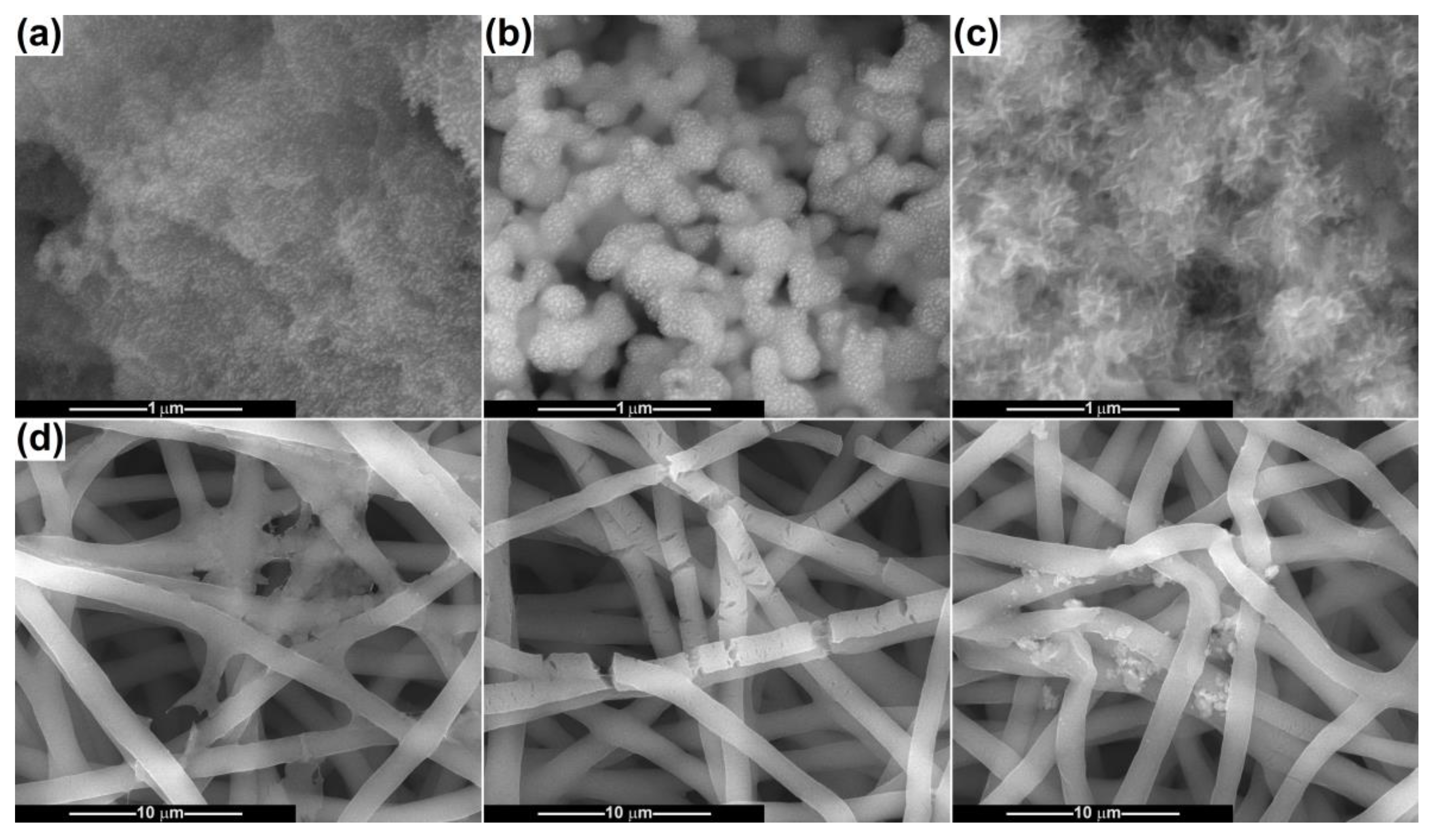
2.1. Characterization of Powders
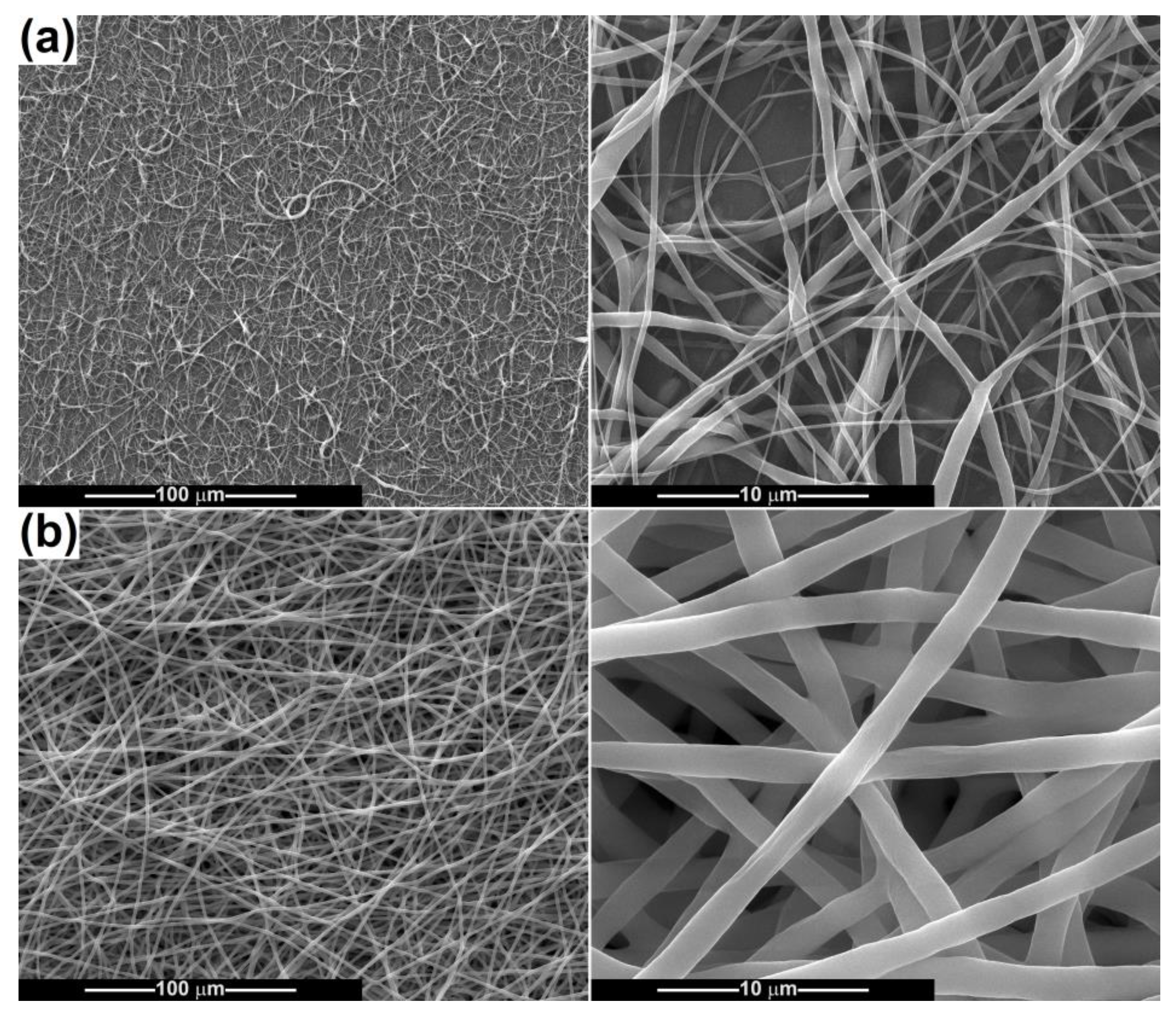
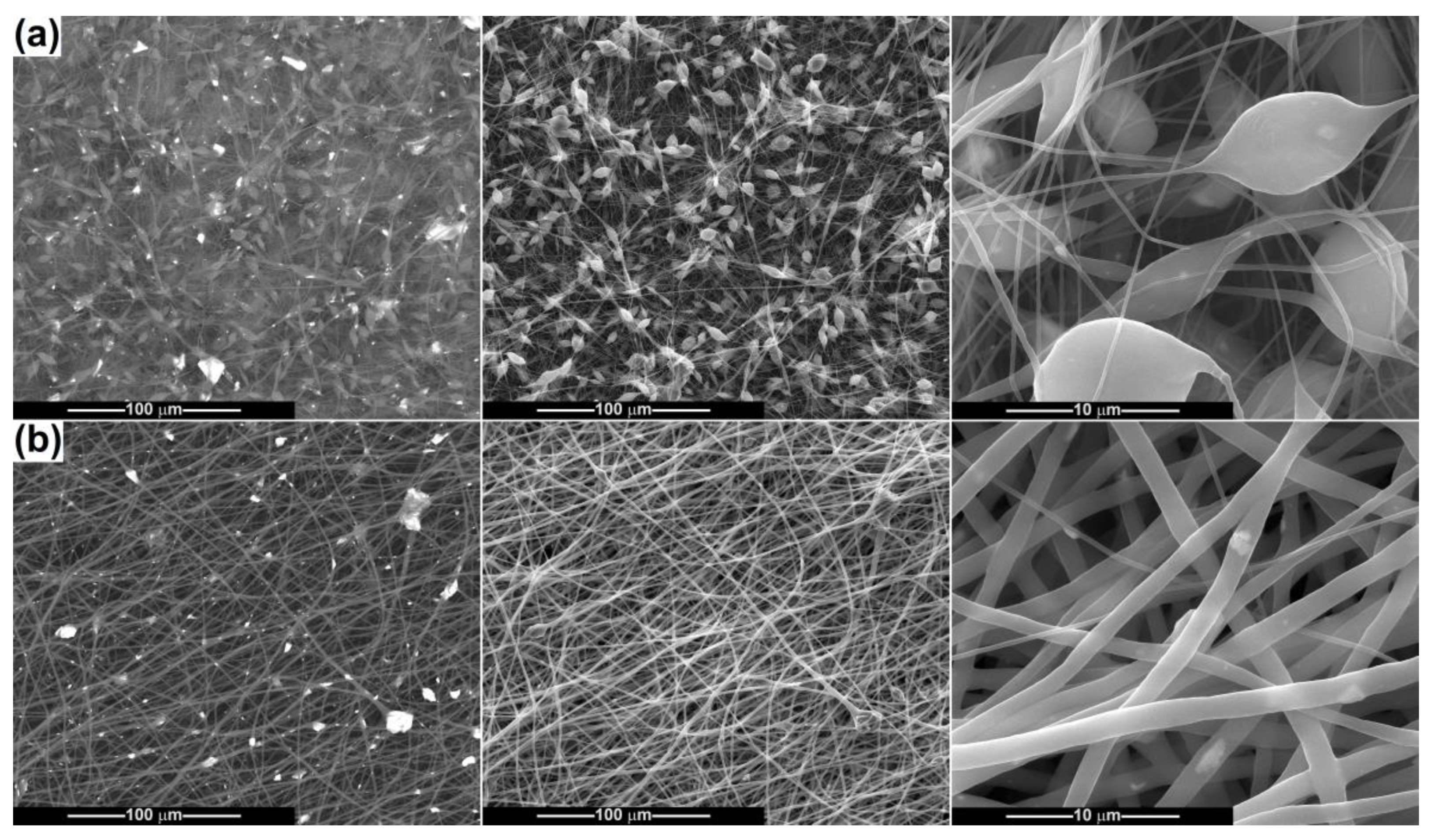
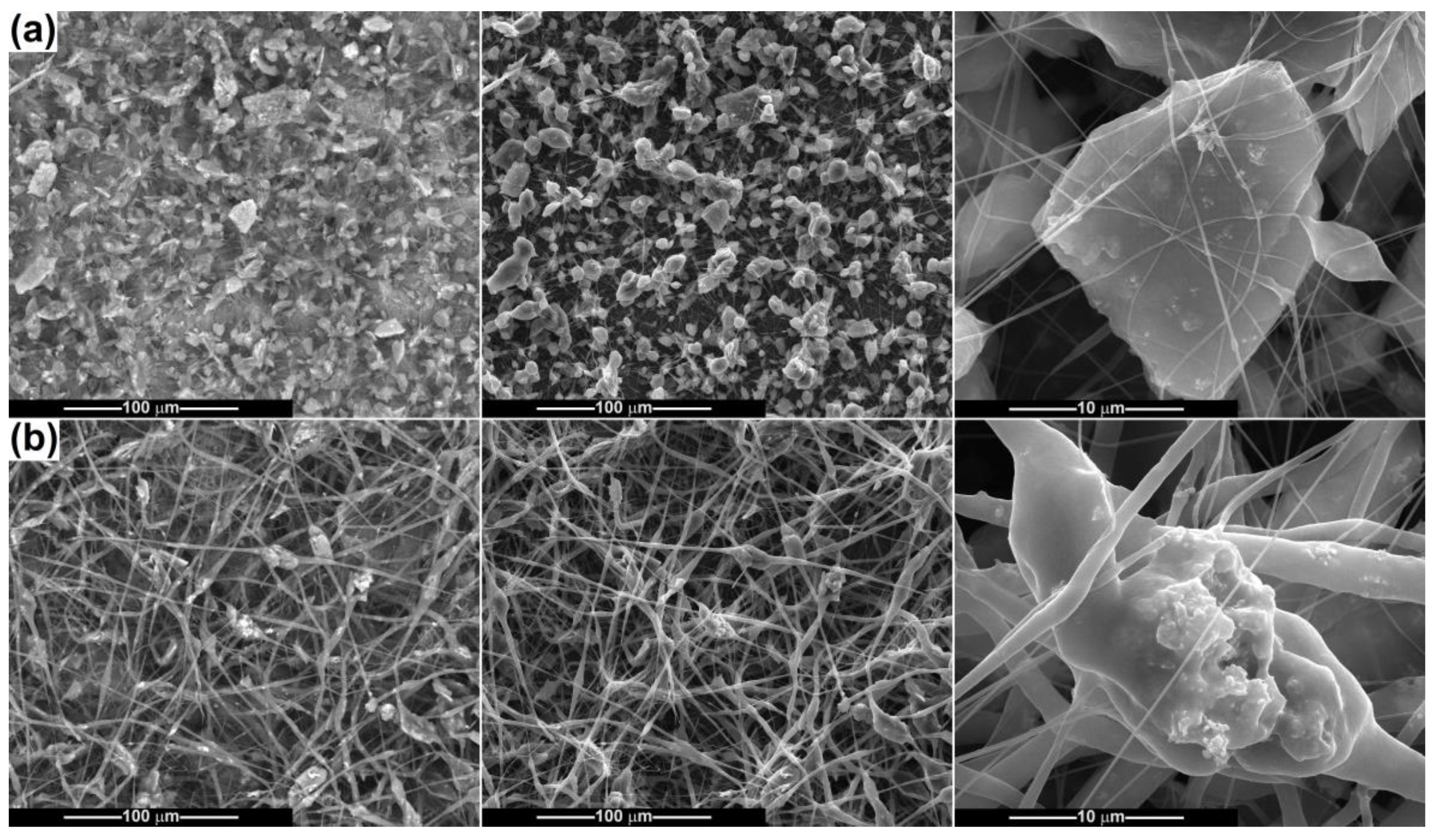
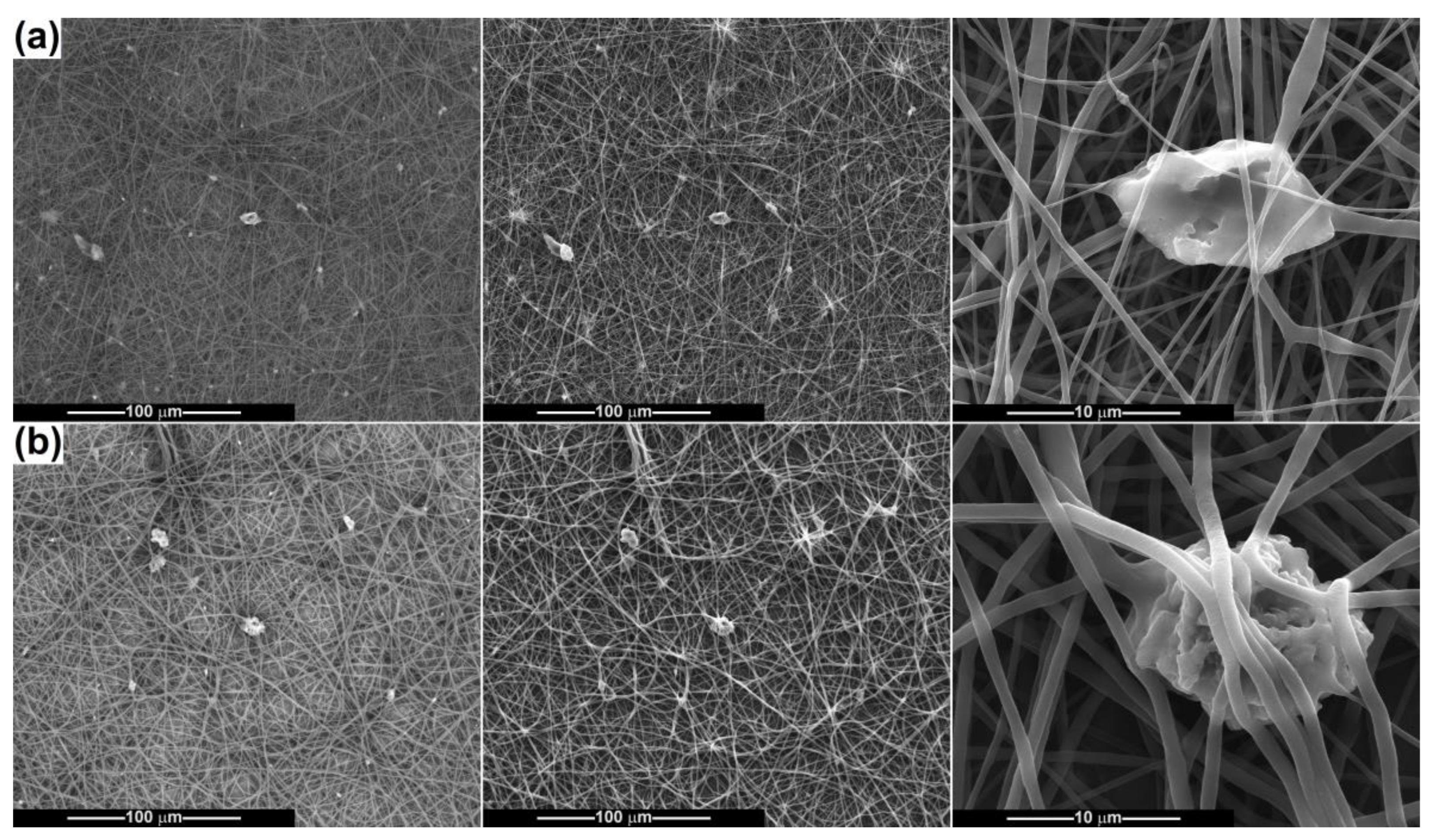
2.2. Scaffold Characterization
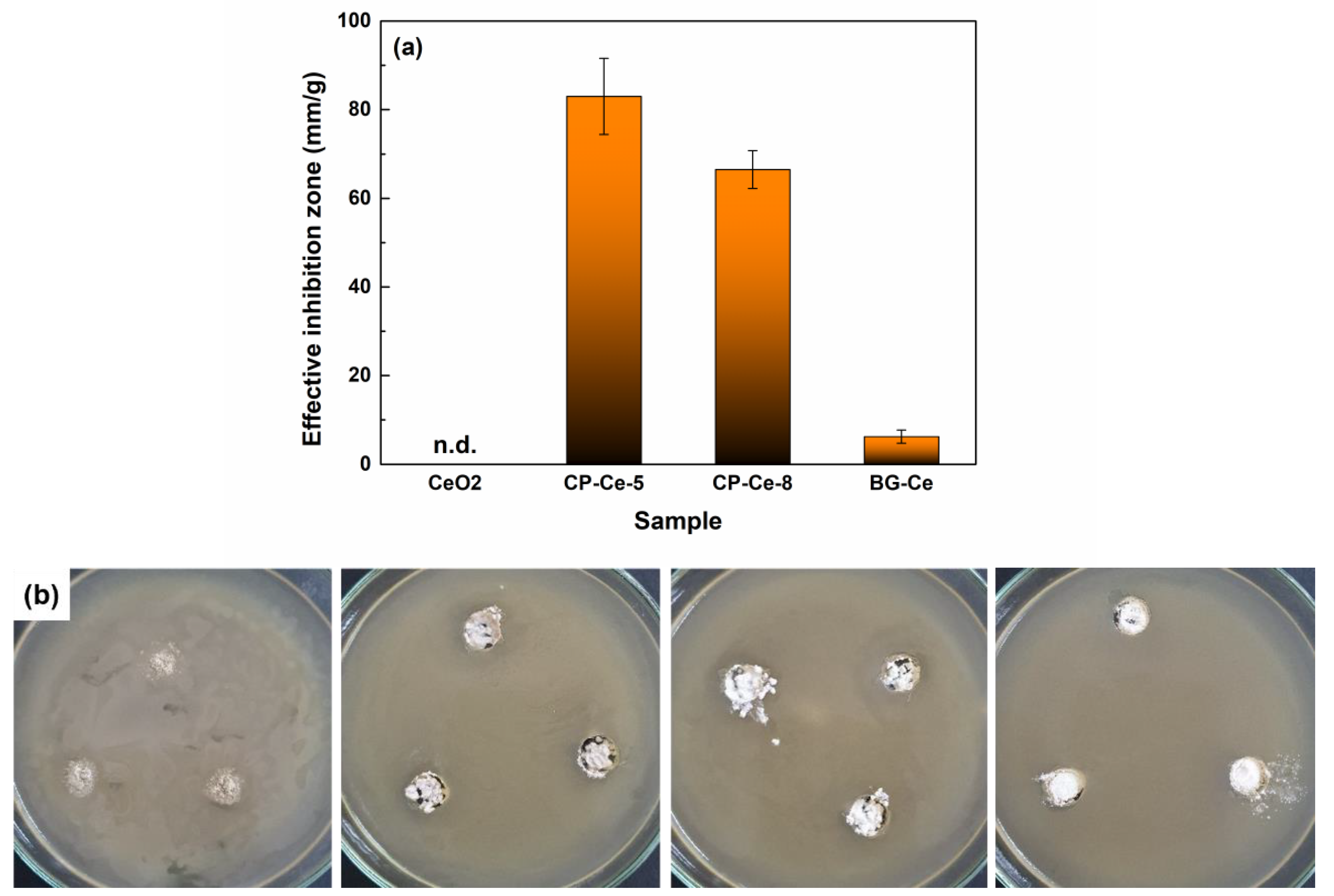
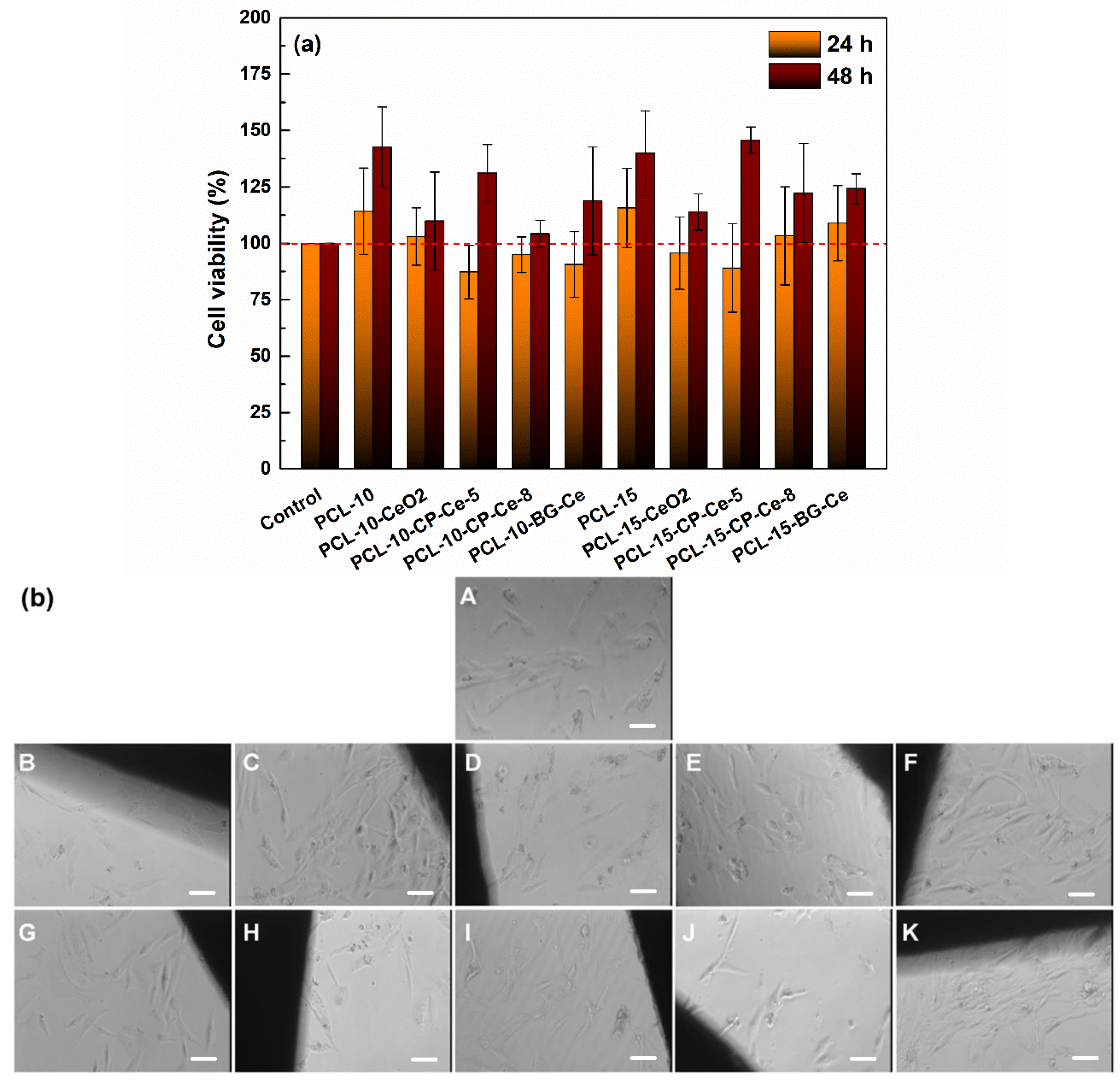
2.3. In Vitro Studies
3. Materials and Methods
3.1. Materials
3.2. Powder Synthesis
3.2.1. CeO2 Synthesis
3.2.2. Calcium Phosphate (CP) Synthesis
3.2.3. Bioglass (BG) Synthesis
3.3. Scaffold Fabrication
3.4. Physicochemical Characterization
3.5. In Vitro Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Elkasabgy, N.A. Biomaterials for tissue engineering applications and current updates in the field: A comprehensive review. AAPS PharmSciTech 2022, 23, 267. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yoo, J.J.; Atala, A. Biomaterials and tissue engineering. In Clinical Regenerative Medicine in Urology; Kim, B.W., Ed.; Springer: Singapore, 2018; pp. 17–51. [Google Scholar]
- Alizadeh-Osgouei, M.; Li, Y.; Wen, C. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact. Mater. 2018, 4, 22–36. [Google Scholar] [CrossRef]
- Forrestal, B.; Case, B.C.; Yerasi, C.; Musallam, A.; Chezar-Azerrad, C.; Waksman, R. Bioresorbable scaffolds: Current technology and future perspectives. Rambam Maimonides Med. J. 2020, 11, e0016. [Google Scholar] [CrossRef]
- Seifert, A.; Gruber, J.; Gbureck, U.; Groll, J. Morphological control of freeze-structured scaffolds by selective temperature and material control in the ice-templating process. Adv. Eng. Mater. 2022, 24, 2100860. [Google Scholar] [CrossRef]
- Pertici, G. Introduction to bioresorbable polymers for biomedical applications. In Bioresorbable Polymers for Biomedical Applications: From Fundamentals to Translational Medicine; Perale, G., Hilborn, J., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 3–29. [Google Scholar]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Abdelaziz, A.G.; Nageh, H.; Abdo, S.M.; Abdalla, M.S.; Amer, A.A.; Abdal-Hay, A.; Barhoum, A. A review of 3D polymeric scaffolds for bone tissue engineering: Principles, fabrication techniques, immunomodulatory roles, and challenges. Bioengineering 2023, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Raina, N.; Pahwa, R.; Khosla, J.K.; Gupta, P.N.; Gupta, M. Polycaprolactone-based materials in wound healing applications. Polym. Bull. 2022, 79, 7041–7063. [Google Scholar] [CrossRef]
- Mozaffari, S.; Seyedabadi, S.; Alemzadeh, E. Anticancer efficiency of doxorubicin and berberine-loaded PCL nanofibers in preventing local breast cancer recurrence. J. Drug Deliv. Sci. Technol. 2022, 67, 102984. [Google Scholar] [CrossRef]
- Liu, H.; Ahlinder, A.; Yassin, M.A.; Finne-Wistrand, A.; Gasser, T.C. Computational and experimental characterization of 3D-printed PCL structures toward the design of soft biological tissue scaffolds. Mater. Des. 2020, 188, 108488. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhou, Y.; Chen, J.; Wan, Q. The application of polycaprolactone in three-dimensional printing scaffolds for bone tissue engineering. Polymers 2021, 13, 2754. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Dias, J.R.; Sousa, A.; Augusto, A.; Bartolo, P.J.; Granja, P.L. Electrospun polycaprolactone (PCL) degradation: An in vitro and in vivo study. Polymers 2022, 14, 3397. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Muthukrishnan, L. An overview on electrospinning and its advancement toward hard and soft tissue engineering applications. Colloid. Polym. Sci. 2022, 300, 875–901. [Google Scholar] [CrossRef] [PubMed]
- Katsogiannis, K.A.G.; Vladisavljevic, G.T.; Georgiadou, S. Porous electrospun polycaprolactone (PCL) fibres by phase separation. Eur. Polym. J. 2015, 69, 284–295. [Google Scholar] [CrossRef]
- Rezaei, F.; Nikiforov, A.; Morent, R.; De Geyter, N. Plasma modification of poly lactic acid solutions to generate high quality electrospun PLA nanofibers. Sci. Rep. 2018, 8, 2241. [Google Scholar] [CrossRef]
- de la Cruz, L.I.S.; Rodriguez, F.J.M.; Velasco-Santos, C.; Martinez-Hernandez, A.; Gutierrez-Sanchez, M. Hydrolytic degradation and morphological characterization of electrospun poly(glycolic acid) [PGA] thin films of different molecular weights containing TiO2 nanoparticles. J. Polym. Res. 2016, 23, 113. [Google Scholar] [CrossRef]
- Hassan, M.I.; Sun, T.; Sultana, N. Fabrication of nanohydroxyapatite/poly(caprolactone) composite microfibers using electrospinning technique for tissue engineering applications. J. Nanomater. 2014, 2014, 209049. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Binulal, N.S.; Deepthy, M.; Jayakumar, R.; Manzoor, K.; Nair, S.V. Preparation, characterization and cell attachment studies of electrospun multi-scale poly(caprolactone) fibrous scaffolds for tissue engineering. J. Macromol. Sci. A 2010, 48, 21–30. [Google Scholar] [CrossRef]
- Natarajan, D.; Ye, Z.; Wang, L.; Ge, L.; Pathak, J.L. Rare earth smart nanomaterials for bone tissue engineering and implantology: Advances, challenges, and prospects. Bioeng. Transl. Med. 2021, 7, e10262. [Google Scholar] [CrossRef]
- Gao, J.; Feng, L.; Chen, B.; Fu, B.; Zhu, M. The role of rare earth elements in bone tissue engineering scaffolds—A review. Compos. B Eng. 2022, 235, 109758. [Google Scholar] [CrossRef]
- Paduraru, A.V.; Musuc, A.M.; Oprea, O.C.; Trusca, R.; Iordache, F.; Vasile, B.S.; Andronescu, E. Synthesis and characterization of photoluminescent Ce(III) and Ce(IV) substituted hydroxyapatite nanomaterials by co-precipitation method: Cytotoxicity and biocompatibility evaluation. Nanomaterials 2021, 11, 1911. [Google Scholar] [CrossRef]
- Wu, C.; Xia, L.; Han, P.; Mao, L.; Wang, J.; Zhai, D.; Fang, B.; Chang, J.; Xiao, Y. Europium-containing mesoporous bioactive glass scaffolds for stimulating in vitro and in vivo osteogenesis. ACS Appl. Mater. Interfaces 2016, 8, 11342–11354. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Smith, C.L.; Feltis, B.N.; Piva, T.J.; Tabatabaie, F.; Harty, P.D.; Gagliardi, F.M.; Platts, K.; Otto, S.; Blencowe, A.; et al. Samarium doped titanium dioxide nanoparticles as theranostic agents in radiation therapy. Phys. Med. 2020, 75, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Fifere, N.; Airinei, A.; Asandulesa, M.; Rotaru, A.; Ursu, E.L.; Doroftei, F. Investigating the vibrational, magnetic and dielectric properties, and antioxidant activity of cerium oxide nanoparticles. Int. J. Mol. Sci. 2022, 23, 13883. [Google Scholar] [CrossRef] [PubMed]
- Qi1, M.; Li, W.; Zheng, X.; Li, X.; Sun, Y.; Wang, Y.; Li, C.; Wang, L. Cerium and its oxidant-based nanomaterials for antibacterial applications: A state-of-the-art review. Front. Mater. 2020, 7, 213. [Google Scholar]
- Zhang, M.; Zhang, C.; Zhai, X.; Luo, F.; Du, Y.; Yan, C. Antibacterial mechanism and activity of cerium oxide nanoparticles. Sci. China Mater. 2019, 62, 1727–1739. [Google Scholar] [CrossRef]
- Pellavio, G.; Sommi, P.; Anselmi-Tamburini, U.; DeMichelis, M.P.; Coniglio, S.; Laforenza, U. Cerium oxide nanoparticles regulate oxidative stress in HeLa cells by increasing the aquaporin-mediated hydrogen peroxide permeability. Int. J. Mol. Sci. 2022, 23, 10837. [Google Scholar] [CrossRef]
- Miri, A.; Darroudi, M.; Sarani, M. Biosynthesis of cerium oxide nanoparticles and its cytotoxicity survey against colon cancer cell line. Appl. Organomet. Chem. 2020, 34, e5308. [Google Scholar] [CrossRef]
- Pop, O.L.; Mesaros, A.; Vodnar, D.C.; Suharoschi, R.; Tabaran, F.; Magerusan, L.; Todor, I.S.; Diaconeasa, Z.; Balint, A.; Ciontea, L.; et al. Cerium oxide nanoparticles and their efficient antibacterial application in vitro against gram-positive and gram-negative pathogens. Nanomaterials 2020, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, K.; Kovarik, T.; Krenek, T.; Docheva, D.; Stich, T.; Pola, J. Recent advances and future perspectives of sol-gel derived porous bioactive glasses: A review. RSC Adv. 2020, 10, 33782–33835. [Google Scholar] [CrossRef]
- Shcherbakov, A.B.; Reukov, V.V.; Yakimansky, A.V.; Krasnopeeva, E.L.; Ivanova, O.S.; Popov, A.L.; Ivanov, V.K. CeO2 nanoparticle-containing polymers for biomedical applications: A review. Polymers 2021, 13, 924. [Google Scholar] [CrossRef] [PubMed]
- Kamphof, R.; Lima, R.N.O.; Schoones, J.W.; Arts, J.J.; Nelissen, R.G.H.H.; Cama, G.; Pijls, B.G.C.W. Antimicrobial activity of ion-substituted calcium phosphates: A systematic review. Heliyon 2023, 9, e16568. [Google Scholar] [CrossRef]
- Janarthanan, G.; Kim, I.G.; Chung, E.J.; Noh, I. Comparative studies on thin polycaprolactone-tricalcium phosphate composite scaffolds and its interaction with mesenchymal stem cells. Biomater. Res. 2019, 23, 1. [Google Scholar] [CrossRef]
- Malavasi, G.; Salvatori, R.; Zambon, A.; Lusvardi, G.; Rigamonti, L.; Chiarini, L.; Anesi, A. Cytocompatibility of potential bioactive cerium-doped glasses based on 45S5. Materials 2019, 12, 594. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, V.; Malavasi, G.; Lusvardi, G.; Zambon, A.; Benedetti, F.; Cerrato, G.; Valeri, S.; Luches, P. Mesoporous bioactive glasses doped with cerium: Investigation over enzymatic-like mimetic activities and bioactivity. Ceram. Int. 2019, 45, 20910–20920. [Google Scholar] [CrossRef]
- Petit, C.; Tulliani, J.M.; Tadier, S.; Meille, S.; Chevalier, J.; Palmero, P. Novel calcium phosphate/PCL graded samples: Design and development in view of biomedical applications. Mater. Sci. Eng. C 2019, 97, 336–346. [Google Scholar] [CrossRef]
- da Fonseca, G.F.; Avelino, S.O.M.; Mello, D.C.R.; do Prado, R.F.; Campos, T.M.B.; de Vasconcellos, L.M.R.; de Sousa Triches, E.; Borges, A.L.S. Scaffolds of PCL combined to bioglass: Synthesis, characterization and biological performance. J. Mater. Sci. Mater. Med. 2020, 31, 41. [Google Scholar] [CrossRef]
- Atkinson, I.; Anghel, E.M.; Petrescu, S.; Seciu, A.M.; Stefan, L.M.; Mocioiu, O.C.; Predoana, L.; Voicescu, M.; Somacescu, S.; Culita, D.; et al. Cerium-containing mesoporous bioactive glasses: Material characterization, in vitro bioactivity, biocompatibility and cytotoxicity evaluation. Micropor. Mesopor. Mater. 2019, 276, 76–88. [Google Scholar] [CrossRef]
- Zheng, K.; Torre, E.; Bari, A.; Taccardi, N.; Cassinelli, C.; Morra, M.; Fiorilli, S.; Vitale-Brovarone, C.; Iviglia, G.; Boccaccini, A.R. Antioxidant mesoporous Ce-doped bioactive glass nanoparticles with anti-inflammatory and pro-osteogenic activities. Mater. Today Bio. 2020, 5, 100041. [Google Scholar] [CrossRef]
- Leu Alexa, R.; Cucuruz, A.; Ghitulica, C.D.; Voicu, G.; Stamat, L.R.; Dinescu, S.; Vlasceanu, G.M.; Stavarache, C.; Ianchis, R.; Iovu, H.; et al. 3D printable composite biomaterials based on GelMA and hydroxyapatite powders doped with cerium ions for bone tissue regeneration. Int. J. Mol. Sci. 2022, 23, 1841. [Google Scholar] [CrossRef]
- Sousa, R.B.; Dametto, A.C.; Sabio, R.M.; de Calvalho, R.A.; Vieira, E.G.; do Amaral Oliveira, A.F.; Ribeiro, L.K.; Barud, H.S.; Silva-Filho, E.C. Cerium-doped calcium phosphates precipitated on bacterial cellulose platform by mineralization. Ceram. Int. 2020, 46, 26985–26990. [Google Scholar] [CrossRef]
- Fernandes, M.S.; Kukulka, E.C.; de Souza, J.R.; Borges, A.L.S.; Campos, T.M.B.; Thim, G.P.; de Vasconcellos, L.M.R. Development and characterization of PCL membranes incorporated with Zn-doped bioactive glass produced by electrospinning for osteogenesis evaluation. J. Polym. Res. 2011, 29, 370. [Google Scholar] [CrossRef]
- Anh, T.T.N.; Tam, L.T.; Thu, V.V.; Le, A.T.; Hung, V.P.; Tam, P.D. Nano-rods structured cerium oxide Platform for cholesterol biosensor. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3886–3893. [Google Scholar] [CrossRef]
- Wang, G.; Mu, Q.; Chen, T.; Wang, Y. Synthesis, characterization and photoluminescence of CeO2 nanoparticles by a facile method at room temperature. J. Alloys Compd. 2010, 493, 202–207. [Google Scholar] [CrossRef]
- Salma, K.; Borodajenko, N.; Plata, A.; Berzina-Cimdina, L.; Stunda, A. Fourier transform infrared spectra of technologically modified calcium phosphates. In Proceedings of the 14th Nordic-Baltic Conference on Biomedical Engineering and Medical Physics, Riga, Latvia, 16–20 June 2008; Katashev, A., Dekhtyar, Y., Spigulis, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 68–71. [Google Scholar]
- Negrea, R.; Busuioc, C.; Constantinoiu, I.; Miu, L.; Enache, C.; Iordache, F.; Jinga, S. Akermanite-based coatings grown by pulsed laser deposition for metallic implants employed in orthopaedics. Surf. Coat. Technol. 2019, 357, 1015–1026. [Google Scholar] [CrossRef]
- Gao, H.; Fang, H.; Wu, Y.; Li, M. Controlled hydrothermal synthesis and optical properties of 3D flower-like CeO2 building with 3D hierarchical porous structure. J. Mater. Sci. Mater. Electron. 2017, 28, 17587–17591. [Google Scholar] [CrossRef]
- Bi, H.; Zhang, L.X.; Xing, Y.; Zhang, P.; Chen, J.J.; Yin, J.; Bie, L.J. Morphology-controlled synthesis of CeO2 nanocrystals and their facet-dependent gas sensing properties. Sens. Actuators B Chem. 2021, 330, 129374. [Google Scholar] [CrossRef]
- Ramjeyanthi, N.; Alagar, M.; Muthuraman, D. Synthesis, structural and optical behavior of cerium oxide nanoparticles by co-precipitation method. Int. J. Sci. Res. Sci. Technol. 2018, 4, 44–51. [Google Scholar]
- Bharggava, R.; Shah, J.; Khan, S.; Kotnala, R.K. Hydroelectric cell based on cerium oxide decorated reduced graphene oxide (CeO2-rG) nanocomposite generates green electricity by room temperature water splitting. Energy Fuels 2020, 34, 13067–13078. [Google Scholar] [CrossRef]
- Atkinson, I.; Seciu-Grama, A.M.; Petrescu, S.; Culita, D.; Mocioiu, O.C.; Voicescu, M.; Mitran, R.A.; Lincu, D.; Prelipcean, A.M.; Craciunescu, O. Cerium-containing mesoporous bioactive glasses (MBGs)-derived scaffolds with drug delivery capability for potential tissue engineering applications. Pharmaceutics 2022, 14, 1169. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Anastasiou, A.; Aslam, Z.; Raif, E.M.; Do, T.; Giannoudis, P.V.; Jha, A. Interrelationships between the structural, spectroscopic, and antibacterial properties of nanoscale (<50 nm) cerium oxides. Sci. Rep. 2021, 11, 20875. [Google Scholar]
- Sahrapeyma, H.; Asefnejad, A.; Azami, M.; Sadroddiny, E. Structural and morphological investigation of polycaprolactone multi-component scaffold containing collagen microsphere/bioglass nanoparticles. Nanomed. Res. J. 2022, 7, 338–349. [Google Scholar]
- Kaur, P.; Singh, K.J.; Yadav, A.K.; Kaur, S.; Kaur, R.; Kaur, S. Growth of bone like hydroxyapatite and cell viability studies on CeO2 doped CaO-P2O5-MgO-SiO2 bioceramics. Mater. Phys. Chem. 2020, 243, 122352. [Google Scholar] [CrossRef]
- Morais, D.S.; Fernandes, S.; Gomes, P.S.; Fernandes, M.H.; Sampaio, P.; Ferraz, M.P.; Santos, J.D.; Lopes, M.A.; Sooraj Hussain, N. Novel cerium doped glass-reinforced hydroxyapatite with antibacterial and osteoconductive properties for bone tissue regeneration. Biomed. Mater. 2015, 10, 055008. [Google Scholar] [CrossRef]
- Varini, E.; Sanchez-Salcedo, S.; Malavasi, G.; Lusvardi, G.; Vallet-Regi, M.; Salinas, A.J. Cerium (III) and (IV) containing mesoporous glasses/alginate beads for bone regeneration: Bioactivity, biocompatibility and reactive oxygen species activity. Mater. Sci. Eng. C 2019, 105, 109971. [Google Scholar] [CrossRef]
- Lusvardi, G.; Sgarbi Stabellini, F.; Salvatori, R. P2O5-free cerium containing glasses: Bioactivity and cytocompatibility evaluation. Materials 2019, 12, 3267. [Google Scholar] [CrossRef]
- Placek, L.M.; Keenan, T.J.; Wren, A.W. Bioactivity of Y2O3 and CeO2 doped SiO2-SrO-Na2O glass-ceramics. J. Biomater. Appl. 2016, 31, 165–180. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]

| No. | Sample Code | Precursor Suspension Composition | |||||
|---|---|---|---|---|---|---|---|
| PCL (g) | CF:DMF 4:1 (mL) | CeO2 (g) | CP-Ce-500 (g) | CP-Ce-800 (g) | BG-Ce (g) | ||
| 1 | PCL-10 | 1.0 | 10 | - | - | - | - |
| 2 | PCL-10-CeO2 | 0.5 | - | - | - | ||
| 3 | PCL-10 CP-Ce-5 | - | 0.5 | - | - | ||
| 4 | PCL-10 CP-Ce-8 | - | - | 0.5 | - | ||
| 5 | PCL-10-BG-Ce | - | - | - | 0.5 | ||
| 6 | PCL-15 | 1.5 | - | - | - | - | |
| 7 | PCL-15-CeO2 | 0.5 | - | - | - | ||
| 8 | PCL-15 CP-Ce-5 | - | 0.5 | - | - | ||
| PCL-15 CP-Ce-8 | - | - | 0.5 | - | |||
| PCL-15-BG-Ce | - | - | - | 0.5 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plocon, C.; Evanghelidis, A.; Enculescu, M.; Isopencu, G.; Oprea, O.; Bacalum, M.; Raileanu, M.; Jinga, S.; Busuioc, C. Development and Characterization of Electrospun Composites Built on Polycaprolactone and Cerium-Containing Phases. Int. J. Mol. Sci. 2023, 24, 14201. https://doi.org/10.3390/ijms241814201
Plocon C, Evanghelidis A, Enculescu M, Isopencu G, Oprea O, Bacalum M, Raileanu M, Jinga S, Busuioc C. Development and Characterization of Electrospun Composites Built on Polycaprolactone and Cerium-Containing Phases. International Journal of Molecular Sciences. 2023; 24(18):14201. https://doi.org/10.3390/ijms241814201
Chicago/Turabian StylePlocon, Cristiana, Alexandru Evanghelidis, Monica Enculescu, Gabriela Isopencu, Ovidiu Oprea, Mihaela Bacalum, Mina Raileanu, Sorin Jinga, and Cristina Busuioc. 2023. "Development and Characterization of Electrospun Composites Built on Polycaprolactone and Cerium-Containing Phases" International Journal of Molecular Sciences 24, no. 18: 14201. https://doi.org/10.3390/ijms241814201
APA StylePlocon, C., Evanghelidis, A., Enculescu, M., Isopencu, G., Oprea, O., Bacalum, M., Raileanu, M., Jinga, S., & Busuioc, C. (2023). Development and Characterization of Electrospun Composites Built on Polycaprolactone and Cerium-Containing Phases. International Journal of Molecular Sciences, 24(18), 14201. https://doi.org/10.3390/ijms241814201








